Abstract
The recent characterization of functional brown adipose tissue in adult humans has opened new perspectives for regulation of energy expenditure with respect to obesity and diabetes. Furthermore, dietary recommendations have taken into account the insufficient dietary intake of ω3 PUFAs and the concomitant excessive intake of ω6 PUFA associated with the occurrence of overweight/obesity. We aimed to study whether ω3 PUFAs could play a role in the recruitment and function of energy-dissipating brown/brite adipocytes. We show that ω3 PUFA supplementation has a beneficial effect on the thermogenic function of adipocytes. In vivo, a low dietary ω6:ω3 ratio improved the thermogenic response of brown and white adipose tissues to β3-adrenergic stimulation. This effect was recapitulated in vitro by PUFA treatment of hMADS adipocytes. We pinpointed the ω6-derived eicosanoid prostaglandin (PG)F2α as the molecular origin because the effects were mimicked with a specific PGF2α receptor agonist. PGF2α level in hMADS adipocytes was reduced in response to ω3 PUFA supplementation. The recruitment of thermogenic adipocytes is influenced by the local quantity of individual oxylipins, which is controlled by the ω6:ω3 ratio of available lipids. In human nutrition, energy homeostasis may thus benefit from the implementation of a more balanced dietary ω6:ω3 ratio.
Keywords: PUFA, oxylipins, prostaglandins, adipose tissue, UCP1
Dietary fats are the source of essential PUFAs; for example, ω6 linoleic acid (LA), a precursor of ω6 arachidonic acid (ARA), and ω3 α-linolenic acid, a precursor of ω3 EPA and DHA. These long-chain PUFAs trigger a variety of biological responses, particularly in adipose tissue, and are required for healthy development (1, 2). New dietary recommendations take into account the insufficient intake of ω3 PUFAs and the excess of ω6 PUFAs, which correlate with overweight/obesity (3–5). Indeed, high ω6:ω3 ratios are positively associated with adiposity of infants at 6 months and 3 and 4 years of age (6–8), and ARA intake correlates positively with BMI and the associated metabolic syndrome (9–13). In fact, diets exhibiting a high ω6:ω3 ratio result in higher ARA bioavailability for the synthesis of ω6 derived oxylipins due to an insufficient compensatory effect of EPA and DHA (14). These ω6 oxygenated derivatives are known to favor inflammatory responses (15), promote energy storage (16), and inhibit energy expenditure (17). These effects are mainly triggered by oxylipins arising from the cyclooxygenase (COX) pathway.
In contrast to the white adipose tissue (WAT) involved in energy storage and release, brown adipose tissue (BAT) is endowed with thermogenic activity and regulates body temperature by dissipating energy through nonshivering thermogenesis (18). This mechanism is mediated by uncoupling protein 1 (UCP1), which uncouples mitochondrial oxygen consumption from energy production. Interestingly, a further population of UCP1-positive adipocytes is present in WAT and is termed brite for “brown in white” or beige adipocytes (19–21). In vivo, brite adipocytes stem from progenitors or emerge by direct conversion of mature white adipocytes (22–24). Several studies have demonstrated the involvement of oxylipins in fat mass development through the modulation of white and brown/brite adipocyte differentiation and activity (25). Mice exposed to diets with high levels of ω6 PUFA during the perinatal period display progressive accumulation of body fat across generations (26). This observation is in agreement with findings that overweight and obesity have increased and develop earlier in life within a given population consuming Westernized diets rich in ω6 PUFAs during the last decades (26). Moreover, oxylipins derived from ω6 PUFA inhibit brite and brown adipocyte activity both in vitro and in vivo (17). ARA lowers the expression and function of UCP1, thus affecting the dissipation of energy in these thermogenic adipocytes. These effects are mediated via COX activities that lead to increased synthesis and release of prostaglandin (PG)E2 and PGF2α, which act as inhibitors of Ucp1-mediated thermogenesis via a calcium-dependent pathway.
It has been shown that healthy adult humans exhibit BAT in the cervical and thoracic part of the body, which consists of islets of energy-dissipating thermogenic adipocytes (27–31). These adipocytes display a gene expression signature comparable to either rodent brown or brite adipocytes depending on the localization and the depth of the analyzed tissue (21, 32–34). As brown and brite adipocytes represent important candidates for controlling body weight, investigations in humans of the regulation of brown/brite adipocyte recruitment and activation are in demand, particularly from a nutritional point of view, because quantitative and qualitative issues of dietary lipids are relevant to increased body weight (3). Because, in mammals, oxylipins govern a large part of the biology of adipose tissue, any dysregulation in their levels may disrupt tissue homeostasis. Thus, it is tempting to assume that prevention of excessive consumption of ω6 fatty acids or reestablishment of a balanced ω6:ω3 PUFA ratio may contribute to reduce excessive adipose tissue development by controlling white adipocyte formation and enhancing brite adipocyte recruitment.
Herein, we aimed to study whether the inhibitory effect of ω6-PUFA LA (via local action of ARA) on brite adipocyte recruitment could be reversed by supplementation with the ω3-PUFA α-linolenic acid associated to its metabolites EPA and DHA. We show, in vivo in mice and in vitro in human cells, that adjustment of a ω6:ω3 PUFA ratio from 30 to 3.7 by supplementation of ω6 PUFA-enriched diet with ω3 PUFAs rescues the inhibitory effect of ω6-derived oxylipins on the activity of brown and brite adipocytes. These effects are mediated through oxylipins via the decreased level of PGF2α.
MATERIALS AND METHODS
Animals and diets
The experiments were conducted in accordance with the French and European regulations (directive 2010/63/EU) for the care and use of research animals and were approved by national experimentation committees (MESR 01947.03). Ten-week-old C57BL/6J male mice from Janvier Laboratory (Le Genest Saint Isle, France) were maintained at thermoneutrality (28 ± 2°C) and 12:12 h light-dark cycles, with ad libitum access to food and water and euthanized between 10 and 11 AM. Mice were fed for 12 weeks with isocaloric ω6- or ω3-supplemented diets (12% energy content as lipids). These experimental diets were prepared from standard chow diets (ref. 2016, Harlan Laboratories, Madison WI). The ω6-supplemented diet was enriched with 0.5% linoleate-ethyl-ester (LA) and 0.7% oleate-ethyl-ester (ratio ω6:ω3 = 30); the ω3-supplemented diet comprised 0.5% LA, 0.54% α-linolenate-ethyl-ester (LNA), 0.08% eicosapentaenoate-ethyl-ester and 0.08% docosahexaenoate-ethyl-ester (ratio ω6:ω3 = 3.7) (see supplemental Table S1 for details) to follow human nutritional recommendations. Safflower oil (0.5%) was added to favor dispersion of ethyl esters in the diet. Fatty acid ethyl esters were from Nu-Chek-Prep (Waterville, MN) and diets were produced by Harlan. Chronic β3-adrenergic receptor stimulation was carried out during the last week of the diet treatment by daily intra-peritoneal injections of CL316,243 (1 mg/kg in saline solution). Control mice were injected with vehicle only. Blood, interscapular BAT (iBAT), epididymal WAT (eWAT), and inguinal subcutaneous WAT (scWAT) were sampled and used for different analyses.
hMADS cell culture
The establishment and characterization of hMADS cells has been described (35–37). In the experiments reported herein, hMADS-3 cells were used between passages 14 and 20. All experiments were performed at least three times using different cultures. Cells were cultured and differentiated as previously described (17, 35). Briefly, cells were induced to differentiate at day 2 postconfluence (designated as day 0) in DMEM/Ham’s F12 media supplemented with 10 µg/ml transferrin, 10 nM insulin, 0.2 nM triiodothyronine, 1 µM dexamethasone, and 500 µM isobutyl-methylxanthine. Two days later, the medium was changed (dexamethasone and isobutyl-methylxanthine omitted) and 100 nM rosiglitazone was added. At day 9, rosiglitazone was withdrawn to enable white adipocyte differentiation but was again included between days 14 and 17 to promote white-to-brite adipocyte conversion as previously described (17, 35). Fatty acids were bound to BSA (0.04% for 15 min at 37°C) prior to addition to culture media.
Oxylipin quantification
In vitro analysis of secreted PGF2α was performed on differentiated cells at day 17 after incubation for 1 or 24 h in fresh culture media. PGF2α was quantified by Elisa Immuno Assay following the manufacturer’s instructions (Cayman, BertinPharma, Montigny le Bretonneux, France).
Quantification of oxylipins was performed at the METATOUL platform (MetaboHUB, INSERM UMR 1048, I2MC, Toulouse, France) by mass spectrometry analysis. All tissues were snap-frozen with liquid nitrogen immediately after collection and stored at −80°C until extraction. Extraction and analysis were performed as previously described (17, 38).
Measurement of oxygen consumption
Oxygen consumption rate (OCR) of hMADS adipocytes was determined at day 17 using an XF24 Extracellular Flux Analyzer (Seahorse Bioscience, Agilent). ATP synthase uncoupled OCR was determined by the addition of 1.2 µM oligomycin A (ATP synthase inhibitor) and maximal OCR by addition of 1 µM FCCP [Carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone as a mitochondrial oxidative phosphorylation uncoupling agent]. Rotenone and Antimycin A (2 µM each) were used to inhibit Complex I- and Complex III-dependent respiration, respectively. Parameters were measured for each individual well using the OCR values as previously described (39).
Histology
Histological analysis was performed as previously described (17). Sections (4 µm) were dewaxed and treated in boiling citrate buffer (10 mM, pH 6.0) for 6 min. Cooled sections were rinsed and then permeabilized in PBS 0.2% triton X-100 at room temperature for 20 min. Sections were saturated in the same buffer containing 3% BSA for 30 min, incubated with perilipin antibody (#RDI-PROGP29, Research Diagnostic Inc., Flanders, NJ) for 1 h, and TRITC-coupled anti-guinea pig antibody for 45 min. Nuclear staining was performed with DAPI.
UCP1 immunohistochemistry was performed following manufacturer’s instructions (LSAB+ system-HRP, Dako, Les Ulis, France) and using goat anti-UCP1 (clone C-17, Santa Cruz, Tebu-bio, Le Perray-en-Yvelines, France).Visualization was performed with an Axiovert microscope and pictures were captured with AxioVision software (Carl Zeiss, Jena, Germany). Lipid droplet diameters (visualized by perilipin staining) were measured using Fiji software (40). Displayed images are representative of the four mice per group analyzed.
Isolation and analysis of RNA
These procedures follow MIQE (Minimum Information for Publication of Quantitative Real-Time PCR Experiments) recommendations (41). Total RNA was extracted using TRI-Reagent kit (Euromedex, Souffelweyersheim, France) according to the manufacturer’s instructions. For RNA isolation from organs, tissues were homogenized in TRI-Reagent using a dispersing instrument (ULTRA TURRAX T25, Ika, Germany). RT-PCR was performed using M-MLV-RT (Promega). SYBR qPCR premix Ex TaqII from Takara (Ozyme, France) was used for quantitative PCR (qPCR), and assays were run on a StepOne Plus ABI real-time PCR machine (PerkinElmer Life and Analytical Sciences, Waltham, MA). The expression of selected genes was normalized to that of the TATA-box binding protein (TBP) and 36B4 housekeeping genes for human genes, and 36B4 and GAPDH for mouse genes, and then quantified using the comparative-ΔCt method. Primer sequences are available upon request.
Statistical analysis
Data are expressed as mean values ± SEM and were analyzed using InStat software (GraphPad Software). Data were analyzed by one-way ANOVA followed by a Student-Newman-Keuls posttest, or Student’s t-test to assess statistical differences between experimental groups. Differences were considered statistically significant with P < 0.01.
RESULTS
ω3 PUFA supplementation makes mice sensitive to β3-adrenergic receptor agonist treatment
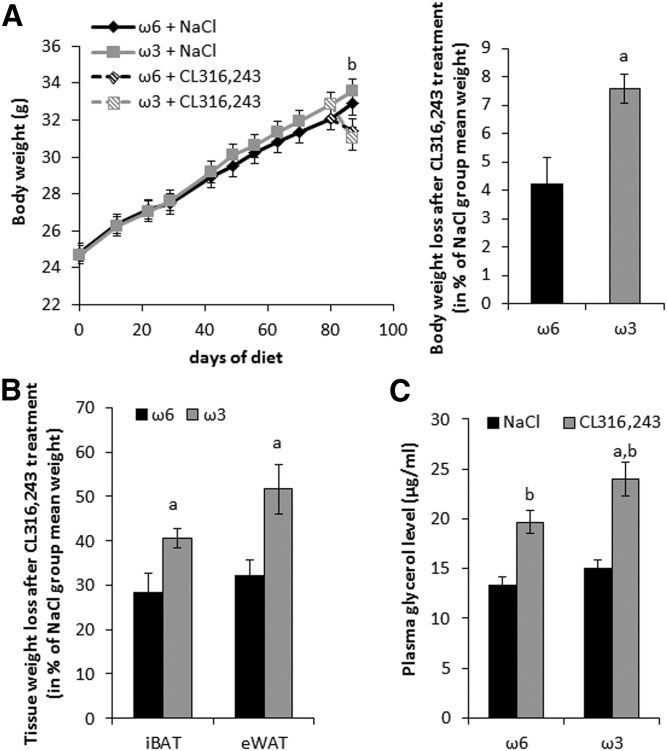
Ten-week-old mice were fed for 12 weeks with an isocaloric standard diet enriched in ω6 PUFAs (ω6 diet, ω6:ω3 = 30), or supplemented with ω3 PUFAs (ω3 diet, ω6:ω3 = 3.7) (supplemental Table S1). There was no difference in body weight (Fig. 1A) and food intake during 12 weeks (ω6 diet, 4.49 g/day vs. ω3 diet, 4.46 g/day per mouse). During the last week, mice received daily injections of the agonist CL316,243 (1 mg/kg) to activate the β3-adrenergic receptor pathway. As expected, such treatment decreased body weight (Fig. 1A) as well as iBAT and eWAT mass (Fig. 1B). Interestingly, ω3 diet-fed mice showed a larger decrease in body mass and adipose tissue mass on CL316,243-treatment compared with ω6 diet-fed mice (Fig. 1A,B). This observation is in line with higher plasma glycerol levels (Fig. 1C). Together, these results indicate a higher sensitivity of ω3 diet-fed mice to CL316,243 treatment.
Fig. 1.
A low dietary ω6:ω3 ratio enhances body weight loss in response to a β3-adrenergic receptor agonist. Mice maintained at 28°C were fed with diets supplemented with ω6 PUFAs or ω3 PUFAs for 12 weeks. CL316,243 or vehicle (NaCl) treatment was performed daily during the last week of feeding. A: Body weight development of mice (left); body weight loss of mice after CL316,243 treatment relative to vehicle treatment (right). B: Interscapular brown adipose tissue (iBAT) and epidydimal white adipose tissue (eWAT) weight loss after CL316,243 treatment. C: Plasma glycerol levels after NaCl or CL316,243 treatment. Data are mean ± SEM, n = 12 mice/group. a, P < 0.01 ω6 versus ω3; b, P < 0.01 NaCl versus CL316,243.
ω3 diet-fed mice showed an improved response to thermogenic stimulation in BAT and scWAT
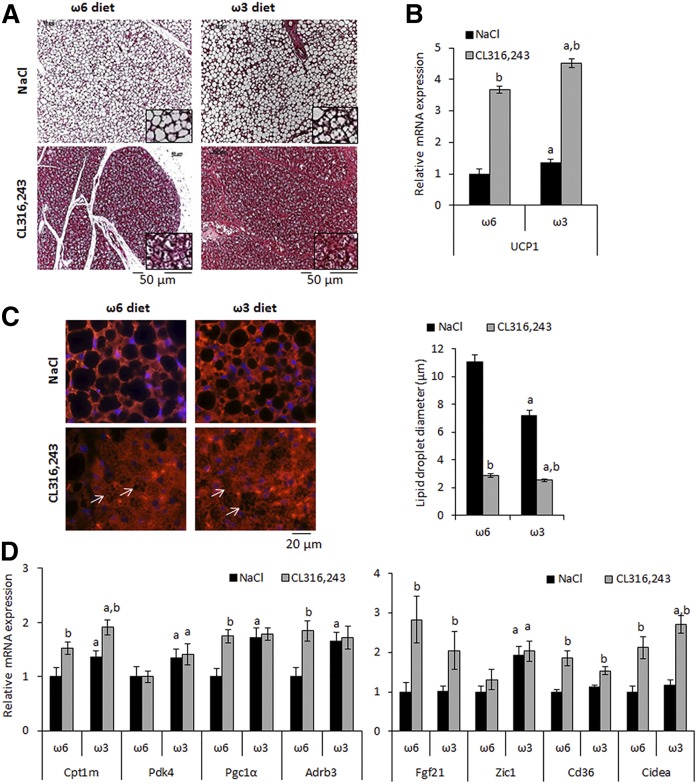
After CL316,243 treatment, mice fed the ω3-diet displayed an increase in BAT mass loss and in plasma glycerol level, suggesting a higher lipolysis capacity. As expected, histological analysis of BAT showed clear activation of brown adipocytes after CL316,243 treatment as indicated by adipocyte morphological changes in the two groups of mice (Fig. 2A). This observation was confirmed by immunostaining for perilipin 1 (PLN1), a known lipid droplet surface protein, which allowed the measurement of droplet diameters. The data showed smaller lipid droplets in BAT of mice treated with CL316,243 (Fig. 2C). Interestingly, the droplet size was smaller in ω3 compared with ω6 diet-fed mice of both treatment groups (Fig. 2C). These morphological modifications were in line with elevated expression of brown adipocyte marker genes in ω3 versus ω6 diet-fed mice (Fig. 2B, D).
Fig. 2.
Morphological and molecular analysis of brown adipose tissue. A: Hematoxylin and eosin staining of paraffin-embedded tissue sections and (B) expression of Ucp1 mRNA. C: PLN1 immunostaining of iBAT sections from mice fed ω6 or ω3-diet. Lipid droplet (white arrows) diameters were evaluated using PLN1 staining; data are mean ± SEM, n = 200 lipid droplets/mouse, 4 mice/ group. D: Expression of brown adipocyte marker mRNAs was determined by RT-qPCR. mRNA expressions are shown as fold increase relative to “NaCl−ω6” values. Data are mean ± SEM, n = 12 mice/group. a, P < 0.01 ω6 versus ω3; b, P < 0.01 NaCl versus CL316,243.
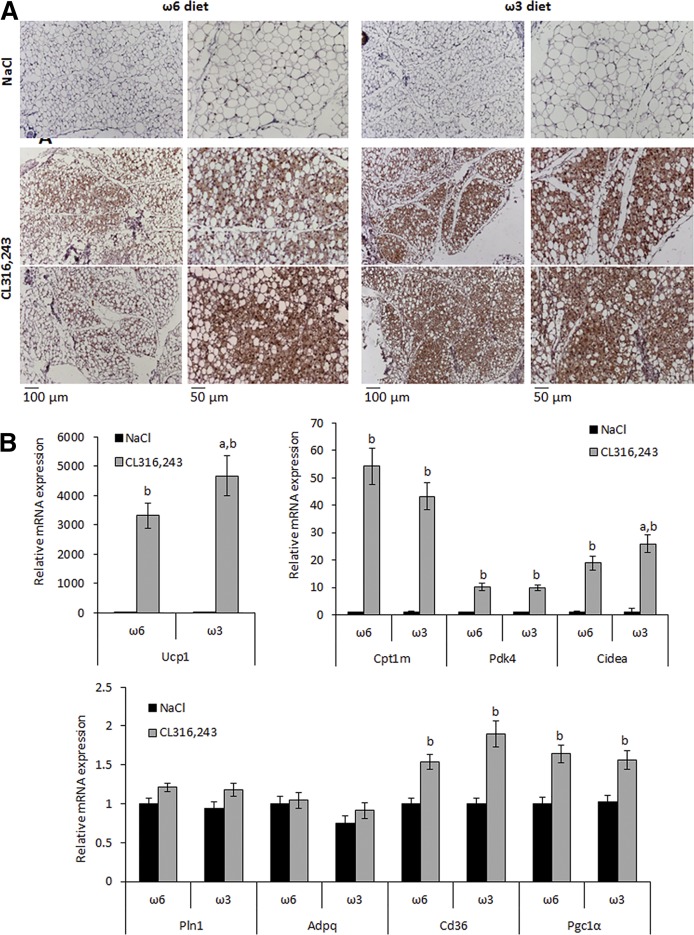
The β3-adrenergic agonist CL316,243 is a potent inducer of brite adipocytes and therefore we hypothesized a contribution of these thermogenic cells to CL316,243-induced plasma glycerol levels and body weight loss. No morphological or gene expression differences were found in scWAT between the two groups of mice in response to vehicle treatment (Fig. 3A, B). However, after CL316,243 treatment, visual examination of sections suggested increased Ucp1-positive adipocytes in scWAT of ω3 diet-fed mice compared with ω6 diet-fed mice (Fig. 3A). This observation was supported by the analysis of Ucp1 mRNA expression, which tended to be increased in the ω3 diet-fed group after CL316,243 treatment. Gene expression of other markers in response to CL316,243 was not affected by the diet (Fig. 3B). As shown in supplemental Fig. S1, eWAT did not display the multiloculated adipocytes that are characteristic of UCP1+ adipocytes. However, a decrease of adipocyte mean diameter after CL316,243 treatment was found in both groups, in agreement with eWAT weight decrease (Fig. 1B).
Fig. 3.
Morphological and molecular analysis of subcutaneous white adipose tissue. A: UCP1 immunohistological analysis of subcutaneous WAT (scWAT) sections from mice fed an ω6 or ω3 diet. Slides were counterstained with hematoxylin and eosin. B: Expression of Ucp1 and brite/white adipocyte marker mRNAs was determined by RT-qPCR. mRNA expressions are shown as fold increase relative to “NaCl−ω6” values. Data are mean ± SEM, n = 12 mice/group. a, P < 0.01 ω6 versus ω3; b, P < 0.01 NaCl versus CL316,243.
Together, these data indicate that the dietary ω6:ω3 ratio affects the induction/activation of thermogenic adipocytes in BAT and scWAT upon stimulation with a β-adrenergic agonist. As dietary fatty acid composition can considerably influence the quality and quantity of fatty acid metabolites (2, 5), this differential response to thermogenic stimulation may be the consequence of modulated eicosanoid level in adipose tissues.
Dietary ω3 PUFA supplementation controls the level of ω6-derived oxylipins
To investigate diet-induced differences in the quantity of ω3- and ω6-derived oxylipins, we quantified the levels of more than 30 selected metabolites in scWAT and iBAT from ω3 and ω6 diet-fed mice. Depending on their origin, oxygenated metabolites derived from ω6 (ARA) or ω3 (EPA and/or DHA) PUFAs, due to COX, LOX, and CYP450 activities (supplemental Fig. S2A, B, left panels, oxylipins) or to COX activity only (right panels, eicosanoids: a group of oxylipins) were analyzed (analyzed oxylipins are detailed in supplemental Table S2).
As expected, ω3 PUFA supplementation allowed higher quantities of their metabolites in BAT and scWAT (supplemental Fig. S2A, B, black columns). In BAT, where the molecular effect of a ω3 diet was the most important, we did not find any significant impact of the diet on ω6-derived oxylipin levels (supplemental Fig. S2A, gray columns). In scWAT, similar data were obtained in untreated mice; however, when the mice were treated with the β3-adrenergic receptor agonist, the decrease in the levels of ω6-derived oxylipins was higher in ω3 diet-fed mice than in ω6-diet fed mice (supplemental Fig. S2).
Analysis of COX-derived eicosanoids showed an increase in EPA-derived eicosanoids in the ω3 diet-fed group and no significant decrease of ω6-derived eicosanoids, except after CL316,243 treatment (supplemental Fig. S2A, B, right panels). Of note, the treatment with CL316,243, a situation where UCP1 activity is increased via the release of fatty acids, led to a decrease in the levels of the various oxygenated metabolites, possibly reflecting the preferential use of PUFAs as fuel for thermogenesis, thus limiting PUFA availability for oxylipin production (Fig. 4 and supplemental Fig. S2).
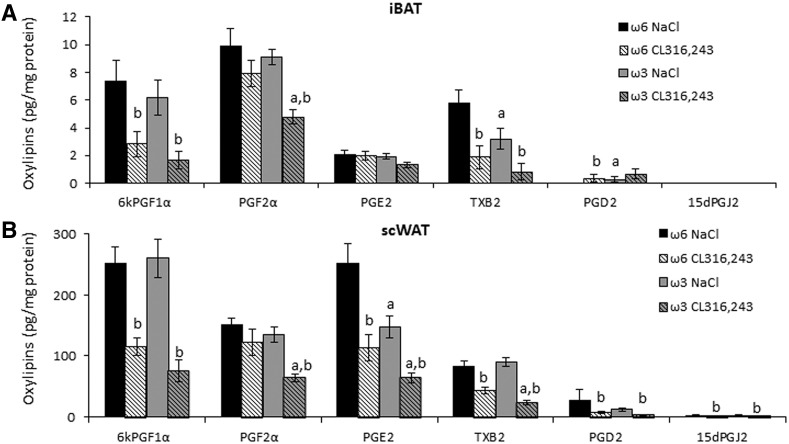
Fig. 4.
Abundance of ω6 PUFA-derived eicosanoids in BAT and WAT. Eicosanoid levels were measured by LC-MS/MS in (A) iBAT and (B) scWAT of vehicle- and CL316,243-treated mice fed with an ω6 or ω3 diet. Data are mean ± SEM, 8 mice/group. a, P < 0.01 ω6 versus ω3 and b, P < 0.01 NaCl versus CL316,243.
Previous work from our laboratory has demonstrated that COX-derived oxylipins are crucial for the formation and activation of thermogenic adipocytes (17, 42). Accordingly, further analyses focused on the levels of individual COX-derived oxylipins [i.e., 6kPGF1α (representative of PGI2), PGF2α, PGE2, TXB2, PGD2, and 15dPGJ2] in BAT and scWAT. The levels of most individual metabolites were similar in iBAT and scWAT of vehicle-treated ω6 diet- and ω3 diet-fed mice, except for a decrease in TXB2 levels in BAT and in PGE2 levels in scWAT (Fig. 4A, B). Treatment with CL316,243 resulted in decreased quantity of these oxylipins in both diet groups in BAT and scWAT, although downregulation of TXB2 and PGE2 was more pronounced in scWAT than iBAT. Interestingly, PGF2α was exempt from this mode of regulation: CL316,243-treatment resulted in a significant decrease in PGF2α level in ω3 diet-fed but not in ω6 diet-fed mice. Thus, the level of this eicosanoid in response to CL316,243-treatment is inversely correlated with Ucp1 expression in BAT and scWAT.
Our previous results demonstrated (17) that PGF2α is a negative regulator of thermogenic adipocyte recruitment. Indeed, the ω6-enriched diet PGF2α level was not affected by CL316,243 treatment (Fig. 4A, B) and correlated with inhibition of brown and brite adipocyte recruitment and activation (Figs. 2, 3). In contrast, for an ω3-enriched diet after CL316,243 treatment, no alteration in brown and brite adipocyte recruitment and activation was found, although a striking decrease in the PGF2α level was observed (Fig. 4A, B).
Taken together, these results demonstrated that diet supplementation with ω3 PUFAs reversed the inhibitory effect of a ω6-enriched diet. This effect could be due to competition between ω6 and ω3 PUFAs at the COX activity level leading in turn to a decrease in PGF2α synthesis. To further investigate the effect of PUFAs and eicosanoids on adipocyte function, we used brite adipocytes derived from hMADS cells as a model system.
EPA reversed the ARA-inhibitory effect in vitro
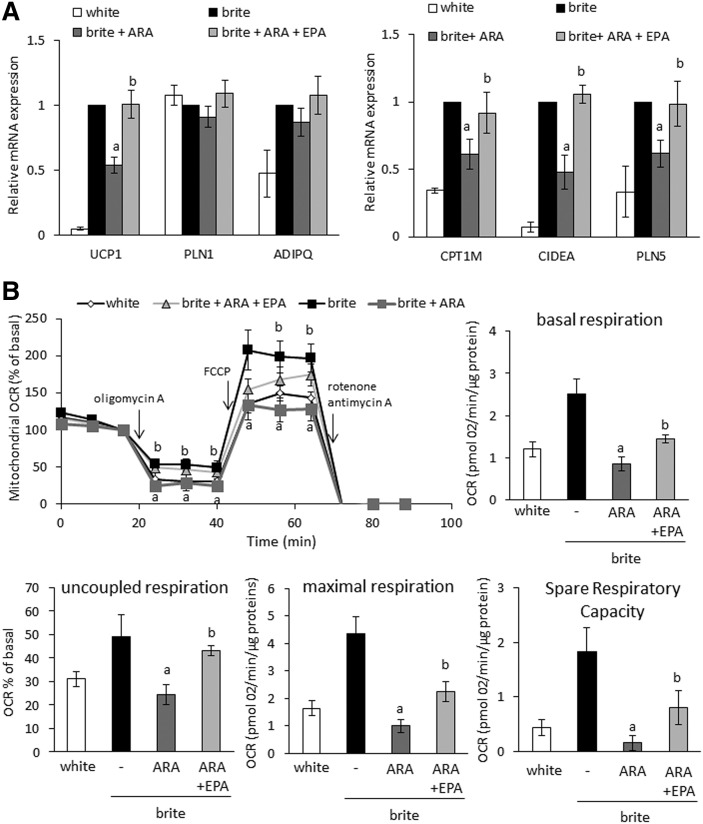
hMADS cells are a human stem cell model that is able to differentiate into white adipocytes and to convert into functional brite adipocytes upon rosiglitazone treatment [Fig. 5A (17, 35)]. As described previously (17), this process can be modified by ω6-PUFA ARA; treatment of hMADS adipocytes during the conversion to brite adipocytes with ARA inhibited the expression of UCP1 and other brite adipocyte markers (CIDEA, CPT1M, PLN5) (Fig. 5A). Such treatment did not affect adipogenesis per se, as the expression of PLN1 and ADIPQ was not affected (Fig. 5A). Treatment of hMADS adipocytes with ω3-PUFA EPA (molar ARA:EPA ratio of 3) reversed the inhibitory effect of ARA on brite adipocyte marker expression (Fig. 5A). This effect was further investigated at the functional level. Oxygen consumption analysis of hMADS brite adipocytes revealed that ARA inhibited all mitochondrial respiration parameters (Fig. 5B), (i.e., basal, uncoupled, and maximal respiration as well as spare respiratory capacity). EPA partially reversed this inhibitory effect of ARA on mitochondrial oxygen consumption, thus affecting the overall thermogenic capacity of these cells (Fig. 5B). No change in adipogenic marker expression was observed when DHA was added as an alternative ω3 PUFA (supplemental Fig. S3) indicating that EPA specifically represents the effective compound within this class of molecules.
Fig. 5.
EPA reversed the effect of ARA on adipocyte browning in vitro. hMADS cells were differentiated into white or brite adipocytes. Brite hMADS adipocytes were treated during the last 3 days of differentiation with 10 µM ARA in the presence or absence of 3.3 µM EPA. A: Expression of adipocyte markers was determined by RT-qPCR and is expressed as fold increase relative to “brite” group values. B: Basal, ATP synthase uncoupled respiration (% of residual respiration after addition of ATP synthase inhibitor oligomycin), and maximal respiration (obtained after addition of the chemical uncoupling agent FCCP) were assessed at the end of treatment to determine the oxygen consumption rate (OCR) of mitochondria. The spare respiratory capacity represents a measure for the full respiratory potential of mitochondria and is calculated as the difference between maximal and basal respiration. Data are mean ± SEM of three (A) or six (B) independent experiments. a, P < 0.01 versus brite; b, P < 0.01 versus brite + ARA.
Taken together, these results indicate that the thermogenic function of adipocytes is positively and negatively affected by EPA (ω3) and ARA (ω6), respectively.
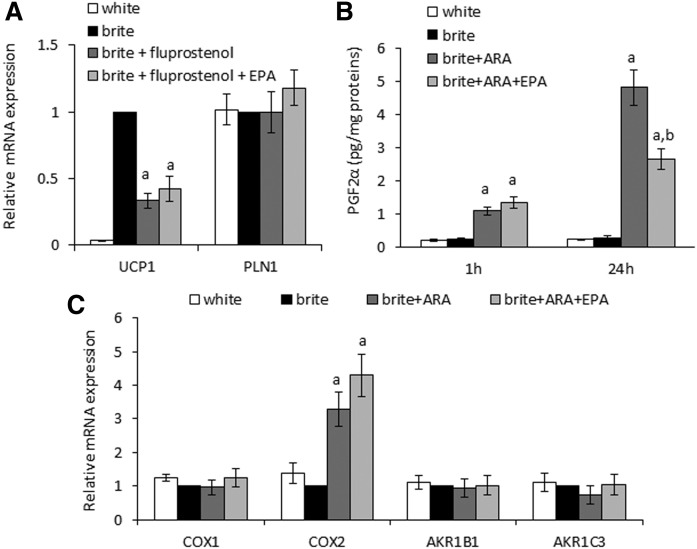
EPA reduced PGF2α synthesis and secretion
To identify the pathway involved in the EPA effect, we used fluprostenol, an agonist of the FP receptor (PGF2α receptor), instead of the precursor ARA during the conversion of white hMADS adipocytes into brite adipocytes. In agreement with our previous work (17), fluprostenol mimicked the effect of ARA by inhibiting the expression of UCP1 mRNA (Fig. 6A). Cotreatment with EPA did not reverse this effect (Fig. 6A), indicating that the UCP1 expression of brite hMADS adipocytes was directly affected by an interaction between the FP receptor and its ω6-derived, endogenous ligand PGF2α. Thus, we hypothesized that EPA reversed the ARA-induced effects in brite hMADS adipocytes by modulating the availability of PGF2α as ligand for its receptor. Accordingly, we measured PGF2α secretion after ARA treatment of brite hMADS adipocytes in the absence or presence of EPA. PGF2α secretion was not altered by EPA during the first hour of treatment. However, a striking decrease in PGF2α levels was observed after 24 h (Fig. 6B). This decrease in quantity was likely not due to a lower expression of the major enzymes involved in PGF2α synthesis. Indeed, the expression of COX-1, COX-2 (allowing the metabolization of ARA to PGG2), AKR1B1, and AKR1C3 (allowing the metabolization of PGG2 to PGF2α) mRNAs was not altered by EPA treatment (Fig. 6C). As both ARA and EPA can be recognized and metabolized by COX-1 and COX-2, EPA most likely blocks PGF2α synthesis by competing with ARA.
Fig. 6.
EPA competes with ARA at the cyclooxygenase level. hMADS cells were differentiated into white or brite adipocytes. A: Brite hMADS adipocytes were treated with 10 nM fluprostenol (agonist of PGF2α receptor) in the presence or absence of 3.3 µM EPA during the last 3 days of adipogenic differentiation. UCP1 and PLN1 were evaluated by RT-qPCR. B: Brite hMADS were exposed to 10 µM ARA in the presence or absence of 3.3 µM EPA for 1 or 24 h. PGF2α was quantified in culture media by Elisa Immuno Assay. C: Brite hMADS adipocytes were treated with 10 µM ARA in the presence or absence of 3.3 µM EPA during the last 3 days of differentiation. Expression of enzymes involved in the metabolization of ARA to PGF2α was evaluated by RT-qPCR. mRNA expressions are expressed as fold increase relative to “brite” values. Data are mean ± SEM of 3 independent experiments. a, P < 0.01 versus brite; b, P < 0.01 versus brite + ARA.
DISCUSSION
The obesogenic effect of ω6 PUFAs, particularly ARA, is thought to originate from their metabolization to oxylipins, thus promoting fat storage and a reduction in energy expenditure (16, 17, 43, 44). However, this system and its regulation appear to underlie an unanticipated complexity. In fact, previous studies demonstrated the ω6 eicosanoid prostacyclin (PGI2, which is derived from ARA via COX-dependent metabolization) as a positive modulator of UCP1 expression and brite adipogenesis in human and murine cellular model systems (42, 45–47). In line with these findings, transgenic mice with constitutive overexpression of COX-2 (the inducible isoform of COX) show enhanced browning of WAT and resistance to diet-induced obesity (46). The complexity of this system becomes apparent in IP receptor (PGI2 receptor) knockout mice, which are protected from ω6 PUFA-induced body and fat mass gain (16). In a similar way, when mice were fed a high-fat diet, the inhibition of COX activities with indomethacin prevented body weight gain, due to decreased fat storage and enhanced recruitment of brite adipocytes in scWAT (15, 43). These examples emphasize the need to increase our current understanding of the pro- and anti-adipogenic properties of PUFAs on the level of oxylipin metabolism to modulate energy balance regulation.
In our previous work, we elucidated in more detail the relationship between ARA-derived eicosanoid level, browning of WAT, and energy expenditure. Mice were fed an ARA-supplemented standard diet and showed impaired brown adipocyte activation and brite adipocyte recruitment in response to CL316,243 treatment, an effect that was attributed to the abundance of PGF2α (17). Using hMADS adipocytes as an in vitro cell model, we demonstrated that this eicosanoid translates its inhibitory effect on the thermogenic capacity of adipocytes via an interaction with its natural cell surface receptor, the FP receptor, on its synthesis from ARA via COX activity (17). Herein we show that this ARA-dependent production of PGF2α is attenuated in the presence of ω3-PUFAs both in vitro in brite hMADS adipocytes and in vivo in thermogenically activated brown and brite adipocytes. We conclude that Ucp1 expression in these models is less affected by the elevated quantity of ω3-derived metabolites but rather depends on the reduced level of individual ω6-derived metabolites (such as PGF2α) with EPA-treatment.
Both ω6 and ω3 PUFAs are transported in the bloodstream between tissues and are incorporated in plasma membrane under the form of phospholipids or as triglycerides within adipocytes (48–50). PUFAs are released into the cell by lipases and metabolized into oxylipins using similar pathways. ω6 and ω3 PUFAs are known to compete at different steps that modulate the availability of their respective metabolites. LA and LNA use the same Δ-desaturases and elongases to generate PUFA metabolites such as ARA, dihomo-γ-linolenic acid, EPA, and DHA (51). They can also be used as substrates via β-oxidation, ω3 PUFAs being more rapidly oxidized compared with ω6 PUFAs and mono-unsaturated fatty acids (52).
Although our data support a model in which dietary PUFAs act on the level of oxylipins, this mechanism is not exclusive in the context of thermogenic adipocyte recruitment, because ω3-PUFAs per se are associated with browning properties (53, 54). For example, when mice or rats are fed an LA-enriched diet, the increase in fat mass can be prevented by LNA supplementation under isolipidic and isocaloric conditions (16, 55, 56). Using this strategy, we demonstrated herein that ω3 PUFA diet supplementation ameliorates brown adipocyte function in response to β-adrenergic stimulation by promoting a more oxidative phenotype and, to a lesser extent, brite adipocyte recruitment. Analysis of PUFA metabolites associated with this improvement showed a decrease in n-2 series prostaglandins levels, especially PGF2α. Altogether, in vivo and in vitro data show that the competition between ω6 and ω3 PUFAs takes place at the level of COX activities, favoring the production of EPA-derived metabolites instead of ARA-derived metabolites. DHA treatment was inefficient. The lack of effect could be due to the fact that DHA does not compete with ARA for metabolite production; however, DHA was described to inhibit activity and expression of COX-2 (57).
Interestingly, this competition between ω6 and ω3 PUFAs does not appear to contribute to the regulation of body weight when mice are fed under thermoneutral conditions but rapidly initiates the mobilization of fat as an energy substrate in response to thermogenic stimulation. This observation may be of particular relevance to the treatment of overweight and obesity in human subjects as humans constantly live in a thermoneutral environment. Thus, adjusting the ω6:ω3-ratio of human nutrition according to dietary recommendations may benefit the recruitment of brown and brite adipocytes associated with other therapeutic strategies to promote a negative energy balance. Supporting the physiological relevance of ω3 PUFA-induced effects, fish oil supplementation has been reported to induce UCP1 expression via the sympathetic nervous system, although this mechanism is restricted to BAT (58, 59).
In conclusion, herein we demonstrate that ω3 PUFA supplementation compensates for the inhibitory effect of ω6 PUFAs on the thermogenic function of adipocytes. We identified the eicosanoid PGF2α as a causal effector, the level of which is influenced by the availability of ω6 and ω3 PUFAs competing at the level of their metabolization. Our data indicate that the dietary ω6:ω3 ratio is a major regulator of adaptive thermogenesis consequently affecting energy homeostasis. These findings are of particular importance in a human nutritional context as the dietary ω6:ω3 ratio is associated with the development of obesity and cardiovascular and inflammatory diseases.
Supplementary Material
Acknowledgments
The authors greatly acknowledge the IRCAN Animal core facilitiy and the Cytomed platform as well as the IBV histology platform. We thank Pauline Le Faouder and Justine Bertrand-Michel from the METATOUL platform (MetaboHUB, INSERM UMR 1048, I2MC, Toulouse, France) for oxylipin analysis. We thank Amanda Balzo and Clémence Auverdin for their technical suport. The manuscript has been corrected by Dr. Brahimi-Horn (EditDocSci, http://cbrahimihorn.free.fr), a native English-speaking scientific editor.
Footnotes
Abbreviations:
- ARA
- arachidonic acid
- BAT
- brown adipose tissue
- COX
- cyclooxygenase
- eWAT
- epididymal WAT
- FP
- Receptor
- PGF2α
- receptor
- iBAT
- interscapular BAT
- LA
- linoleic acid
- LNA
- alpha linolenic acid
- OCR
- oxygen consumption rate
- PG
- prostaglandin
- PLN1
- perilipin 1
- qPCR
- quantitative PCR
- scWAT
- subcutaneous WAT
- UCP
- uncoupling protein 1
- WAT
- white adipose tissue
This work was supported by CNRS, French Agence Nationale de la Recherche, and Deutsche Forschungsgemeinschaft Grant ANR/DFG-15-CE14-0033 “Nutribrite” and Nutricia Research Foundation Grant “2015–26”.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Simopoulos A. P. 2002. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 56: 365–379. [DOI] [PubMed] [Google Scholar]
- 2.Simopoulos A. P. 2016. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 8: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ailhaud G., Massiera F., Weill P., Legrand P., Alessandri J. M., and Guesnet P.. 2006. Temporal changes in dietary fats: role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog. Lipid Res. 45: 203–236. [DOI] [PubMed] [Google Scholar]
- 4.Muhlhausler B. S., and Ailhaud G. P.. 2013. Omega-6 polyunsaturated fatty acids and the early origins of obesity. Curr. Opin. Endocrinol. Diabetes Obes. 20: 56–61. [DOI] [PubMed] [Google Scholar]
- 5.Simopoulos A. P., and DiNicolantonio J. J.. 2016. The importance of a balanced omega-6 to omega-3 ratio in the prevention and management of obesity. Open Heart. 3: e000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donahue S. M., Rifas-Shiman S. L., Gold D. R., Jouni Z. E., Gillman M. W., and Oken E.. 2011. Prenatal fatty acid status and child adiposity at age 3 y: results from a US pregnancy cohort. Am. J. Clin. Nutr. 93: 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moon R. J., Harvey N. C., Robinson S. M., Ntani G., Davies J. H., Inskip H. M., Godfrey K. M., Dennison E. M., Calder P. C., Cooper C., et al. . 2013. Maternal plasma polyunsaturated fatty acid status in late pregnancy is associated with offspring body composition in childhood. J. Clin. Endocrinol. Metab. 98: 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudolph M. C., Young B. E., Lemas D. J., Palmer C. E., Hernandez T. L., Barbour L. A., Friedman J. E., Krebs N. F., and MacLean P. S.. 2017. Early infant adipose deposition is positively associated with the n-6 to n-3 fatty acid ratio in human milk independent of maternal BMI. Int. J. Obes. (Lond.) 41: 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inoue K., Kishida K., Hirata A., Funahashi T., and Shimomura I.. 2013. Low serum eicosapentaenoic acid / arachidonic acid ratio in male subjects with visceral obesity. Nutr. Metab. (Lond.). 10: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savva S. C., Chadjigeorgiou C., Hatzis C., Kyriakakis M., Tsimbinos G., Tornaritis M., and Kafatos A.. 2004. Association of adipose tissue arachidonic acid content with BMI and overweight status in children from Cyprus and Crete. Br. J. Nutr. 91: 643–649. [DOI] [PubMed] [Google Scholar]
- 11.Williams E. S., Baylin A., and Campos H.. 2007. Adipose tissue arachidonic acid and the metabolic syndrome in Costa Rican adults. Clin. Nutr. 26: 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clària J., Nguyen B. T., Madenci A. L., Ozaki C. K., and Serhan C. N.. 2013. Diversity of lipid mediators in human adipose tissue depots. Am. J. Physiol. Cell Physiol. 304: C1141–C1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garaulet M., Perez-Llamas F., Perez-Ayala M., Martinez P., de Medina F. S., Tebar F. J., and Zamora S.. 2001. Site-specific differences in the fatty acid composition of abdominal adipose tissue in an obese population from a Mediterranean area: relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity. Am. J. Clin. Nutr. 74: 585–591. [DOI] [PubMed] [Google Scholar]
- 14.Fischer R., Konkel A., Mehling H., Blossey K., Gapelyuk A., Wessel N., von Schacky C., Dechend R., Muller D. N., Rothe M., et al. . 2014. Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway. J. Lipid Res. 55: 1150–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghoshal S., Trivedi D. B., Graf G. A., and Loftin C. D.. 2011. Cyclooxygenase-2 deficiency attenuates adipose tissue differentiation and inflammation in mice. J. Biol. Chem. 286: 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massiera F., Saint-Marc P., Seydoux J., Murata T., Kobayashi T., Narumiya S., Guesnet P., Amri E. Z., Negrel R., and Ailhaud G.. 2003. Arachidonic acid and prostacyclin signaling promote adipose tissue development: a human health concern? J. Lipid Res. 44: 271–279. [DOI] [PubMed] [Google Scholar]
- 17.Pisani D. F., Ghandour R. A., Beranger G. E., Le Faouder P., Chambard J. C., Giroud M., Vegiopoulos A., Djedaini M., Bertrand-Michel J., Tauc M., et al. . 2014. The omega6-fatty acid, arachidonic acid, regulates the conversion of white to brite adipocyte through a prostaglandin/calcium mediated pathway. Mol. Metab. 3: 834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannon B., and Nedergaard J.. 2004. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84: 277–359. [DOI] [PubMed] [Google Scholar]
- 19.Petrovic N., Walden T. B., Shabalina I. G., Timmons J. A., Cannon B., and Nedergaard J.. 2010. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 285: 7153–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishibashi J., and Seale P.. 2010. Medicine. Beige can be slimming. Science. 328: 1113–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu J., Bostrom P., Sparks L. M., Ye L., Choi J. H., Giang A. H., Khandekar M., Virtanen K. A., Nuutila P., Schaart G., et al. . 2012. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 150: 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbatelli G., Murano I., Madsen L., Hao Q., Jimenez M., Kristiansen K., Giacobino J. P., De Matteis R., and Cinti S.. 2010. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 298: E1244–E1253. [DOI] [PubMed] [Google Scholar]
- 23.Lee Y. H., Mottillo E. P., and Granneman J. G.. 2014. Adipose tissue plasticity from WAT to BAT and in between. Biochim. Biophys. Acta. 1842: 358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenwald M., Perdikari A., Rulicke T., and Wolfrum C.. 2013. Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 15: 659–667. [DOI] [PubMed] [Google Scholar]
- 25.Barquissau V., Ghandour R. A., Ailhaud G., Klingenspor M., Langin D., Amri E. Z., and Pisani D. F.. 2017. Control of adipogenesis by oxylipins, GPCRs and PPARs. Biochimie. 136: 3–11. [DOI] [PubMed] [Google Scholar]
- 26.Massiera F., Barbry P., Guesnet P., Joly A., Luquet S., Moreilhon-Brest C., Mohsen-Kanson T., Amri E. Z., and Ailhaud G.. 2010. A Western-like fat diet is sufficient to induce a gradual enhancement in fat mass over generations. J. Lipid Res. 51: 2352–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cypess A. M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A. B., Kuo F. C., Palmer E. L., Tseng Y. H., Doria A., et al. . 2009. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360: 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nedergaard J., Bengtsson T., and Cannon B.. 2007. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 293: E444–E452. [DOI] [PubMed] [Google Scholar]
- 29.Saito M., Okamatsu-Ogura Y., Matsushita M., Watanabe K., Yoneshiro T., Nio-Kobayashi J., Iwanaga T., Miyagawa M., Kameya T., Nakada K., et al. . 2009. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 58: 1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Marken Lichtenbelt W. D., Vanhommerig J. W., Smulders N. M., Drossaerts J. M., Kemerink G. J., Bouvy N. D., Schrauwen P., and Teule G. J.. 2009. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360: 1500–1508. [DOI] [PubMed] [Google Scholar]
- 31.Virtanen K. A., Lidell M. E., Orava J., Heglind M., Westergren R., Niemi T., Taittonen M., Laine J., Savisto N. J., Enerback S., et al. . 2009. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360: 1518–1525. [DOI] [PubMed] [Google Scholar]
- 32.Cypess A. M., White A. P., Vernochet C., Schulz T. J., Xue R., Sass C. A., Huang T. L., Roberts-Toler C., Weiner L. S., Sze C., et al. . 2013. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat. Med. 19: 635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jespersen N. Z., Larsen T. J., Peijs L., Daugaard S., Homoe P., Loft A., de Jong J., Mathur N., Cannon B., Nedergaard J., et al. . 2013. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 17: 798–805. [DOI] [PubMed] [Google Scholar]
- 34.Sharp L. Z., Shinoda K., Ohno H., Scheel D. W., Tomoda E., Ruiz L., Hu H., Wang L., Pavlova Z., Gilsanz V., et al. . 2012. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 7: e49452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pisani D. F., Djedaini M., Beranger G. E., Elabd C., Scheideler M., Ailhaud G., and Amri E. Z.. 2011. Differentiation of human adipose-derived stem cells into “brite” (brown-in-white) adipocytes. Front. Endocrinol. (Lausanne). 2: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez A. M., Elabd C., Delteil F., Astier J., Vernochet C., Saint-Marc P., Guesnet J., Guezennec A., Amri E. Z., Dani C., et al. . 2004. Adipocyte differentiation of multipotent cells established from human adipose tissue. Biochem. Biophys. Res. Commun. 315: 255–263. [DOI] [PubMed] [Google Scholar]
- 37.Elabd C., Chiellini C., Carmona M., Galitzky J., Cochet O., Petersen R., Penicaud L., Kristiansen K., Bouloumie A., Casteilla L., et al. . 2009. Human multipotent adipose-derived stem cells differentiate into functional brown adipocytes. Stem Cells. 27: 2753–2760. [DOI] [PubMed] [Google Scholar]
- 38.Le Faouder P., Baillif V., Spreadbury I., Motta J. P., Rousset P., Chene G., Guigne C., Terce F., Vanner S., Vergnolle N., et al. . 2013. LC-MS/MS method for rapid and concomitant quantification of pro-inflammatory and pro-resolving polyunsaturated fatty acid metabolites. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 932: 123–133. [DOI] [PubMed] [Google Scholar]
- 39.Brand M. D., and Nicholls D. G.. 2011. Assessing mitochondrial dysfunction in cells. Biochem. J. 435: 297–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. . 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 9: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bustin S. A., Benes V., Garson J. A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M. W., Shipley G. L., et al. . 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55: 611–622. [DOI] [PubMed] [Google Scholar]
- 42.Ghandour R. A., Giroud M., Vegiopoulos A., Herzig S., Ailhaud G., Amri E. Z., and Pisani D. F.. 2016. IP-receptor and PPARs trigger the conversion of human white to brite adipocyte induced by carbaprostacyclin. Biochim. Biophys. Acta. 1861: 285–293. [DOI] [PubMed] [Google Scholar]
- 43.Fjære E., Aune U. L., Roen K., Keenan A. H., Ma T., Borkowski K., Kristensen D. M., Novotny G. W., Mandrup-Poulsen T., Hudson B. D., et al. . 2014. Indomethacin treatment prevents high fat diet-induced obesity and insulin resistance but not glucose intolerance in C57BL/6J mice. J. Biol. Chem. 289: 16032–16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Javadi M., Everts H., Hovenier R., Kocsis S., Lankhorst A. E., Lemmens A. G., Schonewille J. T., Terpstra A. H., and Beynen A. C.. 2004. The effect of six different C18 fatty acids on body fat and energy metabolism in mice. Br. J. Nutr. 92: 391–399. [DOI] [PubMed] [Google Scholar]
- 45.Mössenböck K., Vegiopoulos A., Rose A. J., Sijmonsma T. P., Herzig S., and Schafmeier T.. 2014. Browning of white adipose tissue uncouples glucose uptake from insulin signaling. PLoS One. 9: e110428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vegiopoulos A., Muller-Decker K., Strzoda D., Schmitt I., Chichelnitskiy E., Ostertag A., Berriel Diaz M., Rozman J., Hrabe de Angelis M., Nusing R. M., et al. . 2010. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 328: 1158–1161. [DOI] [PubMed] [Google Scholar]
- 47.Babaei R., Bayindir-Buchhalter I., Meln I., and Vegiopoulos A.. 2017. Immuno-magnetic isolation and thermogenic differentiation of white adipose tissue progenitor cells. Methods Mol. Biol. 1566: 37–48. [DOI] [PubMed] [Google Scholar]
- 48.Fickova M., Hubert P., Cremel G., and Leray C.. 1998. Dietary (n-3) and (n-6) polyunsaturated fatty acids rapidly modify fatty acid composition and insulin effects in rat adipocytes. J. Nutr. 128: 512–519. [DOI] [PubMed] [Google Scholar]
- 49.Herzberg G. R., and Skinner C.. 1997. Differential accumulation and release of long-chain n-3 fatty acids from liver, muscle, and adipose tissue triacylglycerols. Can. J. Physiol. Pharmacol. 75: 945–951. [DOI] [PubMed] [Google Scholar]
- 50.Luo J., Rizkalla S. W., Boillot J., Alamowitch C., Chaib H., Bruzzo F., Desplanque N., Dalix A. M., Durand G., and Slama G.. 1996. Dietary (n-3) polyunsaturated fatty acids improve adipocyte insulin action and glucose metabolism in insulin-resistant rats: relation to membrane fatty acids. J. Nutr. 126: 1951–1958. [DOI] [PubMed] [Google Scholar]
- 51.D’andrea S., Guillou H., Jan S., Catheline D., Thibault J. N., Bouriel M., Rioux V., and Legrand P.. 2002. The same rat Delta6-desaturase not only acts on 18- but also on 24-carbon fatty acids in very-long-chain polyunsaturated fatty acid biosynthesis. Biochem. J. 364: 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cunnane S. C. 2003. Problems with essential fatty acids: time for a new paradigm? Prog. Lipid Res. 42: 544–568. [DOI] [PubMed] [Google Scholar]
- 53.Laiglesia L. M., Lorente-Cebrian S., Prieto-Hontoria P. L., Fernandez-Galilea M., Ribeiro S. M., Sainz N., Martinez J. A., and Moreno-Aliaga M. J.. 2016. Eicosapentaenoic acid promotes mitochondrial biogenesis and beige-like features in subcutaneous adipocytes from overweight subjects. J. Nutr. Biochem. 37: 76–82. [DOI] [PubMed] [Google Scholar]
- 54.Zhao M., and Chen X.. 2014. Eicosapentaenoic acid promotes thermogenic and fatty acid storage capacity in mouse subcutaneous adipocytes. Biochem. Biophys. Res. Commun. 450: 1446–1451. [DOI] [PubMed] [Google Scholar]
- 55.Muhlhausler B. S., Cook-Johnson R., James M., Miljkovic D., Duthoit E., and Gibson R.. 2010. Opposing effects of omega-3 and omega-6 long chain polyunsaturated Fatty acids on the expression of lipogenic genes in omental and retroperitoneal adipose depots in the rat. J. Nutr. Metab. 2010: pii: 927836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martínez-Fernández L., Laiglesia L. M., Huerta A. E., Martinez J. A., and Moreno-Aliaga M. J.. 2015. Omega-3 fatty acids and adipose tissue function in obesity and metabolic syndrome. Prostaglandins Other Lipid Mediat. 121: 24–41. [DOI] [PubMed] [Google Scholar]
- 57.Massaro M., Habib A., Lubrano L., Del Turco S., Lazzerini G., Bourcier T., Weksler B. B., and De Caterina R.. 2006. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP(H) oxidase and PKC epsilon inhibition. Proc. Natl. Acad. Sci. USA. 103: 15184–15189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim M., Goto T., Yu R., Uchida K., Tominaga M., Kano Y., Takahashi N., and Kawada T.. 2015. Fish oil intake induces UCP1 upregulation in brown and white adipose tissue via the sympathetic nervous system. Sci. Rep. 5: 18013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pahlavani M., Razafimanjato F., Ramalingam L., Kalupahana N. S., Moussa H., Scoggin S., and Moustaid-Moussa N.. 2017. Eicosapentaenoic acid regulates brown adipose tissue metabolism in high-fat-fed mice and in clonal brown adipocytes. J. Nutr. Biochem. 39: 101–109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.