Abstract
Platelet granules are unique among secretory vesicles in both their content and their life cycle. Platelets contain three major granule types—dense granules, α-granules, and lysosomes—although other granule types have been reported. Dense granules and α-granules are the most well-studied and the most physiologically important. Platelet granules are formed in large, multilobulated cells, termed megakaryocytes, prior to transport into platelets. The biogenesis of dense granules and α-granules involves common but also distinct pathways. Both are formed from the trans-Golgi network and early endosomes and mature in multivesicular bodies, but the formation of dense granules requires trafficking machinery different from that of α-granules. Following formation in the megakaryocyte body, both granule types are transported through and mature in long proplatelet extensions prior to the release of nascent platelets into the bloodstream. Granules remain stored in circulating platelets until platelet activation triggers the exocytosis of their contents. Soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins, located on both the granules and target membranes, provide the mechanical energy that enables membrane fusion during both granulogenesis and exocytosis. The function of these core fusion engines is controlled by SNARE regulators, which direct the site, timing, and extent to which these SNAREs interact and consequently the resulting membrane fusion. In this review, we assess new developments in the study of platelet granules, from their generation to their exocytosis.
Keywords: Platelets, Granule, Exocytosis, Platelet activation
Introduction
Platelets are anucleate, discoid-shaped blood cells essential for hemostasis, which serves to maintain the integrity of the vasculature upon injury. The functional role of platelets has expanded in recent years to include processes such as inflammation, innate immunity, growth and development, angiogenesis, wound healing, and cancer metastasis 1. Platelet granule exocytosis is central to platelet function and participates in the full repertoire of platelet activities. Platelets contain at least three major types of granules— α-granules, dense granules, and lysosomes—which carry distinct cargos and vary in biogenesis, trafficking, and exocytosis. In addition, platelets have peroxisomes and recently described T granules. This review focuses on the biogenesis of platelet α- and dense granules and mechanisms of their exocytosis.
Platelet granules
α-Granules are unique to platelets and are the most abundant of the platelet granules, numbering 50–80 per platelet 2. These granules measure 200–500 nm in diameter and account for about 10% of platelet volume. They contain mainly proteins, both membrane-associated receptors (for example, αIIbβ3 and P-selectin) and soluble cargo (for example, platelet factor 4 [PF4] and fibrinogen). Proteomic studies have identified more than 300 soluble proteins that are involved in a wide variety of functions, including hemostasis (for example, von Willebrand factor [VWF] and factor V), inflammation (for example, chemokines such as CXCL1 and interleukin-8), and wound healing (for example, vascular endothelial growth factor [VEGF] and fibroblast growth factor [FGF]) 3. The classic representation of α-granules as spherical organelles with a peripheral limiting membrane, a dense nucleoid, and progressively lucent peripheral zones on transmission electron microscopy is probably simplistic and may be in part a preparation artifact. Electron tomography with three-dimensional reconstruction of platelets is notable for a significant percentage of tubular α-granules that generally lack VWF 4. More recent work using transmission electron microscopy and freeze substitution dehydration of resting platelets shows that α-granules are ovoid with a generally homogeneous matrix and that tubes form from α-granules upon activation 5. Thus, whether or not there exists significant structural heterogeneity among α-granules remains to be completely resolved. α-Granule exocytosis is evaluated primarily by plasma membrane expression of P-selectin (CD62P) by flow cytometry or estimation of the release of PF4, VWF, or other granule cargos 6.
Dense granules (also known as δ-granules) are the second most abundant platelet granules, with 3–8 per platelet. They measure about 150 nm in diameter 2. These granules, unique to the platelets, are a subtype of lysosome-related organelles (LROs), a group that also includes melanosomes, lamellar bodies of the type II alveolar cells, and lytic granules of cytotoxic T cells 7. Dense granules mainly contain bioactive amines (for example, serotonin and histamine), adenine nucleotides, polyphosphates, and pyrophosphates as well as high concentrations of cations, particularly calcium. These granules derive their name from their electron-dense appearance on whole mount electron microscopy, which results from their high cation concentrations 8. Dense granule exocytosis is typically evaluated by ADP/ATP release by using luciferase-based luminescence techniques, release of preloaded [ 3H] serotonin, or membrane expression of lysosome-associated membrane protein 2 (LAMP2) or CD63 by flow cytometry 6.
Other platelet granules have been described. Platelets contain about 1–3 lysosomes per platelet and peroxisomes, the platelet-specific function of which remains unclear. Lysosomal exocytosis is typically evaluated by estimation of released lysosomal enzymes such as beta hexosaminidase. An electron-dense granule defined by the presence of Toll-like receptor 9 (TLR9) and protein disulfide isomerase (PDI), termed the T granule, has also been described, although its existence remains controversial 9. PDI and other platelet-borne thiol isomerases have been reported to be packaged within a non-granular compartment derived from the megakaryocyte endoplasmic reticulum (ER), which may be associated with the dense tubular system 10, 11.
Biogenesis of platelet granules
Formation of platelet granules begins in megakaryocytes, but maturation continues in circulating platelets 12, 13. Human platelet granule deficiency syndromes, also referred to as storage pool disorders, and their related murine models have been a major source of study of platelet granulogenesis. Gray platelet syndrome (GPS), an α-granule deficiency disorder, and Hermansky–Pudlak syndrome (HPS), a group of dense granule deficiency syndromes, are two such examples. GPS platelets contain normal dense granules, whereas HPS6 platelets contain normal α-granules, which suggests that these granules have distinct pathways of biogenesis 7, 14, 15. In recent years, many inherited disorders due to defects in transcription factors such as RUNX1, GATA1, FLl1, GFI1b, and ETV6 have been found to impact megakaryopoiesis and impair platelet production and maturation 16– 21. Many of these disorders are associated with one or more granule deficiency states and have helped elucidate the role of these genes in platelet granulogenesis.
α-Granule biogenesis
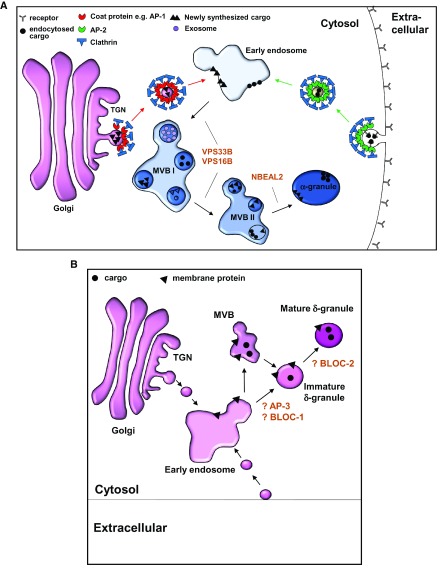
α-Granule proteins derive from both synthetic and endocytic pathways 22. Synthetic pathways traffic translated proteins from ER to α-granules. The endocytic pathway enables megakaryocytes and mature platelets to acquire plasma proteins by the process of endocytosis at the plasma membrane 23. Multiple individual proteins and protein complexes mediate trafficking of these separate pathways ( Figure 1A). Such proteins include coat proteins such as clathrin, adaptor proteins AP1 and AP2, and proteins required for vesicle trafficking, including soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor (SNARE) proteins, SNARE regulators, particularly Sec1/Munc18 proteins, and small GTPases such as Rabs. As a first step, soluble clathrin molecules recruited to either trans-Golgi network (TGN) or plasma membrane self-assemble into a lattice structure and interact with APs to form clathrin-coated pits. Platelets contain clathrin-associated adaptor proteins AP1, AP2, and AP3 24. Since AP2 localizes only to plasma membrane where it functions in the endocytotic pathway and AP3 is critical for lysosomal and LRO trafficking, the deficiency of which leads only to dense granule deficiency as in HPS subtype 2, AP1 is assumed to be employed by the synthetic pathway in α-granule biogenesis, although there is no direct evidence for this or for the role of other coat proteins such as COPI in α-granule biogenesis 25. Vesicles carrying α-granule cargo budding off from either TGN or plasma membrane are subsequently directed to multivesicular bodies (MVBs) via endosomes 26.
Figure 1. Working models of platelet α-granule and dense granule formation in megakaryocytes.
( A) α-Granules derive from two major pathways: synthetic and endocytic. The synthetic pathway originates at the trans-Golgi network (TGN). Soluble clathrin molecules recruited to the TGN self-assemble into a lattice structure and interact with coat proteins, presumed to be adaptor protein 1 (AP1), to form clathrin-coated pits. These pits invaginate to bud off early membrane-bound vesicles that are ultimately directed to early endosomes. Endocytic vesicles originate similarly at the plasma membrane employing adaptor protein 2 (AP2) and ultimately merge into early endosomes. α-Granules mature in multivesicular bodies (MVBs), a process that requires proteins VPS33B, VPS16B, and NBEAL2. ( B) Dense (δ) granules are lysosomal-related organelles, which are derived from the endosomal compartment. The current understanding of biogenesis of dense granule is highly speculative and was extrapolated from the biogenesis of melanosomes. Early endosomes provide input for developing dense granules, which may mature in MVBs. In melanosomes, BLOC1 is required for the exit of tubular structures carrying cargo from the endosomes, which are directed to the developing melanosomes by BLOC2. Alternatively, cargoes can be directed to developing dense granules by an AP3-dependent pathway, which may or may not require BLOC2. BLOC, biogenesis of lysosome-related organelles complex.
MVBs are transient late endosomal structures that contain internal vesicles formed from inward budding of the limiting membrane of the endosome 27. Initially assumed to only direct proteins to be degraded in the lysosomes, these structures are now known to have multiple other functions, including granule trafficking in various cell types. MVBs serve as an intermediate stage of granulogenesis in megakaryocytes 26. α-Granule cargoes from both synthetic and endocytic pathways can be identified in MVBs 26. Both α- and dense granules mature from MVBs but use distinct machinery 26. For example, defects in VPS33B and NBEAL2 lead only to α-granule deficiency but not to dense granule defects. VPS33B, a Sec1/Munc18 protein deficient in arthrogryposis, renal dysfunction, and cholestasis (ARC) syndrome, was the first protein involved in α-granule biogenesis to be identified 28, 29. VPS16B, its partner, works in association 30. Although many platelet-specific details remain poorly understood, the SNARE binding function of VPS33 and VPS16 in vesicular trafficking as a component of two large protein complexes—class C core vacuole/endosome/tethering (CORVET), containing isoforms VPS33B and VPS16B, and homotypic fusion and protein sorting (HOPS), containing isoforms VPS33A and VPS16A—has been characterized in yeast 31. The other proteins of these complexes have membrane-, AP-, and Rab-binding properties, thus bringing together the basic machinery required for endosomal maturation. NBEAL2 deficiency as a cause of GPS was first described in 2011 14, 32, 33. Nbeal2 -/- mice exhibit a phenotype similar to that of patients with GPS, including macrothrombocytopenia, splenomegaly, and myelofibrosis, but the exact molecular function of NBEAL2 is not known 34. It acts at a later state of α-granule development, independently of VPS33B, as Nbeal2 -/- platelets express some P-selectin that externalizes upon platelet activation. NBEAL2 is under direct transcriptional control of GATA1, a mutation in which results in a syndrome similar to GPS, in addition to myelodysplasia 35. It is one of the nine BEACH (beige and Chediak-Higashi) domain-containing proteins, hypothesized to be scaffolds for fission and fusion membrane events 36. Genetic defects in Chediak-Higashi syndrome 1 ( CHS1), another member of this family, lead to platelet dysfunction secondary to dense granule deficiency in addition to immunodeficiency and other manifestations 36.
Protein sorting and packaging into the developing α-granule occur via varying mechanisms dependent on the protein type. Many membrane proteins, such as P-selectin, contain signal peptides that direct them to the developing granule 37. Notably, P-selectin uses distinct signal peptides for trafficking to the α-granules in platelets and Weibel–Palade bodies in endothelial cells 38. Another mechanism is protein aggregation, which is employed by large soluble proteins such as multimerin and VWF 39, 40. VWF self-assembles into large homoaggregates that ultimately form tubular structures occupying a distinct sub-compartment within α-granules 41. Sorting sequences contribute to trafficking of many smaller soluble proteins to α-granules. PF4 is one such protein that has a four-amino acid sequence within its hydrophilic loop that directs it to the maturing α-granule 42. Other examples of small proteins that employ sorting sequences are RANTES and NAP2. Cationic glycosaminoglycans within α-granules may also serve to retain these small chemokines 43. Exogenous proteins are trafficked through an endocytic pathway into α-granules via either receptor-mediated endocytosis or pinocytosis. Fibrinogen, which is internalized via integrin αIIbβ 3, is a classic example of this route, which subsequently uses adaptor protein Disabled-2 for formation of clathrin-coated vesicles 44, 45. Proteins that are incorporated into platelets via pinocytosis include immunoglobulins as well as angiogenesis regulators such as VEGF, endostatin, and FGF 23, 46. Vesicle-associated membrane protein 3 (VAMP-3), a v-SNARE (discussed below), regulates platelet endocytosis. VAMP-3 -/- platelets show impaired αIIbβ 3-mediated fibrinogen uptake 47. In addition, loss of VAMP-3 impairs trafficking of both endocytosed and pinocytosed cargo between Rab4 (early endosomes) and Rab11 (recycling endosomes) positive compartments, although its mechanism remains unclear. Endocytosis of plasma proteins starts in megakaryocytes but continues in mature circulating platelets. For example, platelets from patients with complete factor V deficiency endocytose and release factor V supplemented in transfused plasma for prolonged periods greater than the half-life of factor V 48.
Dense granule biogenesis
Dense granules are platelet-specific LROs 7. These granules are distinct from classic secretory granules in that they are derived from the endosomal system instead of directly from TGN ( Figure 1B). They also share some characteristics with lysosomes as their intra-granular pH is acidic and they possess lysosome-resident proteins, such as the tetraspanin CD63. However, CD63 is not restricted to dense granules in platelets, and the lack of other specific cargoes that can be followed biosynthetically has made evaluation of dense granule biogenesis challenging. There is evidence that early endosomes contribute to dense granule biogenesis 49. In addition, like α-granules, dense granules are believed to be sorted in MVBs, although the only direct evidence of this is the accumulation of CD63 and serotonin in MVBs in megakaryocytes 50. HPS and related disorders together with their murine counterparts have served as a great source of understanding of biogenesis of LROs, and melanosomes are the prototype organelle that has been studied.
In total, at least 10 different HPS genes encode subunits of four distinct ubiquitously present protein complexes: adaptor protein-3 (AP3) and biogenesis of lysosome-related organelles complex (BLOC) 1, 2, and 3 51– 54. These complexes localize mainly to the endosomal compartment and are essential for biogenesis of LROs. Deficiency or alteration in these proteins results in two common manifestations: albinism due to abnormal melanogenesis and a bleeding disorder due to dense granule deficiency. Some HPS subtypes display other manifestations, such as pulmonary fibrosis, inflammatory bowel disease, and immunodeficiency 51. Functions of these individual proteins and protein complexes are being understood with increasing detail. In melanosomes, BLOC1 (complex of HPS7, HPS8, HPS9, Muted, Cappuccino, Snapin, BLOS2, and BLOS3) is required for the exit of melanosome cargoes from endosomes into tubular transport carriers 55. BLOC2 (complex of HPS3, HPS5, and HPS6) directs these carriers specifically to the melanosomes. Alternatively, cargoes can be directed into developing melanosomes in an AP3-dependent pathway, which in turn can be BLOC1-independent or -dependent 55– 58. BLOC3 (complex of HPS1 and 4) functions after cargo delivery in pathways out of melanosomes, specifically in retrieval and recycling of the BLOC1-dependent v-SNARE VAMP-7 59. Owing to concurrence of albinism and dense granule deficiency in HPS, pathways similar to those described above are thought to function in dense granule biogenesis in megakaryocytes, although there is no direct evidence. The exact molecular functions of many of the HPS and related proteins are also being characterized, mainly in melanosomes. HPS9, or Pallidin, a component of BLOC1, is known to interact with syntaxin 13, a SNARE protein involved in vesicle membrane fusion during trafficking 60. BLOC2 constituents HPS3 and HPS6 have been described to bind clathrin and dynactin p150Glued, respectively 61, 62. BLOC3 functions as a guanine nucleotide exchange factor for cell type-specific Rab GTPases, such as Rab32 and Rab38 in melanocytes 63, 64. A direct role of Rab32 and Rab38 in dense granule biogenesis in megakaryocytes has also been implicated 13, 64. RUNX1 mutations lead to dense granule but not α-granule deficiency due to dysregulation of Pallidin (HPS9) transcription 65.
Dense granule contents, such as bioactive amines and adenine nucleotides, are transported into the maturing dense granules via specific membrane pumps, such as vesicular nucleotide transporter (VNUT), which has been proposed as a candidate for ADP and ATP accumulation in dense granules, and multidrug resistance-associated protein 4 (MRP4), which uptakes cAMP into dense granules 66– 68. MRP4 -/- mice show significant platelet dysfunction due to cytosolic accumulation of cAMP and lack of cAMP in dense granules, as do inhibitors of MRP4, such as probenecid 67, 69. York platelet syndrome is characterized by thrombocytopenia and striking giant electron-opaque organelles. It is caused by a calcium-selective release-activated calcium (CRAC) channelopathy, which results in defective calcium storage 70.
Platelet granule exocytosis
Platelet granule exocytosis is a classic example of regulated secretion. Upon agonist stimulation, cargo stored in platelet granules is released, and rates and extent are dependent on the stimulation strength 71. Dense granule exocytosis is fastest and most sensitive to agonists, whereas lysosome exocytosis is slow and requires more stimulation. α-Granule exocytosis is considered to be intermediate. The kinetics and extent of platelet exocytosis vary depending on the concentration and potency of the agonist used, but whether the composition of released cargo follows any agonist-dependent patterns remains controversial 71. The distinct cellular localization of two major platelet v-SNAREs—VAMP-7 and VAMP-8, discussed in greater detail below—suggests a functional heterogeneity in granule exocytosis 72, 73. However, studies extensively characterizing cargo released using multiple agonists, employing both immunoassays and proteomics, suggest that there may not be any thematic patterns of cargo release 74. Thus, whether or not function-specific platelet exocytosis of α-granule subpopulations occurs under physiological conditions remains to be established.
Fusion of vesicle membrane with the plasma membrane is the general scheme of exocytosis in nucleated cells. Platelets follow this general rule but with some atypical features. Platelet granules, which are uniformly distributed throughout the platelet, move centrally upon platelet stimulation and spreading, although this may be artefactual. Second, in addition to fusion with the plasma membrane, most granule exocytosis follows fusion of platelet granules with the open canalicular system (OCS), which are plasma membrane invaginations that increase platelet surface area by at least two- to three-fold 75, 76. α-Granules fuse with the membrane individually as well as in the form of large multi-granular compartments that result from granule–granule fusion. This pattern of granule–granule fusion followed by granule–plasma membrane fusion occurs exclusively in α-granules at higher agonist concentrations 77.
SNAREs
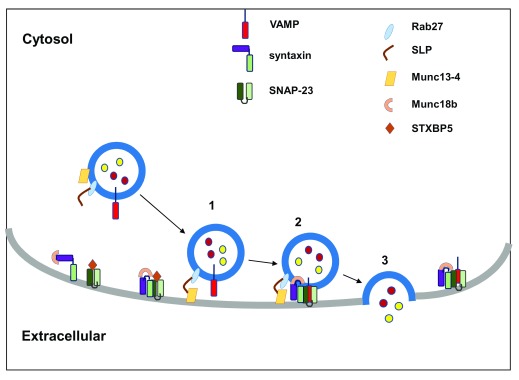
Membrane fusion is facilitated by SNARE proteins, a family of highly conserved eukaryotic proteins essential for vesicle fusion 78. SNARE proteins are classified into two groups on the basis of their location: v-SNAREs, located on the vesicle/granule membrane, and t-SNAREs, located on the target membrane (for example, plasma membrane). Related v- and t-SNAREs interact through SNARE domains, which are α-helices of about 60 amino acids, assembled into amphipathic, heptad repeats. SNAREs can also be classified as R-SNAREs (typically v-SNAREs) or Q-SNAREs (typically t-SNAREs), depending on the presence of an arginine or glutamine residue, respectively, in the central position of the SNARE domain 79. Four SNARE domains—one each from the v-SNARE plus three t-SNAREs—form a coiled-coil structure that brings the two opposing membranes together (for example, granule and plasma membrane) against repulsive electrostatic forces of the two lipid membranes in an aqueous environment ( Figure 2) 80.
Figure 2. SNARE-mediated platelet granule exocytosis.
The pathway of platelet granule exocytosis involves (1) granule docking, (2) priming, and (3) membrane fusion and cargo release. Rab27b and its effectors syntaptotagmin-like protein and Munc13-4 present on vesicle membrane are required for granule docking. Platelet activation promotes conformation change in syntaxins, sequestered by Munc18b in the resting state. This activation results in “priming” with subsequent formation of a four-helical bundle consisting of one R-SNARE provided by VAMP (red) and three Q-SNAREs provided by syntaxin and SNAP-23 (shades of green). In addition, syntaxin binding protein 5 (STXBP5) regulates t-SNARE function by binding syntaxin-SNAP-23 heterodimers. SNARE engagement ultimately leads to formation of the membrane fusion pore and cargo release. SNAP, soluble NSF attachment proteins; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; VAMP, vesicle-associated membrane protein.
VAMPs constitute the largest group of v-SNAREs. Platelets contain VAMP-2, -3, -7, and -8; VAMP-8 is the most abundant and functionally important in human platelets, followed by VAMP-7 81. Loss of VAMP-8 in mice causes defective α- and dense granule exocytosis, and platelet thrombus formation in vivo, without excessive bleeding 82. On the other hand, loss of VAMP-7 in mice leads to defective platelet spreading, and altered α- and dense granule exocytosis, without impacting platelet thrombus formation or bleeding 73. Moreover, VAMP-7 is located peripherally in the spreading platelet whereas VAMP-8 concentrates in the central granulomere 72. These observations suggest a distinct role for these v-SNAREs in platelet function. Interestingly, VAMP-8 has also been linked to early-onset myocardial infarction in genome-wide association studies, suggesting a syndrome of platelet hyper-responsiveness 83. VAMP-3, which is important in the endocytotic pathway of α-granule biogenesis, has minimal function in platelet exocytosis 47. The role of platelet-specific VAMPs has not been well established in granulogenesis.
Of the t-SNAREs, proteomic studies suggest that platelet contains syntaxin 2, 4, 6, 7, 8, 11, 12, 16, 17, and 18 and soluble NSF attachment proteins (SNAPs) 23, 25, and 29 84. Of these, syntaxin 11 and SNAP 23 are the only t-SNAREs found to be essential for platelet granule exocytosis. As with v-SNAREs, most data come from mouse models lacking one or more specific t-SNAREs. Loss of syntaxin 11, which forms complexes with SNAP 23 and VAMP-8, is associated with abnormal exocytosis of all three of the major platelet granules 85. In humans, familial hemophagocytic lymphohistiocytosis type 4 is caused by lack of syntaxin 11 86. Loss of syntaxin 8 has been associated with minor defects in dense granule exocytosis 87.
SNARE regulators
To prevent indiscriminate release of granular content, fusion of vesicle and target membranes is tightly regulated by SNARE regulators. Some SNARE regulators are chaperones (for example, Munc18b), while others promote formation of membrane-fusion complexes and direct where the fusion occurs (for example, Munc13-4, Munc18 isoforms, Rabs, STXBP5/Tomosyn 1, and exocyst complex).
Munc18b is the most important syntaxin chaperone belonging to the Sec/Munc family of proteins present in the platelet, forming specific complexes with t-SNAREs. Munc18b deficiency leads to decreases in platelet levels of syntaxin 11, consistent with its role as a chaperone, resulting in impaired granule exocytosis 88. Homozygous deficiency, as seen in familial hemophagocytic lymphohistiocytosis type 5, leads to severe defects in all platelet granule exocytosis, whereas heterozygous deficiency leads to intermediate defects 89. VPS33B, discussed in α-granule biogenesis above, also belongs to the Sec/Munc family of proteins 29.
Syntaxin binding protein 5 (STXBP5), or tomosyn 1, binds to the cytoskeleton and to t-SNARE heterodimers (syntaxin 11 and SNAP-23) through the presence of a v-SNARE-like domain at its C-terminal. Its deficiency causes defective granule exocytosis, and mice lacking STXBP5 show excessive bleeding 90. Interestingly, STXBP5 negatively regulates VWF release from the endothelial cells, and polymorphisms in STXBP5 gene are associated with increased plasma VWF levels and cardiovascular disease 91.
Rab proteins that belong to the Ras superfamily of GTPases function as master regulators of the complex network of intracellular membrane trafficking pathways 92. Rabs perform this regulatory function by binding to effector proteins in the GTP-bound, or “on”, state 93. Some of these Rab effectors are SNARE regulators. Multiple Rabs, including Rab3b, 6c, and 8, are phosphorylated upon platelet activation, and their inhibition decreases platelet exocytosis 94. Among these, Rab4 is crucial for α-granule exocytosis whereas Rab27b is a key regulator of dense granule biogenesis and exocytosis 95, 96. Munc13-4 is a Rab27b effector protein, essential for dense granule function. Munc13-4 forms calcium-dependent bridges between the dense granule and plasma/OCS membrane, facilitating membrane fusion 97, 98. Rab27 -/- and Munc13-4 -/- platelets have defective dense granule exocytosis and a bleeding diathesis. These platelets also display defective exocytosis of α-granules and lysosomes, which can be overcome by the addition of ADP, a key dense granule component 99. This reversal by ADP, as also occurs in HPS platelets, demonstrates the critical role of autocrine signaling from released dense granule cargo for complete platelet activation 15, 100.
Synatotagmin-like proteins (SLPs), particularly SLP1 and SLP4, that bind calcium/lipids are also known to regulate dense granule exocytosis and may act as calcium sensors. SLP1—which forms a complex with Rap1, a Ras-like GTPase, and RAP1GEF2, its guanine nucleotide exchange factor—is a negative regulator of dense granule release 101. SLP4, a Rab27 effector, on the other hand, is a positive regulator of dense granule release 102.
Tethering complexes, particularly the exocyst complex, which is known to play a role in polarized secretion, may also be involved in the regulation of dense granule exocytosis 103. Exocyst complex is targeted to the plasma membrane by Ral, a Ras-like GTPase, which is expressed in platelets and activated upon platelet stimulation. Blocking of Ral-GTP binding to exocyst complex impairs dense granule exocytosis.
NSF and soluble NSF attachment proteins (SNAPs) are also important regulators of platelet exocytosis 104. These proteins disassemble SNARE complexes to allow recycling of v-SNAREs and t-SNAREs for the next round of membrane fusion 105. The inhibitory effect of nitric oxide on platelet exocytosis is at least partly due to its reversible inhibition of NSF 106.
Many SNAREs and their regulators, such as SNAP23 and Munc18, are known to be protein kinase C substrates, linking platelet activation and ensuing signaling cascades to the exocytosis machinery. Platelet signaling and protein phosphorylation and their role in regulated platelet exocytosis are beyond the scope of this review. The reader is referred to excellent reviews on this topic 107– 109.
Conclusions
Regulated release of platelet granules is central to normal platelet function, which includes a variety of biological processes such as inflammation and immunity, in addition to hemostasis and thrombosis. Human platelet granule deficiency syndromes and their murine models, as well as the study of other cell types such as melanocytes and chromaffin cells 92, have been major sources of understanding of the protein machinery involved in platelet granulogenesis and exocytosis. Despite significant progress in identifying this machinery, many questions remain unanswered. What are the roles in granulopoiesis of the different vesicular trafficking proteins identified by genetic studies? What are the exact platelet-specific functions of SNARE regulators critical for platelet exocytosis, such as STXBP5, Munc13-4, and SLPs? Do platelet α-granules demonstrate a function-specific pattern of release, as may be inferred by evidence of different α-granule pools? How do secondary signaling mechanisms generated upon platelet activation control the distal exocytosis machinery? The answer to these questions will enable a clearer view of the life cycle of platelet granules, which is central to understanding platelet function in varied pathophysiologic processes.
Acknowledgments
The authors gratefully acknowledge the thoughtful review and invaluable input from Michael S. Marks and Walter H. Kahr. The authors acknowledge the many significant contributions to the field that, owing to space restrictions, were not cited in this article.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Walter Kahr, Departments of Paediatrics & Biochemistry, University of Toronto, Toronto, ON, Canada; Division of Haematology/Oncology, Cell Biology Program, The Hospital for Sick Children, Toronto, ON, Canada
Michael Marks, Departments of Pathology & Laboratory Medicine and of Physiology, Children’s Hospital of Philadelphia and University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA
Funding Statement
RF has received support from the National Heart, Lung and Blood Institute (R01 HL125275 and R35 HL135775). AS received support from the Hemostasis and Thrombosis Research Society.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Xu XR, Zhang D, Oswald BE, et al. : Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit Rev Clin Lab Sci. 2016;53(6):409–30. 10.1080/10408363.2016.1200008 [DOI] [PubMed] [Google Scholar]
- 2. White JG: Use of the electron microscope for diagnosis of platelet disorders. Semin Thromb Hemost. 1998;24(2):163–8. 10.1055/s-2007-995836 [DOI] [PubMed] [Google Scholar]
- 3. Maynard DM, Heijnen HF, Gahl WA, et al. : The α-granule proteome: novel proteins in normal and ghost granules in gray platelet syndrome. J Thromb Haemost. 2010;8(8):1786–96. 10.1111/j.1538-7836.2010.03932.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Nispen tot Pannerden H, de Haas F, Geerts W, et al. : The platelet interior revisited: electron tomography reveals tubular alpha-granule subtypes. Blood. 2010;116(7):1147–56. 10.1182/blood-2010-02-268680 [DOI] [PubMed] [Google Scholar]
- 5. Pokrovskaya ID, Aronova MA, Kamykowski JA, et al. : STEM tomography reveals that the canalicular system and α-granules remain separate compartments during early secretion stages in blood platelets. J Thromb Haemost. 2016;14(3):572–84. 10.1111/jth.13225 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Linden MD: Platelet flow cytometry. Methods Mol Biol. 2013;992:241–62. 10.1007/978-1-62703-339-8_18 [DOI] [PubMed] [Google Scholar]
- 7. Ambrosio AL, Di Pietro SM: Storage pool diseases illuminate platelet dense granule biogenesis. Platelets. 2017;28(2):138–46. 10.1080/09537104.2016.1243789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gerrard JM, Rao GH, White JG: The influence of reserpine and ethylenediaminetetraacetic acid (EDTA) on serotonin storage organelles of blood platelets. Am J Pathol. 1977;87(3):633–46. [PMC free article] [PubMed] [Google Scholar]
- 9. Thon JN, Peters CG, Machlus KR, et al. : T granules in human platelets function in TLR9 organization and signaling. J Cell Biol. 2012;198(4):561–74. 10.1083/jcb.201111136 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Crescente M, Pluthero FG, Li L, et al. : Intracellular Trafficking, Localization, and Mobilization of Platelet-Borne Thiol Isomerases. Arterioscler Thromb Vasc Biol. 2016;36(6):1164–73. 10.1161/ATVBAHA.116.307461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Nispen Tot Pannerden HE, van Dijk SM, Du V, et al. : Platelet protein disulfide isomerase is localized in the dense tubular system and does not become surface expressed after activation. Blood. 2009;114(21):4738–40. 10.1182/blood-2009-03-210450 [DOI] [PubMed] [Google Scholar]
- 12. Behnke O: Coated pits and vesicles transfer plasma components to platelet granules. Thromb Haemost. 1989;62(2):718–22. [PubMed] [Google Scholar]
- 13. Hanby HA, Bao J, Noh JY, et al. : Platelet dense granules begin to selectively accumulate mepacrine during proplatelet formation. Blood Adv. 2017;1(19):1478–90. 10.1182/bloodadvances.2017006726 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Albers CA, Cvejic A, Favier R, et al. : Exome sequencing identifies NBEAL2 as the causative gene for gray platelet syndrome. Nat Genet. 2011;43(8):735–7. 10.1038/ng.885 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Meng R, Wu J, Harper DC, et al. : Defective release of α granule and lysosome contents from platelets in mouse Hermansky-Pudlak syndrome models. Blood. 2015;125(10):1623–32. 10.1182/blood-2014-07-586727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Songdej N, Rao AK: Hematopoietic transcription factor mutations: important players in inherited platelet defects. Blood. 2017;129(21):2873–81. 10.1182/blood-2016-11-709881 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Stockley J, Morgan NV, Bem D, et al. : Enrichment of FLI1 and RUNX1 mutations in families with excessive bleeding and platelet dense granule secretion defects. Blood. 2013;122(25):4090–3. 10.1182/blood-2013-06-506873 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Tubman VN, Levine JE, Campagna DR, et al. : X-linked gray platelet syndrome due to a GATA1 Arg216Gln mutation. Blood. 2007;109(8):3297–9. 10.1182/blood-2006-02-004101 [DOI] [PubMed] [Google Scholar]
- 19. Noetzli L, Lo RW, Lee-Sherick AB, et al. : Germline mutations in ETV6 are associated with thrombocytopenia, red cell macrocytosis and predisposition to lymphoblastic leukemia. Nat Genet. 2015;47(5):535–8. 10.1038/ng.3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stevenson WS, Morel-Kopp MC, Chen Q, et al. : GFI1B mutation causes a bleeding disorder with abnormal platelet function. J Thromb Haemost. 2013;11(11):2039–47. 10.1111/jth.12368 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Wang Y, Meng R, Hayes V, et al. : Pleiotropic platelet defects in mice with disrupted FOG1-NuRD interaction. Blood. 2011;118(23):6183–91. 10.1182/blood-2011-06-363580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen CH, Lo RW, Urban D, et al. : α-granule biogenesis: from disease to discovery. Platelets. 2017;28(2):147–54. 10.1080/09537104.2017.1280599 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Banerjee M, Whiteheart SW: The ins and outs of endocytic trafficking in platelet functions. Curr Opin Hematol. 2017;24(5):467–74. 10.1097/MOH.0000000000000366 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Moebius J, Zahedi RP, Lewandrowski U, et al. : The human platelet membrane proteome reveals several new potential membrane proteins. Mol Cell Proteomics. 2005;4(11):1754–61. 10.1074/mcp.M500209-MCP200 [DOI] [PubMed] [Google Scholar]
- 25. Park SY, Guo X: Adaptor protein complexes and intracellular transport. Biosci Rep. 2014;34(4): pii: e00123. 10.1042/BSR20140069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heijnen HF, Debili N, Vainchencker W, et al. : Multivesicular bodies are an intermediate stage in the formation of platelet alpha-granules. Blood. 1998;91(7):2313–25. [PubMed] [Google Scholar]
- 27. Woodman PG, Futter CE: Multivesicular bodies: co-ordinated progression to maturity. Curr Opin Cell Biol. 2008;20(4):408–14. 10.1016/j.ceb.2008.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lo B, Li L, Gissen P, et al. : Requirement of VPS33B, a member of the Sec1/Munc18 protein family, in megakaryocyte and platelet alpha-granule biogenesis. Blood. 2005;106(13):4159–66. 10.1182/blood-2005-04-1356 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Bem D, Smith H, Banushi B, et al. : VPS33B regulates protein sorting into and maturation of α-granule progenitor organelles in mouse megakaryocytes. Blood. 2015;126(2):133–43. 10.1182/blood-2014-12-614677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Urban D, Li L, Christensen H, et al. : The VPS33B-binding protein VPS16B is required in megakaryocyte and platelet α-granule biogenesis. Blood. 2012;120(25):5032–40. 10.1182/blood-2012-05-431205 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Balderhaar HJ, Ungermann C: CORVET and HOPS tethering complexes - coordinators of endosome and lysosome fusion. J Cell Sci. 2013;126(Pt 6):1307–16. 10.1242/jcs.107805 [DOI] [PubMed] [Google Scholar]
- 32. Kahr WH, Hinckley J, Li L, et al. : Mutations in NBEAL2, encoding a BEACH protein, cause gray platelet syndrome. Nat Genet. 2011;43(8):738–40. 10.1038/ng.884 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Gunay-Aygun M, Falik-Zaccai TC, Vilboux T, et al. : NBEAL2 is mutated in gray platelet syndrome and is required for biogenesis of platelet α-granules. Nat Genet. 2011;43(8):732–4. 10.1038/ng.883 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Kahr WH, Lo RW, Li L, et al. : Abnormal megakaryocyte development and platelet function in Nbeal2 -/- mice. Blood. 2013;122(19):3349–58. 10.1182/blood-2013-04-499491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wijgaerts A, Wittevrongel C, Thys C, et al. : The transcription factor GATA1 regulates NBEAL2 expression through a long-distance enhancer. Haematologica. 2017;102(4):695–706. 10.3324/haematol.2016.152777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cullinane AR, Schäffer AA, Huizing M: The BEACH is hot: a LYST of emerging roles for BEACH-domain containing proteins in human disease. Traffic. 2013;14(7):749–66. 10.1111/tra.12069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Disdier M, Morrissey JH, Fugate RD, et al. : Cytoplasmic domain of P-selectin (CD62) contains the signal for sorting into the regulated secretory pathway. Mol Biol Cell. 1992;3(3):309–21. 10.1091/mbc.3.3.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harrison-Lavoie KJ, Michaux G, Hewlett L, et al. : P-selectin and CD63 use different mechanisms for delivery to Weibel-Palade bodies. Traffic. 2006;7(6):647–62. 10.1111/j.1600-0854.2006.00415.x [DOI] [PubMed] [Google Scholar]
- 39. Hayward CP: Platelet multimerin and its proteolytic processing. Thromb Haemost. 1999;82(6):1779–80. [PubMed] [Google Scholar]
- 40. Huang RH, Wang Y, Roth R, et al. : Assembly of Weibel-Palade body-like tubules from N-terminal domains of von Willebrand factor. Proc Natl Acad Sci U S A. 2008;105(2):482–7. 10.1073/pnas.0710079105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cramer EM, Meyer D, le Menn R, et al. : Eccentric localization of von Willebrand factor in an internal structure of platelet alpha-granule resembling that of Weibel-Palade bodies. Blood. 1985;66(3):710–3. [PubMed] [Google Scholar]
- 42. El Golli N, Issertial O, Rosa JP, et al. : Evidence for a granule targeting sequence within platelet factor 4. J Biol Chem. 2005;280(34):30329–35. 10.1074/jbc.M503847200 [DOI] [PubMed] [Google Scholar]
- 43. Woulfe DS, Lilliendahl JK, August S, et al. : Serglycin proteoglycan deletion induces defects in platelet aggregation and thrombus formation in mice. Blood. 2008;111(7):3458–67. 10.1182/blood-2007-07-104703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Handagama P, Bainton DF, Jacques Y, et al. : Kistrin, an integrin antagonist, blocks endocytosis of fibrinogen into guinea pig megakaryocyte and platelet alpha-granules. J Clin Invest. 1993;91(1):193–200. 10.1172/JCI116170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hung WS, Huang CL, Fan JT, et al. : The endocytic adaptor protein Disabled-2 is required for cellular uptake of fibrinogen. Biochim Biophys Acta. 2012;1823(10):1778–88. 10.1016/j.bbamcr.2012.06.008 [DOI] [PubMed] [Google Scholar]
- 46. Klement GL, Yip TT, Cassiola F, et al. : Platelets actively sequester angiogenesis regulators. Blood. 2009;113(12):2835–42. 10.1182/blood-2008-06-159541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Banerjee M, Joshi S, Zhang J, et al. : Cellubrevin/vesicle-associated membrane protein-3-mediated endocytosis and trafficking regulate platelet functions. Blood. 2017;130(26):2872–83. 10.1182/blood-2017-02-768176 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Bouchard BA, Chapin J, Brummel-Ziedins KE, et al. : Platelets and platelet-derived factor Va confer hemostatic competence in complete factor V deficiency. Blood. 2015;125(23):3647–50. 10.1182/blood-2014-07-589580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meng R, Wang Y, Yao Y, et al. : SLC35D3 delivery from megakaryocyte early endosomes is required for platelet dense granule biogenesis and is differentially defective in Hermansky-Pudlak syndrome models. Blood. 2012;120(2):404–14. 10.1182/blood-2011-11-389551 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Youssefian T, Cramer EM: Megakaryocyte dense granule components are sorted in multivesicular bodies. Blood. 2000;95(12):4004–7. [PubMed] [Google Scholar]
- 51. Dell'Angelica EC: The building BLOC(k)s of lysosomes and related organelles. Curr Opin Cell Biol. 2004;16(4):458–64. 10.1016/j.ceb.2004.05.001 [DOI] [PubMed] [Google Scholar]
- 52. Falcón-Pérez JM, Starcevic M, Gautam R, et al. : BLOC-1, a novel complex containing the pallidin and muted proteins involved in the biogenesis of melanosomes and platelet-dense granules. J Biol Chem. 2002;277(31):28191–9. 10.1074/jbc.M204011200 [DOI] [PubMed] [Google Scholar]
- 53. Di Pietro SM, Falcón-Pérez JM, Dell'Angelica EC: Characterization of BLOC-2, a complex containing the Hermansky-Pudlak syndrome proteins HPS3, HPS5 and HPS6. Traffic. 2004;5(4):276–83. 10.1111/j.1600-0854.2004.0171.x [DOI] [PubMed] [Google Scholar]
- 54. Gautam R, Chintala S, Li W, et al. : The Hermansky-Pudlak syndrome 3 (cocoa) protein is a component of the biogenesis of lysosome-related organelles complex-2 (BLOC-2). J Biol Chem. 2004;279(13):12935–42. 10.1074/jbc.M311311200 [DOI] [PubMed] [Google Scholar]
- 55. Sitaram A, Dennis MK, Chaudhuri R, et al. : Differential recognition of a dileucine-based sorting signal by AP-1 and AP-3 reveals a requirement for both BLOC-1 and AP-3 in delivery of OCA2 to melanosomes. Mol Biol Cell. 2012;23(16):3178–92. 10.1091/mbc.E11-06-0509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dennis MK, Mantegazza AR, Snir OL, et al. : BLOC-2 targets recycling endosomal tubules to melanosomes for cargo delivery. J Cell Biol. 2015;209(4):563–77. 10.1083/jcb.201410026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Theos AC, Tenza D, Martina JA, et al. : Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol Biol Cell. 2005;16(11):5356–72. 10.1091/mbc.E05-07-0626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Setty SR, Tenza D, Truschel ST, et al. : BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol Biol Cell. 2007;18(3):768–80. 10.1091/mbc.E06-12-1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dennis MK, Delevoye C, Acosta-Ruiz A, et al. : BLOC-1 and BLOC-3 regulate VAMP7 cycling to and from melanosomes via distinct tubular transport carriers. J Cell Biol. 2016;214(3):293–308. 10.1083/jcb.201605090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Huang L, Kuo YM, Gitschier J: The pallid gene encodes a novel, syntaxin 13-interacting protein involved in platelet storage pool deficiency. Nat Genet. 1999;23(3):329–32. 10.1038/15507 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Helip-Wooley A, Westbroek W, Dorward H, et al. : Association of the Hermansky-Pudlak syndrome type-3 protein with clathrin. BMC Cell Biol. 2005;6:33. 10.1186/1471-2121-6-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li K, Yang L, Zhang C, et al. : HPS6 interacts with dynactin p150 Glued to mediate retrograde trafficking and maturation of lysosomes. J Cell Sci. 2014;127(Pt 21):4574–88. 10.1242/jcs.141978 [DOI] [PubMed] [Google Scholar]
- 63. Gerondopoulos A, Langemeyer L, Liang JR, et al. : BLOC-3 mutated in Hermansky-Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor. Curr Biol. 2012;22(22):2135–9. 10.1016/j.cub.2012.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ambrosio AL, Boyle JA, Di Pietro SM: Mechanism of platelet dense granule biogenesis: study of cargo transport and function of Rab32 and Rab38 in a model system. Blood. 2012;120(19):4072–81. 10.1182/blood-2012-04-420745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mao GF, Goldfinger LE, Fan DC, et al. : Dysregulation of PLDN (pallidin) is a mechanism for platelet dense granule deficiency in RUNX1 haplodeficiency. J Thromb Haemost. 2017;15(4):792–801. 10.1111/jth.13619 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Hiasa M, Togawa N, Miyaji T, et al. : Essential role of vesicular nucleotide transporter in vesicular storage and release of nucleotides in platelets. Physiol Rep. 2014;2(6): pii: e12034. 10.14814/phy2.12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Decouture B, Dreano E, Belleville-Rolland T, et al. : Impaired platelet activation and cAMP homeostasis in MRP4-deficient mice. Blood. 2015;126(15):1823–30. 10.1182/blood-2015-02-631044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jedlitschky G, Tirschmann K, Lubenow LE, et al. : The nucleotide transporter MRP4 (ABCC4) is highly expressed in human platelets and present in dense granules, indicating a role in mediator storage. Blood. 2004;104(12):3603–10. 10.1182/blood-2003-12-4330 [DOI] [PubMed] [Google Scholar]
- 69. Gollapudi S, Kim CH, Tran BN, et al. : Probenecid reverses multidrug resistance in multidrug resistance-associated protein-overexpressing HL60/AR and H69/AR cells but not in P-glycoprotein-overexpressing HL60/Tax and P388/ADR cells. Cancer Chemother Pharmacol. 1997;40(2):150–8. 10.1007/s002800050640 [DOI] [PubMed] [Google Scholar]
- 70. Markello T, Chen D, Kwan JY, et al. : York platelet syndrome is a CRAC channelopathy due to gain-of-function mutations in STIM1. Mol Genet Metab. 2015;114(3):474–82. 10.1016/j.ymgme.2014.12.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jonnalagadda D, Izu LT, Whiteheart SW: Platelet secretion is kinetically heterogeneous in an agonist-responsive manner. Blood. 2012;120(26):5209–16. 10.1182/blood-2012-07-445080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Koseoglu S, Peters CG, Fitch-Tewfik JL, et al. : VAMP-7 links granule exocytosis to actin reorganization during platelet activation. Blood. 2015;126(5):651–60. 10.1182/blood-2014-12-618744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Peters CG, Michelson AD, Flaumenhaft R: Granule exocytosis is required for platelet spreading: differential sorting of α-granules expressing VAMP-7. Blood. 2012;120(1):199–206. 10.1182/blood-2011-10-389247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. van Holten TC, Bleijerveld OB, Wijten P, et al. : Quantitative proteomics analysis reveals similar release profiles following specific PAR-1 or PAR-4 stimulation of platelets. Cardiovasc Res. 2014;103(1):140–6. 10.1093/cvr/cvu113 [DOI] [PubMed] [Google Scholar]
- 75. White JG, Escolar G: The blood platelet open canalicular system: a two-way street. Eur J Cell Biol. 1991;56(2):233–42. [PubMed] [Google Scholar]
- 76. Escolar G, White JG: The platelet open canalicular system: a final common pathway. Blood Cells. 1991;17(3):467–85; discussion 486–95. [PubMed] [Google Scholar]
- 77. Eckly A, Rinckel JY, Proamer F, et al. : Respective contributions of single and compound granule fusion to secretion by activated platelets. Blood. 2016;128(21):2538–49. 10.1182/blood-2016-03-705681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kloepper TH, Kienle CN, Fasshauer D: An elaborate classification of SNARE proteins sheds light on the conservation of the eukaryotic endomembrane system. Mol Biol Cell. 2007;18(9):3463–71. 10.1091/mbc.E07-03-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fasshauer D, Sutton RB, Brunger AT, et al. : Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci U S A. 1998;95(26):15781–6. 10.1073/pnas.95.26.15781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jahn R, Scheller RH: SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7(9):631–43. 10.1038/nrm2002 [DOI] [PubMed] [Google Scholar]
- 81. Dowal L, Yang W, Freeman MR, et al. : Proteomic analysis of palmitoylated platelet proteins. Blood. 2011;118(13):e62–73. 10.1182/blood-2011-05-353078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Graham GJ, Ren Q, Dilks JR, et al. : Endobrevin/VAMP-8-dependent dense granule release mediates thrombus formation in vivo. Blood. 2009;114(5):1083–90. 10.1182/blood-2009-03-210211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shiffman D, Rowland CM, Louie JZ, et al. : Gene variants of VAMP8 and HNRPUL1 are associated with early-onset myocardial infarction. Arterioscler Thromb Vasc Biol. 2006;26(7):1613–8. 10.1161/01.ATV.0000226543.77214.e4 [DOI] [PubMed] [Google Scholar]
- 84. Burkhart JM, Vaudel M, Gambaryan S, et al. : The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood. 2012;120(15):e73–82. 10.1182/blood-2012-04-416594 [DOI] [PubMed] [Google Scholar]
- 85. Ye S, Karim ZA, Al Hawas R, et al. : Syntaxin-11, but not syntaxin-2 or syntaxin-4, is required for platelet secretion. Blood. 2012;120(12):2484–92. 10.1182/blood-2012-05-430603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bryceson YT, Rudd E, Zheng C, et al. : Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 2007;110(6):1906–15. 10.1182/blood-2007-02-074468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Golebiewska EM, Harper MT, Williams CM, et al. : Syntaxin 8 regulates platelet dense granule secretion, aggregation, and thrombus stability. J Biol Chem. 2015;290(3):1536–45. 10.1074/jbc.M114.602615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Al Hawas R, Ren Q, Ye S, et al. : Munc18b/STXBP2 is required for platelet secretion. Blood. 2012;120(12):2493–500. 10.1182/blood-2012-05-430629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Spessott WA, Sanmillan ML, McCormick ME, et al. : Hemophagocytic lymphohistiocytosis caused by dominant-negative mutations in STXBP2 that inhibit SNARE-mediated membrane fusion. Blood. 2015;125(10):1566–77. 10.1182/blood-2014-11-610816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ye S, Huang Y, Joshi S, et al. : Platelet secretion and hemostasis require syntaxin-binding protein STXBP5. J Clin Invest. 2014;124(10):4517–28. 10.1172/JCI75572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. van Loon JE, Leebeek FW, Deckers JW, et al. : Effect of genetic variations in syntaxin-binding protein-5 and syntaxin-2 on von Willebrand factor concentration and cardiovascular risk. Circ Cardiovasc Genet. 2010;3(6):507–12. 10.1161/CIRCGENETICS.110.957407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wandinger-Ness A, Zerial M: Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol. 2014;6(11):a022616. 10.1101/cshperspect.a022616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lee MG, Mishra A, Lambright DG: Structural mechanisms for regulation of membrane traffic by rab GTPases. Traffic. 2009;10(10):1377–89. 10.1111/j.1600-0854.2009.00942.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Karniguian A, Zahraoui A, Tavitian A: Identification of small GTP-binding rab proteins in human platelets: thrombin-induced phosphorylation of rab3B, rab6, and rab8 proteins. Proc Natl Acad Sci U S A. 1993;90(16):7647–51. 10.1073/pnas.90.16.7647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shirakawa R, Yoshioka A, Horiuchi H, et al. : Small GTPase Rab4 regulates Ca 2+-induced alpha-granule secretion in platelets. J Biol Chem. 2000;275(43):33844–9. 10.1074/jbc.M002834200 [DOI] [PubMed] [Google Scholar]
- 96. Tolmachova T, Abrink M, Futter CE, et al. : Rab27b regulates number and secretion of platelet dense granules. Proc Natl Acad Sci U S A. 2007;104(14):5872–7. 10.1073/pnas.0609879104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Boswell KL, James DJ, Esquibel JM, et al. : Munc13-4 reconstitutes calcium-dependent SNARE-mediated membrane fusion. J Cell Biol. 2012;197(2):301–12. 10.1083/jcb.201109132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chicka MC, Ren Q, Richards D, et al. : Role of Munc13-4 as a Ca 2+-dependent tether during platelet secretion. Biochem J. 2016;473(5):627–39. 10.1042/BJ20151150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Harper MT, van den Bosch MT, Hers I, et al. : Platelet dense granule secretion defects may obscure α-granule secretion mechanisms: evidence from Munc13-4-deficient platelets. Blood. 2015;125(19):3034–6. 10.1182/blood-2014-12-618439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sharda A, Kim SH, Jasuja R, et al. : Defective PDI release from platelets and endothelial cells impairs thrombus formation in Hermansky-Pudlak syndrome. Blood. 2015;125(10):1633–42. 10.1182/blood-2014-08-597419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Neumüller O, Hoffmeister M, Babica J, et al. : Synaptotagmin-like protein 1 interacts with the GTPase-activating protein Rap1GAP2 and regulates dense granule secretion in platelets. Blood. 2009;114(7):1396–404. 10.1182/blood-2008-05-155234 [DOI] [PubMed] [Google Scholar]
- 102. Hampson A, O'Connor A, Smolenski A: Synaptotagmin-like protein 4 and Rab8 interact and increase dense granule release in platelets. J Thromb Haemost. 2013;11(1):161–8. 10.1111/jth.12068 [DOI] [PubMed] [Google Scholar]
- 103. Kawato M, Shirakawa R, Kondo H, et al. : Regulation of platelet dense granule secretion by the Ral GTPase-exocyst pathway. J Biol Chem. 2008;283(1):166–74. 10.1074/jbc.M705340200 [DOI] [PubMed] [Google Scholar]
- 104. Polgár J, Reed GL: A critical role for N-ethylmaleimide-sensitive fusion protein (NSF) in platelet granule secretion. Blood. 1999;94(4):1313–8. [PubMed] [Google Scholar]
- 105. Söllner T, Bennett MK, Whiteheart SW, et al. : A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75(3):409–18. 10.1016/0092-8674(93)90376-2 [DOI] [PubMed] [Google Scholar]
- 106. Morrell CN, Matsushita K, Chiles K, et al. : Regulation of platelet granule exocytosis by S-nitrosylation. Proc Natl Acad Sci U S A. 2005;102(10):3782–7. 10.1073/pnas.0408310102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Estevez B, Du X: New Concepts and Mechanisms of Platelet Activation Signaling. Physiology (Bethesda). 2017;32(2):162–77. 10.1152/physiol.00020.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bye AP, Unsworth AJ, Gibbins JM: Platelet signaling: a complex interplay between inhibitory and activatory networks. J Thromb Haemost. 2016;14(5):918–30. 10.1111/jth.13302 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 109. Gurbel PA, Kuliopulos A, Tantry US: G-protein-coupled receptors signaling pathways in new antiplatelet drug development. Arterioscler Thromb Vasc Biol. 2015;35(3):500–12. 10.1161/ATVBAHA.114.303412 [DOI] [PMC free article] [PubMed] [Google Scholar]