Abstract
The incidence of prostate cancer (PCa) within Asian population used to be much lower than in the Western population; however, in recent years the incidence and mortality rate of PCa in some Asian countries have grown rapidly. This collaborative report summarized the latest epidemiology information, risk factors, and racial differences in PCa diagnosis, current status and new trends in surgery management and novel agents for castration-resistant prostate cancer. We believe such information would be helpful in clinical decision making for urologists and oncologists, health-care ministries and medical researchers.
Keywords: Prostate cancer, Asian population, Epidemiology, Risk factors, Racial differences, Surgery management
1. Introduction
Approximately 238,590 new cases of prostate cancer (PCa) were diagnosed in the United States in 2013, causing 29,720 deaths in the same year [1]. During the past few decades, PCa incidence was reported to be much lower in Asian countries [2]; however, with dramatic economic growth and socio-cultural changes leading to an increased life expectancy and westernized lifestyle, the incidence and mortality rates of PCa in some Asian countries have shown a rapidly growing trend [2]. Currently PCa has already ranked the sixth of the most frequent cancers in 2012 among Asian countries [3]. As the aging process of the Asian population is still going on and the benefits of economic development will continue to accelerate lifestyle changes towards Westerners, PCa was predicted to gradually become a more serious healthcare and socio-economic issue. Meanwhile, the average spending on healthcare in most Asian countries was still much lower than that of Western countries [4]. Dealing with such a trend of rapid growth of PCa with a limited budget in most Asian countries is extremely challenging. Here in this report, we not only systematically summarized currently available data, but also collected the first-hand clinical statistics on current diagnostic strategy and emerging treatment options both in surgery management and castration-resistant prostate cancer (CRPC) from Chinese Prostate Cancer Consortium (CPCC) and leading urologists in Asian countries and regions. These data are based on national registry data or from representative/leading hospitals in situations where the former is not available. Thus, cautions should be taken in interpreting these data. Hopefully, we believe such information would be helpful in decision making for urologists and oncologists, healthcare ministries and medical researchers from a clinical prospective.
2. PCa epidemiology in Asia
2.1. Incidence
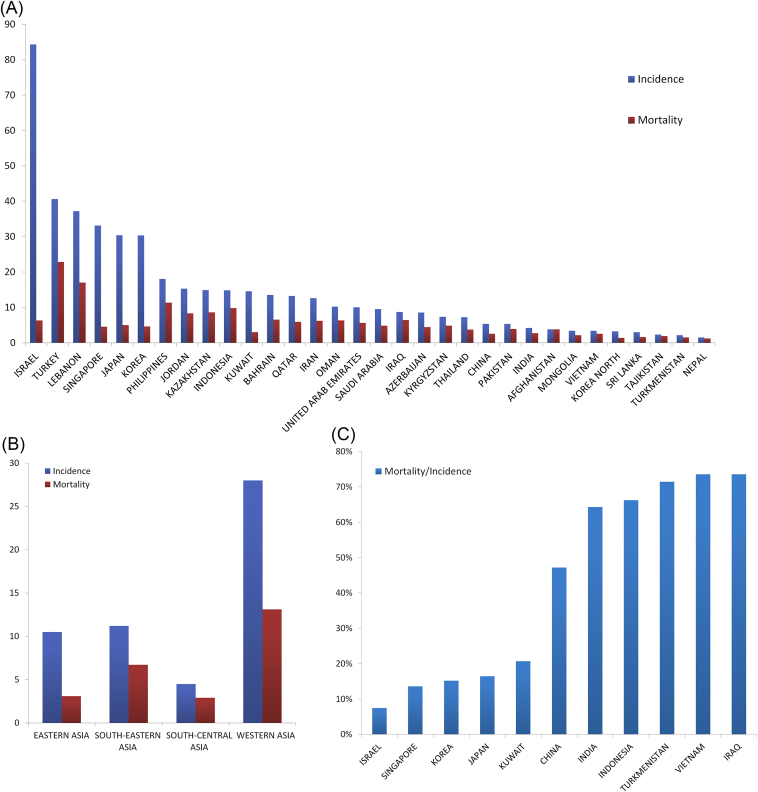
Globally, PCa is the second most common cancer in men and the incidence varies more than 25-fold worldwide; the rates are highest in Australia/New Zealand and Northern America (age standardized rate (ASR) 111.6 and 97.2 per 100,000, respectively), and in Western and Northern Europe [3]. However, after reaching its peak in mid-1900s which is commonly attributed to the widespread use of prostate-specific antigen (PSA) tests, the incidence of PCa in several developed countries (Canada, the United States, United Kingdom, Italy, etc.) has witnessed a decrement in the past few years [5], but still remains higher than that in Asian populations. According to the WHO report in 2012, the incidence rates were estimated at 10.5% and 4.5% in East and South-Central Asia, respectively [3] (Fig. 1A, B). However, PCa incidence in Asian countries has been on a steady increase during the past few decades. Currently, PCa has already become the sixth most frequent cancer in Asian men, with the highest in Western Asia ranking second in all cancers and the lowest in South-Central Asia ranking eighth.
Figure 1.
(A) Incidence and mortality rates (per 100,000) in Asian countries and regions in 2012. (B) Incidence and mortality rates (per 100,000) in different Asian regions in 2012. (C) Mortality/incidence ratios in Asian countries and regions in 2012 (Age specific rate, ASR). Data sources: GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet].
2.2. Mortality
With an estimated 307,000 deaths in 2012, PCa is the fifth leading cause of death from cancer in men globally (6.6% of the total deaths in men). It is still controversy if PSA testing had a significant influence on mortality rates. Less variation in mortality rates was seen worldwide (10-fold from approximately 3 to 30 per 100,000) than is observed for incidence, with the number of deaths from PCa larger in less developed than in more developed regions (165,000 and 142,000, respectively). The average mortality rate of PCa in Asian countries is 3.8 per 100,000. In economically developed regions such as Japan, Singapore, and Korea, these rates are 5, 4.5, and 4.6 per 100,000, respectively [3]. The trends of mortality rates were more variable; with increasing trends observed in China (Hong Kong), Kazakhstan, and Korea, decreasing trends seen in Israel, Japan, Kyrgyzstan, and Uzbekistan, and rather stable trends reported in Singapore and Tajikistan [3].
2.3. Changing trend in epidemiology
As illustrated in a previous review [6], the PCa incidence in developing East Asia countries has been on a rapid rise since the 1990s. For instance, the incidence rate has grown at a rate of 7.2% per year from 2004 to 2009. As the national cancer registry report shows, the incidence was 9.92 per 100,000 in 2009 [7]. The incidence in Korea has also been on a rapid rise from 8.4 per 100,000 in 1999 to 24.4 per 100,000 in 2011 [8]. Considering the similar genetic background between Korea and China, it is predicted that the incidence in China will increase to a level comparable to Korea if China adopts a similar healthcare policy to Korea in PCa detection. However, it is relatively difficult to predict if the incidence will continue to surge in Korea and Japan where this rate has already experienced rapid growth.
2.4. Mortality/incidence ratios
The MR/IR (mortality-to-incidence rate ratio) was as high as 40% in Asia compared to 18% in Europe, 10% in Northern America and 25% worldwide. The lowest MR/IR rate was witnessed in Israel, whereas it is typically much higher in countries with unfavorable economic situations, like Vietnam and Iraq (Fig. 1C). In several developed countries like Japan, Korea and Singapore, although the incidence is higher than other countries, the MR/IR is relatively lower than in that of several Southeast and West Asian countries [3].
3. Risk factors for PCa in Asian men
3.1. Genetic differences in prostate cancer in Asian men
Asians differ significantly with regards to race. East and Southeast Asians share a similar genetic background and are generally known as Mongolians; Northwest and Central Asians are of Turkic, Iranian or Caucasian ethnicity. The South Asians consist of mainly the Indian population which has been considered as a mixed population of Caucasians and Mongolians. As for the West Asian population, some of them are Caucasians and others are Arabians.
There are two types of genetic variations that are important in prostate carcinogenesis; one is somatic alteration in prostate tumors, and the other is germline common polymorphic conferring moderate-to-low risk for developing the disease. For the first type of genetic variation, substantial racial differences have been reported. For instance, the most prevalent gene fusion in Caucasians is the TMPRSS2-ERG fusion, with a prevalence of about 50%. However, much lower frequencies are reported in Asian populations (8%–21%) [9], [10]. Another prominent variation is the PTEN inactivation in PCa patients. PTEN inactivation was reported in 70% of Caucasians but only 34% in Chinese patients [9], [11]. Currently, there is evidence that PTEN loss and TMPRSS2-ERG fusion are related [12]. This may offer a potential explanation for the low incidence of PTEN loss and low expression of TMPRSS2-ERG fusion in the East Asian population. Other mutations, such as KRAS mutations and BRAF copy number gain, are seen more frequently in Asian patients than in the Western population [13], [14]. With the help of the next generation of sequencing technologies, more variations among races will be identified.
Single nucleotide polymorphisms (SNPs) are also different between Asian and Western populations. Genome-wide association studies (GWAS) have identified 76 susceptibility loci associated with PCa risk [15]. Of these, some loci have been validated in Asian population in GWAS and validation studies. To date, three GWAS in Asians were reported [16], [17], [18]. Ten SNPs were identified to be associated with PCa in Japanese and Chinese and 11 SNPs in Tunisians (Table 1). Findings of GWAS in East Asians identified a large portion of SNPs that are identical with Westerners, with some unique SNPs identified. However, reports from GWAS in Arabian populations indicated a relatively large number of SNPs that are different from previous findings [19].
Table 1.
SNPs indentified in genome-wide association studies in Asian populations.
| Locus | SNP | Nearby genes | Reference allele | Effect allele | Effect OR (95%CI) | Ethnic of identification | Reference |
|---|---|---|---|---|---|---|---|
| 2p24 | rs13385191 | C2orf43 | A | G | 1.15 (1.10–1.21) | Japanese | [16] |
| 5p15 | rs12653946 | IRX4 | C | T | 1.26 (1.20–1.33) | Japanese | [16] |
| 6p21 | rs1983891 | FOXP4 | C | T | 1.15 (1.09–1.21) | Japanese | [16] |
| 6q22 | rs339331 | RFX6 | C | T | 1.22 (1.15–1.28) | Japanese | [16] |
| 13q22 | rs9600079 | Unknown | G | T | 1.18 (1.12–1.24) | Japanese | [16] |
| 3p11 | rs2055109 | Unknown | T | C | 1.20 (1.13–1.29) | Japanese | [17] |
| 10q26 | rs2252004 | Unknown | T | G | 1.16 (1.10–1.22) | Japanese | [17] |
| 11q12 | rs1938781 | FAM111A | T | C | 1.16 (1.11–1.21) | Japanese | [17] |
| 9q31 | rs817826 | RAD23B–KLF4 | T | C | 1.41 (1.29–1.54) | Chinese | [18] |
| 19q13 | rs103294 | LILRA3 | T | C | 1.28 (1.21–1.36) | Chinese | [18] |
| 9p24 | rs7045455 | SMARCA2 | T | C | 3.98 (1.97–8.05) | Tunisians | [19] |
| 9p24 | rs12686439 | SMARCA2 | G | A | 3.32 (1.80–6.15) | Tunisians | [19] |
| 9p24 | rs10810919 | SMARCA2 | T | C | 5.03 (2.22–11.4) | Tunisians | [19] |
| 9p24 | rs10963533 | SMARCA2 | T | C | 4.19 (2.01–8.75) | Tunisians | [19] |
| 9p24 | rs10963540 | SMARCA2 | G | A | 4.81 (2.23–10.4) | Tunisians | [19] |
| 17q21 | rs12601982 | STAT5A | A | G | 2.61 (1.67–4.07) | Tunisians | [19] |
| 17q21 | rs8078731 | STAT3 | A | T | 2.47 (1.55–3.93) | Tunisians | [19] |
| 22q13 | rs5750627 | LOC646851 | C | T | 2.40 (1.60–3.60) | Tunisians | [19] |
| 22q13 | rs6001173 | LOC646851 | C | T | 2.32 (1.54–3.49) | Tunisians | [19] |
| 22q13 | rs138702 | SUN2 | T | A | 2.44 (1.60–3.71) | Tunisians | [19] |
| 22q13 | rs138712 | SUN2 | A | G | 2.47 (1.63–3.76) | Tunisians | [19] |
SNPs, single nucleotide polymorphisms; OR, odds ratio, CI, confidential interval.
Such differences in PCa risk-associated SNPs may suggest distinct mechanisms in the development of PCa against different ethnic groups. More importantly and from a clinical relevant point of view, these discrepancies will eventually influence the onset, diagnosis, prognosis and treatment of PCa in the Asian populations.
3.2. Lifestyle changes and PCa in Asian populations
Genetic variations aside, environmental differences may act as an important factor for PCa as well. Asian populations vary greatly on diet and lifestyles. Generally, East and Southeast Asians consume more vegetables, with a lower proportion of animal protein and high-fat food in their daily intake than Western populations [20]. However, the situation has been changing during these past few decades due to economic development.
Firstly, Asian men who immigrate to foreign countries may display a different epidemiologic pattern. For instance, the PCa incidence of Indians who immigrate to Singapore, UK and US were higher compared to Indians in India (9.9, 33.7, 47.4 and 4.6 per 100,000, respectively) [21]. Although the low rate in India may be partially due to under-diagnosis, they may also be due to lifestyle and environmental factors. Secondly, per capita consumption of animal fat in Asian countries has been on an increasing trend since more than 40 years ago. From the 1960s to the 2000s, consumption of animal fat increased by 591.7%, 578.6%, and 128.6%, respectively in Chinese, Korean and Japanese populations [22]. In these countries, nutrition transition played a role in the increasing incidence of colon cancer and PCa during the past few decades. Thirdly, environmental factors, particularly in countries with a Western diet, will keep a relatively high speed of increase in incidence. Other factors are mainly clinical, for example, the variation in access to PSA screening and physicians. All of these might be major contributing factors. In this aspect, incidence in China and India will probably increase substantially owning to the economic development and rapid lifestyle changes [22]. Meanwhile, countries like Japan, Korea and Israel that have a developed economy and a relatively stable lifestyle may present a more stable trend in incidence.
4. Diagnosis of prostate cancer in Asian countries
4.1. PSA-based screening in Asian countries
National PSA screening programs were not carried out in Asian countries, partially due to the lower incidence rate and/or the inadequate financial resources for healthcare in some Asian countries. On a global scale, PCa screening remains controversial. The updated results from the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) did not illustrate a mortality benefit for PSA screening [23]. The European Randomized Study of Screening for Prostate Cancer (ERSPC) demonstrated a 21% reduction in PCa-specific mortality in favor of screening [24]. In most Asian countries, financial consideration has always been a key factor. Based on current governmental spending on healthcare, PSA-based screening would probably not be launched in the near future for the majority of Asian countries.
4.2. Age-specific PSA cut-off value for Asian countries
As it has been shown, the PSA level in Asian populations is reported to be lower than that of Western populations [25]. Age-specific cut-offs have been established for Japanese, Chinese, Indians, Koreans, and Malays [26]. Reported age-specific cut-off values have been summarized in Table 2 [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38]. In general, these cut-off values are higher than that of the Western population, but there are some differences within these countries. For instance, the cut-off values of Malays and Arabians seem to be lower compared with Eastern Asians. In a clinical scenario such consideration should be noted and more importantly, healthcare policy-makers should acknowledge such differences.
Table 2.
Reported age-specific PSA cut-off values for Asian population.
| Study | Country or region | Ref. | Cases | PSA 95%CI (ng/mL) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| <40 | 40–49 | 50–59 | 60–69 | 70–79 | >80 | ||||
| Oesterling et al., 1993 | United States | [28] | 471 | – | 2.5 | 3.5 | 4.5 | 6.5 | – |
| Liu et al., 2008 | Mainland, China | [25] | 8422 | – | 2.15 | 3.2 | 4.1 | 5.37 | – |
| Lin et al., 2004 | Taiwan, China | [27] | 1008 | 1.85 | 2.59 | 3.31 | 5.03 | 5.73 | – |
| Wu and Huang, 2004 | Taiwan, China | [29] | 1236 | – | – | 4.0 | 6.0 | 6.0 | – |
| Choi et al., 2007 | Korea | [30] | 286 | 1.85 | 1.92 | 2.37 | 3.56 | 5.19 | – |
| Oesterling et al., 1995 | Japan | [31] | 335 | – | 2 | 3 | 4 | 5 | – |
| Imai et al., 1995 | Japan | [32] | 3526 | – | 2.1 | 2.9 | 4.0 | 5.2 | 5.9 |
| Malati and Kumari, 2004 | India | [33] | 583 | 0.9 | 1.3 | 1.48 | 1.6 | 2 | 2.47 |
| Abdrabo, 2011 | Sudanese | [34] | 1051 | – | 3 | 3.02 | 3.8 | 8.7 (70–90) | |
| Kamal et al., 2003 | Saudi Arabia | [35] | 567 | 1.03 | 1.62 | 2.09 | 2.86 | 3.17 | |
| Saw and Aw, 2000 | Singapore | [36] | 513 | 1.4 | 1.7 | 2.3 | 4 | 6.3 | 6.6 |
| Lim et al., 2014 | Malay | [26] | 378 | – | 0.6 | 1 | 1.2 | 1.4 | – |
| Kehinde et al., 2005 | Kuwait and Oman | [37] | 396 | – | 0.9 | 1.6 | 2.9 | 5.5 | – |
| Khezri et al., 2009 | Iran | [38] | 930 | – | – | 2.61 | 3.59 | 4.83 | – |
PSA, prostate-specific antigen; Ref., reference; CI, confidential interval.
4.3. Distribution of men who underwent prostate biopsies in different PSA categories
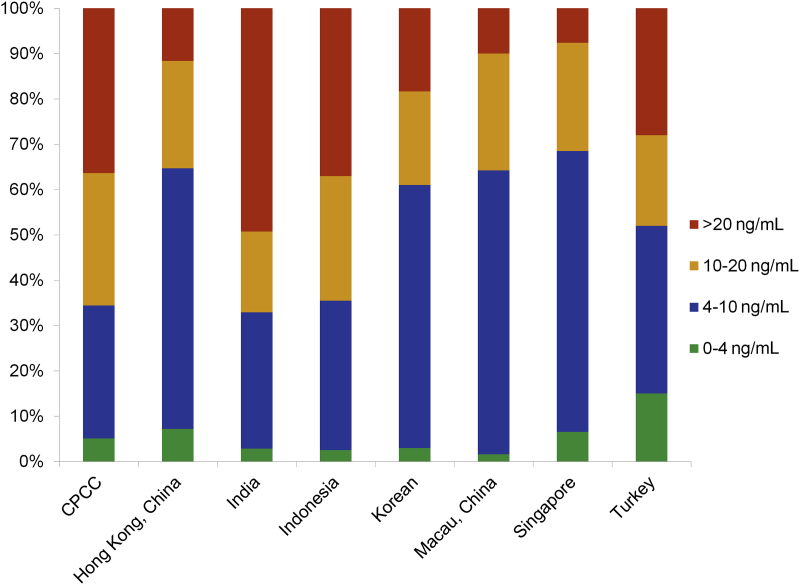
As screening programs in the Western population have led to an increase in the PCa incidence rate starting from the 1990s, PCa patients were diagnosed at a much earlier stage, with most of the patients having a PSA level under 20 ng/mL. In a clinical setting, patients diagnosed in Asian countries such as China and Japan are of an older age with a higher PSA level [39], [40]. In fact, with the joint efforts of participating urology centers, we confirmed the pattern of PSA distribution of patients who underwent biopsies in different Asian countries and further illustrated the characteristics of specific countries (Fig. 2).
Figure 2.
PSA distributions of patients who underwent biopsies in selected Asian countries and regions. Data sources: Mainland, China: Chinese Prostate Cancer Consortium (CPCC) statistics; Hong Kong, China: from HKU (Hong Kong University) and CUHK (The Chinese University of Hong Kong) university hospitals; Singapore: Robotic Transperineal Biopsy of Prostate Database of Singapore General Hospital; Indonesia: Database of Cipto Mangunkusumo Hospital, Faculty of Medicine University of Indonesia. Macau, China: PMID 18304459; Korea: PMID 18158028; India: PMID 25109719.
4.4. PCa detection rate in different PSA categories in different countries
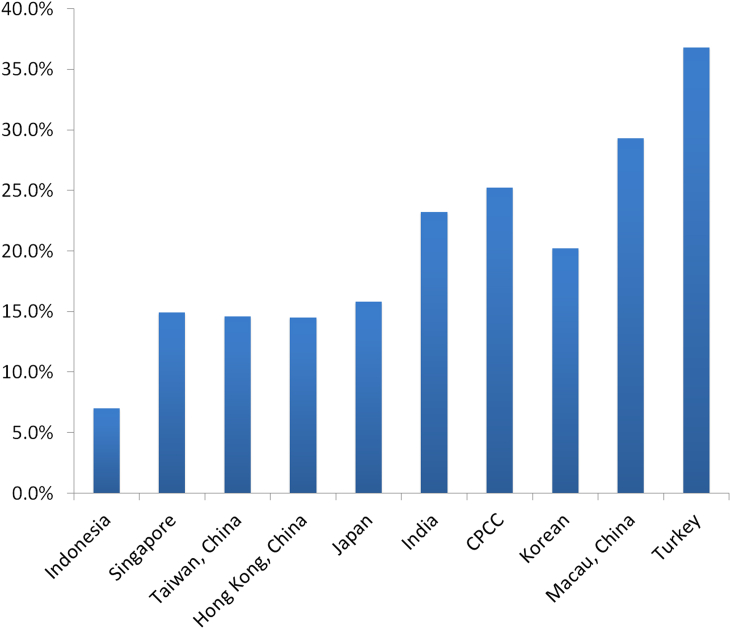
The relationship between detection rate of PCa and PSA levels has been reported to vary distinctively in different countries [41]. In the Asian population, the detection rate of PCa has been shown to be much lower in the diagnostic gray zone of PSA 4–10 ng/mL. Here, we illustrated the detection rate of PCa in this PSA range in different populations of Asia (Fig. 3).
Figure 3.
Prostate cancer detection rate of patients with different PSA levels in Asian countries and regions. Data sources: Indonesia: Hospital data, Cipto Mangunkusumo Hospital, Faculty of Medicine University of Indonesia. (2011–2013); Singapore: Robotic Transperineal Biopsy of Prostate Database of Singapore General Hospital; Taiwan, China: PMID 9610567; Hong Kong, China: from HKU (Hong Kong University) and CUHK (The Chinese University of Hong Kong) university hospitals; Japan: PMID 9861233; India: PMID 25109719; Korea: PMID 18158028; Macau, China: PMID 18304459; Turkey: Ferruh Zorlu et al., Prostate Cancer Incidence in Turkey: An Epidemiological Study, Accepted for publication in the Asian Pacific Journal of Cancer Prevention, Vol 15 (August 19, 2014).
As is shown, although the data sources vary in different countries, a distinct difference lies in the detection rate of PCa between Asians and the Western population [42]. The detection rate is below 25% in most reports except for Macau, China due to the limited number of cases and Turkey mostly Caucasian, which is why they are an exception. The differences in the detection rates indicated strong racial differences and provided further evidence against the universal pattern of using PSA as a screening tool in these populations.
Recently, it has been reported that genetic factors may play in role in the differences of PSA levels between Asians and Western populations. A GWAS in the Japanese population [43] illustrated that SNP rs16856139 is significantly associated with the levels of PSA in the Japanese population, yet the genetic differences in PSA levels between different populations are yet to be unveiled.
4.5. Tumor grade and stage at diagnosis
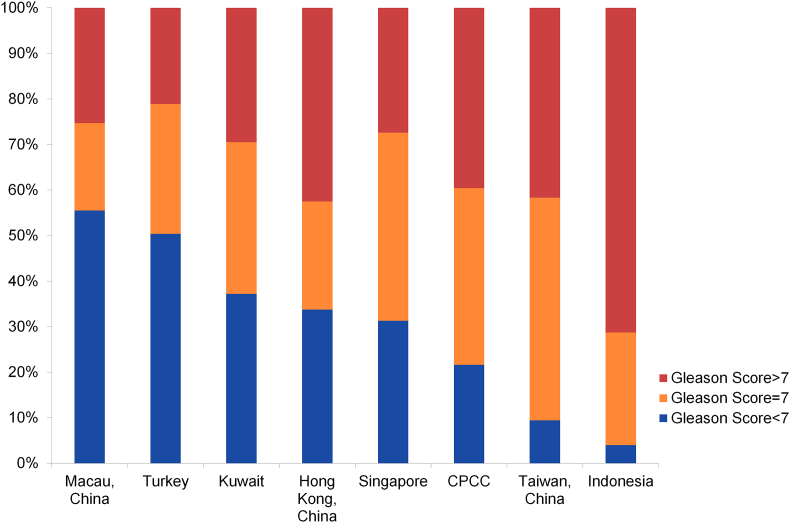
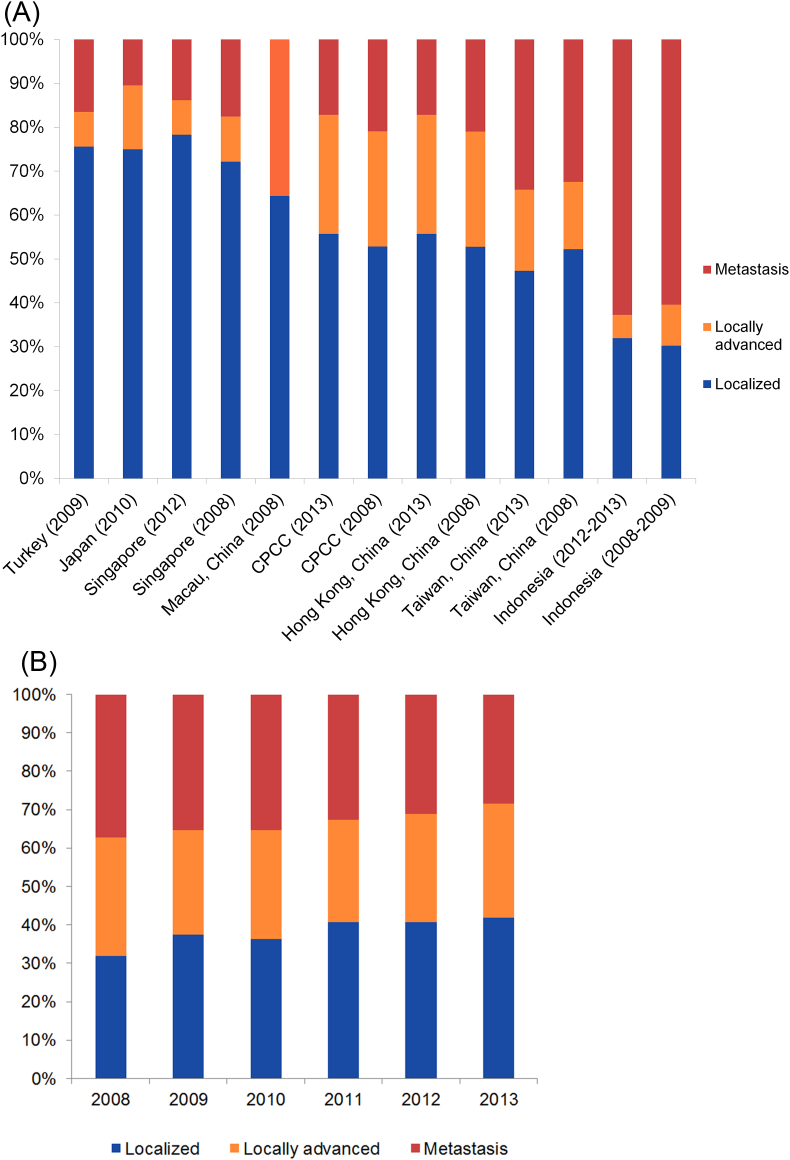
With the application of the PSA tests and PSA-based screening in Western countries, there has been a significant trend of stage migration and a lower Gleason Score in patients at the time of diagnosis. In Asian countries/regions with more developed economic and healthcare situations, there are more patients with early stages and favorable Gleason Scores (Japan, Korea, Singapore, etc.) (Figure 4, Figure 5). However, the majority of patients in China are still diagnosed with high-grade PCa (Gleason Score > 7). PCa of Asian populations generally display a high likelihood of advanced clinical stages compared to Western populations. Similar to that of Western countries, in developing counties of Asia such as China, there is also an emerging trend towards early stages at diagnosis (Fig. 5B). In regions with developed economic environment, such as Hong Kong, Turkey, and Singapore, the rate of localized PCa accounts for more than half of the diagnosed patients (Fig. 5A). However, there are more than 20% of patients diagnosed with metastatic disease in China and only about 40% patients were diagnosed with localized PCa who are favorable to undergo surgical treatments. However, this situation seems to change in recent years as more patients are diagnosed with localized disease in CPCC hospitals than before (Fig. 5B).
Figure 4.
Gleason score of prostate cancer patients at diagnosis in Asian countries and regions. Data sources: Macau, China: PMID 18304459; Turkey: Ferruh Zorlu et al., Prostate Cancer Incidence in Turkey: An Epidemiological Study, Accepted for publication in the Asian Pacific Journal of Cancer Prevention, Vol 15 (August 19, 2014); Kuwait: Database of Mubarak Al-Kabeer Teaching Hospital, Kuwait; Hong Kong, China: from HKU (Hong Kong University) and CUHK (The Chinese University of Hong Kong) university hospitals; Singapore: Cancer Registry Singapore General Hospital; Taiwan, China: PMID 17482934; Indonesia: Hospital data, Cipto Mangunkusumo Hospital, Faculty of Medicine University of Indonesia (2011–2013).
Figure 5.
Stages of diagnosed prostate cancer in (A) Asian countries and regions; (B) Chinese Prostate Cancer Consortium hospitals in past 6 years. Data sources: Turkey: Ferruh Zorlu et al., Prostate Cancer Incidence in Turkey: An Epidemiological Study, Accepted for publication in the Asian Pacific Journal of Cancer Prevention, Vol 15 (August 19, 2014); Japan: Jpn J Clin Oncol 2014 Aug 6. pii: hyu104. (Epub ahead of print); Singapore: Cancer Registry Singapore General Hospital; Macau, China: 55.1% for localized PCa, 30.5% for non-localized PCa and 14.4% not available. PMID 18304459; CPCC: CPCC member hospitals statistics; Hong Kong, China: from HKU (Hong Kong University) and CUHK (The Chinese University of Hong Kong) university hospitals; Taiwan, China: PMID 17482934; Indonesia: Hospital data, Cipto Mangunkusumo Hospital, Faculty of Medicine University of Indonesia. (2011–2013); Kuwait: Database of Mubarak Al-Kabeer Teaching Hospital, Kuwait.
4.6. Emerging novel biomarkers
Currently, a couple of novel biomarkers for PCa have been approved by the United States Food and Drug Administration (FDA). PCa antigen 3 (PCA3) is a long non-coding RNA that is highly expressed specifically in PCa tissue. It has been approved to help decision-making in repeat biopsies [44]. PSA isoform [-2]proPSA (p2psa) is an isoform of serum PSA with the ability to discriminate positive and negative biopsies. P2psa and its derivatives %p2psa and Prostate Health Index (PHI) have proven to be effective in discriminating PCa patients from negative biopsies [45]. Although both biomarkers have been validated in Caucasians, their effectiveness must be validated in Asian population before they can be put to clinical use.
The validation studies have been carried out in the Asian populations [44], [46], [47], [48], [49], [50], [51], [52], [53]. Although PCA3 and p2psa have achieved a comparable diagnostic accuracy with Western reports, their diagnostic accuracy is still limited in patients with PSA of 4–10 ng/mL. On the other hand, as the TMPRSSEGR fusion varies significantly among different races, the lesson it has taught us is that all these biomarkers may vary among ethnicities and there may be better biomarkers for Asian populations as well. In this regard, Asian urologists also performed some pilot studies (Table 3). Biomarkers including metastasis associated in lung adenocarcinoma transcript 1 (MALT-1) [54], genetic score based on SNPs [55], polypeptide neurotransmitter polypeptide neuropeptide-Y (NPY) [56] and serum zinc [57] have been tested as diagnostic and risk stratification biomarkers.
Table 3.
Validation of biomarkers found in the Western population and biomarker research in Asian countries and regions.
| Biomarker | Property | Indication | Ethnic | Study | Ref. | Cases | AUC | Cut-off | Sen. | Spe. |
|---|---|---|---|---|---|---|---|---|---|---|
| PCA3 | Urine long non-coding RNA | Repeat biopsy | Multicentric European | Haese et al., 2008 | [44] | 463 | 0.658 | 35.0 | 47.0 | 72.0 |
| Initial biopsy | Italian | Lazzeri et al., 2013 | [47] | 1026 | 0.733 | 1.7 | 70.4 | 70.1 | ||
| All biopsy | Japanese | Ochiai et al., 2011 | [48] | 105 | NA | 35.0 | 74.3 | 74.6 | ||
| Initial biopsy | Japanese | Ochiai et al., 2013 | [49] | 633 | 0.742 | 35.0 | 66.5 | 71.6 | ||
| Initial biopsy | Chinese (Hong Kong) | Ng et al., 2012 | [50] | 149 | NA | 35.0 | 92.0 | 71.0 | ||
| Initial biopsy | Chinese | Shen et al., 2010 | [51] | 102 | 0.786 | 0.107 (Real-Time PCR) | 62.9 | 90.6 | ||
| PHI | p2PSA/free PSA ± | Initial biopsy PSA 2.0–10.0 ng/mL | Italian | Guazzoni et al., 2011 | [45] | 268 | 0.76 | 48.5 | 90.0 | 42.9 |
| Initial biopsy PSA 2.0–10.0 ng/mL | Chinese | Na et al., 2013 | [52] | 636 | 0.73 | |||||
| Initial biopsy PSA 4–10 ng/mL | Chinese (Hong Kong) | Ng et al., 2014 | [53] | 230 | 0.781 | 26.5 | 90.0 | 45.2 | ||
| Initial biopsy PSA 2.0–10.0 ng/mL | Japanese | Ito et al., 2013 | [54] | 239 | NA | 23.9 | 90.0 | 28.0 | ||
| MALAT-1 | Plasma long non-coding RNA | Initial biopsy PSA 4–10 ng/mL | Chinese | Ren et al., 2013 | [55] | 192 | 0.767 | 867.8 | 43.5 | 81.6 |
| Genetic score | 13 PCa associated SNPs | Initial biopsy (All PSA range) | Chinese | Jiang et al., 2013 | [56] | 667 | 0.63 | 1.5 | 52.0 | 52.0 |
| NPY | Neurotransmitter polypeptide neuropeptide-Y |
Pilot study (All PSA range) | Japanese | Ueda et al., 2013 | [57] | 110 | 0.717 | 5189.0 | 41.5 | 97.8 |
| Serum zinc | Metal ion | Pilot study (PSA 4–10 ng/mL) | Chinese | Li et al., 2005 | [58] | 85 | 0.73 | 100.0 | 90.5 | 32.7 |
PCA3, prostate cancer antigen 3; PHI, prostate health index; Real-Time PCR, real-time polymerase chain reaction; PSA, prostate-specific antigen; MALAT-1, metastasis associated in lung adenocarcinoma transcript; SNPs, single nucleotide polymorphisms; p2PSA: [-2]proPSA; Ref., reference; AUC, area under the curve; Sen., sensitivity; Spe., specificity. NA: not available.
A fragment of MALAT-1, named MD-miniRNA, has proved to be stable in plasma. This fragment has been validated to differentiate PCa and non-PCa patient at a cut-off of 867.8 MD-miniRNA copies per microliter of plasma with the sensitivity of 56.6% and specificity of 84.8% in discriminating PCa patients from non-PCa patients. This is the first time anyone has been able to test fragments of long non-coding RNA in plasma to diagnose diseases. Recently, this approach to diagnose disease has also been validated in patients of gastric cancer [58], illustrating the viability of this approach. Other approaches in identifying novel biomarkers for Asian PCa detection included SNPs, polypeptide and other molecules with promising results [55], [56], [57]. However, research on biomarkers in Asian countries is still behind in both the number of studies and the quality of research compared with Western countries. Because of the heterogenetic nature of PCa, it is predictable that biomarker research will be on a rising trend in Asian population.
5. Surgery management of prostate cancer
5.1. Changes in approach of prostatectomy in Asia
Radical prostatectomy (RP) is a standard treatment option for patients with localized PCa. RP can be performed by either retropubic radical prostatectomy (RRP) or laparoscopic radical prostatectomy (LRP). Anatomic RRP was first performed by Professor Walsh in 1979 [59]. However at that time, few institutes in Asian countries focused their research on PCa. Even the first review of PCa in Japan was only published in 1983. In China, the first anatomic prostatectomy surgeries were performed around the middle of the 1990s.
LRP was first performed in 1992 and received more attention until reported by Schuessler et al. [60]. This time, Asian urologists caught up with this trend relatively faster. A search in the PubMed and CNKI (China National Knowledge Infrastructure) database indicated the first reported LRP from Japan and China was in 2001 and 2002, respectively [61], [62].
The first robotic-assisted radical prostatectomy (RALP) was performed in 2000 by Binder et al. from Frankfurt [63]. RALP was performed in Asian countries from as early as 2003 in Singapore and 2004 in Taiwan, China. In Mainland China, the first RALP was performed in 2007 by Gao et al. [64]. The time span between the application of RALP between Western and Asian urologists has been shorted to only a couple of years. From anatomic RRP to LRP and from LRP to RALP, it is clear that Asian urologists are catching up in surgery methods and the time span between the first cases has shortened.
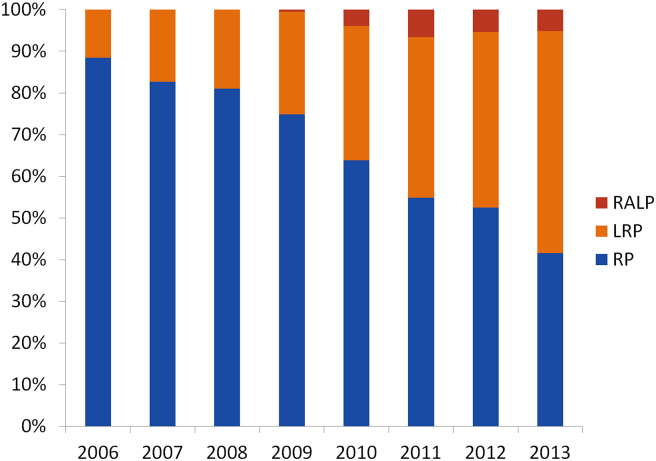
Once again with the collaboration of CPCC members, the current status of the percentage of each surgery approach has been summarized (Fig. 6). One other thing to note is that the data came from both a national level and the representative hospital (in case of failure to obtain a national figure). As is illustrated, the proportion of RRP is reduced whereas LRP takes the largest proportion in the 20 hospitals in CPCC. Although there is a substantial increase in the number of RALP, the proportion of RALP is still fairly low as only three out of 20 hospitals are equipped with da Vinci systems.
Figure 6.
Changes of prostatectomy type in Prostate Cancer Consortium hospitals from 2006 to 2013. Data sources: CPCC member hospitals data. RALP, robotic-assisted radical prostatectomy; LRP, laparoscopic radical prostatectomy; RRP, retropubic radical prostatectomy.
5.2. Current status of the effectiveness of prostatectomy in Asia
Although RP was adopted late in Asia and the number of cases is much smaller compared with Western countries, Asian surgeons have quickly adapted RP techniques. Asian reports of RP results indicated a comparable outcome in terms of preservation of both continence and sexual function to Western reports [65], [66]. In recent years, an increasing number of urologists are not satisfied with just completing the surgery itself but also aimed to achieve the trifecta of RP (cancer free, potency and continence). More approaches have been developed and validated to be viable. For instance, Single-port LRP was preferred by Wen et al. [67] and has been shown to achieve better results in selected patients. Extraperitoneal LRP was preferred by Zheng et al. [66] for its advantages in preventing intra-abdominal complications and combined the advantages of minimally invasive laparoscopy and retropubic open prostatectomy.
There are now several high-volume centers for RP with a well-designed follow-up system that makes the long-term assessment of the surgery result possible in developed countries such as Japan, Korea, Israel and developing countries such as China and India. For instance, Namiki et al. [68] reported the long-term follow-up data for 91 patients in a median follow-up of 102 months. Mishra et al. [69] reported the oncological outcome of LRP in a median follow-up of 6 years. Koo et al. [70] reported a comparison study of RALP and open radical prostatectomy in 1172 patients with a mean follow-up time of 58.4 months. Their result indicated that RALP is as good as open RP in terms of oncological outcomes. Considering the relatively large population in Asia, the number of RP cases is expected to increase fast and there would be more opportunities for urologists to investigate the unique characteristics of RP in Asian men with its own anatomical characteristics.
Furthermore, urologists in Asia also developed some innovative work in RP. Gao et al. [71] performed the first series of single-port transvesical LRP with promising functional outcomes regarding continence and potency. Another innovative approach in RALP is that Korean urologists reported the comparison of Retzius-sparing RALP with conventional RALP [72]. Retzius-sparing RALP was considered as not practical in the past, however, Lim et al. [72] confirmed it would reduce operating time and help to achieve a faster recovery of early continence.
However, it must be recognized that the advances in RP are still limited in Asia when compared with developed Western countries, regardless of the number of cases and techniques. In 2013, the 10th European Conference in Laparoscopic Robotic Surgery in Urology was held in Beijing, China. Well-organized academic meetings would facilitate the communication between urologists in Asia and other countries.
5.3. Trend of robotic-assisted radical prostatectomy in Asia
In recent years, RALP has gained popularity globally with advantages to both patients and urologists. The benefit for patients includes less blood loss, less pain and shorter hospital stay. The benefits for urologists are improved ergonomics, with accurate three-dimensional display, and the ease of use of maneuverable robotic instruments [73].
The literature has shown that RALP had a short learning curve and promising postoperative results, especially with regard to continence recovery [73]. RP is the most common robotically assisted surgical procedure performed in the United States. Reported 5-year biochemical failure-free survival for RALP ranges from 83.3% to 87%, which is better than open RP [73].
The major downside of RALP is the cost. In an ideal scenario, most urologists would be inclined to use this novel technique, but the reality is that the high cost of RALP is often hard to accept by patients in developing countries in Asia, especially when the governmental insurance covers a small or no proportion of the costs of surgery. As is illustrated, the number of da Vinci systems is surging in developed countries such as Japan and Korea but are difficult to find in most other Asian countries at the moment (Table 4). RALP is starting to take its momentum in Mainland China, Turkey and Taiwan (China) in recent years, while Japan has already out-weighted the total number of other Asian countries (Data source: Intuitive Surgical Inc., Shanghai, China). It is predicted that the number of RALP will likely keep this momentum with increases in number of cases in existing centers and the establishment of new da Vinci centers.
Table 4.
Cumulative number of da Vinci Systems in Asian countries and regions.
| Country | 2010 | 2011 | 2012 | 2013 |
|---|---|---|---|---|
| Japan | 32 | 40 | 80 | 159 |
| Korea | 28 | 36 | 38 | 44 |
| Mainland, China | 10 | 13 | 15 | 18 |
| Taiwan, China | 4 | 10 | 15 | 20 |
| India | 6 | 17 | 19 | 20 |
| Hong Kong, China | 7 | 7 | 8 | 8 |
| Singapore | 3 | 6 | 7 | 7 |
| Malaysia | 1 | 3 | 3 | 3 |
| Thailand | 0 | 1 | 4 | 6 |
| Philippines | 0 | 1 | 1 | 2 |
| Indonesia | 0 | 0 | 1 | 1 |
| Total | 108 | 134 | 191 | 288 |
Data sources: Intuitive Surgical Inc., Shanghai, China.
6. Novel agents for CRPC
The prognosis of CRPC patients is rather unfavorable with an overall survival time of 24–36 months [74]. Prior to 2010, the treatment options for CRPC were limited. In the past 3 years, US FDA approved five new agents for CRPC (Table 5). These agents not only expanded the arsenal against CRPC but also enlightened the hope for patients with advanced prostate cancer. Currently, all of these agents are recommended by the AUA guidelines for PCa in different conditions. In Asian countries, with the joint efforts of manufacturers and clinical oncologists, clinical trials have been started in recent years (Fig. 7). Hopefully, with these new agents, patients will have a longer progression-free survival as well as an improved overall rate of survival. However, as there are two sides to every sword, the emergence of these new drugs are beneficial to patients with proper indications but may not always be helpful to the developing Asian countries that already have limited budget for cancer control. In this aspect, both the manufacture and the healthcare ministry of these countries should make individualized policies for each country in order to achieve a win–win situation for all. Currently, there is no leading innovative pharmaceutical research group in Asian countries that could pave the way forward for novel CRPC agents.
Table 5.
Newly FDA-approved agents for CRPC in Asia.
| Agent | Mechanism | FDA approval | Clinical trials in Asia | Clinical trials in USA | Availability on market |
|---|---|---|---|---|---|
| Enzalutamide | Androgen receptor inhibitor | 2012 | 11 | 48 | Korea, Hong Kong, Japan |
| Abiraterone | Androgen biosynthesis inhibitor | 2011 | 20 | 74 | Singapore, Hong Kong, India, Indonesia, Israel, Japan, Jordan, Korea, Kuwait, Malaysia, Saudi Arabia, Taiwan, Turkey |
| Cabazitaxel | Novel taxane chemotherapy | 2010 | 12 | 20 | Singapore, Japan, Turkey, Taiwan, Indonesia |
| Sipuleucel-T | Autologous immunotherapeutic agent | 2010 | 0 | 26 | NA |
| Radium 223 | Alpha particle-emitting radiopharmaceutical | 2013 | 11 | 13 | Korea, Singapore |
Data sources: www.clinicaltrials.gov, searched on Aug 31, 2014.
Figure 7.
Number of completed and on-going clinical trials of five novel agents for CRPC worldwide. Data sources: http://www.clinicaltrials.gov/, searched on Aug 31, 2014.
7. Prospective
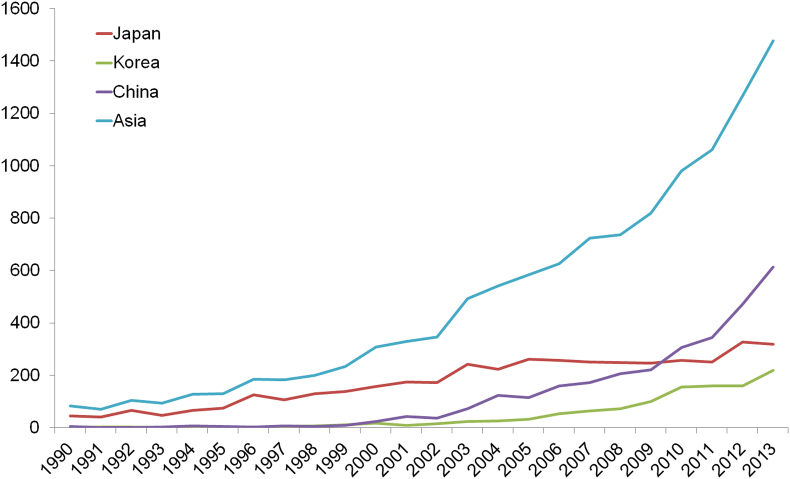
As incidence and mortality rates have continued to increase during the past decades and a trend towards an older population as well as a more Western lifestyle, PCa has now become one of the most prevalent malignancies in Asian countries. A recent autopsy study revealed that incidence of PCa is rather similar in Japanese and Russians [75]. Thus, if the Western strategy of management of PCa is introduced in Asia, it is possible that many more patients will be diagnosed with PCa in Asia. With joint efforts from urologists and researchers across Asia, research of PCa has been surging with more clinical and research articles being published (Fig. 8). With such scientific developments, the management of PCa in diagnosis and treatment has also been improving greatly. It is expected that Asian researchers and urologists will gain a better understanding of prostate cancer in their own countries and find better ways to combat this disease uniquely.
Figure 8.
Prostate-cancer-related research papers by countries. Data sources: http://www.ncbi.nlm.nih.gov/pubmed, searched on Aug 31, 2014.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgment
This work was supported by the Program for Changjiang Scholars and Innovative Research Team in University scheme of the Ministry of Education of China (NO.IRT1111), the National Basic Research Program of China (2012CB518300), the National Natural Science Foundation of China (81101946), the Shanghai Pujiang Program (12PJD008), Prostate Cancer Foundation Young Investigator Award, Shanghai Municipal Health and Family Planning Commission Outstanding Young Investigator (XYQ2013077).
Footnotes
Peer review under responsibility of Chinese Urological Association and SMMU.
References
- 1.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2013. CA: Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Center M.M., Jemal A., Lortet-Tieulent J., Ward E., Ferlay J., Brawley O. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J.S.I., Ervik M., Dikshit R., Eser S., Mathers C., Rebelo M. International Agency for Research on Cancer; Lyon, France: 2013. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase No. 11.http://globocan.iarc.fr [Internet] Available from: [accessed 28.08.14] [Google Scholar]
- 4.Pramesh C.S., Badwe R.A., Borthakur B.B., Chandra M., Raj E.H., Kannan T. Delivery of affordable and equitable cancer care in India. Lancet Oncol. 2014;15:e223–e233. doi: 10.1016/S1470-2045(14)70117-2. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA: Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 6.Ito K. Prostate cancer in Asian men. Nat Rev Urol. 2014;11:197–212. doi: 10.1038/nrurol.2014.42. [DOI] [PubMed] [Google Scholar]
- 7.He J.C.W. China Military Medical Science Press; Beijing: 2013. Chinese cancer registry annual report 2012. [Google Scholar]
- 8.Jung K.W., Won Y.J., Kong H.J., Oh C.M., Lee D.H., Lee J.S. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2011. Cancer Res Treat. 2014;46:109–123. doi: 10.4143/crt.2014.46.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magi-Galluzzi C., Tsusuki T., Elson P., Simmerman K., LaFargue C., Esgueva R. TMPRSS2-ERG gene fusion prevalence and class are significantly different in prostate cancer of Caucasian, African-American and Japanese patients. Prostate. 2011;71:489–497. doi: 10.1002/pros.21265. [DOI] [PubMed] [Google Scholar]
- 10.Ren S., Peng Z., Mao J.H., Yu Y., Yin C., Gao X. RNA-seq analysis of prostate cancer in the Chinese population identifies recurrent gene fusions, cancer-associated long noncoding RNAs and aberrant alternative splicings. Cell Res. 2012;22:806–821. doi: 10.1038/cr.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin M.A., Maher C.A., Chinnaiyan A.M. Common gene rearrangements in prostate cancer. J Clin Oncol. 2011;29:3659–3668. doi: 10.1200/JCO.2011.35.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leinonen K.A., Saramaki O.R., Furusato B., Kimura T., Takahashi H., Egawa S. Loss of PTEN is associated with aggressive behavior in ERG-positive prostate cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:2333–2444. doi: 10.1158/1055-9965.EPI-13-0333-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konishi N., Hiasa Y., Tsuzuki T., Tao M., Enomoto T., Miller G.J. Comparison of ras activation in prostate carcinoma in Japanese and American men. Prostate. 1997;30:53–57. doi: 10.1002/(sici)1097-0045(19970101)30:1<53::aid-pros8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 14.Ren G., Liu X., Mao X., Zhang Y., Stankiewicz E., Hylands L. Identification of frequent BRAF copy number gain and alterations of RAF genes in Chinese prostate cancer. Genes Chromosom Cancer. 2012;51:1014–1023. doi: 10.1002/gcc.21984. [DOI] [PubMed] [Google Scholar]
- 15.Eeles R.A., Olama A.A., Benlloch S., Saunders E.J., Leongamornlert D.A., Tymrakiewicz M. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet. 2013;45:385–391. doi: 10.1038/ng.2560. 91e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takata R., Akamatsu S., Kubo M., Takahashi A., Hosono N., Kawaguchi T. Genome-wide association study identifies five new susceptibility loci for prostate cancer in the Japanese population. Nat Genet. 2010;42:751–754. doi: 10.1038/ng.635. [DOI] [PubMed] [Google Scholar]
- 17.Akamatsu S., Takata R., Haiman C.A., Takahashi A., Inoue T., Kubo M. Common variants at 11q12, 10q26 and 3p11.2 are associated with prostate cancer susceptibility in Japanese. Nat Genet. 2012;44:426–429. doi: 10.1038/ng.1104. S1. [DOI] [PubMed] [Google Scholar]
- 18.Xu J., Mo Z., Ye D., Wang M., Liu F., Jin G. Genome-wide association study in Chinese men identifies two new prostate cancer risk loci at 9q31.2 and 19q13.4. Nat Genet. 2012;44:1231–1235. doi: 10.1038/ng.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shan J., Al-Rumaihi K., Rabah D., Al-Bozom I., Kizhakayil D., Farhat K. Genome scan study of prostate cancer in Arabs: identification of three genomic regions with multiple prostate cancer susceptibility loci in Tunisians. J Transl Med. 2013;11:121. doi: 10.1186/1479-5876-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nam K.C., Jo C., Lee M. Meat products and consumption culture in the East. Meat Sci. 2010;86:95–102. doi: 10.1016/j.meatsci.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Rastogi T., Devesa S., Mangtani P., Mathew A., Cooper N., Kao R. Cancer incidence rates among South Asians in four geographic regions: India, Singapore, UK and US. Int J Epidemiol. 2008;37:147–160. doi: 10.1093/ije/dym219. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J., Dhakal I.B., Zhao Z., Li L. Trends in mortality from cancers of the breast, colon, prostate, esophagus, and stomach in East Asia: role of nutrition transition. Eur J Cancer Prev. 2012;21:480–489. doi: 10.1097/CEJ.0b013e328351c732. [DOI] [PubMed] [Google Scholar]
- 23.Andriole G.L., Crawford E.D., Grubb R.L., 3rd, Buys S.S., Chia D., Church T.R. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroder F.H., Hugosson J., Roobol M.J., Tammela T.L., Ciatto S., Nelen V. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z.Y., Sun Y.H., Xu C.L., Gao X., Zhang L.M., Ren S.C. Age-specific PSA reference ranges in Chinese men without prostate cancer. Asian J Androl. 2009;11:100–103. doi: 10.1038/aja.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim J., Bhoo-Pathy N., Sothilingam S., Malek R., Sundram M., Hisham Bahadzor B. Ethnicity is an independent determinant of age-specific PSA level: findings from a multiethnic Asian setting. PLoS One. 2014;9:e104917. doi: 10.1371/journal.pone.0104917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin T.P., Huang W.J., Chen K.K. Differentiation of benign prostatic hyperplasia from prostate cancer using prostate specific antigen dynamic profile after transrectal prostate biopsy. J Urol. 2004;171:2226–2229. doi: 10.1097/01.ju.0000123988.27122.cb. [DOI] [PubMed] [Google Scholar]
- 28.Oesterling J.E., Jacobsen S.J., Chute C.G., Guess H.A., Girman C.J., Panser L.A. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of age-specific reference ranges. JAMA. 1993;270:860–864. [PubMed] [Google Scholar]
- 29.Wu T.T., Huang J.K. The clinical usefulness of prostate-specific antigen (PSA) level and age-specific PSA reference ranges for detecting prostate cancer in Chinese. Urol Int. 2004;72:208–211. doi: 10.1159/000077116. [DOI] [PubMed] [Google Scholar]
- 30.Choi Y.D., Kang D.R., Nam C.M., Kim Y.S., Cho S.Y., Kim S.J. Age-specific prostate-specific antigen reference ranges in Korean men. Urology. 2007;70:1113–1116. doi: 10.1016/j.urology.2007.07.063. [DOI] [PubMed] [Google Scholar]
- 31.Oesterling J.E., Kumamoto Y., Tsukamoto T., Girman C.J., Guess H.A., Masumori N. Serum prostate-specific antigen in a community-based population of healthy Japanese men: lower values than for similarly aged white men. Br J Urol. 1995;75:347–353. doi: 10.1111/j.1464-410x.1995.tb07347.x. [DOI] [PubMed] [Google Scholar]
- 32.Imai K., Ichinose Y., Kubota Y., Yamanaka H., Sato J. Diagnostic significance of prostate specific antigen and the development of a mass screening system for prostate cancer. J Urol. 1995;154:1085–1089. [PubMed] [Google Scholar]
- 33.Malati T., Kumari G.R. Racial and ethnic variation of PSA in global population: age specific reference intervals for serum prostate specific antigen in healthy South Indian males. Indian J Clin Biochem. 2004;19:132–137. doi: 10.1007/BF02872408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdrabo A.A., Fadlalla A.I., Fadl-Elmula I.M. Age-specific reference range for serum prostate-specific antigen in Sudanese men. Saudi Med J. 2011;32:930–932. [PubMed] [Google Scholar]
- 35.Kamal B.A., Ali G.A., Taha S.A. Prostate specific antigen reference ranges in Saudi men. Saudi Med J. 2003;24:665–668. [PubMed] [Google Scholar]
- 36.Saw S., Aw T.C. Age-related reference intervals for free and total prostate-specific antigen in a Singaporean population. Pathology. 2000;32:245–249. [PubMed] [Google Scholar]
- 37.Kehinde E.O., Mojiminiyi O.A., Sheikh M., Al-Awadi K.A., Daar A.S., Al-Hunayan A. Age-specific reference levels of serum prostate-specific antigen and prostate volume in healthy Arab men. BJU Int. 2005;96:308–312. doi: 10.1111/j.1464-410X.2005.05620.x. [DOI] [PubMed] [Google Scholar]
- 38.Khezri A.A., Shirazi M., Ayatollahi S.M., Lotfi M., Askarian M., Ariafar A. Age specific reference levels of serum prostate-specific antigen, prostate volume and prostate specific antigen density in healthy Iranian men. Iran J Immunol. 2009;6:40–48. [PubMed] [Google Scholar]
- 39.Takahashi H., Epstein J.I., Wakui S., Yamamoto T., Furusato B., Zhang M. Differences in prostate cancer grade, stage, and location in radical prostatectomy specimens from United States and Japan. Prostate. 2014;74:321–325. doi: 10.1002/pros.22754. [DOI] [PubMed] [Google Scholar]
- 40.Peyromaure M., Debre B., Mao K., Zhang G., Wang Y., Sun Z. Management of prostate cancer in China: a multicenter report of 6 institutions. J Urol. 2005;174:1794–1797. doi: 10.1097/01.ju.0000176817.46279.93. [DOI] [PubMed] [Google Scholar]
- 41.Vickers A.J., Cronin A.M., Roobol M.J., Hugosson J., Jones J.S., Kattan M.W. The relationship between prostate-specific antigen and prostate cancer risk: the Prostate Biopsy Collaborative Group. Clin Cancer Res. 2010;16:4374–4381. doi: 10.1158/1078-0432.CCR-10-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimura T. East meets west: ethnic differences in prostate cancer epidemiology between East Asians and Caucasians. Chin J Cancer. 2012;31:421–429. doi: 10.5732/cjc.011.10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terao C., Terada N., Matsuo K., Kawaguchi T., Yoshimura K., Hayashi N. A genome-wide association study of serum levels of prostate-specific antigen in the Japanese population. J Med Genet. 2014;51:530–536. doi: 10.1136/jmedgenet-2014-102423. [DOI] [PubMed] [Google Scholar]
- 44.Haese A., de la Taille A., van Poppel H., Marberger M., Stenzl A., Mulders P.F. Clinical utility of the PCA3 urine assay in European men scheduled for repeat biopsy. Eur Urol. 2008;54:1081–1088. doi: 10.1016/j.eururo.2008.06.071. [DOI] [PubMed] [Google Scholar]
- 45.Guazzoni G., Nava L., Lazzeri M., Scattoni V., Lughezzani G., Maccagnano C. Prostate-specific antigen (PSA) isoform p2PSA significantly improves the prediction of prostate cancer at initial extended prostate biopsies in patients with total PSA between 2.0 and 10 ng/ml: results of a prospective study in a clinical setting. Eur Urol. 2011;60:214–222. doi: 10.1016/j.eururo.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 46.Na R., Ye D., Liu F., Chen H., Qi J., Wu Y. Performance of serum prostate-specific antigen isoform [-2]proPSA (p2PSA) and the prostate health index (PHI) in a Chinese hospital-based biopsy population. Prostate. 2014;74:1569–1575. doi: 10.1002/pros.22876. [DOI] [PubMed] [Google Scholar]
- 47.Lazzeri M., Haese A., Abrate A., de la Taille A., Redorta J.P., McNicholas T. Clinical performance of serum prostate-specific antigen isoform [-2]proPSA (p2PSA) and its derivatives, %p2PSA and the prostate health index (PHI), in men with a family history of prostate cancer: results from a multicentre European study, the PROMEtheuS project. BJU Int. 2013;112:313–321. doi: 10.1111/bju.12217. [DOI] [PubMed] [Google Scholar]
- 48.Ochiai A., Okihara K., Kamoi K., Iwata T., Kawauchi A., Miki T. Prostate cancer gene 3 urine assay for prostate cancer in Japanese men undergoing prostate biopsy. Int J Urol. 2011;18:200–205. doi: 10.1111/j.1442-2042.2010.02711.x. [DOI] [PubMed] [Google Scholar]
- 49.Ochiai A., Okihara K., Kamoi K., Oikawa T., Shimazui T., Murayama S. Clinical utility of the prostate cancer gene 3 (PCA3) urine assay in Japanese men undergoing prostate biopsy. BJU Int. 2013;111:928–933. doi: 10.1111/j.1464-410X.2012.11683.x. [DOI] [PubMed] [Google Scholar]
- 50.Ng C.F., Yeung R., Chiu P.K., Lam N.Y., Chow J., Chan B. The role of urine prostate cancer antigen 3 mRNA levels in the diagnosis of prostate cancer among Hong Kong Chinese patients. Hong Kong Med J. 2012;18:459–465. [PubMed] [Google Scholar]
- 51.Shen M., Chen W., Yu K., Chen Z., Zhou W., Lin X. The diagnostic value of PCA3 gene-based analysis of urine sediments after digital rectal examination for prostate cancer in a Chinese population. Exp Mol Pathol. 2011;90:97–100. doi: 10.1016/j.yexmp.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Ng C.F., Chiu P.K., Lam N.Y., Lam H.C., Lee K.W., Hou S.S. The Prostate Health Index in predicting initial prostate biopsy outcomes in Asian men with prostate-specific antigen levels of 4-10 ng/mL. Int Urol Nephrol. 2014;46:711–717. doi: 10.1007/s11255-013-0582-0. [DOI] [PubMed] [Google Scholar]
- 53.Ito K., Miyakubo M., Sekine Y., Koike H., Matsui H., Shibata Y. Diagnostic significance of [-2]pro-PSA and prostate dimension-adjusted PSA-related indices in men with total PSA in the 2.0–10.0 ng/mL range. World J Urol. 2013;31:305–311. doi: 10.1007/s00345-012-0927-9. [DOI] [PubMed] [Google Scholar]
- 54.Ren S., Wang F., Shen J., Sun Y., Xu W., Lu J. Long non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 derived miniRNA as a novel plasma-based biomarker for diagnosing prostate cancer. Eur J Cancer. 2013;49:2949–2959. doi: 10.1016/j.ejca.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 55.Jiang H., Liu F., Wang Z., Na R., Zhang L., Wu Y. Prediction of prostate cancer from prostate biopsy in Chinese men using a genetic score derived from 24 prostate cancer risk-associated SNPs. Prostate. 2013;73:1651–1659. doi: 10.1002/pros.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ueda K., Tatsuguchi A., Saichi N., Toyama A., Tamura K., Furihata M. Plasma low-molecular-weight proteome profiling identified neuropeptide-Y as a prostate cancer biomarker polypeptide. J Proteome Res. 2013;12:4497–4506. doi: 10.1021/pr400547s. [DOI] [PubMed] [Google Scholar]
- 57.Li X.M., Zhang L., Li J., Li Y., Wang H.L., Ji G.Y. Measurement of serum zinc improves prostate cancer detection efficiency in patients with PSA levels between 4 ng/mL and 10 ng/mL. Asian J Androl. 2005;7:323–328. doi: 10.1111/j.1745-7262.2005.00044.x. [DOI] [PubMed] [Google Scholar]
- 58.Arita T., Ichikawa D., Konishi H., Komatsu S., Shiozaki A., Shoda K. Circulating long non-coding RNAs in plasma of patients with gastric cancer. Anticancer Res. 2013;33:3185–3193. [PubMed] [Google Scholar]
- 59.Reiner W.G., Walsh P.C. An anatomical approach to the surgical management of the dorsal vein and Santorini's plexus during radical retropubic surgery. J Urol. 1979;121:198–200. doi: 10.1016/s0022-5347(17)56718-x. [DOI] [PubMed] [Google Scholar]
- 60.Schuessler W.W., Schulam P.G., Clayman R.V., Kavoussi L.R. Laparoscopic radical prostatectomy: initial short-term experience. Urology. 1997;50:854–857. doi: 10.1016/S0090-4295(97)00543-8. [DOI] [PubMed] [Google Scholar]
- 61.Gao X., Qiu J.G., Wang J.G. Laparoscopic radical prostatectomy: report of the first case. Chin J Urol. 2002;23:59. [Google Scholar]
- 62.Kawabata G., Hara I., Hara S., Isotani S., Sakai Y., Wada Y. Laparoscopic radical prostatectomy: initial 17 case report. Nihon Hinyokika Gakkai zasshi. 2001;92:647–655. doi: 10.5980/jpnjurol1989.92.647. [DOI] [PubMed] [Google Scholar]
- 63.Wolfram M., Brautigam R., Engl T., Bentas W., Heitkamp S., Ostwald M. Robotic-assisted laparoscopic radical prostatectomy: the Frankfurt technique. World J Urol. 2003;21:128–132. doi: 10.1007/s00345-003-0346-z. [DOI] [PubMed] [Google Scholar]
- 64.Wang W., Gao J., Xu X., Dong J., Zhu J. Proceedings of the 16th Chinese Urology Association Annual Meeting, Chengdu, China. Sep 18th, 2009. Extraperitoneal robot-assisted laparoscopic radical prostatectomy. [Google Scholar]
- 65.Chen M.K., Luo Y., Zhang H., Qiu J.G., Wen X.Q., Pang J. Laparoscopic radical prostatectomy plus extended lymph nodes dissection for cases with non-extra node metastatic prostate cancer: 5-year experience in a single Chinese institution. J Cancer Res Clin Oncol. 2013;139:871–878. doi: 10.1007/s00432-013-1395-3. [DOI] [PubMed] [Google Scholar]
- 66.Zheng T., Zhang X., Ma X., Li H.Z., Gao J.P., Cai W. Oncological and functional results of extraperitoneal laparoscopic radical prostatectomy. Oncol Lett. 2012;4:351–357. doi: 10.3892/ol.2012.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wen X.Q., Huang W.T., Situ J., Hu C., Ye C.W., Gao X. Single-port laparoscopic radical prostatectomy: initial experience and technical points to reduce its difficulties. Chin Med J. 2011;124:4092–4095. [PubMed] [Google Scholar]
- 68.Namiki S., Kaiho Y., Mitsuzuka K., Saito H., Yamada S., Nakagawa H. Long-term quality of life after radical prostatectomy: 8-year longitudinal study in Japan. Int J Urol. 2014;21:1220–1226. doi: 10.1111/iju.12586. [DOI] [PubMed] [Google Scholar]
- 69.Mishra S., Agrawal V., Khatri N., Sharma R., Kurien A., Ganpule A. Laparoscopic radical prostatectomy: oncological outcome analysis from a single-center Indian experience of 6 years. Indian J Urol. 2012;28:32–36. doi: 10.4103/0970-1591.94953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koo K.C., Tuliao P., Yoon Y.E., Chung B.H., Hong S.J., Yang S.C. Robot-assisted radical prostatectomy in the Korean population: a 5-year propensity-score matched comparative analysis versus open radical prostatectomy. Int J Urol. 2014;21:781–785. doi: 10.1111/iju.12447. [DOI] [PubMed] [Google Scholar]
- 71.Gao X., Pang J., Si-tu J., Luo Y., Zhang H., Li L.Y. Single-port transvesical laparoscopic radical prostatectomy for organ-confined prostate cancer: technique and outcomes. BJU Int. 2013;112:944–952. doi: 10.1111/bju.12225. [DOI] [PubMed] [Google Scholar]
- 72.Lim S.K., Kim K.H., Shin T.Y., Han W.K., Chung B.H., Hong S.J. Retzius-sparing robot-assisted laparoscopic radical prostatectomy: combining the best of retropubic and perineal approaches. BJU Int. 2014;114:236–244. doi: 10.1111/bju.12705. [DOI] [PubMed] [Google Scholar]
- 73.Ficarra V., Cavalleri S., Novara G., Aragona M., Artibani W. Evidence from robot-assisted laparoscopic radical prostatectomy: a systematic review. Eur Urol. 2007;51:45–56. doi: 10.1016/j.eururo.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 74.Denis L.J. Staging and prognosis of prostate cancer. Eur Urol. 1993;24(Suppl. 2):13–18. doi: 10.1159/000474381. [DOI] [PubMed] [Google Scholar]
- 75.Zlotta A.R., Egawa S., Pushkar D., Govorov A., Kimura T., Kido M. Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. J Natl Cancer Inst. 2013;105:1050–1058. doi: 10.1093/jnci/djt151. [DOI] [PubMed] [Google Scholar]