Abstract
This Editorial highlights a unique focus of this theme issue on the biological perspectives in deriving psychological taxonomies coming from neurochemistry, neuroanatomy, neurophysiology, genetics, psychiatry, developmental and comparative psychology—as contrasted to more common discussions of socio-cultural concepts (personality) and methods (lexical approach). It points out the importance of the distinction between temperament and personality for studies in human and animal differential psychophysiology, psychiatry and psycho-pharmacology, sport and animal practices during the past century. It also highlights the inability of common statistical methods to handle nonlinear, feedback, contingent, dynamical and multi-level relationships between psychophysiological systems of consistent psychological traits discussed in this theme issue.
This article is part of the theme issue ‘Diverse perspectives on diversity: multi-disciplinary approaches to taxonomies of individual differences'.
Keywords: taxonomies, temperament, personality, individual differences, factor analysis
1. Focusing on biologically-based traits: temperament versus personality
The topic of this theme issue is one of the oldest and most fundamental problems of psychology: the classification of biologically-based traits1 in healthy people and their extreme expressions in cases of mental illness. We all know how family upbringing, culture, personal history and socio-economic status (SES) can contribute to individual differences in values, attitudes, manners, skills and habits that manifest as consistent patterns in human behaviour. Yet, at some point all of us start wondering why children in the same family appear to be different from a very early age on, although they have the same parents, teachers, SES and culture (i.e. the same social environment). We often observe that there is something in people that remains rather stably over time, no matter how much training or education is applied. This ‘something’ must come from biological factors.
The oldest theory about types and the biological factors underlying human psychological diversity emerged in a biology-rooted science—medicine—about 2500 years ago. The Greek physician Hippocrates (460–375 BC), often credited as the father of modern medicine, categorized four types of consistent behavioural patterns that resemble descriptors used in modern psychology and psychiatry: impulsive–agitated (choleric), depressed (melancholic), socially detached (phlegmatic) and manic–extraverted (sanguine). Hippocrates (and later Galen, AD 127–132) called these characters temperamentums (Latin for ‘mixtures’) because they suspected that differences in ratios of mixtures of vital bodily fluids (i.e. chemical imbalances) made people consistently different. Two and a half millennia later, neurochemistry, psychiatry and psycho-pharmacology indeed showed that neurochemical imbalances influence the variability in behavioural regulation.
Thus, the concept of temperament refers to neurochemically based individual differences in the regulation of formal dynamical aspects of behaviour. These differences were studied for over 100 years in developmental, clinical, sport and organizational psychology, in psychiatry and, as noted by Rusalov [1], in human and animal differential psychophysiology. This concept was especially useful in the practice of breeding domestic and sport animals (such as dogs, cats and horses) as well as experimental animals for neurochemical and psychopharmacological research, and in research on the impact of stress on farm and sport animals. These differences included the traits that were linked to well-identified neurochemical systems—endurance, impulsivity, mobility, stress reactivity, degree of vocalization, speed of motion, learning capacities (trainability), attentiveness, etc. Since personality theorists expanded their research activities over the past 20 years also to include biologically-based differences, these two concepts became inevitably intertwined. Many studies reported in this theme issue used the term ‘personality’ for biologically-based individual differences, which we also define here as temperamental traits. Let us briefly mention several distinct features that differentiate these concepts of ‘temperament’ and ‘personality’.
First, in line with its original concept, temperament is defined here as neurochemically-based individual differences in behavioural regulation, noted both in pre-cultural individuals (animals, infants) and adult humans, whereas personality is a concept describing individual differences primarily from the socio-cultural perspective specific to humans (figure 1). Many studies into biologically-based individual differences in humans were dictated by the demands for selecting athletes, cosmonauts and top managers, when a candidate's endurance, speed of reaction, impulse control, plasticity for sudden changes in situations and emotional stability were more important for selection than their social experience or attitudes. Studies of the neurophysiological systems underlying these traits have been conducted on animals, in particular, rats, mice, dogs, cats and nonhuman primates, for over a century [1–5], continuing in contemporary investigations of compulsivity, impulsivity [2,6,7], affiliative behaviour and processing of social cues [8–10], attention-related and learning traits [11–13], orientation biases [7,14,15] and especially emotional regulation [6–10,14,16–18]. Behavioural orientation traits, such as sensation seeking, empathy and probabilistic processing, are also present in pre-cultural individuals. As pointed out by Blair [8], mirror neurons linked to empathy were initially found in nonhuman primates, and individual differences in empathy were shown to occur in many other species a well. Hasselmo & Stern [11] describe a mathematical model of the neurophysiology underlying rule learning, which is part of causal thinking and probabilistic processing. Consistent individual differences in rule learning, occurring already in infants, are often described as genetically and biologically-based intellectual abilities in both animals and humans [3]. In short, the traits differentiating between individuals that belong to the same species—physical endurance, speed of actions, sociability, impulsivity, compulsivity, plasticity, emotionality, probabilistic processing, sensation seeking and empathy—are temperament traits having a well-documented biological basis. Some of these traits are also incorporated in structural models of personality but with a socio-cultural perspective.
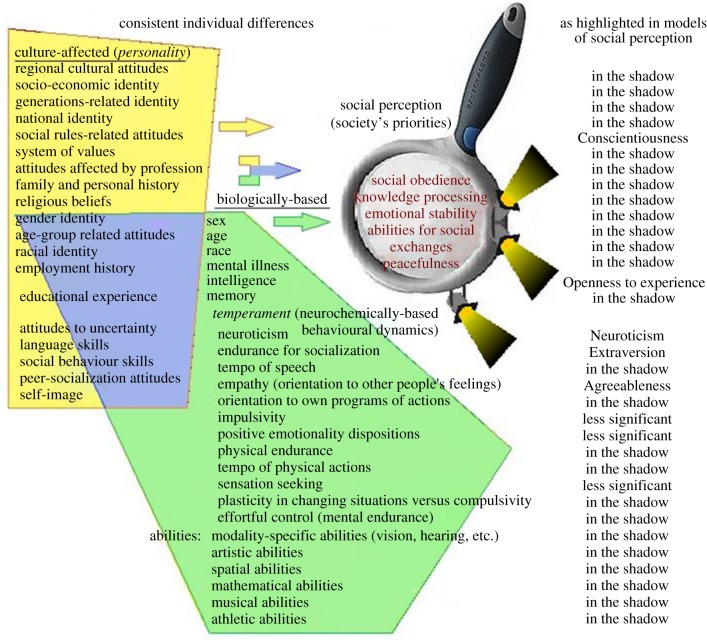
Figure 1.
Differential psychology studies a number of consistent individual differences based on socio-cultural (yellow area) and biological factors (green area). The fact that biological characteristics interact with socio-cultural ones and emerge as components of personality does not devalue the need for biological sciences to study them independently of cultural factors (as, for example, in the case of age and sex). Social perception of individuals (i.e. how people see other people, depicted here as a magnifying glass), as represented by the Five Factor Model (FFM), highlights only some components of personality (spotlights) and downplays many others (labelled here ‘in the shadow’) as listed in the right column of the figure. The resulting list of personality traits is therefore biased toward individual differences prioritized by societies. This list might not be suitable for taxonomies of biologically-based traits related to dynamics in behavioural regulation (temperament) even though it might adequately represent a socio-cultural structure of interpersonal perception.
Second, temperament traits are conceived as individual differences in consistent formal–dynamical aspects of behavioural regulation that show up universally across situations and contexts (endurance, speed of behavioural integration, reactivity, sensitivity to specific reinforcers, emotionality). Many non-temperamental traits conceived as personality, by contrast, are classically considered as comprising the content characteristics shaped by socio-cultural appraisal and interpretation (such as values, particular attitudes, habits and preferences, autobiographical history, self-image, cultural, religious and national identity) [3,4,14,17,19–24].
Third, the most commonly discussed feature of temperamental traits is their relative stability over the life course (if controlled for age-related changes). Personality aspects, on the other hand, such as our systems of values and attitudes, and various aspects of personal identity, often change after dramatic life events. For example, Kagan [14,21,25], one of the pioneers of longitudinal research in temperament, uncovered in his research a relative stability for the temperament trait Neuroticism. This construct, adopted by the Five Factor Model of personality (FFM) as a personality trait, was shown to be biologically based and even having identifiable heritability, confirming that Neuroticism can be conceived as a component of temperament. The impressive works of Netter [7] and Jones & Sloan [9] provided a unique focus on the role of not only monoamines but also hormones, such as cortisol and oxytocin, as contributing factors in temperament traits related to emotional and behavioural reactivity. In line with the findings of Kagan and colleagues using MRI techniques and behavioural observations, Jones & Sloan [9] concluded that ‘foetal and infant development may be the pivotal period for the neocortex to experience and utilize oxytocin as the developmental plasticity’.
Fourth, an important and rather novel criterion separating temperament from personality relating to morality was described by Saucier [5]. Modern personality models describe components of individual differences that are favoured by societies and associated with morality, such as the FFM factors of Conscientiousness and Agreeableness. By contrast, temperament traits of animals and human infants exhibit consistent variations regardless of moral judgement and teachings about morality.
2. Blending concepts referring to biological and socio-cultural aspects of individual differences might be not harmless
Over 40 taxonomic models of temperament and personality have been offered in differential psychology and psychiatry since the birth of these sciences 100 years ago (see [3] for review). We still know very little, however, about the basic neural systems underlying the traits that have been proven to have a biological and neurochemical basis, and a consensus on the list or categorization of these traits has not yet been achieved. One of the challenges slowing progress in the development of taxonomies of temperament and mental disorders is that individual differences in behavioural regulation lie at the crossroads of multiple social and biological factors. All of these factors are strongly entangled. Therefore, special care and a well-developed conceptual framework are needed in their disentanglement for our analytic purposes, which would ensure the accuracy of measurements and the validity of their interpretations for taxonomy development.
A valid scientific approach requires exchange among different disciplines and different perspectives concerning the partitioning of individual differences, respecting differences in terminology, conceptualization and methods. In contemporary personality psychology, social–cultural concepts are mixed with traditional concepts of temperament. This blending has led to at least three unfortunate trends: mixing, missing and misusing scientific concepts.
(a). Mixing concepts
The model most publicized in personality psychology, the FFM of human personality [5,26–36], capitalizes on the stability and biological correlates of two dimensions that are well-known from temperament research (Emotionality and Arousal level), labelled in the FFM as Neuroticism and Extraversion. FFM proponents defend the blending of temperament and personality concepts in their model by referring to the interaction of biologically-based aspects of individual differences with socio-cultural factors (e.g. education, religion, culture, language, family environment) that contribute to an individual's personality. However, an interaction between factors is not an excuse for not differentiating between concepts describing them. For example, sex, age and possible propensities for mental illness, which are also based on neurochemical and neurophysiological systems, interact with socio-cultural factors as well, and so they also contribute to an individual's personality (figure 1). There are sex differences in rates of employment and rule obedience [37]. The interaction between sex and ‘personality traits’ is also coupled with age: there are suggestions of a ‘middle age–middle sex’ effect in verbal and physical abilities, levelling sex differences in these characteristics after the peak of the reproductive period [38]. If we do not blend sex, age and mental illness with personality and instead keep them as independent variables in our studies, then we probably should not do so for neurochemically-based differences, thus temperament, either (figure 1).
Personality psychology uses the factor analysis (FA) of verbal descriptors of human characteristics of mixed origins (biologically- and socially-based). Models, such as the FFM, use correlations among these mixtures of characteristics and this results in mongrel factors which, by default, would not show a valid differentiation between biological systems because the resulting dimensions will be contaminated by pro-social biases of language [3,5,16,26–36,39–45]. Models derived from human language therefore cannot constitute models of biologically-based traits as FFM proponents often claim. Instead, they likely represent dimensions of socio-cultural perception and appraisal, as noted by many authors [3,5,16,26–36,39–41,46].
History has already seen tragic examples of outcomes arising from the insufficient differentiation between socio-cultural and biological factors influencing individual differences. This occurred in the field of intelligence testing, particularly during the massive IQ testing between 1950 and 1980, when scientists claimed that those testing methods allow biological intellectual abilities or learning disabilities to be diagnosed, yet without controlling for socio-cultural factors, such as parental level of education, cultural differences, language barriers, SES, etc. As the result, thousands of low-income people or people from non-English-speaking cultures were labelled with a low IQ-score and lost their chances for employment or higher education. A similar controversy occurred around the employment of women in science based on assumptions that women have inferior intellectual abilities compared to men. These examples illustrate the importance of conceptual separations of social and biological factors contributing to individual differences, even though these factors obviously interact. Otherwise, it might not be harmless to ignore technical concepts such as those used to identify specific temperament traits (figure 1), and to use only concepts in which biologically and socially-based individual differences are blended, such as personality.
(b). Missing concepts
The FFM was initially developed by incorporating concepts studied in temperament research and other fields (Neuroticism, Extraversion, Openness to experience). It also used two further concepts (Agreeableness and Conscientiousness) from the Big Five model of social perception, which was derived from human everyday language using lexical approaches [26,36]. That is, these two models were not developed on the basis of experimental biological methods [26,40,41].
The socio-cultural partitioning of individual differences as reflected in human language, however, is influenced by various pro-social biases that emphasize some individual differences, level others, and also put evaluative moral judgements on many of them [46]. In our theme issue, Saucier [5] points out the contribution of morality to personality traits, and Uher [26,45] and Trofimova [16] point to the redundancy of the FFM, the Big Five Model and other factor-analytic models derived with using merely language-based methods. More specifically, human societies universally highlight individual characteristics that improve functioning of a society as a system but downplay characteristics unrelated to social exchanges that are, however, important for individual's functioning. As a result, social perception is focused on persons' abilities to communicate information (FFM's dimension of Extraversion), to be emotionally stable (Neuroticism), to obey social order and maintain peace (Conscientiousness and Agreeableness), and to learn and deliver knowledge (Openness to experience). Societies give grades and awards to people for socially accepted behaviours and develop other public identifiers of these abilities. Yet, Western societies promote equality between people of different sex, age, blood type, visual capacities, constitution, etc. even though the gender of a person is still a valid subject for psychophysiological studies and is often good-to-know for activities like dating and family planning. Constitution is often important for jobs that depend on certain constitutional types and is also the subject of studies concerning the endocrinology of individual differences. Age is often important to diagnose medical conditions, including psychiatric problems. Several temperament (neurochemically based) traits that are important for individuals' functioning may be less noticed by society (figure 1) even though, as shown in this theme issue, they have well-documented biological bases: plasticity and impulsivity [1–3,6,23,25,41], sensation seeking [10,25], speed of speech and physical actions [1,7,16,24], sustained attention or effortful control [12], sensory sensitivity [15], psychopathy and empathy [3,8], a system of positive emotionality and security [16,18], physical endurance [1,3,23], which, in its weakness, shows up as a main symptom of depressive dispositions [39,47].
(c). Misusing concepts
This theme issue reports on several studies on biological correlates of individual differences as measured by common personality tests [10,25,48]. The use of personality questionnaires mixing social and biological factors for biological investigations of traits may lead to results that are not very consistent [42–45,48,49], and we have to appreciate the practical consequence of theoretical blending of concepts, as described above. Investigating healthy human subjects with drug challenges [7], hormonal monitoring [9], PET scans and application of chemical agents to determine receptor densities of specific neurotransmitters [10], as well with MRI and EEG equipment [1,8,12,13,25] and behavioural tasks [1,7,12] is not only expensive but also challenging in terms of recruitment of volunteers and research ethics. That is why studies such as those reported here, especially by Netter [7], and Farde et al. [10] are so valuable. It is therefore important that we do not squander these resources on explorations of constructs derived from human language but instead use objective methods based on experimental psychology or well-validated questionnaires developed on the basis of findings from biology-rooted sciences such as differential psychophysiology [1,4,9,12,14,15,23–25], psychiatry [8,14,18,39,50] or neurochemistry [3,16,23,24,39,46].
For example, Sallis et al. [48] noted that, in genetic studies, the strongest association of genotypes was found not with specific traits but with years of schooling (i.e. a composite social variable). Yet, when the choice of measurement variables was rooted in well-documented links between specific neurophysiological systems and specific psychological and behavioural individual differences (see, for example, [1,12]), then more consistent associations were found, such as in dispositions for depression or effortful control [12,13,22]. Effortful control is conceived also as a frontal-lobe based ‘processing block’ (Pribram & Luria [51]), intellectual ergonicity (Rusalov [1,23]), mental endurance (Trofimova [3,23]) or more commonly as sustained attention (Hasselmo & Stern [11]). Posner & Rothbart [12] and Hoyniak and colleagues [13] reported links between EEG and MRI measures and effortful control capacities in children, and their life outcomes 30 years later. These links might explain the findings of associations between genotypes and years of schooling [48]. After all, success in school is influenced by learning abilities, self-organization, planning and impulse control, functions classically linked to activity of the frontal cortex. Also, as pointed by Sallis et al. [48], associations were also found between depressive dispositions and genotypes. This coincides with presentation of main symptoms of depression as clinical variations in temperament traits of physical endurance (symptom of fatigue), physical tempo (i.e. motor retardation), sociability (social withdrawal), plasticity (apathy for new tasks), sustained attention (inability to focus), impulsivity, confidence (low self-image) and neuroticism (increasing worries) [39,47]. Well-documented associations of these symptoms and traits with actions of specific neurotransmitters, neuropeptides and hormones [3,7,9,10,16,24,39,47] are reported in the extensive field of psycho-pharmacology; genetic associations and links to the dopaminergic systems are also commonly found in schizophrenia research [48,52]. All this highlights the importance of attention to functionality of neurotransmitters in classifications of human behaviour. Interestingly, none of the factor-analytically derived personality models identified many of these traits, depressive or psychotic dispositions [36]. Structuring neurophysiological, genetic and longitudinal studies in line with neurochemical models of temperament might bring, therefore, more consistent and indicative results.
Unlike many works analysing individual differences at the socio-cultural level of integration (personality), this theme issue gives voice to leading experts investigating the biological level of integration of these differences: neurochemistry [2,6,7,9–11,16], neuroanatomy and neurophysiology [1,8,12,13,15,25], genetics [48,52], psychiatry [8,39] and comparative psychology [2,5,26].
3. Choice of evidence: statistical–psychometric versus conceptual–multi-disciplinary
All scientists studying biologically-based traits appreciate the gravity of the task and look for evidence to support or disprove the classifications proposed. There are, however, five faulty trends in the search for such evidence that are especially common in mainstream psychology:
1. Evidence of psychometric properties of tests is mistakenly considered as evidence of the structure of the actual phenomena under study. In differential psychology, there is a widespread misconception, historically derived from psychometrics, that the best way to discover and verify taxonomies of individual differences would be by using FA. Confirmatory FA and other types of FA are commonly used by psychometricians to ensure that the test scales independently represent the psychological features they purport to measure. Verifying psychometric test properties, however, does not move us any closer to understanding the nature and/or structure of the individual differences measured [26]. By analogy, a weighing scale should reliably measure weight, but this does not constitute a theory of gravitation in itself. The methods that technicians use to tune their equipment differ from the methods theoreticians use to verify the proposed principles of natural phenomena. Technicians may measure millions of weights, but it is theoreticians who have helped us to understand that weight is a consequence of gravity and not fundamental in itself. No matter how many psychometric reports describing factor-analytical structures of specific questionnaires exist, they can only be used to explore the structure of these questionnaires but do not provide any biological evidence [26].
Many researchers are concerned about the psychometric independence of test scales, notably the belief that insufficient independence may be the main reason for inconsistent results [26,28–36,40,41,45,49,53,54]. Independence of scales, however, is a property that psychometricians wish to achieve for their tests; but in real life, independence of characteristics does not exist (as noted by [7,16,26,39,43,44,46,55]). It is not even important for developing taxonomies or diagnoses. Moreover, self-report methods and independence of self-report scales are probably not an issue, because the whole fields of psychiatry and other areas of medicine also rely on patients self-reporting their symptoms during clinical investigations. More important is what people are asked to report and how their answers are grouped, processed and interpreted. When questions related to formal–dynamical features of behavioural regulation (e.g. energetic, speed-related aspects and consistent emotional dispositions) are mixed with questions related to social functioning (e.g. their attitudes to rule obedience, morality), we risk compromising the accuracy of investigations.
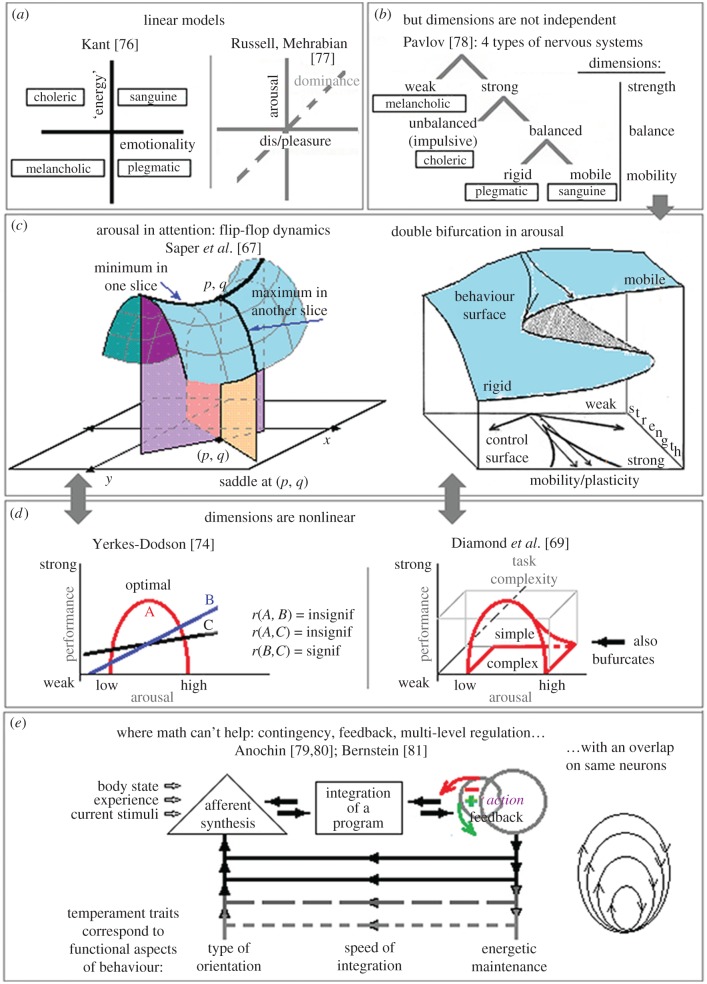
2. Statistical linear models are used as evidence of a structure underlying nonlinear, contingent and feedback processes. FA, mostly used by social scientists, suffers from multiple and serious mathematical limitations, yet these are largely ignored. FA is an easy-to-use statistical method that gives the appearance of grouping variables scientifically into categories. Since its inception [56], it has faced much criticism and has been pretty much ignored outside the social and behavioural sciences. For example, a recent text on statistical methods in physics [57] accorded to it only 7 out of 412 pages, and then dismissed it. Serious limitations of FA have been widely discussed throughout the past century [46,53,55,58–66]. As pointed out by Sulis [39] in this theme issue, FA and its derivative, Structural Equation Modelling (SEM) are linear methods (so-called ‘nonlinear’ FA still uses matrices of linear correlations), whereas most psychological processes show nonlinear relationships among their components [67–73]. Linear methods cannot even approximate the structure of nonlinear phenomena because, by imposing linear structures, they present an incorrect picture. As a relevant example, a linear correlation of the curvilinear function described by the classic Yerkes–Dodson [74] law with any linear ruler will wipe out the nonlinear bell-shape curvature and subsequent additions to this law (figure 2d). Calculating such correlations would show a statistically insignificant effect and therefore will be missed in FA-derived models. The Yerkes–Dodson law, however, relates to the main dimension in all taxonomic models, i.e. arousal. How many other curvilinear effects may have been missed in FA-derived models, we can only but wonder. More sophisticated yet common types of nonlinearity, such as bifurcations (figure 2b,c–d (-right) and saddle-points (figure 2c-left), as well as contingent relations (figure 2e) have also been described in research on arousal systems [24,67–69] and various psychological phenomena associated with individual behavioural differences [70–72]. Subsequent investigations uncovered a bifurcation in the Yerkes–Dodson curve [69] as well: the curve collapsed for simple tasks but still remained in subjectively complex tasks (figure 2d). This nonlinearity cannot be captured by FA. The danger of redundancies in statistically-based approaches is discussed by Sulis [39], Rusalov [45] and Uher [26].
Figure 2.
The progress of formal (mathematical) models in taxonomies: from linear orthogonal dimensions (a) to nonlinear models where functions (y) have multiple responses to the same argument (x) (b–d) to the models described from real neurophysiological data where mathematics is yet to be developed (e).
3. In the description of FA-evidence for specific structures, no attention is given to biases in the data. In FA, the key unit of analysis is the degree of correlations between variables, and FA-based ‘evidence’ does not reflect anything more than a degree of these intercorrelations. This means that FA can identify a large factor of strongly intercorrelated variables only because the researcher has preferred specific types of variables. In this sense, FA is insensitive to biases in the data and in the choice of variables and so some researchers compared FA efficiency to tarot cards [75, p. 144]. For example, the FFM of personality adopted the factor structure of the Big Five Model of social perception based on the lexical approach. It is called lexical not because it used questionnaires but because it used (arbitrarily chosen) thousands of common-language descriptors of psychological differences, in which proximity was analysed using FA. This method, however, has a strong sociability bias by default [3,26–36,40,41,43,44,46]: after all, language (and verbal descriptors) emerged in evolution to bring people together. As a result, we have more words related to pro-social individual differences than to other types of theme, and the prevalence of certain type of descriptors in the list of variables results in a large factor related to socialization. Since this pro-social bias of language is universal, we can expect that in lexical studies the factor of Extraversion will always be one of the largest (figure 3). Moreover, a negativity bias of emotionality and conflation of concepts in everyday concepts and language also affects all resulting lexical models, producing factors of Neuroticism and conflating multiple temperament constructs in one factor, as it happened for the factor of Extraversion [3,46]. Some researchers using the Big Five Model wisely suggested that the FFM might represent dimensions of language-encoded social (peer) perception among people rather than biologically-based ‘human universals’ [3,26,27,31–36,40,41,43,46,54].
Figure 3.
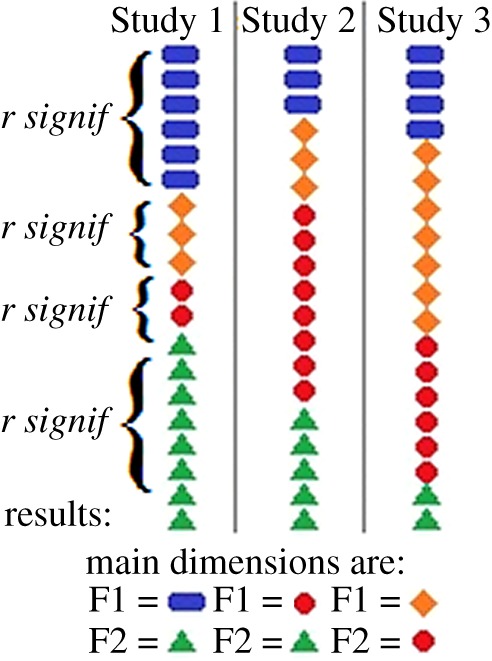
Illustration of the sensitivity of FA to the choice of items. FA groups items on the basis of their intercorrelations. If the lists include significant portions of items related, for example, to socialization (rectangular blue bullets) or negative emotions (triangular green bullets), their proportions and close relationships will create large factors of Extraversion (blue) and Neuroticism (green) (hypothetical Study 1). Changing the lists, however, will resolve in other main factors (Studies 2 and 3).
4. Evidence of cross-cultural similarity of social phenomena is taken as evidence related to biologically-based traits. Researchers working with the FFM often claim to have identified ‘human universals’ and biologically-based traits. They are especially proud of the cross-cultural consistency of at least two FFM dimensions, Extraversion and Neuroticism, as evidence supporting their model. Cross-cultural comparisons of lexical models are, however, irrelevant for explorations of biologically-based systems of individuality. After all, cross-cultural studies measure commonalities between cultures, and not biological systems. As noted above, it is natural to find consistency of a sociability bias of language emerging as the Extraversion factor, and a negativity bias of emotionality emerging as the Neuroticism factor in cross-cultural FFM studies because these biases affect verbal descriptors in all cultures in a similar way [46]. Saucier [5], similar to the position of Uher [36] and Trofimova [3,16,46], pointed out that ‘the lexical approach as formulated by Allport and Odbert, seems to not lead directly to neurophysiological bases of personality (sic)’. A socio-cultural and socio-linguistic framework for classifying consistent individual differences might not be capable of differentiating between biologically-based individual differences in the detail needed, because the social representation of individual differences is often blind to features relevant to biology. Therefore, it is important to continue developing and using specific concepts related to biologically-based differences (i.e. temperament, mental disabilities and abilities).
5. Statistical versus conceptual evidence. Young social scientists often place too much trust in statistical methods, hoping these methods could derive, confirm or disprove taxonomical models of biologically-based traits just by pushing buttons in statistics software. However, FA and other statistical methods often involve arbitrarily selected and subjectively defined variables, while abandoning cases that do not fit well into main trends. In contrast to this, taxonomies should include all cases in all their aspects as Uher [26] elaborated in the concepts of comprehensiveness and representativeness. Moreover, conceptual analysis for the purpose of taxonomy development always includes consideration of the natural principles driving the differentiation between the typological groups of these taxonomies, whereas statistics is blind to such principles. Statistical methods in general are incapable of identifying these underlying principles and cannot substitute for multi-disciplinary analysis: they require researchers to select and define variables prior to statistical processing and cannot do the selection and definition for us.
For these reasons, other more mature sciences do not use statistics to derive their taxonomies, as noted by Sulis [39], although they have collected much more data over the centuries than psychology. In older sciences, the development of taxonomies of natural systems first required the development of a detailed conceptual framework and a large body of observations from boundary disciplines differentiating the features of the phenomena under study. Such conceptual differentiation went hand-in-hand with cross-disciplinary cooperation. For example, the standard model of elementary particles was derived from knowledge in physics, mathematics, cosmology and chemistry; the periodic table of chemical elements was based upon chemistry and was later explained by quantum mechanics; biological taxonomies were derived from findings and principles from reproductive biology, ecology, biochemistry, anatomy and metabolic research; anatomic classifications used principles of functionality, complex fractal structure and ensemble-like dynamics of somatic systems,. Even after centuries of investigations, many of these taxonomies are still works in progress. In this issue, Uher [26] has presented a categorization of methodological approaches used in different fields to select and reduce variables for taxonomy development, discussing advantages and limitations.
4. Principles driving biologically-based traits: interdependent, contingent and multi-level compositions
This theme issue presents studies of individual differences involving multiple methodologies: genetic analysis [48,52], EEG [1,13], evoked potentials [1] and MRI correlates [8,12,25], PET scans for receptor density [10], drug challenges [7], behavioural tasks [1,7,12], combined with questionnaires for parents assessing their children [12] or with self-reports of adults [1,2,7,16,52], cross-cultural and inter-species comparative studies [5,26], profiling clinical samples [3,8,15,39], mathematical modelling and statistical analysis [11,52]. All of these different approaches brought an unexpected and sobering result: components-oriented approaches looking for correlations between identified ‘bits’ of nature (genes, traits, regional brain activity, ‘amount’ of neurotransmitters) might not work [45]. As Cloninger & Zwir [52] point out, ‘evolution and development operate on adaptation of whole organisms or persons, not on individual traits or categories.’
Several key principles for taxonomies are identified by leading scientists in this issue. First, there is the matter of interdependence between components contributing to biologically-based traits, and this speaks against the dimensionality approach presently common in differential psychology. The dimensionality approach that maps psychological types to quadrants within a small number of independent linear dimensions appeared to be a step forward from empirically-derived classifications (that use common behavioural observations). The first known dimensional model was offered by Immanuel Kant in 1798 [76], who plotted Hippocrates' four temperaments into a space of two dimensions (figure 2a). Kant's two dimensions reappeared under different names in practically all existing models of temperament and personality offered in the twentieth century, including that offered by Russell and Mehrabian in 1977 (see [3], for a review). Interestingly, the resemblance between Kant's model or other models of temperament and the FFM's two main dimensions as well as the concept of temperament in general have never been discussed by many FFM proponents. The problem is that the dimensionality approach assumes independence (orthogonality) among dimensions. This orthogonality is mathematically very convenient as it allows plotting psychological types into quadrants and calculating the ‘coordinates’ and values related to specific types. However, it presents a very distorted picture of reality. There is a well-documented interdependence among components (dimensions) of the systems underlying psychological traits, as noted by Netter [7] and Sulis [39] and analysed by Trofimova [16]. This interdependence might underlie the results observed by Farde's and Netter's research groups [7,10], as well as the phenomena of emotional regulation as described by Blair [8] and Jones & Sloan [9]. As seen in the work of Sallis et al. [48], this interdependence between Kant's two main dimensions of energetic and emotional regulation is also found at the genetic level.
Second, as pointed out by Robbins [6], Netter [7], Blair [8], Trofimova [16], Posner & Rothbart [12], Rusalov [1], Hasselmo & Stern [11], neurophysiological systems that are thought to contribute to biologically-based traits have multiple subsystems, and each of these subsystems shows functional specificity and mutual feedback (figure 2e). A similar complexity was found for socio-cultural factors affecting individual differences (e.g. education, SES, cultural and national identity, personal history). These socio-cultural factors appear not to be independent of each other and also have complex coupling with biological factors. Robbins [6] and Trofimova [16] pointed not only to the multiplicity of neurochemical systems of human diversity but also their complex and multi-layered functionality, which can be tuned so as to allow multiple aspects of situations to be addressed simultaneously, but not as independent traits. Cloninger & Zwir [52] and Sallis et al. [48] note the multiplicity principle in genotype analysis. Trofimova [3,16] suggested using the concept of an Ensemble of traits for taxonomies of biologically-based traits instead of a model with independent dimensions, considering them as functional regulatory aspects of behaviour. Ensemble-like relationships are noted, among others, for sensory systems, somatic systems, systems at the molecular level of neurochemical regulation. Many of them have specific identifiable structures, but they act in close coordination with each other. Similar to the division of functionality between vision and hearing or between respiratory and muscular systems in the joint regulation of behavioural acts, it is reasonable to consider the partitioning the properties of nervous systems in accordance with several universal aspects of situations, such as intensity, novelty, complexity, speed of change, safety, emergency and presence of specific reinforcers [1,3,16,39]. It is unlikely that these aspects would emerge as independent dimensions in FA or other statistical models because these methods cannot differentiate them due to the high degree of entanglement between these systems.
Third, as pointed out by Kagan [25], Netter [7], Jones & Sloan [9], Sulis [39], Trofimova [16] and Acevedo et al. [15], the relationships between temperament and the environment are very dynamic during an individual's lifetime. Investigations into the differential contribution of the physical environment, such as physiological state of the mother, nutrition and physical touch (Jones & Sloan [9]) and the social environment, such as culture, perceived social support and status, cannot bring informative measurements without differentiating between neurochemically-based traits (temperament) and traits integrated with social values, appraisals and expectations (personality).
Fourth, there is more to the dynamical complexity of temperament systems than merely its development over the lifespan. Several authors described key dynamical features within neurophysiological systems that could not be captured by linear statistical methods: the importance of variability and noise for the system's stability [11], the constructive and transient nature of processes [16,39,66–71,73] and the differential response of receptors from different locations in the brain contingent on current tasks [2,6,7,10]. Sulis [39] points out that linear correlations and approximations might be partially applicable when stability of the distributions is assumed, but this assumption is not appropriate for natural systems. Similarly, Trofimova [16,73] explores a functional constructivist approach in psychology, emphasizing the generational and transient nature of human behaviour. This approach underlines that all behavioural acts are being created anew based on internal capacities and environmental demands, and therefore she suggests that our taxonomies should include dynamic principles that are reflective of these constructive processes. Trofimova's FET model [3,16] coincides with the principles of diagonal evolution [73] and expands work of Luria and Rusalov [1]. She suggests a taxonomy of temperament traits based on four formal, universal dynamical properties of human behaviour—emotional dispositions, energetic, orientational and dynamic (related to speed of integration) aspects manifested in three neurophysiologically different systems of behavioural regulation (physical, social–verbal and those related to frontal cortical regulation).
Several authors discuss alternative mathematical approaches that would be more adequate for the measurement or modelling of the processes contributing to biologically-based traits than traditional statistical and measurement tools: Mendelian Randomization, which compares distributions of effects rather than single effects [48], cluster analysis based on non-negative matrix factorization as a novel data-mining method [52], new mathematical languages, such as process algebra, non-Kolmogorov probability and functional constructivism [39,73] and variability-noise-based models [11].
5. In search of a common taxonomy of individual differences and mental disorders
Throughout the twentieth century, psychiatrists and clinical psychologists called for establishing a common taxonomy of both healthy and mentally ill people. The focus on temperament in several contributions to this theme issue is based on the premise of a common aetiology of temperament and mental disorders (i.e. neurochemical systems of behavioural regulation). Indeed, if a model of biologically-based traits has been carefully structured so as to be capable of responding to dynamical interrelationships between systems of behavioural regulation in healthy people, then in the presence of mental disorders, which presumably alter these relationships, these profiles should exhibit distinct patterns consistent with Diagnostic and Statistical Manuals of Mental Disorders (DSM/ICD) symptoms of such illness. Therefore, such a taxonomy of healthy people should be testable by investigations of mental disorders if these disorders can be mapped using the descriptors of the same taxonomy [45].
In the latest DSM5 and ICD versions, the main classifications of mental disorders are heavily influenced by a dimensional model of Negative and Positive Affect. Meanwhile, a systemic model or classification of mental disorders should comprise not only emotionality (affect)-related but also non-emotionality-related components reflecting the main functional aspects of behavioural regulation. Indeed, many authors have investigated those aspects that are not related to emotionality but nevertheless may represent at their extremes symptoms of mental illness: impulsivity and compulsivity (i.e. dynamics of integration of behavioural acts [2,6,10,25]); schizophrenia [10,15]; Autistic and Post-Traumatic Stress Disorders [15] (associated with dysfunction in cognitive processing); and psychopathy (as non-empathic behavioural orientation) [8]. One approach to classify systems of behavioural regulation is in terms of the functional aspects of behavioural tasks as described by Rusalov [1], Sulis [39] and Trofimova [16]. Mapping these regulatory systems to a small set of functional features of activities opens up the possibility of a more compact and formal presentation of symptoms of mental disorders that emerge as deficiencies within the neurophysiological systems regulating human behaviour. Emotionality-related traits, however, should also continue to be present at the core of investigations of healthy and clinical samples. Couplings between opioid receptor systems (linked to dispositional emotionality) and monoamine or neuropeptide systems (linked to the regulation of both emotional and non-emotional aspects of behaviour) [16] as well as couplings between hormonal and monoamine systems [7] suggest that emotionality and other aspects of behavioural regulation cannot simply be represented as independent dimensions. The integration of these systems appears to have a logical functional pattern, which is in favour of functional ecology-based, and not a dimensional approach to taxonomies.
In summary, despite intense efforts over several millennia, the task of classifying biologically-based traits and their deviations as mental illnesses continues to be incomplete and challenging. The more knowledge that human-kind has gained, the more challenging this task appears to be, and during the twentieth century, the lists of neuroanatomical and neurochemical systems contributing to biologically-based behavioural traits grew into the hundreds. We are still very far from declaring ‘mission accomplished’ in the classification of these traits, contrary to claims of FFM proponents.
What becomes clear is that taxonomies of these traits cannot be derived on the basis of findings from just one science. Commonplace statistical and other mathematical methods, including nonlinear dynamics, are still rather weak, failing to capture the complexity of the processes that regulate human behaviour. The features of the systems underlying natural phenomena are complex and lie always at the cross-roads of multiple disciplines [45]. Therefore, models that rely on data from just one method or one discipline might be not very informative but datasets from diverse disciplines or methods often have incompatible variance for joint statistical processing. For this reason they should be a subject of analytic discussions to help identify principles for developing taxonomies in the future. However, as always, there is a language barrier (in this case—a multi-disciplinary one). Many branches of psychology, psychiatry, neurochemistry, neuroanatomy, animal biology, endocrinology, philosophy and social sciences approached these matters from their discipline-specific perspectives, and this theme issue is an attempt to represent this diversity of opinions.
For us as editors, it was challenging to group these contributions into chapters because many contributors reflected on several important aspects of consistent individual differences. It was also a delight to discuss these challenging scientific matters with some of the most prominent scientists in the field and to enjoy their competence, intelligence, great sense of work discipline and kind cooperation. We want to especially praise the efforts of Nancy Jones and Aliza Sloan, who continued working on their impressive paper while under attack from hurricane Irma in Florida. At almost the same time, Robert Cloninger, author of one of the earliest neurotransmitter hypotheses of temperament traits, had to handle the consequences of the floods in Texas after hurricane Harvey. We also want to congratulate Petra Netter, a pioneer in research into the role of neurotransmitters in temperament, with her 80th jubilee! Her contribution is a great way to celebrate it, especially as she had to spend a good part of this special year working on it.
We are deeply grateful for very helpful interactions with our Editor at PTRS-B, Helen Eaton, who responded to all of our small and big requests with great care. We hope that after reading this theme issue, scientists from many disciplines will appreciate the complexity of the task of classifying biologically-based individual differences in human behaviour regulation, and will join us in future investigations.
Biographies
Author biographies
 Irina Trofimova conducted research and taught psychology in several Moscow Universities until 1997, and in Brock University and Toronto Mamonides College (Canada) from 1997 to 2000. She assumed her position as a Senior Researcher and an Adjunct Professor within the Collective Intelligence Lab, Department of Psychiatry and Behavioral Neurosciences, McMaster University, Canada in 1997 and holds it currently. She has been also a licensed Psychologist with the Ontario College of Psychologists since 1999 and has been Director of a private psychological practice serving four locations in Southern Ontario since 2004, so her experimental research in temperament theory and cognitive psychology involves clinical and healthy human samples. Her areas of expertise are taxonomies of temperament and personality, functionality of neurotransmitter systems, psychometrics, clinical, evolutionary and cognitive psychology, psychosemantics, psychological assessment and psychological treatment; mathematical modelling in psychology, complex systems theories and diversity theory. She is also a Director of three non-profit psycho-educational documentary projects.
Irina Trofimova conducted research and taught psychology in several Moscow Universities until 1997, and in Brock University and Toronto Mamonides College (Canada) from 1997 to 2000. She assumed her position as a Senior Researcher and an Adjunct Professor within the Collective Intelligence Lab, Department of Psychiatry and Behavioral Neurosciences, McMaster University, Canada in 1997 and holds it currently. She has been also a licensed Psychologist with the Ontario College of Psychologists since 1999 and has been Director of a private psychological practice serving four locations in Southern Ontario since 2004, so her experimental research in temperament theory and cognitive psychology involves clinical and healthy human samples. Her areas of expertise are taxonomies of temperament and personality, functionality of neurotransmitter systems, psychometrics, clinical, evolutionary and cognitive psychology, psychosemantics, psychological assessment and psychological treatment; mathematical modelling in psychology, complex systems theories and diversity theory. She is also a Director of three non-profit psycho-educational documentary projects.
 Trevor Robbins FRS FMedSci FBPs SFBPhS is Professor of Cognitive Neuroscience at the University of Cambridge. He was a Head of the Department of Psychology, Cambridge University for 15 years till 2017. He is also Director of the Behavioural and Clinical Neuroscience Institute, the mission of which is to inter-relate basic and clinical research in psychiatry and neurology. His areas of expertise and research interests are: functionality of brain neurotransmitter systems in human and animal behavior, obsessive-compulsive disorder, functions of fronto-striatal and limbic-striatal systems and their neurochemical modulation by the ascending neurotransmitter systems. Trevor is one of the most cited neuroscientists in the World (H index=177, Web of Science), with over 800 full papers and 8 co-edited books. He is a member of the Board of several scientific journals and scientific committees including the UK MRC. He was elected FRS in 2005 and made a CBE 'for services to medicine' in 2012. Among several awards, he shared the Grete Lundbeck European Brain Research Prize in 2014 and has recently received the Lifetime Achievement Award of the British Association for Psychopharmacology (2015) as well as the Gold Medal of the US Society of Biological Psychiatry (2017), and the Patricia Goldman-Rakic Award for Cognitive Neuroscience (2017).
Trevor Robbins FRS FMedSci FBPs SFBPhS is Professor of Cognitive Neuroscience at the University of Cambridge. He was a Head of the Department of Psychology, Cambridge University for 15 years till 2017. He is also Director of the Behavioural and Clinical Neuroscience Institute, the mission of which is to inter-relate basic and clinical research in psychiatry and neurology. His areas of expertise and research interests are: functionality of brain neurotransmitter systems in human and animal behavior, obsessive-compulsive disorder, functions of fronto-striatal and limbic-striatal systems and their neurochemical modulation by the ascending neurotransmitter systems. Trevor is one of the most cited neuroscientists in the World (H index=177, Web of Science), with over 800 full papers and 8 co-edited books. He is a member of the Board of several scientific journals and scientific committees including the UK MRC. He was elected FRS in 2005 and made a CBE 'for services to medicine' in 2012. Among several awards, he shared the Grete Lundbeck European Brain Research Prize in 2014 and has recently received the Lifetime Achievement Award of the British Association for Psychopharmacology (2015) as well as the Gold Medal of the US Society of Biological Psychiatry (2017), and the Patricia Goldman-Rakic Award for Cognitive Neuroscience (2017).
 William Sulis is Director of the Collective Intelligence Laboratory and an Associate Clinical Professor in the Department of Psychiatry and Behavioral Neurosciences, McMaster University, Canada. He is also an Associate Member, Department of Psychology, McMaster University and Director of the Late Life Memory Clinic, Haldimand War Memorial, Dunnville, Ontario, Canada. He runs a Geriatric Psychiatry practice in Southern Ontario and also conducts academic research in the areas of complex systems, psychiatry and theoretical physics. His areas of expertise are collective intelligence, neurochemical systems regulating human behaviour, mathematical methods and their use in psychology, particularly transient global induced response synchronization and dispositional cellular automata, nonlinear dynamics methods, process theory and developed the process algebra model of quantum mechanics.
William Sulis is Director of the Collective Intelligence Laboratory and an Associate Clinical Professor in the Department of Psychiatry and Behavioral Neurosciences, McMaster University, Canada. He is also an Associate Member, Department of Psychology, McMaster University and Director of the Late Life Memory Clinic, Haldimand War Memorial, Dunnville, Ontario, Canada. He runs a Geriatric Psychiatry practice in Southern Ontario and also conducts academic research in the areas of complex systems, psychiatry and theoretical physics. His areas of expertise are collective intelligence, neurochemical systems regulating human behaviour, mathematical methods and their use in psychology, particularly transient global induced response synchronization and dispositional cellular automata, nonlinear dynamics methods, process theory and developed the process algebra model of quantum mechanics.
 Jana Uher is a Senior Lecturer in Psychology at the University of Greenwich. She was a Senior Research Fellow at the London School of Economics and Political Science (LSE). From 2010–2013, Jana was the Director of the Research Group ‘Comparative Differential and Personality Psychology’ that she founded at the Free University Berlin (FUB), funded by a research grant from the German Science Foundation (DFG). In 2013, she was awarded a Marie Curie Fellowship by the European Commission. Her research is transdisciplinary, concentrating on philosophy-of-science issues of research on individuals from transdisciplinary perspectives reaching across various human cultures and various species. She employs a broad portfolio of methods in studies with human children and adults with different sociocultural backgrounds and more than ten different nonhuman species, in particular primates. Jana Uher has been working at the Max Planck Institute for Evolutionary Anthropology (MPI-EVAN) in Leipzig (2003–2005) and has also been a visiting scholar at the Institute of Cognitive Sciences and Technologies, National Research Council of Italy (ISTC-CNR) in Rome (2011–2013).
Jana Uher is a Senior Lecturer in Psychology at the University of Greenwich. She was a Senior Research Fellow at the London School of Economics and Political Science (LSE). From 2010–2013, Jana was the Director of the Research Group ‘Comparative Differential and Personality Psychology’ that she founded at the Free University Berlin (FUB), funded by a research grant from the German Science Foundation (DFG). In 2013, she was awarded a Marie Curie Fellowship by the European Commission. Her research is transdisciplinary, concentrating on philosophy-of-science issues of research on individuals from transdisciplinary perspectives reaching across various human cultures and various species. She employs a broad portfolio of methods in studies with human children and adults with different sociocultural backgrounds and more than ten different nonhuman species, in particular primates. Jana Uher has been working at the Max Planck Institute for Evolutionary Anthropology (MPI-EVAN) in Leipzig (2003–2005) and has also been a visiting scholar at the Institute of Cognitive Sciences and Technologies, National Research Council of Italy (ISTC-CNR) in Rome (2011–2013).
Endnote
Throughout this Issue, many authors use the expression ‘traits’ and ‘individual differences’ when referring to ‘biologically-based, consistent individual differences in human behavioural regulation’. Since the same trait concepts were used to study both temperament traits and personality traits, the papers do not focus on differences between these two concepts, leaving it for us as Editors to comment on them.
Data accessibility
This article has no additional data.
Authors' contributions
All Editors worked diligently on this Editorial.
Competing interests
We have no competing interests.
Funding
T.W.R.'s research is supported by a Wellcome Trust Senior Investigator Award 104631/Z/14/Z. J.U.'s research was supported by a Marie Curie Fellowship from the European Commission (Grant number 629430).
References
- 1.Rusalov V. 2018. Functional systems theory and the activity-specific approach in psychological taxonomies. Phil. Trans. R. Soc. B 373, 20170166 ( 10.1098/rstb.2017.0166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellu-Hagedorn F, Rivalan M, Fitoussi A, De Deurwaerdère P. 2018. Inter-individual differences in the impulsive/compulsive dimension: deciphering related dopaminergic and serotonergic metabolisms at rest. Phil. Trans. R. Soc. B 373, 20170154 ( 10.1098/rstb.2017.0154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trofimova I. 2016. The interlocking between functional aspects of activities and a neurochemical model of adult temperament. In Temperaments: individual differences, social and environmental influences and impact on quality of life (ed. Arnold MC.), pp. 77–147. New York, NY: Nova Science Publishers, Inc. [Google Scholar]
- 4.Strelau J. 1998. Temperament: a psychological perspective. New York, NY: Plenum. [Google Scholar]
- 5.Saucier G. 2018. Culture, morality and individual differences: comparability and incomparability across species. Phil. Trans. R. Soc. B 373, 20170170 ( 10.1098/rstb.2017.0170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins TW. 2018. Opinion on monoaminergic contributions to traits and temperament. Phil. Trans. R. Soc. B 373, 20170153 ( 10.1098/rstb.2017.0153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Netter P. 2018. Benefits and limitations of drug studies in temperament research: biochemical responses as indicators of temperament. Phil. Trans. R. Soc. B 373, 20170165 ( 10.1098/rstb.2017.0165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blair RJR. 2018. Traits of empathy and anger: implications for psychopathy and other disorders associated with aggression. Phil. Trans. R. Soc. B 373, 20170155 ( 10.1098/rstb.2017.0155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones NA, Sloan A. 2018. Neurohormones and temperament interact during infant development. Phil. Trans. R. Soc. B 373, 20170159 ( 10.1098/rstb.2017.0159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farde L, Plavén-Sigray P, Borg J, Cervenka S. 2018. Brain neuroreceptor density and personality traits: towards dimensional biomarkers for psychiatric disorders. Phil. Trans. R. Soc. B 373, 20170156 ( 10.1098/rstb.2017.0156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasselmo ME, Stern CE. 2018. A network model of behavioural performance in a rule learning task. Phil. Trans. R. Soc. B 373, 20170275 ( 10.1098/rstb.2017.0275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Posner MI, Rothbart MK. 2018. Temperament and brain networks of attention. Phil. Trans. R. Soc. B 373, 20170254 ( 10.1098/rstb.2017.0254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoyniak CP, Petersen IT, Bates JE, Molfese DL. 2018. The neural correlates of temperamental inhibitory control in toddlers. Phil. Trans. R. Soc. B 373, 20170160 ( 10.1098/rstb.2017.0160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kagan J, Snidman N. 2009. The long shadow of temperament. Cambridge, MA: Harvard University Press. [Google Scholar]
- 15.Acevedo B, Aron E, Pospos S, Jessen D. 2018. The functional highly sensitive brain: a review of the brain circuits underlying sensory processing sensitivity and seemingly related disorders. Phil. Trans. R. Soc. B 373, 20170161 ( 10.1098/rstb.2017.0161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trofimova I. 2018. Functionality versus dimensionality in psychological taxonomies, and a puzzle of emotional valence. Phil. Trans. R. Soc. B 373, 20170167 ( 10.1098/rstb.2017.0167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zentner M, Shiner R (eds). 2012. Handbook of temperament. New York, NY: Guilford Publications. [Google Scholar]
- 18.Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD. 1994. The temperament and character inventory (TCI): a guide to its development and use. St. Louis, MO: Center for Psychobiology of Personality, Washington University. [Google Scholar]
- 19.Strelau J, Angleitner A (eds). 1991. Explorations in temperament: international perspectives on theory and measurement. New York, NY: Plenum Press. [Google Scholar]
- 20.Depue R, Fu Y. 2012. Neurobiology and neurochemistry of temperament in adults. In Handbook of temperament (eds Zentner M, Shiner R), pp. 368–399. New York, NY: Guilford. [Google Scholar]
- 21.Kagan J. 1997. Galen's prophecy: temperament in human nature. Boulder, CO: Westview Press. [Google Scholar]
- 22.Rothbart MK, Ahadi SA, Evans DE. 2000. Temperament and personality: origins and outcomes. J. Pers. Soc. Psychol. 78, 122–135. ( 10.1037/0022-3514.78.1.122) [DOI] [PubMed] [Google Scholar]
- 23.Rusalov VM, Trofimova IN. 2007. Structure of temperament and its measurement. Toronto, Canada: PSP (Psychological Services Press). [Google Scholar]
- 24.Trofimova I, Robbins TW. 2016. Temperament and arousal systems: a new synthesis of differential psychology and functional neurochemistry. Neurosci. Biobehav. Rev. 64, 382–402. ( 10.1016/j.neubiorev.2016.03.008) [DOI] [PubMed] [Google Scholar]
- 25.Kagan J. 2018. Perspectives on two temperamental biases. Phil. Trans. R. Soc. B 373, 20170158 ( 10.1098/rstb.2017.0158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uher J. 2018. Taxonomic models of individual differences: a guide to transdisciplinary approaches. Phil. Trans. R. Soc. B 373, 20170171 ( 10.1098/rstb.2017.0171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fahrenberg J. 1991. Differential psychophysiology and the diagnosis of temperament. In Explorations in temperament: international perspectives on theory and measurement (eds Strelau J, Angleitner A), pp. 317–333. London, UK: Plenum. [Google Scholar]
- 28.Eysenck HJ. 1992. Four ways five factors are not basic. Pers. Ind. Diff. 13, 667–673. ( 10.1016/0191-8869(92)90237-J) [DOI] [Google Scholar]
- 29.Matthews G, Gilliland K. 1999. The personality theories of H.J. Eysenck and J.A. Gray: a comparative review. Personal. Ind. Differ. 26, 583–626. ( 10.1016/S0191-8869(98)00158-5) [DOI] [Google Scholar]
- 30.Block J. 2010. The five-factor framing of personality and beyond: some ruminations. Psychol. Inquiry 21, 2–25. ( 10.1080/10478401003596626) [DOI] [Google Scholar]
- 31.Hough L. 1992. The ‘Big Five’ personality variables–construct confusion: description versus prediction. Human Performance 5, 139–155. [Google Scholar]
- 32.Block J. 1995. Going beyond the five factors given: rejoinder to Costa and McCrae and Goldberg and Saucier. Psychol Bul. 117, 226–229. ( 10.1037/0033-2909.117.2.226) [DOI] [Google Scholar]
- 33.Cattell RB. 1995. The fallacy of five factors in the personality sphere. Psychol., 207–208. [Google Scholar]
- 34.Shalizi CR.2007. Three Toed Sloth, October 18. See http:/emg-em,%20a%20Statistical%20Myth.htm .
- 35.Block J. 2001. Millennial contrarianism. J. Res. Personal. 35, 98–107. ( 10.1006/jrpe.2000.2293) [DOI] [Google Scholar]
- 36.Uher J. 2015. Developing ‘personality’ taxonomies: metatheoretical and methodological rationales underlying selection approaches, methods of data generation and reduction principles. Integr. Psychol. Behav. Sci. 49, 531–589. ( 10.1007/s12124-014-9280-4) [DOI] [PubMed] [Google Scholar]
- 37.Trofimova I. 2015. Do psychological sex differences reflect evolutionary bi-sexual partitioning? Am. J. Psychol. 128, 485–514. ( 10.5406/amerjpsyc.128.4.0485) [DOI] [PubMed] [Google Scholar]
- 38.Trofimova I. 2013. A study of the dynamics of sex differences in adulthood. Int. J. Psychol. 48, 1230–1236. ( 10.1080/00207594.2012.756981) [DOI] [PubMed] [Google Scholar]
- 39.Sulis W. 2018. Assessing the continuum between temperament and affective illness: psychiatric and mathematical perspectives. Phil. Trans. R. Soc. B 373, 20170168 ( 10.1098/rstb.2017.0168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyle GJ.2008. Critique of the five-factor model of personality. Humanities & Social Sciences papers. Paper 297. See http://epublications.bond.edu.au/hss_pubs/297 .
- 41.Srivastava S. 2010. The five factor model describes the structure of social perception. Psychol. Inquiry. 21, 69–75. ( 10.1080/10478401003648815) [DOI] [Google Scholar]
- 42.Dubois J, Galdi P, Han Y, Paul LK, Adolphs R. 2017. Predicting personality traits from resting-state fMRI ( 10.1101/215129) [DOI]
- 43.Anderson W. 2014. After phrenology: neural reuse and the interactive brain. Boston, MA: MIT Press. [Google Scholar]
- 44.Vul E, Harris C, Winkielman P, Pashler H. 2009. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Persp. Psychol. Sci. 4/3, 274–290. ( 10.1111/j.1745-6924.2009.01125.x) [DOI] [PubMed] [Google Scholar]
- 45.Uher J, et al. 2018. Diversity in action: exchange of perspectives and reflections on taxonomies of individual differences. Phil. Trans. R. Soc. B 373, 20170172 ( 10.1098/rstb.2017.0172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trofimova I. 2014. Observer bias: how temperament matters in semantic perception of lexical material. PLoS ONE 9, e85677 ( 10.1371/journal.pone.0085677) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trofimova I, Sulis W. 2016. A study of the coupling of FET temperament traits with major depression. Front Psychol. 16, 1848 ( 10.3389/fpsyg.2016.01848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sallis H, Davey Smith G, Munafò MR. 2018. Genetics of biologically based psychological differences. Phil. Trans. R. Soc. B 373, 20170162 ( 10.1098/rstb.2017.0162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts BW, Walton KE, Viechtbauer W. 2006. Patterns of mean-level change in personality traits across the life course: a meta-analysis of longitudinal studies. Psychol Bul. 132, 1–25. ( 10.1037/0033-2909.132.1.1) [DOI] [PubMed] [Google Scholar]
- 50.Zuckerman M. 1994. Behavioural expressions and biosocial bases of sensation seeking. Cambridge, MA: Cambridge University Press. [Google Scholar]
- 51.Pribram KH, Luria AR (eds). 1973. Psychophysiology of the frontal lobes. New York, NY: Academic Press. [Google Scholar]
- 52.Cloninger CR, Zwir I. 2018. What is the natural measurement unit of temperament: single traits or profiles? Phil. Trans. R. Soc. B 373, 20170163 ( 10.1098/rstb.2017.0163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fabrigar L, Wegener DT, McCallum RC, Strahan EJ. 1999. Evaluating the use of exploratory factor analysis in psychological research. Psychol. Methods 4, 272–299. ( 10.1037/1082-989X.4.3.272) [DOI] [Google Scholar]
- 54.Jackson JJ, et al. 2009. Not all conscientiousness scales change alike: a multimethod, multisample study of age differences in the facets of conscientiousness. J. Pers. Soc. Psychol. 96, 446–459. ( 10.1037/a0014156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ertel S. 2013. Factor analysis: healing an ailing model. Gottingen, Germany: University of Gottingen press. [Google Scholar]
- 56.Spearman C. 1904. ‘General intelligence’, objectively determined and measured. Amer. J. Psychol. 15, 201–293. ( 10.2307/1412107) [DOI] [PubMed] [Google Scholar]
- 57.Bohm G, Zech G. 2010. Introduction to Statistics and Data Analysis for Physicists . ( 10.3204/DESY-BOOK/statistics). [DOI]
- 58.Thomson G. 1916. A hierarchy without a general factor. Br. J. Psychol. 8, 271–281. [Google Scholar]
- 59.Thurstone LL. 1934. The vectors of mind. Psychol. Rev. 41, 1–32. ( 10.1037/h0075959) [DOI] [Google Scholar]
- 60.Furfey PH, Daly JF. 1937. A criticism of factor analysis as a technique of social research. Am. Sociol. Rev. 2, 178–186. ( 10.2307/2083471) [DOI] [Google Scholar]
- 61.Creasy M. 1959. Some criticisms of factor analysis with suggestions for alternative methods. Br. J. Psychiat. 105, 755–761. ( 10.1192/bjp.105.440.755) [DOI] [PubMed] [Google Scholar]
- 62.Armstrong JS. 1967. Derivation of theory by means of factor analysis or Tom Swift and his electric factor analysis machine. Am. Statistic. 21, 17–21. [Google Scholar]
- 63.McCroskey J, Young T. 1979. The use and abuse of factor analysis in communication research. Human Commun. Res. 5, 375–382. ( 10.1111/j.1468-2958.1979.tb00651.x) [DOI] [Google Scholar]
- 64.Stewart D. 1981. The application and misapplication of factor analysis in marketing research. J. Market Res. XVIII, 51–62. ( 10.2307/3151313) [DOI] [Google Scholar]
- 65.Yalcin I, Amemiya Y. 2001. Nonlinear factor analysis as a statistical method. Statistic. Sci. 16/3, 275–294. [Google Scholar]
- 66.Trninic V, Jelaska I, Stalec J. 2013. Appropriateness and limitations of factor analysis methods utilized in psychology and kinesiology, Part 2. Phys. Cult. 67, 5–17. [Google Scholar]
- 67.Saper CB, Chou TC, Scammell TE. 2001. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 24, 726–731. ( 10.1016/S0166-2236(00)02002-6) [DOI] [PubMed] [Google Scholar]
- 68.Diamond DM. 2005. Cognitive, endocrine and mechanistic perspectives on non-linear relationships between arousal and brain function. Nonlinear. Biol. Toxicol. Med. 3, 1–7. ( 10.2201/nonlin.003.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diamond DM, Campbell AM, Park CA, Halonen J, Zoladz PR. 2007. The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes–Dodson law. Neural Plast. 60803 ( 10.1155/2007/60803) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guastello S, Gregson RA (eds). 2010. Nonlinear dynamical systems analysis for the behavioral sciences using real data. Boca Raton, FL: Taylor and Francis Group, CRC Press. [Google Scholar]
- 71.Sulis W, Combs A (eds). 1996. Nonlinear dynamics in human behavior. Singapore, Singapore: World Scientific. [Google Scholar]
- 72.Sulis W, Trofimova I (eds). 2001. Nonlinear dynamics in life and social sciences. Amsterdam, The Netherlands: IOS Press. [Google Scholar]
- 73.Trofimova I. 2017. Functional constructivism: in search of formal descriptors. Nonlin. Dynam. Psychol. Life Sci. 21, 441–474. [PubMed] [Google Scholar]
- 74.Yerkes RM, Dodson JD. 1908. The relation of strength of stimuli to rapidity of habit-formation. J. Comp. Neurol. Psychol. 18, 459–482. ( 10.1002/cne.920180503) [DOI] [Google Scholar]
- 75.Norman G, Streiner D. 2003. PDQ Statistics. Hamilton, ON: BC Decker. [Google Scholar]
- 76.Kant I. 1798. Anthropology from a pragmatic point of view [transl. Mary Gregor]. The Hague: Martinus Nijhoff, 1974 (VII). [Google Scholar]
- 77.Russell JA, Mehrabian A. 1977. Evidence for a three-factor theory of emotions. J. Res. Personal. 11, 273–294. ( 10.1016/0092-6566(77)90037-X) [DOI] [Google Scholar]
- 78.Pavlov IP. 1941. Lectures on conditioned reflexes, II. Types of the higher nervous activity, their interdependence with neuroses and psychoses and the physiological mechanism of neurotic and psychotic symptoms. [Translated by Gantt WH.). New York, NY: International Publishers. [Google Scholar]
- 79.Anokhin PK. 1964. Systemogenesis as a general regulator of brain development. In The developing brain (eds Himwich WA, Himwich HE), pp. 54–86. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 80.Anokhin PK. 1975. Biology and neurology of the conditioned reflex. Oxford, UK: Oxford University Press. [Google Scholar]
- 81.Bernstein NA. 1996. Dexterity and its development. In Dexterity and its development (eds Latash ML, Turvey MT), pp. 3–244. Hillsdale, NJ: LEA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.