Abstract
Background
Polyneuropathies (peripheral neuropathies) are the most common type of disorder of the peripheral nervous system in adults, and specifically in the elderly, with an estimated prevalence of 5–8%, depending on age. The options for treatment depend on the cause, which should therefore be identified as precisely as possible by an appropriate diagnostic evaluation.
Methods
This review is based on the current guidelines and on large-scale cohort studies and randomized, controlled trials published from 2000 to 2017, with an emphasis on non-hereditary types of polyneuropathy, that were retrieved by a selective search in PubMed.
Results
Diabetes is the most common cause of polyneuropathy in Europe and North America. Alcohol-associated polyneuropathy has a prevalence of 22–66% among persons with chronic alcoholism. Because of the increasing prevalence of malignant disease and the use of new chemotherapeutic drugs, chemotherapy-induced neuropathies (CIN) have gained in clinical importance; their prevalence is often stated to be 30–40%, with high variation depending on the drug(s) and treatment regimen used. Polyneuropathy can also arise from genetic causes or as a consequence of vitamin deficiency or overdose, exposure to toxic substances and drugs, and a variety of immunological processes. About half of all cases of polyneuropathy are associated with pain. Neuropathic pain can be treated symptomatically with medication. Exercise, physiotherapy, and ergotherapy can also be beneficial, depending on the patient’s symptoms and functional deficits.
Conclusion
A timely diagnosis of the cause of polyneuropathy is a prerequisite for the initiation of appropriate specific treatment. Patients with severe neuropathy of unidentified cause should be referred to a specialized center for a thorough diagnostic evaluation.
Etiology, Diagnosis, and Treatment Options
Polyneuropathies (PNP) are generalized disorders of the peripheral nervous system. With a prevalence of approximately 5%–8%, they represent the commonest disorder in this disease group (1). Since these diseases can have a multitude of etiologies and concomitant disorders, virtually all medical specialties come into contact with polyneuropathy patients.
Methods
This article is based on a selective literature search in PubMed. Publications between 2000 and 2017 with the search terms “neuropathy,” “polyneuropathy,” “diabetic neuropathy,” “alcoholic neuropathy,” “chemotherapy induced neuropathy,” “chronic inflammatory demyelinating polyneuropathy,” “vasculitic neuropathy” were used. Current German and European guidelines were also included. Hereditary neuropathies are dealt with separately in the article by Eggermann et al. (e1) in this issue of Deutsches Ärzteblatt International.
Clinical presentation and diagnostic work-up
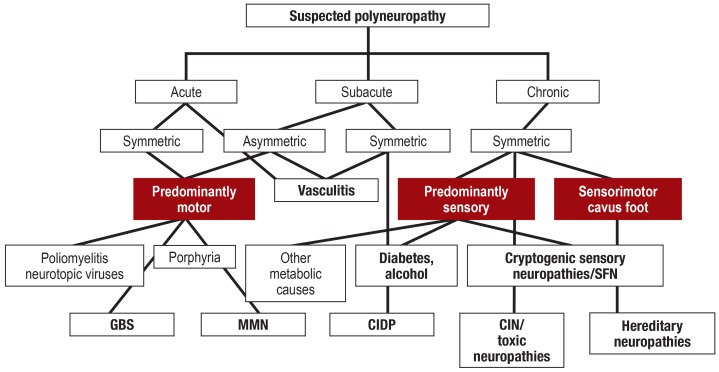
Distal symmetric sensorimotor syndrome is the clinical presentation most frequently seen. A distinction needs to made between polyradiculoneuropathy with proximal and distal involvement of the trunk and cranial nerves and asymmetric mononeuropathy multiplex, in which different nerves are affected simultaneously or sequentially. The main clinical symptoms (table 1) (2) are often key to diagnosis (figure). Depending on the type of nerve fiber involved, either sensory, motor, or autonomic symptoms may form the main focus, whereby a further distinction is then made between negative symptoms, such as paresis or sensory impairment, and positive symptoms such as fasciculations, muscle cramps, or pain
Table 1. Basic diagnostic methods and main clinical symptoms in polyneuropathy (selected from [40, e15]).
| Patient history: personal, system, occupational, social, and family history | |||
| Main symptoms and clinical findings | Symptoms | Clinical findings* | |
| Sensory | Sensation of furriness and numbness | Hypesthesia for various qualities, hypalgesia | |
| Tingling, burning, and cold parasthesia | Heat and cold allodynia | ||
| Burning pain, stinging, electric shock-like pain | Dysesthesia, allodynia | ||
| Gait instability, falls | Sensory ataxia | ||
| Motor | Weakness, muscle loss | Paresis, reduced muscle tone, muscle atrophy, reduced reflexes | |
| Muscle cramps, fasciculations | Muscle cramps on strength testing, fasciculations | ||
| Autonomic | Dry skin | Hypo- and anhidrosis | |
| Body hair loss, skin changes | Trophic disorders | ||
| Sensation of glare | |||
| Bladder dysfunction | |||
| Diarrhea | |||
| Rapid heartbeat | For example, resting tachycardia | ||
| Gastrointestinal symptoms | For example, gastroparesis | ||
| Urogenital symptoms (e.g., impaired micturitionerectile dysfunction) | |||
| Neurophysiology: Neurography and EMG, evoked potential | |||
| Laboratory tests | Basic program | Optional advanced program | |
| CRP, differential blood count, electrolytes, liver and kidney function, protein electrophoresis, immunofixation, ‧Bence Jones proteins, TSH, HbA1c, CDT, vitamin B12 | Holotranscobalamin; vitamins B1, B6 and E, ANA, p- and c-ANCA, cryoglobulins, hepatitis/HIV/Borrelia serology, anti-IgM-GM1, anti-GQ1b, anti-MAG; cerebrospinal fluid analysis, including bacterial and viral serology | ||
| Imaging | Nerve ultrasound and MR neurography | ||
| Biopsy | Nerve biopsy, skin biopsy | ||
| Small-fiber diagnostic methods | Quantitative sensory testing, special evoked potentials, skin biopsy | ||
| Genetics | PMP22, GJB1, MPZ, and MFN2, gene panel, trio exome/genome | ||
On neurological examination, the combination of distal reflex loss and reduced vibration or pinprick sensitivity is a sensitive and specific clinical sign in the diagnosis of polyneuropathy (e22).
* Determine medical and neurological status
ANA, autoantibodies; ANCA, antineutrophil cytoplasmic antibodies; CDT, carbohydrate deficient transferrin; CRP, C-reactive protein; EMG, electromyography;
HbA 1c, adult 1c fraction hemoglobin; HIV, human immunodeficiency virus; MAG, myelin-associated glycoprotein; MR, magnetic resonance; TSH, thyroid-stimulating hormone
Figure.
Simplified diagnostic algorithm for polyneuropathy. This takes only disease courses and clinical presentations into account, without electrophysiology, laboratory tests, and advanced diagnostic methods. Predominantly those forms discussed in the article are shown, with no claim to completeness.
CIDP, chronic inflammatory polyradiculoneuropathy; CIN, chemotherapy-induced neuropathies; GBS, Guillain-Barré syndrome; MMN, multifocal motor neuropathy; SFN, small fiber neuropathy
The primary objective of PNP diagnosis is to promptly and reliably identify: the need for rapid intervention (Guillain-Barré syndrome, vasculitis), as well as treatable etiologies (inflammatory, endocrinological, toxic, nutritive, tumor-related).
Time course is an important parameter, ranging from acute (e.g., Guillain-Barré syndrome) to subacute (e.g., vasculitis) to chronic (e.g., diabetes mellitus) to highly chronic (e.g., hereditary neuropathies). Table 1 summarizes the basic diagnostic methods. In the case of a suspected inflammatory etiology, cerebrospinal fluid analysis is required. Nerve biopsy is only indicated in moderate/severe progressive neuropathy in cases where less invasive methods have failed to yield a diagnosis as yet. Biopsy enables the differentiation between demyelinating and axonal damage, as well as the detection of inflammatory cells or amyloid (e2). The etiology remains unclear in up to 30% of all PNP. A large proportion of these cases are cryptogenic sensory PNP with a good prognosis (e3). In the case of suspected small-fiber neuropathy, the dysfunction associated with which escapes detection on clinical electrophysiology, quantitative sensory testing (QST) and/or skin biopsy are indicated. Here again, laboratory investigations are required to identify the etiology (table 1) (3).
Pathophysiology
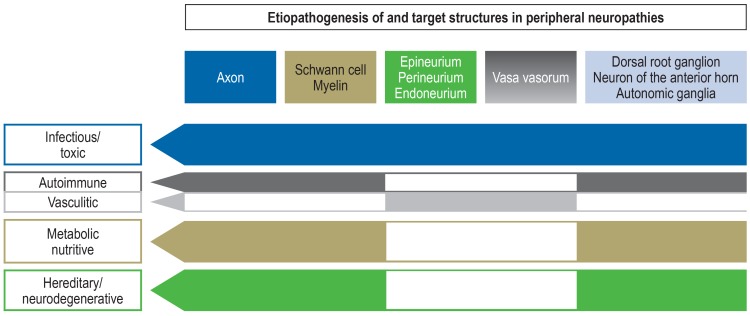
An essential distinction can be made between noxious agents that primarily attack the neurons, i.e., the motoneuron or the dorsal ganglia neuron, and those that disrupt processes in the nerve fiber (axon and Schwann cells) (efigure). The latter are subdivided into effects to the epi- and endoneural blood vessels (vasculitis, peripheral arterial occlusive disease [PAOD]), the medullary sheaths and Ranvier‘s nodes (demyelination, conduction blockage), as well as the axons. Mechanisms of axonal damage are in turn diverse, whereby disorders of axonal transport are considered by some authors to be the most common mechanisms of damage in acquired and hereditary neuropathies (4).
eFigure.
The pathophysiology of polyneuropathy summarized
(modified from Callaghan et al. [40])
Diabetic neuropathy
Due to the pandemic of prediabetes and diabetes, diabetic neuropathy (DN) is the most frequent PNP in Europe and probably worldwide (5). According to national guidelines on the treatment of diabetes in adults, the prevalence of DN is 8%–54% in type 1 diabetes and 13%–46% in type 2 diabetes (6). If DN is suspected, the cause of PNP also needs to be investigated, since there may be other treatable causes besides diabetes (e4). Furthermore, additional factors may also contribute synergistically to the progression of DN (box 1) (5– 7). From a pathophysiological perspective, there is an interplay between the following four factors:
BOX 1. Risk factors for the development of diabetic neuropathy (7).
-
Diabetes-related:
Duration, blood sugar control, retinopathy and nephropathy
-
Vascular:
Arterial hypertension, peripheral arterial occlusive disease, medial calcific sclerosis
-
Nutritive:
Obesity, hyperlipidemia, alcohol, nicotine
-
General:
Age, height, body weight, lack of physical activity
Papanas and Ziegler included all article types on the subject of diabetic polyneuropathy according to their methodological description, irrespective of evidence level (7). Therefore, the data on individual risk factors are of varying quality.
Microcirculatory disorders
Impaired mitochondria and lipid metabolism
Activation of alternative metabolic pathways
Neurotoxic glycated protein formation (5).
The commonest form is distal symmetric PNP, which begins with sensory symptoms (numbness, paresthesia) or in the form of small-fiber neuropathy (pain, loss of temperature perception) (e5). If the clinical picture and electroneurography fail to yield a diagnosis, QST, possibly a skin punch biopsy, or functional diagnostic testing of autonomous nerve fibers are recommended (6). Although motor deficits may occur late in the course of distal symmetric DN, they dominate in other forms, i.e., mononeuropathies such as oculomotor paresis or diabetic amyotrophy/plexopathy. In rare cases, acute painful and autonomic neuropathies, formerly known as insulin neuritis, occur at the start of intensive insulin therapy (8). Given the diversity of these forms of neuropathy, there is no single treatment approach for DN. According to a German national treatment guideline, patients should be advised on all forms and stages of DN in relation to lifestyle, blood sugar control, and foot care. (6). Patients with type 1 as well as type 2 diabetes should undergo individual education on blood glucose control that is tailored to their co-morbidity profile. Treatment consists of the following elements:
Controlling additional risk factors (box 1)
Lifestyle changes, including physical training
Symptom-specific treatment, e.g., pain therapy, vegetative disorders, and diabetic foot syndrome.
Further investigation is required into whether immunotherapies are effective in diabetic amyotrophy (9).
Alcohol-induced polyneuropathy
The prevalence of alcohol-induced PNP among chronic alcoholics is 22%–66%. The duration of alcohol abuse and the lifetime quantity of alcohol consumed represent the two most important factors here. Delta alcoholics are more severely affected than are episodic alcoholics, and women more so than men (10). A quantity of >100 g/day over a number of years is considered likely to be pathogenic for PNP (e6). The pathophysiology of alcohol-induced PNP is a combination of malnutrition, e.g., regarding B vitamins, the direct toxic effects of alcohol, and its degradation products, such as acetaldehydes. Oxidative stress also plays a role. Liver function tests and carbohydrate deficient transferrin (CDT) levels are generally elevated. Macrocytosis is usually also present. Initially, one sees disorders of sensation with and without neuropathic pain. Predominantly distal paresis and vegetative dysfunction may subsequently appear. Neurographically, one sees axonal sensorimotor neuropathy. Neuropathologically, thin nerve fibers are particularly affected (e7), explaining the associated pain. Treatment includes alcohol abstinence and modified dietary habits to correct malnutrition. If abstinence is maintained, neuropathy can resolve within months to years (11, e8).
Chemotherapy-induced neuropathy and other toxic neuropathies
Chemotherapy-induced neuropathy (CIN) is the most frequent neurological side effect of tumor therapy with cytostatic drugs, such as platinum derivatives, vinca alkaloids, taxanes, proteasome inhibitors, as well as modern antibody-based therapies. Due to the rise in cancer and higher long-term survival rates, the incidence of CIN is increasing. The figures vary depending on the substances and regimens used, as well as on the type of assessment; 10%–90 % or 30%–40% are often reported (12). CIN typically starts with sensory deficit symptoms and pain within the first 2 months of therapy and can stabilize or resolve once treatment has been discontinued (12). Whereas, for instance, acute neurotoxic phenomena due to oxaliplatin are reversible in 60%–80% of patients within 2–3 days following administration, persistent structural damage to spinal ganglia and peripheral nerves develops in 73% of cases with increasing treatment duration (13). Platinum- and more rarely vincristine-based therapies can lead to the „coasting“ phenomenon, an initial worsening following discontinuation of the substance (12). Approximately 40% of CIN cases are associated with chronic pain, whereby a neuropathic and a likely secondary myofascial component due to muscular dysfunction may be present (12, 14, e9). In the case of the proteosome inhibitor bortezumib, small-fiber neuropathy is predominantly seen. More recent oncological treatment approaches using immunomodulatory antibodies, the so-called checkpoint inhibitors with the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) or the programmed cell death protein 1 (PD-1) receptor as target structures, can induce acute and chronic immune-mediated neuropathies. These are treated by discontinuing the causative medication and following the guidelines on the treatment of immune-mediated neuropathies (12).
Neurotoxicity depends on the volume of the individual dose, the cumulative total dose, and the duration of chemotherapy. Close clinical monitoring and history taking for PNP symptoms, as well as a clinical neurological examination, are required to adjust the dose and treatment intervals or regimens. Severity and impairment to quality of life can be recorded in a standardized manner (e10, e11). In terms of prevention, the identification of genetic risk factors for specific cytostatic drugs, e.g., platinum or vincristine, could become relevant (12, 13, 15).
Other toxic neuropathies
Numerous drugs and environmental toxins are capable of causing polyneuropathy (box 2). Exposure avoidance and rapid elimination of toxins represent the mainstay of treatment. In the case of heavy metals, elimination can be promoted by complexing agents and forced diuresis (16).
BOX 2. Causes of toxic polyneuropathies (selected).
-
Anti-infective agents
Chloroquine, dapsone, isoniazide, metronidazole, nitrofurantoin, quinolones, dideoxycytidine, thalidomide, other nucleoside analogs
-
Antirheumatic drugs and immunosuppressive drugs
Chloroquine, colchicine, gold, tacrolimus
-
Cardiovascular drugs
Amiodarone, dronedarone, hydralazine, propafenone
-
Psychiatric medication and sedatives
Disulfiram, lithium
-
Other medications
Pyridoxine (vitamin B6), phenytoin
-
Environmental toxins
Arsenic, lead, mercury, thallium, solvents, triorthocresyl phosphate, carbon disulfide, acrylamide
Neuropathies in vitamin deficiency and vitamin overdose
In the case of vitamin B12 deficiency, a subacute clinical picture involving tingling paresthesia of the feet, sensory ataxia, and hypesthesia can appear. Paresis is rare. If untreated, optic atrophy, depression, or dementia may develop (17). Combined myeloneuropathy is present if Aß fibers and the dorsal columns of the spinal cord are involved. This causes increased muscle stretch reflexes and positive pyramidal tract signs, making this form of PNP easier to recognize. Approximately 50% of patients with neurological symptoms show no evidence of macrocytic anemia, which is typical in vitamin B12 deficiency. Vitamin B12 replacement should be initiated as promptly as possible. Vitamin B6 deficiency can cause subacute sensorimotor PNP. Several cases have been described in the form of complications in the treatment of Parkinson‘s disease with an intestinal Duodopa pump (18), as well as following rapid weight loss. Since an overdose of vitamin B6 can also cause PNP, excessive intake should be avoided (19).
Immune-mediated neuropathies
Guillain-Barré syndrome
A patient complaining of ascending paralysis following a gastrointestinal or respiratory infection is highly likely to have Guillain-Barré syndrome (GBS), which requires rapid hospitalization and, in many cases, intensive care treatment. In addition to rapidly ascending tetraparesis, this acute polyradiculoneuritis, with an incidence of 1–2/100 000 cases per year, can cause severe cardiac conduction disorders as well as respiratory muscle failure. Treatment of this autoimmune neuropathy comprises close monitoring, supportive measures, and the administration of intravenous immunoglobulins (IVIg) or plasmapheresis (20) (etable).
eTable. Immunosuppressive treatment of immune-mediated neuropathies and evidence levels (27).
| Agent/method | CIDP | PN | MMN |
Paraproteinemic neuropathy (IgM) |
Vasculitic neuropathy |
| Glucocorticoids | Ib, A | III, GCP | |||
| Immunoglobulins – Intravenous – Subcutaneous |
Ib, A Ib, B |
Ib, A | Ib, GCP | ||
| Plasmapheresis | Ib, A | IIb, 0 | |||
| Azathioprine | IV, 0 | ||||
| Methotrexate | IV, 0 | ||||
| Cyclophosphamide | IV, 0 | IV, 0 | III, GCP | ||
| Rituximab | IV, GCP | IV, GCP |
CIDP, chronic inflammatory polyradiculoneuropathy; GCP, good clinical practice; IgM, immunoglobulin M; MMN, multifocal motor
neuropathy; PN, paranodopathies
Chronic inflammatory polyradiculoneuropathy
In contrast to GBS, which, by definition, reaches its nadir after 4 weeks, chronic inflammatory polyradiculoneuropathy (CIDP) is a chronic autoimmune disease that develops over a period of at least 8 weeks. Its prevalence is reported at 2–3/100 000. The clinical picture consists of symmetrical, primarily motor polyradiculoneuropathy with both distal and proximal muscle weakness, areflexia, paresthesia, and sensory deficits. It generally follows a chronic progressive course and only rarely a relapsing–remitting course. There are forms that have asymmetric distribution, as well as only motor or only sensory impairment (21). Diagnosis is based on the clinical presentation, electrophysiological signs of demyelination, non-obligatory cerebrospinal fluid criteria with less than 10 leukocytes/µL and elevated cerebrospinal fluid protein, as well as the detection of demyelination by means of sural nerve biopsy where appropriate (21). In recent years, nerve ultrasound and magnetic resonance imaging (MRI) have also improved diagnosis and treatment monitoring (e12).
Evidence on the efficacy of glucocorticoids (GC), IVIg, and plasmapheresis is available from controlled trials (22). Due to its high cost and short duration of action, plasmapheresis is predominantly used in cases of acute deterioration (23). Whether GC or IVIg are more cost-effective for long-term treatment remains to be established. Although more patients respond to IVIg initially, GC responders seem to go into remission for longer following treatment discontinuation (e13). According to experts, the selection of IVIg or GC in CIDP is made while taking the expected side effects and costs into consideration (24). When GC are selected, pulse therapy is favored over long-term oral therapy (25). According to the evidence, subcutaneous immunoglobulin therapy is effective (26). However, no preparations have been approved for this indication as yet. None of the other substances for long-term immunotherapy, such as azathioprine, methotrexate, or interferon beta-1a, have been shown in randomized controlled trials to be effective in CIDP (27, 28). Evidence levels are presented in the eTable
Paraproteinemic neuropathies
This term covers all PNP in which a paraprotein is found in patient‘s serum. However, given the frequency of paraproteins, as well as of PNP in old age, this finding is usually a random coincidence that has no implications for the treatment of PNP. The following constellations are an exception to this:
Predominantly distal and motor polyneuropathy with marked signs of demyelination (29) occurs in IgM gammopathy, often with antibody reactivity to myelin-associated glycoprotein. Treatment is initially identical to that of CIDP, although some authors recommend using rituximab (30).
PNP and IgG? paraproteinemia with angiofollicular lymph node hyperplasia (Castleman‘s disease), osteosclerotic bone lesions, or elevated vascular endothelial growth factors can indicate the presence of POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M-protein, and skin changes). The first-line treatment here is autologous stem cell transplantation (31).
In the case of large quantities of paraprotein, one should consider hematological disease. High IgM levels may indicate Waldenstrom‘s disease, while elevated IgG levels may point to myeloma-related AL amyloidosis. Evidence levels are shown in the eTable
Paranodopathies
The term paranodopathies was coined after autoantibodies against paranodal proteins on Ranvier‘s nodes, such as neurofascin-155, contactin-1, and caspr-1, were discovered in patients exhibiting the clinical picture of CIDP. An acute onset, as in GBS, transitioning to chronic disease progression is typical. Clinically, one sees acute, severe, predominantly motor neuropathy that is usually axonal on electrophysiology and often accompanied by action tremor and ataxia. Rituximab is considered the treatment of first choice in this generally IgG4-related immune neuropathy, whereby patients initially also respond to classic CIDP therapy (32, e14, e15).
Multifocal motor neuropathy
The prevalence of multifocal motor neuropathy (MMN) is 0.6–2/100 000 cases. This pure motor neuropathy is characterized by progressive, primarily distal asymmetric paresis and atrophy. It affects men more frequently than women and generally starts in the upper extremities. One typically detects multifocal conduction blocks on motor neurography, independent of physiological constrictions. High serological titers of IgM antibodies against the ganglioside GM1 are detected in 50% of those affected (33). The therapy of choice consists of repeated administration of IVIg. Although subcutaneous immunoglobulin administration is effective, it has not been approved as yet (34). Other immunosuppressants, including GC, are ineffective (27, e16). Evidence levels are shown in the eTable
Vasculitic neuropathies
In the case of progressive, multifocal involvement of various peripheral nerves, as well as in the case of subacute distal symmetric PNP, one must consider vasculitis as a possible cause (efigure). Systemic vasculitis sometimes manifests primarily as PNP, e.g., in microvascular polyangiitis or eosinophilic granulomatosis with polyangiitis. However, isolated vasculitis of the peripheral nervous system is often present (35), making diagnosis only possible by means of nerve biopsy. A combined nerve/muscle/skin biopsy improves the yield (e17, e18). Treatment is performed using GC. In the case of therapy resistance, cyclophosphamide or rituximab are used, as in systemic vasculitides (27) (evidence levels shown in the eTable).
Symptomatic therapy
Treatment of neuropathic pain
Approximately 50% of all polyneuropathies are associated with pain (35, e19, e20). This neuropathic pain is caused—in simplified terms—by spontaneous activity and the sensitization of damaged axons, mediated by overactive sodium channels, as well as the effect of inflammatory mediators and growth factors. Due to the permanent influx of nociceptive information to the spinal cord and brain, the phenomenon of central sensitization may occur there—in addition to the failure of tonic and phasic endogenous pain inhibition (e21). Since, therefore, the mechanisms of neuropathic pain differ fundamentally from those of nociceptive pain, special treatment approaches are needed (36). Pharmacological treatment of neuropathic pain was recently summarized in a meta-analysis that included recommendations (36). Gabapentin, pregabalin, duloxetine, and tricyclika are the drugs of first choice, whereby attention needs to be paid to the different indications and the side-effect profile (table 2). Topical therapies such as lidocaine or capsaicin patches can be helpful in well-defined areas of pain (37, 38).
Table 2. Treatment of neuropathic pain*.
| Active agent |
Evidence level; recommendation |
Mode of action/comment |
| Gabapentin/ pregabalin |
Ia; drug of 1st choice | Especially in the case of central sensitization (hyperalgesia, allodynia) |
| Tricyclic antidepressants (e.g., amitriptyline) |
Ia; drug of 1st choice | Sodium channel blockers and serotonine-noradrenaline reuptake inhibitors |
| Duloxetine/ venlafaxine |
Ia; duloxetine Ib; venlafaxine drug of 1 st choice |
Serotonine-noradrenaline reuptake inhibitors; duloxetine approved in G for pain due to diabetic neuropathy; venlafaxine off-label |
| Capsaicin 8% | Ib; drug of 2nd choice | In focal pain syndrome |
| Lidocaine (plaster) | IIb; drug of 2nd choice | In focal pain syndrome; approved for postherpetic neuralgia |
| Tramadol | IIb; drug of 2nd choice | Weak opioid; habituation; nausea |
| Strong opioids | IIb; drug of 3rd choice | Note: opioid-induced hyperalgesia, habituation and abuse; scant longterm data |
| Botulinum toxin A (subcutaneous) |
III; drug of 2nd choice | Evidence still insufficient for broad use; off-label in G |
| Carbamazepine/ oxcarbazepine/ lamotrigine |
Ib; carbamazepine/ oxcarbazepine IV; lamotrigine |
Drug of 1st choice in neuralgia (e.g., trigeminal neuralgia); lamotrigine off-label |
*Modified from Finnerup (36); G, Germany
Physiotherapy, ergotherapy, and training therapy
Physiotherapy for neuropathies is guided by symptoms and functional deficits. It includes exercises that improve stability during standing and walking and which train balance, coordination, and proprioception. In the case of paresis, the objective is to increase muscle strength and function and to maintain or restore muscular balance in order to prevent deformities and contractures. Physical and balneological therapy methods can also be used. If hand function is impaired, occupational therapy is indicated, complemented where necessary by the deployment of appropriate aid devices. Sporting activity within the context of preserved function is desirable. Since the proximal muscle groups are barely affected for a long time in many patients, these can be trained. Ergometer and resistance training three times a week has a positive effect on fitness and muscle strength in CIDP (39).
Key messages.
At a prevalence of around 5%–8%, polyneuropathies represent the most common disorders of the peripheral nervous system in adults.
In addition to distal, symmetrical sensorimotor neuropathy, which is comparatively common, there are also polyradiculoneuropathies with additional proximal involvement, as well as asymmetric forms such as mononeuritis multiplex, in which different nerves are affected either simultaneously or sequentially.
The aim of diagnosis is to identify and treat neurological emergencies, e.g., Guillain-Barré syndrome or vasculitis, or to detect preventable causes.
The diagnosis is established primarily by patient history of disease onset and course. The affected systems can be identified by clinical examination of the type of distribution and severity, electrophysiological tests (axonal, demyelinating), and laboratory tests for diabetes, vitamin deficiency, alcohol abuse, and autoantibodies.
In Germany, diabetes is the most common cause of polyneuropathy. Other important causes include chemotherapy, alcohol abuse, autoimmune processes, and genetic mutations.
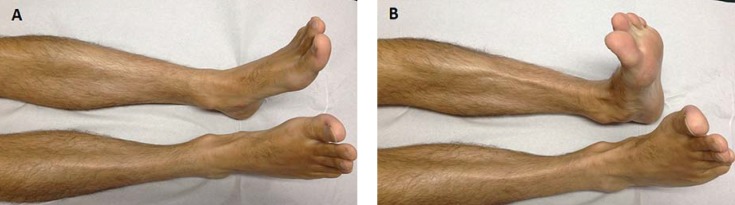
eFigure:
Feet and lower legs of a patient with vasculitic polyneuropathy
(A) Thinning of the small foot muscles as a sign of a subacute to chronic process
(B) Acute-onset right-foot extensor paresis
Acknowledgments
Translated from the original German by Christine Schaefer-Tsorpatzidis.
Footnotes
Conflict of interest statement
Prof. Sommer received consulting fees from Air Liquide, Astellas, Baxter/Baxalta, CSL Behring, and Genzyme, LFB. She received lecture fees from Baxalta, Genzyme, Kedrion, Novartis und Pfizer. Study support (third-party funds) was provided by Kedrion and CSL Behring.
PD Dr. Geber received consulting fees from Pfizer.
Prof. Young received consulting fees, congress and travel expenses as well as study support (third-party funds) from Pharnext.
Prof. Birklein received lecture fees from Pfizer and study support (third-party funding) from Pfizer und Lilly.
Prof. Schoser received reimbursement of congress fees and travel expenses, as well as lecture fees from CSL Behring.
Prof. Forst declares that there are no conflicts of interest.
References
- 1.Hanewinckel R, Drenthen J, van Oijen M, Hofman A, van Doorn PA, Ikram MA. Prevalence of polyneuropathy in the general middle-aged and elderly population. Neurology. 2016;87:1892–1898. doi: 10.1212/WNL.0000000000003293. [DOI] [PubMed] [Google Scholar]
- 2.Deutsche Gesellschaft für Neurologie. S1 Leitlinie Diagnostik bei Polyneuropathien. www.dgn.org/leitlinien/2331-ll-44-2012-diagnostik-bei-polyneuropathien (last accessed on 8 December 2017) [Google Scholar]
- 3.Sommer C, Üçeyler N. Small-Fiber-Neuropathien. Klin Neurophysiol. 2017;48:63–72. [Google Scholar]
- 4.Prior R, van Helleputte L, Benoy V, van den Bosch L. Defective axonal transport: a common pathological mechanism in inherited and acquired peripheral neuropathies. Neurobiol Dis. 2017;105:300–320. doi: 10.1016/j.nbd.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Feldman EL, Nave KA, Jensen TS, Bennett DL. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. 2017;93:1296–1313. doi: 10.1016/j.neuron.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bundesärztekammer (BÄK) Kassenärztliche Bundesvereinigung (KBV), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF) Nationale Versorgungsleitlinie Neuropathie bei Diabetes im Erwachsenenalter Stand 01.07.2016. www.awmf.org/leitlinien/detail/ll/nvl-001e.html (last accessed on 8 December 2017) [Google Scholar]
- 7.Papanas N, Ziegler D. Risk factors and comorbidities in diabetic neuropathy: an update 2015. Rev Diabet Stud. 2015;12:48–62. doi: 10.1900/RDS.2015.12.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbons CH. Treatment-induced neuropathy of diabetes. Curr Diab Rep. 2017;17 doi: 10.1007/s11892-017-0960-6. [DOI] [PubMed] [Google Scholar]
- 9.Chan YC, Lo YL, Chan ES. Immunotherapy for diabetic amyotrophy. Cochrane Database Syst Rev. 2017;7 doi: 10.1002/14651858.CD006521.pub4. CD006521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chopra K, Tiwari V. Alcoholic neuropathy: possible mechanisms and future treatment possibilities. Br J Clin Pharmacol. 2012;73:348–362. doi: 10.1111/j.1365-2125.2011.04111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillbom M, Wennberg A. Prognosis of alcoholic peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1984;47:699–703. doi: 10.1136/jnnp.47.7.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staff NP, Grisold A, Grisold W, Windebank AJ. Chemotherapy-induced peripheral neuropathy: a current review. Ann Neurol. 2017;81:772–781. doi: 10.1002/ana.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avan A, Postma TJ, Ceresa C, et al. Platinum-induced neurotoxicity and preventive strategies: past, present, and future. Oncologist. 2015;20:411–432. doi: 10.1634/theoncologist.2014-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geber C, Breimhorst M, Burbach B, et al. Pain in chemotherapy-induced neuropathy—more than neuropathic? Pain. 2013;154:2877–2887. doi: 10.1016/j.pain.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 15.Diouf B, Crews KR, Lew G, et al. Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. JAMA. 2015;313:815–823. doi: 10.1001/jama.2015.0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Little AA, Albers JW. Clinical description of toxic neuropathies. Handb Clin Neurol. 2015;131:253–296. doi: 10.1016/B978-0-444-62627-1.00015-9. [DOI] [PubMed] [Google Scholar]
- 17.Kumar N. Neurologic aspects of cobalamin (B12) deficiency. Handb Clin Neurol. 2014;120:915–926. doi: 10.1016/B978-0-7020-4087-0.00060-7. [DOI] [PubMed] [Google Scholar]
- 18.Santos-Garcia D, de la Fuente-Fernandez R, Valldeoriola F, et al. Polyneuropathy while on duodenal levodopa infusion in Parkinson‘s disease patients: we must be alert. J Neurol. 2012;259:1668–1672. doi: 10.1007/s00415-011-6396-z. [DOI] [PubMed] [Google Scholar]
- 19.Ghavanini AA, Kimpinski K. Revisiting the evidence for neuropathy caused by pyridoxine deficiency and excess. J Clin Neuromuscul Dis. 2014;16:25–31. doi: 10.1097/CND.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 20.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barre syndrome. Lancet. 2016;388:717–727. doi: 10.1016/S0140-6736(16)00339-1. [DOI] [PubMed] [Google Scholar]
- 21.Joint Task Force of the European Federation of Neurological Societies (EFNS) and the Peripheral Nerve Society (PNS) European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of paraproteinemic demyelinating neuropathies. Report of a Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. J Peripher Nerv Syst. 2010;15:185–195. doi: 10.1111/j.1529-8027.2010.00278.x. [DOI] [PubMed] [Google Scholar]
- 22.Oaklander AL, Lunn MP, Hughes RA, van Schaik IN, Frost C, Chalk CH. Treatments for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): an overview of systematic reviews. Cochrane Database Syst Rev. 2017;1 doi: 10.1002/14651858.CD010369.pub2. CD010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehndiratta MM, Hughes RA, Pritchard J. Plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD003906.pub4. CD003906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nobile-Orazio E, Gallia F, Terenghi F, Bianco M. Comparing treatment options for chronic inflammatory neuropathies and choosing the right treatment plan. Expert Rev Neurother. 2017;17:755–765. doi: 10.1080/14737175.2017.1340832. [DOI] [PubMed] [Google Scholar]
- 25.Press R, Hiew FL, Rajabally YA. Steroids for chronic inflammatory demyelinating polyradiculoneuropathy: evidence base and clinical practice. Acta Neurol Scand. 2016;133:228–238. doi: 10.1111/ane.12519. [DOI] [PubMed] [Google Scholar]
- 26.Racosta JM, Sposato LA, Kimpinski K. Subcutaneous versus intravenous immunoglobulin for chronic autoimmune neuropathies: a meta-analysis. Muscle Nerve. 2017;55:802–809. doi: 10.1002/mus.25409. [DOI] [PubMed] [Google Scholar]
- 27.Deutsche Gesellschaft für Neurologie. S2e Leitlinie Therapie akuter und chronischer immunvermittelter Neuropathien und Neuritiden. www.dgn.org/leitlinien/2338-ll-45-2012-therapie-akuter-und-chronischer-immunvermittelter-neuropathien-und-neuritiden (last accessed on 8 December 2017) [Google Scholar]
- 28.Mahdi-Rogers M, Brassington R, Gunn AA, van Doorn PA, Hughes RA. Immunomodulatory treatment other than corticosteroids, immunoglobulin and plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2017;5 doi: 10.1002/14651858.CD003280.pub5. CD003280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katz JS, Saperstein DS, Gronseth G, Amato AA, Barohn RJ. Distal acquired demyelinating symmetric neuropathy. Neurology. 2000;54:615–620. doi: 10.1212/wnl.54.3.615. [DOI] [PubMed] [Google Scholar]
- 30.Campagnolo M, Zambello R, Nobile-Orazio E, et al. IgM MGUS and Waldenstrom-associated anti-MAG neuropathies display similar response to rituximab therapy. J Neurol Neurosurg Psychiatry. 2017 doi: 10.1136/jnnp-2017-315736. [DOI] [PubMed] [Google Scholar]
- 31.Dispenzieri A. POEMS syndrome: 2017 update on diagnosis, risk stratification, and management. Am J Hematol. 2017;92:814–829. doi: 10.1002/ajh.24802. [DOI] [PubMed] [Google Scholar]
- 32.Querol L, Devaux J, Rojas-Garcia R, Illa I. Autoantibodies in chronic inflammatory neuropathies: diagnostic and therapeutic implications. Nat Rev Neurol. 2017;13:533–547. doi: 10.1038/nrneurol.2017.84. [DOI] [PubMed] [Google Scholar]
- 33.Vlam L, van der Pol WL, Cats EA, et al. Multifocal motor neuropathy: diagnosis, pathogenesis and treatment strategies. Nat Rev Neurol. 2011;8:48–58. doi: 10.1038/nrneurol.2011.175. [DOI] [PubMed] [Google Scholar]
- 34.Kumar A, Patwa HS, Nowak RJ. Immunoglobulin therapy in the treatment of multifocal motor neuropathy. J Neurol Sci. 2017;375:190–197. doi: 10.1016/j.jns.2017.01.061. [DOI] [PubMed] [Google Scholar]
- 35.Collins MP, Hadden RD. The nonsystemic vasculitic neuropathies. Nat Rev Neurol. 2017;13:302–316. doi: 10.1038/nrneurol.2017.42. [DOI] [PubMed] [Google Scholar]
- 36.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Treede RD, Wagner T, Kern KU, et al. Mechanism- and experience-based strategies to optimize treatment response to the capsaicin 8% cutaneous patch in patients with localized neuropathic pain. Curr Med Res Opin. 2013;29:527–538. doi: 10.1185/03007995.2013.781019. [DOI] [PubMed] [Google Scholar]
- 38.Sommer C, Cruccu G. Topical treatment of peripheral neuropathic pain: applying the evidence. J Pain Symptom Manage. 2017;53:614–629. doi: 10.1016/j.jpainsymman.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Markvardsen LH, Overgaard K, Heje K, et al. Resistance training and aerobic training improve muscle strength and aerobic capacity in chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2018;57:70–76. doi: 10.1002/mus.25652. [DOI] [PubMed] [Google Scholar]
- 40.Callaghan BC, Price RS, Chen KS, Feldman EL. The importance of rare subtypes in diagnosis and treatment of peripheral neuropathy: a review. JAMA Neurol. 2015;72:1510–1518. doi: 10.1001/jamaneurol.2015.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Eggermann K, Gess B, Häusler M, Weis J, Hahn A, Kurth I. Hereditary neuropathies: clinical presentation and genetic panel diagnosis. Dtsch Arztebl Int. 2018;115:91–97. doi: 10.3238/arztebl.2018.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Sommer CL, Brandner S, Dyck PJ, et al. Peripheral Nerve Society guideline on processing and evaluation of nerve biopsies on processing and evaluation of nerve biopsies. J Peripher Nerv Syst. 2010;15:164–175. doi: 10.1111/j.1529-8027.2010.00276.x. [DOI] [PubMed] [Google Scholar]
- E3.Pasnoor M, Dimachkie MM, Barohn RJ. Cryptogenic sensory polyneuropathy. Neurol Clin. 2013;31:463–476. doi: 10.1016/j.ncl.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Rajabally YA, Stettner M, Kieseier BC, Hartung HP, Malik RA. CIDP and other inflammatory neuropathies in diabetes—diagnosis and management. Nat Rev Neurol. 2017;13:599–611. doi: 10.1038/nrneurol.2017.123. [DOI] [PubMed] [Google Scholar]
- E5.Cortez M, Singleton JR, Smith AG. Glucose intolerance, metabolic syndrome, and neuropathy. Handb Clin Neurol. 2014;126:109–122. doi: 10.1016/B978-0-444-53480-4.00009-6. [DOI] [PubMed] [Google Scholar]
- E6.Koike H, Iijima M, Sugiura M, et al. Alcoholic neuropathy is clinicopathologically distinct from thiamine-deficiency neuropathy. Ann Neurol. 2003;54:19–29. doi: 10.1002/ana.10550. [DOI] [PubMed] [Google Scholar]
- E7.Koike H, Mori K, Misu K, et al. Painful alcoholic polyneuropathy with predominant small-fiber loss and normal thiamine status. Neurology. 2001;56:1727–1732. doi: 10.1212/wnl.56.12.1727. [DOI] [PubMed] [Google Scholar]
- E8.Schuchardt V. [Alcohol and the peripheral nervous system] Ther Umsch. 2000;57:196–199. doi: 10.1024/0040-5930.57.4.196. [DOI] [PubMed] [Google Scholar]
- E9.Kautio AL, Haanpaa M, Kautiainen H, Kalso E, Saarto T. Burden of chemotherapy-induced neuropathy—a cross-sectional study. Support Care Cancer. 2011;19:1991–1996. doi: 10.1007/s00520-010-1043-2. [DOI] [PubMed] [Google Scholar]
- E10.Common Terminology Criteria for Adverse Events (CTCAE) v4.0. 2016. ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (last accessed on 8 December 2017) [Google Scholar]
- E11.Postma TJ, Aaronson NK, Heimans JJ, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer. 2005;41:1135–1139. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- E12.Pitarokoili K, Schlamann M, Kerasnoudis A, Gold R, Yoon MS. Comparison of clinical, electrophysiological, sonographic and MRI features in CIDP. J Neurol Sci. 2015;357:198–203. doi: 10.1016/j.jns.2015.07.030. [DOI] [PubMed] [Google Scholar]
- E13.Nobile-Orazio E, Cocito D, Jann S, et al. Frequency and time to relapse after discontinuing 6-month therapy with IVIg or pulsed methylprednisolone in CIDP. J Neurol Neurosurg Psychiatry. 2015;86:729–734. doi: 10.1136/jnnp-2013-307515. [DOI] [PubMed] [Google Scholar]
- E14.Doppler K, Appeltshauser L, Villmann C, et al. Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathy. Brain. 2016;139:2617–2630. doi: 10.1093/brain/aww189. [DOI] [PubMed] [Google Scholar]
- E15.Doppler K, Appeltshauser L, Wilhelmi K, et al. Destruction of paranodal architecture in inflammatory neuropathy with anticontactin-1 autoantibodies. J Neurol Neurosurg Psychiatry. 2015;86:720–728. doi: 10.1136/jnnp-2014-309916. [DOI] [PubMed] [Google Scholar]
- E16.Joint Task Force of the European Federation of Neurological Societies (EFNS) and the Peripheral Nerve Society (PNS) European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of multifocal motor neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. J Peripher Nerv Syst. 2010;15:295–301. doi: 10.1111/j.1529-8027.2010.00290.x. [DOI] [PubMed] [Google Scholar]
- E17.Üçeyler N, Devigili G, Toyka KV, Sommer C. Skin biopsy as an additional diagnostic tool in non-systemic vasculitic neuropathy. Acta Neuropathol. 2010;120:109–116. doi: 10.1007/s00401-010-0662-5. [DOI] [PubMed] [Google Scholar]
- E18.Vrancken AF, Gathier CS, Cats EA, Notermans NC, Collins MP. The additional yield of combined nerve/muscle biopsy in vasculitic neuropathy. Eur J Neurol. 2011;18:49–58. doi: 10.1111/j.1468-1331.2010.03041.x. [DOI] [PubMed] [Google Scholar]
- E19.Üçeyler N, Rogausch JP, Toyka KV, Sommer C. Differential expression of cytokines in painful and painless neuropathies. Neurology. 2007;69:42–49. doi: 10.1212/01.wnl.0000265062.92340.a5. [DOI] [PubMed] [Google Scholar]
- E20.Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34:2220–2224. doi: 10.2337/dc11-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E21.Birklein F, Baron R, Gaul C, et al. Schmerz - ein vernachlässigtes neurologisches Thema. Nervenarzt. 2016;87:609–615. doi: 10.1007/s00115-016-0113-1. [DOI] [PubMed] [Google Scholar]
- E22.Abraham A, Alabdali M, Alsulaiman A, et al. The sensitivity and specificity of the neurological examination in polyneuropathy patients with clinical and electrophysiological correlations. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171597. e0171597. [DOI] [PMC free article] [PubMed] [Google Scholar]