Abstract
Objective
Despite a multitude of minimally invasive surgical options available for benign prostatic enlargement, open simple prostatectomy (OSP) remains the standard for large prostates (typically greater than 100 g). OSP, however, is associated with significant morbidity. Recently, a few reports touting robotic application to simple prostatectomy have been published. Herein, we reviewed our series of robotic assisted laparoscopic simple suprapubic prostatectomy (RALSSP) and detailed modifications in our technique as our experience increased.
Methods
All RALSSP cases performed between January 2013 and January 2014 were reviewed for demographics, pre-operative features, and perioperative outcomes. All parameters were tabulated and mean values were calculated. Student's t-test was utilized with p < 0.05 deemed significant. Details regarding surgical technique were reviewed and highlighted.
Results
Fifteen patients underwent RALSSP during this period. Mean age of these men was 68.7 years. Mean body mass index (BMI) was 28.5 kg/m2. American Society of Anesthesiologists (ASA) score was on average 2.6. Average International Prostate Symptom Score (IPSS) was 16.2 with the majority of men experiencing some adverse clinical sequela of such benign prostatic hyperplasia (BPH). For those patients not in retention, preoperative post-void residual (PVR) was 428 mL. All patients underwent successful RALSSP without need for conversion or need for blood transfusion. Mean estimated blood loss (EBL) was 290 mL. Five patients underwent other concurrent procedures (e.g., cystolithotomy). Mean length of hospital stay (LOS) was 2.4 days and only five patients required continuous bladder irrigation (CBI) postoperatively. Postoperative PVR improved to a mean of 33 mL and IPSS improved to 4.5 (p < 0.001). No major complications were identified. Adaptation of low transverse cystotomy, utilization of a robotic tenaculum in the #3 arm with its control by a surgeon on a second console, and the utilization of mucosal advancement have all subjectively aided in performance of RALSSP and perioperative outcomes.
Conclusion
RALSSP allows for feasible performance of prostate adenoma enucleation with low risk of blood transfusion, short LOS, and significant improvement in IPSS and PVR; all while maintaining a minimally invasive approach. The use of a robotic tenaculum controlled by the secondary console and the mucosal advancement facilitate excellent outcomes and may play a role in minimizing hematuria and need for CBI.
Keywords: Robotic assisted laparoscopic simple suprapubic prostatectomy, Benign prostatic hyperplasia, Blood loss, Hospital stay
1. Introduction
A large variety of options to treat obstructive voiding symptoms and hematuria due to benign prostatic hyperplasia (BPH) has developed over the last decade. Given the patient population requiring such therapy, elderly men often with severe cardiovascular conditions and other co-morbidities, each new medication or procedure has had the goal of reducing the invasiveness and morbidity of treatment while maintaining effective outcomes [1]. As a result, the use of various techniques such as laser vaporization and enucleation, plasma vaporization, bipolar resection [2], and prostate embolization [3] have proliferated. Despite these advancements, the standard treatment for obstructive urinary symptoms due to large-gland (greater than 100 g) BPH remains open simple prostatectomy (OSP) [4].
Minimally invasive techniques emulating OSP have recently been developed and may improve perioperative morbidity with equivalent treatment outcomes. Robotic assisted laparoscopic simple suprapubic prostatectomy (RALSSP) has previously been described as a novel alternative to OSP [5]. We describe our initial experience with RALSSP and modifications we have adapted our technique to improve efficiency.
2. Patients and methods
2.1. Patient demographics and outcomes
A retrospective chart review was conducted on all patients who underwent RALSSP between January 2013 and January 2014. Outpatient records were reviewed for age, body mass index (BMI), prostate specific antigen (PSA) value, International Prostate Symptom Score (IPSS), prostate volume (cm3), post-void residual (PVR), and BPH related complications (urinary retention, need for indwelling foley catheter, urinary tract infection, gross hematuria, bladder outlet obstruction with evidence of bilateral hydronephrosis). The formula for volume of an ellipsoid (length × width × height × π/6) was utilized to approximate the preoperative prostate volume with such measurements obtained from transrectal ultrasound, MRI, or CT [6]. Inpatient charts were reviewed for perioperative parameters including: operative details for procedure, operative time, estimated blood loss (EBL), American Society of Anesthesiologists (ASA) score, and intraoperative complications. Pathology was reviewed for histology, concomitant pathology, and size of adenoma enucleated. Patient outcomes were reviewed.
2.2. Procedure details
Patients with a large prostatic gland precluding transurethral approach, or those with other pathology necessitating a transperitoneal approach (e.g., large bladder stones or large bladder diverticula), were educated regarding the risks and benefits and consented for RALSSP. All patients were cleared for surgery by their primary care physicians and/or cardiologists and the pre-surgical testing clinic. No bowel prep was required.
After induction of general anesthesia and administration of an intravenous first generation cephalosporin (or other culture directed antibiotic) the patient was secured the operating table with all pressure-points well padded. All patients tolerated the dorsal lithotomy position in steep Trendelenberg. Based on surgeon preference, a cystoscopy and bilateral ureteral stent placement is performed to better identify the ureteral orfices ahead of the RALSSP. Pneumoperitoneum was established utilizing Veress needle technique and set to 15 mmHg. A 12-mm port (for the laparoscope) is placed peri-umbilically and two 8-mm robotic trocar ports are placed 10 cm lateral, on either side, to the camera port. Two assistant ports are positioned on the patient's left side; a 12-mm airseal trocar, 10 cm lateral to the left sided robotic port, and a 5-mm port 8 cm superior and 4 cm lateral to the camera port.
After the robot is docked, the anterior peritoneum is incised on the lateral aspect of both medial umbilical ligaments in order to create the space of Retzius. Both medial umbilical ligaments and the urachus are transected superior to the dome of the bladder and the bladder is effectively dropped posteriorly. The prostate is cleared of fat anteriorly in order to facilitate identification of its anatomy. The Foley catheter is also advanced and retracted in order to delineate the bladder neck. A transverse cystotomy 2 cm cephalad to the bladder neck allows for adequate exposure of the prostate gland within the bladder. Both ureteral orfices are identified. If bladder stones are present, they are grasped and placed into an endocatch bag for subsequent extraction at this point. The mucosa overlying the prostate is scored, roughly 1–2 cm caudal to the trigone and this incision is continued circumferentially towards the anterior aspect of the prostate. Early in our series we utilized a 2-0 vicryl stay suture placed into the adenoma in order to aid in retraction, but we have since abandoned this and adopted the utilization of the robotic tenaculum for such retraction (Fig. 1). The assistant aids in suctioning of any blood and urine and with retraction of the prostatic capsule to allow for optimal visualization of the proper plane between the prostatic adenoma and the prostatic capsule. Diathermy is utilized to dissect along the contour of the prostate in this fashion, with advancement made incrementally around the periphery of the gland until the apex is transected from the urethra and the prostatic adenoma is completely freed. The adenoma is then placed into the endo-catch bag and placed aside for subsequent extraction. Remaining is the prostatic fossa (Fig. 2). 2-0 V-loc suture is then utilized to obtain hemostasis with a running whip stitch that incorporates the bladder neck vessels, classically located at the 7 O-clock and 5 O-clock position at the level of the bladder neck. A subsequent vesico-urethral advancement is performed utilizing a 3-0 V-loc suture to obliterate the space in the posterior prostatic fossa and facilitate subsequent foley catheter insertion or exchange (Fig. 3). A 24-Fr 3-way Foley catheter is then inserted and the cystotomy is repaired in two layers (running 3-0 V-loc for the mucosa/muscularis and an imbricating 2-0 V-loc for the serosa/muscularis). Irrigation of the foley catheter confirms a water-tight closure, the absence of any other capsular perforations, and ensures patency of the catheter.
Figure 1.
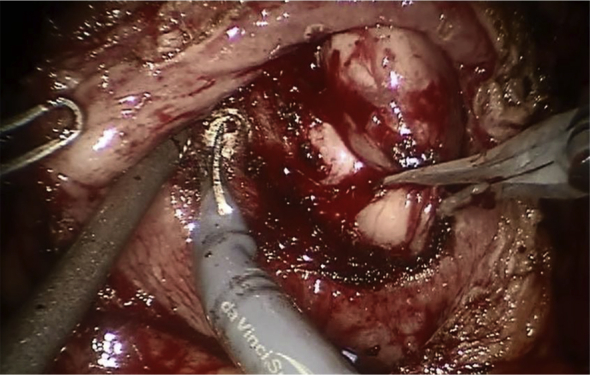
Tenaculum retracting adenoma.
Figure 2.
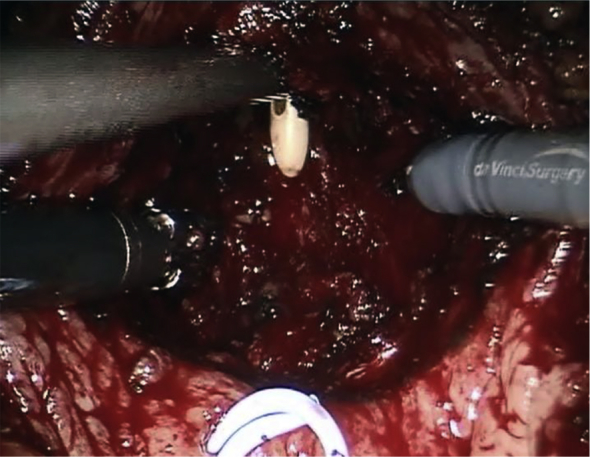
Open prostatic fossa.
Figure 3.
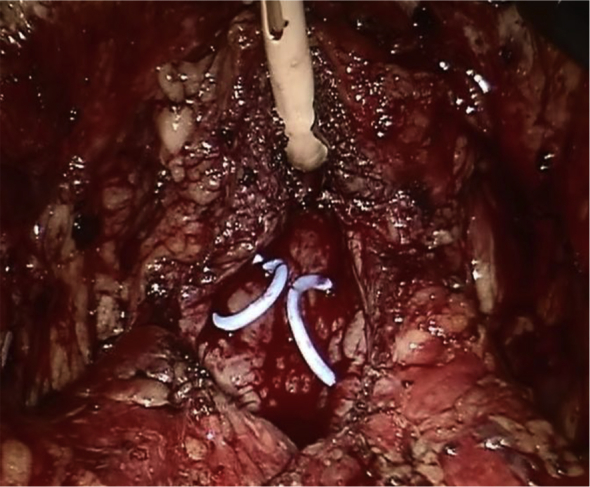
Obliterated fossa.
3. Results
Fifteen patients underwent RALSSP during the study duration. Patients were of a mean age of 68.7 years (range: 58–78 years), mean BMI of 28.5 kg/m2 (range: 23–39 kg/m2), and a mean ASA score of 2.6 (range 2–4). Average prostate volume was 156 cm3 (range: 61–255 cm3) and average PSA was 10.81 mg/dL (range:4.17–17.50 mg/dL). Mean preoperative PVR was 428 mL (range: 35–1054 mL). Many patients suffered from untoward sequela of BPH prior to treatment: six (40%) had a history of urinary retention, four (27%) were catheter dependent, seven (47%) had a history of urinary tract infections, four (27%) had gross hematuria, three (20%) had bladder stones, and one (7%) had a large bladder diverticulum. Four (27%) patients in our cohort underwent, unsuccessfully, prior surgical treatments of their BPH.
Perioperative data are highlighted in Table 1. Mean operative time was 189 min (range: 127–289 min). Mean estimated blood loss (EBL) was 290 mL (range: 100–500 mL). Mean length of hospital stay (LOS) was 2.4 days (range: 1–6 days, median 2 days). Change in hemoglobin was noted to be −1.1 ng/dL (range: −2.4 to −0.3 ng/dL). No patients in our series required a blood transfusion or conversion to open surgery. Only five (33%) patients of the 15 required continuous bladder irrigation (CBI) postoperatively. Mean Foley catheter duration was 8.67 days (range: 6–20 days). One intraoperative complication was identified. A significant capsular perforation, which was not repairable due to the friability of the prostatic capsule necessitated a formal vesico-urethral anastamosis be performed (similar to the von-Velthoven technique utilized for radical prostatectomy). Only two postoperative complications were identified (one Clavien grade 1 and one Clavien grade 2). One patient demonstrated erythema at the extraction port site and was treated with antibiotics for 2 weeks. Another patient (ASA 4) developed urosepsis postoperatively and required readmission and hospitalization. He also developed Clostridium difficile colitis but ultimately made a full recovery. No patients developed gross hematuria after discharge requiring catheterization or treatment.
Table 1.
Perioperative outcomes (n = 15).
| Variable | Value |
|---|---|
| Operative time (min)a | 189 (127–289) |
| EBL (mL)a | 290 (100–500) |
| Concurrent proceduresb | 5 (33.3) |
| Blood transfusionsb | 0 (0) |
| Conversionsb | 0 (0) |
| Length of hospital stay (d)c | 2.4 (1–6), 2 |
| No. patients requiring CBIb | 5 (33.3) |
| Length of catheter duration (d)a | 8.67 (6–20) |
| Preoperative HGB (g/dL)a | 12.6 (8.6–16.2) |
| Postoperative day#1 HGB (g/dL)a | 11.5 (8.3–14.5) |
| Postoperative PVR (mL)c | 33 (0–100), 25 |
| IPSS score postoperativelyc | 4.5 (0–8), 5 |
| Intraoperative complicationsb,d | 1 (6.67) |
| Complications (Clavien-Dindo)b | |
| Grade 1e | 1 |
| Grade 2f | 1 |
EBL, estimated blood loss; CBI, continuous bladder irrigation; HGB, hemoglobin; PVR, post-void residual.
Data presented as mean (range).
Data presented as No. (%).
Data presented as mean (range), median.
Large prostatic capsular perforation.
Superficial wound infection-Rx w abx.
Urosepsis and Clostridium difficile infection requiring hospitalization.
3.1. Specimens and pathology
Mean weight of prostate adenoma excised is 110 g. All specimens were consistent with BPH with only one specimen containing Gleason 3 + 3 prostate cancer in less than 5% of the enucleated specimen.
3.2. Functional outcomes
All patients reported significant improvement in lower urinary tract symptoms. PVR significantly improved to a mean of 33 mL (range 0–100 mL, paired t-test, p < 0.001). IPSS scores improved from mean of 16.2 to 4.5 (paired t-test, p < 0.001). Three patients developed stress urinary incontinence beyond 3 months, all of whom reported it to be “mild” with pad use ranging from one to three pads per day. Two patients complained of decrease in erectile function and were improved with the use of oral phosphodiesterase-5 inhibitors.
4. Discussion
Despite the myriad of surgical procedures available for the treatment of BPH, few options remain available for large glands (>100 g). OSP, either suprapubic or retropubic, were long unchallenged as the sole treatment options for the surgical extirpation of such large prostate glands. In addition, such approaches have allowed for the treatment of associated sequela of such BPH, such as bladder stones or diverticula. Unfortunately, the surgical incision necessary for the OSP and the associated bleeding from within the prostatic fossa are often associated with significant morbidity, need for blood transfusion, and convalescence.
Several centers have attempted to adopt a minimally invasive approach to simple prostatectomy with the promise of decreasing morbidity. Laparoscopic simple prostatectomy was first described by Mariano et al. [7] in 2002 with much enthusiasm. A subsequent cohort comparison however demonstrated that laparoscopic simple prostatectomy was associated with a higher transfusion rate compared with open [8]. The technical demands of laparoscopic suturing for hemostasis were certainly felt to be a factor for this outcome.
Numerous groups have since utilized the da Vinci robotic surgical platform to overcome such difficulties. With its associated magnified vision and articulating instruments, the da Vinci robot has been touted as the modality par excellence for minimally invasive surgery that requires complex intracorporeal reconstruction/suturing.
Several groups have recently published their experience with robotic simple prostatectomy [5], [9], [10], [11], [12], [13], [14], [15]. Table 2 summarizes their associated findings. Interestingly, there are many variations to the technique that can be elucidated from the literature. First, for simple suprapubic prostatectomies, access to the bladder is typically performed in a longitudinal fashion when performed open—with stay sutures placed for retraction of the bladder wall on either side. While this has persisted in some of the reports, many have favored a transverse incision just proximal to the bladder neck. We have found that this negates the need for stay suture retraction and allows excellent exposure of the adenoma. Secondly, some reports suggest the need to suture the venous plexus of Santorini; we have not found this to be necessary as exhibited by the minimal blood loss in our series. Thirdly, we have previously utilized a 2-0 vicryl suture placed into the adenoma for retraction, as described in earlier series. We have found that this suture often rips through the adenoma and may require multiple replacements. Recently we adapted the use of a robotic tenaculum which allows for firm retraction of the adenoma that can be easily repositioned. After the adenoma is removed, a 2-0 V-loc suture is utilized to obliterate the space within the prostatic fossa and attain hemostasis in a running fashion. A 3-0 V-loc suture is then utilized to anastomose the posterior bladder neck to the posterior membranous urethra. We presume that this aids in hemostasis and allows for more facile placement of Foley catheter.
Table 2.
Summary of prior studies.
| Study | n | Prostate size (preoperative) (mL)a | Prostate adenoma resected weight (g)a | EBL (mL)a | Transfusion rate (%) | LOS (d)a | Foley duration (d)a |
|---|---|---|---|---|---|---|---|
| Vora et al. [5] | 13 | 163 | 127 | 219 | 0 | 2.7 | 8.8 |
| Sotelo et al. [9] | 7 | 78 | 51 | 298 | 14 | 1.4 | 7 |
| John et al. [10] | 13 | 100 | 82 | 500 | 0 | 6 | 6 |
| Uffort and Jensen [11] | 15 | 71 | 46 | 139 | 0 | 2.5 | 4.6 |
| Sutherland et al. [12] | 9 | 137 | 112 | 206 | 0 | 1.3 | 13 |
| Coelho et al. [13] | 6 | 157 | 145 | 208 | 0 | 1 | 4.8 |
| Matei et al. [14] | 35 | 107 | 87 | 121 | 0 | 3.2 | 7.4 |
| Leslie et al. [15] | 25 | 150 | 88 | 143 | 4 | 4 | 9 |
| Current study | 15 | 157 | 110 | 290 | 0 | 2.4 | 8.67 |
Data presented as mean values. EBL, estimated blood loss; LOS, length of hospital stay.
As described above our technique has undergone many adjustments which, we find facilitate the surgery. First we abandoned the utilization of a longitudinal cystotomy for the caudal transverse cystotomy. The low-transverse cystotomy allows for a smaller incision in the bladder while the Trendelenberg position allows the superior bladder to drop out of the way obviating the need for stay sutures. Based on such excellent exposure afforded by the low transverse cystotomy, the facile visualization of the trigonal ridge and the ureteral orfices has negated our need to perform intraoperative cystoscopy and ureteral stent insertion, as was commonly performed early in our experience. Secondly we have abandoned the use of stay sutures placed into the prostate adenoma for the robotic tenaculum. The robotic tenaculum in the #3 arm (can be exchanged for the #1 arm if access to the right dissection plane is difficult) allows for excellent traction of the gland with, in our opinion, less risk of tearing through the adenoma or the need for adjustment of the stay suture as more adenoma is delivered. The third technical implementation, is allowing for an assistant surgeon on the second robotic console to control the robotic tenaculum. This facilitates what we call “dynamic traction” and decreases the need for repetitive clutching by the surgeon to adjust the traction as progress is being made. While, we do not advocate for this when an available assistant is not available, we are convinced that second robotic console control of the robotic tenaculum decreases time necessary to repetitively toggle control to the fourth arm to provide appropriate traction. We believe that these techniques have resulted in the improvement of several operative parameters (EBL and OR time) as our experience has increased.
Our report reveals outcomes similar to previous reports on RALSSP. Though our report is limited by small cohort, this article highlights unique attributes of our technique and its rapid evolution over our initial experience. Ideally, such outcomes should be compared to open suprapubic prostatectomy, though the paucity of such cases precludes fruitful comparison at our institution. Further, Holmium Laser Enucleation of the Prostate (HoLEP) has enjoyed significant attention recently as a minimally invasive alternative to open surprapubic prostatectomy, and ideally comparision of RALSSP to HoLEP should be included. However, HoLEP is technically difficult and not offered in many centers, whereas the da Vinci robotic platform has become widespread. Nonetheless, this report represents one of the larger series on RALSSP demonstrating feasibility and superiority compared to historical open simple prostatectomy series outcomes.
5. Conclusion
RALSSP mimics open simple prostatectomy with improvements in morbidity, blood loss, and convalescence, though our study does not provide a direct comparison. The use of a low transverse cystotomy and the robotic tenaculum (if possible controlled by the surgeon at the second console allowing for dynamic retraction) facilitates the procedure. Functional outcomes are acceptable.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Chinese Urological Association and SMMU.
References
- 1.Bhojani N., Gandaglia G., Sood A., Rai A., Pucheril D., Chang S.L. Morbidity and mortality after benign prostatic hyperplasia surgery: data from the american college of surgeons national surgical quality improvement program. J Endourol. 2014;28:831–840. doi: 10.1089/end.2013.0805. [DOI] [PubMed] [Google Scholar]
- 2.Health Quality Ontario Energy delivery systems for treatment of benign prostatic hyperplasia: an evidence-based analysis. Ont Health Technol Assess Ser. 2006;6:1–121. [PMC free article] [PubMed] [Google Scholar]
- 3.Pisco J.M., Pinheiro L.C., Bilhim T., Duarte M., Mendes J.R., Oliveira A.G. Prostatic arterial embolization to treat benign prostatic hyperplasia. J Vasc Interv Radiol. 2011;22:11–19. doi: 10.1016/j.jvir.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 4.Suer E., Gokce I., Yaman O., Anafarta K., Göğüş O. Open prostatectomy is still a valid option for large prostates: a high-volume, single-center experience. Urology. 2008;72:90–94. doi: 10.1016/j.urology.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Vora A., Mittal S., Hwang J., Bandi G. Robot-assisted simple prostatectomy: multi-institutional outcomes for glands larger than 100 grams. J Endourol. 2012;26:499–502. doi: 10.1089/end.2011.0562. [DOI] [PubMed] [Google Scholar]
- 6.Weiss B.E., Wein A.J., Malkowicz S.B., Guzzo T.J. Comparison of prostate volume measured by transrectal ultrasound and magnetic resonance imaging: is transrectal ultrasound suitable to determine which patients should undergo active surveillance? Urol Oncol. 2013;31:1436–1440. doi: 10.1016/j.urolonc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Mariano M.B., Graziottin T.M., Tefilli M.V. Laparoscopic prostatectomy with vascular control for benign prostatic hyperplasia. J Urol. 2002;167:2528–2529. [PubMed] [Google Scholar]
- 8.McCullough T.C., Heldwein F.L., Soon S.J., Galiano M., Barret E., Cathelineau X. Laparoscopic versus open simple prostatectomy: an evaluation of morbidity. J Endourol. 2009;23:129–133. doi: 10.1089/end.2008.0401. [DOI] [PubMed] [Google Scholar]
- 9.Sotelo R., Clavijo R., Carmona O., Garcia A., Banda E., Miranda M. Robotic simple prostatectomy. J Urol. 2008;179:513–515. doi: 10.1016/j.juro.2007.09.065. [DOI] [PubMed] [Google Scholar]
- 10.John H., Bucher C., Engel N., Fischer B., Fehr J.L. Preperitoneal robotic prostate adenomectomy. Urology. 2009;73:811–815. doi: 10.1016/j.urology.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 11.Uffort E.E., Jensen J.C. Robotic-assisted laparoscopic simple prostatectomy: an alternative minimal invasive approach for prostate adenoma. J Robot Surg. 2010;4:7–10. doi: 10.1007/s11701-010-0180-4. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland D.E., Perez D.S., Weeks D.C. Robot-assisted simple prostatectomy for severe benign prostatic hyperplasia. J Endourol. 2011;25:641–644. doi: 10.1089/end.2010.0528. [DOI] [PubMed] [Google Scholar]
- 13.Coelho R.F., Chauhan S., Sivaraman A., Palmer K.J., Orvieto M.A., Rocco B. Modified technique of robotic-assisted simple prostatectomy: advantages of a vesico-urethral anastomosis. BJU Int. 2012;109:426–433. doi: 10.1111/j.1464-410X.2011.010401.x. [DOI] [PubMed] [Google Scholar]
- 14.Matei D.V., Brescia A., Mazzoleni F., Spinelli M., Musi G., Melegari S. Robot-assisted simple prostatectomy (RASP): does it make sense? BJU Int. 2012;110(11 Pt C):E972–E979. doi: 10.1111/j.1464-410X.2012.11192.x. [DOI] [PubMed] [Google Scholar]
- 15.Leslie S., de Castro Abreu A.L., Chopra S., Ramos P., Park D., Berger A.K. Transvesical robotic simple prostatectomy: initial clinical experience. Eur Urol. 2014;66:321–329. doi: 10.1016/j.eururo.2013.12.020. [DOI] [PubMed] [Google Scholar]


