Abstract
Age is the single greatest risk factor for many diseases, including oral diseases. Despite this, a majority of preclinical oral health research has not adequately considered the importance of aging in research aimed at the mechanistic understanding of oral disease. Here, we have attempted to provide insights from animal studies in the geroscience field and apply them in the context of oral health research. In particular, we discuss the relationship between the biology of aging and mechanisms of oral disease. We also present a framework for defining and utilizing age-appropriate rodents and present experimental design considerations, such as the number of age-points used and the importance of genetic background. While focused primarily on rodent models, alternative animal models that may be particularly useful for studies of oral health during aging, such as companion dogs and marmoset monkeys, are also discussed. We hope that such information will aid in the design of future preclinical studies of geriatric dental health, thus allowing more reliability for translation of such studies to age-associated oral disease in people.
Keywords: Dentistry, Periodontal disease, Rapamycin, mTOR, Mice, Dogs, Marmosets, Dry mouth, Oral cancer, Hallmarks of aging, Xerostomia, Oral microbiome, Inflammation
Introduction
The population in the USA and other developed nations is rapidly aging (Lutz et al., 2008) with recent demographic trends suggesting that the fraction of Americans age 65 and older will double by 2060 (Ortman et al., 2014). One consequence of this “silver tsunami” is a dramatic increase in the prevalence of the many diseases for which age is the greatest risk factor (Perry, 2010). Included among these are all of the major causes of mortality in developed nations, including cardiovascular disease, most cancers, kidney disease, and dementias (Pitt & Kaeberlein, 2015; Kaeberlein, 2013).
The field of geroscience seeks to understand the biological mechanisms that underlie this relationship between aging and chronic disability and disease (Sierra & Kohanski, 2017; Kennedy & , S.L. Berger, A. Brunet, J. Campisi, A.M. Cuervo, E.S. Epel, C. Franceschi, G.J. Lithgow, R.I. Morimoto, J.E. Pessin, T.A. Rando, A. Richardson, E.E. Schadt, T. Wyss-Coray, F. Sierra, 2014). Several Hallmarks of Aging have been identified which represent conserved molecular processes and pathways that contribute to age-related cellular, tissue, and organismal declines in function (Lopez-Otin et al., 2013). Interventions targeting these hallmarks of aging have been developed and found to increase lifespan and reduce age-related diseases in laboratory models (Kaeberlein et al., 2015). For example, the drug rapamycin is a specific inhibitor of the mTOR complex 1 that increases lifespan and delays age-related functional declines in evolutionarily diverse laboratory organisms including yeast, nematodes, fruit flies, and mice (Johnson et al., 2015; Johnson et al., 2013). Initial evidence indicates that rapamycin can also delay at least some aspects of aging in clinical studies, including restoration of cardiac function in healthy middle-aged companion dogs (Urfer et al., 2017a) and immune function in healthy elderly people (Mannick et al., 2014). These observations have contributed to growing optimism that advances in geroscience research can be harnessed to delay the onset and progression of multiple age-associated diseases and improve healthspan outside of a laboratory setting (Kaeberlein, 2017).
In contrast to major killers such as heart disease and cancer, the relationship between biological aging and oral health has received less attention among the broader biomedical and geroscience research communities. We believe that this relationship could be particularly important for both healthspan and lifespan, as age is the major risk factor for several oral health conditions ( (Guiglia et al., 2010), (Razak et al., 2014)), including oral cancer (Gil-Montoya et al., 2015a), xerostomia (dry mouth) (Navazesh, 2002), and periodontal disease. These chronic conditions can significantly detract from quality of life for the elderly and have been linked to increased risk for a variety of co-morbidities. A lack of studies in pre-clinical animal models that take into account the impact of aging may contribute to our limited understanding of the relationships between the hallmarks of aging and oral biology. We propose that development and appropriate utilization of animal models for oral geroscience research will allow for a better understanding of mechanisms leading to oral disease and may facilitate development of interventions to improve oral health in the elderly.
Aging and diseases of the oral cavity
Aging is associated with several pathological changes that likely contribute to declining oral health. These include increased systemic and local inflammation (“inflammaging”), immune decline and senescence, stem cell dysfunction, bone loss, and dysregulation of the microbiome (Lopez-Otin et al., 2013; De Martinis et al., 2005; Gruver et al., 2007; Yatsunenko & , F.E. Rey, M.J. Manary, I. Trehan, M.G. Dominguez-Bello, M. Contreras, M. Magris, G. Hidalgo, R.N. Baldassano, A.P. Anokhin, A.C. Heath, B. Warner, J. Reeder, J. Kuczynski, J.G. Caporaso, C.A. Lozupone, C. Lauber, J.C. Clemente, D. Knights, R. Knight, J.I. Gordon, 2012). The risk for several common oral conditions such as xerostomia and periodontal disease increase dramatically with age and can have a major impact on quality of life for the elderly. Xerostomia, or dry mouth, for example affects over 30% of patients older than 65. This condition is primarily related to medications but also from other comorbid age-related conditions such as Parkinson’s disease, Alzheimer’s disease, and diabetes (Yellowitz & Schneiderman, 2014; Levy, 2007). Dry mouth can lead to root surface cavities, cracked lips, and fissured tongue. Patients with salivary hypofunction experience taste disturbance and have difficulty masticating and swallowing, as saliva is necessary to prepare food for digestion (Gil-Montoya et al., 2015b). Saliva is also important for oropharyngeal health, as it aids in function and preservation of the oral and gastrointestinal environment.
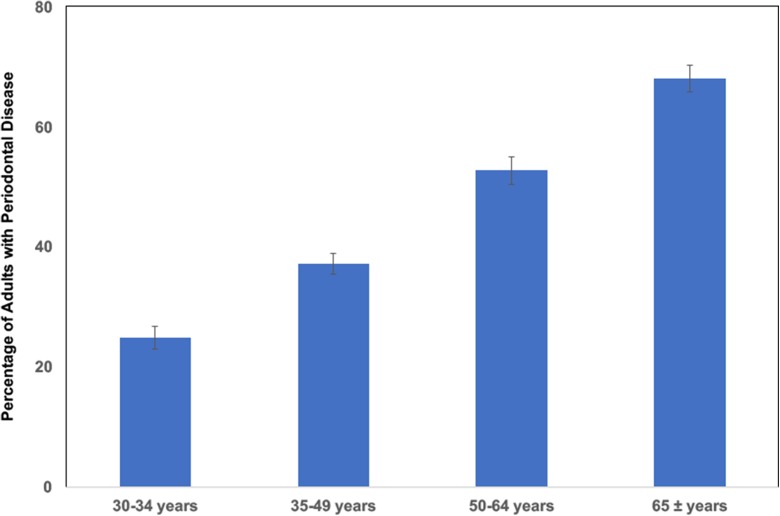
Periodontal disease is a major age-associated oral health concern in the elderly, resulting in alveolar bone loss and tissue degradation (Eke et al., 2012). Indeed, recent estimates suggest that approximately 70% of people in the USA have clinical periodontal disease by the time they reach age 65 (Fig. 1). Some of these patients will end up losing their teeth and will ultimately be fitted for a dental prosthesis. Along with periodontal disease, the lack of saliva will also affect the dental prosthesis, contributing to denture sores and compromised retention of the prosthesis.
Fig. 1.
Periodontal disease is an age-associated disorder. The percentage of adults in USA with clinical periodontitis is shown for different age groups (mean, 24 teeth). Periodontitis is defined by a combination of periodontal probing depth and clinical attachment loss from six different sites per tooth on all teeth. All data were derived from the Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009–2012 (Eke et al., 2015 )
From an economic standpoint, treatment of oral disease is costly, as it is the fourth most expensive disease in most industrialized countries, with periodontitis alone estimated to account for more than 50 billion dollars per year in lost productivity in the USA (Tonetti et al., 2017). Importantly, oral disease is strongly associated with several age-associated co-morbidities, which account for up to 75% of healthcare spending altogether (Petersen et al., 2005). For example, people with periodontal disease are at higher risk of developing other age-related conditions such as heart disease, diabetes, and Alzheimer’s disease (Razak et al., 2014; Gil-Montoya et al., 2015b; Kim & Amar, 2006). Whether this relationship is causal or not remains unclear; however, what is clear is that these diseases share a single greatest risk factor: old age (Kaeberlein, 2013).
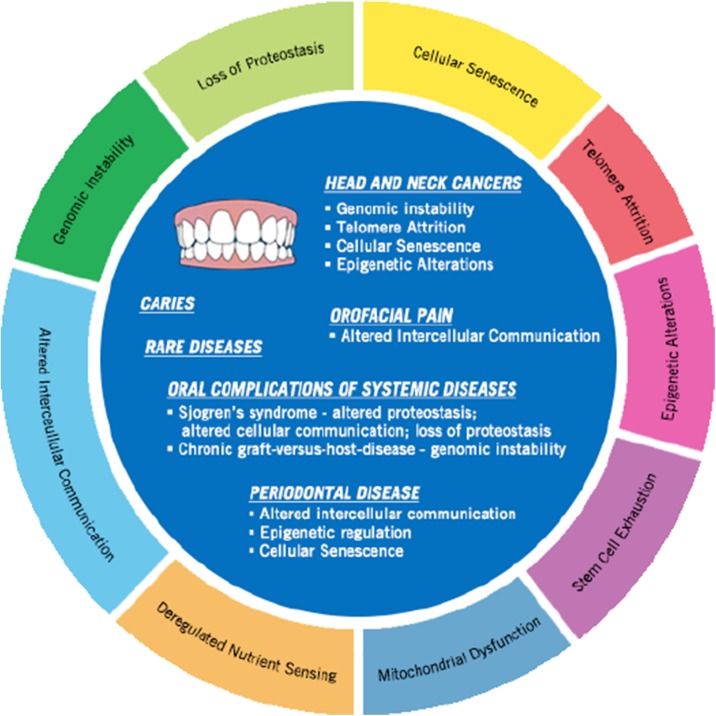
The geroscience community has defined and categorized cellular and molecular hallmarks of aging (Lopez-Otin et al., 2013). These include genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. These aging hallmarks can also be linked to oral health (Fig. 2). Common oral conditions with age include periodontal disease, oral cancer, candidiasis, and xerostomia, which can lead to other dental conditions like root caries (Gil-Montoya et al., 2015a; Gonsalves et al., 2008). For example, oral cancer alone can be attributed to multiple hallmarks of aging including genomic instability (Bhattacharya & , R. Roy, A.M. Snijders, G. Hamilton, J. Paquette, T. Tokuyasu, H. Bengtsson, R.C.K. Jordan, A.B. Olshen, D. Pinkel, B.L. Schmidt, D.G. Albertson, 2011), epigenetic alteration, and telomere attrition (Sumida & Hamakawa, 2001). Candida species are present in the normal oral flora, but conditions such as immunodeficiencies, malnutrition, or xerostomia, can lead to risk in overgrowth in the elderly leading to infection (Flevari et al., 2013).
Fig. 2.
Age-related oral diseases share molecular links with the hallmarks of aging. Highlighting a few oral health conditions in relationship to aging hallmarks (Bhattacharya & , R. Roy, A.M. Snijders, G. Hamilton, J. Paquette, T. Tokuyasu, H. Bengtsson, R.C.K. Jordan, A.B. Olshen, D. Pinkel, B.L. Schmidt, D.G. Albertson, 2011; Sumida & Hamakawa, 2001; Thevaranjan et al., 2017; Barrera et al., 2016; Khan et al., 2010; Yin & Chung, 2011; Parkinson et al., 2016; Lindroth & Park, 2013; Paul et al., 2015; Ballestar, 2011)
Current research on periodontitis is largely limited to clinical studies seeking to characterize changes in the oral cavity during disease and to preclinical studies using animal models. One limitation of most such preclinical studies is that they almost exclusively utilize artificial disease models in young rodents, thereby losing the contribution of the aged systemic and local environment. We suggest that the failure to utilize aged animals for preclinical studies of oral disease limits the clinical relevance of such studies. One goal of the Geroscience Initiative is to raise awareness of the importance of understanding the contribution of aging biology to age-related diseases (Sierra & Kohanski, 2017; Kennedy & , S.L. Berger, A. Brunet, J. Campisi, A.M. Cuervo, E.S. Epel, C. Franceschi, G.J. Lithgow, R.I. Morimoto, J.E. Pessin, T.A. Rando, A. Richardson, E.E. Schadt, T. Wyss-Coray, F. Sierra, 2014). In this context, oral health research could potentially benefit from a greater consideration of the importance of aging biology to oral disease.
Use of animal models
Animal models have proven useful for the investigation of a variety of mechanisms underlying oral diseases. Although there is no single model species that recapitulates all aspects of human oral structure and function, studies using non-human primates, dogs, and rodents have provided important insights into mechanisms impacting oral health. A discussion on non-human primates and dogs will occur in later sections, but first, we will focus on rodent models, where a majority of preclinical oral research has occurred. The periodontal anatomy of rodent molars is similar to humans, and for this reason, mice, in particular, have been proven useful for investigating a variety of mechanisms underlying oral diseases (Struillou et al., 2010; Yamasaki et al., 1979). For instance, relationships between immune function, microbial changes within the oral cavity, and inflammation have been established through studies performed in rodent models (Struillou et al., 2010; Oz & Puleo, 2011a; Graves et al., 2008; Polak et al., 2009). In contrast, one way that rodents differ substantially from humans is that their incisors lack a root structure and grow continuously throughout life, with only the front of the incisors having enamel. It is therefore important to keep in mind these anatomical differences and similarities when considering which aspects of oral anatomy and health can be appropriately modeled in rodents.
Common techniques used in rodent models of oral disease include placement of ligatures in the gingival sulcus, oral gavage with periodontal pathogens, and dextran sulfate sodium treatment (DSS) to induce innate immune damage to the gastrointestinal tissues which can result in oral mucosal inflammation and alveolar bone loss (Oz & Puleo, 2011b). For example, oral infection with Porphyromonas gingivalis and Fusobacterium nucleatum in a 4-week old rodent model revealed more severe alveolar bone loss and inflammatory response when both bacteria were combined (Li & , H. Yang, Y. Ding, R. Aprecio, W. Zhang, Q. Wang, Y. Li, 2013). To model inflammatory bowel disease and periodontal disease progression in patients, 11–12 week old BALB/c mice were treated with oral delivery of DSS, which induced IBD including diarrhea, anemia, dilation of the stomach wall, dysregulated hepatic concentration, and severe alveolar bone loss (Oz et al., 2010). The ligature model allows biofilm accumulation, disrupts the gingival epithelium, and enhances bone loss. For example, a recent study utilizing this method revealed that P. gingivalis exacerbates RANKL-dependent alveolar bone resorption via TLR2/TLR4 signaling in 8–10-week-old mice (Lin et al., 2014).
It must be noted, however, that many of these studies reflect the biology of disease modeled in young adult or still-developing, juvenile mice that may not reflect the biology of naturally developing human oral disease, which most often occurs in an aged individual who may already be experiencing declines in immune function and other physiological systems. Despite many important discoveries, we speculate that progress in understanding the mechanisms of age-associated periodontal disease have been hampered by a lack of appropriately designed preclinical research on oral health in the context of biological aging. We note that there are currently no guidelines or consensus on the appropriate animal models or age of animals used in such studies. Development of such guidelines is essential to assure that conclusions drawn represent relevant biological mechanisms. To move the geriatric dental health field forward, it is important to understand how to properly utilize rodent models to study aging. Here, we describe several considerations based on knowledge in the field of geroscience that we hope will aid in design and execution of preclinical studies of geriatric dental health in rodent models.
Defining an “old” mouse
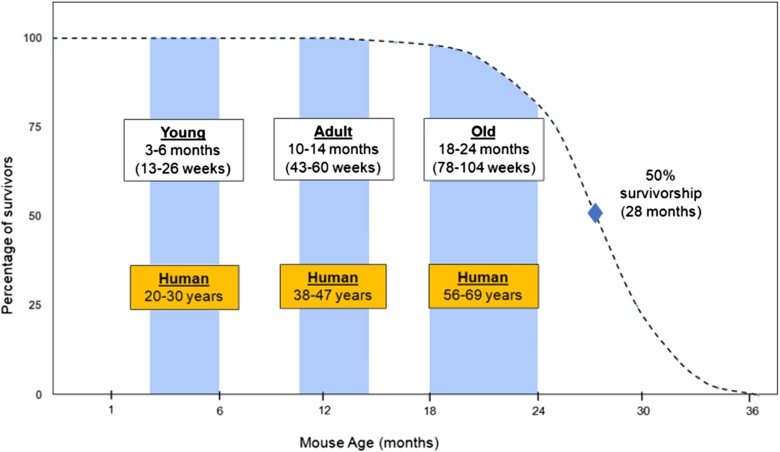
It is our observation that many studies utilizing rodents within the oral health research field are consistently defining “aged” and “old” incorrectly. The National Institute on Aging (NIA) and The Mouse in Biomedical Research (Flurkey et al., 2007) suggest that “old” are mice 18–24 months of age, middle-age is 10–14 months, and young age is 3–6 months. The median lifespan for C57BL/6JNia mice, for example, is approximately 25 months for females and 27 months for males. In order to model human geriatric dental health in mice, the animals used for these studies should be roughly similar in biological age to older people (Fig. 3). For instance, a 3-month-old young mouse may be roughly equivalent to 20-year-old human, while an 18-month-old mouse will be approximately equivalent to a 56–60-year-old human. If the goal is to understand mechanisms of oral disease that are particularly important in the elderly, even older mice (perhaps up to 30 months of age) should be used; however, it is important to consider the differences between pathological changes due to disease progression versus changes associated with normative aging, particularly when utilizing animals at advanced ages. Thus, we suggest that mice at least 18 months of age, and preferably 20–24 months of age, are appropriate for most rodent studies of age-related oral health.
Fig. 3.
Mouse age is an important consideration when modeling human age-associated disease. A typical survival curve for C57BL/6JNia mice maintained in the laboratory (adapted from 42). A mouse is mostly developmentally mature and can be considered a young adult by about 6 months of age. Most geroscience studies would not consider a mouse to be old until 18–24 months of age. A rough comparison of human age ranges is shown for different mouse ages. When studying or modeling a disease process that is most prevalent in elderly people, it is strongly recommended that older mice be used, in order to recapitulate the contributions of physiological changes that only occur in an aged animal
It is important to not only utilize mice that are biologically old in oral health research but to also use correct terminology to avoid confusion and misinterpretation by other researchers. As an example, one recent study reported an increase in Th17 cells in the mouse gingiva, which was determined not to be dependent on commensal bacteria colonization, by comparing 8-week (1–2 month)-old mice to 24-week (5–6 month)-old mice (Dutzan et al., 2017). Throughout the report, the 1–2-month-old mice are referred to as “young,” while the 5–6-month-old mice are referred to as “middle-aged,” “old,” and “aged,” none of which are biologically accurate terms. It is also important to note that 1–2-month-old mice are still developing and experiencing rapid body weight gain (perhaps akin to teenagers) while, as shown in Fig. 3, 5–6-month-old mice are roughly the biological equivalent of a 25–30-year-old person. Thus, one potential explanation for the lack of changes observed in the commensal oral bacteria in this study is that the oldest age group studied was really only young adult.
In this regard, it will be of particular interest going forward to determine how the oral microbiome changes in laboratory mice in response to normal aging and interventions that modulate aging rate. Based on published studies of the changes in the gut microbiome during aging in rodents (Flemer et al., 2017; Bitto et al., 2016; Thevaranjan et al., 2017; Langille et al., 2014), we anticipate that large changes in the oral microbiome will also occur in aging mice, but it will require sampling from biologically old animals to detect these important changes. Indeed, a comparative analysis of the oral and gut microbiota as a function of age could be particularly informative. As changes in the oral microbiome can influence the gut microbiome, both microbial populations will likely be affected by age-related changes in immunity and inflammation.
Analysis of multiple age groups is optimal
It is also common to see preclinical dental research studies where conclusions are drawn after comparing only two age groups, which authors report as “young” and “old.” It has become recognized within the geroscience community, however, that multiple age-points are highly preferable to a simple pairwise comparison of “young” versus “old.” Utilizing multiple age points is important because not all age-related physiological changes or disease states occur with identical kinetics or in a linear fashion. For example, some age-associated molecular changes or declines in function can be detected relatively early in middle-age, while others may not be present in a large percentage of the population until very late in life. A good example of a three age-point design is provided by the series of studies performed at the Jackson Laboratory examining lifespan and healthspan phenotypes across 31 inbred laboratory strains of mice, in which mice were phenotyped at 6 months (young), 12 months (middle-aged), and > 18 months (old) (Ackert-Bicknell et al., 2015).
A specific example of the importance of multiple age-points from the dental health literature is provided by Shaik-Dasthagirisaheb et al. (Shaik-Dasthagirisaheb & Kantarci, 2010), who showed that when comparing 2-month-old and 1-year-old mice, there is no significant difference in production of IL-6 and TNF-α in bone marrow-derived macrophages challenged with Porphyromonas gingivalis, a keystone periodontal pathogen; however, when a third age-group is added at 2 years of age, a significant difference is observed. In another study, we recently published that alveolar bone levels decline only a very slight amount in C57BL6/JNia mice between 2 and 3 months of age and 9–10 months of age; however, by 22–24 months, a substantial decline in alveolar bone levels has occurred (An et al. 2017). These data are consistent with a prior study by Liang et al. (Liang et al., 2010) who showed loss of alveolar bone in 18-month-old mice compared to 2-month-old mice. A simple pairwise comparison of 2-month-old mice to 9–10-month-old mice would not have uncovered this large age-related decline in alveolar bone, however.
We recognize that comparison of three different age groups may not be feasible in every study and that important information can be obtained from pairwise comparisons of young and old mice, as long as the aged mice are truly biologically old (> 18 months of age). In such cases, interpretation should be tempered by the limited number of age groups spanned, however, and even with three age groups, it may not be possible to detect all of the relevant age-related changes or to accurately model the kinetics of such changes. In every case, the biological age of the animals (Fig. 3) should be considered, especially when extrapolating results for potential implications for human oral health and medicine.
Choice of strain background
There is currently little consensus in the geroscience field as to what the best genetic background is for studies of aging biology in mice. Currently, the most commonly used strain backgrounds for aging studies are C57BL/6J and C57BL/6JNia (note that these are not identical) (Mekada et al., 2009; Simon et al., 2013). This is due in part to the widespread use of these strains in biomedical research and the fact that the NIA has provided aged C57BL/6JNia animals to the research community for many years though the NIA Aged Rodent Colony. More recently, genetically heterogeneous UMHET3 mice have become a “gold standard” of sorts for lifespan studies, due to their exclusive use in the NIA Interventions Testing Program (Miller et al., 2007; Nadon et al., 2008).
While there is no “right” choice of strain background, it is important to keep in mind that different mouse strains do differ somewhat in lifespan and disease burden. Some strains, for example, are particularly prone to certain forms of cancer. For example, Balb/C mice have a 44% incidence rate of lymphoma at 13 months of age, while SJL/J mice have a 91% incidence rate of reticulum cell sarcoma at the same age (Altman & Dorothy, 1979). With respect to longevity, there are wide ranges among mouse strains for both median and maximum lifespan and interesting sexual dimorphisms that can go both directions. For example, BALB/cBY median lifespan for males has been reported as 707 and 757 days for females, while C57BL/6J median lifespan has been reported as 901 days for males and 866 days for females, but these values also vary from study to study (Altman & Dorothy, 1979; Yuan et al., 2009). In another study of 31 inbred mouse strains, median lifespan ranged from 251 to 964 days across the different strains, with correspondingly high variance in a variety of age-associated phenotypic measures (Yuan et al., 2009). The Jackson Laboratory Mouse Phenome Database contains a large number of age-related measures for many different mouse strains and can be a useful resource when designing studies of aging biology in mice (Bogue et al., 2016).
Additional animal models
While rodents will undoubtedly continue to serve as the premier pre-clinical models for geroscience and dental health research, companion animals may offer a powerful additional model for understanding the factors and mechanisms that contribute to human age-associated disease. Pet dogs, in particular, are emerging as a premier model for geroscience research (Kaeberlein, 2016; Creevy et al., 2016; Urfer et al., 2017b). Several factors make companion dogs a powerful animal model for understanding age-related diseases, including their accelerated rates of aging (compared to people), their unique genetic architecture, and the wealth of clinical veterinary knowledge and expertise (Kaeberlein et al., 2016). In addition, companion dogs display a high prevalence of oral diseases (Albuquerque et al., 2012), which makes them a suitable model to study the relationship between oral health and aging. It is additionally worth noting that while dogs and mice are approximately equally evolutionarily distant from humans, companion dogs share many aspects of the human environment. This environmental variation and similarity to humans is impossible to recapitulate in a captive animal model.
In the dental research fields, such as in the field of periodontics, dogs, especially captive beagle dogs, have been extensively utilized to elucidate mechanisms of periodontal disease due to their similarity to human periodontal tissues and tooth sizes. Although differences exist, such as not having occlusal contacts in the premolar teeth or open contacts between the teeth, the prevalence of gingivitis and periodontitis increases in severity with age in dogs even faster than in humans with similar etiologic factors (Struillou et al., 2010). Studies in beagle dogs have shown that during a plaque-induced model, beagle dogs develop calculus, have loss of attachment or periodontal tissue breakdown, and bone loss (Lindhe et al., 1975; Sorensen et al., 1980; Aukhil et al., 1988). Experimental periodontitis induced by ligatures in beagle dogs has revealed osteoclasts appearing during later stages of inflammation (Shibutani et al., 1997) and that IL-11 is capable of slowing the progression of attachment and bone loss (Martuscelli et al., 2000). Although these studies, as well as many others not discussed here, have provided useful information to the field of periodontics, considering the lifespan of beagle dogs to be 11–12 years, with 10% survival age about 16 years (Albert et al., 1994), the majority of these studies suffer from the same limitations of those described above in mice, in that they have been performed on young animals.
In addition to dogs, non-human primates like Macaca nemestrina have served as a model for studies for periodontal disease to investigate periodontal microbial-host interactions, as they harbor similar periodontal pathogens and exhibit clinical hallmarks of periodontitis comparable to humans (Struillou et al., 2010; Persson et al., 1993). Further, non-human primates have also served an important role in geroscience research, most notably for long-term studies of caloric restriction in captive rhesus monkeys (Colman et al., 2009; Colman et al., 2014; Mattison et al., 2012). In addition to the many challenges associated with long-term captive studies in M. nemestrina and rhesus monkeys, these types of studies are also extremely expensive and difficult to replicate due to the long life spans (> 30 years on average) of these animals. More recently, shorter-lived marmosets have emerged as a potentially important non-human primate model in geroscience (Tardif et al., 2011; Salmon, 2016), and it would be of high interest to better understand on how the oral health of marmosets changes during aging.
Conclusion
Geroscience research has progressed rapidly in the past few years, and there is growing recognition that most major diseases are interconnected through the biology of aging (Kaeberlein, 2017). Historically, NIH-funded geroscience has been primarily supported through the NIA. Recently, the National Institute of Dental and Craniofacial Research (NIDCR) has also begun to promote collaborations to improve the oral health of older adults living the USA by “addressing knowledge gaps in the etiology and management of dental, oral and craniofacial diseases associated with aging.” This represents an important opportunity to better understand the role of aging biology in oral health and disease. Here, we have attempted to provide some insights from the geroscience field to facilitate oral health studies, primarily in rodent models. We hope that these suggestions will stimulate further discussion and help our colleagues to design more informative experiments such that the conclusions may translate more reliably to age-associated oral diseases in people.
Acknowledgements
JA was supported by the NIH/NIDCR F30DE027254, the ARCS Foundation, and the Magnuson Scholarship. This work was supported by a Glenn Award to MK from the Glenn Foundation for Medical Research. The authors thank Alessandro Bitto, Silvan Urfer, and Matthew An for reading the manuscript and for giving critical comments on the manuscript.
References
- Ackert-Bicknell CL, Anderson LC, Sheehan S, Hill WG, Chang B, Churchill GA, Chesler EJ, Korstanje R, Peters LL. Aging research using mouse models. Curr Protoc Mouse Biol. 2015;5(2):95–133. doi: 10.1002/9780470942390.mo140195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert RE, Benjamin SA, Shukla R. Life span and cancer mortality in the beagle dog and humans. Mech Ageing Dev. 1994;74(3):149–159. doi: 10.1016/0047-6374(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Albuquerque C, Morinha F, Requicha J, Martins T, Dias I, Guedes-Pinto H, Bastos E, Viegas C. Canine periodontitis: the dog as an important model for periodontal studies. Vet J. 2012;191(3):299–305. doi: 10.1016/j.tvjl.2011.08.017. [DOI] [PubMed] [Google Scholar]
- P. Altman, K. Dorothy, Inbred and genetically defined strains of laboratory animals part 1: mouse and rat. 418 (1979)
- An JY et al (2017) Rapamycin treatment attenuates age-associated periodontitis in mice. Geroscience. 10.1007/s11357-017-9994-6 [DOI] [PMC free article] [PubMed]
- Aukhil I, Schaberg TV, Greco GW, Simpson DM. Surgical versus non-surgical treatment and recurrent periodontal disease in beagle dogs. J Clin Periodontol. 1988;15(2):99–105. doi: 10.1111/j.1600-051X.1988.tb01001.x. [DOI] [PubMed] [Google Scholar]
- Ballestar E. Epigenetic alterations in autoimmune rheumatic diseases. Nat Rev Rheumatol. 2011;7(5):263–271. doi: 10.1038/nrrheum.2011.16. [DOI] [PubMed] [Google Scholar]
- Barrera MJ, Aguilera S, Castro I, Cortés J, Bahamondes V, Quest AFG, Molina C, González S, Hermoso M, Urzúa U, Leyton C, González MJ. Pro-inflammatory cytokines enhance ERAD and ATF6α pathway activity in salivary glands of Sjögren’s syndrome patients. J Autoimmun. 2016;75:68–81. doi: 10.1016/j.jaut.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Roy R, Snijders AM, Hamilton G, Paquette J, Tokuyasu T, Bengtsson H, Jordan RCK, Olshen AB, Pinkel D, Schmidt BL, Albertson DG. Two distinct routes to oral cancer differing in genome instability and risk for cervical node metastasis. Clin Cancer Res. 2011;17(22):7024–7034. doi: 10.1158/1078-0432.CCR-11-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitto A, et al. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. elife. 2016;5:e16351. doi: 10.7554/eLife.16351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogue MA, Peters LL, Paigen B, Korstanje R, Yuan R, Ackert-Bicknell C, Grubb SC, Churchill GA, Chesler EJ. Accessing data resources in the mouse phenome database for genetic analysis of murine life span and health span. J Gerontol A Biol Sci Med Sci. 2016;71(2):170–177. doi: 10.1093/gerona/glu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, et al. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creevy KE, Austad SN, Hoffman JM, O'Neill DG, Promislow DE. The companion dog as a model for the longevity dividend. Cold Spring Harb Perspect Med. 2016;6(1):a026633. doi: 10.1101/cshperspect.a026633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martinis M, Franceschi C, Monti D, Ginaldi L. in. FEBS Lett. 2005;579(10):2035–2039. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- Dutzan N, Abusleme L, Bridgeman H, Greenwell-Wild T, Zangerle-Murray T, Fife ME, Bouladoux N, Linley H, Brenchley L, Wemyss K, Calderon G, Hong BY, Break TJ, Bowdish DME, Lionakis MS, Jones SA, Trinchieri G, Diaz PI, Belkaid Y, Konkel JE, Moutsopoulos NM. On-going mechanical damage from mastication drives homeostatic Th17 cell responses at the oral barrier. Immunity. 2017;46(1):133–147. doi: 10.1016/j.immuni.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Dye B, Wei L, Thornton-Evans G, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD, Genco RJ. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015;86(5):611–622. doi: 10.1902/jop.2015.140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemer B et al (2017) Fecal microbiota variation across the lifespan of the healthy laboratory rat. Gut Microbes:1–12 [DOI] [PMC free article] [PubMed]
- Flevari A, Theodorakopoulou M, Velegraki A, Armaganidis A, Dimopoulos G. Treatment of invasive candidiasis in the elderly: a review. Clin Interv Aging. 2013;8:1199–1208. doi: 10.2147/CIA.S39120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- K. Flurkey, J. M. Currer, D. E. Harrison, in The Mouse in Biomedical Research. (2007), pp. 637–672
- Gil-Montoya JA, de Mello ALF, Barrios R, Gonzalez-Moles MA, Bravo M. Clinical Interventions in Aging. 2015;10:461–467. doi: 10.2147/CIA.S54630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Montoya JA, de Mello AL, Barrios R, Gonzalez-Moles MA, Bravo M. Oral health in the elderly patient and its impact on general well-being: a nonsystematic review. Clin Interv Aging. 2015;10:461–467. doi: 10.2147/CIA.S54630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- W. C. Gonsalves, A. S. Wrightson, R. G. Henry, in Am Fam Physician. (2008), vol. 78, pp. 845–852 [PubMed]
- Graves DT, Fine D, Teng YT, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35(2):89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol. 2007;211(2):144–156. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiglia R, Musciotto A, Compilato D, Procaccini M, Russo L, Ciavarella D, Muzio L, Cannone V, Pepe I, D'Angelo M, Campisi G. Aging and oral health: effects in hard and soft tissues. Curr Pharm Des. 2010;16(6):619–630. doi: 10.2174/138161210790883813. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493(7432):338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Sangesland M, Kaeberlein M, Rabinovitch PS. Modulating mTOR in aging and health. Interdiscip Top Gerontol. 2015;40:107–127. doi: 10.1159/000364974. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M. Longevity and aging. F1000prime reports. 2013;5:5. doi: 10.12703/P5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M. The biology of aging: citizen scientists and their pets as a bridge between research on model organisms and human subjects. Vet Pathol. 2016;53(2):291–298. doi: 10.1177/0300985815591082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M (2017) Translational geroscience: a new paradigm for 21st century medicine. Translat Med Aging. 10.1016/j.tma.2017.09.004 [DOI] [PMC free article] [PubMed]
- Kaeberlein M, Rabinovitch PS, Martin GM. Healthy aging: the ultimate preventative medicine. Science. 2015;350(6265):1191–1193. doi: 10.1126/science.aad3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Creevy KE, Promislow DE. The dog aging project: translational geroscience in companion animals. Mamm Genome: Off J Intl Mamm Genome Soc. 2016;27:279–288. doi: 10.1007/s00335-016-9638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan FM, Sy S, Louie P, Ugarte-Torres A, Berka N, Sinclair GD, Stewart DA, Russell JA, Storek J. Genomic instability after allogeneic hematopoietic cell transplantation is frequent in oral mucosa, particularly in patients with a history of chronic graft-versus-host disease, and rare in nasal mucosa. Blood. 2010;116(10):1803–1806. doi: 10.1182/blood-2009-10-249201. [DOI] [PubMed] [Google Scholar]
- Kim J, Amar S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 2006;94(1):10–21. doi: 10.1007/s10266-006-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langille MG, et al. Microbial shifts in the aging mouse gut. Microbiome. 2014;2(1):50. doi: 10.1186/s40168-014-0050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G. The relationship of Parkinson disease with aging. Arch Neurol. 2007;64(9):1242–1246. doi: 10.1001/archneur.64.9.1242. [DOI] [PubMed] [Google Scholar]
- Li H, Yang H, Ding Y, Aprecio R, Zhang W, Wang Q, Li Y. Experimental periodontitis induced by Porphyromonas gingivalis does not alter the onset or severity of diabetes in mice. J Periodontal Res. 2013;48(5):582–590. doi: 10.1111/jre.12041. [DOI] [PubMed] [Google Scholar]
- Liang S, Hosur KB, Domon H, Hajishengallis G. Periodontal inflammation and bone loss in aged mice. J Periodontal Res. 2010;45(4):574–578. doi: 10.1111/j.1600-0765.2009.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Bi L, Yu X, Kawai T, Taubman MA, Shen B, Han X. Porphyromonas gingivalis exacerbates ligature-induced, RANKLdependent alveolar bone resorption via differential regulation of toll-like receptor 2 (TLR2) and TLR4. Infect Immun. 2014;82(10):4127–4134. doi: 10.1128/IAI.02084-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindhe J, Hamp SE, Loe H. Plaque induced periodontal disease in beagle dogs. A 4-year clinical, roentgenographical and histometrical study. J Periodontal Res. 1975;10(5):243–255. doi: 10.1111/j.1600-0765.1975.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Lindroth AM, Park YJ (2013) Epigenetic biomarkers: a step forward for understanding periodontitis. J Periodontal Implant Sci. 43(3):111–120. 10.5051/jpis.2013.43.3.111 [DOI] [PMC free article] [PubMed]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz W, Sanderson W, Scherbov S. The coming acceleration of global population ageing. Nature. 2008;451(7179):716–719. doi: 10.1038/nature06516. [DOI] [PubMed] [Google Scholar]
- Mannick JB, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6:268ra179. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- Martuscelli G, Fiorellini JP, Crohin CC, Howell TH. The effect of interleukin-11 on the progression of ligature-induced periodontal disease in the beagle dog. J Periodontol. 2000;71(4):573–578. doi: 10.1902/jop.2000.71.4.573. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489(7415):318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekada K, et al. Genetic differences among C57BL/6 substrains. Exp Anim. 2009;58(2):141–149. doi: 10.1538/expanim.58.141. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Strong R. An aging interventions testing program: study design and interim report. Aging Cell. 2007;6(4):565–575. doi: 10.1111/j.1474-9726.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- Nadon NL, Strong R, Miller RA, Nelson J, Javors M, Sharp ZD, Peralba JM, Harrison DE. Design of aging intervention studies: the NIA interventions testing program. Age (Dordr) 2008;30(4):187–199. doi: 10.1007/s11357-008-9048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazesh M. Dry mouth: aging and oral health. Compend Contin Educ Dent. 2002;23(10 Suppl):41–48. [PubMed] [Google Scholar]
- Ortman JM, Velkoff VA, Hogan H (2014) An aging nation: the older population in the United States. Curr Popul Reports
- Oz HS, Puleo DA. Animal models for periodontal disease. J Biomed Biotechnol. 2011;2011:754857. doi: 10.1155/2011/754857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- H. S. Oz, D. A. Puleo, Animal models for periodontal disease. Journal of biomedicine & biotechnology2011b, 754857 (2011) [DOI] [PMC free article] [PubMed]
- Oz HS, Chen T, Ebersole JL. A model for chronic mucosal inflammation in IBD and periodontitis. Dig Dis Sci. 2010;55(8):2194–2202. doi: 10.1007/s10620-009-1031-x. [DOI] [PubMed] [Google Scholar]
- E. K. Parkinson, E. L. James, S. S. Prime, in Gerontology. (2016), vol. 62, pp. 417–424 [DOI] [PubMed]
- Paul DS, Jones A, Sellar RS, Mayor NP, Feber A, Webster AP, Afonso N, Sergeant R, Szydlo RM, Apperley JF, Widschwendter M, Mackinnon S, Marsh SGE, Madrigal JA, Rakyan VK, Peggs KS, Beck S. A donor-specific epigenetic classifier for acute graft-versus-host disease severity in hematopoietic stem cell transplantation. Genome Medicine. 2015;7(1):128. doi: 10.1186/s13073-015-0246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DP (2010) Introduction to aging, cancer, and age-related diseases. Ann N Y Acad Sci1197, vii-x [DOI] [PubMed]
- Persson GR, Engel LD, Moncla BJ, Page RC. Macaca nemestrina: a non-human primate model for studies of periodontal disease. J Periodontal Res. 1993;28(4):294–300. doi: 10.1111/j.1600-0765.1993.tb02096.x. [DOI] [PubMed] [Google Scholar]
- P. E. Petersen, D. Bourgeois, H. Ogawa, S. Estupinan-Day, C. Ndiaye, in Bull World Health Organ. (2005), vol. 83, pp. 661–669 [PMC free article] [PubMed]
- Pitt JN, Kaeberlein M (2015) Why is aging conserved and what can we do about it? PLoS Biol 13(4):e1002131. 10.1371/journal.pbio.1002131. [DOI] [PMC free article] [PubMed]
- Polak D, Wilensky A, Shapira L, Halabi A, Goldstein D, Weiss EI, Houri-Haddad Y. Mouse model of experimental periodontitis induced by Porphyromonas gingivalis/fusobacterium nucleatum infection: bone loss and host response. J Clin Periodontol. 2009;36(5):406–410. doi: 10.1111/j.1600-051X.2009.01393.x. [DOI] [PubMed] [Google Scholar]
- Razak PA, Richard KM, Thankachan RP, Hafiz KA, Kumar KN, Sameer KM. Geriatric oral health: a review article. J Int Oral Health. 2014;6(6):110–116. [PMC free article] [PubMed] [Google Scholar]
- Salmon AB. Moving toward ‘common’ use of the marmoset as a non-human primate aging model. Pathobiology Aging Age Relat Dis. 2016;6(1):32758. doi: 10.3402/pba.v6.32758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaik-Dasthagirisaheb YB, Kantarci A, Gibson iii FC (2010) Immune response of macrophages from young and aged mice to the oral pathogenic bacterium Porphyromonas gingivalis. Immun Ageing 7:15. 10.1186/1742-4933-7-15. [DOI] [PMC free article] [PubMed]
- Shibutani T, Murahashi Y, Tsukada E, Iwayama Y, Heersche JN. Experimentally induced periodontitis in beagle dogs causes rapid increases in osteoclastic resorption of alveolar bone. J Periodontol. 1997;68(4):385–391. doi: 10.1902/jop.1997.68.4.385. [DOI] [PubMed] [Google Scholar]
- Sierra F, Kohanski R (2017) Geroscience and the trans-NIH GeroScience Interest Group. GSIG Geroscience 39(1):1–5. 10.1007/s11357-016-9954-6 [DOI] [PMC free article] [PubMed]
- Simon MM, Greenaway S, White JK, Fuchs H, Gailus-Durner V, Wells S, Sorg T, Wong K, Bedu E, Cartwright EJ, Dacquin R, Djebali S, Estabel J, Graw J, Ingham NJ, Jackson IJ, Lengeling A, Mandillo S, Marvel J, Meziane H, Preitner F, Puk O, Roux M, Adams DJ, Atkins S, Ayadi A, Becker L, Blake A, Brooker D, Cater H, Champy MF, Combe R, Danecek P, di Fenza A, Gates H, Gerdin AK, Golini E, Hancock JM, Hans W, Hölter SM, Hough T, Jurdic P, Keane TM, Morgan H, Müller W, Neff F, Nicholson G, Pasche B, Roberson LA, Rozman J, Sanderson M, Santos L, Selloum M, Shannon C, Southwell A, Tocchini-Valentini GP, Vancollie VE, Westerberg H, Wurst W, Zi M, Yalcin B, Ramirez-Solis R, Steel KP, Mallon AM, de Angelis M, Herault Y, Brown SDM. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol. 2013;14(7):R82. doi: 10.1186/gb-2013-14-7-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen WP, Loe H, Ramfjord SP. Periodontal disease in the beagle dog. A cross sectional clinical study. J Periodontal Res. 1980;15(4):380–389. doi: 10.1111/j.1600-0765.1980.tb00295.x. [DOI] [PubMed] [Google Scholar]
- Struillou X, Boutigny H, Soueidan A, Layrolle P. Experimental animal models in periodontology: a review. Open Dent J. 2010;4:37–47. doi: 10.2174/1874210601004010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida T, Hamakawa H. in. Oral Oncol. 2001;37(4):333–340. doi: 10.1016/S1368-8375(00)00132-9. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Mansfield KG, Ratnam R, Ross CN, Ziegler TE. The marmoset as a model of aging and age-related diseases. ILAR J/Natl Res Council, Inst Lab Anim Res. 2011;52(1):54–65. doi: 10.1093/ilar.52.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevaranjan N, et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21:455–466. doi: 10.1016/j.chom.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonetti MS, Jepsen S, Jin L, Otomo-Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J Clin Periodontol. 2017;44(5):456–462. doi: 10.1111/jcpe.12732. [DOI] [PubMed] [Google Scholar]
- Urfer SR, Kaeberlein TL, Mailheau S, Bergman PJ, Creevy KE, Promislow DEL, Kaeberlein M. A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. Geroscience. 2017;39(2):117–127. doi: 10.1007/s11357-017-9972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urfer SR, Kaeberlein TL, Mailheau S, Bergman PJ, Creevy KE, Promislow DEL, Kaeberlein M. Asymptomatic heart valve dysfunction in healthy middle-aged companion dogs and its implications for cardiac aging. Geroscience. 2017;39(1):43–50. doi: 10.1007/s11357-016-9956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki A, Nikai H, Niitani K, Ijuhin N. Ultrastructure of the junctional epithelium of germfree rat gingiva. J Periodontol. 1979;50(12):641–648. doi: 10.1902/jop.1979.50.12.641. [DOI] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellowitz JA, Schneiderman MT. Elder’s oral health crisis. J Evid Based Dent Pract. 2014;14:191–200. doi: 10.1016/j.jebdp.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Yin L, Chung WO. Epigenetic regulation of human β-defensin 2 and CC chemokine ligand 20 expression in gingival epithelial cells in response to oral bacteria. Mucosal Immunol. 2011;4(4):409–419. doi: 10.1038/mi.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R, Tsaih SW, Petkova SB, de Evsikova CM, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, Rosen CJ, Sundberg JP, Harrison DE, Churchill GA, Paigen B. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell. 2009;8(3):277–287. doi: 10.1111/j.1474-9726.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]