Abstract
The decline in basic and instrumental activities of daily living (BADLs and IADLs, respectively) is a well-established clinical hallmark of dementia. Growing evidence has shown that systemic subclinical inflammation may be related to functional impairment. We evaluated the possible association between low-grade systemic inflammation and functional disability in older individuals affected by dementia. We explored the association between high-sensitivity C-reactive protein (hs-CRP) levels and BADLs/IADLs in older individuals affected by late onset Alzheimer’s disease (LOAD; n 110), “mixed” dementia (n 135), or mild cognitive impairment (MCI; n 258), and compared them with 75 normal Controls. Independent of age, gender, comorbidity, and other potential confounders, higher hs-CRP was significantly associated with poorer BADLs (loss ≥ 1 function) in people with LOAD (odds ratio [OR] 3.14, 95% confidence interval [CI], 1.33–7.33) and mixed dementia (OR 2.48, 95%CI 1.12–5.55), but not in those with MCI (OR 1.38, 95%CI 0.83–2.45) or Controls (OR 2.98, 95%CI 0.54–10.10). No association emerged between hs-CRP and IADLs in any of the sub-group. Our data suggest that systemic low-grade inflammation may contribute to functional disability in older patients with dementia.
Electronic supplementary material
The online version of this article (10.1007/s11357-018-0010-6) contains supplementary material, which is available to authorized users.
Keywords: Inflammation, Dementia, Alzheimer’s disease, Mixed dementia, Functional abilities
Introduction
Chronic low-grade inflammation is regarded as one of the most relevant features characterizing aging and related diseases. In particular, increased levels of inflammatory markers in periphery have been widely associated with dementia in a number of studies. Accordingly, we found higher plasma IL-1beta and TNF-alpha levels in older patients with late onset Alzheimer’s disease (LOAD) or vascular dementia (VAD) compared to controls (Zuliani et al. 2007b). This study, along with many others, suggests that, in LOAD and VAD, inflammatory processes do not merely take place in the brain but also at systemic level, and it may play a role in disease progression and cognitive decline (Tan et al. 2007; Holmes et al. 2009; Cervellati et al. 2016).
As cognitive deterioration progresses, patients with LOAD or VAD experience a gradual loss in functional performance. Accordingly, both instrumental (IADLs) and basic (BADLs) ADLs (i.e., two basic aspects of functional status in the elderly) are currently included as essential clinical markers to distinguish mild cognitive impairment (MCI) from full-blown dementia (Knopman et al. 2003; American Psychiatric Association 2013).
It is known that a persistent low-grade systemic inflammation, even if subclinical, can affect physical and functional ability in older people (Kuo et al. 2006; Brinkley et al. 2009). Indeed, this condition, even when it is subclinical, has a number of consequences; for example, it leads to an accelerated protein catabolism and might negatively influence muscle strength (Cannon 1995; Kuo et al. 2006), which in turn relate to functional decline and disability (Kuo et al. 2006). High-sensitive C-reactive protein (hs-CRP) is the marker of choice for the evaluation of systemic inflammation and the most assessed in clinical studies, including those exploring the association between disability and inflammatory processes.
Homocysteine is another marker that is often measured in clinical practice because of its well-established association with cardiovascular diseases (CVDs) and the risk of stroke and dementia (Maccioni et al. 2010; Cervellati et al. 2014; Ganguly and Alam 2015). High peripheral levels of this thiol have been also found in association with poor physical performance in the general older population, independent of vitamins B12 and folate (i.e., major determinants of homocysteine concentration) (Kado et al. 2002; van Schoor et al. 2012). Various mechanisms have been hypothesized to explain the above-observed correlation. The most plausible hypothesis is based on the potential pro-oxidant and pro-inflammatory activity of homocysteine, that can alter muscle homeostasis “by inhibiting repair after muscle tissue injury and by promoting muscle proteolysis”(van Schoor et al. 2012).
Regardless of the concept that a decline in functional performance is an intrinsic aspect of the clinical progression of dementia, the possible association of systemic inflammation and homocysteine with physical function has not been yet deeply explored on these patients.
In light of these premises, the main aim to this study was to explore the association between hs-CRP and homocysteine serum levels and BADLs or IADLs in a large sample of older individuals affected by LOAD, mixed dementia, or MCI, and compared them to a sample of cognitively healthy controls.
Materials and methods
Subjects
Elderly Caucasian outpatients (≥ 65 years) consecutively admitted from 2006 to date to the Memory Clinic, Department of Internal Medicine, S. Anna University Hospital, Ferrara, Italy, have been enrolled. This research protocol was carried out accordingly to the Declaration of Helsinki (World Medical Association, http://www.wma.net) and the European Guidelines for Good Clinical Practice (European Medicines Agency, http://www.ema. europa.eu). The research protocol did not modify the routine clinical/diagnostic protocols implemented for the diagnosis of cognitive impairment/dementia in the memory clinic, nor conditioned any decision about the treatments of the enrolled individuals. All the participants (and/or their caregiver if demented) were informed about the research project and signed an informed consent.
Subjects affected by severe congestive heart failure (New York Heart Association class III–IV), severe liver or kidney disease, severe chronic obstructive pulmonary disease, and cancer were excluded. There were no evidences of acute illnesses at the time of clinical observation and blood sampling. No subject was taking NSAIDS, antibiotics, or steroids at the time of recruitment. Personal data and medical history were collected by trained personnel from eligible patients and/or caregivers. General and neuropsychological examination was carried as previously described (Cervellati et al. 2015a). Clinical chemistry analyses were routinely performed to exclude causes of secondary cognitive impairment. These analyses included serum B-12 vitamin, serum folate, liver function tests including ammonia, kidney function tests, thyroid function tests, complete blood cell count, and arterial oxygen saturation. Trained geriatricians made the diagnosis of dementia as described elsewhere (Cervellati et al. 2015b).
For the present study, only individuals with complete demographic, health status, and functional status information were included. The sample included:
One hundred ten individuals with mild-moderate late onset Alzheimer’s disease (LOAD) according to the National Institute of Neurological Disorders and Stroke—Alzheimer’s Disease and Related Disorders Association criteria (MMSE range 18–23) (CDR 1–2)
One hundred thirty-five with mild-moderate “mixed” dementia (MIXED). In these patients, a definite diagnosis of probable LOAD or VAD was not possible since both the clinical characteristics of LOAD and VAD were present. In particular, while CT scan or MRI demonstrated significant cerebrovascular disease, the evolution of the symptoms was slow and progressive (MMSE range 18–23) (CDR 1–2)
Two hundred fifty-eight individuals with mild cognitive impairment (MCI), defined as presence of short/long-term memory impairment, with/without impairment in other single or multiple cognitive domains, in an individual who did not meet the standardized criteria for dementia. The majority of these individuals were affected by amnestic multi-domain MCI. Subjects with MCI due to known causes (e.g., major depression, severe vitamin B-12 deficiency) were excluded (MMSE range 23–26)
Seventy-five with normal cognitive performance (MMSE range 26–29)
Functional status assessments
Functional status was measured at two levels:
Basic activity daily living (BADLs), assessed by considering the following six tasks: bathing, dressing, toileting, maintaining continence, feeding, and transferring (maximum score 6/6) (Katz et al. 1970).
Instrumental activities of daily living (IADLs), evaluated by assessing six tasks as follows: use of telephone, managing shopping, medications, finances, and use of transportation (maximum score 8/8) (Katz et al. 1970).
Both scales were scored as sum of items on which participants reported difficulty in performing a task, and scores on each scale were dichotomized as 0–1 (no difficulties vs 1 or more difficulties).
Assessment of serum hs-CRP and homocysteine levels
Venous blood was collected from subjects upon an overnight fast, between 8.30 and 9.30 A.M., and blood was centrifuged at 3000 rpm for 10 min. The isolated serum was then aliquoted and stored at − 80 °C until analysis.
Determination of hs-CRP level was based on a high-sensitivity enzyme-linked immunosorbent assay, using purified protein and polyclonal anti-CRP antibodies. The minimum detectable concentration was 0.03 mg/L. The average of two measures performed on each sample was used in the analysis. Both the intra-assay and inter-assay coefficients of variation were around 5%.
High hs-CRP was defined in the presence of serum levels > 0.5 mg/dL.
Homocysteine level was determined by the Liquid Stable (LS) 2-Part Homocysteine Reagent (Axis-Shield Diagnostics Ltd., UK) using ROCHE COBAS INTEGRA 800 chemistry analyzer following the manufacturer’s instructions. The concentrations of homocysteine, determined in reference to the calibration curve, were expressed in μmol/L. The intra-assay CV was 1.5%, whereas the inter-assay CV was 2.6%. Serum levels of vitamin B12 and folate were measured by routine laboratory methods.
Statistical analysis
Analysis of variance (ANOVA) with Sidak as post hoc test (for normal variables), Kruskall-Wallis with Wilcoxon-Mann-Whitney test followed by Bonferroni adjustment (non-normal variables), or chi-square test (categorical variables) was used for comparing the general characteristics of the sample. Analysis of covariance (ANCOVA) was performed to test whether the differences revealed at univariate analysis were independent of potential confounding factors. To examine the association between dichotomous IADLs or BADLs (loss of ≥ 1 activity) and hs-CRP (Log transformed to approach normal distribution) or homocysteine, univariate and multivariate logistic regression analyses were computed. Covariates included in the multi-adjusted models were as follows: age, sex (M/F), hypertension (Y/N), cardiovascular diseases (Y/N), diabetes (Y/N), stroke (Y/N), smoking habit (current/never), MMSE, hemoglobin, serum creatinine, and total cholesterol (plus vitamin B12 and folate for multivariate analysis involving homocysteine).
In all analyses, alpha level of 0.05 was accepted as statistically significant.
Results
Table 1 provides a summary of the socio-demographic, lifestyle, health status, and main laboratory characteristics of the study sample (n = 578). Females were prevalent in all the groups and reached the highest relative percentage in LOAD group (p < 0.01 compared to all). CONTROLS were slightly younger (p < 0.05 vs MIXED) and displayed a lower prevalence of CVD and stroke compared to the other groups (p < 0.001 vs MCI and MIXED). On the contrary, no significant difference in diabetes and hypertension frequency was found across the sample groups (p < 0.01 for all pairwise comparisons). As expected, LOAD and MIXED patients had significantly lower MMSE and greater disability, both in BADLs and IADLs, compared with CONTROLS and MCI (p < 0.01 for both pairwise comparisons). Patients with either the two types of dementia had significantly higher serum levels of hs-CRP compared to CONTROLS and MCI (p < 0.05 for both). On the contrary, only one significant difference was observed for homocysteine, with higher levels found in MIXED dementia compared to CONTROLS (p < 0.001). Of note, the differences in hs-CRP levels, but not that in homocysteine, were still significant after controlling for age, sex, smoking, and comorbidities.
Table 1.
Main characteristics of the sample according to diagnosis
| CONTROLS (n = 75) |
MCI (n = 258) |
LOAD (n = 110) |
MIXED (n = 135) |
|
|---|---|---|---|---|
| Age (years)* | 76 (69–80)c | 77 (73–81) | 78 (73–82) | 79 (75–83) |
| Sex (females, %)* | 60b | 56b | 74c | 62 |
| MMSE score* | 28 (26–29)b,c | 25 (23–26)b,c | 21 (18–23) | 21 (17–23) |
| Current smokers (%) | 6 | 6 | 9 | 5 |
| Hypertension (%) | 59 | 64 | 59 | 66 |
| Diabetes (%) | 17 | 16 | 16 | 16 |
| CVD (%)* | 12a,c | 19b | 13 c | 22 |
| Stroke (%)* | 3a,c | 7 b | 0 c | 8 |
| Creatinine (mg/dL)* | 0.8 (0.7–1.0) | 0.9 (0.7–1.1) | 0.9 (0.7–1.0) | 1.0 (0.8–1.1) |
| Total cholesterol | 213 ± 39 | 206 ± 41 | 212 ± 43 | 208 ± 43 |
| Hemoglobin | 13.7 ± 1.2a,b | 13.2 ± 1.7 | 13.0 ± 1.5 | 13.3 ± 1.4 |
| Vitamin B12 (pmol/L) | 363 (296–514) | 343 (263–466) | 334 (247–446) | 338 (250–444) |
| Folate (nmol/L) | 6.6 (5–8.7) | 6 (4.6–7.9) | 5.5 (4.4–7.3) | 5.8 (4.7–8.1) |
| IADLs* | 8 (7–8)a,b,c | 8 (4–8)b,c | 4 (2–6) | 4 (1–6) |
| BADLs* | 6 (5–6)b,c | 6 (5–6)b,c | 5 (4–6) | 5 (4–6) |
| Hs-CRP (mg/dL)# | 0.13 (0.06–0.24)b,c | 0.16 (0.09–0.40)b,c | 0.30 (0.11–0.63) | 0.24 (0.10–0.47) |
| Homocysteine (μmol/L) | 13 (9–18)c | 15 (11–22) | 17 (13–22) | 15 (11–22) |
Mean ± SD for normally distributed variables; median (interquartile range) for not-normally distributed variables. post hoc test: a: p < 0.05 vs MCI; b: p < 0.05 vs LOAD; c: p < 0.05 vs MIXED
MCI mild cognitive impairment, LOAD late onset Alzheimer’s disease, CVD cardiovascular disease, IADL instrumental activities of daily living, BADL basic activity of daily living
*p < 0.05 Kruskal-Wallis (median) or Chi-squared test (prevalence)
#p < 0.05 with ANCOVA (covariates: age, sex, smoking, hypertension, diabetes, stroke, and CVD)
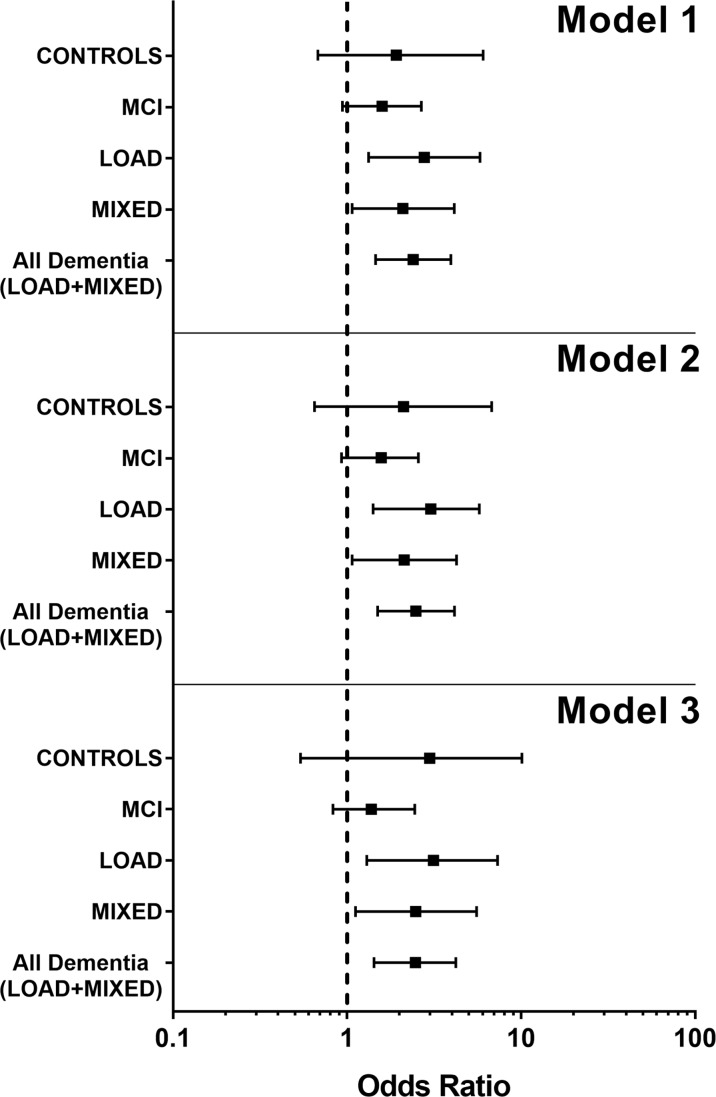
Simple and multi-adjusted associations between hs-CRP or homocysteine and functional status scores were determined with each subset of the sample (Table 2 and supplementary Table 1). From the analysis of the subsamples, different association between hs-CRP and the two disability measures emerged. The loss of ≥ 1 BADL was significantly and independently associated with high hs-CRP in people with dementia but not in CONTROLS and (or) MCI (Fig. 1); considering the two types of dementia examined in this study, the levels of hs-CRP seemed more closely associated with BADLs in LOAD than in MIXED dementia (Fig. 1). On the contrary, IADLs difficulties were not associated with hs-CRP levels, neither in people with dementia nor in CONTROLS. The only exception in this trend was the MCI group, where the association remained significant after controlling for age and sex (p < 0.05), but disappeared after adding the other covariates to the analysis. Notably, MMSE score was negatively associated with the risk of disability in BADLs (p < 0.01) and IADLs (p < 0.001) (the higher the MMSE score, the lower the risk of disability), but was not correlated with hs-CRP levels (supplementary Table 2).
Table 2.
Associations of (log10) hs-CRP (explanatory variables) and BADLs or IADLs (response variables) as assessed by simple and multivariate logistic regression analysis
| Response* variables | CONTROLS n = 75# |
MCI n = 258 |
LOAD n = 110 |
MIXED n = 135 |
All dementia n = 245 |
|---|---|---|---|---|---|
| ≥ 1 lost BADLs | |||||
| Model 1 | 1.92 (0.68–6.05) | 1.59 (0.94–2.67) | 2.78 (1.33–5.81) | 2.09 (1.07–4.13) | 2.40 (1.46–3.95) |
| Model 2 | 2.11 (0.65–6.78) | 1.57 (0.93–2.57) | 3.03 (1.41–5.75) | 2.13 (1.07–4.26) | 2.49 (1.50–4.14) |
| Model 3 | 2.98 (0.54–10.10) | 1.38 (0.83–2.45) | 3.14 (1.30–7.33) | 2.48 (1.12–5.55) | 2.47 1.43–4.22) |
| ≥ 1 lost IADLs# | |||||
| Model 1 | 2.36 (0.76–7.37) | 2.43 (1.08–5.41) | 0.82 (0.31–2.19) | 2.58 (0.51–12.80) | 1.16 (0.50–2.69) |
| Model 2 | 1.44 (0.40–5.15) | 2.73 (1.18–6.32) | 0.85 (0.33–2.19) | 3.87 (0.72–18.50) | 1.26 (0.56–2.87) |
| Model 3 | 1.87 (0.40–7.59) | 2.05 (0.84–5.30) | 0.58 (0.14–2.58) | 5.00 (0.35–72.41) | 1.59 (0.60–4.23) |
Within the table, the italicized terms correspond to the significant models
Model 1: unadjusted; Model 2: adjusted for age and gender; Model 3: adjusted for age, gender, MMSE, diabetes, hypertension, stroke, cardiovascular disease, smoking, creatinine, hemoglobin, and total cholesterol levels
BADLs basic activities of daily living; IADLs instrumental activities of daily living, hs-CRP, high sensitivity C-reactive protein; MCI mild cognitive impairment; LOAD late onset Alzheimer disease; MIXED mixed dementia
*Results of logistic regression computed for BADLs and IADLs are reported as odds ratio (95% confidence intervals)
#IADLs were assessed in 362/578 sample subjects (CONTROLS, n = 71; MCI, n = 135; LOAD, n = 87; MIXED, n = 69)
Fig. 1.
Forest plot showing the ODDS ratio as a measure of association between BADL decline (≥ 1 lost BADLs) and hs-CRP in the considered groups of subjects. Model 1 represents the unadjusted model, Model 2 the model adjusted for age and gender, and Model 3 represents the model adjusted for all covariates and possible confounding factors (see Table 2 for reference). As can be seen, in all the three models, the decline in BADL activities was significantly associated with inflammation only in the LOAD, MIXED, and All Dementia groups, with the LOAD group showing the greater association. The ODDS ratio is represented by the square in the center; the horizontal bar shows the range of ODDS ratio
Homocysteine showed the same trend of hs-CRP, with higher levels of the marker associated with higher BADL or AIDL (supplementary Table 3). However, unlike hs-CRP, the associations between homocysteine and either of the two functional status scores were not significant, even before the adjustment for covariates (including vitamin B12 and folate). The only exception was represented by the, although barely, significant and independent relationship between homocysteine and BADL observed in MCI subsample (odds ratio, 95% confidence 1.02, 1.00–1.05). Finally, no significant association was observed between homocysteine and MMSE (data not shown).
Discussion
It is well recognized that the cognitive decline associated with dementia is a very important determinant for the development of functional disability. However, the etiology of disability in the elderly is much more complex, and involves processes of non-physical (i.e., cognitive, social, behavioral) and physical decline. A wealth of evidence points to chronic subclinical systemic inflammation as one of the organic abnormalities leading to functional impairment, and interestingly, this “latent” condition has been associated with dementia. One of the proposed mechanisms underlying the adverse effects of inflammation to functional status, and to general health, is the mutual and close connection between this condition and oxidative stress (Pecorelli et al. 2016). It is well-known that homocysteine stimulates the production of reactive oxygen species (ROS), and pro-inflammatory cytokines, which have a well-established role in the pathogenesis of many pathological conditions, including dementia (Ganguly and Alam 2015). Besides, hyperhomocysteinemia has been found to be related with worse physical performance in some recent large population-based studies (Kado et al. 2002; Soumaré et al. 2006; van Schoor et al. 2012). In this light, we evaluated the possible contribution of systemic inflammation and homocysteine to BADLs/IADLs deficits in patients with dementia or MCI.
The principal finding of our study was that hs-CRP levels were directly associated with disability in BADLs among demented individuals and, within this group, more closely in those affected by LOAD compared to MIXED. Noteworthy, these associations were independent of important confounders including MMSE score, demographic factors, comorbidity, and other parameters known to affect the functional status. Different data emerged from the analyses of IADLs. Indeed, loss in IADLs was significantly associated with hs-CRP levels (p < 0.01 for both unadjusted and age-sex adjusted models), but this association disappeared after multivariate adjustment.
Differently from hs-CRP, homocysteine did not show any relevant associations with the disability scores considered in the study. The apparent discrepancy with the aforementioned results might be due to evident differences in sample size, demographic, and clinical characteristics of the subjects (only cognitively normal, and in one case highly functional (Kado et al. 2002)), and in the tests used for the assessment of functional status (which were more specifically addressed to the determination of muscle strength, balance, and coordination (van Schoor et al. 2012)).
A number of studies previously reported significant associations between peripheral markers of inflammation (such as tumor necrosis factor, interleukin-6, CRP) and measures of physical functioning in community dwelling elderly people (Ferrucci et al. 1999; Reuben et al. 2002; Ravaglia et al. 2004; Kuo et al. 2006; Aiello et al. 2008; Haren et al. 2010) or exclusively in demented patients (Zuliani et al. 2007a). Nevertheless, only Aiello et al. considered dementia as a factor influencing the association between CRP and BADLs/IADLs (Aiello et al. 2008); these authors did not assess the “weight” of dementia (only 5% of the sample) on the risk, but simply included it in multivariate analysis (Aiello et al. 2008). Interestingly, regardless of differences in study design and sample characteristics, the effect size reported by Aiello et al. was comparable to ours, considering the whole sample (see supplementary Table 2) (our study BADLs, OR 1.98; IADLs, OR 2.16; Aiello et al.’s study: BADLs, OR 1.92; IADL, OR 1.98). Also, previous findings from our group on a smaller cohort of individuals suffering from dementia were grossly in line with the present data. Indeed, we found a significant positive association between interleukin-6, a well-known upstream regulator of CRP, and decline in functional status in these patients (Zuliani et al. 2007a).
Similarly, Haren et al. by examining the relationship between CRP and functional status in a sample of late middle-aged African Americans, reported results similar to ours (BADLs, OR 2.13; IADLs, OR 2.13) (Haren et al. 2010). Noteworthy, since they included subjects with MMSE < 16, an undefined number of people with dementia was included in their study. In contrast, effect sizes for the CRP association were larger in our cohort compared to National Health and Nutrition Examination Survey (NHANES) (BADLs, OR 1.11, IADLs, OR 1.18) (Kuo et al. 2006). These discrepancies might depend from the difference in the selection criteria; while the NHANES sample is representative of community dwelling elderly living in the USA (Kuo et al. 2006), our data cannot be generalized, since subjects were outpatients of an Italian hospital memory clinic. However, the data of Kuo et al. (2006) can help us to interpret our findings. From multivariate analysis, they found that muscle power and/or walking speed mediated the association between disability and elevated CRP. This effect might be even stronger in people with dementia, since cognitive impairment is closely related to decline in strength, to development of functional disability, and to the loss of independence (Njegovan et al. 2001; Auyeung et al. 2008).
The relationship we found between BADLs and hs-CRP is clearly supportive of this hypothesis, while the lack of association with IADLs might be explained by the intrinsic differences between these two measures of functional status. By definition, IADLs consist of more complex activities (i.e., using public transportation, managing finances, shopping, etc.) compared to BADLs, which includes self-maintenance skills such as bathing or eating (Jekel et al. 2015). As a consequence, IADLs need more composite neuropsychological processing capacity and are more directly associated with brain cognitive functions (Agüero-Torres et al. 2002). Accordingly, it is tempting to speculate that BADLs might be more susceptible than other functional abilities to the deteriorative effect of peripheral and persistent pathophysiological conditions, such as systemic chronic inflammation. Thus, in full-blown dementia, where the impairment of cognitive functions has (by definition) already harmed the ability to perform the more complex tasks, such as IADLs, the possible additional negative effect of systemic inflammation may reveal mostly in BADLs.
An opposite trend was observed in the MCI group; in these patients, a much larger contribution of inflammation to IADLs impairment was observed, compared to that BADLs. Actually, in line with previous studies (Mariani et al. 2008; Ahn et al. 2009; Pedrosa et al. 2010; Jekel et al. 2015), we found that some deficits in instrumental, but not in basal activities, may be present in MCI (Table 2). Thus, patients with MCI had “intermediate” cognitive, but also functional, performance between healthy individuals and patients with dementia. Our findings seem to suggest that in this clinical condition, the degree of cognitive deficit is not sufficiently severe to completely mask the possible contribution of systemic inflammation to the impairment of most complex functional activities (IADLs).
Interestingly, as reviewed by Jekel and colleagues (Jekel et al. 2015), the IADL deficits are more pronounced in the MCI subjects with greater risk of conversion to LOAD. Thus, it might be reasonably hypothesized that systemic inflammation could influence the probability of conversion to dementia also through detrimental interactions with functional ability.
Finally, some important limitations of the study must be emphasized. First, the cross-sectional design precludes the ability to determine any cause-and-effect relationships between the measured variables. Second, we did not measure either other pro-inflammatory cytokines, such as interleukin-6, tumor necrosis factor, or anti- or pro-inflammatory lipids, such as leukotrienes and prostaglandins, which could provide a more exhaustive picture of the inflammatory state. However, we purposely chose to measure only hs-CRP for several reasons: (i.) high clinical and analytical accuracy, (ii.) low cost, and (iii.) it is the marker of inflammation most assessed in clinical routine, especially in geriatric patients. Third, some important potential confounders (e.g., body mass index and serum albumin) were not evaluated in this study. Fourth, we are aware that the relatively small size of subsamples may have affected the reliability of the observed associations.
In conclusion, our results suggest that low-grade systemic inflammation may contribute to the loss in functional performance in elderly patients affected by cognitive impairment, independent of possible confounding factors. In particular, systemic inflammation seems to be associated with loss of functions in IADLs in subjects with MCI, and with loss of BADLs in patient affected by dementia. A cause-effect relationship between inflammatory state and development of functional disability might be definitely ascertained by longitudinal studies.
Electronic supplementary material
(DOCX 21 kb)
Compliance with ethical standards
Conflict of interest
The authors declare that they no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s11357-018-0010-6) contains supplementary material, which is available to authorized users.
References
- Agüero-Torres H, Thomas VS, Winblad B, Fratiglioni L. The impact of somatic and cognitive disorders on the functional status of the elderly. J Clin Epidemiol. 2002;55(10):1007–1012. doi: 10.1016/S0895-4356(02)00461-4. [DOI] [PubMed] [Google Scholar]
- Ahn IS, Kim J-H, Kim S, Chung JW, Kim H, Kang HS, Kim DK. Impairment of instrumental activities of daily living in patients with mild cognitive impairment. Psychiatry Investig. 2009;6(3):180–184. doi: 10.4306/pi.2009.6.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello AE, Haan MN, Pierce CM, Simanek AM, Liang J. Persistent infection, inflammation, and functional impairment in older Latinos. J Gerontol A Biol Sci Med Sci. 2008;63(6):610–618. doi: 10.1093/gerona/63.6.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (5th ed.)
- Auyeung TW, Kwok T, Lee J, Leung PC, Leung J, Woo J. Functional decline in cognitive impairment—the relationship between physical and cognitive function. Neuroepidemiology. 2008;31(3):167–173. doi: 10.1159/000154929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley TE, Leng X, Miller ME, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64:455–461. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JG (1995) Cytokines in aging and muscle homeostasis. J Gerontol A Biol Sci Med Sci 50 Spec No:120–3 [DOI] [PubMed]
- Cervellati C, Romani A, Seripa D, Cremonini E, Bosi C, Magon S, Passaro A, Bergamini CM, Pilotto A, Zuliani G. Oxidative balance, homocysteine, and uric acid levels in older patients with late onset Alzheimer’s disease or vascular dementia. J Neurol Sci. 2014;337(1-2):156–161. doi: 10.1016/j.jns.2013.11.041. [DOI] [PubMed] [Google Scholar]
- Cervellati C, Romani A, Bergamini CM, Bosi C, Sanz JM, Passaro A, Zuliani G. PON-1 and ferroxidase activities in older patients with mild cognitive impairment, late onset Alzheimer’s disease or vascular dementia. Clin Chem Lab Med. 2015;53(7):1049–1056. doi: 10.1515/cclm-2014-0803. [DOI] [PubMed] [Google Scholar]
- Cervellati C, Trentini A, Romani A, Bellini T, Bosi C, Ortolani B, Zurlo A, Passaro A, Seripa D, Zuliani G. Serum paraoxonase and arylesterase activities of paraoxonase-1 (PON-1), mild cognitive impairment, and 2-year conversion to dementia: a pilot study. J Neurochem. 2015;135(2):395–401. doi: 10.1111/jnc.13240. [DOI] [PubMed] [Google Scholar]
- Cervellati C, Wood PL, Romani A, Valacchi G, Squerzanti M, Sanz JM, Ortolani B, Zuliani G. Oxidative challenge in Alzheimer’s disease: state of knowledge and future needs. J Investig Med. 2016;64(1):21–32. doi: 10.1136/jim-2015-000017. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47(6):639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14(1):6. doi: 10.1186/1475-2891-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haren MT, Malmstrom TK, Miller DK, Patrick P, Perry HM, Herning MM, Banks WA, Morley JE. Higher C-reactive protein and soluble tumor necrosis factor receptor levels are associated with poor physical function and disability: a cross-sectional analysis of a cohort of late middle-aged African Americans. J Gerontol A Biol Sci Med Sci. 2010;65(3):274–281. doi: 10.1093/gerona/glp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, Culliford D, Perry VH. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73(10):768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jekel K, Damian M, Wattmo C, Hausner L, Bullock R, Connelly PJ, Dubois B, Eriksdotter M, Ewers M, Graessel E, Kramberger MG, Law E, Mecocci P, Molinuevo JL, Nygård L, Olde-Rikkert MGM, Orgogozo JM, Pasquier F, Peres K, Salmon E, Sikkes SAM, Sobow T, Spiegel R, Tsolaki M, Winblad B, Frölich L. Mild cognitive impairment and deficits in instrumental activities of daily living: a systematic review. Alzheimers Res Ther. 2015;7(1):17. doi: 10.1186/s13195-015-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado DM, Bucur A, Selhub J, Rowe JW, Seeman T. Homocysteine levels and decline in physical function: MacArthur studies of successful aging. Am J Med. 2002;113(7):537–542. doi: 10.1016/S0002-9343(02)01269-X. [DOI] [PubMed] [Google Scholar]
- Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10(1 Part 1):20–30. doi: 10.1093/geront/10.1_Part_1.20. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Boeve BF, Petersen RC. Essentials of the proper diagnoses of mild cognitive impairment, dementia, and major subtypes of dementia. Mayo Clin Proc. 2003;78(10):1290–1308. doi: 10.4065/78.10.1290. [DOI] [PubMed] [Google Scholar]
- Kuo H-K, Bean JF, Yen C-J, Leveille SG. Linking C-reactive protein to late-life disability in the National Health and Nutrition Examination Survey (NHANES) 1999-2002. J Gerontol A Biol Sci Med Sci. 2006;61(4):380–387. doi: 10.1093/gerona/61.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni RB, Farías G, Morales I, Navarrete L. The revitalized tau hypothesis on Alzheimer’s disease. Arch Med Res. 2010;41(3):226–231. doi: 10.1016/j.arcmed.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Mariani E, Monastero R, Ercolani S, Rinaldi P, Mangialasche F, Costanzi E, Vitale DF, Senin U, Mecocci P, for the ReGAl Study Group Influence of comorbidity and cognitive status on instrumental activities of daily living in amnestic mild cognitive impairment: results from the ReGAl project. Int J Geriatr Psychiatry. 2008;23(5):523–530. doi: 10.1002/gps.1932. [DOI] [PubMed] [Google Scholar]
- Njegovan V, Hing MM, Mitchell SL, Molnar FJ. The hierarchy of functional loss associated with cognitive decline in older persons. J Gerontol A Biol Sci Med Sci. 2001;56(10):M638–M643. doi: 10.1093/gerona/56.10.M638. [DOI] [PubMed] [Google Scholar]
- Pecorelli A, Cervellati C, Hayek J, Valacchi G. OxInflammation in Rett syndrome. Int J Biochem Cell Biol. 2016;81(Pt B):246–253. doi: 10.1016/j.biocel.2016.07.015. [DOI] [PubMed] [Google Scholar]
- Pedrosa H, De Sa A, Guerreiro M, et al. Functional evaluation distinguishes MCI patients from healthy elderly people—the ADCS/MCI/ADL scale. J Nutr Health Aging. 2010;14(8):703–709. doi: 10.1007/s12603-010-0102-1. [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Brunetti N, Martelli M, Talerico T, Bastagli L, Muscari A, Mariani E. Peripheral blood markers of inflammation and functional impairment in elderly community-dwellers. Exp Gerontol. 2004;39(9):1415–1422. doi: 10.1016/j.exger.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Reuben DB, Cheh AI, Harris TB, Ferrucci L, Rowe JW, Tracy RP, Seeman TE. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc. 2002;50(4):638–644. doi: 10.1046/j.1532-5415.2002.50157.x. [DOI] [PubMed] [Google Scholar]
- Soumaré A, Elbaz A, Ducros V, et al. Cross-sectional association between homocysteine and motor function in the elderly. Neurology. 2006;67(6):985–990. doi: 10.1212/01.wnl.0000237325.16502.08. [DOI] [PubMed] [Google Scholar]
- Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, Benjamin EJ, Au R, Kiel DP, Wolf PA, Seshadri S. Inflammatory markers and the risk of Alzheimer disease: the Framingham study. Neurology. 2007;68(22):1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- van Schoor NM, Swart KMA, Pluijm SMF, Visser M, Simsek S, Smulders Y, Lips P. Cross-sectional and longitudinal association between homocysteine, vitamin B12 and physical performance in older persons. Eur J Clin Nutr. 2012;66(2):174–181. doi: 10.1038/ejcn.2011.151. [DOI] [PubMed] [Google Scholar]
- Zuliani G, Guerra G, Ranzini M, Rossi L, Munari MR, Zurlo A, Blè A, Volpato S, Atti AR, Fellin R. High interleukin-6 plasma levels are associated with functional impairment in older patients with vascular dementia. Int J Geriatr Psychiatry. 2007;22(4):305–311. doi: 10.1002/gps.1674. [DOI] [PubMed] [Google Scholar]
- Zuliani G, Ranzini M, Guerra G, Rossi L, Munari MR, Zurlo A, Volpato S, Atti AR, Blè A, Fellin R. Plasma cytokines profile in older subjects with late onset Alzheimer’s disease or vascular dementia. J Psychiatr Res. 2007;41(8):686–693. doi: 10.1016/j.jpsychires.2006.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 21 kb)