Abstract
Background
Renal cell carcinoma account for 3% of all cancers, with peak incidence between 60 and 70 years of age predominantly affecting male population. Renal carcinoma is the most common malignancy of kidney constitutes for 80–90% of renal neoplasm with an overall 45% five years survival rate. Majority are diagnosed incidentally during investigation for other disease process of abdomen. Classical triad of gross hematuria, pain and palpable mass in abdomen is rare accounting to only 6–10%. Treatment of early stages of disease i.e. localized disease is partial or radical nephrectomy. Most common metastasis in RCC occurs to lung, followed by bone involvement in 20–35%, lymph nodes, liver, adrenal gland and brain. In metastatic disease median survival rate of patient is about eight months with 50% mortality rate within first year of life, five years survival rate is 10%. Skeletal metastasis are very destructive in patients with renal cell carcinoma compromising bone integrity leading to skeletal related events including pains, impending fractures, nerve compressions, hypercalcemia and even pathological fractures which may require surgical interventions and other therapy. In addition to skeletal complications, presence of bone metastases in RCC has negative impact on progression free survival and overall survival of patients treated with systemic therapies.
Objective
In this review we discuss pathophysiology of tumor metastasis, diagnosis, management and Case examples of metastatic renal cell carcinoma.
Conclusion
Incidence of metastatic renal carcinoma is increasing. Overall prognosis of patient with advanced RCC is poor, emphasizing the importance of early detection and prompt treatment of primary lesion in its early stage. Advancement in targeted therapy in recent decades had made some improvement in treatment of SREs and has helped in improving patent's quality of life but still we are in need of further improvement in treatment modalities to cure disease thereby decreasing morbidity and mortality.
Keywords: Renal cell carcinoma, Nephrectomy, Pathological fracture, Impending fracture, Metastasis
Highlights
-
•
Renal cell carcinoma account for 3% of all cancers.
-
•
It is a very destructive that may compromise bone integrity.
-
•
Most common metastasis in renal cell carcinoma occurs to lung, followed by bone , lymph nodes, liver, adrenal gland and brain.
-
•
Most common metastasis in renal cell carcinoma occurs to lung, followed by bone involvement in 20–35%, lymph nodes, liver, adrenal gland and brain.
-
•
In metastatic disease median survival rate of patient is about eight months with 50% mortality rate within first year of life, five years survival rate is 10.
1. Introduction
Renal cell carcinoma account for 3% of all cancers and commonly occurs in western countries [1]. Its peak incidence found between 60 and 70 years of age and is more common in men than women. Renal carcinoma is the most common malignancy of kidney constitutes for 80–90% of renal neoplasm with an overall 45% five years survival rate. Renal cell carcinoma [RCC] is subdivided into clear cell, papillary and chromophobe but clear cell variety is the most common. Due to increased use of modern diagnostic modalities of choice like ultrasound and CT scan, diagnosis of RCCs has increased in early stages [2] and majority are diagnosed incidentally during investigation for other disease process of abdomen [4]. Classical triad of gross hematuria, pain and palpable mass in abdomen is rare accounting to only 6–10% [3]. Ultrasound and cross sectional imaging like CT scan and MRI are needed to establish diagnosis. Treatment of early stages of disease i.e. localized disease is partial or radical nephrectomy. Recurrent lesion [>10 years] is rare in RCC [4]. The recurrence rate are about 10.5%–21.6% at 15 and 20 years respectively as described by Miyao et al. [5].
Most common metastasis in RCC occurs to lung, followed by bone involvement in 20–35% [6], lymph nodes, liver, adrenal gland and brain. In metastatic disease median survival rate of patient is about eight months [7] with 50% mortality rate within first year of life, five years survival rate is 10% [8].
Skeletal metastasis is very destructive in patients with renal cell carcinoma leading to mainly osteolytic lesions that compromise bone integrity and negatively impact patients outcome. Skeletal involvement in RCC is associated with skeletal related events [SRE] including pains, impending fractures, nerve compressions, hypercalcemia and even pathological fractures which may require surgical interventions and other therapy [9, 10]. Swanson et al. studied 947 patients with renal cell carcinoma and skeletal metastasis was found in 26.7% of patients which mostly involved spine, pelvis and proximal femur [11]. In addition to skeletal complications, presence of bone metastases in RCC has negative impact on progression free survival and overall survival of patients treated with systemic therapies.
Santoni et al. investigated patients with bone metastases from RCC and found patients' age, ECOG performance status, histology, MSKCC prognostic score, presence of concomitant metastasis and time from nephrectomy to bone metastases [TTBM] to be significant factors associated with prognosis [12].
Kume et al. analyzed 94 patients with mRCC to bone and on a multivariate analysis found that sarcomatoid differentiation of RCC, vertebral bone involvement, extra-osseous metastases, alkaline phosphatase >1.5 times normal and C-reactive protein >0.3 mg/dl were significant risk factors that adversely effect overall survival [13].
2. Pathophysiology of tumor metastasis
Skeletal metastatic lesions are divided into three types: Osteolytic, osteoblastic and mixed. Activity of osteoclasts is responsible for osteolytic lesion and their activating mechanism varies according to different types of primary malignancies. Osteoclasts are derived from hematopoietic stem cells [monocyte-macrophage lineage] and mainly they resorb mineralized bone matrix by creating microenvironment and ultimately undergo apoptosis. In normal metabolism, bone micro-environment enhances osteoclast production by forming different molecules like macrophage colony stimulating factors and receptor activator of nuclear factor kB [RANK] and its ligand [RANKL] by stromal cells, osteoblast, activated T-cells, tumor cells and osteoclast precursor cells. Bone metastases develop by occupation of bone erythropoietic system by cancer cells. Interaction of tumor cells and bone micro-environment induces immune cells to release factors that attract and stimulate osteoclasts thereby causing increased bone turnover and destruction [9].
The discovery and characterization of the essential cytokines for osteoclast biology, receptor activator of nuclear factor [NF]-kB ligand [RANKL], its receptor RANK, and its decoy receptor osteoprotegerin [OPG] have led to a concept of bone metabolism. With accumulating evidence of the role of the OPG/RANKL/RANK system in normal skeletal physiology, it became clear that many clinically relevant metabolic bone diseases in humans, including inflammatory bone diseases e.g., rheumatoid arthritis, malignant bone tumors e.g., myeloma or osteolytic metastases and different forms of osteoporosis are related to, or caused by, alterations of the OPG/RANKL/RANK system [9].
3. Bone cells and immune system
A complex system of interaction exists between bone and body immune system at molecular level. This includes RANK, RANKL and natural decoy receptor osteoprotegerin [OPG]. Higher serum ratio of RANKL/OPG promotes osteoclastogensis [14]. Mikami at el [15]. stated that expression of RANKL and RANK is directly related to stage of primary lesion and metastasis to bone and other organ.
4. Bone cells and renal cell carcinoma
The pathogenesis of skeletal metastasis in RCC is same as for breast cancer. A vicious cycle exists between tumor cells and bone. Osteoclast activation due to presence of malignant cells lead to bone destruction with secretion of different bone-derived growth factors and cytokines which facilitate cancer cell proliferation and enhance tumor growth. These include transforming growth factor-beta [TGF-β], fibroblast growth factors [FGF], insulin like growth factors and bone morphogenic protein and many more. These factors not only stimulate the local growth of RCC cells but also circulate and stimulate remote metastatic growth [16]. Tumor cells are responsible for release of prostaglandins, activated vitamin D, tumor necrosis factor [TNF], para-thyroid hormone and its related peptide, these activates osteoblast and stromal cells on bone marrow by interacting through RANKL system and ultimately stimulates osteoclast activity.
5. Osseus metastasis in RCC and role of different molecular mediators
Bone is a source of numerous growth factors, thus enabling survival of metastatic tumor cells. Kominsky et al. [17] studied that RCC bone metastasis cells can be stimulated by transforming growth factor-beta1 [TGF-1] in vivo. This interaction increases tumor growth and bone destruction. They also concluded that inhibition of TGF-1 helpful in treatment of RCC bone metastasis.
Weber et al. [18] demonstrated that growth factor signaling pathway which includes epidermal growth factor receptors [EGF-R] and transforming growth factor beta receptor [TGF-betaR] play a vital role in RCC- activated osteoclast bone resorption and inhibition of this signaling pathway decreases RCC bone metastasis.
Joeckel et al. [19] demonstrated a positive relationship between extracellular calcium concentration and RCC with the help of calcium sensing receptor [CaSR]. Higher expression was found in RCC and calcium treatment leads to increase RCC proliferation.
PTHrP is a polypeptide and is released by normal as well as malignant cells, regulating growth, differentiation and death. Massfelder et al. [20] studied that blocking of PTHrP with antibodies or antagonizing PTHrP receptors increases cell death in RCC. Talon et al. [21] demonstrated blocking PTHrP system can be employed for therapeutic treatment of RCC in clinical setting.
There are many more molecules which involves in regulation of renal cell carcinoma, immune system and skeletal metastasis. These include isuline mRNA binding protein-3 [IMP3][22], caderrin-11 [23], AKT/integrin-5 signaling system [24], MicroRNAs [miRNAs] [25] and matritase [26]. Studies are still needed for better understanding of more signaling pathways to improve prognosis of RCC.
6. Diagnosis of RCC skeletal metastasis
Early diagnosis and prompt treatment reduces long term skeletal complications [6]. Like other malignancies, bone scan is the imaging modality of choice to determine metastatic bone growth for patient with RCC. Plain radiograph shows pure lytic lesion and helpful to identify impending or established fracture.
Bone scan shows osteoblastic activity in the form of hot spots [27]. In osteolytic lesion, compensatory osteoblastic activities increase, hence producing hotspots even in lytic lesion. Therefore early lesions in RCC have difficulty in detection by bone scan. Patient may present with hyper-calcaemia, spinal cord or nerve root compression, pain with impending fracture or pathologic fracture. Non-symptomatic patients produce few positive results. Hence bone scintigraphy is more helpful in symptomatic patients.
Positron emission tomography [PET] and whole body MRI are also used for diagnosis of lesion. It can quantify lesion and is helpful for monitoring during followup. Some studies may describe that PET may replace bone scanning but it is expensive [28].
MRI is the investigation of choice in lesion with cord compression. Studies have proved superiority of MRI over PET in detecting renal bone metastasis besides non-requirement of any labeling agent [radiopharmaceutical Drug].
7. Management
Metastatic lesions are commonly encountered with local pain, spinal cord compression/deficit, fracture and hypercalcaemiaof malignancy. Goal of treatment includes prevention of theses complication, pain palliation and improvement in quality of life [29]. Management of lesion involves adequate history and physical examination and metastatic workup. Multidisciplinary approach should de needed, comprising of orthopedic surgeon, urologist, radiologist, pathologist, radiation oncologist and medical oncologist.
8. Medical treatment
Tumor cells produce activation of osteoclast through RANKL signaling pathways and other molecular mechanism, as described earlier. Medical management is aimed to prevent these signaling pathways and ultimately prevent activation of osteoclast. Two classes of drug are commonly used to suppress osteoclast mediated bone resorption. These include: bisphosphonate and denosumab.
9. Bisphosphonate
Development of bisphosphonate has improved quality of life of patients and widely used treatment of different malignancies including RCC. It inhibits osteoclastic activity thereby decreasing bone resorption. Various clinical trials have been conducted to determine safety and efficacy of the drug [30]. Favorable results were obtained in the form of reduction in skeletal related events [SREs] like pain reduction [31]. Different types of bisphosphonate have been prescribed for SREs prevention including clodronate, pamidronate, ibandronate and zoledronic acid. Zoledronic acid is a potent inhibitor of osteolytic activity and studied in various clinical trials for use in reducing SREs in patients with metastatic lesions from breast [32], castration-resistant prostate cancer [33], lung and RCC [34]. Now Zoledronic acid is widely used for treatment of metastatic bone disease [35] however, compared to bone metastases from other tumors, this drug has been under-utilized for management of metastases in RCC [36].
A study conducted by Broom et al. [37] demonstrated improved results of adding Zoledronic acid to targeted therapy [everolimus and zoledronic acid] in patient with RCC bone metastasis. After 12 weeks of administration, median progression-free survival [PFS] was 7.5 months with everolimus plus zoledronic acid and 5.4 months with everolimus. The median time to first SRE was 9.6 months among patient on combination therapy and 5.2 months on single therapeutic agent [everolimus][38]. They concluded that addition of zoledronic acid to everolimus significantly reduced bone resorption and may prolong tumor control. Zoledronic acid also induces apoptotic cell death of renal cancer cell lines in vitro [36].
Both pre-clinical and clinical data suggests that bisphosphonates have potential direct antitumor effects and prevent tumor progression of RCC. The also improve the outcome i.e. response rate, progression free survival and overall survival of systematic targeted therapy for mRCC [39].
Mckay et al. did a pooled analysis from clinical trials database [largest to-date] and evaluated the impact of bone metastases and bisphosphonate therapy on outcomes in mRCC. The study included 2749 patients treated with different modern agents of targeted therapy i.e. Sunitinib, Sorafenib, Axitinib, Temsirolimus, Temsirolimus + INF-α or INF-α alone. Presence of bone metastases was associated with shorter overall survival in all risk groups compared to those without metastases [13.2 vs 20.2 months] and bisphosphonate therapy did not improve progression free survival or overall survival compared to those who did not receive bisphosphonates [40].
Patient should be counseled about adverse event of therapy. Flu like symptoms is common in acute phase and can be managed with simple analgesia. Renal toxicity can occur and can be managed with dose adjustment and require special consideration in case of RCC [41]. A potentially serious complication is osteonecrosis of jaw which is rare [42] but common in combination therapy [43,44]. It is recommended that patients should have a regular dental examination before and during treatment [45].
10. Denosumab
As discussed previously, RANK-L is a potent mediator for osteoclastic mediated bone destruction and inhibition of this signaling pathway system decreases bone resorption. Denosumab is a human monoclonal antibody that binds to RANKL and inhibits bone resorption in patients with advanced cancers and those with failure of bisphosphonate treatment.
A large phase III trial including 800 patients with >100 patients with bone metastases from RCC demonstrated that denosumab is non-inferior to zoledronic acid with regard to overall survival, disease progression and adverse events [46].
Lipton et al. [47]analyzed 3 randomized phase III trials to evaluate efficacy and safety of denosumab versus zolendronic acid for patients with bone metastasis [48,49]. Denosumab was found to be superior to Zoledronic acid in delaying time to first SRE by a median 8.21 months and reduced the risk of first SRE by 17%. Denosumab did not require monitoring, dose modification, or withholding based on renal functions and it was not associated acute phase reactions. However hypo-calcaemia was more common with denosumab. Rate of osteonecrosis was similar in both drugs.
11. Other drugs
11.1. Systemic therapy for metastatic disease
Several systemic agents are available for treatment for metastatic renal cell carcinoma. Medroxy-progesterone [Progestational agents] have been found to have symptomatic improvement with very little antitumor effect, with evidene of 5–6% overall response rate [50]. RCC are chemo and radio-resistant tumors. Combination of chemotherapy and cytokine has not produced any drastic difference, so the scope of chemotherapy is very narrow in metastatic renal cell carcinoma. Immunotherapy with Interlukin 2 and Interferon-a [IFN-a] has modestre result with 7%–27% response rate. [51], patients with good Motzer criteria, clear cell variety and only lung metastasis has the best response.
11.2. Targeted therapy
Advancements in the molecular biology of RCC has led to the better understanding of biological pathways and their relationship to tumor progression, inhibition of those pathways by targeted therapy can be beneficial in tumor control. These agents include multikinase inhibitors like Sunitinib and Sorafenib, humanized monoclonal antibody i.e. Bevcizumab and mTOR inhibitors like Temsirolimus. Vascular endothelial growth factor [VEGF] with its receptor and mammalian target of rapamycin [mTOR] pathways as relevant therapeutic targets to renal cell carcinoma.
VHL is a tumor suppressor gene that has a pivotal role in pathogenesis of RCC. The product of gene [pVHL] mediates the cellular responses to oxygen deprivation [52].
Under normoxic conditions, pVHL recognizes the hydroxylated hypoxia inducible factor [HIF]and targets it to degradation. Its mutation leads to formation of defective von Hippel-Landau protein and HIF [hypoxia-inducible factor] is not degraded leading to accumulation with the cell. This leads to activation of pro-angiogenic genes with over expression of VEGF, platelet-derived growth factor [PDGF], transforming growth factor alpha [TGF-α] and basic fibroblast growth factor [b-FGF] genes, which in turn promotes tumor angiogenesis, proliferation and metastasis. Activation of the mTOR pathway also leads to HIF production and is associated with angiogenesis. mTOR inhibitors act proximally by decreasing the levels of HIF rather than on pro-angiogenic factors.
12. VEGF inhibiting drugs
12.1. Sunitinib
Sunitinib is an inhibitor of the tyrosine-kinase fraction of the VEGF family of receptors. Motzer et al. in a phase III trial compared sunitinib with interferon alfa and showed objective response rate of 39% vs 8% and progression free survival 11 months vs 5 months. Overall survival data reported 26.4 months versus 21.8 months for Sunitinib-treated vs interferon-treated patients respectively [53]. Sunitinib has emerged as a front-line standard of care in metastatic renal cell carcinoma with a better response rate balanced for toxicity. In a mouse model, Maita et al. showed inhibitory effect of Sunitinib against progression of renal cell cancer bone metastasis [54]. Karaca et al. demonstrated a reduction in para-neoplastic hypercalcemia with Sunitinib therapy for metastatic RCC which may indirectly reflect bone positive effect [55].
Zolnierek et al. analyzed data from 3 randomized trials and two other studies comparing the effect of interferon alpha, sunitinib and sorafenib on occurrence and progression of bone metastases in RCC and found sunitinib to be more effective than other 2 drugs at decreasing the formation and prolongation of time to occurrence of new bone lesions [56]. Xiaolin et al. found that oligometastatic state of bone metastatsis [with less than 5 sites] treated with sunitinib had a favorable outcome for renal cell carcinoma [57].
12.2. Sorafenib
Sorafenib is a small molecule inhibitor of VEGF and related receptors, and also inhibits an intracellular signaling enzyme raf kinase. In a phase III trial, patients with treatment-refractory metastatic renal cell carcinoma noted improvement in progression-free survival of 5.5 months in the sorafenib group versus 2.8 months in the placebo group [58].
12.3. Pazopanib
It is an oral angiogenesis inhibitor targeting VEGF receptor, PDGF receptor, and c-KIT. In a recent prospective randomized trial of pazopanib versus placebo in treatment naive or cytokine-treated mRCC patients, there was a significant improvement in progression-free survival and tumor response, 9.2 months vs 4.2 months [59].
12.4. Bevacizumab
Bevacizumab is a monoclonal antibody that binds and neutralises circulating VEGF protein. Its activity is popularized after large multicentre trials making its mark as a front line therapy in low and intermediate risk groups. In a phase 3 trial Escudier et al. compared bevacizumab plus IFN-α with IFN-a monotherapy. The median overall response rate was 31% versus 13% for IFN-α only. Median progression-free survival increased significantly from 5.4 months with IFN-α to 10.2 months for bevacizumab plus IFN-α [60].
13. mTOR inhibiting drugs
13.1. Temsirolimus
Temsirolimus is an inhibitor of specific mammalian target of rapamycin [mTOR], a molecule implicated in several tumor promoting intracellular signaling pathways.Hudes et al. in a phase III trial, randomized patients with high risk metastatic renal cell carcinoma to receive first-line treatment with temsirolimus or IFN-a monotherapy or temsirolimus + IFN-α. In the temsirolimus group, overall survival was 10.9 mo versus 7.3 mo in the IFN-α group [61].In patients treated with combined temsirolimus plus IFN-a, overall survival was not significantly improved.
13.2. Everolimus
Everolimus is an oral mTOR inhibitor. Motzer et al. in a phase 3 study compared everolimus versus placebo in metastatic renal cell carcinoma, who had failed previous targeted theapies along with best supportive care. The median progression-free survival was 4 months with everolimus versus 1.9 months with placebo [62].
13.3. Effects of targeted agents on bone metastases in RCC
Bone metastases require special consideration regarding lesion measurability according to Response Evaluation Criteria in Solid Tumor [RECIST] criteria [63]. Bone scans and plain x-ray films are not considered adequate imaging technique to measure bone lesions while cross sectional imaging like CT or MRI are considered for evaluation of lesions if soft tissue component meets the definition of measure ability. In many studies, the assessment of bone metastases are subjective rendering comparisons difficult. In many clinical trials using targeted therapy in mRCC, patients with bone-only metastases were excluded as no objective bone endpoints have been measured [39].
13.4. Surgical management
Patient with isolated bony metastasis, radical surgery has long term response. Patient has better survival with absence of pathological fracture, single metastasis and tumor free resection [64].For multiple metastases to bone, orthopedic intervention provides palliation. Surgery in such circumstances is helpful in reducing risk of impending fracture, to treat pathological fracture, emergency spinal decompression and to decrease spinal instability [6].
Metastasis from renal cell are very aggressive, osteolytic lesions with cortical destruction without periosteal reaction. Spine and long bones are exposed to pathological fracture with very little potential for spontaneous union.
14. Angioembolization
Metastases from renal cell carcinoma are very hypervascular in more than 65% cases equally similar to the primary neoplasm in vascularity [65]. Several reports also demonstrated the effectiveness of transcatheter intra-arterial embolization of the hypervascular bone metastases. Pre-operative embolization can contribute to reduction of pain, decrease the blood loss during surgery and transfusion requirements. Chatziioannou et al., in 2000 noted mean blood loss of 535 ± 390 ml in patients with complete embolization as compared to 1247 ± 1047 ml with incomplete embolization, concluding that successful embolization leads to significant reduction of blood loss during surgery [66].
15. Nephrectomy
Cyto-reductive nephrectomy is performed in patients with metastatic renal cell carcinomato decrease the systemic issues including pain, hypercalcemia, hypertension and erythrocytosis. Data from the pre-targeted therapy era showed that cytoreductive nephrectomy had a role in metastatic renal cell carcinoma, increasing life expectancy by approximately 6 months in selected patients only and showed regression of metastatic lesions in 1–2% cases [67]. The role of adjuvant nephrectomy for the purpose of inducing spontaneous regression of metastatic lesions is questionable in the era of targeted therapy.
16. Resection of metastatic foci
A solitary metastasis with renal cell carcinoma is rare and has a reported incidence of less than 5% [68]. Resection of metastatic foci is also a surgical option that can be considered in selected patients. Resection of the primary tumor and solitary metastasis should be considered as this treatment can produce some long term survivors. Surgery for metastatic foci may also be considered for palliation, in presence of large lesions, progressive bony destruction, severe uncontrollable pain, instability or impending fracture.
17. Impending and pathologic fractures
Any skeletal lesion may cause a pathologic fracture. Certain criteria for selecting patients for prophylactic fixation have gradually evolved. Early efforts were based solely on retrospective observations of pathologic fractures in the proximal femur and hip, as this area is related to significant morbidity and mortality.
The first set of combined guidelines for prophylactic fixation of proximal femur were presented in 1986 as: [1] greater than 50% cortical destruction seen on CT, [2] a lytic lesion of the proximal femur > 2.5 cm in diameter and [3] avulsion of the lesser trochanter [69]. While these guidelines were helpful for lytic lesions of the femur, they failed to account for other patterns of mixed or permeative lesions and would not be readily applied to other sites. These guidelines could not account for the lesions suitable for nonsurgical managements and adjuvant therapy [65].
Mirels proposed a scoring system to recommend for or against the prophylactic internal fixation of impending fractures. It is based on four characteristics, site of lesion, nature of lesion, size of lesion and pain [70]. All the features were assigned progressive scores ranging from 1 to 3. Prophylactic fixation is highly indicated for a lesion with an overall score of 9 or greater (Table 1).
Table 1.
Mirel's scoring system [71].
| Score | Site of lesion | Size of lesion | Nature of lesion | Pain |
|---|---|---|---|---|
| 1 | Upper limb | <1/3 of cortex | Blastic | Mild |
| 2 | Lower limb | 1/3-2/3 of cortex | Mixed | Moderate |
| 3 | Trochanteric region | >2/3 of cortex | Lytic | Functional |
With the evolution of treatment modalities the survival of these patients with metastatic disease benefitting from these palliative procedures is improving. Management must be individualized keeping the balance between benefits of surgery and risks associated with operating on a patient with limited life expectancy and suboptimal medical condition. All extremities must be carefully examined before embarking on a course of treatment. Isolated fractures in upper extremities which are non weight bearing can be managed with palliative radiotherapy, casting or bracing. Whereas patients with additional lower-extremity lesions requires greater use of the upper extremities for transfers and crutch or walker-assisted ambulation, which justifies the surgical intervention [71].
The goals of surgical fixation are to relieve pain, improve function, facilitate nursing care and improve psychological well-being. This requires a different approach from that used for non-neoplastic lesions, as bony union almost never occurs without surgery and radiotherapeutic treatment.These patients do not tolerate multiple procedures so immediate fixation must be obtained at the time of surgery. The basic principle of surgical management is internal fixation or prosthetic replacement combined with PMMA. Cementation permits immediate stability and early mobilization and pain reduction. At least 2 months of survival should be expected for surgery to be indicated [72]. The use of local cryotherapy, using liquid nitrogen, at the time of surgery, or polymethyl methacrylate, can also be considered [73]. There is a suggestion that the number of viable tumor cells may be reduced by the heat generated by polymethyl methacrylate, thereby decreasing the chance of local recurrence.
Prostheses can be used in certain circumstances and reconstruction of areas not amenable to internal fixation, including the articular surfaces, the proximal femur, the proximal tibia, and the proximal humerus [74]. These devices can be used in arthroplasty for the hip, knee and shoulder and modular diaphysis replacement systems for segmental long bone defects. Improvement in design of these prosthesis has expanded the horizon of these replacement arthroplasty. Even for low-demand patients, internal fixation of impending and pathological fractures of the femoral head and neck has an unacceptably high risk of failure due to the high stresses across the proximal femur.Peri-trochanteric lesions can be reconstructed using a calcar replacement and proximal femur replacement hip implants to address bone loss and high stresses [75]. Certain principles should be followed when the prosthetic replacement is considered; patient's estimated survival should exceed the time needed for recovery from the operation. The reconstruction should be stable enough to permit full weight-bearing immediately after the procedure and must be durable enough to last the expected lifetime of the patient. Planned reconstruction should address areas of weakened bone that are present at operation, as well as areas that are likely to be weakened subsequently.
18. Case examples
18.1. Case 1
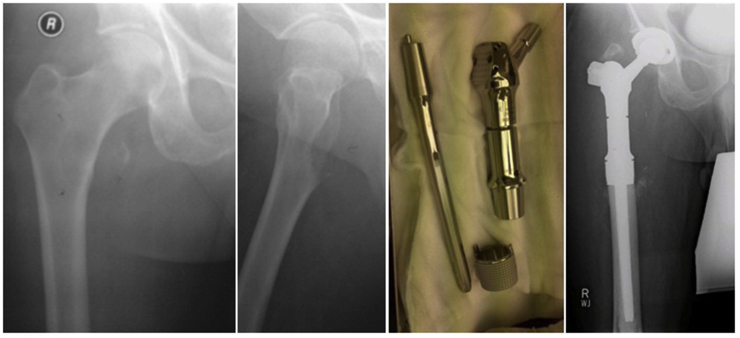
55 year gentleman with history of radical nephrectomy for renal cell carcinoma presented with solitary painful metastasis in right peritrochanteric region managed with wide margin resection and prosthetic reconstruction with proximal femoral replacement hip Arthroplasty Fig. 1.
Fig. 1.
Case No 1.
18.2. Case 2
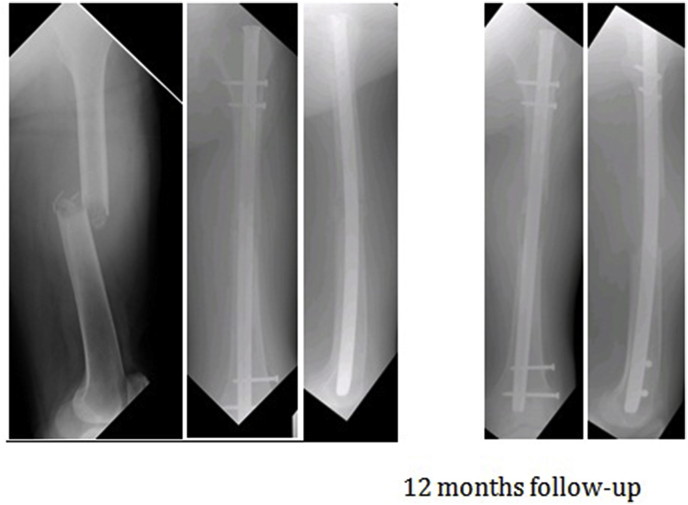
80 year old gentleman known case of RCC, post nephrectomy, underwent intramedullary nailing of left femur as a palliative procedure for pathologic fracture. 12 month follow-up xray demonstrates resorption of bone Fig. 2.
Fig. 2.
Case No 2.
18.3. Case 3
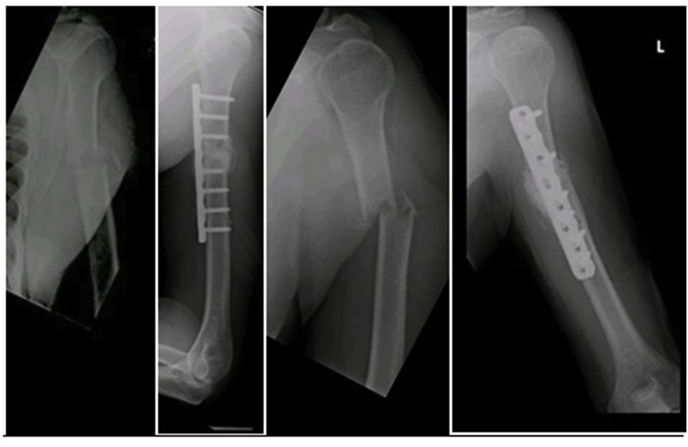
75 year gentleman, known case of metastastatic renal cell carcinoma with deposits to lungs and bone, underwent left nephrectomy 7 years back, sustained pathological proximal humerus fracture managed by internal fixation with poly methyl methacrylate Fig. 3.
Fig. 3.
Case No 3.
19. Radiotherapy[RT]
Major role of radiotherapy is to provide palliative treatment. It is very effective in painful bone metastasis and nerve or spinal cord decompression. Although tumor is considered as a radioresistant but advances in radiotherapy technologies such as intensity modulation RT, stereotactic body RT and stereotactic radiosurgery [SRS], have made this possible to deliver high doses of radiation with local control may exceed 90% for SRS. Thus this provides excellent palliation [76,77]. A study conducted by Reichel et al. [78] revealed radiotherapy has promising effects in short term pain control, preventing fractures and avoiding need for surgery in renal cell carcinoma patients with multiple bone metastasis.
Treatment of inoperable painful metastatic lesions is considered conservative which may employ radiotherapy, chemotherapy and analgesia [79]. Recently minimally invasive techniques are being used to control painful lesions. These techniques may include ethanol [80], laser, cryo-ablation [81], microwave [82] and radiofrequency ablation [83].
20. Conclusion
Incidence of metastatic renal carcinoma is increasing with increasing incidence of primacy lesion. Studies show 85 %of patient with metastatic RCC may experience SREs related complications in their life with a mean no. of >2 events per patient [84]. These events increase economic burden, decrease quality of life and ultimately enhance morbidity and mortality. Overall prognosis of patient with advanced RCC is poor; this emphasizes on importance of early detection and prompt treatment of primary lesion in its early stage. Advancement in targeted therapy in recent decades had made some improvement in treatment of SREs and has helped in improving patent's quality of life but still we are in need of further improvement in treatment modalities to cure disease thereby decreasing morbidity and mortality.
Ethical approval
Not applicable.
Funding
Not applicable.
Author contribution
Masood Umer: study design, writing.
Yasir Mohib: study design, writing.
Muhammed Atif: writing.
Muhammed Nazim: writing.
Conflicts of interest
NO Conflict of interest.
Guarantor
Masood Umer.
Yasir Mohib.
Research registration Unique Identifying number (UIN)
Not applicable.
Trial registry number – ISRCTN
Not applicable.
Contributor Information
Masood Umer, Email: masood.umer@aku.edu.
Yasir Mohib, Email: Yasir.mohib@aku.edu.
Muhammad Nazim, Email: muhammad.nazim@aku.edu.
References
- 1.Ferlay J., Bray F., Pisani P., Parkin D. IARCPress; Lyon: 2004. Cancer Incidence, Mortality and Prevalence Worldwide. IARC Cancer Base No. 5. [Google Scholar]
- 2.Patard J.J., Rodriguez A., Rioux-Leclercq N., Guille F., Lobel B. Prognostic significance of the mode of detection in renal tumours. BJU Int. 2002;90(4):358–363. doi: 10.1046/j.1464-410x.2002.02910.x. [DOI] [PubMed] [Google Scholar]
- 3.Patard J.J., Leray E., Rodriguez A., Rioux-Leclercq N., Guille F., Lobel B. Correlation between symptom graduation, tumor characteristics and survival in renal cell carcinoma. Eur. Urol. 2003;44(2):226–232. doi: 10.1016/s0302-2838(03)00216-1. [DOI] [PubMed] [Google Scholar]
- 4.McNichols D.W., Segura J.W., DeWeerd J.H. Renal cell carcinoma: long-term survival and late recurrence. J. Urol. 1981;126(1):17–23. doi: 10.1016/s0022-5347(17)54359-1. [DOI] [PubMed] [Google Scholar]
- 5.Miyao N., Naito S., Ozono S., Shinohara N., Masumori N., Igarashi T. Late recurrence of renal cell carcinoma: retrospective and collaborative study of the Japanese Society of Renal Cancer. Urology. 2011;77(2):379–384. doi: 10.1016/j.urology.2010.07.462. [DOI] [PubMed] [Google Scholar]
- 6.Kozlowski J.M. Management of distant solitary recurrence in the patient with renal cancer. Contralateral kidney and other sites. The Urol. Clin. North Am. 1994;21(4):601–624. [PubMed] [Google Scholar]
- 7.Rini B.I. Stabilization of disease in patients with metastatic renal cell carcinoma using sorafenib. Nat. Clin. Pract. Oncol. 2006;3(11):602–603. doi: 10.1038/ncponc0634. [DOI] [PubMed] [Google Scholar]
- 8.Motzer R.J., Bacik J., Mazumdar M. Prognostic factors for survival of patients with stage IV renal cell carcinoma. Clin. Canc. Res. 2004;10(18) doi: 10.1158/1078-0432.CCR-040031. 6302S–3S. [DOI] [PubMed] [Google Scholar]
- 9.Beuselinck B., Oudard S., Rixe O., Wolter P., Blesius A., Ayllon J. Negative impact of bone metastasis on outcome in clear-cell renal cell carcinoma treated with sunitinib. Ann. Oncol. 2010;22(4):794–800. doi: 10.1093/annonc/mdq554. [DOI] [PubMed] [Google Scholar]
- 10.Patil S., Figlin R., Hutson T., Michaelson M., Négrier S., Kim S. Prognostic factors for progression-free and overall survival with sunitinib targeted therapy and with cytokine as first-line therapy in patients with metastatic renal cell carcinoma. Ann. Oncol. 2010;22(2):295–300. doi: 10.1093/annonc/mdq342. [DOI] [PubMed] [Google Scholar]
- 11.Swanson D.A., Orovan W.L., Johnson D.E., Giacco G. Osseous metastases secondary to renal cell carcinoma. Urology. 1981;18(6):556–561. doi: 10.1016/0090-4295(81)90455-6. [DOI] [PubMed] [Google Scholar]
- 12.Santoni M., Conti A., Procopio G., Porta C., Ibrahim T., Barni S. Bone metastases in patients with metastatic renal cell carcinoma: are they always associated with poor prognosis? J. Exp. Clin. Canc. Res. 2015;34(1):10. doi: 10.1186/s13046-015-0122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kume H., Kakutani S., Yamada Y., Shinohara M., Tominaga T., Suzuki M. Prognostic factors for renal cell carcinoma with bone metastasis: who are the long-term survivors? J. Urol. 2011;185(5):1611–1614. doi: 10.1016/j.juro.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 14.Hofbauer L.C., Khosla S., Dunstan C.R., Lacey D.L., Boyle W.J., Riggs B.L. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2000;15(1):2–12. doi: 10.1359/jbmr.2000.15.1.2. [DOI] [PubMed] [Google Scholar]
- 15.Mikami S., Katsube K., Oya M., Ishida M., Kosaka T., Mizuno R. Increased RANKL expression is related to tumour migration and metastasis of renal cell carcinomas. J. Pathol. 2009;218(4):530–539. doi: 10.1002/path.2567. [DOI] [PubMed] [Google Scholar]
- 16.Hauschka P.V., Mavrakos A.E., Iafrati M.D., Doleman S.E., Klagsbrun M. Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-Sepharose. J. Biol. Chem. 1986;261(27):12665–12674. [PubMed] [Google Scholar]
- 17.Kominsky S.L., Doucet M., Brady K., Weber K.L. TGF-beta promotes the establishment of renal cell carcinoma bone metastasis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2007;22(1):37–44. doi: 10.1359/jbmr.061005. [DOI] [PubMed] [Google Scholar]
- 18.Weber K., Doucet M., Kominsky S. Renal cell carcinoma bone metastasis–elucidating the molecular targets. Canc. Metastasis Rev. 2007;26(3–4):691–704. doi: 10.1007/s10555-007-9090-y. [DOI] [PubMed] [Google Scholar]
- 19.Joeckel E., Haber T., Prawitt D., Junker K., Hampel C., Thuroff J.W. High calcium concentration in bones promotes bone metastasis in renal cell carcinomas expressing calcium-sensing receptor. Mol. Canc. 2014;13:42. doi: 10.1186/1476-4598-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massfelder T., Lang H., Schordan E., Lindner V., Rothhut S., Welsch S. Parathyroid hormone-related protein is an essential growth factor for human clear cell renal carcinoma and a target for the von Hippel-Lindau tumor suppressor gene. Canc. Res. 2004;64(1):180–188. doi: 10.1158/0008-5472.can-03-1968. [DOI] [PubMed] [Google Scholar]
- 21.Talon I., Lindner V., Sourbier C., Schordan E., Rothhut S., Barthelmebs M. Antitumor effect of parathyroid hormone-related protein neutralizing antibody in human renal cell carcinoma in vitro and in vivo. Carcinogenesis. 2006;27(1):73–83. doi: 10.1093/carcin/bgi203. [DOI] [PubMed] [Google Scholar]
- 22.Xie C., Li Y., Li Q., Chen Y., Yao J., Yin G. Increased insulin mRNA binding Protein-3 expression correlates with vascular enhancement of renal cell carcinoma by intravenous contrast-CT and is associated with bone metastasis. J. Bone Oncol. 2015;4(3):69–76. doi: 10.1016/j.jbo.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satcher R.L., Pan T., Cheng C.J., Lee Y.C., Lin S.C., Yu G. Cadherin-11 in renal cell carcinoma bone metastasis. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0089880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haber T., Jockel E., Roos F.C., Junker K., Prawitt D., Hampel C. Bone metastasis in renal cell carcinoma is preprogrammed in the primary tumor and caused by AKT and integrin alpha5 signaling. J. Urol. 2015;194(2):539–546. doi: 10.1016/j.juro.2015.01.079. [DOI] [PubMed] [Google Scholar]
- 25.Wotschofsky Z., Liep J., Meyer H.A., Jung M., Wagner I., Disch A.C. Identification of metastamirs as metastasis-associated microRNAs in clear cell renal cell carcinomas. Int. J. Biol. Sci. 2012;8(10):1363–1374. doi: 10.7150/ijbs.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukai S., Yorita K., Kawagoe Y., Katayama Y., Nakahara K., Kamibeppu T. Matriptase and MET are prominently expressed at the site of bone metastasis in renal cell carcinoma: immunohistochemical analysis. Hum. Cell. 2015;28(1):44–50. doi: 10.1007/s13577-014-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sohaib S., Cook G., Allen S., Hughes M., Eisen T., Gore M. Comparison of whole-body MRI and bone scintigraphy in the detection of bone metastases in renal cancer. British J. Radiol. 2014 doi: 10.1259/bjr/52773262. [DOI] [PubMed] [Google Scholar]
- 28.Wood S.L., Brown J.E. Skeletal metastasis in renal cell carcinoma: current and future management options. Canc. Treat Rev. 2012;38(4):284–291. doi: 10.1016/j.ctrv.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Zekri J., Ahmed N., Coleman R.E., Hancock B.W. The skeletal metastatic complications of renal cell carcinoma. Int. J. Oncol. 2001;19(2):379–382. doi: 10.3892/ijo.19.2.379. [DOI] [PubMed] [Google Scholar]
- 30.Dhillon S., Lyseng-Williamson K.A. Zoledronic acid : a review of its use in the management of bone metastases of malignancy. Drugs. 2008;68(4):507–534. doi: 10.2165/00003495-200868040-00010. [DOI] [PubMed] [Google Scholar]
- 31.Rosen L.S., Gordon D., Tchekmedyian S., Yanagihara R., Hirsh V., Krzakowski M. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial–the zoledronic acid lung cancer and other solid tumors study group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003;21(16):3150–3157. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 32.Kohno N., Aogi K., Minami H., Nakamura S., Asaga T., Iino Y. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J. Clin. Oncol. 2005;23(15):3314–3321. doi: 10.1200/JCO.2005.05.116. [DOI] [PubMed] [Google Scholar]
- 33.Saad F., Gleason D.M., Murray R., Tchekmedyian S., Venner P., Lacombe L. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J. Nat. Cancer Inst. 2004;96(11):879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 34.Rosen L.S., Gordon D., Tchekmedyian N.S., Yanagihara R., Hirsh V., Krzakowski M. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors. Cancer. 2004;100(12):2613–2621. doi: 10.1002/cncr.20308. [DOI] [PubMed] [Google Scholar]
- 35.Costa L., Major P.P. Effect of bisphosphonates on pain and quality of life in patients with bone metastases. Nat. Clin. Pract. Oncol. 2009;6(3):163–174. doi: 10.1038/ncponc1323. [DOI] [PubMed] [Google Scholar]
- 36.Ullén A., Schwarz S., Lennartsson L., Kälkner K.-M., Sandström P., Costa F. Zoledronic acid induces caspase-dependent apoptosis in renal cancer cell lines. Scand. J. Urol. Nephrol. 2009;43(2):98–103. doi: 10.1080/00365590802475904. [DOI] [PubMed] [Google Scholar]
- 37.Broom R.J., Hinder V., Sharples K., Proctor J., Duffey S., Pollard S. Everolimus and zoledronic acid in patients with renal cell carcinoma with bone metastases: a randomized first-line phase II trial. Clin. Genitourin. Canc. 2015;13(1):50–58. doi: 10.1016/j.clgc.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Alcaraz A., Gonzalez-Lopez R., Morote J., de la Piedra C., Meseguer C., Esteban E. Biochemical markers of bone turnover and clinical outcome in patients with renal cell and bladder carcinoma with bone metastases following treatment with zoledronic acid: the TUGAMO study. British J. cancer. 2013;109(1):121–130. doi: 10.1038/bjc.2013.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keizman D., Ish-Shalom M., Maimon N., Gottfried M. Are bisphosphonates an indispensable tool in the era of targeted therapy for renal cell carcinoma and bone metastases? World J. Urol. 2014;32(1):39–45. doi: 10.1007/s00345-013-1059-6. [DOI] [PubMed] [Google Scholar]
- 40.McKay R.R., Lin X., Perkins J.J., Heng D.Y., Simantov R., Choueiri T.K. Prognostic significance of bone metastases and bisphosphonate therapy in patients with renal cell carcinoma. Eur. Urol. 2014;66(3):502–509. doi: 10.1016/j.eururo.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munns C.F., Rajab M.H., Hong J., Briody J., Hogler W., McQuade M. Acute phase response and mineral status following low dose intravenous zoledronic acid in children. Bone. 2007;41(3):366–370. doi: 10.1016/j.bone.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Ayllon J., Launay-Vacher V., Medioni J., Cros C., Spano J.P., Oudard S. Osteonecrosis of the jaw under bisphosphonate and antiangiogenic therapies: cumulative toxicity profile? Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2009;20(3):600–601. doi: 10.1093/annonc/mdn788. [DOI] [PubMed] [Google Scholar]
- 43.Hasegawa Y., Mita K., Matsubara A., Ohdan H. Multidisciplinary treatment including sorafenib stabilized the bone metastases of renal cell carcinoma in an immunosuppressed renal transplant recipient. Int. J. Clin. Oncol. 2009;14(5):465–467. doi: 10.1007/s10147-008-0868-x. [DOI] [PubMed] [Google Scholar]
- 44.Brunello A., Saia G., Bedogni A., Scaglione D., Basso U. Worsening of osteonecrosis of the jaw during treatment with sunitinib in a patient with metastatic renal cell carcinoma. Bone. 2009;44(1):173–175. doi: 10.1016/j.bone.2008.08.132. [DOI] [PubMed] [Google Scholar]
- 45.Weitzman R., Sauter N., Eriksen E.F., Tarassoff P.G., Lacerna L.V., Dias R. Critical review: updated recommendations for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in cancer patients—may 2006. Crit. Rev. Oncol. Hematol. 2007;62(2):148–152. doi: 10.1016/j.critrevonc.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Henry D.H., Costa L., Goldwasser F., Hirsh V., Hungria V., Prausova J. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 2011;29(9):1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 47.Lipton A., Fizazi K., Stopeck A.T., Henry D.H., Brown J.E., Yardley D.A. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur. J. cancer (Oxford, England : 1990) 2012;48(16):3082–3092. doi: 10.1016/j.ejca.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Stopeck A.T., Lipton A., Body J.J., Steger G.G., Tonkin K., de Boer R.H. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010;28(35):5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 49.Henry D.H., Costa L., Goldwasser F., Hirsh V., Hungria V., Prausova J. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011;29(9):1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 50.Patard J.-J., Rioux-Leclercq N., Fergelot P. Understanding the importance of smart drugs in renal cell carcinoma. Eur. Urol. 2006;49(4):633–643. doi: 10.1016/j.eururo.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 51.Coppin C., Porzsolt F., Awa A., Kumpf J., Coldman A., Wilt T. Immunotherapy for advanced renal cell cancer. Cochrane Database Syst. Rev. 2004;1 doi: 10.1002/14651858.CD001425.pub2. [DOI] [PubMed] [Google Scholar]
- 52.Jiang Y., Zhang W., Kondo K., Klco J.M., Martin T.B.S., Dufault M.R. Gene expression profiling in a renal cell carcinoma cell line: dissecting VHL and hypoxia-dependent pathways. Mol. Canc. Res. 2003;1(6):453–462. [PubMed] [Google Scholar]
- 53.Motzer R.J., Hutson T.E., Tomczak P., Michaelson M.D., Bukowski R.M., Oudard S. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2009;27(22):3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maita S., Yuasa T., Tsuchiya N., Mitobe Y., Narita S., Horikawa Y. Antitumor effect of sunitinib against skeletal metastatic renal cell carcinoma through inhibition of osteoclast function. Int. J. Canc. 2012;130(3):677–684. doi: 10.1002/ijc.26034. [DOI] [PubMed] [Google Scholar]
- 55.Karaca H., Lale A., Dikilitas M., Ozkan M., Er O. Recovery of paraneoplastic hypercalcemia by sunitinib treatment for renal cell carcinoma: a case report and review of the literature. Med. Oncol. 2010;27(3):1023–1026. doi: 10.1007/s12032-009-9327-4. [DOI] [PubMed] [Google Scholar]
- 56.Żołnierek J., Nurzyński P., Langiewicz P., Oborska S., Waśko-Grabowska A., Kuszatal E. Efficacy of targeted therapy in patients with renal cell carcinoma with pre-existing or new bone metastases. J. Canc. Res. Clin. Oncol. 2010;136(3):371–378. doi: 10.1007/s00432-009-0664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu X., Gu W., Zhang H., Zhu Y., Shi G., Ye D. Oligometastatic state predicts a favorable outcome for renal cell carcinoma patients with bone metastasis under the treatment of Sunitinib. Oncotarget. 2016;7(18):26879. doi: 10.18632/oncotarget.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Escudier B., Eisen T., Stadler W.M., Szczylik C., Oudard S., Siebels M. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 59.Sternberg C.N., Davis I.D., Mardiak J., Szczylik C., Lee E., Wagstaff J. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J. Clin. Oncol. 2010;28(6):1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 60.Escudier B., Pluzanska A., Koralewski P., Ravaud A., Bracarda S., Szczylik C. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2008;370(9605):2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 61.Hudes G., Carducci M., Tomczak P., Dutcher J., Figlin R., Kapoor A. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N. Engl. J. Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 62.Motzer R.J., Escudier B., Oudard S., Hutson T.E., Porta C., Bracarda S. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 63.Nishino M., Jagannathan J.P., Ramaiya N.H., Van den Abbeele A.D. Revised RECIST guideline version 1.1: what oncologists want to know and what radiologists need to know. Am. J. Roentgenol. 2010;195(2):281–289. doi: 10.2214/AJR.09.4110. [DOI] [PubMed] [Google Scholar]
- 64.Szendrői A., Dinya E., Kardos M., Szász A.M., Németh Z., Áts K. Prognostic factors and survival of renal clear cell carcinoma patients with bone metastases. Pathol. Oncol. Res. 2010;16(1):29–38. doi: 10.1007/s12253-009-9184-7. [DOI] [PubMed] [Google Scholar]
- 65.Takahashi A., Sasaki H., Kim S.J., Tobisu K-i, Kakizoe T., Tsukamoto T. Markedly increased amounts of messenger RNAs for vascular endothelial growth factor and placenta growth factor in renal cell carcinoma associated with angiogenesis. Canc. Res. 1994;54(15):4233–4237. [PubMed] [Google Scholar]
- 66.Chatziioannou A., Johnson M., Pneumaticos S., Lawrence D., Carrasco C.H. Preoperative embolization of bone metastases from renal cell carcinoma. Eur. Radiol. 2000;10(4):593–596. doi: 10.1007/s003300050969. [DOI] [PubMed] [Google Scholar]
- 67.Giberti C., Oneto F., Martorana G., Rovida S., Carmignani G. Radical nephrectomy for renal cell carcinoma: long-term results and prognostic factors on a series of 328 cases. Eur. Urol. 1997;31(1):40–48. doi: 10.1159/000474416. [DOI] [PubMed] [Google Scholar]
- 68.Kavolius J., Mastorakos D., Pavlovich C., Russo P., Burt M., Brady M.S. Resection of metastatic renal cell carcinoma. J. Clin. Oncol. 1998;16(6):2261–2266. doi: 10.1200/JCO.1998.16.6.2261. [DOI] [PubMed] [Google Scholar]
- 69.Hardman P., Robb J., Kerr G., Rodger A., MacFarlane A. The value of internal fixation and radiotherapy in the management of upper and lower limb bone metastases. Clin. Oncol. 1992;4(4):244–248. doi: 10.1016/s0936-6555(05)81063-5. [DOI] [PubMed] [Google Scholar]
- 70.Damron T.A., Morgan H., Prakash D., Grant W., Aronowitz J., Heiner J. Critical evaluation of Mirels' rating system for impending pathologic fractures. Clin. Orthop. Relat. Res. 2003;415:S201–S207. doi: 10.1097/01.blo.0000093842.72468.73. [DOI] [PubMed] [Google Scholar]
- 71.Mirels H. The classic: metastatic disease in long bones a proposed scoring system for diagnosing impending pathologic fractures. Clin. Orthop. Relat. Res. 2003;415:S4–S13. doi: 10.1097/01.blo.0000093045.56370.dd. [DOI] [PubMed] [Google Scholar]
- 72.Ward W.G., Holsenbeck S., Dorey F.J., Spang J., Howe D. Metastatic disease of the femur: surgical treatment. Clin. Orthop. Relat. Res. 2003;415:S230–S244. doi: 10.1097/01.blo.0000093849.72468.82. [DOI] [PubMed] [Google Scholar]
- 73.Marcove R.C., Sadrieh J., Huvos A.G., Grabstald H. Cryosurgery in the treatment of solitary or multiple bone metastases from renal cell carcinoma. J. Urol. 1972;108(4):540–547. doi: 10.1016/s0022-5347(17)60797-3. [DOI] [PubMed] [Google Scholar]
- 74.Bickels J., Dadia S., Lidar Z. Surgical management of metastatic bone disease. JBJS. 2009;91(6):1503–1516. doi: 10.2106/JBJS.H.00175. [DOI] [PubMed] [Google Scholar]
- 75.Jacofsky D.J., Haidukewych G.J. Management of pathologic fractures of the proximal femur: state of the art. J. Orthop. Trauma. 2004;18(7):459–469. doi: 10.1097/00005131-200408000-00013. [DOI] [PubMed] [Google Scholar]
- 76.Yamada Y., Bilsky M.H., Lovelock D.M., Venkatraman E.S., Toner S., Johnson J. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int. J. Radiat. Oncol. Biol. Phys. 2008;71(2):484–490. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 77.Basile A., Giuliano G., Scuderi V., Motta S., Crisafi R., Coppolino F. Cementoplasty in the management of painful extraspinal bone metastases: our experience. La Radiologia medica. 2008;113(7):1018–1028. doi: 10.1007/s11547-008-0314-1. [DOI] [PubMed] [Google Scholar]
- 78.Reichel L.M., Pohar S., Heiner J., Buzaianu E.M., Damron T.A. Radiotherapy to bone has utility in multifocal metastatic renal carcinoma. Clin. Orthop. Relat. Res. 2007;459:133–138. doi: 10.1097/BLO.0b013e3180616594. [DOI] [PubMed] [Google Scholar]
- 79.Fizazi K., Lipton A., Mariette X., Body J.J., Rahim Y., Gralow J.R. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009;27(10):1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 80.Gangi A., Kastler B., Klinkert A., Dietemann J.L. Injection of alcohol into bone metastases under CT guidance. J. Comput. Assist. Tomogr. 1994;18(6):932–935. doi: 10.1097/00004728-199411000-00016. [DOI] [PubMed] [Google Scholar]
- 81.Callstrom M.R., Atwell T.D., Charboneau J.W., Farrell M.A., Goetz M.P., Rubin J. Painful metastases involving bone: percutaneous image-guided cryoablation–prospective trial interim analysis. Radiology. 2006;241(2):572–580. doi: 10.1148/radiol.2412051247. [DOI] [PubMed] [Google Scholar]
- 82.Simon C.J., Dupuy D.E., Mayo-Smith W.W. vol. 25. Publication of the Radiological Society of North America, Inc.; 2005. pp. S69–S83. (Microwave Ablation: Principles and Applications. Radiographics : a Review). Suppl 1. [DOI] [PubMed] [Google Scholar]
- 83.Toyota N., Naito A., Kakizawa H., Hieda M., Hirai N., Tachikake T. Radiofrequency ablation therapy combined with cementoplasty for painful bone metastases: initial experience. Cardiovas. Intervent. Radiol. 2005;28(5):578–583. doi: 10.1007/s00270-004-0208-0. [DOI] [PubMed] [Google Scholar]
- 84.Woodward E., Jagdev S., McParland L., Clark K., Gregory W., Newsham A. Skeletal complications and survival in renal cancer patients with bone metastases. Bone. 2011;48(1):160–166. doi: 10.1016/j.bone.2010.09.008. [DOI] [PubMed] [Google Scholar]