Abstract
Several impulse control disorders such as ADHD, mania, personality disorders or substance abuse share common behavioural traits, like impulsiveness, risk-taking or inflexible behaviour. These disorders are treated with drugs targeting dopamine (DA) and/or serotonin (5-HT). However, the patient's monoamine imbalance that these neurotransmitters compensate is unclear. This study aims to investigate the patterns of DA and 5-HT metabolisms at rest within selected brain regions related to inter-individual variability in six main components of impulsivity/compulsivity (anticipatory hyperactivity, premature responses, delay discounting, risk-taking, perseveration, flexibility). Rats with adaptive and highly inadaptive behaviours were identified in each task and a sensitive biochemical approach allowed mapping of post-mortem endogenous monoamine tissue content in 20 brain areas. Distinct patterns of 5-HT and DA metabolisms were revealed according to the behavioural traits. Except for hyperactive responses, lower control of actions was mainly associated with a lower DA or 5-HT metabolism in prefrontal and/or subcortical areas (i.e. in orbitofrontal cortex (DA), amygdala and anterior cingulate cortex (5-HT) for inflexible and risk-prone rats). Our results reveal the complex nature of behavioural traits related to impulse control disorders through their associated monoaminergic networks at rest, paving the way for understanding the link between mental disorders and drug therapeutic actions.
This article is part of the theme issue ‘Diverse perspectives on diversity: multi-disciplinary approaches to taxonomies of individual differences'.
Keywords: monoamine tissue content, serotonin, dopamine, impulsivity, risk-taking, inflexibility
1. Introduction
Dimensional traits that are continuous with ‘normal’ conditions are thought to characterize most mental disorders [1]. Impulsive/compulsive symptoms occur in several impulse control disorders such as ADHD, mania, personality disorders, pathological gambling or substance abuse and could represent neurocognitive endophenotypes [2], i.e. behavioural and cognitive changes associated with discrete deficits in defined neural systems, these deficits being present in first degree relatives of patients without psychiatric disorder. Impulsivity is a multifactorial trait that has been defined as ‘actions which are poorly conceived, prematurely expressed, unduly risky or inappropriate to the situation’ [3, p.23]. In experimental research, impulse-related behaviours have been divided into different constructs that encompass risk-taking: impulsive choices (inability to wait for a delayed greater benefit) and impulsive actions (inability to withhold a response, i.e. premature responses or anticipatory hyperactivity) [4]. The compulsivity construct refers to ‘actions which persist inappropriate to the situation, have no obvious relationship to the overall goal and which often result in undesirable consequences' [4, pp. 681–682]. Compulsivity is typically demonstrated by inflexible behaviour (i.e. during reversal learning) and by resistance to extinction as shown by perseverative behaviours. Compulsivity, sometimes confused with impulsivity, is quite different in nature: it reflects a maladaptive engagement in stimulus–response habit learning [5], as opposed to goal-directed behaviours that characterize impulsive behaviours. These two constructs are mediated by overlapping as well as distinct neural substrates and serotonin (5-HT) and dopamine (DA) interact across these circuits to modulate aspects of impulsivity and compulsivity [2,6,7]. The substantial gap in our knowledge of the neurobiology that underlies these mental disorders’ symptoms derives in part from the lack of consideration of a dimensional and trans-nosological view of mental disorders in experimental researches. In an attempt to explore the neural basis of the impulsivity/compulsivity dimension, we investigated inter-individual variability in six main components of impulsivity/compulsivity: anticipatory hyperactivity, perseverations, premature responses, delay discounting, behavioural flexibility and risk-taking. Highly adaptive versus maladaptive individuals were selected for each behaviour and their neurochemical signatures were characterized through the patterns of dopaminergic and serotonergic turnovers and metabolisms at rest within a wide number of brain regions of interest.
2. Material and methods
(a). Behaviour
Subjects and behavioural methods are described in detail in the electronic supplementary material.
(i). Impulsive actions: premature responses
The fixed consecutive number of 16 lever press schedule (FCN16) measures behavioural inhibition in operant chambers by testing the rat's ability to carry out a long chain of sequential lever presses before obtaining a reward [8]. The schedule required a fixed minimum number of 16 responses on one lever (FCN lever), before a response on the second lever (reinforcement lever) resulted in the delivery of one food pellet. A cue light is maintained on until the completion of the sequence is reached, thus predicting reward availability upon pressing the reinforcement lever. Impulsivity was reflected by the proportion of prematurely ended chains of presses on the FCN lever. These chains reset the count and were not rewarded.
Data measure: The last FCN16 session was analysed because it revealed the largest inter-individual behavioural differences. Impulsivity was measured by the proportion of prematurely ended chains. The number of sessions needed to reach the test phase (learning score) was also considered. The distribution of the mean number of chains of lever presses according to their length was analysed.
(ii). Impulsive/compulsive actions: anticipatory hyperactivity and perseveration
The multiple fixed-interval/extinction schedules of reinforcement (FI-EXT) were performed in operant chambers equipped with one lever. Two periods of fixed-interval schedule of reinforcement (FI) alternated with two periods of extinction (EXT) (FI-EXT–FI-EXT). Impulsive responses corresponded to lever presses during FI, prior to light presentation that allowed food delivering upon pressing the lever, a reflection of anticipatory activity. Perseveration (compulsive actions) was indicated by lever presses during EXT, when no reward can be obtained (chamber's light switched off).
Data measure: The time-course of mean number of lever presses during each FI and each EXT conditions was recorded.
(iii). Impulsive choice: delay discounting
The delay discounting task (DDT) measures impulsive choice in an operant chamber by assessing the preference for an immediate small reward (one pellet, when pressing the L1 lever) over a larger one delivered after a delay (five pellets, when pressing the L5 lever). The delay preceding the delivery of the larger reinforcement was progressively increased between daily sessions by 10 s from 0 to 40 s according to a criterion of stability and was fixed for a given session.
Data measure: Percentage of L5 choice, total mean number of lever presses, and presses during the delay and time-out periods were measured. These parameters were calculated for each delay as the mean of the last two stable sessions.
(iv). Behavioural flexibility
Behavioural flexibility was measured in a rat gambling task (RGT) that requires successive choices among four options in an operant cage adapted from the 5-CSRTT [9–11]. Rats could freely choose between four nose-poke holes to obtain food pellets. RGT measures, across successive trials, the ability to make the most advantageous choices. In this task, the contingencies associated with a higher immediate gain are disadvantageous in the long run, due to unpredictable penalties. Behavioural flexibility was measured the following day by reversing contingencies (advantageous/disadvantageous outcomes are spatially exchanged): maintaining the choice of the same location reveals behavioural inflexibility (a compulsive-like behaviour), whereas shifting choices reflects detection of the change and behavioural flexibility.
Data measure: Performances were calculated as the mean percentage of reversed choices, for the preferred contingency during the RGT. The behaviours were classified into three categories: flexible behaviour, with progressive reversion towards the new location of their favourite options (more than 50% of reversed choices), inflexible behaviour with perseveration of previously learned choices instead of reversing choices (less than 15% of reversed choices), the remaining being intermediate behaviour.
(v). Risk-taking
The light–dark emergence test allows assessment of spontaneous risk-taking behaviour in rats [10,11]. Exiting from a dark, safe compartment to a brightly illuminated one is a risky and stressful situation for a rat.
Data measure: From the rat's first entrance into the dark box, the latency to emerge from this compartment to the illuminated one was recorded (600 s cut-off). Risk assessments were evaluated by number of body stretches and by head protrusions in the light compartment, with at least the hind limb remaining in the safe compartment. Total time spent in the dark compartment was also measured. An index of risk-taking was calculated including these three parameters (number of risk assessments, latency to emerge and time spent in safe compartment).
(b). Analysis of inter-individual differences
For each behavioural task, performances were split into subgroups to assess inter-individual differences in behaviour. The interest of this approach is to identify and describe in detail the opposite behaviours of individuals, within a normal population, for one particular behaviour (risk taker or risk avoider; impulsive or non-impulsive; flexible or inflexible) and to reveal differences in basal monoamine functions related to this behavioural trait.
Rats were classified according to their scores in the most representative parameter measured in a given task. Selection was made by extracting subgroups of individuals with low or high scores according to the upper and the lower terciles in each task (n = 12 ± 2), the remainder constituting an intermediate group (n = 12 ± 2). This arbitrary separation offers the advantage of magnifying differences between subjects [12–14]. In the case that a rat from the intermediate animals had an identical score to the following rat from an extreme tercile, it was included in this latter group. The behaviour and basal monoamine functions of the two extreme subgroups were then compared.
(c). Tissue processing for histological dissection and chromatographic analyses of post-mortem samples
Rats were sacrificed one month after being tested. Brains were removed rapidly, frozen using liquid nitrogen and stored until use at −80°C. After thawing, dissection of brain areas was performed on a cryostat in the frontal plane. The procedures were published [15]. The monoamine content, the variability of which can reliably be measured in the rat [15], was evaluated in the brain areas encompassing enlarged prefronto–subcortical network. Tissue dosages of monoamines and their metabolites were performed using high-pressure liquid chromatography coupled to electrochemical detection (see electronic supplementary material). Basal dopaminergic and serotonergic turnover corresponded to the ratio of the tissue content of 3,4-dihydroxyphenylacetic acid (DOPAC) versus DA (DOPAC/DA) and 5-hydroxyindole-3-acetic acid (5-HIAA) versus 5-HT (5-HIAA/5-HT), respectively. The full neurochemical database for the molecules and turnovers has already been reported [15] in the following areas: the dorsolateral and lateral parts of the orbitofrontal cortex (DLO/LO), the medial and ventral parts of the orbitofrontal cortex (MO/VO), the infralimbic (IL) and the prelimbic (PL) cortices, the motor cortex (M2), the anterior (aCg) and posterior (pCg) cingulate cortices, the anterior (aIns) and posterior (pIns) insular cortices. Subcortical measures were made in the hippocampus (HPC), subthalamic nucleus (STN), central nucleus (CE) and basolateral nucleus (BLA) of the amygdala, nucleus accumbens core and shell, and the striatum (ventromedial (VMS), ventrolateral (VLS), dorsolateral (DLS), dorsomedial (DMS)).
(d). Data analysis
Comparisons of behavioural scores were made using two-way analysis of variance (group factor and repeated-measures (RM) for sessions) followed by post hoc comparisons (Newman–Keuls; NK test), when appropriate (Statistica, Statsoft v. 7.1). Mean scores for flexible behaviour in the RGT-reversal task and mean percentage of choice for the large reinforcement in the DDT were compared with indifference level (50%) using a two-tailed t-test. The normality of the variable distribution was verified using Shapiro–Wilk's test. Student's t-tests were used to compare subgroup scores for behaviour or basal monoamine functions (mean ± s.e.m.). Correlations between behavioural scores, or between basal monoamine levels and behavioural scores were made using the parametric Bravais–Pearson's correlation test (r) (Statistica, Statsoft v. 7.1). All comparisons were made at the 5% level. Additional information is provided in electronic supplementary material.
3. Results
As shown previously [11], no correlation was observed between indexes of risk-seeking and behavioural flexibility (r = 0.31; d.f. = 31, ns). These two behavioural traits were unrelated to scores of impulsivity with only one exception: risk seeking was positively correlated with perseverative behaviour during EXT (r = 0.34; n = 34, p < 0.05): rats showing perseverative behaviour were risk-takers or had an intermediate score for risk, except one. No correlation was observed between the different impulsive responses, except between the two measures assessing the capacity to withhold a response: anticipatory hyperactivity in the FI and perseveration during EXT, measured during the same task. These two parameters were positively correlated (r = 0.65; d.f. = 34, p < 0.001). However, we analysed separately inter-individual differences in these two distinct tasks because the ability to extinguish behaviour during EXT, and not during FI, has been related to higher working memory capacities [13], suggesting differences in underlying capacities. Among the 35 rats tested, 19 were identically classified in EXT and FI (54%), but 10 rats changed to a higher impulsive score during EXT compared to FI whereas six rats changed to a lower impulsive score.
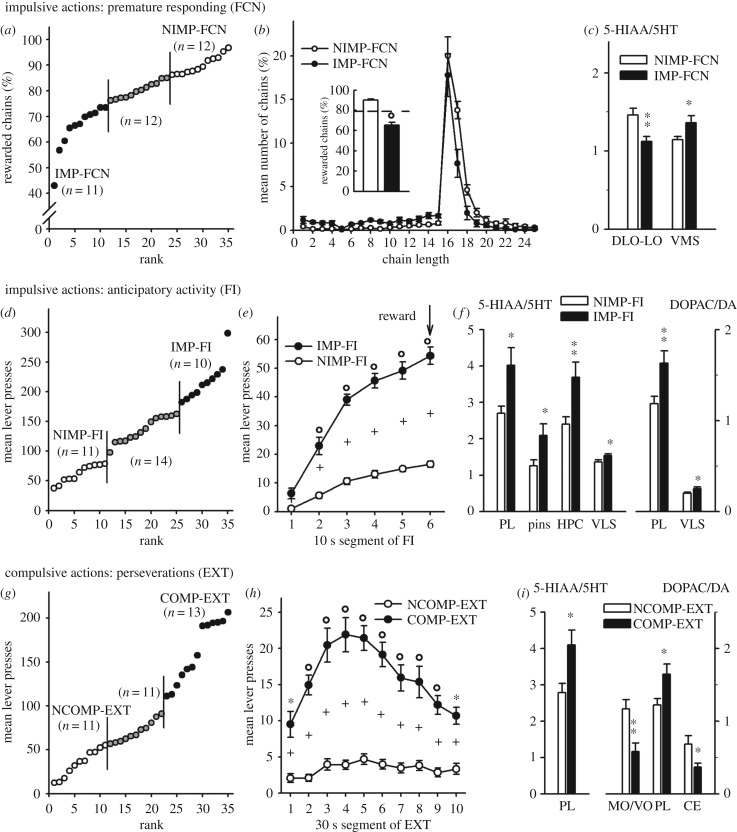
(a). Differences in basal monoaminergic metabolisms associated with premature responses in the FCN task
Rats were split into two subgroups according to their mean percentage of rewarded chains of lever presses in the FCN task: impulsive rats with a low percentage of rewarded chains (less than 75%, n = 11, IMP-FCN), and non-impulsive rats with a high percentage (more than 85%, n = 12, NIMP-FCN) (figure 1a). The lower score was 43% and the maximum being 97%. The chain length distribution reveals a marked peak of chains of 16 responses, which corresponds to the optimal response (figure 1b). Although both IMP and NIMP-FCN groups demonstrated a similar peak, the number of unrewarded chains (less than 16) was higher for IMP-FCN whereas it was lower for rewarded chains (greater than 16), resulting in a lower proportion of rewarded chains (IMP-FCN: 65.3% ± 2.7; NIMP-FCN: 90% ± 1.1; t = 8.78, d.f. = 21, p < 0.001) (figure 1b). No correlation between learning score and proportion of rewarded chains could be shown (r = 0.04, d.f. = 34, ns) showing that premature responding is unrelated to learning deficits. Both groups reached the FCN-16 test condition at the same rate (learning scores: IMP-FCN = 12.4 ± 0.5 sessions; NIMP-FCN = 12.6 ± 0.7 sessions, t = 0.14, d.f. = 21, ns).
Figure 1.
Inter-individual differences in impulsive/compulsive actions and monoamines turnovers at rest. The figure reports the inter-individual differences in fixed consecutive number of 16 lever press schedule (FCN16) (a), in the anticipatory activity (d) or in perseverative behaviour (g), the mean behavioural performances in subgroups for each task (b,e,h) and the corresponding regional changes of serotonergic and dopaminergic turnovers (mean ± s.e.m.) measured across 20 brain regions (c,f,i). Premature responses in FCN (a) are reflected by the percentage of rewarded chains distinguishing rats with impulsive (IMP-FCN, scores <75% presses) and non-impulsive behaviour (NIMP-FCN, scores >85%); (b) frequency distribution (%) of chain length and mean percentage of rewarded chains (insert) of the two groups. In the anticipatory activity during FI (d), rats displayed impulsive (IMP-FI, scores >180 presses) and non-impulsive behaviour (NIMP-FI, scores <80 presses); (e) mean number of lever presses by each group during the 1 min FI component as a function of 10 s segments of the FI period. Perseverative behaviour (g) of rats during EXT highlighted compulsive (COMP-EXT, scores >110 presses) and non-compulsive behaviours (NCOMP-EXT, scores <55 presses); (i) mean number of lever presses by each group during the 5 min EXT component as a function of 30 s segments of the EXT period. In (b,e,h) dotted line or crosses indicate median score. °p < 0.001; **p < 0.01; *p < 0.05 (Student's t test).
Among the 20 brain regions investigated for the dopaminergic and serotonergic turnovers, only the regions in which differences were significant are reported in the figures. The origin of the difference in turnover (metabolite and/or neurotransmitter content) is indicated in parenthesis whenever significant differences were observed (see electronic supplementary material, table S2 for details). We occasionally made reference to the table 3 of the supplementary material reporting the results for each molecule in each constituted subgroup.
Basal serotonergic turnovers were lower for IMP-FCN compared to NIMP-FCN in DLO/LO (t = 3.11, d.f. = 20, p < 0.01), and higher in VMS (t = 2.43, d.f. = 18, p < 0.05) (figure 1c). The 5-HT and 5-HIAA contents were not significantly different in DLO/LO and VMS, but were both higher in LO for IMP-FCN (t = 3.48, d.f. = 15, p < 0.01; t = 2.1, d.f. = 21, p < 0.05). Conversely, DA and DOPAC were lower in core for IMP-FCN (t = 2.84, d.f. = 19, p < 0.02; t = 2.7, d.f. = 19, p < 0.02). A positive correlation was found between percentage of rewarded chains and serotonergic turnover in DLO–LO (r = 0.38, d.f. = 31, p < 0.05), and a negative correlation in VMS (r = 0.38, d.f. = 33, p < 0.05).
(b). Differences in basal monoaminergic metabolisms associated with anticipatory hyperactivity during FI
Large inter-individual differences were observed in activity during FI with a minimum mean of lever presses of 41 and a maximum of 298. Rats were split into two subgroups according to their mean scores during FI: impulsive rats with a high level of lever presses (greater than 180 lever presses, n = 10, IMP-FI), and non-impulsive rats with a low level of lever presses (less than 80, n = 11, NIMP-FI) (figure 1d). The time course of their activity within a 1-min fixed interval is represented in figure 1e. Although both groups significantly increased their number of lever presses during FI (NIMP, F5,50 = 66.7, p < 0.001: IMP, F5,45 = 81.3, p < 0.001), the mean lever presses of IMP-FI (217.5 ± 10.6) was 3.5 times higher than that of NIMP-FI (61.9 ± 4.5), the median score being 131.5 lever presses. The two groups did not differ in activity level during the first 10 s of the FI period (NK, ns) and then, IMP-FI developed readily a much higher level of activity until food delivery. Consequently, the mean number of visits to the empty tray of NIMP-FI (79.7 ± 12.2 visits) was significantly higher compared to IMP-FI (35.3 ± 6.1 visits) (t = 3.26; d.f. = 19, p < 0.01). No significant difference between the two groups was found between the latency to collect food once delivered (IMP-FI: 0.92 ± 0.28 s; NIMP-FI: 0.83 ± 0.42 s; t = 0.24, d.f. = 19, ns).
Basal serotonergic turnovers were higher in IMP-FI compared to NIMP-FI in PL (t = 2.51, d.f. = 16, p = 0.05; increased 5-HIAA content), pins (t = 2.3, d.f. = 16, p < 0.05, increased 5-HT content), HPC (t = 3.03, d.f. = 15, p < 0.01) and VLS (t = 2.27, d.f. = 19, p < 0.05). Basal dopaminergic turnovers were higher in IMP-FI compared to NIMP-FI in PL (t = 2.86, d.f. = 17, p = 0.01) and VLS (t = 2.28, d.f. = 19, p < 0.05) (figure 1f). DLO 5-HT and 5-HIAA contents were lower in IMP-FI (t = 3.2, d.f. = 15, p < 0.01; t = 3.05, d.f. = 18, p < 0.01). Positive correlations were found between activity level and serotonergic turnover in HPC (r = 0.47, d.f. = 27, p < 0.02), and VLS (r = 0.37, d.f. = 34, p < 0.05).
(c). Differences in basal monoaminergic metabolisms associated with perseverative behaviour during extinction
Large inter-individual differences were observed in activity during EXT with a minimum mean of lever presses of 12 and a maximum of 206. Rats were split into two subgroups according to their mean scores during EXT: compulsive rats with a high level of lever presses (greater than 110 lever presses, n = 13, COMP-EXT), and non-compulsive rats with a low level of lever presses (less than 55, n = 11, NCOMP-EXT) (figure 1g). The time course of their activity within 5 min of EXT is represented on figure 1h. The two groups differed in activity level all along the EXT period. Both groups significantly increased their number of lever presses during EXT (NCOMP, F9,90 = 2.79, p < 0.01; IMP, F9,108 = 7.71, p < 0.001). However, NCOMP-EXT activity remained very low whereas COMP-EXT activity increased rapidly to reach a plateau after 1.5 min, and then decreased progressively. The mean lever presses of COMP-EXT (161.6 ± 9.8) was 4.7 times higher than that of NCOMP-EXT (34.2 ± 4.6), the median score being 72.7 lever presses. The mean number of visits to the empty tray did not significantly differ between groups (COMP-EXT: 21 ± 1.4 visits; NCOMP-EXT: 28 ± 5.4 visits) (t = 1.32; d.f. = 22, ns). The mean number of lever presses during FI and EXT were positively correlated (r = 0.65, d.f. = 34, p < 0.001).
One significant difference was revealed between COMP-EXT and NCOMP-EXT for basal serotonergic turnovers (figure 1i): it was higher in COMP-EXT compared to NCOMP-EXT in PL (t = 2.28, d.f. = 17, p < 0.05; increased 5-HIAA content). Basal dopaminergic turnovers were higher in COMP-EXT compared to NCOMP-EXT in PL (t = 2.27, d.f. = 18, p < 0.05), and lower in both MO/VO (t = 3.38, d.f. = 15, p < 0.01; increased DA content) and CE (t = 2.5, d.f. = 21, p < 0.05) (figure 1i). A negative correlation was found between compulsivity level and dopaminergic turnover in MO/VO (r = −0.53, d.f. = 22, p < 0.01), and a positive one with dopaminergic turnover in VLS (r = 0.42, d.f. = 34, p < 0.02).
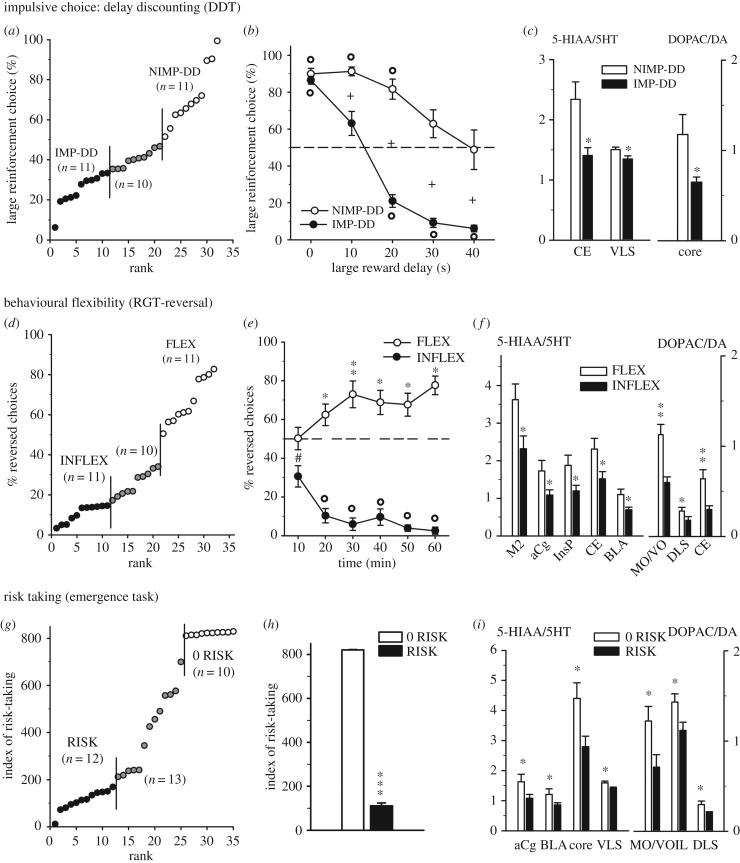
(d). Differences in basal monoaminergic metabolisms associated with impulsive choices in the delay discounting task
Large inter-individual differences were observed in the mean proportion of choices for the large delayed reward at delays 10 to 40 s, with a minimum mean percentage of choice of 6% and a maximum of 99% (figure 2a). Rats were split into two subgroups according to their mean percentage of choice for the large reward from delay 10 to 40 s: impulsive rats with a low proportion of choices for the large reward (less than 35, n = 11, IMP-DD), and non-impulsive rats with a high proportion for the large reward (greater than 53, n = 10, NIMP-DD). As expected, following the training period, animals largely preferred the lever delivering the large reward when no delay was imposed (IMP-DD: 86.3 ± 3.8%; NIMP-DD: 86.5 ± 2.2%). The preference progressively shifted towards the small reward as the delay increased for NIMP-DD whereas this preference shifted much more rapidly for IMP-DD. The two groups largely differed in the time lapse at which they no longer preferred pressing for the large reward (breakpoint): as early as the 10 s delay for IMP-DD and at the 30 s delay for NIMP-DD (figure 2b). The mean percentages of choice for the large reward from delay 10 s to 40 s for the two groups are represented in figure 2b. No correlation was found between choice for the larger reward and the total number of sessions required to reach each step of the test (r = 0.15, d.f. = 31, ns).
Figure 2.
Inter-individual differences in impulsive/compulsive actions and monoamines turnovers at rest. The figure reports the inter-individual differences in the DDT (a), the rat gambling task (RGT-reversal, d) and the emergence task (g), the mean behavioural performances in subgroups for each task (b,e,h) and the corresponding regional changes of serotonergic and dopaminergic turnovers (mean ± s.e.m.) measured across 20 brain regions (c,f,i). In the DDT (a), impulsive choice was reflected by the mean percentage of choice for the large reinforcement when a delay (from 10 to 40 s) occurs, highlighting impulsive (IMP-DD, scores <35%) and non-impulsive behaviour (NIMP-DD, scores >53%); (b) Percentage choice of the two groups for the large reinforcement according to the length of the delay before obtaining it. Comparisons with chance level (50%, dotted line): significant differences with chance for scores above or below 50% show a preference for the large or the small reward respectively. Flexibility index in the RGT-reversal (d) showed inflexible (INFLEX) and flexible behaviour (FLEX); (e) time-course of reversed choice percentage in the RGT-reversal task for FLEX and INFLEX. Dotted line indicates 50% level (no preference). The index of risk-taking in the emergence task (g) showed risk-prone (RISK, scores <170) and risk avoiders (NORISK, scores >810); (h) Mean values of the risk-taking index for RISK and 0RISK (s). °p < 0.001; **p < 0.01; *p < 0.05 (Student's t test).
Basal serotonergic turnovers were lower in IMP-DD compared to NIMP-DD in CE (t = 2.80, d.f. = 17, p < 0.02; figure 2c) and in VLS (t = 2.26, d.f. = 20, p < 0.05). Basal dopaminergic turnover was lower in IMP-DD compared to NIMP-DD in core (t = 32.3, d.f. = 18, p < 0.05, figure 2c).
(e). Differences in basal monoaminergic metabolisms associated with behavioural flexibility capacities in the RGT
Rats were split into two subgroups: rats with flexible behaviour (n = 11; FLEX) during the RGT-reversal, with a score of reversed choices above 50%, and rats with inflexible behaviour (n = 15; INFLEX), with a score of reversed choices below 15% (figure 2d). The time-course of the percentage of reversed choices during the RGT-reversal for FLEX and INFLEX rats is represented on figure 2e. During the RGT-reversal, FLEX rats started to sample the options randomly before reorienting their choices toward the ones that they preferred during the RGT, until the end of the task. Conversely, INFLEX rats persisted to choose the same location, independently of the reversed contingencies, and remained on these options all along the test. Three rats that did not display preference for any particular option during the RGT were discarded from the analysis.
Basal serotonergic turnovers were lower in INFLEX compared to FLEX in M2 (t = 2.32, d.f. = 15, p < 0.05), aCg (t = 2.14, d.f. = 16, p < 0.05), pins (t = 2.11, d.f. = 17, p = 0.05) and amygdala (CE, t = 2.37, d.f. = 18, p < 0.05; BLA, t = 2.54, d.f. = 17, p < 0.05). Basal dopaminergic turnovers were lower in INFLEX compared to FLEX in MO/VO (t = 3.07, d.f. = 13, p < 0.01; increased DA content), DLS (t = 2.22, d.f. = 19, p < 0.05; increased DA content) and CE (t = 2.83, d.f. = 20, p = 0.01) (figure 2f).
Positive correlations were found between the index of flexibility and dopaminergic turnover in MO/VO (r = 0.61, d.f. = 20, p < 0.01), and DLS (r = 0.41, d.f. = 30, p < 0.05) as well as serotonergic turnover in BLA (r = 0.48, d.f. = 27, p < 0.02), CE (r = 0.40, d.f. = 27, p < 0.05), M2 (r = 0.41, d.f. = 22, p = 0.05) and aCg (r = 0.49, d.f. = 25, p < 0.02).
(f). Differences in basal monoaminergic metabolisms associated with risk-taking in the light–dark emergence task
Large inter-individual differences were observed in the index of risk-taking, some rats remaining in the dark compartment during the whole duration of the task, some others exiting this compartment in less than 30 s. Rats were split into two subgroups according to this index: risk-prone rats with a low index (less than 170, n = 11, RISK), and risk avoiders with a high index (greater than 810, n = 10, 0RISK) (figure 2g). Risk-taking index was calculated on the basis of the number of risk assessments, the latency to emerge into the risky compartment and the total time spent in the lightened compartment. The scores of the two groups are represented on figure 2h.
Basal serotonergic turnovers were lower in RISK compared to 0RISK in aCg (t = 2.07, d.f. = 16, p = 0.05; figure 2i; increased 5-HT content), BLA (t = 2.19, d.f. = 16, p < 0.05; decreased 5-HIAA content), core (t = 2.65, d.f. = 18, p < 0.02) and VLS (t = 2.6, d.f. = 20, p < 0.02). Basal dopaminergic turnovers were significantly lower in RISK compared to 0RISK in MO/VO (t = 2.44, d.f. = 11, p < 0.05, figure 2i), IL (t = 2.42, d.f. = 20, p < 0.05; lower increase in DOPAC compared to DA) and DLS (t = 2.53, d.f. = 20, p < 0.02; fig. 7f). DA and DOPAC contents were higher in PL (t = 2.28, d.f. = 16, p < 0.05; t = 2.93, d.f. = 18, p < 0.01) and lower in shell (t = 3.1, d.f. = 17, p < 0.01; t = 3.02, d.f. = 17, p < 0.01) for RISK. Significant correlations were found between the index of risk-taking and dopaminergic turnover in MO/VO (r= 0.48, d.f. = 22, p = 0.02), IL (r = 0.35, d.f. = 32, p < 0.05), and pCg (r = 0.47, d.f. = 22, p < 0.05) as well as serotonergic turnover in aCg (r = 0.49; d.f. = 25, p < 0.02), CE (r = 0.41, d.f. = 27, p < 0.05) and BLA (r = 0.48, d.f. = 27, p < 0.02).
4. Discussion
Inter-individual differences in behaviour within a large sample of outbred rats enabled to highlight the complex phenotype related to the impulsivity/compulsivity dimension. The main finding is that distinct components of this dimension show specific neurochemical signatures on dopaminergic and serotonergic metabolisms in a subset of brain regions involved in cognition, confirming the complexity of the underlying neurochemical substrates. The classification of individuals into three groups according to their scores, allowed us to compare individuals with opposite performances. Highly adaptive versus maladaptive behaviours in each of the components were observed, along an almost continuous distribution of performance. These differences are unrelated to learning capacities or motivation for food, as shown by similar learning of the tasks or latency to collect food between groups. It is noteworthy that most of the behaviours studied were uncorrelated: individuals exhibiting impulsive actions in one test do not necessarily display impulsive responses in another test. This evidence is pointed out by the distinct neurochemical profiles sustaining each of these traits. These data agree with the multimodal aspect of the impulsivity/compulsivity dimension [2] and the notion that each paradigm models specific components possibly revealing substrates of related psychiatric disorders [16].
The comparison of individuals with opposite behavioural responses in each task revealed neurochemical differences in serotonergic turnovers and/or dopaminergic turnovers in restricted brain regions. Overall, we report contrasting patterns of variations according to the behavioural traits: lower serotonergic and dopaminergic turnovers in rats making impulsive choices, inflexible or risk-prone rats in some brain regions and higher turnovers in rats showing impulsive anticipatory responses in other regions (PL and VLS notably). By contrast, opposite changes between cortical (LO-DLO) and striatal (VMS) serotonergic turnovers were reported in premature responders. Rats showing perseverative activity in the extinction paradigm and inflexible rats in the RGT-reversal had reduced dopaminergic turnovers in MO/VO and CE, thus suggesting a hypodopaminergic function in these brain regions in compulsive-like behaviours. Taken individually, some of these results are in accordance with the literature: e.g. the involvement of MO/VO and CE in reversal learning and cognitive flexibility [17,18]; a reduced serotonergic function that tends to promote inflexible behaviours [19] or the decrease in core dopaminergic turnover in impulsive choice (delay discounting) [20]. Our results agree with our previous work on poor decision-making [21] and a growing literature showing that serotonergic and dopaminergic systems tune behavioural traits [2,4,6]. Whether some patterns of variations within networks in monoaminergic activity could represent a predisposition for an individual to impulsivity/compulsivity is still elusive. Beyond the involvement of one or two specific structures, the notion of network seems particularly important. For instance, combined increases in PL dopaminergic/serotonergic metabolisms have been observed in both anticipatory activity and perseverations but the other brain regions in which differences in these two distinct behavioural tasks are reported, are completely different.
While our differential approach is not hypothesis-driven but purely descriptive, it highlights also some specific turnovers' modifications away from the expected modifications (i.e. the decrease in CE serotonergic turnover in rats displaying impulsive choices in the DDT). The functional meaning of these modifications is unclear and tends to suggest a wider network sustaining the behavioural traits as previously hypothesized [2,4,6].
It has to be kept in mind that the neurochemical data correspond to the metabolism at rest obtained one month after the completion of all behavioural tests. These differences in metabolism cannot represent dynamic monoaminergic activities during the tests. The variations of the turnovers among subgroups likely witness distinct maturations in discrete loci encompassing the neurobiological networks recruited in each behavioural task. Indeed, in addition to specific distribution of serotonergic and dopaminergic neurons in raphe nuclei or in dopaminergic areas of mesencephalic complex [22,23], terminal fields of monoaminergic neurons establish local interactions which participate in their regulation independently from their mesencephalic origin [15,24,25]. The variations of the turnovers were due to changes in the level of the neurotransmitter (e.g. DA in MO/VO), the metabolite (e.g. 5-HIAA in PL) or none specifically. In some cases, no modification of the turnover is reported because both the neurotransmitter and its metabolite significantly differ in the same direction, suggesting a difference in general activity in these structures (e.g. higher PL and lower shell DA and DOPAC for risk-taking; higher LO 5-HT and 5-HIAA with lower DA and DOPAC in core for premature responders) (electronic supplementary material, table S3).
The therapeutic approach of impulse control disorders relies on monoaminergic based treatments. The mechanism of action of these treatments is still poorly understood and the drug adherence variable in-between individuals [2]. Based on our results and those previously published [2,25], abnormal response in a specific component of impulsive/compulsive dimension is associated with patterns of monoaminergic imbalances within fronto–subcortical circuits. The therapeutic efficacy of monoaminergic drugs could be related to the compensation of monoaminergic imbalances, or the creation of a new one. Nonetheless, their efficacy would be associated with counterproductive outcomes because of their action on other circuits and behavioural traits. Thus, a better determination of components within a dimension and their underlying neurobiological substrate is required to ameliorate the therapeutic approach of mental disorders according to the individuals.
5. Conclusion
Our results reveal the complex nature of behavioural traits related to impulse control disorders through their associated monoaminergic networks at rest. These results pave the way for understanding the link between mental disorders and drug therapeutic actions.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
The authors wish to thank Matteo Laqui technical support, Stéphane Lelgouach for animal care and Dr. Martine Cador for kind support.
Ethics
All procedures were conducted in strict accordance with the 2010-63-EU and with approval of the Bordeaux University Animal Care and Use Committee (permit number: 5012087-A).
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
M.R. and F.D.H. carried out the behavioural laboratory work and collected data; A.F. and P.D.D. carried out the biochemical laboratory work and collected data; F.D.H. conceived of and designed the study and carried out statistical analyses; F.D.H. and P.D.D. wrote the manuscript.
Competing interests
We have no competing interests.
Funding
This work was supported and funded by CNRS, Univ. of Bordeaux, Conseil Régional d'Aquitaine, a fellowship from the French Government to M.R. and from the Fondation pour la Vocation Bleustein-Blanchet to A.F.
References
- 1.Hyman SE. 2007. Can neuroscience be integrated into the DSM–V? Nat. Rev. Neurosci. 8, 725–732. ( 10.1038/nrn2218) [DOI] [PubMed] [Google Scholar]
- 2.Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD. 2012. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn. Sci. 16, 81–91. ( 10.1016/j.tics.2011.11.009) [DOI] [PubMed] [Google Scholar]
- 3.Daruna J, Barnes P. 1993. The impulsive client: theory, research and treatment. In A neurodevelopmental view of impulsivity and its relationship to the superfactors of personality (eds McCown W, Johnson J, Shure M), p. 23. Washington, DC: American Psychological Association. [Google Scholar]
- 4.Dalley JW, Everitt BJ, Robbins TW. 2011. Impulsivity, compulsivity, and top-down cognitive control. Neuron 69, 680–694. ( 10.1016/j.neuron.2011.01.020) [DOI] [PubMed] [Google Scholar]
- 5.Balleine BW, O'Doherty JP. 2010. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35, 48–69. ( 10.1038/npp.2009.131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eagle DM, Baunez C. 2010. Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci. Biobehav. Rev. 34, 50–72. ( 10.1016/j.neubiorev.2009.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fineberg NA, et al. 2010. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology 35, 591–604. ( 10.1038/npp.2009.185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivalan M, Grégoire S, Dellu-Hagedorn F. 2007. Reduction of impulsivity with amphetamine in an appetitive fixed consecutive number schedule with cue for optimal performance in rats. Psychopharmacology 192, 171–182. ( 10.1007/s00213-007-0702-6) [DOI] [PubMed] [Google Scholar]
- 9.de Visser L, et al. 2011. Rodent versions of the iowa gambling task: opportunities and challenges for the understanding of decision-making. Front. Neurosci. 5, 109 ( 10.3389/fnins.2011.00109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivalan M, Ahmed SH, Dellu-Hagedorn F. 2009. Risk-prone individuals prefer the wrong options on a rat version of the iowa gambling task. Biol. Psychiatry 66, 743–749. ( 10.1016/j.biopsych.2009.04.008) [DOI] [PubMed] [Google Scholar]
- 11.Rivalan M, Valton V, Series P, Marchand AR, Dellu-Hagedorn F. 2013. Elucidating poor decision-making in a rat gambling task. PLoS ONE 8, e82052 ( 10.1371/journal.pone.0082052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dellu-Hagedorn F. 2005. Spontaneous individual differences in cognitive performances of young adult rats predict locomotor response to amphetamine. Neurobiol. Learn. Mem. 83, 43–47. ( 10.1016/j.nlm.2004.07.002) [DOI] [PubMed] [Google Scholar]
- 13.Dellu-Hagedorn F. 2006. Relationship between impulsivity, hyperactivity and working memory: a differential analysis in the rat. Behav. Brain Funct. 2, 10 ( 10.1186/1744-9081-2-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregoire S, Rivalan M, Le Moine C, Dellu-Hagedorn F. 2012. The synergy of working memory and inhibitory control: behavioral, pharmacological and neural functional evidences. Neurobiol. Learn. Mem. 97, 202–212. ( 10.1016/j.nlm.2011.12.003) [DOI] [PubMed] [Google Scholar]
- 15.Fitoussi A, Dellu-Hagedorn F, De Deurwaerdere P. 2013. Monoamines tissue content analysis reveals restricted and site-specific correlations in brain regions involved in cognition. Neuroscience 255, 233–245. ( 10.1016/j.neuroscience.2013.09.059) [DOI] [PubMed] [Google Scholar]
- 16.Dalley JW, Mar AC, Economidou D, Robbins TW. 2008. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol. Biochem. Behav. 90, 250–260. ( 10.1016/j.pbb.2007.12.021) [DOI] [PubMed] [Google Scholar]
- 17.Schoenbaum G, Chiba AA, Gallagher M. 2000. Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training. J. Neurosci. 20, 5179–5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young JJ, Shapiro ML. 2009. Double dissociation and hierarchical organization of strategy switches and reversals in the rat PFC. Behav. Neurosci. 123, 1028–1035. ( 10.1037/a0016822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. 2004. Cognitive inflexibility after prefrontal serotonin depletion. Science 304, 878–880. ( 10.1126/science.1094987) [DOI] [PubMed] [Google Scholar]
- 20.Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ. 2004. Limbic corticostriatal systems and delayed reinforcement. Ann. N. Y. Acad. Sci. 1021, 33–50. ( 10.1196/annals.1308.004) [DOI] [PubMed] [Google Scholar]
- 21.Fitoussi A, Le Moine C, De Deurwaerdere P, Laqui M, Rivalan M, Cador M, Dellu-Hagedorn F. 2015. Prefronto-subcortical imbalance characterizes poor decision-making: neurochemical and neural functional evidences in rats. Brain Struct. Funct. 220, 3485–3496. ( 10.1007/s00429-014-0868-8) [DOI] [PubMed] [Google Scholar]
- 22.Bjorklund A, Dunnett SB. 2007. Dopamine neuron systems in the brain: an update. Trends Neurosci. 30, 194–202. ( 10.1016/j.tins.2007.03.006) [DOI] [PubMed] [Google Scholar]
- 23.Hale MW, Lowry CA. 2011. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl.) 213, 243–264. ( 10.1007/s00213-010-2089-z) [DOI] [PubMed] [Google Scholar]
- 24.Dellu-Hagedorn F, Fitoussi A, De Deurwaerdere P. 2017. Correlative analysis of dopaminergic and serotonergic metabolism across the brain to study monoaminergic function and interaction. J. Neurosci. Methods 280, 54–63. ( 10.1016/j.jneumeth.2017.01.020) [DOI] [PubMed] [Google Scholar]
- 25.De Deurwaerdere P, Di Giovanni G. 2017. Serotonergic modulation of the activity of mesencephalic dopaminergic systems: therapeutic implications. Prog. Neurobiol. 151, 175–236. ( 10.1016/j.pneurobio.2016.03.004) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.