Abstract
Individual differences in behaviour characterize humans and animals alike. A hot field in behavioural ecology asks why this variation in ‘personality’ evolved. Theory posits that selection favours the integration of ‘intrinsic state’ and behaviour. Metabolism, hormones, energetic reserves and structural size have particularly been proposed as states covarying with behaviour among-individuals, either genetically or through plasticity integration. We conducted a meta-analysis estimating the amount of among-individual variation in behaviour attributable to variation in state. Our literature search showed that only 22% of the studies claiming to estimate individual-level associations between state and behaviour actually did so. Our meta-analysis revealed that relatively aggressive, bold, explorative and/or active individuals had relatively high metabolic rates, hormone levels, body weights and/or body sizes. The proportion of among-individual variation common to state and behaviour was nevertheless small (approx. 5%). This means that (i) adaptive explanations involving intrinsic states fail to explain much individual variation in behaviour, (ii) empiricists should consider nonlinear, additive or interactive effects of (multiple) intrinsic states, (iii) explanations not involving intrinsic states might be important, or (iv) empirical tests of state-dependent personality theory were inappropriate. Our meta-analysis highlights the importance of feedback between empiricists and theoreticians in the study of adaptive behavioural variation.
Keywords: state-dependent behaviour, among-individual variation, within-individual variation, repeatability, correlation, multi-variate mixed-effects modelling
1. Introduction
Over the past decade, it has become evident that repeatedly expressed behaviours differ more substantially among individuals than previously assumed [1,2]. Evidence is also accumulating that individuals differ in whole suites of behaviours [3], similar to how humans vary in personality [4]. Current research is further revealing individual variation in behaviour as a key factor in many ecological [5–9] and evolutionary processes [10–13]. Despite this progress, we still have limited understanding of why repeatable individual variation in behaviour has evolved. This is not due to a lack of adaptive theory but rather to empiricists long lagging behind in putting model assumptions and predictions to the test [14–16]. Recently, however, empirical tests have accumulated in the literature (e.g. [17,18]), implying that formal meta-analytical reviews can now be conducted to summarize available data, and elicit productive feedback between empiricists and theoreticians [14,16].
Behavioural ecologists have developed a body of theory explaining individual behaviour from an adaptive perspective. Many theoretical [19–24] and conceptual [25–31] models explain individual differences in behaviour as resulting from individual differences in intrinsic ‘state’ (defined as internal features of the individual affecting optimal behaviour [32]) or from adaptive positive feedback loops between state and behaviour [15], in both cases implying that selection may favour the adaptive integration of state and behaviour. Empirical tests of theory have recently focused on measuring behavioural and intrinsic state variables within the same set of individuals, typically focusing on ‘risky’ behaviours (defined as behaviours affecting resource acquisition at the cost of increased mortality [20,33]) and state variables such as metabolic rate (e.g. [34–37]), plasma hormone levels (e.g. [38–41]), body mass (e.g. [42–45]) or body size (e.g. [46,47]).
The idea that individual differences in above-mentioned intrinsic states underpin or have coevolved with individual variation in behaviour is extremely popular in the behavioural ecology literature, yet an authoritative review of empirical evidence is missing. Key questions are whether these state variables harbour repeatable individual variation (a key assumption), and whether such repeatable differences in state are in turn—regardless of presumed cause–effect relationships—important statistical predictors of repeatable differences in behaviour (a key prediction). A recent meta-analysis has already verified the assumption that many intrinsic state variables are—like behavioural traits [1]—individually repeatable [48]. This means that if such among-individual differences in state and behaviour are strongly integrated, we would predict strong among-individual correlations between states and behaviours.
Behavioural and intrinsic state variables do not harbour just among-individual variation, but typically also substantial within-individual variation [49–51], which represents an important obstacle in addressing this hot question. The ‘state-dependent personality’ hypothesis explicitly posits that state and behaviour covary among individuals. By contrast, state and behaviours also covary within individuals due to other processes such as plasticity integration or correlated measurement errors [51]. This implies that a firm test of theory requires datasets that unambiguously partition among- from within-individual patterns of covariance between state and behaviour. Appropriate tests of theory therefore must present repeated measures data for both state and behaviour, and apply advanced multi-level statistical techniques to partition among- from within-individual patterns of covariance between state and behaviour [51]. We present here a meta-analysis that uses such high-quality data, thereby firmly evaluating empirical evidence for state-dependent personality theory.
2. Methods
(a). Collection of meta-analytical data
We conducted a literature search in the Web of Science and Scopus on 23 November 2017 to retrieve papers presenting among-individual correlations between behaviours and internal state variables playing a key role in adaptive state-dependent personality theory (metabolic rate, hormone levels, body mass and body size) using the PRISMA (i.e. preferred reporting items for systematic reviews and meta-analyses) method [52] (electronic supplementary material, figure S1). Body mass and body size were treated as distinct state variables (i) because the former is influenced both by structural size and the amount of energetic reserves [53] while the latter is not, and (ii) because adaptive behaviour models imply that energetic reserves and size have distinct effects on behaviour [32]. We used a broad range of search terms to identify papers focusing on among-individual associations between mentioned intrinsic state variables and behaviour (search terms detailed in electronic supplementary material, text S1). We primarily focused on terms like ‘animal personality’, ‘behavioural syndrome’, ‘pace-of-life’ and ‘coping style’ since studies using those terms generally present among-individual rather than unpartitioned (‘raw’) phenotypic correlations. Notably, more general search terms (e.g. ‘behavior’ or ‘behaviour’) typically instead retrieved unpartitioned estimates, and this was also generally the case for studies published prior to the year (approx. 2010) when multivariate mixed-effect modelling approaches (enabling the unbiased estimation of among-individual correlations) were becoming established in behavioural ecology [51,54]. We also screened papers cited in table 1 of each of two key review papers [27,30] to retrieve additional studies documenting individual-level correlations between behaviour and metabolism. In our Web of Science search, we used ‘Behavioral sciences’, ‘Ecology’, ‘Endocrinology and metabolism’, ‘Evolutionary biology’, ‘Physiology’ and ‘Zoology’ as topic fields. In our Scopus search, we used ‘Agricultural and Biological Sciences’ as topic field. These searches altogether retrieved 1086 papers. We also acquired information from an additional 16 studies from other sources. Those included re-analyses (either by P.T.N. or the authors of the focal study) of five unpublished and six published datasets to acquire estimates of among-individual correlations not presented in the original publications. We excluded two published estimates of among-individual correlations that were >|1| [42], because such values are outside the natural range and cannot be z-transformed (a requirement for inclusion in our meta-analysis, detailed below). Our searches retrieved a total of 146 among-individual correlations between state and behaviour (detailed in electronic supplementary material, table S1).
Table 1.
Data transformations and sampling variance calculations applied to published data; r represents the focal correlation coefficient, n the number of individuals, SE the standard error, and 95% CI the 95% confidence or credible interval.
| description | equation | equation |
|---|---|---|
| Fisher's r to Z transformation (Zr) | 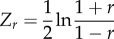 |
equation (2.1) |
| back-transformation Z to r (r) | 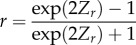 |
equation (2.2) |
| SE-based calculation of sampling variance (VarZr) | 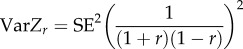 |
equation (2.3) |
| 95% CI to SE transformation | 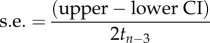 |
equation (2.4) |
We changed the sign of 25 correlation coefficients (for 13 positive and 12 negative correlations; electronic supplementary material, table S1) to ensure that higher values indicated more ‘proactive’ behaviour (sensu [55]), higher levels of boldness, activity, exploration or aggressiveness, and lower levels of docility. By doing so, we ensured that the sign of the untransformed correlation coefficients was biologically interpretable. Adaptive theory notably predicts that both the sign and direction of correlations between state and behaviour may vary as a function of ecological conditions [15]; our statistical analyses (detailed below) both estimated and controlled for such biological variation in effect sizes (‘heterogeneity analyses’, detailed below). Our main interest was in estimating the fraction of among-individual variance common to state and behaviour (see Introduction), and we therefore focused on absolute values of (squared) correlations. Biological variation in sign of correlations would be indicated by absolute values having greater effect sizes compared to untransformed ones.
(b). Statistical methods
We focused on estimating the squared average absolute magnitude of the among-individual correlation (|r|2) between state and behaviour, as this metric represents the proportion of among-individual variance in behaviour (‘personality’) that is attributable (in a statistical sense) to among-individual variation in state. To achieve this, we applied the ‘analyse-then-transform’ approach [56], consisting of estimating the posterior distribution of the average z-transformed correlation coefficient (rZ; equation (2.1), table 1), back-transforming this posterior to normal correlation coefficients (r; equation (2.2), table 1), folding the latter posterior to return the absolute average magnitude of the correlations (|r|) (eq. (7) in [56]), and squaring the folded posterior distribution to estimate |r|2. This approach is more accurate than the alternative ‘transform-then-analyse’ approach [56,57], where correlations are transformed into absolute values prior to analysis. For each focal posterior distribution (i.e. of r, |r| or |r|2), we estimated the mode and 95% credible intervals (95% CIs). We also estimated total heterogeneity (I2 total), residual heterogeneity (I2 residual) and study heterogeneity (I2 study; the proportion of variance among effect sizes explained by a study identity variance component) while statistically controlling for sampling error variance [58] (electronic supplementary material, table S2).
We applied standard multilevel meta-analytic models (i.e. intercept models) to estimates of among-individual correlations between behaviour and each of the four types of state-variable (metabolic rate, hormone levels, body mass and body size) separately, estimating their global effect sizes. We controlled for sampling variance in all models as doing so controls for statistical noise (e.g. differences in sample size across estimates) and thereby greatly increases the precision of estimated effect size [56,57]. Sampling variance was calculated (using equation (2.3), table 1) from the standard error reported for each correlation estimate (95% confidence/credible Intervals were transformed into standard errors prior to calculating sampling variance if reported instead using equation (2.4), table 1). The following random effects were considered in our models: study identity (n = 30 studies), species identity (n = 21 species) and phylogeny. Unfortunately, we were unable to simultaneously include all of these effects into our statistical models as this led to model convergence issues. This was probably the case because 71% (15/21) of the studies had unique species (electronic supplementary material, table S1). Reassuringly, models fitting only study, species or phylogeny produced the same general results (electronic supplementary material, table S3). In the main text, we arbitrarily chose to present the model controlling for study identity effects. This model suitably avoids pseudo-replication caused by the inclusion of repeated observations of estimates within the same study. The standard meta-analytical models were run using the MCMCglmm package [59] in the statistical environment R 3.1.3. [60]. We ran 3 300 000 iterations per model, from which we discarded the initial 300 000 (burn-in period). Each iteration chain was sampled at an interval of 1000 iterations, which resulted in a low autocorrelation among samples (always ≤0.04). Estimates with 95% credible intervals (Cls) not overlapping with zero were viewed as indicating statistically significant effects.
(c). Publication bias
We constructed funnel plots, fitting precision (i.e. the inverse of sampling variance) versus meta-analytic residuals (derived from our standard meta-analytical model that was conditioned for sampling variance and study identity), to test for publication bias in correlation coefficients [61]. We used Egger's regression analysis to test whether the distribution of estimates was more asymmetrical than expected by chance [62]. Following [61], meta-analytic residuals were calculated using the MCMCglmm R package [59], and Egger's regression conducted using the R package metaphor [63]. Funnel plot was symmetrical, suggesting no publication bias (electronic supplementary material, figure S2), which Egger's regression confirmed statistically (p = 0.904). Moreover, a trim-and-fill test [64] indicated no missing studies on either side of the funnel plot (p = 0.500).
3. Results
The average among-individual correlation between intrinsic state and behaviour differed from zero (r = [mean, 95% CIs] 0.101, 0.011; 0.185) (table 2). The positive value implied that relatively aggressive, bold, explorative and/or active (i.e. ‘pro-active’) individuals had relatively high metabolic rates, hormone levels, body weights and/or body sizes. This assessment was confirmed when we ran analyses for each of the four types of intrinsic state variable separately: their point estimates were all positive (table 2). The absolute average correlation was significant and (by definition) positive (|r| = 0.216, 0.168; 0.278) providing conclusive evidence for the existence of ‘state-dependent personality’. The estimate of |r| was about twice as high as the estimate of r owing to substantial among-study heterogeneity (see below) in the sign of state-behaviour correlations (electronic supplementary material, table S2). Among-individual variation in intrinsic state, importantly, explained (in a statistical sense) only a very minor proportion of the among-individual variation in behaviour (|r|2 = 0.047, 0.028; 0.076) (table 2). This meta-analytical finding implies that repeatable variation in intrinsic state and behaviour overlap only to a minor extent (i.e. 4.7%).
Table 2.
Estimates (mode) of r (correlation coefficient), |r| (absolute value of correlation coefficient) and |r|2 (squared absolute value of correlation coefficient) between behaviour and state (all states combined) and between behaviour and the four state variables separately from models controlling for study identity. We present here point mode estimates with 95% CIs (in brackets) derived from standard multilevel meta-analytic models.
| r | |r| | |r|2 | |
|---|---|---|---|
| all states combined | 0.101 (0.011; 0.185) | 0.216 (0.168; 0.278) | 0.047 (0.028; 0.076) |
| body mass | 0.074 (−0.056; 0.210) | 0.215 (0.149; 0.335) | 0.039 (0.018; 0.107) |
| body size | 0.018 (−0.062; 0.127) | 0.132 (0.077; 0.201) | 0.017 (0.005; 0.039) |
| metabolic rate | 0.226 (−0.057; 0.439) | 0.337 (0.205; 0.545) | 0.113 (0.039; 0.290) |
| hormone levels | 0.064 (−0.139; 0.234) | 0.141 (0.042; 0.316) | 0.020 (0.001; 0.097) |
Neither type of correlation coefficient (r, |r|) differed between the four types of state-variable (metabolism, hormones, body mass, body size), nor did the proportion of among-individual variation common to both state and behaviour (|r|2) differ between classes of state. Large credible intervals associated with each of the four point estimates (table 2), notably, indicated that subtle differences would not have been detectable.
The total heterogeneity was ‘high’ (80%; electronic supplementary material, table S2) following Higgins & Thompson [58] classification (i.e. 25%: small, 50%: medium, 75%: high). Our estimate was thus well within the limits of total heterogeneity expected in ecological studies [65]. Statistical noise or sampling error, (i.e. sampling variance) explained 20% (total variance − total heterogeneity; 100% − 80%) of the total variance in estimates of r. Study-level heterogeneity was at the medium level (46%), indicating that estimates (i.e. the strength and/or sign of correlations) differed, on average, between studies (electronic supplementary material, table S2). Residual heterogeneity was small (34%; electronic supplementary material, table S2) indicating that the variation in r was relatively small within studies. Moreover, phylogeny explained relatively much variation in state-behaviour correlations among studies (65%; electronic supplementary material, table S2), indicative of evolutionary signals on associations between state and behaviour. This finding implies, as predicted by ecological theory [15], the existence of genetic variation among species in either phenotypic plasticity (underpinning positive state–behaviour feedback loops) or genetic correlations between state and behaviour.
4. Discussion
Our study supports predictions of state-dependent personality theory as our meta-analysis showed that intrinsic state explained significant variation in behaviour among individuals. Our meta-analysis revealed that relatively aggressive, bold, explorative and/or non-docile (i.e. ‘pro-active’) individuals were also characterized by relatively high metabolic rates, hormone levels, body weights and/or structural body sizes. This finding does not suggest any specific cause–effect relationship (e.g. state affecting behaviour). Instead, it demonstrates that intrinsic state and behaviour are, on average, integrated among individuals, whether proximately underpinned by phenotypic plasticity (e.g. positive feedback loops) or by genetic correlations between intrinsic states and behaviour. Variation in intrinsic state thus ‘explains’ variation in personality in a statistical sense. Our meta-analysis also showed that each intrinsic state variable explained at best 3–8% (95% CIs) of the variation in ‘personality’ (sensu [51]). This implies either (i) that intrinsic states explain only a modest portion of the standing individual variation in behaviour, (ii) that nonlinear, additive or interactive effects of (multiple) intrinsic state variables are (as predicted by theory) instead important, (iii) that other explanations (e.g. extrinsic states) should be considered, or (iv) that empirical tests of theory are somehow inappropriate [66,67]. Our study thereby represents an important first step in furthering productive interactions between empiricists and theoreticians in explaining repeatable individual variation in behaviour from an adaptive perspective.
Our meta-analysis focused on among-individual correlations between behaviour and circulating hormones, metabolism, body weight, or structural body size. While we demonstrated that these types of intrinsic state variables individually did not explain much variation in ‘personality’, this does not mean that explanations involving state are unimportant. First, other state variables, whether intrinsic (e.g. immune defence, morphology) or extrinsic (e.g. behaviour of conspecifics, competitive regimes, predation risk), may need to be considered. Second, theory often predicts threshold effects, or other nonlinear relationships, between state and behaviour [19,68–71]. Empiricists instead primarily estimate linear associations (possibly because bivariate mixed effects models only enable the estimations of linear covariances). Effect sizes based on correlation coefficients reviewed here may thus underestimate true effect sizes. Therefore, in some cases proper testing of the theory might require estimating nonlinear associations between state and behaviour. Third, the combined (‘additive’) effects of the four intrinsic state variables studied here may actually explain as much as 18.8% of the variation in personality (i.e. 4 × 4.7%), though this would require that the four types of intrinsic state variable varied independently (which may be unlikely). A test of this idea would necessitate studies, few of which exist to date, quantifying various intrinsic state variables simultaneously. Studying multiple intrinsic state variables simultaneously would also enable testing a third explanation, positing that internal state variables interactively affect individual-level behaviour. Finally, there is considerable debate in the literature on whether the proxies of intrinsic states reviewed here are appropriate proxies of the intrinsic state variables considered by adaptive theory. For example, hormone receptor density, affinity, or specificity, greatly influence the effects of circulating hormone levels on the phenotype and may represent better proxies for hormonal state [72–74]. Similarly, body mass is often used as a measure of body reserves but is often also conflated with aspects of physiological condition and structural size.
The measurement theoretical arguments made above are also applicable to the choice of behaviour measured as part of empirical tests of state-dependent personality theory [66,67]. State-dependent personality models, for example, largely focus on ‘risky’ behaviours (i.e. behaviours that facilitate the acquisition of resources at the cost of increased mortality [20,22,33]). Our meta-analysis instead included estimates of among-individual correlations between intrinsic state variables and any empirically studied behaviour. This was not the cause of the low effect size because there was no notable increase in the proportion of among-individual variance in behaviour explained by state when we only included studies of risky behaviours (i.e. exploration, activity, boldness and aggression; n = 112 estimates) in our standard meta-analytic model (|r|2 = 0.053, 0.030–0.091). In summary, an important role for individual variation in intrinsic state in explaining personality variation would require more complex explanations than empiricists currently tend to consider. At the same time, our findings may also imply that alternative, state-independent, explanations for personality (e.g. mutation-selection balance), are more important than currently appreciated.
Our literature search also brought to the foreground a somewhat worrying pattern of scientific conduct that has repeatedly been highlighted in the personality literature [14,16] but has largely been ignored, and thereby obstructs scientific progress. That is, despite the huge amount of empirical literature on the topic of ‘animal personalities’ and ‘behavioural syndromes’, we found surprisingly few studies presenting appropriate empirical tests of theory. Unfortunately, this was not due to a paucity of empirical studies claiming to report relationships between intrinsic state and ‘personality’: our search identified 145 of such studies. Instead, the vast majority of studies claiming to report among-individual level estimates (113 out of 145 studies; 78%) reported unpartitioned phenotypic associations instead. These estimates were typically based on intrinsic states and/or behaviours measured once or, if repeated measures of traits existed, using state as a model covariate in a way that does not allow the separation of among- and within-individual level effects. Only a minority (32 out of 145 studies; 22%) used a combination of sampling design (i.e. repeated measures design) and statistical methods that allowed for the calculation of among-individual correlations indicative of associations between state and personality. Making matters worse, half of those latter studies (16 out of 32; 50%) reported simplistic statistical approximations of among-individual correlations that are known to be biased towards within-individual correlations [75–77]. Two types of approximations were used in particular: (i) correlations between individual-mean values or best linear unbiased predictors (BLUPs), or (ii) mean values or BLUPs of state fitted as a covariate explaining variation in behaviour. Consequently, only about 11% of the studies (16 out of 147) reported among-individual correlations unambiguously indicative of state-dependent personality. Fortunately, there are specific situations where unpartitioned phenotypic correlations, or statistical approximations of among-individual correlations (detailed above), provide unbiased estimates of among-individual correlations, namely when within- and among-individual correlations are identical [51]. We tested this assertion by additionally applying our meta-analysis to estimates of within-individual correlations. These analyses confirmed our suspicion that studies reporting phenotypic correlations cannot be used to test adaptive state-dependent personality theory: absolute within-individual correlations between intrinsic state and behaviour were significantly weaker than their among-individual counterparts (electronic supplementary material, text S2). This implies that phenotypic correlations represent biased, attenuated, estimates of among-individual correlations between state and behaviour [51,78], and highlights the importance of estimating the appropriate parameters for testing theory [16].
In conclusion, our meta-analysis supports adaptive personality theory predicting the adaptive integration of intrinsic state and behaviour among individuals. Our meta-analysis also showed that each intrinsic state variable explained (in a statistical sense) relatively little variation in ‘personality’. Finally, our meta-analysis revealed that much of the current empirical work (unknowingly) fails to appropriately test adaptive personality theory. A revival of interactions between empiricists and theoreticians seeking to explain individual behaviour from an adaptive perspective is therefore required to further this hot area in evolutionary behavioural ecology.
Supplementary Material
Acknowledgements
We thank Peter Biro, Anja Gunther, Chang Han, Ralf Kurvers, Indrikis Krams, Maria Moiron and Ariane Mutzel for providing access to the unpublished and published data, and Nick DiRienzo and Maria Moiron for helpful comments on an early version of the manuscript. We also thank Daniel Noble for the assistance with coding required to estimate absolute correlation coefficients and Shinichi Nakagawa for deriving equation (2.3) in table 1. Finally, we would like to thank Anne Charmantier, Barney Luttbeg, Simona Kralj-Fišer and one anonymous reviewer for constructive comments during review process.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
P.T.N. and N.J.D. designed the study, and drafted the manuscript, together. P.T.N. collected the data and carried out the statistical analysis. Both authors approved publication.
Competing interests
We declare we have no competing interests.
Funding
P.T.N. was funded by Deutsche Forschungsgemeinschaft (DFG grant no. NI 1539/1-1) and N.J.D. by the Ludwig-Maximilians University, Munich. This work contains data collected from other papers and thus, live animals were not used.
References
- 1.Bell AM, Hankison SJ, Laskowski KL. 2009. The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783. ( 10.1016/j.anbehav.2008.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dochtermann NA, Schwab T, Sih A. 2015. The contribution of additive genetic variation to personality variation: heritability of personality. Proc. R. Soc. B 282, 20142201 ( 10.1098/rspb.2014.2201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garamszegi LZ, Markó G, Herczeg G. 2012. A meta-analysis of correlated behaviours with implications for behavioural syndromes: mean effect size, publication bias, phylogenetic effects and the role of mediator variables. Evol. Ecol. 26, 1213–1235. ( 10.1007/s10682-012-9589-8) [DOI] [Google Scholar]
- 4.Gosling SD, Vazire S. 2002. Are we barking up the right tree? Evaluating a comparative approach to personality. J. Res. Pers. 36, 607–614. ( 10.1016/S0092-6566(02)00511-1) [DOI] [Google Scholar]
- 5.Chapple DG, Simmonds SM, Wong BBM. 2012. Can behavioral and personality traits influence the success of unintentional species introductions? Trends Ecol. Evol. 27, 57–62. ( 10.1016/j.tree.2011.09.010) [DOI] [PubMed] [Google Scholar]
- 6.Fogarty S, Cote J, Sih A. 2011. Social personality polymorphism and the spread of invasive species: a model. Am. Nat. 177, 273–287. ( 10.1086/658174) [DOI] [PubMed] [Google Scholar]
- 7.Duckworth RA, Badyaev AV. 2007. Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc. Natl Acad. Sci. USA 104, 15 017–15 022. ( 10.1073/pnas.0706174104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duckworth RA, Belloni V, Anderson SR. 2015. Cycles of species replacement emerge from locally induced maternal effects on offspring behavior in a passerine bird. Science 347, 875–877. ( 10.1126/science.1260154) [DOI] [PubMed] [Google Scholar]
- 9.Keiser CN, Pruitt JN. 2014. Personality composition is more important than group size in determining collective foraging behaviour in the wild. Proc. R. Soc. B 281, 20141424 ( 10.1098/rspb.2014.1424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santostefano F, Wilson AJ, Niemelä PT, Dingemanse NJ.. 2017. Behavioural mediators of genetic life-history trade-offs in field crickets. Proc. R. Soc. B 284, 20171567 ( 10.1098/rspb.2017.1567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dochtermann NA, Dingemanse NJ. 2013. Behavioral syndromes as evolutionary constraints. Behav. Ecol. 24, 806–811. ( 10.1093/beheco/art002) [DOI] [Google Scholar]
- 12.McNamara JM, Stephens PA, Dall SRX, Houston AI. 2009. Evolution of trust and trustworthiness: social awareness favours personality differences. Proc. R. Soc. B 276, 605–613. ( 10.1098/rspb.2008.1182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf M, Weissing FJ. 2012. Animal personalities: consequences for ecology and evolution. Trends Ecol. Evol. 27, 452–461. ( 10.1016/j.tree.2012.05.001) [DOI] [PubMed] [Google Scholar]
- 14.Dall SRX, Griffith SC. 2014. An empiricist guide to animal personality variation in ecology and evolution. Front. Ecol. Evol. 2, 3 ( 10.3389/fevo.2014.00003) [DOI] [Google Scholar]
- 15.Sih A, Mathot KJ, Moirón M, Montiglio PO, Wolf M, Dingemanse NJ. 2015. Animal personality and state-behaviour feedbacks: a review and guide for empiricists. Trends Ecol. Evol. 30, 50–60. ( 10.1016/j.tree.2014.11.004) [DOI] [PubMed] [Google Scholar]
- 16.Dingemanse NJ, Wolf M. 2010. Recent models for adaptive personality differences: a review. Phil. Trans. R. Soc. B 365, 3947–3958. ( 10.1098/rstb.2010.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laskowski KL, Pruitt JN. 2014. Evidence of social niche construction: persistent and repeated social interactions generate stronger personalities in a social spider. Proc. R. Soc. B 281, 20133166 ( 10.1098/rspb.2013.3166) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Nicolaus M, Tinbergen JM, Bouwman KM, Michler SPM, Ubels R, Both C, Kempenaers B, Dingemanse NJ. 2012. Experimental evidence for adaptive personalities in a wild passerine bird. Proc. R. Soc. B 279, 4885–4892. ( 10.1098/rspb.2012.1936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McElreath R, Strimling P. 2006. How noisy information and individual asymmetries can make ‘personality’ an adaptation: a simple model. Anim. Behav. 72, 1135–1139. ( 10.1016/j.anbehav.2006.04.001) [DOI] [Google Scholar]
- 20.Wolf M, van Doorn GS, Leimar O, Weissing FJ. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584. ( 10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- 21.Houston AI. 2010. Evolutionary models of metabolism, behaviour and personality. Phil. Trans. R. Soc. B 365, 3969–3975. ( 10.1098/rstb.2010.0161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luttbeg B, Sih A. 2010. Risk, resources and state-dependent adaptive behavioural syndromes. Phil. Trans. R. Soc. B 365, 3977–3990. ( 10.1098/rstb.2010.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf M, McNamara JM. 2012. On the evolution of personalities via frequency-dependent selection. Am. Nat. 179, 679–692. ( 10.1086/665656) [DOI] [PubMed] [Google Scholar]
- 24.Mathot KJ, Dall SRX. 2013. Metabolic rates can drive individual differences in information and insurance use under the risk of starvation. Am. Nat. 182, 611–620. ( 10.1086/673300) [DOI] [PubMed] [Google Scholar]
- 25.Careau V, Thomas D, Humphries MM, Réale D. 2008. Energy metabolism and animal personality. Oikos 117, 641–653. ( 10.1111/j.0030-1299.2008.16513.x) [DOI] [Google Scholar]
- 26.Øverli Ø, Sørensen C, Pulman KGT, Pottinger TG, Korzan W, Summers CH, Nilsson GE. 2007. Evolutionary background for stress-coping styles: relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates. Neurosci. Biobehav. Rev. 31, 396–412. ( 10.1016/j.neubiorev.2006.10.006) [DOI] [PubMed] [Google Scholar]
- 27.Biro PA, Stamps JA. 2010. Do consistent individual differences in metabolic rate promote consistent individual differences in behavior? Trends Ecol. Evol. 25, 653–659. ( 10.1016/j.tree.2010.08.003) [DOI] [PubMed] [Google Scholar]
- 28.Coppens CM, de Boer SF, Koolhaas JM. 2010. Coping styles and behavioural flexibility: towards underlying mechanisms. Phil. Trans. R. Soc. B 365, 4021–4028. ( 10.1098/rstb.2010.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koolhaas JM, de Boer SF, Coppens CM, Buwalda B. 2010. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front. Neuroendocrinol. 31, 307–321. ( 10.1016/j.yfrne.2010.04.001) [DOI] [PubMed] [Google Scholar]
- 30.Mathot KJ, Dingemanse NJ. 2015. Energetics and behavior: unrequited needs and new directions. Trends Ecol. Evol. 30, 199–206. ( 10.1016/j.tree.2015.01.010) [DOI] [PubMed] [Google Scholar]
- 31.Hau M, Casagrande S, Ouyang JQ, Baugh AT. 2016. Glucocorticoid-mediated phenotypes in vertebrates: multilevel variation and evolution. Adv. Study Behav. 48, 41–115. ( 10.1016/bs.asb.2016.01.002) [DOI] [Google Scholar]
- 32.Houston AI, McNamara JM. 1999. Models of adaptive behaviour: an approach based on state. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 33.Stamps JA. 2007. Growth-mortality tradeoffs and ‘personality traits’ in animals. Ecol. Lett. 10, 355–363. ( 10.1111/j.1461-0248.2007.01034.x) [DOI] [PubMed] [Google Scholar]
- 34.Gifford ME, Clay TA, Careau V. 2014. Individual (Co)variation in standard metabolic rate, feeding rate, and exploratory behavior in wild-caught semiaquatic salamanders. Physiol. Biochem. Zool. 87, 384–396. ( 10.1086/675974) [DOI] [PubMed] [Google Scholar]
- 35.Careau V, Montiglio PO, Garant D, Pelletier F, Speakman JR, Humphries MM, Réale D. 2015. Energy expenditure and personality in wild chipmunks. Behav. Ecol. Sociobiol. 69, 653–661. ( 10.1007/s00265-015-1876-2) [DOI] [Google Scholar]
- 36.Royauté R, Greenlee K, Baldwin M, Dochtermann NA. 2015. Behaviour, metabolism and size: phenotypic modularity or integration in Acheta domesticus? Anim. Behav. 110, 163–169. ( 10.1016/j.anbehav.2015.09.027) [DOI] [Google Scholar]
- 37.Krams IA, et al. 2017. Metabolic rate associates with, but does not generate covariation between, behaviours in western stutter-trilling crickets, Gryllus integer. Proc. R. Soc. B 284, 20162481 ( 10.1098/rspb.2016.2481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrari C, Pasquaretta C, Carere C, Cavallone E, von Hardenberg A, Réale D. 2013. Testing for the presence of coping styles in a wild mammal. Anim. Behav. 85, 1385–1396. ( 10.1016/j.anbehav.2013.03.030) [DOI] [Google Scholar]
- 39.Boulton K, Couto E, Grimmer AJ, Earley RL, Canario AVM, Wilson AJ, Walling CA. 2015. How integrated are behavioral and endocrine stress response traits? A repeated measures approach to testing the stress-coping style model. Ecol. Evol. 5, 618–633. ( 10.1002/ece3.1395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dosmann AJ, Brooks KC, Mateo JM. 2015. Within-individual correlations reveal link between a behavioral syndrome, condition, and cortisol in free-ranging Belding's ground squirrels. Ethology 121, 125–134. ( 10.1111/eth.12320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iserbyt A, Eens M, Baetens W, Vermeulen A, Müller W. 2017. Within- and between-individual (co)variance partitioning reveals limited pleiotropic effects of testosterone on immune function, sexual signaling, and parental investment. Behav. Ecol. Sociobiol. 71, 74 ( 10.1007/s00265-017-2308-2) [DOI] [Google Scholar]
- 42.Wilson AJ, Grimmer A, Rosenthal GG. 2013. Causes and consequences of contest outcome: aggressiveness, dominance and growth in the sheepshead swordtail, Xiphophorus birchmanni. Behav. Ecol. Sociobiol. 67, 1151–1161. ( 10.1007/s00265-013-1540-7) [DOI] [Google Scholar]
- 43.Wilson AJ, de Boer M, Arnott G, Grimmer A. 2011. Integrating personality research and animal contest theory: aggressiveness in the green swordtail Xiphophorus helleri. PLoS ONE 6, e28024 ( 10.1371/journal.pone.0028024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han CS, Jäger HY, Dingemanse NJ. 2016. Individuality in nutritional preferences: a multi-level approach in field crickets. Sci. Rep. 6, 29071 ( 10.1038/srep29071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelleher SR, Silla AJ, Dingemanse NJ, Byrne PG. 2017. Body size predicts between-individual differences in exploration behaviour in the southern corroboree frog. Anim. Behav. 129, 161–170. ( 10.1016/j.anbehav.2017.05.013) [DOI] [Google Scholar]
- 46.Kluen E, Siitari H, Brommer JE. 2013. Testing for between individual correlations of personality and physiological traits in a wild bird. Behav. Ecol. Sociobiol. 68, 205–213. ( 10.1007/s00265-013-1635-1) [DOI] [Google Scholar]
- 47.White SJ, Kells TJ, Wilson AJ. 2016. Metabolism, personality and pace of life in the Trinidadian guppy, Poecilia reticulata. Behaviour 153, 1517–1543. ( 10.1163/1568539X-00003375) [DOI] [Google Scholar]
- 48.Holtmann B, Lagisz M, Nakagawa S. 2017. Metabolic rates, and not hormone levels, are a likely mediator of between-individual differences in behaviour: a meta-analysis. Funct. Ecol. 31, 685–696. ( 10.1111/1365-2435.12779) [DOI] [Google Scholar]
- 49.Brommer JE. 2013. On between-individual and residual (co)variances in the study of animal personality: are you willing to take the ‘individual gambit’? Behav. Ecol. Sociobiol. 67, 1027–1032. ( 10.1007/s00265-013-1527-4) [DOI] [Google Scholar]
- 50.Dingemanse NJ, Kazem AJN, Réale D, Wright J. 2010. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89. ( 10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- 51.Dingemanse NJ, Dochtermann NA. 2013. Quantifying individual variation in behaviour: mixed-effect modelling approaches. J. Anim. Ecol. 82, 39–54. ( 10.1111/1365-2656.12013) [DOI] [PubMed] [Google Scholar]
- 52.Liberati A, et al. 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 151, W65–W94. ( 10.7326/0003-4819-151-4-200908180-00136) [DOI] [PubMed] [Google Scholar]
- 53.Piersma T, Davidson NC. 1991. Confusion of mass and size. Auk 108, 441–443. [Google Scholar]
- 54.Wilson AJ, Réale D, Clements MN, Morrissey MM, Postma E, Walling CA, Kruuk LEB, Nussey DH. 2010. An ecologist's guide to the animal model. J. Anim. Ecol. 79, 13–26. ( 10.1111/j.1365-2656.2009.01639.x) [DOI] [PubMed] [Google Scholar]
- 55.Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. ( 10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 56.Morrissey MB. 2016. Meta-analysis of magnitudes, differences and variation in evolutionary parameters. J. Evol. Biol. 29, 1882–1904. ( 10.1111/jeb.12950) [DOI] [PubMed] [Google Scholar]
- 57.Nakagawa S, Lagisz M. 2016. Visualizing unbiased and biased unweighted meta-analyses. J. Evol. Biol. 29, 1914–1916. ( 10.1111/jeb.12945) [DOI] [PubMed] [Google Scholar]
- 58.Higgins JPT, Thompson SG. 2002. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558. ( 10.1002/sim.1186) [DOI] [PubMed] [Google Scholar]
- 59.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.1002/ana.22635)20808728 [DOI] [Google Scholar]
- 60.R core team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 61.Nakagawa S, Santos ESA. 2012. Methodological issues and advances in biological meta-analysis. Evol. Ecol. 26, 1253–1274. ( 10.1007/s10682-012-9555-5) [DOI] [Google Scholar]
- 62.Stuck AE, et al. 1998. Bias in meta-analysis detected by a simple, graphical. BMJ 316, 469 ( 10.1136/bmj.316.7129.469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. ( 10.18637/jss.v036.i03) [DOI] [Google Scholar]
- 64.Duval S, Tweedie R. 2000. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–463. ( 10.1111/j.0006-341X.2000.00455.x) [DOI] [PubMed] [Google Scholar]
- 65.Senior AM, Grueber CE, Kamiya T, Lagisz M, O'Dwyer K, Santos ESA, Nakagawa S. 2016. Heterogeneity in ecological and evolutionary meta-analyses: its magnitude and implications. Ecology 97, 3293–3299. ( 10.1002/ecy.1591) [DOI] [PubMed] [Google Scholar]
- 66.Carter AJ, Feeney WE, Marshall HH, Cowlishaw G, Heinsohn R. 2013. Animal personality: what are behavioural ecologists measuring? Biol. Rev. 88, 465–475. ( 10.1111/brv.12007) [DOI] [PubMed] [Google Scholar]
- 67.Houle D, Pélabon C, Wagner GP, Hansen TF. 2011. Measurement and meaning in biology. Q. Rev. Biol. 86, 3–34. ( 10.1086/658408) [DOI] [PubMed] [Google Scholar]
- 68.Hutchinson JMC, McNamara JM. 2000. Ways to test stochastic dynamic programming models empirically. Anim. Behav. 59, 665–676. ( 10.1006/anbe.1999.1362) [DOI] [PubMed] [Google Scholar]
- 69.McNamara JM, Mace RH, Houston AI. 1987. Optimal daily routines of singing and foraging in a bird singing to attract a mate. Behav. Ecol. Sociobiol. 20, 399–405. ( 10.1007/BF00302982) [DOI] [Google Scholar]
- 70.McNamara JM, Houston AI, Lima SL. 1994. Foraging routines of small birds in winter: a theoretical investigation. J. Avian Biol. 25, 287–302. ( 10.2307/3677276) [DOI] [Google Scholar]
- 71.Luttbeg B. 2017. Re-examining the causes and meaning of the risk allocation hypothesis. Am. Nat. 189, 644–656. ( 10.1086/691470) [DOI] [PubMed] [Google Scholar]
- 72.Dufty AM, Clobert J, Møller AP. 2002. Hormones, developmental plasticity and adaptation. Trends Ecol. Evol. 17, 190–196. ( 10.1016/S0169-5347(02)02498-9) [DOI] [Google Scholar]
- 73.Ball GF, Balthazart J. 2008. Individual variation and the endocrine regulation of behaviour and physiology in birds: a cellular/molecular perspective. Phil. Trans. R. Soc. B 363, 1699–1710. ( 10.1098/rstb.2007.0010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adkins-Regan E. 2005. Hormones and animal social behavior. Princeton, NJ: Princeton University Press. [Google Scholar]
- 75.Hadfield JD, Wilson AJ, Garant D, Sheldon BC, Kruuk LEB. 2010. The misuse of BLUP in ecology and evolution. Am. Nat. 175, 116–125. ( 10.1086/648604) [DOI] [PubMed] [Google Scholar]
- 76.Dingemanse NJ, Dochtermann NA, Nakagawa S. 2012. Defining behavioural syndromes and the role of ‘syndrome deviation’ in understanding their evolution. Behav. Ecol. Sociobiol. 66, 1543–1548. ( 10.1007/s00265-012-1416-2) [DOI] [Google Scholar]
- 77.Lüdtke O, Marsh HW, Robitzsch A, Trautwein U, Asparouhov T, Muthén B. 2008. The multilevel latent covariate model: a new, more reliable approach to group-level effects in contextual studies. Psychol. Methods 13, 203–229. ( 10.1037/a0012869) [DOI] [PubMed] [Google Scholar]
- 78.Adolph SC, Hardin JS. 2007. Estimating phenotypic correlations: correcting for bias due to intraindividual variability. Funct. Ecol. 21, 178–184. ( 10.1111/j.1365-2435.2006.01209.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.


