Abstract
The current study examined the association between effortful control and a well-studied neural index of self-regulation, the N2 event-related potential (ERP) component, in toddlers. Participants included 107 toddlers (44 girls) assessed at 30, 36 and 42 months of age. Participants completed a Go/NoGo task while electroencephalography data were recorded. The study focused on the N2 ERP component. Parent-reported effortful control was examined in association with the NoGo N2 ERP component. Findings suggest a positive association between the NoGo N2 component and the inhibitory control subscale of the wider effortful control dimension, suggesting that the N2 component may index processes associated with temperamental effortful control.
This article is part of the theme issue ‘Diverse perspectives on diversity: multi-disciplinary approaches to taxonomies of individual differences’.
Keywords: event-related potentials, effortful control, early childhood
1. Introduction
Research on neural biomarkers in early childhood has led to improved understanding of the early correlates of developing psychopathology [1]. Similarly, child temperament—early-emerging, biologically based, individual differences in reactivity and self-regulation—has been shown to be associated with emerging psychopathology [2–4]. However, relatively little research has focused on understanding how these two markers of risk for psychopathology are associated with one another in early childhood. To fill this gap in the literature, this study examines the association between temperamental regulation and a well-studied neural biomarker for dysregulation, the N2 event-related potential (ERP) component, in toddlers.
2. Temperamental effortful control
Temperament describes individuals' tendencies when reacting to changes in their internal and external environment (i.e. reactivity) and their capacity to modulate this reactivity (i.e. self-regulation). Temperament, as measured in childhood, is frequently subdivided into three broad dimensions, including surgency/extraversion and negative affectivity, both of which describe individual differences in reactivity, and effortful control [5], which describes individual differences in the capacity to modulate reactivity. While self-regulatory processes are likely associated with all three dimensions of temperament, individual differences in self-regulation are best described via the dimension of effortful control. Effortful control includes a variety of processes, including the capacity to inhibit a prepotent response, the capacity to execute goal-directed behaviours, and the capacity for strategic allocation of attention. Children with poor effortful control have been shown to be at increased risk for externalizing problems [6,7], academic difficulties [8] and social problems [9].
Temperamental effortful control can be measured using a variety of techniques, including parent-report measures, laboratory or home observations, and laboratory tasks [10]. Effortful control has been shown to improve dramatically across childhood, from reliance on caregivers for regulation in infancy to the more self-initiated deployment of regulatory strategies in childhood [10]. The toddler to preschool years are characterized by particularly rapid improvements in the skills associated with effortful control [10,11]. However, despite mean-level improvements in performance on effortful control tasks across childhood, as an aspect of temperament, it typically shows rank-order stability across development [4].
3. The neural correlates of effortful control
Research suggests that an important root of effortful control is in the executive attention network, a well-specified neural network that underlies the self-initiated deployment of attention and other higher-order cognitive abilities [12,13]. The regions that comprise this network, including the anterior cingulate cortex (ACC) and regions of the lateral prefrontal cortex (PFC), are thought to underlie effortful control abilities [10,13]. These brain regions monitor and regulate activation in the networks of brain responsible for reactivity, emotional expression and motoric behaviours [14,15]. Although the ACC and lateral PFC are active in infancy, it is during the late toddler and preschool period that they begin to take on the regulatory characteristics of what will become their adult functionality [16]. The development of these prefrontal brain regions theoretically underlies improved self-regulation across development. However, empirical studies are needed to assess such processes. To study neural activation in toddlers, the most feasible methodology is electroencephalography (EEG) and corresponding ERPs. ERPs represent large-scale, synchronous neural activity that is time-locked to stimulus presentation. Although ERPs lack the spatial resolution of magnetic resonance imaging, they have high temporal resolution, and are better suited to study rapidly occurring neural processes, like effortful control.
4. The N2 event-related potential component
The N2 ERP component is the second negative deflection in the waveform that occurs from approximately 200 to 400 ms post-stimulus across fronto-central electrodes. The N2 component has been elicited in both adults and children and is thought to index aspects of cognitive control [17], particularly response inhibition capacities. The amplitude of the N2 component is larger (more negative) in response to NoGo stimuli (in which inhibition is required) than to Go stimuli (in which activation is required). This feature of the N2 led researchers to theorize that the N2 component reflects response inhibition capacities. Poorer response inhibition, thought to be indexed by larger N2 amplitudes, has been associated with externalizing behaviour problems in childhood [18], leading some researchers to propose that the N2 component is a biomarker for dysregulation.
Go/NoGo (GNG) tasks are frequently used to assess the N2 component. This task includes two stimuli: a Go stimulus, which is paired with response activation (e.g. a button press), and a NoGo stimulus, which is paired with response inhibition. To establish a prepotent tendency to respond, thereby making the inhibition task more difficult, the Go stimuli are often presented more frequently than the NoGo stimuli. Several studies that have used source localization techniques to identify the neural generators of the N2 component elicited during a GNG task have suggested that the N2 component can be localized to the ACC, orbitofrontal cortex, ventral PFC and dorsolateral PFC [19–21]. Both of these prefrontal regions are thought to underlie response inhibition capacities specifically, as well as executive functioning more broadly. There is notable overlap between skills encompassed within executive functions and effortful control, such that differences might actually reflect the different disciplines from which each construct emerged [22]. Hence, it is possible that the N2 component could index the neural correlates of effortful control.
To examine this possibility, several research teams have examined the association between the N2 component and effortful control abilities. Across these studies, a somewhat contradictory pattern of findings has emerged. Using a GNG task, Wiersema & Roeyers [23] found that, in school-aged children, NoGo N2 amplitudes were negatively associated with an aspect of parent-reported effortful control (attentional shifting), such that children with larger, more negative NoGo N2 amplitudes tended to have better attentional shifting skills [23]. Notably, however, parent-reported levels of other types of effortful control, including attentional focusing, impulsivity and persistence, were not found to be associated with N2 amplitudes [23]. Alternatively, using a flanker task, Buss et al. [24] found that smaller, less negative N2 amplitudes during incongruent trials in 4–8-year-old children were associated with higher levels of parent-reported effortful control. Similarly, in a sample of preschool-age children, Rueberry et al. [23] found that the difference in amplitude between the N2 in the Go and NoGo conditions (Go–NoGo) was positively associated with performance on a battery of effortful control tasks, such that children with a greater difference between Go and NoGo N2 amplitudes performed better on effortful control tasks. As a larger difference between Go and NoGo N2 amplitudes is thought to reflect more advanced conflict detection capacities, this finding aligns with expectations of how the N2 component should theoretically be associated with effortful control. However, the sparse, but conflicting, findings of the studies highlighted above suggest that more research is needed to explore the association between effortful control and the N2 component.
5. Current study
The current study examined the neural correlates of effortful control in toddlers, by examining the association between effortful control and the N2 ERP component. This is the first such study, to our knowledge, to focus on toddlerhood. As the toddler and preschool years are characterized by substantial improvements in executive functioning, this is an especially important era during which to examine the neural correlates of effortful control/executive functioning. Because previous findings with older children have been contradictory, we used theory to guide our hypothesis. Based on findings that suggest that more mature response inhibition capacities are associated with smaller, less negative NoGo N2 amplitudes [17], we expected higher levels of parent-reported effortful control to be associated with less negative NoGo N2 amplitudes.
6. Methods
For a description of the methods of the current study, see the electronic supplementary material, appendix S1.
7. Results
Descriptive statistics for all variables included in analysis are presented in electronic supplementary material, table S2. Only correlations with the temperament scales for inhibitory control, attentional control and effortful control are presented in electronic supplementary material, table S2; correlations with the two other subscales of the effortful control composite, low intensity pleasure and perceptual sensitivity, were not significant (−0.01 < r < 0.05, p > 0.05), so they are not considered further.
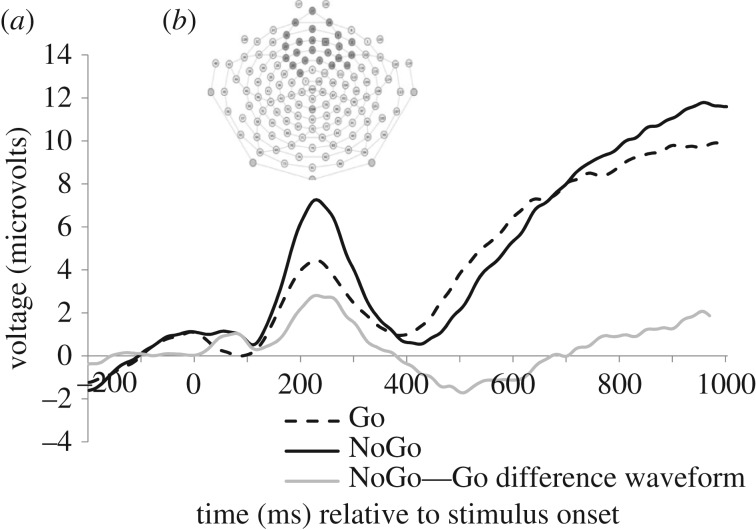
Grand-averaged waveforms for the Go and NoGo conditions are presented in figure 1a. The N2 elicited to NoGo trials (M = −2.96 µV) was significantly more negative than the N2 to Go trials (M = −0.51 µV; t[156] = −2.56, p = 0.01). We calculated the N2 effect by subtracting Go N2 amplitudes from NoGo N2 amplitudes (i.e. NoGo–Go), such that a larger difference between NoGo and Go N2 amplitudes was represented by a more negative N2 effect score. Correlations between Go and NoGo N2 amplitude, the N2 effect, behavioural performance on the Fish–Sharks task, and child temperament are presented in electronic supplementary material, table S2.
Figure 1.
(a) Grand-averaged N2 waveforms across the (b) fronto-central electrode group determined via temporospatial principal component analysis (PCA) to correspond with the N2 component. The waveform depicted represents the mean waveform from those electrodes with a 0.4 or greater factor loading onto the PCA component reflecting the N2; electrodes were averaged with equal, unit weighting.
Behavioural performance on the Fish–Sharks task, as indexed by the per cent of correct NoGo trials, was correlated with N2 effect (r[157] = 0.25, p = 0.002). Findings suggest that better performance on the inhibition trials of the Fish–Sharks task was associated with a smaller difference between Go and NoGo N2 amplitudes. In a follow-up test, we found a negative association between Go N2 amplitudes and behavioural performance (r[157] = −0.27, p = 0.001), such that enhanced Go N2 amplitudes were associated with better performance on the Go/NoGo task. As the Go N2 component is not thought to be associated with response inhibition, this correlation was not investigated further.
The inhibitory control and attentional control subscales of the children's behaviour questionnaire (CBQ) were significantly, positively associated with NoGo N2 amplitudes (r[142] = 0.23, p = 0.007 and r[142] = 0.17, p = 0.04, respectively), such that children with higher levels of parent-reported inhibitory and attentional control showed less negative (smaller) NoGo N2 amplitudes. As less negative NoGo N2 amplitudes have been associated with more mature response inhibition [17], these findings suggest that better temperamental inhibitory and attentional control is associated with a neural response pattern indicative of better response inhibition capacities. Given that the higher-order effortful control scale comprised both the inhibitory control and attentional control subscales, but also the low intensity pleasure and perceptual sensitivity subscales, it is understandable that a trend association emerged between the effortful control scale and NoGo N2 amplitude (r[142] = 0.16, p = 0.056). Although worth mentioning, we did not further investigate this association because it did not meet traditional thresholds for statistical significance. No significant association was found between the N2 effect and child temperament. Additionally, in a follow-up test, we found no association between Go N2 amplitudes and child temperament.
The three significant associations (behavioural performance and the N2 effect; temperamental inhibitory control and NoGo N2 amplitudes; attentional control and NoGo N2 amplitudes) were further tested using multiple regression, controlling for the number of trials kept in the NoGo condition, child age (in months) and child sex. These associations are presented in table 1. When these associations were further examined using nested regression to account for the longitudinal dependency in the data, two effects (behavioural performance and the N2 effect; temperamental inhibitory control and NoGo N2 amplitudes) remained significant. However, the association between attentional control and NoGo N2 amplitudes became a trend rather than statistically significant when accounting for longitudinal dependency (p = 0.054).
Table 1.
Multiple regression analyses predicting (a) NoGo N2 amplitude with inhibitory control, attentional control and control variables and (b) the N2 effect (Go–NoGo) with NoGo trials per cent correct and control variables.
| (a) NoGo N2 amplitude | B | β | s.e. | p-value |
|---|---|---|---|---|
| CBQ inhibitory control | 2.91 | 0.25 | 1.09 | <0.01 |
| control variables | ||||
| # NoGo trials included | −0.16 | −0.06 | 0.23 | 0.48 |
| Age (months) | −3.07 | −0.11 | 2.70 | 0.26 |
| Sexa | −1.42 | −0.07 | 1.82 | 0.44 |
| F4,118 = 2.24, p = 0.06, R2 = 0.07 | ||||
| CBQ attentional control | 1.97 | 0.21 | 0.91 | <0.05 |
| control variables | ||||
| # NoGo trials included | −0.11 | −0.05 | 0.23 | 0.62 |
| Age (months) | −3.36 | −0.04 | 2.74 | 0.22 |
| Sexa | −0.79 | −0.11 | 1.79 | 0.66 |
| F4,118 = 1.62, p = 0.17, R2 = 0.05 | ||||
| (b) N2 effect | B | β | s.e. | p-value |
|---|---|---|---|---|
| NoGo per cent correct | 0.25 | 0.30 | 0.09 | <0.01 |
| control variables | ||||
| # NoGo trials included | −0.40 | −0.12 | 0.36 | 0.27 |
| Age (months) | −6.27 | −0.15 | 3.56 | 0.08 |
| Sexa | −2.15 | −0.08 | 2.23 | 0.34 |
| F4,125 = 3.30, p < 0.05, R2 = 0.10 | ||||
a0 = male, 1 = female.
8. Discussion
The current study's findings replicate and extend the existing literature on the neural correlates of effortful control. Our findings suggest an association between the NoGo N2 ERP component, an index of response inhibition and temperamental inhibitory control. Less negative NoGo N2 amplitudes were associated with better parent-reported inhibitory control, a scale thought to index a child's ability to suppress inappropriate responses when directed. The association between the attentional control subscale, a scale thought to index the child's capacity to maintain appropriate attention on task-relevant stimuli, and the NoGo N2 ERP component was initially significant, but was no longer significant when accounting for covariates and nested data, so while this suggests that the N2 may also be associated with attentional control, the current study did not show this association at a p < 0.05 level. Additionally, the other subscales comprising the effortful control construct (low intensity pleasure and perceptual sensitivity) were not associated with the N2 ERP component, suggesting a potentially unique association between the N2 and the more regulatory aspects of effortful control. This was expected given that the low intensity pleasure and perceptual sensitivity subscales assess preference for lower levels of stimulation and attention to minute environmental details (respectively), and such traits are not thought to be related to the N2 component.
Our findings replicate the Buss et al. [24] finding that the N2 component indexes some aspects of effortful control in childhood, while supplementing these findings in two important ways. First, our use of a different task to elicit the N2 component suggests that the N2 component, across paradigms, indexes a neural process related to inhibitory control. Additionally, the current study extends the findings of Buss et al. [24] to a sample of toddlers. Toddlerhood is a period of rapid improvements in effortful control. Given the importance of toddlerhood for the development of effortful control abilities, the identification of a potential neural biomarker at this age could have important practical implications for both our understanding of the normative development of effortful control as well as our understanding of emerging deficits in effortful control. Our findings did not replicate those of Wiersema & Royers [23], who found a positive association between the NoGo N2 and certain aspects of effortful control in middle childhood, or Ruberry et al. [25], who found that conflict monitoring capacities, as represented by the Go–NoGo difference waveform, were associated with effortful control. These divergent findings could be due to differences in the age of the sample [23] or differences in the measurement of effortful control [25]. More research will be needed to understand why contradictory findings characterize this literature. Additionally, our findings provide support for the hypothesis that the executive attention system, which is thought to functionally underlie the N2 component, supports effortful control abilities in very early childhood.
Our findings contribute to the development of taxonomies of individual differences based on neurobiological correlates in two ways. First, they provide support for the role of the executive attention network as a neural network underlying effortful control capacities, such that dysfunction in this network might underlie dysregulated behaviour in children with low levels of effortful control. Next, our findings suggest that efforts to develop taxonomies of individual differences can and should incorporate young children, examining the application of developed taxonomies to early childhood.
Among the strengths of this study, it is the first, to our knowledge, to examine a plausible electrophysiological marker for effortful control in very early childhood. Additionally, this study also provides electrophysiological evidence for the executive attention network's role in supporting effortful control in toddlerhood. Additionally, the study has a large sample when compared with many ERP studies of young children, which enables a more stable estimate of covariations between the study's measures. The study also has limitations. Although this study contributes to a literature examining the brain networks underlying effortful control, ERPs do not provide conclusive information about the brain regions underlying the components we examined. Our inferences from source localization studies with older children and adults about the brain regions involved in the N2 and task performance have to be somewhat tentative, because we cannot be sure how applicable these studies are to toddlers. Future studies, using novel imaging techniques with good spatial resolution (e.g. near-infrared spectroscopy), could better articulate the neural regions associated with effortful control.
In summary, the findings of the current study support an association between the N2 ERP component and parent-reported effortful control in toddlers, in which smaller, less negative NoGo N2 amplitudes were associated with better effortful control. These findings add to an existing literature examining the neural correlates of temperament in both childhood, focusing on an understudied age group, and toddlers, for whom effortful control abilities are developing rapidly.
Supplementary Material
Supplementary Material
Supplementary Material
Ethics
All procedures approved and monitored by the Institutional Review Board at Indiana University.
Data accessibility
The datasets supporting this article have been uploaded to Open Science Framework at the following link: https://osf.io/9nzev/ [26].
Authors' contributions
All four authors met all of the requirements for authorship.
Competing interests
We have no competing interests.
Funding
This work has been funded by grant nos. HD073202 and HD007475-17 from the NICHD, 1 F31 MH100814-01A1 from the NIMH, 1342962 from the NSF, and from Indiana University.
References
- 1.Banaschewski T, Brandeis D. 2007. Annotation: what electrical brain activity tells us about brain function that other techniques cannot tell us – a child psychiatric perspective. J. Child Psychol. Psychiatry 48, 415–435. ( 10.1111/j.1469-7610.2006.01681.x) [DOI] [PubMed] [Google Scholar]
- 2.Bates JE, Schermerhorn AC, Petersen IT. 2013. Temperament concepts in developmental psychopathology. In Handbook of developmental psychopathology (eds Rudolph K, Lewis M), pp. 311–329. New York, NY: Springer. [Google Scholar]
- 3.Nigg JT. 2006. Temperament and developmental psychopathology. J. Child Psychol. Psychiatry 47, 395–422. ( 10.1111/j.1469-7610.2006.01612.x) [DOI] [PubMed] [Google Scholar]
- 4.Rothbart MK, Bates JE. 2006. Temperament. In Handbook of child psychology, 6th edn, vol. 3 (ed. Eisenberg N.), pp. 99–166. Hoboken, NJ: Wiley. [Google Scholar]
- 5.Rothbart MK, Ahadi SA, Hershey KL, Fisher P. 2001. Investigations of temperament at three to seven years: the children's behavior questionnaire. Child Dev. 72, 1394–1408. ( 10.1111/1467-8624.00355) [DOI] [PubMed] [Google Scholar]
- 6.Olson SL, Sameroff AJ, Kerr DCR, Lopez NL, Wellman HM. 2005. Developmental foundations of externalizing problems in young children: the role of effortful control. Dev. Psychopathol. 17, 25–45. (doi:10.10170S0954579405050029) [DOI] [PubMed] [Google Scholar]
- 7.Murray KT, Kochanska G. 2002. Effortful control: factor structure and relation to externalizing and internalizing behaviors. J. Abnorm. Child Psychol. 30, 503–514. ( 10.1023/A:1019821031523) [DOI] [PubMed] [Google Scholar]
- 8.Allan NP, Lonigan CJ. 2011. Examining the dimensionality of effortful control in preschool children and its relation to academic and socioemotional indicators. Dev. Psychol. 47, 905–915. ( 10.1037/a0023748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orta IM, Corapci F, Yagmurlu B, Aksan N. 2013. The mediational role of effortful control and emotional dysregulation in the link between maternal responsiveness and Turkish preschoolers' social competency and externalizing symptoms. Infant Child Dev. 22, 459–479. ( 10.1002/icd.1806) [DOI] [Google Scholar]
- 10.Rueda R. 2012. Effortful control. In Handbook of temperament (eds Zentner M, Shiner RL), pp. 145–167. New York, NY: Guilford Press. [Google Scholar]
- 11.Rothbart MK, Sheese BE, Posner MI. 2007. Executive attention and effortful control: linking temperament, brain networks, and genes. Child Dev. Perspect. 1, 2–7. ( 10.1111/j.1750-8606.2007.00002.x) [DOI] [Google Scholar]
- 12.Posner MI, Rothbart MK. 2000. Developing mechanisms of self-regulation. Dev. Psychopathol. 12, 427–441. ( 10.1017/s0954579400003096) [DOI] [PubMed] [Google Scholar]
- 13.Posner MI, Rothbart MK. 2009. Toward a physical basis of attention and self-regulation. Phys. Life Rev. 6, 103–120. ( 10.1016/j.plrev.2009.02.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luna B, Padmanabhan A, O'Hearn K. 2010. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 72, 101–113. ( 10.1016/j.bandc.2009.08.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White LK, Lamm C, Helfinstein SM, Fox NA. 2012. Neurobiology and neurochemistry of temperament in children. In Handbook of temperament (eds Zentner M, Shiner RL), pp. 347–367. New York, NY: Guilford Press. [Google Scholar]
- 16.Diamond A. 2002. Normal development of prefrontal cortex from birth to young adulthood: cognitive functions, anatomy, and biochemistry. In Principles of frontal lobe function (eds Stuss DT, Knight RT), pp. 466–503. New York, NY: Oxford University Press. [Google Scholar]
- 17.Hoyniak CP. 2017. Changes in the NoGo N2 event-related potential component across childhood: a systematic review and meta-analysis. Dev. Neuropsychol. 42, 1–24. ( 10.1080/87565641.2016.1247162) [DOI] [PubMed] [Google Scholar]
- 18.Smith JL, Johnstone SJ, Barry RJ. 2004. Inhibitory processing during the Go/NoGo task: an ERP analysis of children with attention-deficit/hyperactivity disorder. Clin. Neurophysiol. 115, 1320–1331. ( 10.1016/j.clinph.2003.12.027) [DOI] [PubMed] [Google Scholar]
- 19.Bokura H, Yamaguchi S, Kobayashi S. 2001. Electrophysiological correlates for response inhibition in a Go/NoGo task. Clin. Neurophysiol. 112, 2224–2232. ( 10.1016/S1388-2457(01)00691-5) [DOI] [PubMed] [Google Scholar]
- 20.Nieuwenhuis S, Yeung N, Van Den Wildenberg W, Ridderinkhof KR. 2003. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cogn. Affect. Behav. Neurosci. 3, 17–26. ( 10.3758/CABN.3.1.17) [DOI] [PubMed] [Google Scholar]
- 21.Lavric A, Pizzagalli DA, Forstmeier S. 2004. When ‘go’ and ‘nogo’ are equally frequent: ERP components and cortical tomography. Eur. J. Neurosci. 20, 2483–2488. ( 10.1111/j.1460-9568.2004.03683.x) [DOI] [PubMed] [Google Scholar]
- 22.Zhou Q, Chen SH, Main A. 2011. Commonalities and differences in the research on children's effortful control and executive function: a call for an integrated model of self-regulation. Child Dev. Perspect. 6, 112–121. ( 10.1111/j.1750-8606.2011.00176.x) [DOI] [Google Scholar]
- 23.Wiersema JR, Roeyers H. 2009. ERP correlates of effortful control in children with varying levels of ADHD symptoms: temperament and psychopathology. J. Abnorm. Child Psychol. 37, 327–336. ( 10.1007/s10802-008-9288-7) [DOI] [PubMed] [Google Scholar]
- 24.Buss KA, Dennis TA, Brooker RJ, Sippel LM. 2011. An ERP study of conflict monitoring in 4–8-year old children: associations with temperament. Dev. Cogn. Neurosci. 1, 131–140. ( 10.1016/j.dcn.2010.12.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruberry EJ, Lengua LJ, Crocker LH, Bruce J, Upshaw MB, Sommerville JA. 2017. Income, neural executive processes, and preschool children's executive control. Dev. Psychopathol. 29, 143–154. ( 10.1017/S095457941600002X) [DOI] [PubMed] [Google Scholar]
- 26.Hoyniak CP, Petersen IT, Bates JE, Molfese DL. 2017. Data from: The neural correlates of temperamental inhibitory control in toddlers OSF Repository. (https://osf.io/9nzev/). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hoyniak CP, Petersen IT, Bates JE, Molfese DL. 2017. Data from: The neural correlates of temperamental inhibitory control in toddlers OSF Repository. (https://osf.io/9nzev/). [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded to Open Science Framework at the following link: https://osf.io/9nzev/ [26].