Abstract
Objectives
Vocal fold granulomas are benign lesions of the larynx commonly caused by gastroesophageal reflux, intubation, and phonotrauma. Current medical therapy includes inhaled corticosteroids to target inflammation that leads to granuloma formation. Particle sizes of commonly prescribed inhalers range over 1–4 μm. The study objective was to use computational fluid dynamics to investigate deposition patterns over a range of particle sizes of inhaled corticosteroids targeting the larynx and vocal fold granulomas.
Study Design
Retrospective, case-specific computational study.
Setting
Tertiary academic center.
Subjects/Methods
A three-dimensional anatomically realistic computational model of a normal adult airway from mouth-to-trachea was constructed from three computed tomography scans. Virtual granulomas of varying sizes and positions along the vocal fold were incorporated into the base model. Assuming steady-state, inspiratory, turbulent airflow at 30 L/min, computational fluid dynamics was used to simulate respiratory transport and deposition of inhaled corticosteroid particles ranging over 1–20 μm.
Results
Laryngeal deposition in the base model peaked for particle sizes 8–10 μm (2.8–3.5%). Ideal sizes ranged over 6–10, 7–13, and 7–14 μm for small, medium, and large granuloma sizes, respectively. Glottic deposition was maximal at 10.8% for 9 μm-sized particles for the large posterior granuloma, 3x the normal model (3.5%).
Conclusion
As the virtual granuloma size increased and the location became more posterior, glottic deposition and ideal particle size generally increased. This preliminary study suggests that inhalers with larger particle sizes, such as fluticasone propionate DPI, may improve laryngeal drug deposition. Most commercially available inhalers have smaller particles than suggested here.
Keywords: Computational fluid dynamics (CFD), inhaled corticosteroids, vocal fold granuloma
Introduction
Vocal fold granulomas are benign epithelial lesions and were first reported in 1928 by Chevalier Jackson as “contact ulcers of the larynx”1,2. Generally accepted etiologies include phonotrauma, gastroesophageal reflux (GERD), intubation, and surgical trauma. Various treatments for vocal fold granulomas include gastric acid suppression, speech therapy, intra-lesional or inhaled steroid therapy, botulinum toxin injections, or some combination of the aforementioned3–6. Recent use of the in-office potassium titanyl phosphate (KTP) laser has also been described7. Surgical excision is typically reserved for patients who fail medical therapy, present with large symptomatic granulomas, or acute airway obstruction5,7.
Vocal fold granulomas can be difficult to treat given their remote location and propensity to recur, particularly following surgical excision. Inhaled corticosteroids are a non-invasive topical approach to target the reactive, inflammatory process behind granuloma formation8. The use of inhaled corticosteroids to treat vocal fold granulomas was first introduced in the literature by Roh and colleagues in 19999. They reported that the use of inhaled budesonide resulted in 95% resolution of intubation induced granulomas9. This was followed by Hillel et al. in 2010 who reported a complete response in 69% of patients treated with the concurrent use of a proton pump inhibitor and inhaled triamcinolone10.
The most commonly prescribed inhaled corticosteroids include fluticasone propionate dry powder inhaler (DPI) (Advair DISKUS, GlaxoSmithKline, Research Triangle Park, NC), budesonide formeterol fumarate dyhydrate hydrofluoroalkane (HFA) (Symbicort, AstraZeneca, London, England), mometasone furote DPI (Asmanex, Merck Sharp & Dohme Corp, Kenilworth, NJ), fluticasone propionate HFA (Flovent, GlaxoSmithKline, Research Triangle Park, NC), beclomethasone dipropionate HFA (QVAR, IVAX Pharmaceuticals, The Netherlands) and ciclesonide HFA (Alvesco, SUNovion Pharmaceuticals, Marlborough, MA), and range in particle size from 1.1 to 4.0 μm in aerodynamic diameter11–15 (Table 1). Particle sizes, in addition to airway diameter and inhalation rates, are known to influence the location of the particle deposition site along the airway16. There is no consensus on optimal qualitative and quantitative aspects of an inhaler in order to target the vocal fold granulomas in the larynx. With increasing clinical use of these medications, there is a need for data assessing differential laryngeal deposition and the ideal particle size for treatment.
Table 1.
Inhaled Corticosteroid Particle Sizes11–15,40,41
Commonly prescribed inhaled corticosteroids and particle sizes.
| Inhaled Corticosteroid | Particle Size MMAD (μm) |
|---|---|
| Ciclesonide HFA (Alvesco) | 1.0 |
| Beclomethasone dipropionate HFA (QVAR) | 1.1 |
| Fluticasone propionate HFA (Flovent HFA) | 2.4 |
| Mometasone furoate DPI (Asmanex) | 3.7 |
| Budesonide formeterol furmarate dihydrate HFA (Symbicort) | 3.7 |
| Fluticasone propionate DPI (Advair DISKUS) | 4.0 |
Advair, GlaxoSmithKline, Research Triangle Park, NC
Symbicort, AstraZeneca, London, England
Asmanex, Merck Sharp & Dohme Corp, Kenilworth, NJ
Flovent, GlaxoSmithKline, Research Triangle Park, NC
QVAR, IVAX Pharmaceuticals, The Netherlands
Alvesco, SUNovion Pharmaceuticals, Marlborough, MA
Numerical simulations using computational fluid dynamics (CFD) methods have been demonstrated to be an effective and reproducible route towards tracking particle deposition in the adult respiratory tract. Prior investigations in extrathoracic airways focused primarily on the entire larynx17,18. Computational particle tracking similar to our proposed study has been used to predict regional particle deposition in a nasal model, with in vitro validation in a replica cast model19. Simulations of inhaled corticosteroid deposition on the vocal folds, and specifically on vocal fold granulomas are yet to be reported.
In the current work, we employed CFD tracking to simulate and quantify inhaled particle deposition on the larynx for a wide range of particle sizes (1–20 μm), including those commercially available (1–4 μm). In addition, we aimed to investigate particle size ranges that may potentially result in maximal glottic deposition in the setting of vocal fold granulomas and how topical deposition may be affected by granuloma sizes and position.
Materials and Methods
3D Model Creation
This study used existing, de-identified patient data and was approved with exempt status by the Institutional Review Board (IRB) at the University of North Carolina at Chapel Hill. Computed tomography (CT) scans from two male subjects, 56 and 67 years old respectively, were retrospectively selected, de-identified, and combined to generate a three-dimensional (3D) reconstruction of a normal adult airway. The first study subject had subglottic stenosis and two CT scans of the neck, one with ideal mouth opening for virtual placement of an inhaler and one with ideal vocal fold positioning for inhalation. The CT scans were imported and edited in Mimics™ 18.0 (Materialize Inc., Plymouth, MI, USA). The two models from the first study subject were seamlessly joined without any alterations in airway diameter. To avoid confounding effects from subglottic stenosis, this reconstruction was joined with an upper tracheal reconstruction from a CT scan of the second study subject with a healthy subglottis and trachea. To match cross-sectional area at the mid-subglottal joining region, the healthy upper tracheal reconstruction was scaled by 70% and 85% in the left-to-right and anterior-to-posterior directions, respectively. The final, hybrid model assured reasonable representation of a normal airway.
A CT-based reconstruction of a hydrofluoroalkane (HFA) inhaler was virtually fitted into the open mouth of the 3D airway which was then exported in stereolithography (STL) format and imported into ICEM-CFD™ 15.0 (ANSYS Inc., Canonsburg, PA, USA), a computer-aided design and meshing software. Using ICEM-CFD™, a glottic deposition area was designated, including the glottis and a small portion of the distal supraglottis, based on anatomic landmarks identified on the CT scans (Fig. 1).
Figure 1.
(A) Side view of airway model. (B) Enlarged view of glottic deposition, dark line at the level of the vocal folds. (C) Cross section of the computational mesh at the vocal folds. (D) Outline of vocal fold cross section used for computing hydraulic diameter.
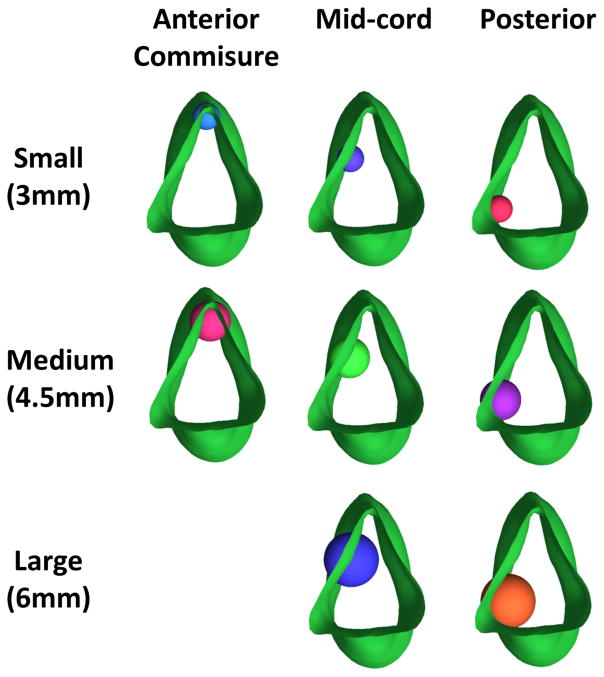
Through the design tools in ICEM-CFD™, virtual granulomas were constructed as semi-spherical protrusions incorporated into the vocal fold area of the normal laryngeal model. Three different granuloma sizes were created: small, medium, and large, with diameters of 3, 4, and 6.5 mm, respectively. We chose the large granuloma to represent 50% of length of the vocal fold of our model, with medium and small granulomas equivalent to 30% and 20% of vocal fold length, respectively.
The granulomas were positioned at three different locations along the length of the membranous vocal fold: the anterior commissure, the mid vocal fold, and the posterior vocal fold (Fig. 2). We chose to investigate granulomas at different lengths along the vocal fold to determine if inhaled corticosteroids have preferential deposition locations there, since such findings would have potential therapeutic and preventative applications. Nine models were constructed and used for further analysis, including the normal “base” model. Note that we omitted a model with the large anterior commissure granuloma, owing to its unrealistic size and the resultant anatomic non-viability for such a growth at that location.
Figure 2.
Top view of glottic area showing the placement of spheres along the vocal folds to represent three granuloma sizes and locations.
For each of the models, using protocols based on mesh refinement studies,20,21 a computational grid of 6 million unstructured tetrahedral elements filling the inhaler-airway reconstruction was created in ICEM-CFD™, with four layers of 0.1 mm graded prism cells at the airway-tissue interfaces. Mesh quality was found sufficient for reliable numerical performance.
Flow and Particle Transport Simulations
Steady-state inspiratory airflow was simulated using the CFD software package Fluent™ (ANSYS Inc. Canonsburg, PA). The airflow rate was set at 30 L/min22–25 under pressure-driven conditions, with air entering through the nose and the inhaler. Particles with density of 1 gm/cm3 and aerodynamic diameters ranging from 1 to 20 μm were released passively from 25,679 locations spread evenly over a planer cross-section at the inhaler outlet and tracked using Fluent’s™ Discrete Phase Model (DPM). Results were not sensitive to increasing numbers of release locations (details to follow in the results section).
Reynolds number (Re) is a critical marker of the airflow. A ratio of the convective inertia of the flow to its viscosity, it is calculated as Re = ρ v Dh /μ, where ρ is the inspired air density (1.204 kg/m3), v is the flow velocity at a cross-section, Dh is the cross-sectional hydraulic diameter, and μ is the dynamic viscosity of air (1.825 × 10−5 kg/m·s). Re of 1900 – 3100 have been observed for time-variable glottal motion during human breathing26. Airflow through a pipe, which is a simplistic idealization for tracheal flow, develops turbulence beyond Re ≈ 270027–29. The average Re for the 9 current models (= 3740) exceeded these thresholds and ranged from 3429 to 4348, suggesting sustained turbulence in inspiratory airflow.
Feasibility of the 3D meshes to run turbulence modeling schemes was checked using y+ a model-specific parameter that detects turbulence possibility close to the wall and ascertains if the mesh is fine enough to resolve such flow scales. It is computed as , where τw is the wall shear stress, Δ is the normal distance from the wall to the mesh element center, and v is the kinematic viscosity of air30. Smaller values (y+ < 5) imply laminar sub-layers in near-wall regions, while y+ >30 suggests fully turbulent layers. In our simulations, y+ averaged 4.35 and confirmed mesh adequacy31 for implementing the shear-stress transport based k-ω model with low Re corrections, using Reynolds-averaged Navier Stokes (RANS) equations. Similar modeling frameworks have been shown to agree with in vivo data for monodisperse aerosol deposition in mouth-throat models.32,33
In this study, the CFD-based inhaled particle transport findings were reported as the percent of the number of particles released of each size (25,679) that deposited in the glottic deposition area (Fig. 1). Based on the numerical results, the “ideal” particle size was computationally determined for the individual granuloma types. This was reported as a range (e.g. 6–10 μm) by dividing the maximum deposition fraction among the 20 particle sizes for each granuloma case into quartiles and including all sizes whose deposition fraction fell within the top quartile (assumed to be within 25% of the maximum deposition fraction).
Results
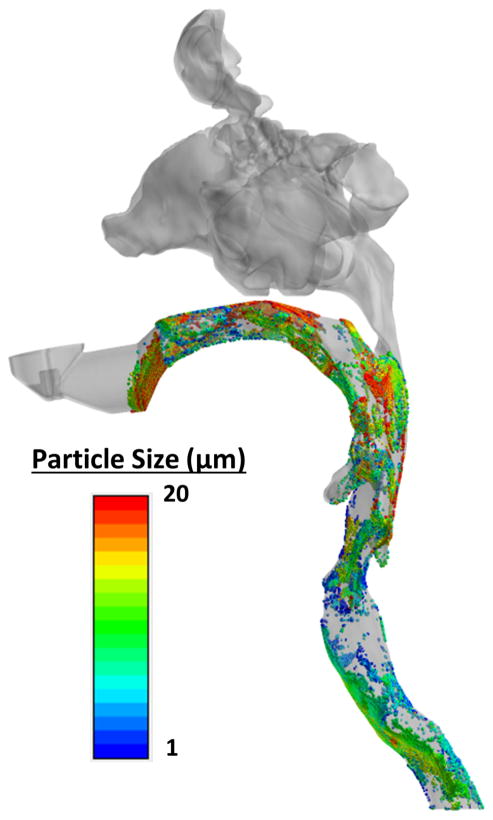
In the normal or “base” model, larger particles (16–20 μm) were predicted to deposit in the mouth, oropharynx, and proximal supraglottis, while smaller particle sizes (1–15 μm) were more dispersed throughout the airway (Fig. 3) or escaped into the trachea and lungs. A maximum glottic deposition of 3.5% was predicted at 9 μm and minimum deposition of 0.3% was predicted at the 18 μm particle size (Fig. 4).
Figure 3.
Inhaled particle deposition predicted in the normal model for particle sizes 1–20 μm.
Figure 4.
Predicted particle deposition fractions in the normal glottis.
The granuloma models revealed greater glottic deposition than the base model at all sizes and positions for all particle sizes except 1 μm, with the exception of the small anterior commissure granuloma. The small anterior granuloma had minimal effect on glottic deposition with a maximal deposition of 3.49% at 7 μm compared to 3.50% at 9 μm for the normal model (Fig. 5). The large, posterior granuloma had the greatest percentage of glottic deposition of 10.8% at 9 μm, 3x that of the normal model (3.5%). The small anterior granuloma had the lowest particle deposition (0.02%) at 18 μm. Within each granuloma size (small, medium, large) the more posteriorly located granulomas had the largest glottic deposition and the anterior granulomas had the smallest particle deposition (Fig. 5).
Figure 5.
Predicted glottic deposition for all locations of small, medium, and large granulomas.
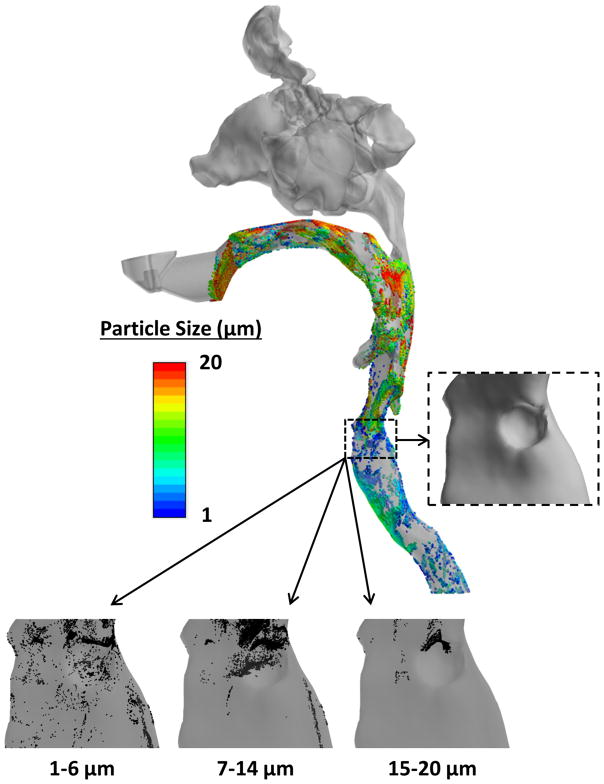
The range of particle sizes that targeted the simulated normal glottis most effectively was 8–10 μm (2.8–3.5%). Ideal particle size was influenced by the presence of a granuloma. As the granulomas grew in size, and their locations moved posteriorly along the vocal fold, the ideal particle size to target the larynx generally increased (Fig. 6). In addition, the span of the particle size range also tended to increase. The large, posterior granuloma had a wider range of ideal sizes (7–14 μm), compared to 8–10 μm for the normal model and 6–10 μm for the small, anterior granuloma (see Fig. 7).
Figure 6.
Ideal particle size range for each granuloma case. From left to right, the granulomas increase in size and more posterior location along the length of the vocal fold. Abbreviations: SAC, small anterior commissure; SMC, small mid cord; SP, small posterior; MAC, medium anterior commissure, MMC, medium mid cord; MP, medium posterior; LMC, large mid cord; LP, large posterior.
Figure 7.
Side view of large posterior granuloma airway model showing representative deposition of particles from 1 to 20 μm. Note: 7–14 μm represents range with highest deposition.
Within the particle size range of the commercially available inhaled corticosteroids (1–4 μm), glottic deposition was largest for the 4 μm particle size amongst all granuloma cases. The large posterior granuloma had the largest glottic deposition of the 4 μm particle size (3.13% vs. 1.69% for the base model).
In order to address potential variability between multiple particle simulations, we performed a sensitivity analysis by varying the total number of particles released from the inhaler outlet. This entailed changing the number of release locations on the inhaler outlet. For three representative particle sizes (2, 4, 8 μm), simulations were run using approximately 5000, 10000, 25000, 50000, and 100000 release locations. The statistical variability in deposition was miniscule. Standard deviations of the percentage of total number of inhaled particles deposited on the supraglottis, glottis, and granuloma ranged between 0.016%–0.75% for 2 μm particles, 0.011%–0.083% for 4 μm particles, and 0.023%–0.287% for 8 μm particles. An anatomy-based investigation of the statistical variability would require consideration of multiple study subjects, which was beyond the scope of the current work.
Discussion
The introduction of inhaled corticosteroids in the treatment of granulomas provides a safe and effective therapeutic option with minimal side effects. Inhaled corticosteroids were designed to treat disease of the terminal bronchi and lungs, such as chronic obstructive pulmonary disease and asthma. In such a setting, laryngeal deposition can be an unwanted side effect and lead to steroid induced laryngitis. In treating vocal fold granulomas, the goal is the opposite, to specifically target the granuloma and glottis. Our study was designed to initiate investigation of the clinical question: which inhaled corticosteroid would be ideal to target the larynx and how this may be affected by the presence of a granuloma?
While preliminary, the results presented here provide an interesting first look at the possibility that certain particle sizes may preferentially target the vocal folds and granulomas. The available prescribed inhaled corticosteroids have particles sizes ranging over 1–4 μm, and are smaller than the particle size that best targeted the glottis in our study. While all particle sizes have some proportion of laryngeal deposition, small particle size is ideal for traversing the larynx to target the terminal bronchi, which has been the goal of the commercially available inhaled corticosteroids. In our computational model, within the 1–4 μm range, the 4 μm size had the largest glottic deposition amongst all our simulated granuloma cases, suggesting that an inhaler with a larger particle size may improve targeting the larynx (i.e. fluticasone propionate DPI Advair DISKUS). This may also help explain the clinical observation that this product anecdotally produces more frequent steroid induced laryngitis than other preparations34.
In our model, particles that targeted the glottis constituted a range of much larger inhaled particle sizes (8–10 μm) than those commercially available. Presence of a granuloma (specifically, larger more posteriorly located granuloma) appeared to increase glottic deposition of inhaled particles. In addition, the larger granulomas had a wider range of predicted ideal particle size (7–14 vs. 8–10 μm), which suggests that prescribing an inhaled corticosteroid for a larger granuloma could be less preferential.
The development of our model necessitated a series of assumptions. During model construction, we assumed that the virtual placement of the inhaler in the model was an adequate approximation of an actual position according to usage instructions, and that our semi-spherical granuloma constructions approximated actual granuloma profiles protruding into the airspace at the vocal fold. We also assumed that our model approximated vocal fold position during inhaler use. The exact position of the vocal folds during inhaler use may be variable, but was assumed to be in an inhaled, slightly abducted position in our model.
Inspiration and expiration clearly have acceleratory and deceleratory components. However, as a simplistic assumption, the steady-state condition can be considered a reliable approximation for the brief single-cycle inspiratory span over which the inhaled particles are tracked in our numerical models. Our preliminary work and other existing findings on time-dependent simulations of sinonasal flows35,36 lend support to the assumption of steady state airflow framework for resting breathing.
The current study has limited clinical application at this stage, as it did not account for individual variations in anatomy, inhalation rates, and airflow, which subsequently may lead to inter-individual fluctuations in the airflow. By passively releasing the particles from the outlet of the inhaler, we assumed that particle velocity owing to the inhaler ejection forces had diminished to zero once the particles reached the outlet, and we further assumed that the particles did not interact with each other, affect inspiratory airflow, or re-enter the airspace after touching an airway wall. Comparison of our results with models constructed from imaging of actual vocal fold granulomas, clinically confirmed vocal fold position during inhaler use, and extension to other individuals and airflow rates are needed for assessment of these effects.
Our simulations represent a computational model of the human airway. The airway is dynamic and although CFD studies using conventional CT-based geometries have been proven to be effective and accurate for predicting airflow and transport, these methods produce static simulations37,38. Miyawaki et al. demonstrated a 7% difference of particle deposition between static vs. dynamic CFD analysis of the adult human airway39. Despite the relatively small variance, the translation of this difference to our model and the clinical setting is yet to be determined.
Conclusions
This pilot study used CFD techniques to predict the ideal inhaled particle size range for targeted laryngeal deposition in the setting of vocal fold granulomas in one subject. The numerical simulations suggested that in general, the presence of a granuloma facilitated localized glottic deposition of the inhaled particles. As the size of the granuloma increased, and with an increasingly posterior location along the vocal fold, glottic deposition and the ideal particle size for targeted delivery generally increased. The most commonly prescribed inhalers release particle sizes (1–4 μm) which are smaller than our model-based predictions on the “ideal” sizes to target the glottis (8–10 μm). The findings, while preliminary, are compelling and warrant further investigations. These include validating our CFD numerical results in an in vitro 3D printed laryngeal model and accounting for the dynamic nature of the human airway with in vivo studies. Eventually, this line of research may lead to the development of novel inhalers designed to produce particles customized for therapeutic laryngeal delivery.
Acknowledgments
Financial support: Research reported in this publication was supported in part by the National Heart, Lung and Blood Institute of the National Centers of Health under award number R01HL122154. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This research effort was supported in part by the National Heart, Lung and Blood Institute of the National Centers of Health under award number R01HL122154. Presented content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors gratefully acknowledge the technical assistance of Nichole Witten.
Abbreviations used
- GERD
gastroesophageal reflux
- KTP
potassium titanyl phosphate
- CFD
computational fluid dynamics
- CT
computed tomography
- MMAD
mass median aerodynamic diameter
- HFA
hydrofluoroalkane
- DPI
dry powder inhaler
- STL
stereolithography
Footnotes
The study was presented at the American Laryngological Association – Combined Otolaryngology Spring Meetings (COSM) in San Diego, CA in April 2017.
Level of evidence: NA
Disclosures
Conflict of Interest: One co-author (JSK) receives partial salary support and funds for supplies and travel from Applied Research Associates, Inc. for a separate, unrelated project. One co-author (RNS) has consulted for Bryan Medical, which has no product involved in this study.
References
- 1.CJ Contact Ulcer of the Larynx. Ann Otol Rhinol Laryngol. 1928;37:227–230. [Google Scholar]
- 2.Jackson C, Jackson CL. Contact ulcer of the larynx. Archives of Otolaryngology. 1935;22(1):1–15. [Google Scholar]
- 3.Fink DS, Achkar J, Franco RA, Song PC. Interarytenoid botulinum toxin injection for recalcitrant vocal process granuloma. Laryngoscope. 2013;123(12):3084–3087. doi: 10.1002/lary.23915. [DOI] [PubMed] [Google Scholar]
- 4.Karkos PD, George M, Van Der Veen J, et al. Vocal process granulomas: a systematic review of treatment. Ann Otol Rhinol Laryngol. 2014;123(5):314–320. doi: 10.1177/0003489414525921. [DOI] [PubMed] [Google Scholar]
- 5.Hong-Gang D, He-Juan J, Chun-Quan Z, Guo-Kang F. Surgery and proton pump inhibitors for treatment of vocal process granulomas. Eur Arch Otorhinolaryngol. 2013;270(11):2921–2926. doi: 10.1007/s00405-013-2527-8. [DOI] [PubMed] [Google Scholar]
- 6.Yilmaz T, Suslu N, Atay G, Ozer S, Gunaydin RO, Bajin MD. Recurrent contact granuloma: experience with excision and botulinum toxin injection. JAMA Otolaryngol Head Neck Surg. 2013;139(6):579–583. doi: 10.1001/jamaoto.2013.3186. [DOI] [PubMed] [Google Scholar]
- 7.Mascarella MA, Young J. In-Office Excision En Masse of a Vocal Process Granuloma Using the Potassium-Titanyl-Phosphate Laser. J Voice. 2016;30(1):93–95. doi: 10.1016/j.jvoice.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 8.McFerran DJ, Abdullah V, Gallimore AP, Pringle MB, Croft CB. Vocal process granulomata. J Laryngol Otol. 1994;108(3):216–220. doi: 10.1017/s0022215100126337. [DOI] [PubMed] [Google Scholar]
- 9.Roh HJ, Goh EK, Chon KM, Wang SG. Topical inhalant steroid (budesonide, Pulmicort nasal) therapy in intubation granuloma. J Laryngol Otol. 1999;113(5):427–432. doi: 10.1017/s0022215100144147. [DOI] [PubMed] [Google Scholar]
- 10.Hillel AT, Lin LM, Samlan R, Starmer H, Leahy K, Flint PW. Inhaled triamcinolone with proton pump inhibitor for treatment of vocal process granulomas: a series of 67 granulomas. Ann Otol Rhinol Laryngol. 2010;119(5):325–330. doi: 10.1177/000348941011900509. [DOI] [PubMed] [Google Scholar]
- 11.Leach C, Colice GL, Luskin A. Particle size of inhaled corticosteroids: does it matter? J Allergy Clin Immunol. 2009;124(6 Suppl):S88–93. doi: 10.1016/j.jaci.2009.09.050. [DOI] [PubMed] [Google Scholar]
- 12.Newman S, Salmon A, Nave R, Drollmann A. High lung deposition of 99mTc-labeled ciclesonide administered via HFA-MDI to patients with asthma. Respir Med. 2006;100(3):375–384. doi: 10.1016/j.rmed.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 13.Yang TT, Li S, Wyka B, Kenyon D. Drug delivery performance of the mometasone furoate dry powder inhaler. J Aerosol Med. 2001;14(4):487–494. doi: 10.1089/08942680152744695. [DOI] [PubMed] [Google Scholar]
- 14.Cripps A, Riebe M, Schulze M, Woodhouse R. Pharmaceutical transition to non-CFC pressurized metered dose inhalers. Respir Med. 2000;94(Suppl B):S3–9. [PubMed] [Google Scholar]
- 15.Leach CL, Davidson PJ, Boudreau RJ. Improved airway targeting with the CFC-free HFA-beclomethasone metered-dose inhaler compared with CFC-beclomethasone. Eur Respir J. 1998;12(6):1346–1353. doi: 10.1183/09031936.98.12061346. [DOI] [PubMed] [Google Scholar]
- 16.Stahlhofen W, Rudolf G, James AC. Intercomparison of Experimental Regional Aerosol Deposition Data. Journal of Aerosol Medicine. 1989;2(3):285–308. [Google Scholar]
- 17.Rahimi-Gorji M, Gorji TB, Gorji-Bandpy M. Details of regional particle deposition and airflow structures in a realistic model of human tracheobronchial airways: two-phase flow simulation. Comput Biol Med. 2016;74:1–17. doi: 10.1016/j.compbiomed.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Ghalati PF, Keshavarzian E, Abouali O, Faramarzi A, Tu J, Shakibafard A. Numerical analysis of micro-and nano-particle deposition in a realistic human upper airway. Computers in Biology and Medicine. 2012;42(1):39–49. doi: 10.1016/j.compbiomed.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Schroeter JD, Tewksbury EW, Wong BA, Kimbell JS. Experimental measurements and computational predictions of regional particle deposition in a sectional nasal model. J Aerosol Med Pulm Drug Deliv. 2015;28(1):20–29. doi: 10.1089/jamp.2013.1084. [DOI] [PubMed] [Google Scholar]
- 20.Frank-Ito DO, Wofford M, Schroeter JD, Kimbell JS. Influence of Mesh Density on Airflow and Particle Deposition in Sinonasal Airway Modeling. J Aerosol Med Pulm Drug Deliv. 2015 doi: 10.1089/jamp.2014.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basu S, Witten N, Kimbell J. Influence of localized mesh refinement on numerical simulations of post-surgical sinonasal airflow. Paper presented at: Journal of aerosol medicine and pulmonary drug delivery; 2017. [Google Scholar]
- 22.Pedersen S, Hansen O, Fuglsang G. Influence of inspiratory flow rate upon the effect of a Turbuhaler. Archives of disease in childhood. 1990;65(3):308–310. doi: 10.1136/adc.65.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basheti IA, Bosnic-Anticevich SZ, Armour CL, Reddel HK. Checklists for dry powder inhaler technique: a review and recommendations. Respiratory care. 2013 doi: 10.4187/respcare.02342. respcare. 02342. [DOI] [PubMed] [Google Scholar]
- 24.Hawksworth G, James L, Chrystyn H. Characterization of the inspiratory manoeuvre when asthmatics inhale through a Turbohaler pre-and post-counselling in a community pharmacy. Respiratory medicine. 2000;94(5):501–504. doi: 10.1053/rmed.1999.0768. [DOI] [PubMed] [Google Scholar]
- 25.Engel T, Heinig J, Madsen F, Nikander K. Peak inspiratory flow and inspiratory vital capacity of patients with asthma measured with and without a new dry-powder inhaler device (Turbuhaler) European Respiratory Journal. 1990;3(9):1037–1041. [PubMed] [Google Scholar]
- 26.Scheinherr A, Bailly L, Boiron O, et al. Realistic glottal motion and airflow rate during human breathing. Medical engineering & physics. 2015;37(9):829–839. doi: 10.1016/j.medengphy.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Darbyshire A, Mullin T. Transition to turbulence in constant-mass-flux pipe flow. Journal of Fluid Mechanics. 1995;289:83–114. [Google Scholar]
- 28.Wygnanski IJCF. On transition in a pipe. Part 1. The origin of puffs and slugs and the flow in a turbulent slug. Journal of Fluid Mechanics. 1973;59(2):281–335. [Google Scholar]
- 29.Draad AAKG, Nieuwstadt FT. Laminar–turbulent transition in pipe flow for Newtonian and non-Newtonian fluids. Journal of Fluid Mechanics. 1998;377:267–312. [Google Scholar]
- 30.Pope SB. Turbulent Flows. New York: Cambridge University Press; 2000. [Google Scholar]
- 31.Walenga RL, Tian G, Hindle M, Yelverton J, Dodson K, Longest PW. Variability in nose-to-lung aerosol delivery. Journal of aerosol science. 2014;78:11–29. doi: 10.1016/j.jaerosci.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longest PW, Tian G, Khajeh-Hosseini-Dalasm N, Hindle M. Validating Whole-Airway CFD Predictions of DPI Aerosol Deposition at Multiple Flow Rates. Journal of aerosol medicine and pulmonary drug delivery. 2016;29(6):461–481. doi: 10.1089/jamp.2015.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matida E, Finlay W, Lange C, Grgic B. Improved numerical simulation of aerosol deposition in an idealized mouth–throat. Journal of aerosol science. 2004;35(1):1–19. [Google Scholar]
- 34.DelGaudio JM. Steroid inhaler laryngitis: dysphonia caused by inhaled fluticasone therapy. Arch Otolaryngol Head Neck Surg. 2002;128(6):677–681. doi: 10.1001/archotol.128.6.677. [DOI] [PubMed] [Google Scholar]
- 35.Bahmanzadeh HAO, Ahmadi G. Unsteady particle tracking of micro-particle deposition in the human nasal cavity under cyclic inspiratory flow. Journal of Aerosol Science. 2016;101:86–103. [Google Scholar]
- 36.Chen XB, Lee HP, Chong VFH, Wang DY. Aerodynamic characteristics inside the rhino-sinonasal cavity after functional endoscopic sinus surgery. American journal of rhinology & allergy. 2011;25(6):388–392. doi: 10.2500/ajra.2011.25.3669. [DOI] [PubMed] [Google Scholar]
- 37.Choi J, Xia G, Tawhai MH, Hoffman EA, Lin CL. Numerical study of high-frequency oscillatory air flow and convective mixing in a CT-based human airway model. Ann Biomed Eng. 2010;38(12):3550–3571. doi: 10.1007/s10439-010-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyawaki S, Tawhai MH, Hoffman EA, Lin CL. Effect of carrier gas properties on aerosol distribution in a CT-based human airway numerical model. Ann Biomed Eng. 2012;40(7):1495–1507. doi: 10.1007/s10439-011-0503-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyawaki S, Hoffman EA, Lin CL. Effect of static vs. dynamic imaging on particle transport in CT-based numerical models of human central airways. J Aerosol Sci. 2016;100:129–139. doi: 10.1016/j.jaerosci.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamin WE, Genz T, Roeder S, et al. Mass output and particle size distribution of glucocorticosteroids emitted from different inhalation devices depending on various inspiratory parameters. J Aerosol Med. 2002;15(1):65–73. doi: 10.1089/08942680252908593. [DOI] [PubMed] [Google Scholar]
- 41.Leach CL, Bethke TD, Boudreau RJ, et al. Two-dimensional and three-dimensional imaging show ciclesonide has high lung deposition and peripheral distribution: a nonrandomized study in healthy volunteers. J Aerosol Med. 2006;19(2):117–126. doi: 10.1089/jam.2006.19.117. [DOI] [PubMed] [Google Scholar]