Abstract
Purpose
The aim was to systematically extrapolate the occurrence, risk factors, prognostic characteristics, management and outcome of bone metastases (BM) and skeletal related events (SREs) of breast cancer survivors in the real world clinical setting.
Methods
A systematic literature search of PubMed, Web of Science, EMBASE OvidSP and EBSCO Academic Search Complete was conducted. Published prospective and retrospective papers investigating BM and SREs in breast cancer patients in non-trial settings were identified and systematically reviewed.
Results
Twenty-four studies met the inclusion criteria. Incidences of BM based on new diagnosis, length of BM-free interval (BMFI) and number and sites of BM were detected by 17 of 24 studies. Seven studies included in the review were subjected to analyses of risk factors for BM. Developments of SREs regarding the occurrence ratio of total and specific SREs, SERs-free interval (SREFI) and the first-line therapy for SREs were observed in 16 of 24 studies. Out of 5 studies, we extracted uni- and multivariate analysis of risk factor for SREs and out of 16 studies - predictors for survival in breast cancer patients with BM.
Conclusions
BM and SREs are common problems in non-trial breast cancer populations. Patient demographics, clinical stage, tumor pathological type, molecular receptors status are significantly risk factors for incidence of BM, SREs and the survival. The unique characteristics of BM and SREs in breast cancer patients should be taken into account in future randomized controlled trials, as to optimize individual treatment options and assure a maximally long good quality of life.
Keywords: Breast cancer, Bone metastases, Skeletal related events, Real-world data, Systematic review
1. Background
Breast cancer is the most common cancer in females worldwide [1], [2]. Significant progress in prophylaxis, diagnosis and management of breast cancer has been made, especially in the last decade [3], [4]. However, female deaths by breast cancer did not decrease since advances in treatment merely compensated for the increasing incidence originating from demographical development and lifestyle changes [5], [6]. Distant metastases are still the leading cause of death in breast cancer patients [7], [8].
Bone is the most frequent site of breast cancer metastasis [9], [10]. At the time of diagnosis of breast cancer approximately 5–6% of women present themselves with bone metastases (BM). In advanced stages of breast cancer, about 65–75% of patients eventually develop BM [11], [12]. BM is associated with accelerated bone resorption leading to increased morbidity due to a range of skeletal-related events (SREs) including bone pain (BP), pathological fracture (PF), spinal cord compression (SCC), tumor-induced hypercalcemia (TIH) and surgery or radiation therapy (RT) to bone [13]. Not surprisingly, SREs often worsen quality of life, performance status, and independent functioning. Studies have demonstrated that at least one SRE occurs in nearly 50% of patients with bone metastases of breast cancer [14], [15]. Given the high prevalence of breast cancer, the population wide burden of BM is considerable. Thus, it is of immense important to analyze SREs and BM in the context of diagnosis, therapy and follow up.
Management options of breast cancer are based on surgical interventions, RT, neo- and adjuvant chemotherapy (ChT), hormonal therapy (HT) or molecular-targeted therapy (MT). The optimal, personalized management however, varies between patients according to cancer entity and physical status of the patient. In consequence comparability of patients with BM in clinical trials is limited. Furthermore few single-center, multi-center, and population-based studies specifically reporting BM and SREs exist. So far the systematic review or meta-analysis of these data is lacking. Therefore, we conducted review, focusing on incidence, risk factors, prognostic characteristics, management and outcome of BM and SREs in breast cancer patients. Our data provide the first coherent dataset that can be used for adjustments in care of breast cancer patients with BM and SREs in order to assure the best possible outcome, as well as to avoid an over-or under-treatment with BM and SREs.
2. Methods
Several breast surgeons, a medical oncologist and a medical statistician formed the panel to develop the search, selection, and review strategies, based on guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [16], [17].
2.1. Sources and search strategy
Literature research was performed independently by two trained reviewers (GF.H. and E.B.) using Pubmed, Web of Science, EMBASE OvidSP and EBSCO Academic Search Complete for articles published between 2000 and 2017 on English-language studies related to breast cancer, BM and SREs. The search was conducted using Medical Subject Headings (MeSH) or keywords, and when appropriate, search terms. Search terms were Boolean search criteria and included “Breast Neoplasms”, “Breast Cancer”, “Breast Carcinoma” OR “Breast Tumor*” and “Bone metastases”, “Bone metastasis”, “Metastasis of Bone”, “Metastases of Bone”, “Skeletal metastases”, “Skeletal metastasis”, “Skeletal complication*” OR “Skeletal-related event*”. Further manuscripts were identified from reference lists of the primary papers. The last search was performed on June 11th, 2017. Detailed search methods are provided in the supplemental file (Appendix A, Appendix B, Appendix C, Appendix D).
2.2. Eligibility criteria
The records obtained from the literature search, containing titles and abstracts of the reviews, were exported into Refworks. First, duplicates were identified and removed from the pool of bibliographic records. Then, two trained investigators (GF.H. and E.B.) independently screened all retrieved abstracts and titles to determine articles that were ‘‘potentially’’ and deemed ‘‘relevant’’ references. Afterwards, two further reviewers (W.Y., H.W.) independently reviewed the full articles, using the following inclusion criteria: (1) single-center, multi-center or population-based clinical studies, focusing on breast cancer patients with BM; (2) studies that provided clinical information and specific data on the outcome of patients with BM from breast cancer. Studies were excluded if (1) they were single case reports, regular reviews or systematic review articles; (2) clinical trials focusing on breast cancer treatment; (3) studies on metastatic breast cancer focusing on visceral metastases; (4) investigating other cancers besides metastatic breast cancer. Disagreements were resolved by consulting with three additional reviewers (W.Z., Z.Y., H.Z.). When studies of overlapping groups of patients were identified, only the most recent studies were retained, with the notable exception of earlier studies presenting analyses that were not repeated in the most recent study.
2.3. Quality assessment
Two reviewers (C.Z. and GX.H.) independently assessed the quality of all included studies according to the Newcastle-Ottawa Scale (NOS) [18], [19]. The NOS has been developed to assess the quality of case–control and cohort studies, containing three parameters of quality that included: (1) selection; (2) comparability; and (3) exposure/ outcome assessment. Studies that achieved five or more points were considered to be of high quality. Any discrepancies between reviewers were addressed by a joint reevaluation of the original article.
2.4. Data abstraction
Two investigators (GF.H. and C.Z.) independently abstracted the data from the included articles. First author's name, publication year of the article, patients’ data (demographics, tumor characteristics, BM) were extracted from each study. Any univariate and multivariate analysis for risk factors for BM and SREs or prognostic factors affecting survival in patients with breast cancer BM were tabulated. Whenever possible, diagnostics of BM, development of SREs, treatment information and prognostic outcomes were extracted. If these data were not mentioned explicitly in the manuscripts (e.g. number of SREs), they were extrapolated from graphs, tabulated proportions of events or from subgroup analyses. Any disagreements were discussed to reach a consensus agreement.
3. Results
3.1. Literature search
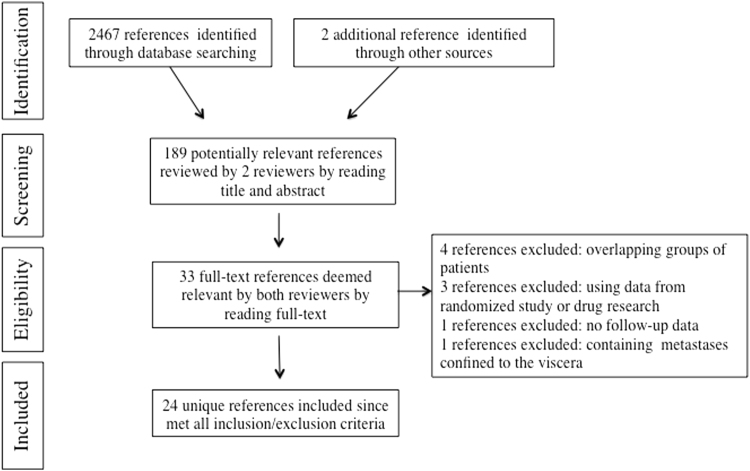
The literature search yielded 2469 bibliographic records. Of this initial pool of records, 2280 were excluded after the first screen of the titles and abstracts. Following the full-text review, 156 studies were rejected for being out of scope. Of the remaining 33 records, nine were removed applying the exclusion criteria. The final set of bibliographic records reviewed was composed of 24 studies [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43] (Fig. 1).
Fig. 1.
PRISMA flow diagram detailing the strategy adopted for the literature search described in this article.
3.2. Assessment of methodologic quality
The results of the quality assessment according to the NOS are shown in supplemental file (Appendix E). In total, 24 studies were included and all of which were assessed as high quality: One study [24] was rated with a NOS score of six, eight studies [23], [26], [28], [33], [36], [37], [41], [42] with a NOS score of seven, six studies [21], [22], [32], [34], [39], [40] with a NOS score of eight, seven studies [20], [25], [27], [31], [35], [38], [43] with a NOS score of nine, and two studies [29], [30] with a NOS score of ten.
3.3. Characteristics of the studies
Characteristics of the studies regarding study type and sample size, BM occurrence rates, patient demographics, tumor histopathological findings and clinical stage, estrogen receptor (ER) expression status, progesterone receptor (PR) expression status, epidermal growth factor receptor 2 (HER2) status, follow-up period are described in Table 1. The 24 studies selected according to the inclusion criteria were published between 2000 and 2016. The median follow-up period ranged from 1.12 [21] to 12.50 years [20]. The BM occurrence rates ranged from 4.1% [40] to 30% [30]. The number of patients enrolled ranged from 48 [37] to 7189 patients [32], of whom only one was male [23]. The median patients age at the time of diagnosis of breast cancer ranged from 46 [38] to 75 [32]. Premenopausal status reported in eight studies ranged from 13% [39] to 80% [30]. In total, hormone receptor (ER and/or PR) positive breast cancer was most common, followed by HER2 (ER-PR-) positive breast cancer and triple negative breast cancer (TNBC). At the time of diagnosis, most breast cancer patients had TNM stage I-III. Synchronous bone-only metastases at the time of diagnosis were reported in four studies [22], [33], [34], [39].
Table 1.
Cohort characteristics of patients at time of diagnosis of breast cancer included studies.
| Refs. | Year | Country | Study | THC | Total N | BM N (%) | Median/Mean Age (range) | M/F | Menopausal Status N (%) | ER//PR/HER2 N (%) | Stage N (%) | Median/Mean (range) F/U yr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liede A [20]a | 2016 | Canada | Single-center, prospective | 1987–2000 | 2097 | 257 (13.2) | 54.3(22–94) | F | NA | ER 1402(66.9), PR 1149(54.8), HER2 291(13.9) | I 352(16.8), II 807(38.5), III 611(29.1), NA 326(15.6) | 12.50(0.06–27) |
| Cetin K [21] | 2015 | Denmark | Population-based, retrospective | 1997–2011 | NA | 2427 (NA) | 63(28–97) | F | NA | ER 1859(76), PR NA, HER2 393(16) | I-III 1821(75), IV 606(25) | 1.12b |
| Dibekoglu C [22] | 2015 | Turkey | Single-center, retrospective | 1993–2006 | NA | 139 (NA) | 50(23–81) | F | Pre- 56(40) | ER 107(77), PR 78(56), HER2 31(22) | I-III 95(68), IV (BM) 44(32) | 3.42(0.67–12.67)b |
| Bollen L [23]c | 2015 | Netherlands | Multi-center, retrospective | 2005–2012 | NA | 111 (NA) | 59.9 | F 110, M 1 | NA | HR+ 76(68), HER2+ 11(10), TNBC 24(22) | NA | 3.20(0.60–5.50)b |
| Foerster R [24]c | 2015 | Germany | Single-center, retrospective | 2000–2012 | NA | 92 (NA) | 60.8 | NA | NA | HR+ 63(68), HER2+ 9(10), TNBC 20(22) | NA | NA |
| Harries M [25]a | 2014 | U.K. | Single-center, retrospective | 1976–2006 | 7064 | 1589 (22) | NA | F | NA | ER 332(21), PR 531(33), HER2 478(30) | NA | 8.40 |
| Steinauer K [26] | 2014 | Switzerland | Single-center, retrospective | 1990–2009 | NA | 237 (NA) | 63(28–91) | F | NA | NA | NA | NA |
| Yamashiro H [27]a | 2014 | Japan | Multi-center, retrospective | 2003–2005 | 1708 | 193 (11.3) | 58(24–93) | F | Pre- 384(22.5) | ER 1099(64.3), PR 892(52.2), HER2 280(16.4) | I 336(19.7), II 963(56.4), III 226(15.6), IV 39(2.3) | 5.71(0.04–8.12) |
| Arican A [28] | 2014 | Turkey | Multi-center, cross-sectional | 2010–2011 | NA | 1026 (NA) | 54(22–87) | F | Pre- 801(78) | NA | I 44(4), II 267(26), III 331(32), IV 346(34) | 1.30(0.00–13.70) |
| Kuchuk I [29]d | 2013 | Canada | Single-center, retrospective | 2008–2012 | 2096 | 195 (9.3) | 56 | NA | NA | ER 150(85), PR 126(72), HER2 33(19) | I 21(12), II 38(22), III 39(22), IV 74(42), NA 3(2) | 4.30(0.02–39.34) |
| Chen J [30] | 2013 | China | Single-center, retrospective | 2006–2009 | 360 | 108 (30) | NA | F | Pre- 87(80) | ER 81(75), PR 58 (54), HER2 38(35) | I 14(13), II 48(44), III 46(43) | NA |
| Sung GA [31] | 2013 | Korea. | Single-center, retrospective | 1991–2011 | NA | 110 (NA) | NA | NA | NA | ER 77(70), PR NA, HER2 NA | I 10(9), II 38(35), III 43(39), IV 19(17) | 4.60(3.22–5.99) |
| Sathiakumar N [32] | 2012 | U.S. | Population-based, retrospective | 1999–2006 | 98260 | 7189 (7.3) | 75 | F | NA | NA | I-III 4215(59), IV 2705(38), NA 269(4) | 2.30 |
| Niikura N [33]e | 2011 | U.S. | Single-center, retrospective | 1997–2008 | NA | 351 (NA) | NA | NA | Pre- 143(41) | HR+ 263(75), HER2+ 63(19), TNBC 21(6) | I-III 190(54), IV (BM) 161(46) | 2.75(0.33–11.91) |
| Su JL [34]e | 2011 | Korea. | Single-center, retrospective | 1994–2007 | NA | 146 (NA) | 47(18–76) | NA | NA | HR+ 124(85), HER2+ 12(8), TNBC 10(7) | I 15(10), II 50(34), III 57(39), IV (BM) 24(17) | 6.25(2.33–10.33) |
| Koizumi M [35] | 2010 | Japan | Single-center, prospective | 1989–1998 | 5023 | 690 (13.7) | NA | F | Pre- 356(52) | ER 225(33), PR 324(50), HER2 NA | I 55(8), II 313(45), III 267(39), IV (other than BM) 53(8) | NA |
| Trinkaus M [36] | 2010 | Canada | Two-center, retrospective | 1999–2005 | NA | 87 (NA) | 51 | NA | NA | HR+ 65(75), HER2+ 22(25) | NA | NA |
| Irawan C [37] | 2008 | Indonesia | Single-center, cross-sectional | 1998–2002 | 197 | 48 (24.3) | 47(46–50) | NA | NA | ER 7(17), PR 2(4), HER2 8(8) | NA | – |
| Yavas O [38]f | 2007 | Turkey | Single-center, retrospective | 1996–2003 | 2857 | 248 (8.7) | 46(23–76) | F | Pre- 130(52) | ER 142(57), PR 163(66), HER2 NA | I-III 248(100) | 4.21 |
| Cazzaniga ME [39] | 2006 | Italy | Multi-center, prospective | 2000–2001 | NA | 459 (NA) | 60(28–94) | F | Pre- 60(13) | ER 272(59), PR NA, HER2 NA | I-III NA, IV (BM) 95(21) | 2.33(0.17–3.58)a |
| Briasoulis E [40]g | 2004 | Greece | Two-center, retrospective | 1986–2000 | 2514 | 104 (4.1) | 58(26–79) | F | Pre- 14(13) | ER/PR 65(63), HER2 NA | I-III 60(58), IV 44(42) | NA |
| James JJ [41] | 2003 | U.K. | Single-center, retrospective | 1997–2001 | NA | 212 (NA) | NA | NA | NA | ER 157(74), PR NA, HER2 NA | NA | NA |
| Plunkett TA [42] | 2000 | U.K. | Single-center, retrospective | 1975–1991 | NA | 859 (NA) | NA | NA | NA | NA | NA | NA |
| Domchek SM [43] | 2000 | U.S. | Single-center, retrospective | 1981–1991 | NA | 718 (NA) | NA | F | NA | ER 359(50), PR NA, HER2 NA | I-III 531(74), IV 187(26) | 8.92 |
THC, time horizon covered; BM, bone metastases; ER, estrogen receptor positive; PR, progesterone receptor positive; HER2: human epidermal growth factor receptor 2 positive; HR+, hormone receptor positive (ER and/or PR) breast cancer; HER2+, HER2 (ER-PR-) breast cancer; TNBC, triple negative breast cancer; Pre-, premenopausal; F, female; NA, not available; F/U, follow-up.
The cohort characteristics is of total patients.
Median/mean follow-up from the time of bone metastases diagnosis until the date of death, emigration or end of follow-up.
Included only spinal bone metastases patients.
176 BM patients were included for further analysis.
Included bone-only metastases patients.
Included only patients with first metastases in bone.
Included only patients with metastatic disease remaining confined to bone for a minimum of 24 months.
3.4. Incidences of BM
Incidences of BM reported as new diagnosis of BM, the length of BM-free interval (BMFI), the number and sites of BM, organ metastases other than BM could be extracted from 17 studies (Table 2). In these 17 studies, the most common imaging modality for diagnosing the BM was bone scintigraphy (BS), followed by computed tomography (CT), magnetic resonance imaging (MRI) and direct radiography (DR). Positron emission tomography-CT (PET/CT) was reported as a diagnostic method for BM in two studies [27], [31]. Bone biopsy (Bb) and fine-needle aspiration biopsy (FNAB) were used as diagnostic methods in only one study [28]. The mean time of length of BMFI ranged from 0.91 [23] to 4.20 years [36].
Table 2.
Incidences of bone metastases in breast cancer patients in included studies.
| Refs. | BM N (%) | BM Diagnosis | BMFI (range) yr (%) | N (%) | Site(s) N (%) | Spread to other organ(s) N (%) |
|---|---|---|---|---|---|---|
| Cetin K [21] | 2427 (NA) | NA | 1.85 | NA | NA | Other metastases 1292 (53) |
| Dibekoglu C [22] | 139 (NA) | BS, DR, CT, MRI | 2.91(0.75–17) | NA | Spine 26(19), femur 11(8), hip 4(3)) | NA |
| Bollen L [23] | 111 (NA) | NA | 0.91a, 1.75b | NA | Spine 111(100) | Visceral and/or brain metastases 56 (50) |
| Foerster R [24] | 92 (NA) | CT | NA | M 59(64) | Spine 92(100) | Liver 28(18), lung 26(17), brain 6(7) |
| Harries M [25] | 1589 (22) | NA | < 0.75(25), < 2(50) | NA | NA | NA |
| Yamashiro H [27] | 193 911.3) | BS, CT, MRI, PET/CT | NA | NA | Spine 112(58), pelvis 55(28), sternum 43(22), rib 34(18), femur 18(9), skull 12(6) | NA |
| Arican A [28] | 1026 (NA) | BS, DR, CT, MRI, Bb, FNAB | NA | NA | NA | NA |
| Kuchuk I [29]c | 195 (9.3) | NA | 1.92 | NA | Thoracic-spine 146(83), lumbar-spine 137(78) | Visceral metastases 123(70), brain 39(23) |
| Chen J [30] | 108 (30) | BS, DR, CT, MRI | NA | NA | Thorax > spine > pelvis > limbs > skull | NA |
| Sung GA [31] | 110 (NA) | BS, MRI, PET/CT | 2.58 | M 78(71) | NA | NA |
| Sathiakumar N [32] | 7189 (7.3) | NA | 1.45 | NA | Spine 328(5), hip 163(2), femur 87(1)d | NA |
| Sun JL [34] | 146 (NA) | NA | 3.08(2.25–3.83) | M 112(77) | Spine 81(55), pelvis 62(43), rib 53(36), sternum 26(18), femur 26(18), humerus 5(4) | Lung 28(19), liver 22(15), brain 12(8), pleura 11(8) |
| Trinkaus M [36] | 87 (NA) | NA | 4.20 | M 77(91) | NA | At diagnosis, visceral metastases 57(65) |
| Cazzaniga ME [39] | 459 (NA) | BS, DR, CT, MRI | BM 2.15 (0–24.9) | M 288(63) | Spine 96(21) | cNBM 86(19), pNBM 125(27) |
| Briasoulis E [40] | 104 (4.1) | BS | 3.17(0.67–13.33) | M 78(75) | Spine 61(63), rib 21(20), pelvis 16(15), stemum 16(15), scalp 8(8), long bones 9(9) | NA |
| Plunkett TA [42] | 859 (NA) | BS | NA | NA | NA | Pleuro-pulmonary 237(28), liver 111(13) |
| Domchek SM [43] | 718 (NA) | BS, DR | < 1 (65), 1–3 (30) | NA | NA | At diagnosis, visceral metastases 452(73) |
BM, bone metastases; BMFI, bone metastasis-free interval; BS, bone scintigraphy; DR, direct radiography; CT, computed tomography; MRI, magnetic resonance imaging; PET/CT, positron emission tomography-CT; Bb, bone biopsy; FNAB, fine-needle aspiration biopsy; cNMB, concomitant bone metastases and nonskeletal metastases; pNBM, previous nonskeletal metastases.
NA, not available.
TNBC, tripe negtive breast cancer.
HR+, hormone receptor positive breast cancer.
176 BM patients were included for further analysis.
Sites of pathological fracture.
3.5. Analysis of risk factors for BM
Seven of the included 24 studies were subjected to analyses of risk factors for BM. Five studies performed both univariate and multivariate analyses [20], [25], [27], [30], [35], while in two further studies only univariate analysis was performed [37], [41]. A summary is illustrated in Table 3. Increased risk for BM was associated with young age, higher TNM stages and higher grades [25], [27], [30], [35], [41]. The menopausal state did not seem to affect BM formation [27], [35]. Three studies [25], [35], [37] indicated that invasive lobular carcinoma was an independent risk factor for developing BM: Harries et al. [25] showed in their multivariate analysis a HR of 1.26 (1.03–1.55). Regarding the impact of hormone receptor status (ER, PR, HER2) on BM, results were inconsistent: some reports [30], [41] suggested that ER and or PR positivity had susceptibility to BM; whereas other data [25], [35], [37] claimed that ER and PR status did not affect the formation of BM. One study [27] even showed that BM was less likely to occur in PR-positive, and most likely to occur in HER2-positive breast cancer patients.
Table 3.
Univariate and/or multivariate analysing risk for developing BM.
| Refs. | Independent risk factors in UA | Independent risk factors in MA | Results |
|---|---|---|---|
| Liede A [20] | Age, ER, PR, HER2, chemotherapy, tamoxifen, radiotherapy, size, grade, lymph nodes | Same to UA | Age, hormone receptor status, tumor size, grade and lymph node involvement at diagnosis were identified as independent predictors of BM, either as the first distant recurrence or any BM. |
| Harries M [25] | Age, year of diagnosis, T, N, tumor grade, histological type, ER/PR/HER2 status. | Same to UA | Incidence of BM was significantly higher in younger women, T > 5 cm, higher tumor grade, lobular carcinoma and N positive > 4, not affected by ER/PR/HER2 status. |
| Yamashiro H [27] | Age, performance status, menopausal status, T, N, M, clinical stage, histological/nuclear grade, PR, HER2, AST, ALT, ALP, CA153, CEA, type of surgery, lymphovascular invasion, tumor subtype. | Same to UA | In UA, all except age, performance status, menopausal status were significantly correlated; In MA, clinical stage, N, PR, and tumor subtype correlated statistically significantly with BM. |
| Chen J [30] | Age, menopausal status, N, clinical stage, histological grade, ER/PR/HER2 status. | ER/PR, histological grade | ER/PR status [ER(+) vs. ER(–), χ2 = 4.328, P = 0.037; ER(+)PR(+) vs. ER(+)PR(–), χ2 = 4.425, P = 0.035] and histological grade (χ2 = 7.131, P = 0.028) were significantly associated with BM. |
| Koizumi M [35] | Age, menopausal status, T, N (pN and axillary N), histology, ER/PR, adjuvant therapy. | Age, T, pN, histology, adjuvant therapy | In UA, all except menstruation status, ER and PR were significantly correlated; In MA, age, T, pN, histology and adjuvant therapy were significantly correlated. |
| Irawan C [37] | Age, hormonal contraceptives, histopathological type, ER/PR/HER2 status, cathepsin D. | NA | No significant correlation was found between the use of hormonal contraceptives, ER/PR/HER2 status, and cathepsin D and BM, except histopathological type (p = 0.011). |
| James JJ [41] | Age, histological grade, lymph node stage, T, histopathological type. | NA | There was a significant association between BM and lower grade tumors (P = 0.019), ER-positive tumors (P < 0.0001) and the lymph node stage of the primary tumor (P = 0.047). |
BM, bone metastases; T, tumor size; N, nodal status; pN, pathologic nodal status; M, metastatic status; ER, estrogen receptor; PR, progesterone receptor; HER2: human epidermal growth factor receptor 2; UA, uivariate analysis; MA, mltivariate analysis; NA, not available.
3.6. Developments of SREs
Breast cancer patients predominantly present with osteolytic BM, which leads SREs, included BP, PF, SCC, and TIH. The most frequent therapeutic measures include bone-modifying agents (BMAs), RT and surgery. Characteristics of SREs regarding the occurrence, the ratio of total and specific SREs, SERs-free interval (SREFI) and the first-line therapy are summarized in Table 4. The majority of patients with SREs received BMA as first systemic treatment after the diagnosis of bone disease [22], [24], [28]. Some patients also received ChT, HT or MT for visceral metastases at the diagnosis of SREs [24], [29], [31]. Patients with SREs such as BP, PF, SCC were more likely to receive RT or a surgical intervention [21], [40], [42].
Table 4.
Developments of skeletal-related events in breast cancer patients in included studies.
| Refs. | SREs N(%) | SREFI (range) yr | BP N (%) | PF N (%) | SCC N (%) | TIH N (%) | RT N (%) | Sur N (%) | First-line Therapy for BM N (%) |
|---|---|---|---|---|---|---|---|---|---|
| Cetin K [21] | NA | NA | NA | 67(3) | 63(3) | NA | 484(20) | 63(3) | RT 484(20), Sur 63(3) |
| Dibekoglu C [22] | NA | 3.41 (0.66–12.67) | NA | 41 (30) | NA | NA | NA | NA | BMA 139(100), HT 69(50), ChT 18(13), ChT+HT 42(30), MT 2(1.4) |
| Bollen L [23] | NA | NA | NA | NA | NA | NA | 67 (60) | 21(19) | RT 69(62), Sur 21(19) |
| Foerster R [24] | NA | NA | NA | 6 (7) | NA | NA | 92 (100) | NA | BMA 85(92), RT 92(100), ChT 53(58) |
| Steinauer K [26] | NA | NA | NA | 35 (15) | NA | NA | 137 (58) | 66(28) | BMA 170(71), RT 108(46), Sur 37(16), RT+Sur 29(12), ChT 49(21), HT 60(25), ChT+HT 100(42) |
| Yamashiro H [27] | 133(68.9) | 0.068 | NA | NA | NA | NA | NA | NA | NA |
| Arican A [28] | NA | NA | 22(2) | NA | NA | NA | 580(57) | 36(4) | BMA 985(96), RT 580(57), Sur 36(4), ChT 271(26), HT 107(10) |
| Kuchuk I [29] | NA | 0.15 | 71(40) | 53(35) | 14(9) | 18(12) | 132(85) | 20(13) | BMA 155(88), RT 132(85), Sur 20(13), ChT 119 (68), HT 135(77), MT 25(13) |
| Sung GA [31] | NA | NA | NA | NA | NA | NA | 80(73) | NA | BMA 45(41), RT 80(73), ChT 99(90), HT 45(41) |
| Sathiakumar N [32] | 3319(46) | NA | 785(29) | 303(11) | NA | 1616(59) | 29(1) | RT 1616(59), Sur 29(1) | |
| Sun JL [34] | NA | NA | NA | NA | NA | NA | 47(32) | NA | BMA 100(69), RT 19(13), ChT 27(19), RT+ChT 15(10), RT+HT 13(9), HT 54(37), ChT+MT 6(4) |
| Trinkaus M [36] | NA | 0.85a, 2.4b | 49(56) | 10(12) | 3(3) | 8(9) | 69(79) | NA | NA |
| Cazzaniga ME [39] | NA | 205 (45) | 5 (1) | NA | 2 (0.4) | NA | NA | BMA 310(68), RT 172(38), ChT 195(42), HT 96(21), ChT +HT 104(23) | |
| Briasoulis E [40] | 13(13) | NA | NA | 6(6) | 7(7) | NA | 104(100) | NA | BMA 70(67), RT 104(100), ChT 61(59), HT 53(51) |
| Plunkett TA [42] | NA | NA | 576(67) | 296(35) | 64(8) | 162(19) | 576(67) | NA | RT 576(67) |
| Domchek SM [43] | 369(51) | 2.25 | NA | 57(8) | 61(9) | 73(10) | 293(41) | 56(8) | BMA 310(4), RT 293(41), Sur 56(8) |
SREs, skeletal-related events; SREFI, skeletal-related events-free interval; BP: bone pain; PF, pathological fractures; SCC, spinal cord compression; TIH, tumor-induced hypercalcemia; BMA, bone-modifying agents; RT, radiation therapy; HT, hormonal therapy; MT, molecular-targeted therapy; ChT, chemotherapy; Sur, surgery; yr, year.
NA, not available; F/U, follow-up.
With osteoporosis.
Without osteoporosis.
3.7. Analysis of risk factors for SREs
A summary of the univariate and multivariate analysis of risk factor for SREs is shown in Table 5. As for the occurrence of SREs, two single factor [22], [43] and two multi-factor articles [29], [36]. One article [22] specifically discussed the PF, while other [36], [43] focused on the occurrence of SREs were analyzed. Main risk factors for SREs were the clinical stage of the disease, age, menstrual status, tissue grade and molecular classification [27], [29]. Moreover, BM in osteoporotic patients, occurrence of BM as first metastases, multiple BM, disease free interval (DFI) less than 3 years and presence of BM longer than 2 years [29], [36], [43]. In terms of molecular markers, patients with positive Ca153 and ALP (combined) more prone to SREs [22], [27], [43].
Table 5.
Univariate and/or multivariate analysis of risk factors for developing skeletal-related events.
| Refs. | Independent risk factors in UA | Independent risk factors in MA | Results |
|---|---|---|---|
| Dibekoglu C [22] | Age, menopausal status, BM development time, CA153, ER/PR/HER2 status, hormone sensitivity. | NA | Hormone sensitivity, high CA153 levels and positive HER2 status are slight risk factors for bone fractures |
| Yamashiro H [27] | Age, performance status, menopausal status, T, N, M, clinical stage, histological/nuclear grade, PR, HER2, AST, ALT, ALP, CA153, CEA, type of surgery, lymphovascular invasion, tumor subtype. | Same to UA | All correlated with BM at statistically significant levels in the UA, except menopausal status, histological/nuclear grade, ALP, PR; In MA, only clinical stage and N were statistically significant independent risk factors. |
| Kuchuk I [29] | NA | Age, ER/PR/HER2 status, number of BM, duration of BM, timing of BMA initiation from BM diagnosis, timing of BMA administration. | Patients with BM for 2-yr or longer, with 5 or more BM had a higher risk to develop SREs. Age and hormone receptor status were not statistically significant. |
| Trinkaus M [36] | NA | Osteoporosis at time of BM, a SRE prior to i.v. BP use, a solitary or multiple BM, location of BM, sites of visceral disease. | Osteoporosis at time of BM (HR = 2.8, 95%CI 1.0–7.6, P = 0.045); bone only disease (HR = 3.0, 95% CI 1.4–6.2, P = 0.003). |
| Domchek SM [43] | Age, race, ER status, histology, type of therapy, DFI, laboratory values obtained at the time of diagnosis of metastatic disease, site of metastatic disease at initial presentation. | Same to UA | In UA, BM at time of diagnosis of metastatic disease (P < 0.001), abnormal ALP value (P = 0.004), and DFI of 3-yr (P < 0.047) were statistically significant. In MA, BM at time of diagnosis of metastatic disease (P < 0.001) were statistically significant;In the no-bone group, a DFI of < 3-yr were more likely to develop a SRE (P = 0.005). |
SREs, skeletal-related events; BM, bone metastases; ER, estrogen receptor; PR, progesterone receptor; HER2: human epidermal growth factor receptor 2; BP, bisphosphonates; AST, aspartate transaminase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; CA153, carbohydrate antigen 153; CEA, carcino embryonie antigen; DFI, disease free interval; NA, not available.
3.8. Analysis of survival in breast cancer patients with BM
Sixteen studies evaluated predictors of survival in breast cancer patients with BM. Results are summarized in Table 6: In univariate and/or multivariate analysis, the main predictors included age, menopausal status, clinical stage, histological type, ER/PR/HER2 status, Karnofsky Performance Status score (KPS), BMFI, number of BM, visceral metastases and treatment received. After the initial diagnosis of BM, the median survival for breast cancer patients was ranging from 2.1 [42] to 4 years [22]. However many trials did not strictly divide between patients with bone-only vs. multiple metastatic sites. The latter had a far worse survival and recurrences were more frequent. Since a number of prognostic factors at breast cancer diagnosis retained prognostic significance for survival following the first diagnosis of metastatic breast cancer, patients with bone-only metastasis of breast cancer with favorable tumor characteristics had a better outcome [20], [25], [41].
Table 6.
Univariate and/or mltivariate analysis of survival of breast cancer patients in included studies.
| Refs. | Independent variables in the UA | Independent variables in the MA | Results |
|---|---|---|---|
| Liede A [20] | First site of metastasis, visceral metastases subsequent to BM, | NA | Survival of first site of BM vs synchronous bone and visceral metastases vs visceral metastasis occurred first: |
| 3-yr: 35.1% vs 26.2% vs 18.1%; 5-yr: 12.5% vs 14.1% vs 8.3%. | |||
| The HR for dying with visceral metastases after BM vs BM only: 2.70 (95%CI 1.88–3.87; P < 0.0001) | |||
| Cetin K [21] | NA | Age, ER status, level of comorbidity, presence/absence of other distant metastases at or prior to diagnosis of BM, stage, BMFI | BoS decreased with more advanced stage (IV vs. I-III (adjusted HR = 2.12, 95%CI 1.71–2.62); BoS was highest with a BMFI < 1-yr, however, it increased with longer BMFI for BMFI ≥1-yr. |
| Dibekoglu C [22] | Bone fractures | NA | BoS was not different in patients with or without bone fractures, MBoS: 4-yr vs. 3.25-yr, P = 0.65. |
| Bollen L [23] | Molecular phenotype | NA | Patients with SBM from TNBC have a shorter survival than from RPBC (0.56-yr vs. 1.88-yra, P < 0.001). |
| Foerster R [24] | NA | Age, PSS, ChT prior to RT, number of metastases, local response, concomitant BP, orthopedic corset, PF prior to RT | An age > 50-yr (P < 0.001, HR = 1.036(95%CI 1.015–1.057)), the presence of a single BM (P = 0.002, HR = 0.469, 95%CI 0.292–0.753) and TNBC (P < 0.001, HR = 1.068, 95%CI 0.933–1.125) were identified as independent prognostic factors for BoS. |
| Harries M [25] | Metastases sits | NA | MBoS: bone-only metastases vs. visceral and bone metastases (2.3-yr vs. < 0.91-yr) |
| Steinauer K [26] | Non-systemic locoregional therapy | NA | RT and/or Sur improved MBoS (2.29-yr vs. 1.625-yr, P < 0.001). |
| Chen J [30] | Histological grade, ER status | Age, menopausal status, clinical staging, N, histological grade ER/PR/HER2 status, BMFI | In UA, low-grade and ER positive BC showed significantly prolonged BoS compared with those with high-grade or ER negative ones (χ2 = 0.705, P = 0.019); In MA, ER status (χ2 = 8.315, P = 0.004) and BMFI (χ2 = 6.863, P = 0.009) were independent prognostic factors for BoS, and a histological grade wasn’t one (χ2 = 0.767, P = 0.381). |
| Sung GA [31] | Age, T, N, ER status, histologic grade, BMFI, number of BM, BMA, RT, ChT, HT | ER status, BMFI, number of BM, BAM | In UA, lower N (P = 0.006), BMFI ≥2-yr (P < 0.001), ER positivity (P = 0.027), solitary BM (P < 0.001), HT (P = 0.222), BMA (P < 0.001) showed significantly prolonged BoS; In MA, ER positivity (HR = 0.51, 95%CI 0.28–0.94), solitary BM (HR = 0.32, 95%CI 0.14–0.72), BAM (HR = 0.18, 95%CI 0.07–0.43) were significantly associated with longer BoS. |
| Sathiakumar N [32] | Age, race/ethnicity, stage at cancer diagnosis, PSS, BM, SREs | Age, race/ethnicity, PSS, BM, SREs | In UA, HRs for risk of death were 4.9 (95% CI 4.7–5.1) and 6.2 (95% CI 5.9–6.5), respectively, for women with BM but no SREs and for women with BM plus SREs, compared with women without BM; In MA, HR was 1.5 (95% CI 1.4–1.6) for women with BM plus SREs, compared with women with BM but without SRE. |
| Niikura N [33] | Age, menopausal status, timing of BM diagnosis, DFI, PSS, ER/HER2 status, nuclear grade, number of metastases, bone pain | Treatment, timing of BM diagnosis, PSS, number of metastases, BP | In UA, the time of their primary breast cancer diagnosis, a single metastasis, asymptomatic bone disease, performance status of 0–1 had a longer PFS or/and BoS; In MA, Trastuzumab led to no difference in the BoS among patients with HER2+. |
| Yavas O [38] | Age, menopausal status, T, N, histological type and grade, HR status, LVI, skin involvement, BMFI, additional nonosseous metastatic sites | The same to UA | In UA, T, N, HR status, LVI, skin involvement, additional nonosseous metastatic sites, BMFI had significant prognostic values; In MA, T, HR status, LVI, additional nonosseous metastatic sites were found to have prognostic significance. |
| Cazzaniga ME [39] | The metastatic sites | NA | The 2-yr probability for death was 0.74 (95% CI 0.67–0.79) for BM, 0.38 (95%CI 0.25–0.51) for previous nonskeletal BM and 0.56(95%CI 0.46–0.66) for concomitant nonskeletal BM (P < 0.0001). |
| Briasoulis E [40] | Histological tumor type and grade, M, number of BM | NA | No association noted between MBoS and histological tumor type and grade, M, number of BM. |
| James JJ [41] | Age, ER status, histological grade, additional metastatic sites other than bone, number of hotspots on bone scan, CA153, CEA, radiographic appearance of BM, histological tumor type, N, T, ESR | BMFI, absence of metastases at sites other than bone, ER, CEA CA153 | In UA, ER status (P < 0.0003), histological grade (P < 0.034), additional metastatic sites other than bone (P < 0.0004), age (P < 0.0003), number of hotspots on bone scan (P = 0.040), CA153 (P = 0.0026), CEA (P = 0.017) were found as independent prognostic factors for BoS; In MA, BMFI, additional metastatic sites other than bone, ER status and serological tumor marker levels all independently contributed to BoS. |
| Plunkett TA [42] | The metastatic sites | NA | MBoS: longest in BM only, shortest in BM plus liver metastases (2.1-yr vs.0.46-yr) |
BM, bone metastases; SBM, spinal bone metastases; BMFI, bone metastasis-free interval; SREs, skeletal-related events; BC, breast cancer; RPBC, receptor positive breast cancer; TNBC, triple negative breast cancer; PSS, performance status score; T, tumor size; N, nodal status; M, metastatic status; ER, estrogen receptor; PR, progesterone receptor; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; BMA, bone-modifying agents; RT, radiation therapy; HT, hormonal therapy; ChT, chemotherapy; BoS, bone metastases over survival; MBoS, median bone metastases over survival; PFS, progression-free survival; BP, bisphosphonates; CA153, carbohydrate antigen 153; CEA, carcino embryonie antigen; ESR, erythrocyte sedimentation rate; LVI, lymphovascular invasion; DFI, disease-free interval; vs., versus; HR, hazards ratio; UA, uivariate analysis; MA, mltivariate analysis; NA, not available.
BoS was defined as the time from initial diagnosis of BM until death from any cause; MBoS was defined as the median time from initial diagnosis of BM until death from any cause; PFS was defined as the time interval from diagnosis of BM to progression, death, or the last follow-up date, whichever occurred first.
Survival time was calculated between start of treatment for the spinal metastasis and date of death or last follow-up moment recorded.
4. Discussion
To date, although recent population-based research has improved our empirical understanding of the occurrence of BM secondary to breast cancer, and have quantified the impact of BM and subsequent SREs on breast cancer survival, important gaps in the data remain [20], [32]. Especially real-world data on prognostic and predictive factors among patients’, tumor's and treatment's characteristics are limited. Given the heterogeneity of patients with breast cancer, such information may help to select the most appropriate treatment strategy and thereby improving patient outcome and cost effectiveness [21], [44].
We therefore conducted a systematic review and analysis in order to describe the incidence, identify risk factors for BM and SREs and their prognostic value in breast cancer patients. In addition, we have focused on BMFI and SREFI, as well as radiologic imaging and the first line therapy. Our aim was to provide results that will improve understanding of prognostic and predictive factors may enable delivery of a more personalized treatment for the individual patient and a more cost-effective use of health care resources [45]. Our analysis included 24 studies, 22 of which were of a retrospective nature. The demographic analysis, as well as the analysis of patients’ characteristics (distribution of the age, tumor type etc.), was reflecting the general population, thus the real world data.
4.1. Characteristics and risk factors of BM
The incidence of BM in breast cancer patients remained at about 70% over decades from early 1960s to 1990s [46]. Over the last 30 years, advances in early detection and evolving treatment options led to a noticeable fall in incidence [47]. Overall, the occurrence rate of BM in breast cancer patients in our study ranged from 4.1% [40] to 30% [30]. BM diagnosis was made mostly via scintigraphy and CT scans [48]. Moreover, BM most commonly affected the axial skeleton [49]. The reason for this phenomenon has not been fully understood yet, however, it is assumable that molecular and cellular biological characteristics of the tumor cells and the tissues to which they metastasize are of importance and influence the pattern of metastatic spread [50]. Several risk factors of BM showed conflicting or non-definitive associations, especially the hormone receptor status [20], [30], [35], [37]. The high heterogeneity in study populations, cancer treatment, and study methodology may explain the conflicting results. Interestingly, our analysis showed that the menopausal status had no influence on the development of BM – suggesting that the protective influence of estrogen on bones density was not protecting against tumor spread in the bones [27], [35]. Other factors such as gene signatures, molecular changes along tumorigenesis and therapy, as well as the bone turnover might also influence the development of BM [51]. The development of algorithms to determine each patient's individual risk for BM should in turn trigger the use of specific therapies in order to increase bone disease free interval. Further research on this topic is, therefore, extremely relevant for the daily practice [25], [52].
4.2. Characteristics and risk factors of SREs
In randomized trials in advanced breast cancer, one of the major SREs occurs on average every 3–6 months after the onset of BM. In the studies included in our review, the mean SREFI, however, ranged from 25 days [27] to 3.41 years [22]. There are two main potential explanations for the significant differences. One reason could be that former studies [27] included patients with metastatic breast cancer with metastases other than BM and who were at an intermediate or high risk of recurrence, while the later studies [22] focused on bone-only metastatic patients and BF as the main SREs. BP however is also a very imminent SRE that causes limitations of the quality of life (QoF) and develops into a chronic condition [29], [53], [54].
Based on the real world data, we were able to identify a number of risk factors for SREs occurrence in BM breast cancer patients. This observation is in accordance with a general knowledge. Regrettably, major trials did not mention important characteristics of the patients, such as low body mass index (BMI), presence or absence of sarcopenia or cachexia, intake of analgetics etc. In other cancers, such as multiple myeloma, these factors have shown a prognostic significance for the development of SREs [55], [56]. Further research on these aspects is warranted.
4.3. BMAs Management of BM
Most BM patients in our study had been treated with BMAs. The approved BMAs include bisphosphonates such as pamidronate, zoledronic acid, and denosumab etc. The current American Society of Clinical Oncology (ASCO) evidence-based clinical practice guidelines for the use of bisphosphonates in breast cancer recommend a therapy for patients with evidence of metastatic bone destruction in order to prevent further destruction and SREs [57]. Nevertheless, there are a number of open questions in clinical practice, which are still not covered by the guidelines, for example, no clear recommendations on the treatment duration, dosage-modification and administration frequency. Additionally, the adjuvant administration of bisphosphonates is still controversial since long-term observations on risk profiles, especially for elderly and multi-morbid patients, are sparse [58]. Recently, however, an extremely positive effect has been shown: Adding zoledronate in postmenopausal women under letrozole for example improved the DFS significantly, showing less subsequent BM in the immediate-zoledronate group versus the delayed-zoledronate group [59]. Moreover, for patients at an increased risk of anticancer therapy induced loss of bone mass, an osteoprotection with bisphosphonates are to be critically discussed [60]. Currently, there are several studies under run, such as the REDUSE study of the Swiss Schweizerische Arbeitsgemeinschaft für Klinische Krebsforschung (SAKK, Swiss Group for Clinical Cancer Research).
It must be noted that comparison of the benefits of BMAs between real world and clinical trial populations is important, for a number of reasons. Firstly, the frequency of radiological investigations is likely significantly less for patients treated in routine clinical practice compared to those entering clinical trials. [27]. In addition, clinical trial patients usually have a better performance status due to restrictive inclusion criteria. Moreover, most of the patients enrolled in BMAs trials have metastatic disease confined to skeleton, while in real world, patients often suffer from a more spreaded disease. It must also be noted that bisphosphonates are relatively expensive supportive care drugs, and the criterion of percentage (person-year) for the use of bisphosphonates to prevent BM should be required with regard to cost-effectiveness [61], [62].
4.4. Prognostic value of BM
Establishing the prognostic value of BM in breast cancer patients faces tremendous challenges. Firstly, trials mostly focus on anticancer treatment options and outcomes, predominantly in non-metastatic patients. Secondly, a concept of randomized studies focusing on survival in breast cancer patients with only BM would demand very strict eligibility criteria, extrapolation from various accompanying factors such as comorbidities, demographics etc. and presumably not achieve a representative n-number (since bone-only metastasis are believed to occur in 17–37% of patients with distant metastasis). In addition, nearly all metastatic patients have received some kind of treatment prior to the diagnosis of BM. Advanced breast cancer is per se very complex in a molecular, biological and therapeutic aspect. Patients not only suffer from BM, but can also be entrapped in a vicious circle of aggressive therapies with bone thinning as side effect and resulting susceptibility to SREs [63], [64]. As for the survival, predictive factors show a certain overlap with risk factors of developing BM and SREs: age, menopausal status, clinical stage, histological type, BMFI, number of BM, visceral metastases and treatment received. Our analysis also identified ER/PR/HER2 status and KPS as prognostic factors.
4.5. Limitations, advantage and future recommendations
Along the study, we have stated that necessary quality and data comparability is not powerful enough to conduct a meta-analysis. Incidences of BM and SREs were not comparable among trials, neither were the risk factors and survival data. Nevertheless, we have been able to systematically and qualitatively review the data according to NOS guidelines and provided important information about the current real world situation. We have also underlined the need of a well-designed, retrospective and prospective study, focusing on the incidence and therapy of BM and SREs in breast cancer patients, as to better understand the efficacy of anticancer management and antiresorptive therapies in this specific breast cancer population. Open questions remain, such as an optimal time of commencing and duration of bisphosphonate therapy, influence of life-style factors (physical activity, cessation of smoking, alcohol abstinence), efficacy of supplements of Vitamin D3 and calcium etc. Long-term data on risk profiles, especially for elderly and multimorbid patients, are needed. Last but not least, so far there are no data that would correlate the BMI with possible outcomes and severity of SREs in BM patients.
Acknowledgments and funding information
This publication was made possible in part by Key Medical Specialty Fund Projects of Shanghai (ZK2015A07); National Natural Science Foundation of China (NSFC) grants 81502267; Youth Fundation of Zhongshan Hospital grants 2016ZSQZ54; General Scientific Research foundation of Shanghai Mental Health Center grants 2017-YJ-10; 2014 Annual Science and Technology Innovation Plan of Action of Shanghai Science and Techology Committee Funding grants 14411950302. Its contents are solely the responsibility of the authors and do not ne- cessarily represent the official views of the NCFS, Zhongshan Hospital, Shanghai Mental Health Center or Shanghai Science and Teachology Committee.
Acknowledgments
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Ethical standards
Experiments comply with the current laws of China.
Appendix A. Pubmed search strategies
| Search | Search strings | No. of articles |
|---|---|---|
| #1 | Search "Breast Neoplasms"[Mesh] OR “Breast Neoplasm” OR “Breast Cancer” OR “Breast Carcinoma” OR “Breast Tumor*” OR “Neoplasm*, Breast” OR “Tumor*, Breast” OR “mammary carcinoma” OR “mammae cancer” OR “mammary cancer” OR “carcinoma mammae” | 322520 |
| #2 | Search "Bone metastases" OR "Bone metastasis" OR "metastasis of Bone" OR "metastases of Bone" OR “Skeletal metastases” OR “Skeletal metastasis” OR “Skeletal complication*” OR “skeletal-related event*” | 44143 |
| #3 | Search #1 AND #2 | 8664 |
| #4 | Search #3 NOT (Clinical trial* [pt] OR Bibliography [pt] OR Comment [pt] OR Historical Article [pt] OR Interview [pt] OR Review [pt] OR Letter [pt] OR Newspaper Article [pt] OR Case Report* OR Books and Documents [pt] OR Clinical Study [pt] OR Guideline [pt] OR Systematic Review* [pt]) | 5429 |
| #5 | Search #4 Limits: Humans, English | 3781 |
| #6 | Search #5 Limits: Publication Date from January 1st, 2000 to June 11, 2017 | 2314 |
Appendix B. Web of Science search strategies
| Search | Search strings | No. of articles |
|---|---|---|
| #1 | Search TS = ("Breast Neoplasms"[Mesh] OR “Breast Neoplasm” OR “Breast Cancer” OR “Breast Carcinoma” OR “Breast Tumor*” OR “Neoplasm*, Breast” OR “Tumor*, Breast” OR “mammary carcinoma” OR “mammae cancer” OR “mammary cancer” OR “carcinoma mammae”) | 438485 |
| #2 | Search TS = ("Bone metastases" OR "Bone metastasis" OR "metastasis of Bone" OR "metastases of Bone" OR “Skeletal metastases” OR “Skeletal metastasis” OR “Skeletal complication*” OR “skeletal-related event*”) | 17633 |
| #3 | Search #1 AND #2 | 4118 |
| #4 | Search #3 AND DT = Article | 3194 |
| #5 | Search #4 AND English AND PY = (2000–2017) | 2160 |
Appendix C. EMBASE OvidSP search strategies
| Search | Search strings | No. of articles |
|---|---|---|
| #1 | Search ("Breast Neoplasms"[Mesh] OR “Breast Neoplasm” OR “Breast Cancer” OR “Breast Carcinoma” OR “Breast Tumor*” OR “Neoplasm*, Breast” OR “Tumor*, Breast” OR “Mammary Carcinoma” OR “Mammae Cancer” OR “Mammary Cancer” OR “Carcinoma Mammae”) {No Related Terms} | 10079 |
| #2 | Search ("Bone metastases" OR "Bone metastasis" OR "metastasis of Bone" OR "metastases of Bone" OR “Skeletal metastases” OR “Skeletal metastasis” OR “Skeletal complication*” OR “skeletal-related event*”) {No Related Terms} | 10057 |
| #3 | Search #1 AND #2 {No Related Terms} | 4065 |
| #4 | Search #5 NOT (Clinical trial* [pt] OR Bibliography [pt] OR Comment [pt] OR Historical Article [pt] OR Interview [pt] OR Review [pt] OR Letter [pt] OR Newspaper Article [pt] OR Case Report* OR Books and Documents [pt] OR Clinical Study [pt] OR Guideline [pt] OR Systematic Review* [pt]) {No Related Terms} | 3139 |
| #5 | Search #3 Limits: Humans, English | 2004 |
| #6 | Search #4 Limits: Publication Date from January 1st, 2000 to June 11, 2017 | 1432 |
Appendix D. EBSCO search strategies
| Search | Search Strings | No. of Articles |
|---|---|---|
| #1 | Search "Breast Neoplasms"[Mesh] OR “Breast Neoplasm” OR “Breast Cancer” OR “Breast Carcinoma” OR “Breast Tumor*” OR “Neoplasm*, Breast” OR “Tumor*, Breast” OR “mammary carcinoma” OR “mammae cancer” OR “mammary cancer” OR “carcinoma mammae” | 480419 |
| #2 | Search "Bone metastases" OR "Bone metastasis" OR "metastasis of Bone" OR "metastases of Bone" OR “Skeletal metastases” OR “Skeletal metastasis” OR “Skeletal complication*” OR “skeletal-related event*” | 25931 |
| #3 | Search #1 AND #2 | 5704 |
| #4 | #3 Limiters Year of Publication: 2000–2017; Language: English; Full Text; Document Type* | 2267 |
Appendix E. Quality assessment according to the Newcastle-Ottawa
| Study | Case-Control Star Template |
|||
|---|---|---|---|---|
| Selection | Comparability | Exposure | Total | |
| Liede A [20] | 4 | 2 | 3 | 9 |
| Cetin K [21] | 3 | 4 | 2 | 8 |
| Dibekoglu C [22] | 3 | 4 | 2 | 8 |
| Bollen L [23] | 2 | 2 | 3 | 7 |
| Foerster R [24] | 2 | 2 | 2 | 6 |
| Harries M [25] | 4 | 2 | 3 | 9 |
| Steinauer K [26] | 3 | 2 | 2 | 7 |
| Yamashiro H [27] | 4 | 2 | 3 | 9 |
| Arican A [28] | 3 | 2 | 2 | 7 |
| Kuchuk I [29] | 4 | 3 | 3 | 10 |
| Chen J [30] | 4 | 4 | 2 | 10 |
| Sung GA [31] | 3 | 3 | 3 | 9 |
| Sathiakumar N [32] | 4 | 2 | 2 | 8 |
| Niikura N [33] | 2 | 3 | 2 | 7 |
| Sun JL [34] | 2 | 3 | 3 | 8 |
| Koizumi M [35] | 4 | 3 | 2 | 9 |
| Trinkaus M [36] | 3 | 2 | 2 | 7 |
| Irawan C [37] | 3 | 2 | 2 | 7 |
| Yavas O [38] | 3 | 3 | 3 | 9 |
| Cazzaniga ME [39] | 3 | 3 | 2 | 8 |
| Briasoulis E [40] | 3 | 3 | 2 | 8 |
| James JJ [41] | 3 | 2 | 2 | 7 |
| Plunkett TA [42] | 3 | 2 | 2 | 7 |
| Domchek SM [43] | 3 | 3 | 3 | 9 |
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Fan L., Strasser-Weippl K., Li J.J., Louis J. St, Finkelstein D.M., Yu K.D. Breast cancer in China. Lancet Oncol. 2014;15(7):279–289. doi: 10.1016/S1470-2045(13)70567-9. [DOI] [PubMed] [Google Scholar]
- 3.Colzani E., Liljegren A., Johansson A.L., Adolfsson J., Hellborg H., Hall P.F. Prognosis of patients with breast cancer: causes of death and effects of time since diagnosis, age, and tumor characteristics. J. Clin. Oncol. 2011;29(30):4014–4021. doi: 10.1200/JCO.2010.32.6462. [DOI] [PubMed] [Google Scholar]
- 4.McGuire A., Brown J.A., Malone C., McLaughlin R., Kerin M.J. Effects of age on the detection and management of breast cancer. Cancers. 2015;7(2):908–929. doi: 10.3390/cancers7020815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris H.R., Bergkvist L., Wolk A. Adherence to the world cancer research fund/american institute for cancer research recommendations and breast cancer risk. Int. J. Cancer. 2016;138(11):2657–2664. doi: 10.1002/ijc.30015. [DOI] [PubMed] [Google Scholar]
- 6.Kellen E., Vansant G., Christiaens M.R., Neven P., Van Limbergen E. Lifestyle changes and breast cancer prognosis: a review. Breast Cancer Res. Treat. 2009;114(1):13–22. doi: 10.1007/s10549-008-9990-8. [DOI] [PubMed] [Google Scholar]
- 7.Lim B., Hortobagyi G.N. Current challenges of metastatic breast cancer. Cancer Metastasis Rev. 2016;35(4):495–514. doi: 10.1007/s10555-016-9636-y. [DOI] [PubMed] [Google Scholar]
- 8.Senkus E., Łacko A. Over-treatment in metastatic breast cancer. Breast. 2017;31:309–317. doi: 10.1016/j.breast.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Li S., Peng Y., Weinhandl E.D., Blaes A.H., Cetin K., Chia V.M. Estimated number of prevalent cases of metastatic bone disease in the us adult population. Clin. Epidemiol. 2012;4:87–93. doi: 10.2147/CLEP.S28339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mundy G.R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer. 2002;2(8):584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 11.Manders K., van de Poll-Franse L.V., Creemers G.J., Vreugdenhil G., van der Sangen M.J., Nieuwenhuijzen G.A. Clinical management of women with metastatic breast cancer: a descriptive study according to age group. BMC Cancer. 2006;6:179. doi: 10.1186/1471-2407-6-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harries M., Taylor A., Holmberg L., Agbaje O., Garmo H., Kabilan S. Incidence of bone metastases and survival after a diagnosis of bone metastases in breast cancer patients. Cancer Epidemiol. 2014;38(4):427–434. doi: 10.1016/j.canep.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Svendsen M.L., Gammelager H., Svaerke C., Yong M., Chia V.M., Christiansen C.F. Hospital visits among women with skeletal-related events secondary to breast cancer and bone metastases: a nationwide population-based cohort study in Denmark. Clin. Epidemiol. 2013;5:97–103. doi: 10.2147/CLEP.S42325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipton A., Steger G.G., Figueroa J., Alvarado C., Solal-Celigny P., Body J.J. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J. Clin. Oncol. 2007;25(28):4431–4437. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- 15.Stopeck A.T., Lipton A., Body J.J., Steger G.G., Tonkin K., de Boer R.H. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J. Clin. Oncol. 2010;28(35):5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 16.Knobloch K., Yoon U., Vogt P.M. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J. Craniomaxillofac Surg. 2011;39(2):91–92. doi: 10.1016/j.jcms.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Hutton B., Salanti G., Caldwell D.M., Chaimani A., Schmid C.H., Cameron C. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 18.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 19.Castillo J.J., Dalia S., Pascual S.K. Association between red blood cell transfusions and development of non-Hodgkin lym- phoma: a meta-analysis of observational studies. Blood. 2010;116:2897–2907. doi: 10.1182/blood-2010-03-276683. [DOI] [PubMed] [Google Scholar]
- 20.Liede A., Jerzak K.J., Hernandez R.K., Wade S.W., Sun P., Narod S.A. The incidence of bone metastasis after early-stage breast cancer in Canada. Breast Cancer Res. Treat. 2016;156(3):587–595. doi: 10.1007/s10549-016-3782-3. [DOI] [PubMed] [Google Scholar]
- 21.Cetin K., Christiansen C.F., Svaerke C., Jacobsen J.B., Sorensen H.T. Survival in patients with breast cancer with bone metastasis: a Danish population-based cohort study on the prognostic impact of initial stage of disease at breast cancer diagnosis and length of the bone metastasis-free interval. BMJ Open. 2015;5(4):e007702. doi: 10.1136/bmjopen-2015-007702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dibekoglu C., Turanli S., Karaman N., Ozcelik K.C., Erdogan O. Bone fracture in breast cancer patients with isolated bone metastasis. Chirugia. 2015;110(1):43–48. [PubMed] [Google Scholar]
- 23.Bollen L., Wibmer C., Wang M., van der Linden Y.M., Leithner A., Bunger C.E. Molecular phenotype is associated with survival in breast cancer patients with spinal bone metastases. Clin. Exp. Metastasis. 2015;32(1):1–5. doi: 10.1007/s10585-014-9685-y. [DOI] [PubMed] [Google Scholar]
- 24.Foerster R., Bruckner T., Bostel T., Schlampp I., Debus J., Rief H. Prognostic factors for survival of women with unstable spinal bone metastases from breast cancer. Radiat. Oncol. 2015;10:144. doi: 10.1186/s13014-015-0458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harries M., Taylor A., Holmberg L., Agbaje O., Garmo H., Kabilan S. Incidence of bone metastases and survival after a diagnosis of bone metastases in breast cancer patients. Cancer Epidemiol. 2014;38(4):427–434.5. doi: 10.1016/j.canep.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Steinauer K., Huang D.J., Eppenberger-Castori S., Amann E., Guth U. Bone metastases in breast cancer: frequency, metastatic pattern and non-systemic locoregional therapy. J. Bone Oncol. 2014;3(2):54–60. doi: 10.1016/j.jbo.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashiro H., Takada M., Nakatani E., Imai S., Yamauchi A., Tsuyuki S. Prevalence and risk factors of bone metastasis and skeletal related events in patients with primary breast cancer in Japan. Int J. Clin. Oncol. 2014;19(5):852–862. doi: 10.1007/s10147-013-0643-5. [DOI] [PubMed] [Google Scholar]
- 28.Arican A., Bozkurt T., Bozcuk H., Demirkan B., Buyukberber S., Alkis N. A cross-sectional survey of the diagnosis and management of bone metastasis in breast cancer patients in Turkey. Support Care Cancer. 2014;22(10):2629–2634. doi: 10.1007/s00520-014-2253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuchuk I., Hutton B., Moretto P., Ng T., Addison C.L., Clemons M. Incidence, consequences and treatment of bone metastases in breast cancer patients - experience from a single cancer centre. J. Bone Oncol. 2013;2(4):137–144. doi: 10.1016/j.jbo.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J., Zhu S., Xie X.Z., Guo S.F., Tong L.Q., Zhou S. Analysis of clinicopathological factors associated with bone metastasis in breast cancer. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013;33(1):122–125. doi: 10.1007/s11596-013-1083-1. [DOI] [PubMed] [Google Scholar]
- 31.Ahn S.G., Lee H.M., Cho S.H., Lee S.A., Hwang S.H., Jeong J. Prognostic factors for patients with bone-only metastasis in breast cancer. Yonsei Med. J. 2013;54(5):1168–1177. doi: 10.3349/ymj.2013.54.5.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sathiakumar N., Delzell E., Morrisey M.A., Falkson C., Yong M., Chia V. Mortality following bone metastasis and skeletal-related events among women with breast cancer: a population-based analysis of U.S. Medicare beneficiaries, 1999–2006. Breast Cancer Res. Treat. 2012;131(1):231–238. doi: 10.1007/s10549-011-1721-x. [DOI] [PubMed] [Google Scholar]
- 33.Niikura N., Liu J., Hayash N., Palla S.L., Tokuda Y., Hortobagyi G.N. Treatment outcome and prognostic factors for patients with bone-only metastases of breast cancer: a single-institution retrospective analysis. Oncologist. 2011;16(2):155–164. doi: 10.1634/theoncologist.2010-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S.J., Park S., Ahn H.K., Yi J.H., Cho E.Y., Sun J.M. Implications of bone-only metastases in breast cancer: favorable preference with excellent outcomes of hormone receptor positive breast cancer. Cancer Res. Treat. 2011;43(2):89–95. doi: 10.4143/crt.2011.43.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koizumi M., Yoshimoto M., Kasumi F., Iwase T. An open cohort study of bone metastasis incidence following surgery in breast cancer patients. BMC Cancer. 2010;10:381. doi: 10.1186/1471-2407-10-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trinkaus M., Simmons C., Myers J., Dranatisaris G., Clemons M. Skeletal-related events (SREs) in breast cancer patients with bone metastases treated in the nontrial setting. Support Care Cancer. 2010;18(2):197–203. doi: 10.1007/s00520-009-0645-z. [DOI] [PubMed] [Google Scholar]
- 37.Irawan C., Hukom R., Prayogo N. Factors associated with bone metastasis in breast cancer: a preliminary study in an Indonesian population. Acta Med. Indones. 2008;40(4):178–180. [PubMed] [Google Scholar]
- 38.Yavas O., Hayran M., Ozisik Y. Factors affecting survival in breast cancer patients following bone metastasis. Tumori. 2007;93(6):580–586. doi: 10.1177/030089160709300611. [DOI] [PubMed] [Google Scholar]
- 39.Cazzaniga M.E., Dogliotti L., Cascinu S., Barni S., Labianca R., Chiara S. Diagnosis, management and clinical outcome of bone metastases in breast cancer patients: results from a prospective, multicenter study. Oncology. 2006;71(5–6):374–381. doi: 10.1159/000107772. [DOI] [PubMed] [Google Scholar]
- 40.Briasoulis E., Karavasilis V., Kostadima L., Ignatiadis M., Fountzilas G., Pavlidis N. Metastatic breast carcinoma confined to bone: portrait of a clinical entity. Cancer. 2004;101(7):1524–1528. doi: 10.1002/cncr.20545. [DOI] [PubMed] [Google Scholar]
- 41.James J.J., Evans A.J., Pinder S.E., Gutteridge E., Cheung K.L., Chan S. Bone metastases from breast carcinoma: histopathological - radiological correlations and prognostic features. Br. J. Cancer. 2003;89(4):660–665. doi: 10.1038/sj.bjc.6601198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plunkett T.A., Smith P., Rubens R.D. Risk of complications from bone metastases in breast cancer. implications for management. Eur. J. Cancer. 2000;36(4):476–482. doi: 10.1016/s0959-8049(99)00331-7. [DOI] [PubMed] [Google Scholar]
- 43.Domchek S.M., Younger J., Finkelstein D.M., Seiden M.V. Predictors of skeletal complications in patients with metastatic breast carcinoma. Cancer. 2000;89(2):363–368. doi: 10.1002/1097-0142(20000715)89:2<363::aid-cncr22>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 44.Baird R.D., Caldas C. Genetic heterogeneity in breast cancer: the road to personalized medicine? BMC Med. 2013;11:151. doi: 10.1186/1741-7015-11-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coleman R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006;12(20 Pt 2):6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 46.Coleman R.E., Rubens R.D. The clinical course of bone metastases from breast cancer. Br. J. Cancer. 1987;55(1):61–66. doi: 10.1038/bjc.1987.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Group EBCTC, Clarke M., Coates A.S., Darby S.C., Davies C., Gelber R.D. Adjuvant chemotherapy in oestrogen-receptor-poor breast cancer: patient-level meta-analysis of randomised trials. Lancet. 2008;371(9606):29–40. doi: 10.1016/S0140-6736(08)60069-0. [DOI] [PubMed] [Google Scholar]
- 48.Houssami N., Costelloe C.M. Imaging bone metastases in breast cancer: evidence on comparative test accuracy. Ann. Oncol. 2012;23(4):834–843. doi: 10.1093/annonc/mdr397. [DOI] [PubMed] [Google Scholar]
- 49.van der Pol C.B., Schweitzer M.E., Di Primio G., Sampaio M.L., Kielar A., Clemons M. Breast cancer and bone metastases: the association of axial skeleton MRI findings with skeletal-related events and survival. Breast Cancer Res. Treat. 2014;46(3):583–589. doi: 10.1007/s10549-014-3046-z. [DOI] [PubMed] [Google Scholar]
- 50.Suva L.J., Griffin R.J., Makhoul I. Mechanisms of bone metastases of breast cancer. Endocr. Relat. Cancer. 2009;16(3):703–713. doi: 10.1677/ERC-09-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casimiro S., Luis I., Fernandes A., Pires R., Pinto A., Gouveia A.G. Analysis of a bone metastasis gene expression signature in patients with bone metastasis from solid tumors. Clin. Exp. Metastasis. 2012;29(2):155–164. doi: 10.1007/s10585-011-9438-0. [DOI] [PubMed] [Google Scholar]
- 52.Lin N.U., Vanderplas A., Hughes M.E., Theriault R.L., Edge S.B., Wong Y.N. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive. Cancer Netw., Cancer. 2012;118(22):5463–5472. doi: 10.1002/cncr.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Front D., Schneck S.O., Frankel A., Robinson E. Bone metastases and bone pain in breast cancer. Are they closely associated? JAMA. 1979;242(16):1747–1748. [PubMed] [Google Scholar]
- 54.Sabino M.A., Mantyh P.W. Pathophysiology of bone cancer pain. J. Support Oncol. 2005;3(1):15–24. [PubMed] [Google Scholar]
- 55.Palumbo A., Anderson K. Multiple myeloma. N. Engl. J. Med. 2011;364(11):1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 56.Terpos E., Berenson J., Raje N., Roodman G.D. Management of bone disease in multiple myeloma. Expert Rev. Hematol. 2014;7(1):113–125. doi: 10.1586/17474086.2013.874943. [DOI] [PubMed] [Google Scholar]
- 57.Van Poznak C.H., Temin S., Yee G.C., Janjan N.A., Barlow W.E., Biermann J.S. American Society of Clinical O. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J. Clin. Oncol. 2011;29(9):1221–1227. doi: 10.1200/JCO.2010.32.5209. [DOI] [PubMed] [Google Scholar]
- 58.Winter M.C., Coleman R.E. Bisphosphonates in the adjuvant treatment of breast cancer. Clin. Oncol. (R. Coll. Radiol.) 2013;25(2):135–145. doi: 10.1016/j.clon.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 59.Coleman R., de Boer R., Eidtmann H., Llombart A., Davidson N., Neven P. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann. Oncol. 2013;24(2):398–405. doi: 10.1093/annonc/mds277. [DOI] [PubMed] [Google Scholar]
- 60.Salmen J., Banys-Paluchowski M., Fehm T. Bone-Targeted Therapy. Geburtshilfe Frauenheilkd. 2015;75(6):584–587. doi: 10.1055/s-0035-1546151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delea T.E., Taneja C., Sofrygin O., Kaura S., Gnant M. Cost-effectiveness of zoledronic acid plus endocrine therapy in premenopausal women with hormone-responsive early breast cancer. Clin. Breast Cancer. 2010;10(4):267–274. doi: 10.3816/CBC.2010.n.034. [DOI] [PubMed] [Google Scholar]
- 62.Felix J., Andreozzi V., Soares M., Borrego P., Gervasio H., Moreira A. Hospital resource utilization and treatment cost of skeletal-related events in patients with metastatic breast or prostate cancer: estimation for the Portuguese National Health System. Value Health. 2011;14(4):499–505. doi: 10.1016/j.jval.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 63.Hadji P., Body J.J., Aapro M.S., Brufsky A., Coleman R.E., Guise T. Practical guidance for the management of aromatase inhibitor-associated bone loss. Ann. Oncol. 2008;19(8):1407–1416. doi: 10.1093/annonc/mdn164. [DOI] [PubMed] [Google Scholar]
- 64.Coleman R.E., Guise T.A., Lipton A., Roodman G.D., Berenson J.R., Body J.J. Advancing treatment for metastatic bone cancer: consensus recommendations from the Second Cambridge Conference. Clin. Cancer Res. 2008;14(20):6387–6395. doi: 10.1158/1078-0432.CCR-08-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]