Abstract
How flowering plants have recurrently evolved from hermaphroditism to separate sexes (dioecy) is a central question in evolutionary biology. Here, we investigate whether diallelic self-incompatibility (DSI) is associated with sexual specialization in the polygamous common ash (Fraxinus excelsior), which would ultimately facilitate the evolution towards dioecy. Using interspecific crosses, we provide evidence of strong relationships between the DSI system and sexual phenotype. The reproductive system in F. excelsior that was previously viewed as polygamy (co-occurrence of unisexuals and hermaphrodites with varying degrees of allocation to the male and female functions) and thus appears to actually behave as a subdioecious system. Hermaphrodites and females belong to one SI group and functionally reproduce as females, whereas males and male-biased hermaphrodites belong to the other SI group and are functionally males. Our results offer an alternative mechanism for the evolution of sexual specialization in flowering plants.
Keywords: plant reproductive system, polygamy/trioecy, functional gender, dioecy
1. Introduction
(a). Introduction to sexual diversity and dioecy
One of the striking characteristics of flowering plants is their extreme diversity of sexual systems in terms of the distribution of male and female reproductive roles among individuals of the same species. The sexual phenotype of an individual plant is based on its investment in the male (stamens, pollen) and female (pistils, ovules) reproductive organs, which defines the individual as either unisexual (i.e. male or female) or hermaphrodite. For our purposes, the term ‘hermaphrodite’ indicates the occurrence of both male and female sexual organs within a single plant, regardless of the distribution of these organs among the individual's flowers.
(b). Evolution of dioecy from hermaphroditism
Evolutionarily speaking, complete sexual specialization (dioecy) has evolved from a hermaphroditic ancestral state many times [1]. The most studied evolutionary pathway to dioecy involves two successive steps: first, the establishment of either female or male unisexuals in a hermaphroditic population (gynodioecy and androdioecy, respectively); and second, a more or less progressive sexual specialization of hermaphrodites towards the other reproductive function [2,3]. Other pathways involve the progressive evolution of some hermaphrodites towards increased maleness and others towards increased femaleness through disruptive selection [4]. Several studies have emphasized the importance of this progressive pathway, although it has not been so intensively investigated in theoretical studies [5,6]. In some cases, sexual divergence may be facilitated by the pre-occurrence of two categories of hermaphrodites. For instance, in heterodichogamous species, half of the individuals phenologically reproduce first as males and the other half as females [4,7]; similarly, in distylous species, hermaphrodites have either long styles and short stamens or short styles and long stamens [8]. Both of these reproductive systems facilitate crosses between hermaphrodites with different phenology or floral morphs. Furthermore, in most distylous species, a self-incompatibility (SI) system is associated with morphs, thus effectively restricting mating to between—rather than within—morphs [9–11]. One of the two classes of hermaphroditic mating partners then typically evolves towards pure male and the other towards pure female phenotypes [12].
In these scenarios, phenological/morphological differences between the two categories of hermaphrodites are thought to play an important role in the evolution of sexual specialization. Outside the group of flowering plants, there are however multiple pieces of evidence that homomorphic (not associated with morphological differences) SI systems, generally called mating types, are associated with the evolution of such sexual differentiation: anisogamy in the green algae is associated with ancestral mating types [13]; several species of basidiomycetes fungi have ‘sexual chromosomes’ [14]; and organelles show unilateral inheritance in isogamous yeast [15], green [16] and brown algae [17]. In this study, we address the following question: can homomorphic SI play a role in the transition from hermaphroditism to dioecy? More specifically, can progressive sexual specialization of each SI group lead to sexually differentiated types?
Fraxinus excelsior (Oleaceae) is a good candidate to test this hypothesis for three reasons. First, F. excelsior has puzzled biologists for some time because individuals of this species show continuous variation in the relative expression of their male and female organs (a complex and rare reproductive system defined as polygamy) [18], with sexual phenotypes being stable over time [19]. Second, recent studies have demonstrated the existence of homomorphic diallelic SI (DSI) systems in a closely related species, Fraxinus ornus [20], suggesting its possible existence in F. excelsior. Third, the phylogeny of the Fraxininae tribe shows several transitions from hermaphroditism to sexual specialization, including evolution to dioecy, androdioecy and, in the section Fraxinus, transitions towards polygamy [21]. This study sets out to test whether DSI occurs in F. excelsior, and investigates its association with sexual phenotype. We hypothesize that if a homomorphic DSI system facilitates the evolution to dioecy, then we should observe a clear association between the SI group in hermaphrodites and resource allocation to either the male or female function. Our results demonstrate that DSI turns the apparent polygamy of F. excelsior into functional subdioecy, with one group of plants reproducing essentially as males and the other group as females. Our findings shed new light on the evolution of plant sexual systems.
2. Material and methods
(a). Study system
Common ash (F. excelsior L., Oleaceae) is a wind-pollinated deciduous forest tree distributed throughout Europe and Asia Minor. In France, flowering occurs in early spring (March–April) for three to four weeks, before the leaves emerge (May). Fraxinus excelsior belongs to the Oleaceae family that harbours two androdioecious (co-occurrence of males and hermaphrodites within populations) species Phillyrea angustifolia L. and F. ornus L. in which SI has been investigated [20,22]. These species share a homomorphic sporophytic DSI system. Self-incompatible hermaphroditic individuals belong to one of two homomorphic SI groups (named G1 and G2): G1 (respectively, G2) individuals can only sire seeds on G2 (respectively, G1) hermaphrodites because cross-pollination between individuals of the same group elicits an incompatibility response [22]. The DSI system has been conserved in both species, although they belong to two different subtribes, and cross-species pollination tests have demonstrated that the recognition specificities currently segregating in the two species are identical [20].
(b). Plant material and sexual phenotyping
Two samples of trees from the Western and Central Europe part of the genetic distribution of the species [23] were used in the present study. The first, referred to as the ‘Orléans’ collection, includes 27 genotypes selected from natural stands in Normandy (France), grafted and planted in a private nursery in Alençon (France) in 1990 and, in replicate, in an experimental nursery in Orléans (France) in 2001 (see detailed description in electronic supplementary material S1 and table S1). Highly robust sexual phenotyping has been performed for over 10 years on this collection (electronic supplementary material, table S1) [19]. Albert et al. [19] used seven categories of sexual phenotypes based on the frequencies of male, female and hermaphrodite flowers: (i) male plants with male flowers only; (ii) andromonoecious plants with male flowers and less than 50% hermaphrodite flowers; (iii) andromonoecious plants with male flowers and more than 50% hermaphrodite flowers; (iv) hermaphrodite plants, with only hermaphrodite flowers; (v) gynomonoecious plants with female flowers and more than 50% hermaphrodite flowers; (vi) gynomonoecious plants with female flowers and less than 50% hermaphrodite flowers; and (vii) female plants with female flowers only [19]. The second sample, referred to as the ‘Cevennes’ collection, is made up of two natural populations, Camprieu and Aures, located in the Cevennes National Park (southern France). These two populations are found 600 km from the natural sites where the genotypes from the Orléans collection were sampled, at an altitude that prevents hybridization with Fraxinus angustifolia [24]. The sexual phenotype was characterized on the Cevennes sample in 2016 by assigning each flowering tree (n = 33 and 28 trees in Camprieu and Aures, respectively) to one of the seven classes defined in [19]. The same survey was scheduled in 2017, but only five trees could be scored due to a very low flowering rate (see the electronic supplementary material, table S2). For these trees, we analysed the sexual value averaged over the 2 years. In addition, all trees from both samples were scored for an estimation of previous seed production in November 2015 (see detailed description in electronic supplementary material S1, and tables S1 and S2).
(c). Compatibility/incompatibility assessments using stigma tests
We applied the experimental approaches developed in P. angustifolia and F. ornus [20,22] to assess the SI system in F. excelsior and characterize its relationship with sexual phenotype. On all tested F. excelsior individuals (Orléans and Cevennes collections), we performed interspecific stigma tests using P. angustifolia and/or F. ornus individuals previously assigned to either the G1 or G2 SI group [20,22] and we scored cross-compatibility based on pollen tube growth on stigmas. One major advantage of the cross-species pollination tests is that F. excelsior plants can be assigned to a SI group by crossing them with hermaphrodites (producing functional pollen and receptive stigmas) from the other two species. This test can verify pollen and stigma functionality of every F. excelsior individual in our collection and fully investigate male and female fertility and its association with DSI. Based on these results, each tree was assigned to either the G1 or the G2 group. In addition, intraspecific stigma tests were performed on a subsample of plants (n = 21) to verify that F. excelsior individuals assigned to one SI group based on interspecific tests were not compatible, but can mate with plants from the other group. In most cases, tested F. excelsior were used either as pollen donors or pollen recipients, based on their sexual phenotype (availability of pollen/receptive stigmas). It was possible to perform reciprocal stigma tests on six genotypes, using them both as pollen donors and recipients. Finally, self-pollination tests were performed on 10 plants. Details of the crossing design are provided in the electronic supplementary material, table S3, and the method for scoring cross-compatibility is detailed in electronic supplementary material S1.
Because the three species are not fully synchronized for their flowering phenology, and because the period of receptivity of stigma from a given individual is limited in time, we performed some of the stigma tests with pollen collected in 2015 or early in 2016 and stored at –80°C [20] (as described in electronic supplementary material S1) until application on recipient stigmas. This procedure allowed us to synchronize viable pollen and receptive stigmas between tested F. excelsior genotypes and the P. angustifolia and/or F. ornus testers.
(d). Statistical analyses
Because the Orléans and the Cevennes datasets have different structures, we statistically analysed the two datasets separately. To investigate the link between sexual phenotype and SI in the Orléans sample, we used the Mann–Whitney–Wilcoxon test to analyse the effect of the SI group on the average sexual phenotypic value from Albert et al. [19] and on fruiting intensity. Regarding the two natural populations from Cevennes, we analysed the effect of the SI group, population and their interaction on sexual phenotype by performing a generalized linear model [25]. In this analysis, population was treated as a fixed factor to test whether variation of sexual phenotype and its potential link with SI was similar between the two populations. Similarly, we tested the effects of SI group, population and their interaction on the fruiting value with a general linear model. Note that residuals of statistical models were not normally distributed even after data transformation; below we present the statistics obtained on untransformed data.
3. Results
(a). Fraxinus excelsior is self-incompatible and shows the same DSI system as Phillyrea angustifolia and Fraxinus ornus
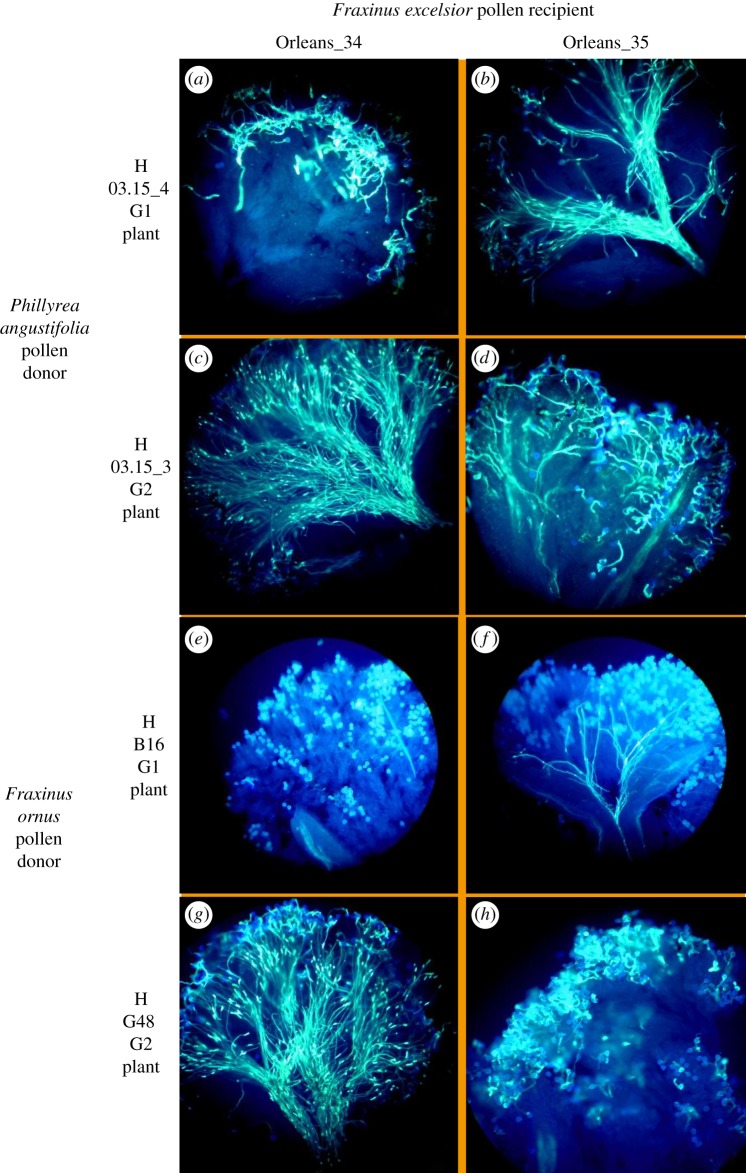
For every tested F. excelsior individual, we scored (i) unambiguous and repeatable incompatibility reactions when crossed with F. ornus and P. angustifolia from one group, and (ii) unambiguous and repeatable compatibility reactions when crossed with plants from the other group (figure 1). Importantly, this clear-cut result demonstrates that the pollen and stigmas used in this experiment were respectively viable and receptive, and that trans-generic and trans-specific pollen can germinate and elicit compatible and incompatible responses. Moreover, tests performed on individuals that were used as both pollen donors and recipients always provided results consistent with the rest of the experiment. Based on these results, it was possible to assign every tested F. excelsior individual to either the G1 or the G2 group. No individual compatible with both groups was found, rejecting the possibility of a third incompatibility group (electronic supplementary material, table S3). Intraspecific stigma tests showed complete consistency with these results: F. excelsior individuals assigned to the G1 (respectively, G2) group showed compatibility with plants from the G2 (respectively, G1) group only (electronic supplementary material, figure S1). Finally, self-pollination—tested in 10 cases (7 G1 and 3 G2)—always showed a typical incompatibility reaction (electronic supplementary material, table S3 and figure S1).
Figure 1.
Results of interspecific stigma tests between Fraxinus excelsior and Fraxinus ornus/Phillyrea angustifolia individuals previously assigned to either G1 or G2 SI group. Photos (b,c,f,g) show typical compatibility reactions, with several pollen tubes growing in the stigma tissue and converging towards the style. Photos (a,d,e,h) show typical incompatibility reactions. In this example, Orleans_34 was assigned to the G1 group, and Orleans_35 was assigned to G2. (Online version in colour.)
(b). Association between sexual phenotype and SI group
(i). The distribution of sexual phenotypic values shows discontinuity
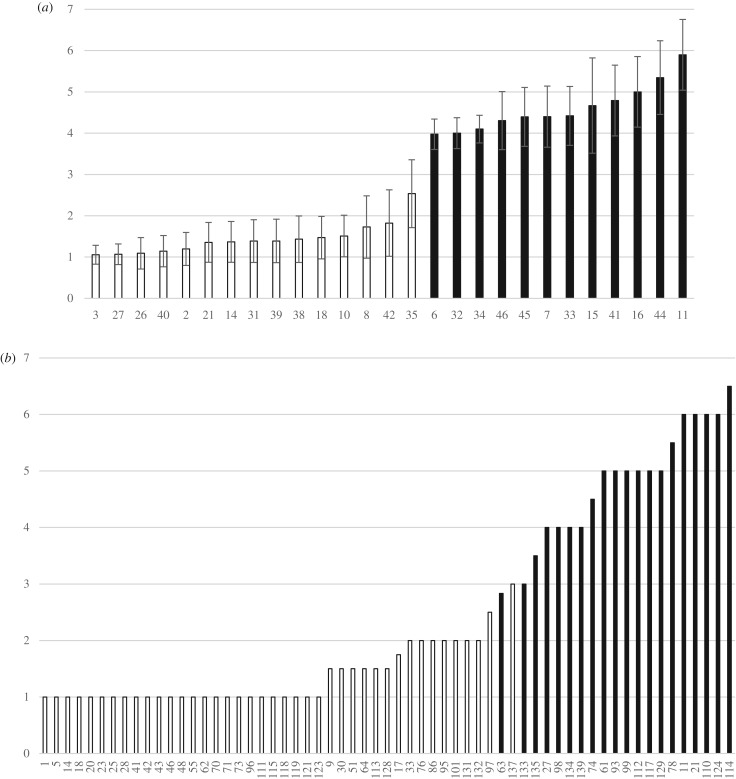
In both collections, scores for sexual phenotypes varied from 1 (pure males) to 6 (gynomonoecious trees with a majority of female flowers; figure 2). Phenotyping of the Cevennes collection was based on a single (rarely two) flowering season(s), but the sexual phenotypes available in the Orléans collection were based on the average values for each clone (genet), with scores for several ramets and years (more than 60 observations per genet on average), providing an accurate picture of the distribution of sexual phenotypes. In this dataset, the distribution of sexual phenotype varied continuously among genets. Relative differences between two genets ordered by average sexual phenotype ranged from 0.001 to 0.15, with however a break in the middle of the distribution (figure 2a). A relative difference of 0.39 was found between genotypes 42 and 35 and the highest difference (0.56) occurred between genotypes 35 and 6. This discontinuity occurred around the sexual phenotype value 3 (andromonoecious with few male flowers) that was only occasionally observed (8% of observations; see the electronic supplementary material, table S1 for details).
Figure 2.
Distribution of sexual phenotype scores from 1 (pure male) to 7 (pure female) in (a) Orléans and (b) Cevennes according to the assigned SI group (black: G1, white: G2) for each studied individual. Bars in (a) are for standard errors among clones and year replicates. In Cevennes (b), most individuals were phenotyped only in 2016.
(ii). A strong association was found between DSI groups and sexual phenotype value in both samples
In the Orléans collection, all 15 male and andromonoecious genotypes (with sexual phenotypic values of less than 3) belonged to the G2 incompatibility group, and all 12 genotypes classified as hermaphrodites or gynomonoecious (sexual phenotypic value greater than 3) belonged to the G1 group (group effect on phenotype: W = 180, p = 1.15 × 10–7; figure 2a). There was no overlap in the distribution of the two incompatibility phenotypes along the continuous distribution of the sexual phenotype values. The discontinuity in the distribution of sexual phenotypic values coincides with the transition between the two SI groups. Finally, sexual lability over ramets and years was higher in the G1 group than in G2 (average difference between the two extreme values within each clone = 2.92 and 1.73 for G1 and G2, respectively; Wilcoxon rank sum test: W = 148, p = 0.003).
Similarly, in the Cevennes collection, we observed a strong association between low average sexual values (maleness) and the G2 group (41 genotypes) on the one hand, and high average sexual values (femaleness) and G1 (20 genotypes) on the other hand (effect of SI group on sex: F1,57 = 206.05, p < 10−4, with no significant effect of the population nor of the population × SI group interaction; figure 2b). Even though sexual phenotyping was based on a single year of observation in this sample, we observed a very limited overlap of the groups, involving individuals Cevennes_137 (G2, average sexual phenotype = 3), Cevennes_63 and Cevennes_133 (G1, average sexual phenotype = 2.84 and 3, respectively; figure 2b).
(iii). Fruiting is essentially carried out by one of the two groups
In Orléans, we recorded a strong association between SI group and the amount of fruits produced (SI group effect on fruiting: W = 180, p = 4.26 × 10−6; electronic supplementary material, table S1 and figure S2). Among the 15 G2 plants, 12 produced no fruit the previous year, and three produced fruits in very low density (score = 1). By contrast, all G1 plants produced fruits, with intensity varying from 2 to 5 (average intensity = 3.42). Similarly, in the Cevennes populations, we found that all 20 G1 individuals that were flowering in 2016 had produced fruits in 2015 (average fruiting intensity = 3.65), whereas under the same conditions, only two out of the 41 G2 plants had produced fruits in 2015 (average intensity = 0.05), and in low quantity (SI group effect on fruiting intensity: F1,57 = 263.19, p < 10−4, with no significant effect of population or interaction with SI group; see also electronic supplementary material, table S2 and figure S2). Interestingly, the overlap between the two groups observed in these populations disappeared when considering fruiting: the G2 individual (Cevennes_137) that showed a relatively high sexual score produced only rare fruits, whereas the two G1 individuals (Cevennes_63 and Cevennes_133) that had a sexual score equal or lower showed a much higher fruiting intensity (figure 2b and electronic supplementary material, table S2 and figure S2).
(iv). Pollen and stigmas are functional in both groups
When considering the 32 G1 individuals identified in this study, 10 were used as pollen donors in a test with a G2 individual and showed functional pollen. This includes the ‘most female-like individuals' (e.g. Cevennes_21, Cevennes_124 and Orleans_15, respectively, scored with sexual phenotype values of 6, 6 and 4.67). Among the 56 G2 individuals, Cevennes_017 (sexual phenotype 2.5) was used as a pollen recipient in a test with a G1 individuals, and Orleans_31 (sexual phenotype 1.38) was used as pollen recipient with a male from P. angustifolia (compatible with both SI groups). Both showed functional stigma receptivity during these tests.
4. Discussion
(a). Self-incompatibility in Fraxinus excelsior and its link with sex
Our study unambiguously demonstrates that F. excelsior is a true self-incompatible species, consistent with previous population genetics studies that have demonstrated the predominantly outcrossing behaviour of the species [26]. The low selfing rates sometimes reported in controlled crosses can be explained by ‘leaky’ SI, similar to what has been reported in two other Oleaceae species: androdioecious P. angustifolia [27] and hermaphroditic Olea europaea [28].
Not only is F. excelsior self-incompatible, but its SI is based on the exact same system as the one previously identified in P. angustifolia [22], F. ornus [20] and more recently in O. europaea [28]. First, incompatibility reactions were observed based on cross-species stigma tests, regardless of whether F. excelsior was used as the maternal or the paternal parent. Second, males from P. angustifolia were compatible with F. excelsior G1 and G2 hermaphrodites (electronic supplementary material, table S3). This is similar to the system previously described in P. angustifolia, in which males are compatible with G1 and G2 hermaphrodites [22] (see below). These results confirm the fascinating evolutionary stability of DSI. Finally, no individual was compatible with testers of both groups, ruling out the possibility of a third SI group and attesting to the occurrence of DSI in F. excelsior.
In P. angustifolia, F. ornus and O. europaea, the DSI system is homomorphic with no differences in floral morphology between hermaphrodites of the two groups [22]. In F. excelsior, in contrast, we found a clear association between sexual phenotype and SI group. Interaction between DSI and sexual phenotype is not a novelty in the Oleaceae family, but the nature of the interaction observed in the present study differs from what is known in other species. In the androdiecious Oleaceae species P. angustifolia and F. ornus, the male determinant is genetically independent from the SI locus but fully linked to a genetic determinant epistatic over SI [27]. This confers compatibility with hermaphrodites from both SI groups to all males, whatever their genotype at the SI locus, and eliminates the reproductive disadvantage that males face due to the loss of female function [22,29–31]. By contrast, males of F. excelsior are constrained by the DSI: they belong to the G2 SI group and can only mate with hermaphrodites and females (or almost females) of the G1 group. In F. excelsior, the genetic association between the DSI locus and genes coding for sex is thus different from the other known species. Consequently, the evolutionary dynamics of the sexual system also differs from other documented cases in the family, because unisexuals do not benefit from a mating advantage over hermaphrodites through compatibility. Therefore, the question of how sterility mutations have been selected for within each SI group needs to be addressed.
(b). In the light of DSI, a complex sexual system (polygamy) turns out to be functional subdioecy
Our results show that, in spite of its apparent complexity, the sexual system of F. excelsior can be viewed as subdioecy. Indeed, although no pure females were found among sampled trees, the continuous variation from pure males to (almost) pure females has been previously interpreted as a complex version of trioecy, with some individuals only or mainly investing in male function, others mainly investing in female function and a large range of hermaphrodites [19]. The association between DSI and sexual phenotype completely changes our interpretation of this sexual system. The only way for a G1 genotype to sire seeds through pollen is to fertilize ovules carried by G2 and vice versa. Because the vast majority of fruits observed in natural populations is carried by G1 plants, this strongly suggests that most pollination events occur between G2 trees as pollen donors and G1 as recipients. This means that G2 hermaphrodites have a functional gender strongly biased towards maleness and that the various hermaphrodites from the G1 group, even those producing substantial quantities of male gametes, should mainly function as females.
This separation of functional gender shines a different light on the intriguing results from previous studies based on paternity analyses on progenies in F. excelsior. In those studies, several hermaphrodites had been hand-pollinated with a mixture of pollen collected on male and hermaphrodite trees. Hermaphrodites never sired any seeds in any of the studied crosses [32–34]. Until now, these results were—by default—attributed to subtle (and non-confirmed) differences in pollen vigour, quantity and/or timing of pollen release [35,36]. The existence of DSI in F. excelsior suggests that differences in pollination efficiency reported in these studies were likely driven by SI, with most hermaphrodites belonging to a single SI group (G1), rendering them incompatible with each other, and few andromonoecious G2 individuals producing pollen fully compatible with G1 recipients. On a more practical note, our results also provide valuable information for guiding the genetic management of F. excelsior given that the species is currently threatened in almost all of its native range by the invasive fungus Hymenoscyphus fraxineus [37]. Designing appropriate conservation, selection and crossing schemes will undoubtedly benefit from these results. Finally, this work was carried out on only two collections, both belonging to the Western–Central Europe part of the species's distribution. One important extension of this study is to investigate whether this association between sexual phenotype and DSI also occurs in northern and eastern areas of the species’ distribution.
The situation that we report here provides an accurate illustration of the concepts and ideas developed by Lloyd [38,39] on plant functional gender that should be differentiated from sexual phenotype. As underlined afterwards by Charnov [40], flower type may be a very imperfect predictor of functional gender. This assertion rings particularly true in F. excelsior, because individuals phenotyped as hermaphrodites have their functional gender constrained by DSI and appear to reproduce primarily through only one sexual function (either male or female). Lloyd also stated that virtually all plant species should be either gender monomophic or gender dimorphic [38,39]. The current study also conforms to this view: even an apparently complex sexual system can indeed operate as an essentially dimorphic system in terms of functional gender.
(c). Evolution towards dioecy or stable polygamy?
We recorded some sexual specialization of hermaphrodites based on the frequency of male, female and hermaphrodite flowers within inflorescences, with stronger investment in male (respectively, female) function in the G2 (respectively, G1) group. Interestingly, (imperfect) sexual specialization may also occur at another level, because some anthers of reduced size were recorded in bisexual flowers, but exclusively on gynomonoecious plants belonging to G1 group (data not shown).
However, in spite of this sexual specialization, several results indicate that male and female functions have been maintained in both SI groups. We currently know very little about the quantitative performance of pollen and ovule/seeds in the two groups, but according to our study: (i) plants from the G1 group all produce pollen (and some of them in non-negligible quantities); (ii) some plants from the G2 group produce a few ovules; (iii) pollen from G1 plants and stigmas from G2 are functional in pre-zygotic tests; and (iv) a minority of G2 plants were found to produce a few fruits, which contained viable embryos (germination tests performed on two G2 genotypes; data not shown). Thus, although plants from the G1 (respectively, G2) group primarily function as females (respectively, males), phenotypic sexual specialization appears incomplete. Noteworthily, incomplete specialization is particularly common in the G1 group: although the sexual phenotype of G2 plants seems more consistent with their male-biased functional gender (they produce either no or very few ovules), some G1 plants produce large amounts of pollen, seemingly contradicting their strongly female-biased functional gender. However, although G2 plants seem to be more strongly specialized in male function, some of them appear to produce a few fruits. This incomplete specialization can be linked with the phenomenon of labile sex expression in (sub)dioecious plants, also referred to as ‘leaky dioecy’ [5]. Such leakiness in flowering plants is apparently more common in males (inconstant males), frequently interpreted as evidence for the gynodioecy pathway [41]. Here, we document another example of leaky (sub)dioecy, where females seem more ‘inconstant’ than males, which may reflect an alternative evolutionary pathway.
This incomplete sexual specialization opens the question of the evolutionary stability of this reproductive system. Co-occurrence of unisexuals and hermaphrodites can be stable under specific conditions. In particular, the maintenance of both males and females with hermaphrodites (trioecious species) depends on three main mechanisms: the relative reproductive advantage of unisexuals relative to hermaphrodites, pollen limitation of females and inbreeding depression suffered by hermaphrodites. Maurice & Fleming [42] showed that trioecy is stable only if there is pollen limitation, because hermaphrodites benefit from reproductive assurance through self-fertilization, and if the reproductive advantage of unisexuals is large enough, through inbreeding depression in hermaphrodites or resource reallocation in unisexuals. The coexistence of unisexuals and hermaphrodites in F. excelsior is puzzling because hermaphrodites are self-incompatible: they cannot benefit from reproductive assurance through self-fertilization and they do not suffer from inbreeding depression. For now, nothing is known about a possible resource reallocation that would provide unisexuals with a reproductive advantage over hermaphrodites. Two alternative evolutionary scenarios can be proposed. First, this reproductive system is a transient state towards dioecy and any future mutations that decrease the investment in the male (respectively, female) function in the G1 (respectively, G2) group will be selected for. Second, the existence of two SI groups makes the maintenance of different sexual phenotypes possible because it limits the siring success of hermaphrodites and males (it has been shown that the existence of two SI groups broadens the conditions for the maintenance of androdioecy [30,31]). Determining which hypothesis is valid needs further theoretical work.
This study enhances the idea that DSI is associated with the evolution of sexual specialization in the Oleaceae family. Previous studies have shown that an association between DSI and female sterility allows males to be maintained along with hermaphrodites in two androdioecious species [20,27,30,31], although conditions for such reproductive systems are extremely restrictive otherwise. Here, we demonstrate that an association between DSI and sexual phenotype in F. excelsior deeply affects plant functional gender. Our results provide evidence that sexual specialization can evolve from hermaphroditism without passing through an intermediate stage such as gyno- or androdioecy, thereby revealing a role for DSI in the evolution of reproductive systems in flowering plants. Whether or not this reproductive system will ultimately evolve to full dioecy requires further investigation (and time!).
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Christophe Borel and the INRA GBFOR experimental unit (UE 0995) for pollen collection and tree care and maintenance. We also thank Lemonier nurseries for providing access to their seed orchard. Part of the field and laboratory work for incompatibility phenotyping was done at the Terrains d'Expériences facility (Labex CeMEB ANR-10-LABX-0004-CeMEB) at the Centre d'Ecologie Fonctionnelle et Evolutive (CEFE, CNRS) with the help of Thierry Mathieu and David Degueldre. We are very grateful to Marie-Pierre Dubois for providing access to the microscopy facilities at the SMGE (Service des Marqueurs Génétiques en Ecologie) facility (CEFE). We warmly thank the town of Saint Sauveur Camprieu in Gard, Sylvette Boulet (Landowne) and Cyril Turc (sheep farmer) in Aures, for their help in locating populations and providing access to sampling sites. We are very grateful to Jacques Lepart from CEFE, to Joël Mathez from Montpellier University, Franz Hopkins from the Cevennes Natural Park for facilitating access to trees in natural sites at the optimal time in regard to their phenology.
Data accessibility
This article has no additional data.
Authors' contributions
P.S.-L. and P.V. developed, designed and coordinated the study; they carried out stigma tests, participated in data analysis, interpreted the results and wrote the manuscript. A.D. coordinated the collection of data and plant material in Orleans, and managed funds to recruit S.Be.; S.Be. carried out stigma tests, estimated the fruit production and seed germination rates, participated in data analysis and helped interpret the results, S.Bi. helped develop and design the experiment and interpret results, B.A. and P.-H.G. coordinated access to trees in Alençon for sexual phenotyping and helped interpret results; M.D. wrote the manuscript, carried out statistical analyses and interpreted results. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This research was supported by the French National Research Agency through the project ‘TRANS’ (ANR-11-BSV7- 013-03) and by the French Ministry of Agriculture and Food (Convention MAAF—CRGF/INRA 2015 2016). It is a contribution to the CPER research project CLIMIBIO. The authors thank the French Ministry for Higher Education and Research, the Hauts-de-France Regional Council and the European Funds for Regional Economic Development for their financial support for this project.
References
- 1.Renner SS. 2014. The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an updated online database. Am. J. Bot. 101, 1588–1596. ( 10.3732/ajb.1400196) [DOI] [PubMed] [Google Scholar]
- 2.Barrett SC. 2010. Understanding plant reproductive diversity. Phil. Trans. R. Soc. B 365, 99–109. ( 10.1098/rstb.2009.0199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charlesworth B, Charlesworth D. 1978. A model for the evolution of dioecy and gynodioecy. Am. Nat. 112, 975–997. ( 10.1086/283342) [DOI] [Google Scholar]
- 4.Pannell JR, Verdu M. 2006. The evolution of gender specialization from dimorphic hermaphroditism: paths from heterodichogamy to gynodioecy and androdioecy. Evolution 60, 660–673. ( 10.1554/05-481.1) [DOI] [PubMed] [Google Scholar]
- 5.Käfer J, Marais GA, Pannell JR. 2017. On the rarity of dioecy in flowering plants. Mol. Ecol. 26, 1225–1241. ( 10.1111/mec.14020) [DOI] [PubMed] [Google Scholar]
- 6.Renner S, Ricklefs RE. 1995. Dioecy and its correlates in the flowering plants. Am. J. Bot. 82, 596–606. ( 10.2307/2445418) [DOI] [Google Scholar]
- 7.Renner S, Won H. 2001. Repeated evolution of dioecy from monoecy in Siparunaceae (Laurales). Syst. Biol. 50, 700–712. ( 10.1080/106351501753328820) [DOI] [PubMed] [Google Scholar]
- 8.Barrett SC, Shore JS. 2008. New insights on heterostyly: comparative biology, ecology and genetics. In Self incompatibility in flowering plants: evolution, diversity and mechanisms (ed. Franklin-Tong VE.), pp. 3–32. Berlin, Germany: Springer. [Google Scholar]
- 9.Baker H. 1958. Studies in the reproductive biology of West African Rubiaceae. J. West Afr. Sci. Assoc. 4, 9–24. [Google Scholar]
- 10.Barrett S. 1992. Heterostylous genetic polymorphisms: model systems for evolutionary analysis. In Evolution and function of heterostyly (ed. Barrett S.), pp. 1–29. Berlin, Germany: Springer. [Google Scholar]
- 11.Ornduff R. 1966. The origin of dioecism from heterostyly in Nymphoides (Menyanthaceae). Evolution 20, 309–314. ( 10.1111/j.1558-5646.1966.tb03368.x) [DOI] [PubMed] [Google Scholar]
- 12.Li A-M, Wu X-Q, Zhang D-X, Barrett SCH. 2010. Cryptic dioecy in Mussaenda pubescens (Rubiaceae): a species with stigma-height dimorphism. Ann. Bot. 106, 521–531. ( 10.1093/aob/mcq146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferris P, et al. 2010. Evolution of an expanded sex-determining locus in Volvox. Science 328, 351–354. ( 10.1126/science.1186222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badouin H, et al. 2017. Widespread selective sweeps throughout the genome of model plant pathogenic fungi and identification of effector candidates. Mol. Ecol. 26, 2041–2062. ( 10.1111/mec.13976) [DOI] [PubMed] [Google Scholar]
- 15.Birky CW., Jr 2001. The inheritance of genes in mitochondria and chloroplasts: laws, mechanisms, and models. Annu. Rev. Genet. 35, 125–148. ( 10.1146/annurev.genet.35.102401.090231) [DOI] [PubMed] [Google Scholar]
- 16.Boynton JE, Harris EH, Burkhart BD, Lamerson PM, Gillham NW. 1987. Transmission of mitochondrial and chloroplast genomes in crosses of Chlamydomonas. Proc. Natl Acad. Sci. USA 84, 2391–2395. ( 10.1073/pnas.84.8.2391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters AF, Scornet D, Müller DG, Kloareg B, Cock JM. 2004. Inheritance of organelles in artificial hybrids of the isogamous multicellular chromist alga Ectocarpus siliculosus (Phaeophyceae). Eur. J. Phycol. 39, 235–242. ( 10.1080/09670260410001683241) [DOI] [Google Scholar]
- 18.Wallander E. 2001. Evolution of wind-pollination in Fraxinus (Oleaceae): an ecophylogentic approach. PhD thesis, Göteborg University.
- 19.Albert B, Morand-Prieur M-É, Brachet S, Gouyon P-H, Frascaria-Lacoste N, Raquin C. 2013. Sex expression and reproductive biology in a tree species, Fraxinus excelsior L. C. R. Biol. 336, 479–485. ( 10.1016/j.crvi.2013.08.004) [DOI] [PubMed] [Google Scholar]
- 20.Vernet P, Lepercq P, Billiard S, Bourceaux A, Lepart J, Dommée B, Saumitou-Laprade P. 2016. Evidence for the long-term maintenance of a rare self-incompatibility system in Oleaceae. New Phytol. 210, 1408–1417. ( 10.1111/nph.13872) [DOI] [PubMed] [Google Scholar]
- 21.Wallander E. 2008. Systematics of Fraxinus (Oleaceae) and evolution of dioecy. Plant Syst. Evol. 273, 25–49. ( 10.1007/s00606-008-0005-3) [DOI] [Google Scholar]
- 22.Saumitou-Laprade P, Vernet P, Vassiliadis C, Hoareau Y, de Magny G, Dommée B, Lepart J. 2010. A self-incompatibility system explains high male frequencies in an androdioecious plant. Science 327, 1648–1650. ( 10.1126/science.1186687) [DOI] [PubMed] [Google Scholar]
- 23.Heuertz M, Hausman J-F, Hardy OJ, Vendramin GG, Frascaria-Lacoste N, Vekemans X, Fenster C. 2004. Nuclear microsatellites reveal contrasting patterns of genetic structure between western and southeastern European populations of the common ash (Fraxinus excelsior L). Evolution 58, 976–988. [DOI] [PubMed] [Google Scholar]
- 24.Fernández-Manjarrés J, Gerard P, Dufour J, Raquin C, Frascaria-Lacoste N. 2006. Differential patterns of morphological and molecular hybridization between Fraxinus excelsior L. and Fraxinus angustifolia Vahl (Oleaceae) in eastern and western France. Mol. Ecol. 15, 3245–3257. ( 10.1111/j.1365-294X.2006.02975.x) [DOI] [PubMed] [Google Scholar]
- 25.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. (doi:10.18637) [Google Scholar]
- 26.Bacles CF, Burczyk J, Lowe AJ, Ennos RA, Williams R. 2005. Historical and contemporary mating patterns in remnant populations of the forest tree Fraxinus excelsior L. Evolution 59, 979–990. [PubMed] [Google Scholar]
- 27.Billiard S, Husse L, Lepercq P, Godé C, Bourceaux A, Lepart J, Vernet P, Saumitou-Laprade P. 2015. Selfish male-determining element favors the transition from hermaphroditism to androdioecy. Evolution 69, 683–693. ( 10.1111/evo.12613) [DOI] [PubMed] [Google Scholar]
- 28.Saumitou-Laprade P, et al. 2017. Elucidation of the genetic architecture of self-incompatibility in olive: evolutionary consequences and perspectives for orchard management. Evol. Appl. 10, 867–880. ( 10.1111/eva.12457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Billiard S, López-Villavicencio M, Devier B, Hood ME, Fairhead C, Giraud T. 2011. Having sex, yes, but with whom? Inferences from fungi on the evolution of anisogamy and mating types. Biol. Rev. 86, 421–442. ( 10.1111/j.1469-185X.2010.00153.x) [DOI] [PubMed] [Google Scholar]
- 30.Husse L, Billiard S, Lepart J, Vernet P, Saumitou-Laprade P. 2013. A one-locus model of androdioecy with two homomorphic self-incompatibility groups: expected vs. observed male frequencies. J. Evol. Biol. 26, 1269–1280. ( 10.1111/jeb.12124) [DOI] [PubMed] [Google Scholar]
- 31.Pannell JR, Korbecka G. 2010. Mating-system evolution: rise of the irresistible males. Curr. Biol. 20, R482–R484. ( 10.1016/j.cub.2010.04.033) [DOI] [PubMed] [Google Scholar]
- 32.Bochenek GM, Eriksen B. 2011. First come, first served: delayed fertilization does not enhance pollen competition in a wind-pollinated tree, Fraxinus excelsior L. (Oleaceae). Int. J. Plant Sci. 172, 60–69. ( 10.1086/657298) [DOI] [Google Scholar]
- 33.Morand-Prieur M-E, Raquin C, Shykoff JA, Frascaria-Lacoste N. 2003. Males outcompete hermaphrodites for seed siring success in controlled crosses in the polygamous Fraxinus excelsior (Oleaceae). Am. J. Bot. 90, 949–953. ( 10.3732/ajb.90.6.949) [DOI] [PubMed] [Google Scholar]
- 34.Tal O.2006. Comparative flowering ecology of Fraxinus excelsior, Acer platanoides, Acer pseudoplatanus and Tilia cordata in the canopy of Leipzig's floodplain forest. PhD thesis, Leipzig University.
- 35.FRAXIGEN. 2005. Ash species in Europe: biological characteristics and practical guidelines for sustainable use. Oxford, UK: Oxford Forestry Institute, University of Oxford. [Google Scholar]
- 36.Wallander E. 2001. Evolution of wind-pollination and gender specialisation in Oleaceae: exaptations and adaptations. Unpublished paper, Göteborg University. [Google Scholar]
- 37.Marçais B, Husson C, Caël O, Dowkiw A, Saintonge F-X, Delahaye L, Collet C, Chandelier A. 2017. Estimation of ash mortality induced by Hymenoscyphus fraxineus in France and Belgium. Balt. Forestry 23, 159–167. [Google Scholar]
- 38.Lloyd DG. 1980. The distributions of gender in four angiosperm species illustrating two evolutionary pathways to dioecy. Evolution 34, 123–134. ( 10.1111/j.1558-5646.1980.tb04795.x) [DOI] [PubMed] [Google Scholar]
- 39.Lloyd DG. 1980. Sexual strategies in plants III. A quantitative method for describing the gender of plants. N. Z. J. Bot. 18, 103–108. ( 10.1080/0028825X.1980.10427235) [DOI] [Google Scholar]
- 40.Charnov EL. 1982. The theory of sex allocation. Princeton, NJ: Princeton University Press. [Google Scholar]
- 41.Ehlers BK, Bataillon T. 2007. ‘Inconstant males’ and the maintenance of labile sex expression in subdioecious plants. New Phytol. 174, 194–211. ( 10.1111/j.1469-8137.2007.01975.x) [DOI] [PubMed] [Google Scholar]
- 42.Maurice S, Fleming TH. 1995. The effect of pollen limitation on plant reproductive systems and the maintenance of sexual polymorphisms. Oikos 74, 55–60. ( 10.2307/3545674) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.