Abstract
Although monosomy X is the most common karyotype in patients with Turner Syndrome, presence of Y chromosome material has been observed in about 10% of patients. Y chromosome material in patients with Turner syndrome poses an increased risk of gonadoblastoma and malignant transformation. We report a woman with a diagnosis of Turner syndrome at 12yo, without signs of virilization, and karyotype reported as 46,X,del(X)(q13). At 26yo, cytogenetic studies indicated the patient to be mosaic for monosomy X and a cell line that contained a duplicated Yq chromosome. Bilateral gonadectomy was performed and revealed streak gonads, without evidence of gonadoblastoma. Histological analysis showed ovarian stromal cells with few primordial tubal structures. FISH performed on streak gonadal tissue showed a heterogeneous distribution of SRY, with exclusive localization to the primordial tubal structures. DNA extraction from the gonadal tissue showed a 6.5% prevalence of SRY by microarray analysis, contrasting the 86% prevalence in the peripheral blood sample. This indicates that the overall gonadal sex appears to be determined by the majority gonosome complement in gonadal tissue in cases of sex chromosome mosaicism. This case also raises questions regarding malignancy risk associated with Y prevalence and tubal structures in gonadal tissue.
Keywords: Turner Syndrome, Gonadoblastoma, Y chromosome, Gonosome, Prevalence, Tubal structures
Background
Turner syndrome is a common chromosome abnormality occurring in approximately 1:2000 live female births [1]. The clinical features are highly variable and can be confirmed by chromosome analysis, which usually demonstrates complete or mosaic monosomy of the X chromosome. Although haploinsufficiency due to monosomy X is the most common karyotype found in Turner syndrome (50–60%), other structural abnormalities in the X chromosome, such as ring chromosomes, isochromosomes of the long arm, and deletion of the short arm have been reported [2] [3]. Additionally, there have been reports of a Y cell line in about 10% of patients [4], as well as complex karyotypes with derivative X chromosome material [2].
The presence of a Y chromosome material in patients with Turner Syndrome is of integral importance as it increases the risk of developing a gonadoblastoma, with an estimated risk of 7–10% [5]. However, Y chromosome material is not always evident by standard karyotype analysis, and may require further study using the newer cytogenomic techniques such as FISH and chromosomal microarray. Testing for Y chromosome material is suggested for patients who have signs of virilization, or if a marker chromosome is found on karyotype [6]. Here we report a case of a young woman with Turner Syndrome. Detailed genetic analysis demonstrated mosaicism with the majority of cells showing a duplicated Yq chromosome and the minority of cells showing monosomy X. Y chromosome material was also found in the gonads, exclusively localized to primitive tubal cells, although there were no signs of phenotypic virilization.
Case Presentation
The patient is a 26 year old woman of Ashkenazi descent diagnosed clinically with Turner syndrome at age 12. She was born full term with no complications to a G1P0 mother. She had never been hospitalized and had no medical issues at time of presentation.
Karyotype analysis was performed at age 12 by a commercial laboratory and was reported as 46,X,del(X)(q13). A pelvic ultrasound was performed showing small ovaries and a uterus. She was also found to have aortic insufficiency and a bicuspid valve. She received treatment with growth hormone starting at age 12 until age 16, and underwent induction of puberty with oral estrogen supplementation at age 18.
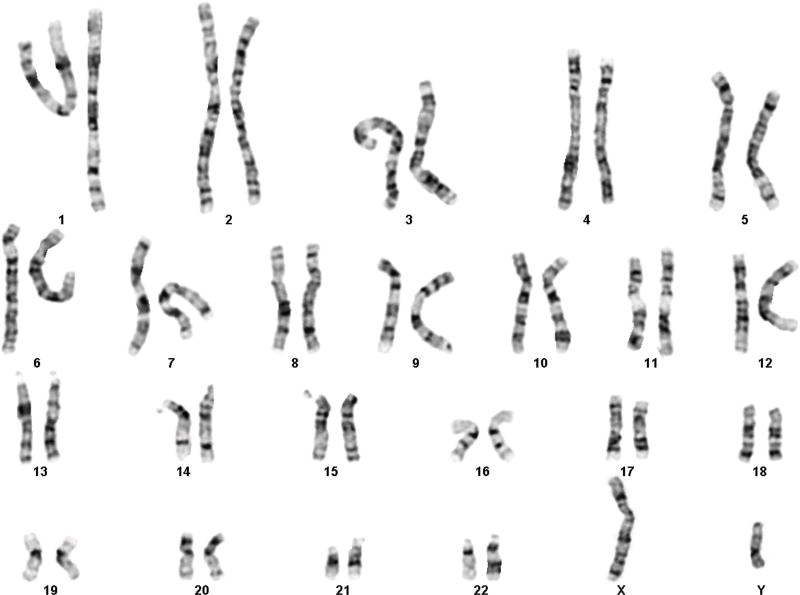
At age 26, physical exam showed height of 155.4cm, weight 120kg. She had normal chest shape, with Tanner V breasts and pubic hair. No evidence of clitoral enlargement or posterior labial adhesions were noted. Vaginal mucosa was estrogenized. Extremities were notable for scooped nails, short broad thumb, and short 4th and 5th toes. She was referred to a reproductive endocrinology program for fertility workup and hormone replacement therapy. At that time, cytogenetic testing was repeated and included karyotype and FISH analysis. Cytogenetic analysis revealed an abnormal mosaic karyotype with approximately 12% of cells showing Monosomy X (45,X) and the majority of cells (88%) showing a male genetic constitution with the Y chromosome containing a duplication of the entire euchromatic region of the long arm (46,X,dup(Y)(q.11.21q11.23) (Figure 1). The level of mosaicism was estimated from both G-banded and FISH studies which included a total of 250 cells analyzed.
Figure 1.
G-banded karyotype showing 46,X,dup(Y)(q11.21q11.23)
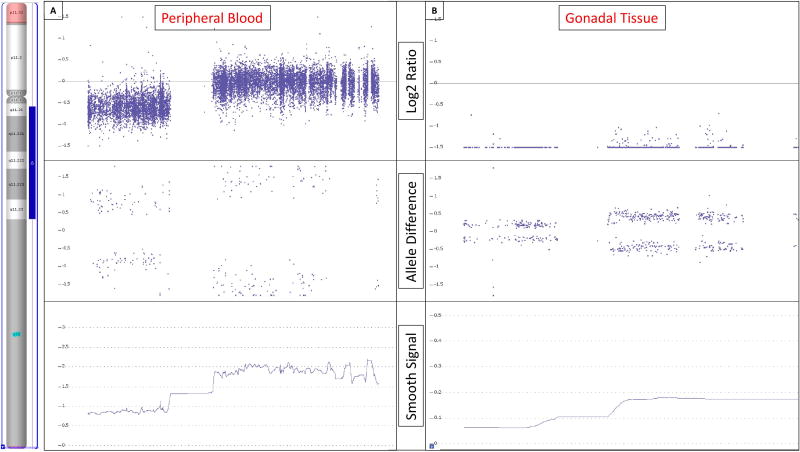
Chromosomal SNP microarray analysis was also performed with 2,696,550 probe targets and revealed arr[7] (X)x1,Yp11.31p11.2(2,650,141–10,073,965)x1[8],Yq11.21q11.23 (13,800,734–28,799,937)x2[9] (Figure 2a). This was interpreted together with the karyotype results as an estimated 14% mosaic loss of the Y chromosome, with Y chromosomal material present in 86% of the cells, with a duplication of the entire long arm euchromatic region from positions 13,800,734–28,799,937.This genetic constitution is equivalent to the 45,X/46,X,dup(Y)(q11.21q11.23) karyotype reported by standard G-band analysis.
Figure 2.
Chromosome microarray analysis results. The region that is duplicated on the long arm of the Y chromosome is indicated by the blue bar in the ideogram on the left. [A] Chromosome microarray analysis performed on DNA extracted from peripheral blood showed an 86% prevalence of Y chromosome material with a duplication of the entire euchromatic region of the the long arm (blue bar). The duplication is identified by the higher Log2 ratio observed for probes in the long arm compared to those in the short arm as seen in the Log2 Ratio panel. [B] Chromosome microarray analysis performed on DNA extracted from gonadal tissue paraffin sections showed the same duplicated Y chromosome but at a 6.5% prevalence. The Log2 ratios in panel B are distinctly diminished compared to panel A, indicating a very low presence of Y-chromosomal material. However, the Log2 ratio in the long arm remains noticeably increased over the short arm, representing the same structural Y chromosome as seen in peripheral blood but at a much lower prevalence. The allele difference panels indicates the genotype for each SNP probe. For normal copy number of 2, there are only 3 possible SNP combinations, AA, AB and BB which are plotted on the allele difference graph. When there is a single copy of a region (copy number of 1), the genotype options are either A or B and thus only two distinct tracks are visible on the allele difference graph. When the same region is duplicated, as in the long arm of the Y chromosome, there remains only 2 genotypes but they are represented as AA and BB. The smooth signal copy number panels indicate the exact copy number of each probe. This panel is helpful in identifying mosaicism which is evident when the smooth signal for multiple consecutive probes lies between an integer, e.g. between 1 and 2. The level of mosaicism in peripheral blood is markedly higher compared to that observed in gonadal tissue.
Pelvic MRI showed a small uterus, and the ovaries could not be definitively identified.
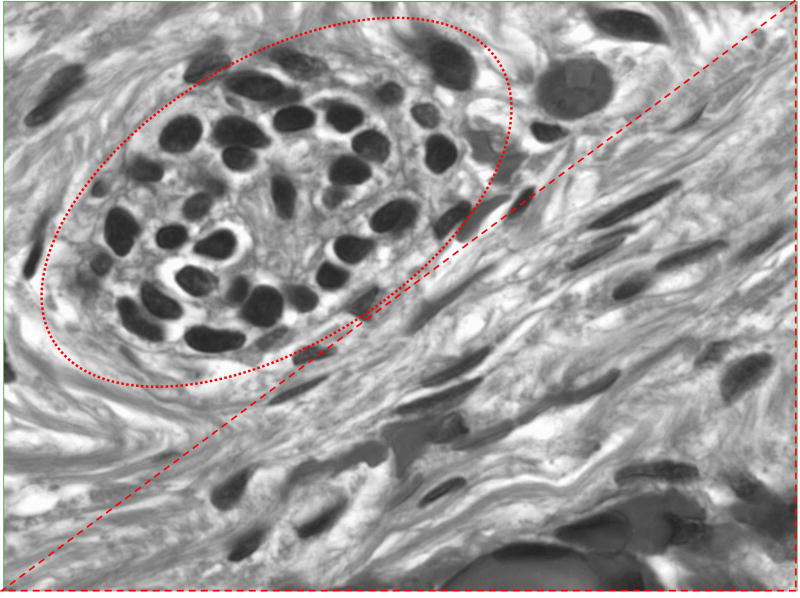
Due to the risk for gonadoblastoma, the patient underwent bilateral gonadectomy. Gross pathology showed right and left fallopian tubes with streak gonads. Dissection revealed ovarian stromal cells with conspicuous nuclei and scanty cytoplasm with few primordial tubal structures and calcification (Figure 3). No evidence of gonadoblastoma was seen.
Figure 3.
Microscopic dissection of streak gonadal tissue. Tubal structures at top left (red oval) with wavy ovarian stroma at bottom right (red triangle).
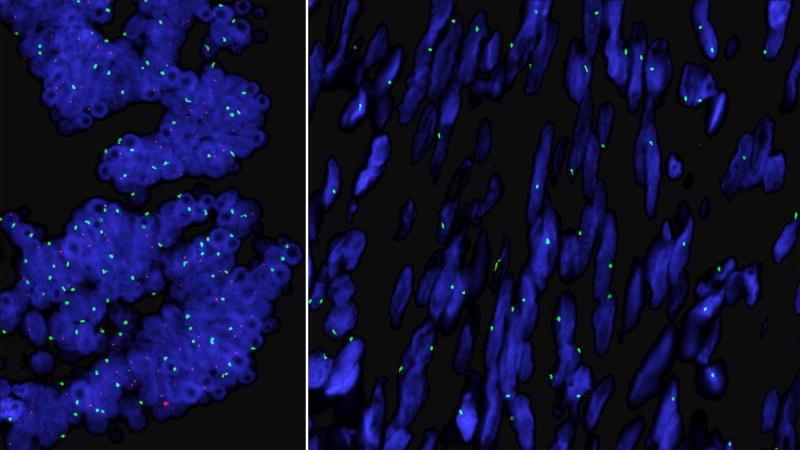
FISH using probes specific for the centromere of the X chromosome and the SRY region of the Y chromosome was performed on streak gonads and showed a heterogeneous distribution of SRY, localized exclusively to the areas with tubular remnants. SRY was absent in all ovarian stromal cells which showed the presence of only one X chromosome (Figure 4). Chromosomal microarray analysis performed on DNA extracted from gonadal tissue paraffin sections showed approximately 6.5% prevalence of the Y material (Figure 2b). The 46,X,dup(Y)(q11.21q11.23) cells thus appeared to be contained exclusively within the tubular tissue while the 45,X cells were isolated to the stromal tissue.
Figure 4.
FISH using probes for the centromere of the X chromosome (green) and the SRY region on the short arm of the Y chromosome (red). SRY is present only in the tubal structures (left panel) while the ovarian stroma contains a single X chromosome with no SRY (right panel).
Discussion
Multiple studies have shown the presence of previously undetected Y chromosome material in patients with Turner syndromes [4] [10] [11] [12]. One study in Denmark found that 14 of 114 women, mean age 27 +/− 13, with Turner syndrome confirmed by karyotype were found to have Y chromosomal material on repeat genetic testing with PCR, 7 of whom did not have the Y material identified on original karyotyping [5]. Similar findings have been shown in Korean [13], Brazilian[10] and Hungarian [14] populations. Some studies have proposed that all patients with Turner syndrome and 45,X karyotypes carry a second chromosomal cell line, difficult to detect with standard testing [15]. In some cases, due to a low percentage of mosaicism, this may be due to an insufficient number of cells having been tested, or an insufficient number of tissues having been sampled. In our patient, despite the high percentage of Y chromosome material found in the peripheral sample (86%), the duplicated Y cell line was mistakenly identified as a deleted X chromosome on the initial karyotype analysis, and reported as 46,X,del(X)q13. Standard karyotyping is not always the best way to identify cryptic Y chromosome material, and observation of any structural aberration of the sex chromosomes should therefore be followed with molecular cytogenetic investigation such as FISH and/or an array based diagnostic. Although microarray is not the gold standard under current clinical practice guidelines, given the discrepancy of the original karyotype in this case, microarray was performed to delineate the breakpoints, and rule out any cryptic complexity.
It is important to accurately identify the precise Turner karyotype as it correlates with the severity of the Turner phenotype and specific sequelae [2]. For example, haploinsufficiency of genes on the long arm of the X chromosome is associated with gonadal dysgenesis, while patients with a deletion of the short arm usually have normal ovarian function [16]. Similarly, identifying a Y-chromosome line in a patient with Turner syndrome is important due to the risk of gonadoblastoma, estimated to be about 7–10% [5]. Gonadoblastoma is an in situ germ cell neoplasm, associated with an increased risk of developing invasive malignant germ cell tumors. These tumors are found in more than 30% of patients with XY gonadal dysgenesis and in 15–20% of those with mixed X/XY gonadal dysgenesis [11] [17]. Conventional karyotype analysis may not reveal such findings, putting the patient at risk for malignant transformation if missed. PCR and FISH analysis may reveal information that is not discernable by conventional karyotyping, and as suggested by newer studies, should be used for enhanced screening of patients with Turner syndrome, particularly in those with marker chromosomes and even in those without signs of virilization [18] [11] [10] [4].
FISH for SRY alone however is not always adequate, and an array based diagnostic should strongly be considered. Although SRY is important for other aspects of sexual differentiation, the SRY region of the Y chromosome has been found lacking in many patients with gonadoblastoma [19]. More recent literature suggests that FISH studies on patients with Turner syndrome should include probes that specifically target the SRY, TSPY, and DYZ3 gene regions to more adequately assess for the presence of cryptic Y chromosome material [7] [8] [9] [20]. In the present case, since the microarray analysis revealed that all regions of the Y chromosome were present in the dup(Y)(q11.21q11.23) cell line, the SRY probe was used as a marker for the presence of the aberrant Y chromosome.
Mizuno and colleagues [21] reported a case of mixed gonadal dysgenesis (45,X/46,XY) in a phenotypic male whose right sided streak gonad was negative for SRY despite SRY presence in the peripheral sample. Conversely, Bisat and colleagues [22] reported a case in which Y chromosome material was discovered in the gonad but was absent in the peripheral sample of a girl with Turner syndrome and virilization. Identifying Y chromosome material in the peripheral blood therefore does not necessarily predict its presence in gonadal tissue [23], and cannot be used as a proxy for gonadal prevalence. Our phenotypically female subject had an 86% prevalence of Y chromosome material in the peripheral blood and only a 6.5% prevalence in the gonadal tissue, verifying that mosaic distribution differs between tissues. Guedes [23] reported a similar case of a woman with 45,X/46,X,idic(Yp) mosaicism who had a 97% Y chromosome prevalence in her peripheral lymphocytes, but only a 40% prevalence in the streak gonads.
Alvarez-Nava and colleagues [24] reported 3 subjects with the same karyotype, 45,X/46,X,+mar (Y) but 3 distinct phenotypic presentations. It was the gonosomal karyotype found in the majority of cells in the gonadal tissue that was correlated with the patients’ phenotypic characteristics. Mazzanti and colleagues [11] reported 5 cases of gonadectomized Turner patients in which Y chromosome prevalence was lower in the gonadal tissue than in the blood. Identifying the prevalent gonosome karyotype is important, considering that it appears to influence phenotypic presentation [23] [25] [26], and also likely impacts the risk of malignancy [27] [17]. However, until the gonadal prevalence of Y chromosome material can be directly correlated with risk of malignancy, current management of such cases with prophylactic gonadectomy is most prudent.
Streak gonads are a type of dysgenetic gonad characterized primarily by ovarian stroma without sex cord or germ cell elements, though there may also be presence of other cellular types, such as rete structures [28]. In cases of Turner syndrome where there is evidence of Y chromosome material, streak gonads may also contain tubular structures indicative of potential testes [29] in addition to gonadoblastoma nests. Microscopic analysis of the streak gonads in our subject contained sheets of ovarian stroma along with areas showing tubule histology. The distribution of SRY in the gonadal sample correlated exclusively with the areas containing these tubal cells, while the areas with ovarian stroma showed the 45,X karyotype. A similar distribution was reported by Salas Cortes [30] in patients with 46,XY pure gonadal dysgenesis. In the streak gonads of these patients, SRY was found in the nuclei of the rete and tubular cells. It has also been reported that there is a higher prevalence of Y chromosome material in gonadoblastoma cells as compared with the surrounding non tumor cells [31], perhaps suggesting an association between Y chromosomal distribution or concentration and malignancy risk. It is also interesting that in our subject, evidence of tubal formation was seen in the streak gonad despite only a 6.5% prevalence of Y material. As we cannot currently use Y prevalence and distribution in the gonad, or the mere presence of tubal structures, to predict specific risk of developing gonadoblastoma and malignant transformation, we felt that gonadectomy in this patient was certainly justified, and the most prudent course of management. Whether this pattern of expression confers risk of malignancy is unclear and further investigation in this area is warranted.
Established facts.
Although monosomy X is the most common karyotype in Turner Syndrome, Y chromosome material has been reported in about 10% of patients.
The presence of Y chromosome material confers a risk of gonadoblastoma.
Gonadoblastoma cells contain a high prevalence of Y chromosome material.
Novel Insights.
FISH and/or microarray based studies should be considered for any patient with a structurally abnormal X chromosome, despite absence of virilization.
Y prevalence may differ significantly in peripheral blood and gonadal tissue
The gonosomal complement that makes up the majority of cells in gonadal tissue determines sexual phenotypic presentation
When Y chromosome material is present in the gonadal tissue of a woman with Turner syndrome, it is likely to localize exclusively in tubal structures in the gonad and be absent from any ovarian stroma that is present.
Tubal structures may be present in the gonad despite a relatively low prevalence of Y chromosome material within the tissue itself.
Acknowledgments
Funding Source/Acknowledgements: NIH/NIDDK T32DK065522 (TGB & SEO, P.I.)
Footnotes
Ethical Considerations: As per Columbia University’s Institutional Review Board, case reports of a single patient that do not contain unique identifiers do not require IRB approval. As per the privacy office, this case has been de-identified and contains no unique identifiers. Policy has been attached for your convenience.
Conflict of Interest: None of the authors have any conflicts of interest to declare.
References
- 1.Stockholm K, Juul S, Juel K, Naeraa RW, Gravholt CH. Prevalence, incidence, diagnostic delay, and mortality in Turner Syndrome. The Journal of clinical endocrinology and metabolism. 2006;91:3897–3902. doi: 10.1210/jc.2006-0558. [DOI] [PubMed] [Google Scholar]
- 2.Bispo A, Dos Santos LO, Burégio-Frota P, Galdino MB, Duarte AR, Leal GF, Araújo J, Gomes B, Soares-Ventura EM, Muniz MT, Santos N. Effect of chromosome constitution variations on the expression of Turner phenotype. Genet Mol Res. 2013;12(4):4243–4250. doi: 10.4238/2013.March.13.13. [DOI] [PubMed] [Google Scholar]
- 3.Saenger P, Wikland KA, Conway GS. Recommendations for the diagnosis and management of Turner syndrome. The Journal of clinical endocrinology and metabolism. 2001;86(7):3061–9. doi: 10.1210/jcem.86.7.7683. [DOI] [PubMed] [Google Scholar]
- 4.Cortés-Gutiérrez EI, Herrera-Bartolo R, Dávila-Rodríguez MI, Palacios-Saucedo GC, Vargas-Villarreal J, Romero-Villarreal JB. Molecular detection of cryptic Ychromosomal material in patients with Turner syndrome. Oncology Reports. 2012;28:1205–1210. doi: 10.3892/or.2012.1916. [DOI] [PubMed] [Google Scholar]
- 5.Gravholt C, Fedder J, Naeraa RW, Müller J. T Occurrence of gonadoblastoma in females with Turner syndrome and Y chromosome material: a population study. he Journal of clinical endocrinology and metabolism. 2000;85(9):3199–202. doi: 10.1210/jcem.85.9.6800. [DOI] [PubMed] [Google Scholar]
- 6.Zinn A, Page DC. Turner syndrome and the Y chromosome. In: Hibi I, Takano K, editors. Basic and clinical approach to Turner syndrome. Amsterdam: Elsevier; 1993. pp. 49–56. [Google Scholar]
- 7.Ackermann A, Bamba V. Current controversies in turner syndrome: Genetic testing, assisted reproduction, and cardiovascular risks. Journal of Clinical and Translational Endocrinology. 2014;1(3):61–65. doi: 10.1016/j.jcte.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kersemaekers A, Honecker F, Stoop H, Cools M, Molier M, Wolffenbuttel K, Bokemeyer C, Li Y, Lau YF, Oosterhuis JW, Looijenga LH. Identification of germ cells at risk for neoplastic transformation in gonadoblastoma: an immunohistochemical study for OCT3/4 and TSPY. Human Pathology. 2005;36(512–521) doi: 10.1016/j.humpath.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Hertel JD, Huettner P, Dehner L, Pfeifer J. The Chromosome Y-linked testis specific protein locus TSPY1 is characteristically present in gonadoblastoma. Human Pathology. 2010;41:1544–1549. doi: 10.1016/j.humpath.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Bianco B, Lipay MV, Melaragno MI, Guedes AD, Verreschi IT. Detection of hidden Y mosaicism in Turner's syndrome: importance in the prevention of gonadoblastoma. J Pediatr Endocrinol Metab. 2006;19(9):1113–7. doi: 10.1515/jpem.2006.19.9.1113. [DOI] [PubMed] [Google Scholar]
- 11.Mazzanti L, Cicognani A, Baldazzi L, Bergamaschi R, Scarano E, Strocchi S, Nicoletti A, Mencarelli F, Pittalis M, Forabosco A, Cacciari E. Gonadoblastoma in Turner Syndrome and Y chromosome derived material. Am J Med Genet A. 2005;135:150–154. doi: 10.1002/ajmg.a.30569. [DOI] [PubMed] [Google Scholar]
- 12.Bispo A, Buregio-Frota O, Oliveira dose Santos L, Leal GF, Duarte AR, Araujo J, Cavalcante da Silva V, Muniz MT, Liehr T, Santos N. Y chromosome in Turner syndrome: detection of hidden mosaicism and the report of a rare X;Y translocation case. Reprod Fertil Dev. 2014;26:1176–82. doi: 10.1071/RD13207. [DOI] [PubMed] [Google Scholar]
- 13.Kim H, Shin JH, Jung WY, Lee JN. Identification of Y chromosome by Molecular Analysis in Patients with Turner Syndrome. Korean J Lab Med. 2006;26(2):131–136. doi: 10.3343/kjlm.2006.26.2.131. [DOI] [PubMed] [Google Scholar]
- 14.Sallai A, Solyom J, Dobos M, Szabó J, Halász Z, Ságodi L, Niederland T, Kozári A, Bertalan R, Ugocsai P, Fekete G. Y chromosome markers in Turner Syndrome: screening of 130 patients. J Endocrinol Invest. 2010;33:222–227. doi: 10.1007/BF03345783. [DOI] [PubMed] [Google Scholar]
- 15.Held K, Kerber S, Kaminsky E. Mosaicism in 45,X Turner syndrome: does survival in early pregnancy depend on the presence of two sex chromosomes. Human genetics. 1992;88(3):288–94. doi: 10.1007/BF00197261. [DOI] [PubMed] [Google Scholar]
- 16.Davison R, Fox M, Conway GS. Mapping of the POF1 locus and identification of putative genes fro premature ovarian failure. Mol. Hum. Reprod. 2000;6:314–318. doi: 10.1093/molehr/6.4.314. [DOI] [PubMed] [Google Scholar]
- 17.Verp M, Simpson JL. Abnormal sexual differentiation and neoplasia. Cancer genetics and cytogenetics. 1987;25(2):191–218. doi: 10.1016/0165-4608(87)90180-4. [DOI] [PubMed] [Google Scholar]
- 18.Chu C, Connor JM, Donaldson MD, Kelnar CJ, Smail PJ, Greene SA. Detection of Y mosaicism in patients with Turner's syndrome. J med Genet. 1995;32(578–580) doi: 10.1136/jmg.32.7.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kido T, Lau YF. Roles of the Y chromosome genes in human cancers. Asian Journal of Andrology. 2015;17:373–380. doi: 10.4103/1008-682X.150842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Marqui A, da Silva-Grecco RL, Balarin MA. Prevalence of Y-chromosome sequences and gonadoblastoma in Turner syndrome. Revista paulista de pediatria : orgão oficial da Sociedade de Pediatria de São Paulo. 2016;34(1):114–21. doi: 10.1016/j.rppede.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizuno K, Kojima Y, Tozawa K, Sasaki S, Hayashi Y, Kohri K. Molecular evaluation of the SRY gene for gonads of patients with mixed gonadal dysgenesis. International journal of urology: Official journal of the Japanese Urological Association. 2005;12(7):673–6. doi: 10.1111/j.1442-2042.2005.01119.x. [DOI] [PubMed] [Google Scholar]
- 22.Bisat T, May K, Litwer S, Broecker B. Y chromosome mosaicism in the gonads, but not in the blood, of a girl with the Turner phenotype and virilized external genitalia. Clinical genetics. 1993;44(3):142–5. doi: 10.1111/j.1399-0004.1993.tb03865.x. [DOI] [PubMed] [Google Scholar]
- 23.Guedes A, Bianco B, Lipay MV, Brunoni D, de Lourdes Chauffaille M, Verreschi IT. Determination of the sexual phenotype in a child with 45,X/46,X,Idic(Yp) mosaicism: importance of the relative proportion of the 45,X line in gonadal tissue. American journal of medical genetics. 2006;Part A. 140A(17):1871–5. doi: 10.1002/ajmg.a.31363. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez-Nava F, Soto M, Martínez MC, Prieto M, Alvarez Z. FISH and PCR analyses in three patients with 45,X/46,X,idic(Y) karyotype: clinical and pathologic spectrum. Annales de génétique. 2003;46(4):443–8. doi: 10.1016/s0003-3995(03)00016-9. [DOI] [PubMed] [Google Scholar]
- 25.Kelly T, Franko JB, Rogol A, Golden WL. Discordant phenotypes and 45,X/46,X,idic(Y) Journal of medical genetics. 1998;35(10):862–864. doi: 10.1136/jmg.35.10.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Telvi L, Lebbar A, Del Pino O, Barbet JP, Chaussain JL. 45,X/46,XY mosaicism: report of 27 cases. Pediatrics. 1999;104(2):304–8. doi: 10.1542/peds.104.2.304. [DOI] [PubMed] [Google Scholar]
- 27.Scully R. Gonadoblastoma. A review of 74 cases. Cancer. 1970;25(6):1340–56. doi: 10.1002/1097-0142(197006)25:6<1340::aid-cncr2820250612>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 28.Boyd T. Disorders of Sexual Differentiation. Current Concepts of Pediatric Pathology. Vol. 3. Surgical Pathology Clinics; 2010. [DOI] [PubMed] [Google Scholar]
- 29.Vilain E, Jaubert F, Fellous M, McElreavey K. Pathology of 46,XY pure gonadal dysgenesis: absence of testis differentiation associated with mutations in the testis-determining factor. Differentiation;research in biological diversity. 1993;52(2):151–9. doi: 10.1111/j.1432-0436.1993.tb00625.x. [DOI] [PubMed] [Google Scholar]
- 30.Salas-Cortés L, Jaubert F, Nihoul-Feketé C, Brauner R, Rosemblatt M, Fellous M. SRY protein is expressed in ovotestis and streak gonads from human sex-reversal. Cytogenetics and cell genetics. 2000;91:212–6. doi: 10.1159/000056847. [DOI] [PubMed] [Google Scholar]
- 31.Iezzoni J, Von Kap-Herr C, Golden WL, Gaffey MJ. Gonadoblastomas in 45,X/46,XY mosaicism: analysis of Y chromosome distribution by fluorescence in situ hybridization. American journal of clinical pathology. 1997;108(2):197–201. doi: 10.1093/ajcp/108.2.197. [DOI] [PubMed] [Google Scholar]