Abstract
Species of Nigrospora commonly occur as plant pathogens, endophytes or saprobes, and have been shown to be extremely interesting for the discovery of novel metabolites. The familial placement, as well as phylogenetic relationships among Nigrospora species remain ambiguous. In this study, Nigrospora (= Khusia) is confirmed as a monophyletic genus belonging to Apiosporaceae (Xylariales), based on a phylogeny inferred from LSU sequence data. A multi-locus phylogeny based on ITS, TEF1-α and TUB2, in conjunction with morphological characters, host associations, and ecological data was employed for species delimitation in Nigrospora, as well as identification of 165 recently collected isolates from China, and three from Europe. In total 13 novelties are proposed including 12 new species and 1 new combination. Five species are re-described based on an examination of type specimens and/or fresh collections. New species described in this paper include: N. aurantiaca, N. bambusae, N. camelliae-sinensis, N. chinensis, N. guilinensis, N. hainanensis, N. lacticolonia, N. osmanthi, N. pyriformis, N. rubi, N. vesicularis and N. zimmermanii. Furthermore, N. vietnamensis is transferred to Arthrinium. Our results indicate a high level of species diversity within Nigrospora, with a general lack in host specificity. Taxa that cluster basal in Nigrospora have wide host ranges, whereas those that diverged later tend to have narrow host ranges. The currently available data suggest, therefore, that the general evolutionary direction in the genus Nigrospora is from a wide to a narrow host range.
Keywords: Apiosporaceae, Ascomycota, phylogeny, species delimitation, systematics
INTRODUCTION
Nigrospora is an important genus of fungal ascomycetes with a cosmopolitan distribution and wide host range. Nigrospora species have been isolated as endophytes from leaves and stems of various plants, or as saprobes from detritus, dead larvae or leaf litter (Mason 1927, Wu et al. 2009, Thalavaipandian et al. 2011, Uzor et al. 2015). Nigrospora species have also been commonly recorded as plant pathogens on many important economic crops, fruits and ornamentals. Examples include N. oryzae causing stem blight on Brassica juncea in India (Sharma et al. 2013), N. sphaerica causing leaf blight on Camellia sinensis in China (Liu et al. 2015) and N. musae causing ‘squirter’ disease on bananas (Jones & Stover 2000). In addition, N. sphaerica is an opportunistic pathogen causing onychomycosis in humans (De Hoog et al. 2000, Fan et al. 2009) and corneal ulcer (Kindo et al. 2014).
Nigrospora species are also commonly isolated from the indoor environment. Webster (1952) demonstrated that N. sphaerica has a violent spore discharge mechanism, that can forcibly project its spores to a distance of up to 2 cm vertically, and 6.7 cm horizontally. The study by Wu et al. (2004) also showed that Nigrospora spores are one of the more dominant groups in the atmosphere, being associated with dust storms. Moreover, some Nigrospora spores are responsible for a Type I allergic response, seasonal rhinitis (hay fever), asthma or respiratory allergic diseases (Santo-Pietro 2006, Khan & Karuppayil 2012, Saha & Bhattacharya 2015).
Nigrospora is regarded as extremely interesting as a source of natural products and because of its potential industrial applications (Chen et al. 2016). Metabolites produced by N. sacchari showed remarkable herbicidal activity in the treatment of intact greenhouse-grown plants (Fukushima et al. 1998), while Phomalactone produced by N. spherica was found to be an active constituent against mosquitoes (Meepagala et al. 2015). Moreover, some extrolites produced by N. sphaerica exhibited antibacterial activities against the growth of methicillin-resistant Staphylococcus aureus (MRSA) and Klebsiella pneumonia cells (Ibrahim et al. 2015).
The generic name Nigrospora was first introduced by Zimmerman (1902) for N. panici, which was isolated as an endophyte from leaves of Panicum amphibium in Java, Indonesia. Later, Mason (1927) transferred several black-spored hyphomycetes occurring on monocotyledonous hosts to Nigrospora, including N. oryzae (= Monotospora oryzae), N. sphaerica (= Trichosporum sphaericum), N. arundinacea (= Hadrotrichum arundinaceum) and N. sacchari (= Glenospora sacchari). Mason (1927) further pointed out that the Indonesian fungus, N. javanica (Palm 1918), occurs on maize, rice and wheat, and is a synonym of N. panici. However, type specimens from both taxa have been lost, and thus a direct morphological comparison and molecular analysis is not possible. Nigrospora gallarum (= Basisporium gallarum) and N. gorlenkoana were previously regarded as synonyms of N. oryzae in MycoBank due to their similar conidial morphology. Presently, there are 15 recognised species listed in MycoBank, but the familial placement of the genus remains unresolved. Barnett & Hunter (1998) placed Nigrospora in Dematiaceae (Moniliales) based on its conidial characters, while Kirk et al. (2008) assigned Nigrospora and its Khuskia sexual morph to the Trichosphaeriaceae (Trichosphaeriales).
The objectives of the present study were therefore to:
1. resolve the higher order phylogenetic placement of Nigrospora;
2. infer the phylogenetic and evolutionary relationships of Nigrospora species based on multi-locus DNA sequence data (ITS, TEF1-α, TUB2) analyses; and
3. identify 165 Nigrospora strains collected in China and three strains from Europe to species level.
MATERIALS AND METHODS
Collection, isolation and herbarium specimens
Diseased and healthy plant tissues were collected from Camellia sinensis, Musa paradisiaca and several other unidentified plant hosts in eight Chinese provinces (Fujian, Guangxi, Guizhou, Hainan, Hubei, Jiangxi, Tibet and Yunnan). Isolates associated with leaf spots were cultured using both single spore and tissue isolation methods. The single spore isolation protocol of Zhang et al. (2013) was adopted by using quarter strength potato dextrose agar (1/4 PDA; 9.75g Difco PDA, 15g Difco agar and 1L distilled water) with antibiotics (Sodium ampicillin and Streptomycin sulfate). Fungal endophytes were isolated by cutting four fragments (2 × 2 mm) per leaf from the apex, base and lateral sides; samples were surface sterilised with 75 % ethanol for 1 min, 5 % NaClO for 30 s; and then rinsed in sterile distilled water for 1 min. Leaf pieces were dried between sterilised paper towels and then plated onto 1/4 PDA.
All cultures are preserved in the LC culture collection (personal culture collection of Lei Cai housed in the Institute of Microbiology, Chinese Academy of Sciences). Type specimens were deposited in the Mycological Herbarium of Microbiology Institute, Chinese Academy of Sciences, Beijing, China (HMAS), with ex-type living cultures deposited in the China General Microbiological Culture Collection Center (CGMCC) and the Agricultural Culture Collection of China (ACCC). New descriptions and nomenclature were deposited in MycoBank (www.MycoBank.org; Crous et al. 2004).
Loan requests of type specimens were sent to 22 herbaria, viz. B, BIOT, BO, BZ, FIPIA, IARI, IPA, K, KRB, LE, LIL, LP, MEL, MELU, PAD, PAS, PDA, PEUFR, RO, UFP, URM, VLA. Four types of Nigrospora species were received from K, i.e. N. oryzae (= Monotospora oryzae, IMI 99832), N. sphaerica (= Trichosporum sphaericum, IMI 103253), N. arundinacea (= Hadrotrichum arundinaceum, K(M) 203264) and Khuskia oryzae (sexual morph of N. oryzae, IMI 79239).
Morphology
Cultures were incubated on PDA for 7 d at 25 °C to measure diagonal growth. To enhance sporulation, 5 mm diam plugs from the margin of actively growing cultures were transferred to the centre of 9 cm diam Petri dishes containing synthetic nutrient-poor agar medium (SNA; Nirenberg 1976) at 28 °C. Morphological descriptions were based on cultures sporulating on SNA. The shape and size of microscopic structures were observed using a light microscope and colonies were assessed according to the colour charts of Rayner (1970). At least 50 conidiogenous cells and conidia were measured to calculate the mean size.
DNA extraction, PCR amplification and sequencing
Fresh fungal mycelia grown on PDA for 7 d at 25 °C were scraped from the colony margin and used for genomic DNA extraction using a modified CTAB protocol as described in Guo et al. (2000). PCR amplification and sequencing of the large subunit (LSU) rDNA using the primer pair LR0R/LR5 and the 5.8S nuclear ribosomal gene with the two flanking transcribed spacers (ITS) using primer pair ITS1/ITS4 was performed (Vilgalys & Hester 1990, White et al. 1990). Part of the translation elongation factor 1-alpha (TEF1-α) was amplified and sequenced using primer pair EF1-728F (Carbone & Kohn 1999) and EF-2 (O’Donnell et al. 1998). Bt-2a and Bt-2b (Glass & Donaldson 1995) were used for the Beta-tubulin fragment (TUB2). PCR was performed in a 25 μL reaction containing 18.95 μL double distilled water, 2.5 μL 10 × PCR buffer, 0.3 μL dNTP mix (2.5 mM), 1 μL per primer (10 mM), 1 μL DNA template, 0.25 μL Taq DNA polymerase (Genstar). Amplification conditions for ITS, LSU and TEF1-α followed Crous et al. (2013) and for TUB2, Lee et al. (2004). Purification and sequencing of PCR amplicons were carried out at the SinoGenoMax Company, Beijing. DNA sequences were generated with upper surface and reverse primers to obtain consensus sequences analysed with MEGA v. 6.0 (Tamura et al. 2013).
Phylogenetic analysis
LSU sequences of Nigrospora and similar sequences from related genera obtained from GenBank (Table 1, 2) were analysed to resolve the higher order phylogenetic placement of Nigrospora. Single locus and concatenated gene trees were inferred from ITS, TUB2 and TEF1-α (Table 1) using Bayesian and Maximum-likelihood analyses to help delimit species in Nigrospora. Sequences were aligned using an online version of MAFFT v. 7 (Katoh & Standley 2013). Ambiguous regions were excluded from the analyses and gaps were treated as missing data. A 70 % neighbour-joining (NJ) reciprocal bootstrap method with maximum-likelihood distance was applied to check the congruence of the individual loci in the multi-locus dataset (Mason-Gamer & Kellogg 1996).
Table 1.
Strains of the Nigrospora species used in this study with details about host and location, and GenBank accessions of the sequences generated.
| Species | Accession numbers1,2 | Host | Locality | GenBank accession numbers3 |
|||
|---|---|---|---|---|---|---|---|
| ITS | LSU | TUB2 | TEF1-α | ||||
| N. aurantiaca | CGMCC 3.18130* = LC 7302 | Nelumbo sp. (leaf) | China | KX986064 | KX986098 | KY019465 | KY019295 |
| LC 7034 | Musa paradisiaca | China | KX986093 | – | KY019598 | KY019394 | |
| N. bambusae | CGMCC 3.18327* = LC 7114 | Bamboo (leaf) | China | KY385307 | – | KY385319 | KY385313 |
| LC 7244 | Bamboo (leaf) | China | KY385306 | – | KY385320 | KY385314 | |
| LC 7245 | Bamboo (leaf) | China | KY385305 | – | KY385321 | KY385315 | |
| N. camelliae-sinensis | LC 2710 | Castanopsis sp. | China | KX985957 | – | KY019484 | KY019310 |
| LC 3287 | Camellia sinensis | China | KX985975 | – | KY019502 | KY019323 | |
| LC 3496 | Camellia sinensis | China | KX985985 | – | KY019510 | KY019327 | |
| CGMCC 3.18125* = LC 3500 | Camellia sinensis | China | KX985986 | KX986103 | KY019460 | KY019293 | |
| LC 4460 | Castanopsis sp. | China | KX986015 | – | KY019538 | KY019353 | |
| LC 6304 | Camellia sinensis | China | KX986045 | – | KY019566 | KY019370 | |
| LC 6984 | Musa paradisiaca (leaf) | China | KX986080 | – | KY019587 | KY019387 | |
| LC 6684 | Camellia sinensis | China | KX986046 | – | KY019570 | KY019449 | |
| LC 6989 | Musa paradisiaca (leaf) | China | KX986083 | – | KY019590 | KY019453 | |
| LC 6992 | Musa paradisiaca (leaf) | China | KX986084 | – | KY019591 | KY019389 | |
| LC 7018 | Musa paradisiaca (leaf) | China | KX986089 | – | KY019595 | KY019392 | |
| LC 7044 | Musa paradisiaca (leaf) | China | KX986095 | – | KY019600 | KY019395 | |
| N. chinensis | LC 2696 | Lindera aggregata | China | KX985947 | – | KY019474 | KY019424 |
| LC 3085 | Camellia sinensis | China | KX985970 | – | KY019497 | KY019427 | |
| LC 3175 | Camellia sinensis | China | KX985972 | – | KY019499 | KY019428 | |
| LC 3275 | Camellia sinensis | China | KX985973 | – | KY019500 | KY019429 | |
| LC 3286 | Camellia sinensis | China | KX985974 | – | KY019501 | KY019430 | |
| LC 3293 | Camellia sinensis | China | KX985977 | – | KY019504 | KY019431 | |
| LC 3400 | Camellia sinensis | China | KX985979 | – | KY019505 | KY019432 | |
| LC 3441 | Camellia sinensis | China | KX985981 | – | KY019507 | KY019433 | |
| LC 3493 | Camellia sinensis | China | KX985984 | – | KY019509 | KY019434 | |
| LC 4364 | Aucuba japonica | China | KX986011 | – | KY019534 | KY019435 | |
| LC 4433 | Castanopsis sp. | China | KX986013 | – | KY019536 | KY019436 | |
| LC 4463 | Unknown host plant | China | KX986016 | – | KY019539 | KY019437 | |
| LC 4554 | Unknown host plant | China | KX986018 | – | KY019541 | KY019439 | |
| LC 4555 | Unknown host plant | China | KX986019 | – | KY019542 | KY019440 | |
| LC 4558 | Unknown host plant | China | KX986020 | – | KY019543 | KY019441 | |
| LC 4565 | Itea sp. | China | KX986021 | – | KY019544 | KY019442 | |
| CGMCC 3.18127* = LC 4575 | Machilus breviflora | China | KX986023 | KX986107 | KY019462 | KY019422 | |
| LC 4593 | Machilus duthiei | China | KX986024 | – | KY019546 | KY019443 | |
| LC 4619 | Osmanthus sp. | China | KX986025 | – | KY019547 | KY019444 | |
| LC 4660 | Quercus sp. | China | KX986026 | – | KY019548 | KY019445 | |
| LC 4673 | Smilax ocreata | China | KX986028 | – | KY019550 | KY019446 | |
| LC 6631 | Camellia sinensis | China | KX986043 | – | KY019569 | KY019448 | |
| LC 6851 | Unknown host plant | China | KX986049 | – | KY019579 | KY019450 | |
| LC 6972 | Musa paradisiaca | China | KX986078 | – | KY019585 | KY019451 | |
| LC 6998 | Musa paradisiaca (leaf) | China | KX986086 | – | KY019593 | KY019391 | |
| LC 7026 | Musa paradisiaca (leaf) | China | KX986090 | – | KY019596 | KY019393 | |
| N. gorlenkoana | CBS 480.73* | Vitis vinifera | Kazakhstan | KX986048 | KX986109 | KY019456 | KY019420 |
| N. guilinensis | LC 7301 | Nelumbo sp. (stem) | China | KX986063 | – | KY019608 | KY019404 |
| CGMCC 3.18124* = LC 3481 | Camellia sinensis | China | KX985983 | KX986113 | KY019459 | KY019292 | |
| N. hainanensis | CGMCC 3.18129* = LC 7030 | Musa paradisiaca (leaf) | China | KX986091 | KX986112 | KY019464 | KY019415 |
| LC 6979 | Musa paradisiaca (leaf) | China | KX986079 | – | KY019586 | KY019416 | |
| LC 7031 | Musa paradisiaca (leaf) | China | KX986092 | – | KY019597 | KY019417 | |
| LC 7042 | Musa paradisiaca (leaf) | China | KX986094 | – | KY019599 | KY019418 | |
| N . lacticolonia | CGMCC 3.18123* = LC 3324 | Camellia sinensis | China | KX985978 | KX986105 | KY019458 | KY019291 |
| LC 7009 | Musa paradisiaca (leaf) | China | KX986087 | – | KY019594 | KY019454 | |
| N. musae | CBS 319.34* | Musa paradisiaca (fruit) | Australia | KX986076 | KX986110 | KY019455 | KY019419 |
| LC 6385 | Camellia sinensis | China | KX986042 | – | KY019567 | KY019371 | |
| N. oryzae | LC 6759 | Oryza sativa | China | KX986054 | – | KY019572 | KY019374 |
| LC 6760 | Oryza sativa | China | KX986055 | – | KY019573 | KY019375 | |
| LC 6761 | Oryza sativa | China | KX986056 | – | KY019574 | KY019376 | |
| LC 6762 | Oryza sativa | China | KX986057 | – | KY019575 | KY019377 | |
| LC 6763 | Oryza sativa | China | KX986058 | – | KY019576 | KY019378 | |
| LC 6764 | Oryza sativa | China | KX986059 | – | KY019577 | KY019379 | |
| LC 6765 | Oryza sativa | China | KX986060 | – | – | KY019380 | |
| LC 6893 | Oryza sativa | China | KX986050 | – | KY019580 | KY019382 | |
| LC 7293 | Nelumbo sp. (leaf) | China | KX985931 | – | KY019601 | KY019396 | |
| LC 7297 | Nelumbo sp. (leaf) | China | KX985936 | – | KY019605 | KY019400 | |
| LC 6029 | Nelumbo sp. (leaf) | China | KX985938 | – | KY019564 | KY019368 | |
| LC 7299 | Nelumbo sp. (leaf) | China | KX98606 | – | KY019607 | KY019402 | |
| LC 7300 | Nelumbo sp. (leaf) | China | KX986062 | – | – | KY019403 | |
| LC 7305 | Nelumbo sp. (leaf) | China | KX986067 | – | KY019611 | KY019407 | |
| LC 7306 | Nelumbo sp. (leaf) | China | KX986068 | – | KY019612 | KY019408 | |
| LC 7307 | Nelumbo sp. (leaf) | China | KX986069 | – | KY019613 | KY019409 | |
| LC 7308 | Nelumbo sp. (leaf) | China | KX986070 | – | KY019614 | KY019410 | |
| LC 7309 | Nelumbo sp. (leaf) | China | KX986071 | – | KY019615 | KY019411 | |
| LC 7310 | Nelumbo sp. (leaf) | China | KX986072 | – | KY019616 | KY019412 | |
| LC 7311 | Nelumbo sp. (leaf) | China | KX986073 | – | KY019617 | KY019413 | |
| LC 6766 | Oryza sativa L. | China | KX986074 | – | KY019578 | KY019381 | |
| LC 6566 | Oryza sativa L. | China | KX986075 | – | KY019568 | KY019372 | |
| LC 2689 | Rhododendron sp. | China | KX985942 | – | KY019469 | KY019423 | |
| LC 2693 | Neolitsea sp. | China | KX985944 | KX986101 | KY019471 | KY019299 | |
| LC 2695 | Rubus reflexus | China | KX985946 | – | KY019473 | KY019301 | |
| LC 2699 | Hamamelis mollis | China | KX985949 | – | KY019476 | KY019303 | |
| LC 2702 | Rubus sp. | China | KX985950 | – | KY019477 | KY019304 | |
| LC 2704 | Rhododendron sp. | China | KX985951 | – | KY019478 | KY019425 | |
| LC 2706 | Rhododendron sp. | China | KX985953 | – | KY019480 | KY019306 | |
| LC 2707 | Rhododendron simiarum | China | KX985954 | – | KY019481 | KY019307 | |
| LC 2708 | Rhododendron sp. | China | KX985955 | – | KY019482 | KY019308 | |
| LC 2709 | Rhododendron simiarum | China | KX985956 | – | KY019483 | KY019309 | |
| LC 2712 | Castanopsis sp. | China | KX985958 | – | KY019485 | KY019311 | |
| LC 2724 | Symplocos zizyphoides | China | KX985959 | – | KY019486 | KY019312 | |
| LC 2744 | Symplocos zizyphoides | China | KX985961 | – | KY019488 | KY019314 | |
| LC 2749 | Ternstroemia sp. | China | KX985962 | – | KY019489 | KY019315 | |
| LC 2752 | Osmanthus sp. | China | KX985963 | – | KY019490 | KY019316 | |
| LC 2972 | Tutcheria microcarpa | China | KX985967 | – | KY019494 | KY019320 | |
| LC 2991 | Cleyera japonica | China | KX985969 | – | KY019496 | KY019321 | |
| LC 3690 | Symplocos zizyphoides | China | KX985987 | – | KY019511 | KY019328 | |
| LC 3695 | Osmanthus fragrans | China | KX985988 | – | KY019512 | KY019329 | |
| LC 4260 | Rhododendron sp. | China | KX985991 | – | KY019515 | KY019332 | |
| LC 4265 | Rhododendron sp. | China | KX985994 | – | KY019518 | KY019335 | |
| LC 4273 | Cephalotaxus sinensis | China | KX985995 | – | KY019519 | KY019336 | |
| LC 4275 | Rhododendron sp. | China | KX985997 | – | KY019521 | KY019338 | |
| LC 4281 | Rhododendron sp. | China | KX985999 | – | KY019523 | KY019340 | |
| LC 4294 | Daphniphyllum macropodum | China | KX986002 | – | KY019526 | KY019343 | |
| LC 4295 | Daphniphyllum macropodum | China | KX986003 | – | KY019527 | KY019344 | |
| LC 4320 | Daphniphyllum oldhamii | China | KX986006 | – | KY019530 | KY019347 | |
| LC 4327 | Camellia sp. | China | KX986007 | – | KY019531 | KY019348 | |
| LC 4338 | Camellia sp. | China | KX986008 | – | KY019532 | KY019349 | |
| LC 4345 | Camellia sp. | China | KX986009 | – | KY019533 | KY019350 | |
| LC 4679 | Osmanthus sp. | China | KX986029 | – | KY019551 | KY019356 | |
| LC 4680 | Camellia sinensis | China | KX986030 | – | KY019552 | KY019357 | |
| LC 4961 | Pittosporum illicioides | China | KX986031 | – | KY019553 | KY019358 | |
| LC 5181 | Pentactina rupicola | China | KX986032 | – | KY019554 | KY019359 | |
| LC 5243 | Submerged wood | China | KX986033 | – | KY019555 | KY019360 | |
| LC 5964 | Submerged wood | China | KX986037 | – | KY019559 | KY019447 | |
| LC 5965 | Submerged wood | China | KX986038 | – | KY019560 | KY019364 | |
| LC 5982 | Submerged wood | China | KX986040 | – | KY019562 | KY019366 | |
| LC 5999 | Submerged wood | China | KX986041 | – | KY019563 | KY019367 | |
| LC 6923 | Oryza sativa L. | China | KX986051 | – | KY019581 | KY019383 | |
| LC 6955 | Oryza sativa L. | China | KX986052 | – | KY019582 | KY019384 | |
| LC 6957 | Oryza sativa L. | China | KX986053 | – | KY019583 | KY019385 | |
| N. osmanthi | CGMCC 3.18126* = LC 4350 | Osmanthus sp. | China | KX986010 | KX986106 | KY019461 | KY019421 |
| LC 4487 | Hedera nepalensis | China | KX986017 | – | KY019540 | KY019438 | |
| N. pyriformis | CGMCC 3.18122* = LC 2045 | Citrus sinensis | China | KX985940 | KX986100 | KY019457 | KY019290 |
| LC 2688 | Lindera aggregata | China | KX985941 | – | KY019468 | KY019297 | |
| LC 2690 | Rosa sp. | China | KX985943 | – | KY019470 | KY019298 | |
| LC 2694 | Rubus reflexus | China | KX985945 | – | KY019472 | KY019300 | |
| LC 3099 | Camellia sinensis | China | KX985971 | – | KY019498 | KY019322 | |
| LC 3292 | Camellia sinensis | China | KX985976 | – | KY019503 | KY019324 | |
| LC 4669 | Castanopsis sp. | China | KX986027 | – | KY019549 | KY019355 | |
| LC 6985 | Musa paradisiaca (leaf) | China | KX986081 | – | KY019588 | KY019388 | |
| LC 6988 | Musa paradisiaca (leaf) | China | KX986082 | – | KY019589 | KY019452 | |
| N. rubi | CGMCC 3.18326* = LC 2698 | Rubus sp. | China | KX985948 | KX986102 | KY019475 | KY019302 |
| N. sphaerica | LC 7294 | Nelumbo sp. (leaf) | China | KX985932 | – | KY019602 | KY019397 |
| LC 7295 | Nelumbo sp. (leaf) | China | KX985933 | – | KY019603 | KY019398 | |
| LC 7296 | Nelumbo sp. (leaf) | China | KX985934 | – | KY019604 | KY019399 | |
| LC 7312 | Nelumbo sp. (leaf) | China | KX985935 | – | KY019618 | KY019414 | |
| LC 7298 | Nelumbo sp. (leaf) | China | KX985937 | KX986097 | KY019606 | KY019401 | |
| LC 7303 | Nelumbo sp. (leaf) | China | KX986065 | – | KY019609 | KY019405 | |
| LC 7304 | Nelumbo sp. (leaf) | China | KX986066 | – | KY019610 | KY019406 | |
| LC 2705 | Rosa sp. | China | KX985952 | – | KY019479 | KY019305 | |
| LC 2839 | Harpullia longipetala | China | KX985964 | – | KY019491 | KY019317 | |
| LC 2840 | Harpullia longipetala | China | KX985965 | – | KY019492 | KY019318 | |
| LC 2958 | Cleyera japonica | China | KX985966 | – | KY019493 | KY019319 | |
| LC 2983 | Camellia sp. | China | KX985968 | – | KY019495 | KY019426 | |
| LC 3420 | Camellia sinensis | China | KX985980 | – | KY019506 | KY019325 | |
| LC 3477 | Camellia sinensis | China | KX985982 | – | KY019508 | KY019326 | |
| LC 4174 | Rhododendron arboreum | China | KX985989 | – | KY019513 | KY019330 | |
| LC 4241 | Deutzia sp. | China | KX985990 | – | KY019514 | KY019331 | |
| LC 4263 | Rhododendron arboreum | China | KX985992 | – | KY019516 | KY019333 | |
| LC 4264 | Rhododendron arboreum | China | KX985993 | – | KY019517 | KY019334 | |
| LC 4274 | Rhododendron arboreum | China | KX985996 | – | KY019520 | KY019337 | |
| LC 4278 | Rhododendron arboreum | China | KX985998 | – | KY019522 | KY019339 | |
| LC 4291 | Rhododendron arboreum | China | KX986000 | – | KY019524 | KY019341 | |
| LC 4293 | Rhododendron arboreum | China | KX986001 | – | KY019525 | KY019342 | |
| LC 4303 | Rhododendron arboreum | China | KX986004 | – | KY019528 | KY019345 | |
| LC 4307 | Rhododendron arboreum | China | KX986005 | – | KY019529 | KY019346 | |
| LC 4372 | Rhododendron arboreum | China | KX986012 | – | KY019535 | KY019351 | |
| LC 4447 | Unknown host plant | China | KX986014 | – | KY019537 | KY019352 | |
| LC 5901 | Submerged wood | China | KX986034 | – | KY019556 | KY019361 | |
| LC 5932 | Submerged wood | China | KX986035 | – | KY019557 | KY019362 | |
| LC 5944 | Submerged wood | China | KX986036 | – | KY019558 | KY019363 | |
| LC 5966 | Submerged wood | China | KX986039 | – | KY019561 | KY019365 | |
| LC 6294 | Camellia sinensis | China | KX986044 | – | KY019565 | KY019369 | |
| LC 6969 | Musa paradisiaca (leaf) | China | KX986077 | – | KY019584 | KY019386 | |
| LC 6996 | Musa paradisiaca (leaf) | China | KX986085 | – | KY019592 | KY019390 | |
| Nigrospora sp. 1 | LC 2725 | Symplocos zizyphoides | China | KX985960 | KX986104 | KY019487 | KY019313 |
| LC 4566 | Lithocarpus sp. | China | KX986022 | – | KY019545 | KY019354 | |
| Nigrospora sp. 2 | LC 6704 | Camellia sinensis | China | KX986047 | KX986108 | KY019571 | KY019373 |
| N. vesicularis | LC 0322 | Unknown host plant | Thailand | KX985939 | – | KY019467 | KY019296 |
| CGMCC 3.18128* = LC 7010 | Musa paradisiaca (leaf) | China | KX986088 | KX986099 | KY019463 | KY019294 | |
| N. zimmermanii | CBS 167.26 | Unknown | Unknown | KY385308 | – | KY385318 | KY385312 |
| CBS 290.62* | Saccharum officinarum (leaf) | Ecuador | KY385309 | – | KY385317 | KY385311 | |
| CBS 984.69 | Saccharum officinarum (leaf) | Brazil | KY385310 | – | KY385322 | KY385316 | |
| A. vietnamensis | IMI 99670* | Citrus sinensis | Vietnam | KX986096 | KX986111 | KY019466 | – |
1 CGMCC = China General Microbiological Culture Collection, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China; CBS = Culture Collection of the Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; IMI = Culture Collection of CABI Europe UK Centre, Egham, UK; LC = working collection of Lei Cai, housed at the Institute of Microbiology, Chinese Academy of Sciences, Beijing, China.
2* = ex-type culture.
3 ITS = internal transcribed spacers and intervening 5.8S nrDNA; LSU = 28S nrRNA gene; TUB2 = Beta-tubulin; TEF1-α: translation elongation factor 1-alpha.
Table 2.
GenBank accession numbers of the sequences used for the LSU analyses of Xylariales and Amphisphaeriales.
| Taxon name | Culture accession no. | GenBank accessions LSU |
|---|---|---|
| Adisciso tricellulare | NBRC 32705 | NG 042334 |
| Adisciso yakushimense | MAFF 242774 | AB 593721 |
| Amphibambusa bambusicola | MFLUCC 11-0617 | KP 744474 |
| Amphisphaeria sorbi | MFLUCC 13-0721 | KP 744475 |
| Amphisphaeria umbrina | HKUCC 994 | AF 452029 |
| Apiosordaria verruculosa | F152365 | AY346258 |
| Apiospora setosa | ATCC 58184 | AY 346259 |
| Apiospora tintinnabula | ICMP 6889-96 | DQ 810217 |
| Arecophila bambusae | HKUCC 4794 | AF 452038 |
| Arthrinium arundinis | CBS 106.12 | KF 144927 |
| CBS 114316 | KF 144928 | |
| Arthrinium aureum | CBS 244.83 | KF 144935 |
| Arthrinium hydei | CBS 114990 | KF 144936 |
| Arthrinium kogelbergense | CBS 113333 | KF 144938 |
| Arthrinium malaysianum | CBS 102053 | KF 144942 |
| CBS251.29 | KF 144943 | |
| Arthrinium marii | CBS 497.90 | KF 144947 |
| Arthrinium ovatum | CBS 115042 | KF 144950 |
| Arthrinium phaeospermum | CBS 114314 | KF 144951 |
| CBS 114318 | KF 144954 | |
| Arthrinium phragmites | CPC 18900 | KF 144956 |
| Arthrinium pseudosinense | CPC 21546 | KF 144957 |
| Arthrinium pseudospegazzinii | CBS 102052 | KF 144958 |
| Arthrinium pterospermum | CPC 20193 | KF 144960 |
| Arthrinium rasikravindrii | CBS 337.61 | KF 144961 |
| Arthrinium sacchari | CBS 212.30 | KF 144962 |
| CBS 372.67 | KF 144964 | |
| Arthrinium saccharicola | CBS 191.73 | KF 144966 |
| CBS 463.83 | KF 144968 | |
| Arthrinium xenocordella | CBS 478.86 | KF 144970 |
| CBS595.66 | KF 144971 | |
| Atrotorquata spartii | MFLUCC 13-0444 | KP 325443 |
| Bartalinia robillardoides | CBS 122705 | KJ 710438 |
| MFLUCC 12-0070 | KR 559738 | |
| Broomella vitalbae | MFLUCC 13-0798 | KP 757749 |
| MFLUCC 14-1000 | KP 757750 | |
| Cainia anthoxanthis | MFLUCC 15-0539 | KR 092777 |
| Cainia graminis | MFLUCC 15-0540 | KR 092781 |
| CBS 136.62 | AF 431949 | |
| Ciferriascosea fluctamurum | MFLUCC 15-0541 | KR 092778 |
| Ciferriascosea rectamurum | MFLUCC 15-0542 | KR 092776 |
| Ciliochorella castaneae | HHUF 28799 | AB 433277 |
| Clypeosphaeria uniseptata | – | AY 083830 |
| HKUCC 6349 | DQ 810219 | |
| Coniocessia maxima | Co117 | GU 553344 |
| Coniocessia nodulisporioides | CBS281.77 | AJ 875224 |
| Creosphaeria sassafras | CM AT-018 | DQ 840056 |
| Cryptodiaporthe aesculi | AFTOL-ID 1238 | DQ 836905 |
| Diatrype disciformis | MFLUCC 15–0538 | KR 092784 |
| Diatrype palmicola | MFLUCC 11-0018 | KP 744481 |
| Discosia artocreas | NBRC 8975 | AB 593705 |
| Discosia neofraxinea | MFLU 15-0375 | KR 072672 |
| Discosia pini | MAFF 410149 | AB 593708 |
| Discostroma fuscellum | MFLUCC 14-0052 | KT 005514 |
| Discostroma tostum | NBRC 32626 | AB 593727 |
| Dyrithiopsis lakefuxianensis | HKUCC 7303 | AF 452047 |
| Eutypa flavovirens | MFLUCC 13-0625 | KR 092774 |
| Hyalotiella rubi | MFLUCC 13-0660 | KR 092775 |
| Hyalotiella spartii | MFLUCC 13-0397 | KP 757752 |
| Hyponectria buxi | UME 31430 | AY 083834 |
| Kretzschmaria deusta | CBS 163.93 | KT 281896 |
| Lepteutypa cupressi | IMI 052255 | AF 382379 |
| Lopadostoma americanum | HV-2014h LG8 | KC 774568 |
| Lopadostoma dryophilum | LG21 | KC 774570 |
| Lopadostoma fagi | HV-2014f LF1 | KC 774575 |
| Lopadostoma quercicola | HV-2014a LG27 | KC 774610 |
| Lopadostoma turgidum | LT2 | KC 774618 |
| Monochaetia kansensis | PSHI2004Endo1032 | DQ 534037 |
| PSHI2004Endo1030 | DQ 534035 | |
| Neopestalotiopsis aotearoa | CBS 367.54 | KM 116247 |
| Neopestalotiopsis formicarum | CBS 362.72 | KM 116248 |
| Ophiodiaporthe cyatheae | YMJ 1364 | JX 570891 |
| Pestalotiopsis knightiae | CBS 114138 | KM 116227 |
| Pestalotiopsis malayana | CBS 102220 | KM 116238 |
| Phlogicylindrium eucalyptorum | CBS 111689 | KF 251708 |
| Phlogicylindrium uniforme | CBS 131312 | JQ 044445 |
| Podosordaria tulasnei | CBS 128.80 | KT 281897 |
| Poronia punctata | CBS 656.78 | KT 281900 |
| Pseudomassaria chondrospora | MFLUCC 15-0545 | KR 092779 |
| PC1 | JF 44098 | |
| Pseudomassaria sepincoliformis | CBS 129022 | JF 440984 |
| Pseudopestalotiopsis cocos | CBS 272.29 | KM 116276 |
| Pseudopestalotiopsis theae | MFLUCC 12-0055 | KM 116282 |
| Sarcostroma restionis | CBS 118154 | DQ 278924 |
| Sarcoxylon compunctum | CBS 359.61 | KT 281898 |
| Seimatosporium cornii | MFLUCC 14-0467 | KR 559739 |
| Seimatosporium eucalypti | CPC 156 | JN 871209 |
| Seimatosporium ficeae | SGL002 | KR 920686 |
| Seimatosporium hypericinum | NBRC 32647 | AB 593737 |
| Seimatosporium rhombisporum | MFLUCC 15-0543 | KR 092780 |
| Seimatosporium rosae | MFLUCC 14-0621 | KT 198727 |
| Seiridium cardinale | CBS 172.56 | AF 382376 |
| Seiridium papillatum | CBS 340.97 | DQ 414531 |
| Seiridium phylicae | CPC 19965 | KC 005809 |
| Seynesia erumpens | SMH 1291 | AF 279410 |
| Sordaria fimicola | HKUCC 3714 | AF 132330 |
| Truncatella angustata | ICMP 7062 | AF 382383 |
| Truncatella hartigii | CBS 118148 | DQ 278928 |
| Truncatella laurocerasi | ICMP 11214 | AF 382385 |
| Truncatella restionacearum | CMW 18755 | DQ 278929 |
| Truncatella spartii | MFLUCC 13-0397 | KR 092782 |
| MFLUCC 15-0573 | KR 092783 | |
| Vialaea mangifia | MFLUCC 12-0808 | KF 724975 |
| Vialaea minutella | BRIP 56959 | KC 181924 |
| Xylaria polymorpha | MUCL 49884 | KT 281899 |
| Xylaria obovata | MFLUCC 13-0115 | KR 049089 |
| Zetiasplozna acaciae | CPC 23421 | KJ 869206 |
The best nucleotide substitution model of each locus used for MrBayes v. 3.2.1 (Ronquist et al. 2012), was calculated with jModelTest v. 2.1.4 (Posada 2008). Posterior probabilities (PP) (Zhaxybayeva & Gogarten 2002) were determined by Markov Chain Monte Carlo sampling (MCMC) under the estimated model of evolution. Four simultaneous Markov chains were run for 10 M generations and trees were sampled every 1 000 generations. The run was stopped automatically when the average standard deviation of split frequencies fell below 0.01. The first 25 % of trees, which represented the burn-in phase of the analyses, were discarded and the remaining trees were used for calculating PP in the majority rule consensus tree. Maximum-likelihood analyses including 1 000 bootstrap replicates were conducted using RAxML v. 7.2.6 (Stamatakis & Alachiotis 2010). A general time reversible model (GTR) was applied with a gamma-distributed rate variation. Novel sequences generated in this study were deposited in GenBank (Table 1), the final matrices used for phylogenetic analyses in TreeBASE (www.treebase.org; accession number S20829).
Fungus host distribution analysis
To better illustrate the distribution of Nigrospora species on different hosts, a heatmap was plotted using the ‘pheatmap’ package in R (R Development Core Team 2015), on the basis of data from this study and the USDA fungal database (Farr & Rossman 2017).
RESULTS
Phylogeny
The manually adjusted LSU alignment dataset contained 123 sequences from 110 taxa, in which 897 characters including alignment gaps (available in TreeBASE) were used in the phylogenetic analysis. According to the results of jModeltest v. 2.1.4, the GTR+I+G model was chosen for MrBayes. The phylogeny resulting from the analysis of LSU sequence data is shown in Fig. 1. All strains of Nigrospora formed a sister clade, with high statistical support to Arthrinium, indicating that Nigrospora belongs to Apiosporaceae, Xylariales. The two genera, however, are clearly phylogenetically distinct (Fig 1). The ex-type strain of Nigrospora vietnamensis (IMI 99670) is nested within Arthrinium and appeared conspecific to A. malaysianum.
Fig. 1.
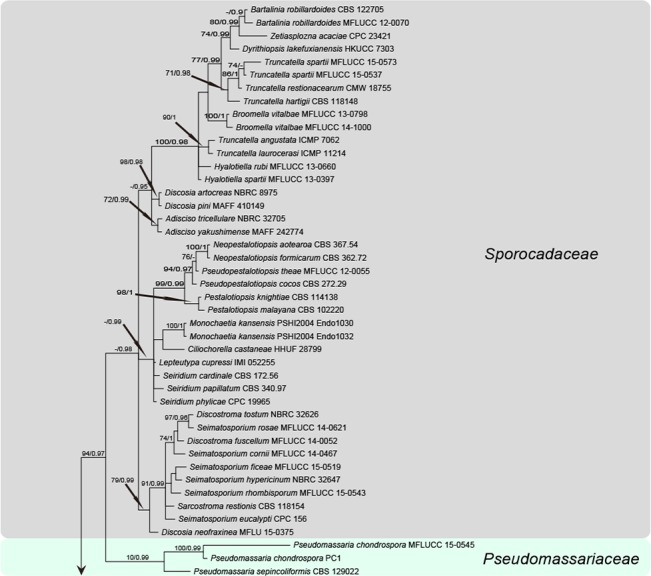
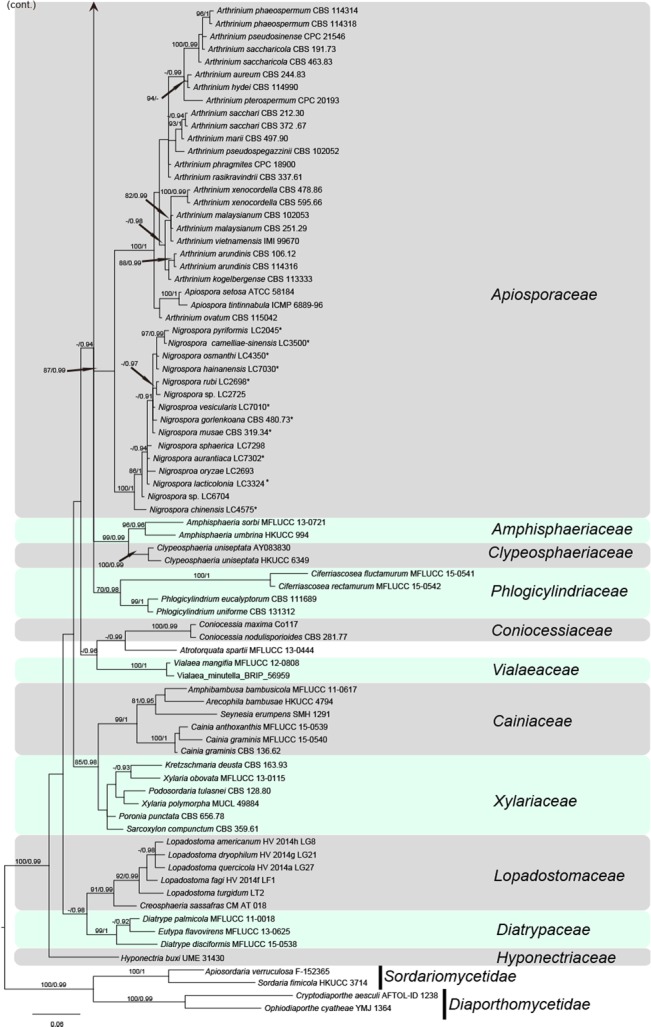
Phylogenetic tree based on the LSU sequences generated from a Maximum likelihood phylogenetic analysis. Bootstrap support values (> 70 %) and posterior probabilities (> 0.9) are given at the nodes. The tree is rooted to Sordariomycetidae (Apiosordaria verruculosa F-152365 and Sordaria fimicola HKUCC 3714) and Diaportheomycetidae (Cryptodiaporthe aesculi AFTOL-ID 1238 and Ophiodiaporthe cyatheae YMJ 1364).
The 70 % neighbour-joining (NJ) reciprocal bootstrap method with maximum-likelihood distance confirmed that single gene trees of Nigrospora inferred from ITS, TUB2 and TEF1-α datasets were congruent (results not shown). The concatenated dataset of ITS, TUB2 and TEF1-α contained 62 strains representing each clade of Nigrospora with reference to single locus trees, and Arthrinium malaysianum CBS 102053 as outgroup. A total of 1 581 characters including gaps (available in TreeBASE) were included in this dataset. For the Bayesian analyses, the best-fit models TIM1ef+I+G, TPM2uf+G, TrN+I+G were set for ITS, TUB2 and TEF1-α, respectively. The concatenated gene tree (Fig. 2) is congruent with the single-locus gene trees (ITS, TUB2 and TEF1-α) and comprises 18 species representing clades with high bootstrap and posterior probability supports values.
Fig. 2.
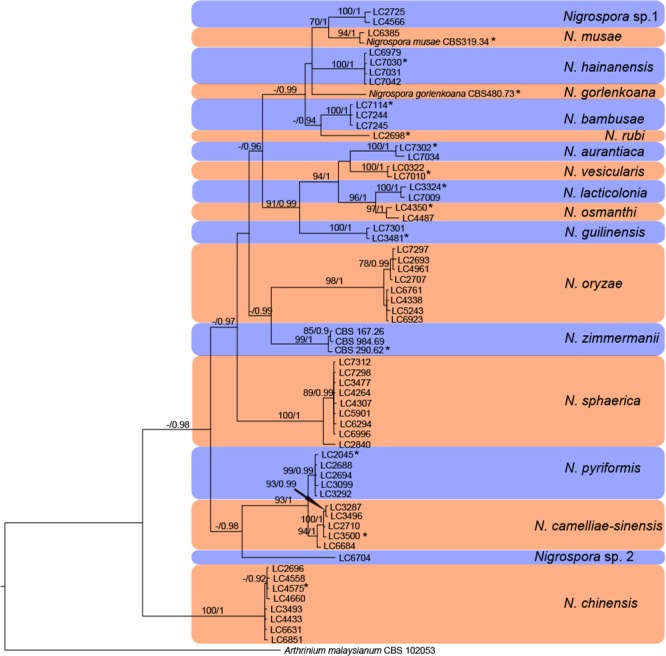
Multilocus phylogenetic tree based on the combined ITS, TEF1-α and TUB2 sequences alignment generated from a Maximum likelihood phylogenetic analysis. Bootstrap support values (> 70 %) and posterior probabilities (> 0.9) are given at the nodes (MLB/PP). The tree is rooted with Arthrinium malaysianum. The novel species are highlighted (* indicates the ex-type cultures).
Fungus host distribution
Nigrospora species appear to be widely distributed on various hosts. Among which, N. sphaerica, N. oryzae and N. chinensis are the three most ubiquitous species. For example, N. sphaerica has been reported from 40 different host genera including Zea, Andropogon and Cymbopogon, while N. oryzae has been reported from 20 different genera including Oryza, Zea and Phyllostachys. Host genera such as Musa and Camellia appear to be amongst the most preferred hosts for Nigrospora, having 10 and 8 Nigrospora species reported on each respective host genus. Eight species, i.e., N. arundinacea, N. canescens, N. gorlenkoana, N. gossypii, N. javanica, N. maydis, N. padwickii, and N. panici, are hitherto only known from one host genus each.
TAXONOMY
Nigrospora Zimm., Centralbl. Bakteriol. Parasitenk., 1. Abth. 8: 220. 1902
Type species. Nigrospora panici Zimm., Centralbl. Bakteriol. Parasitenk., 1. Abth. 8: 220. 1902.
Synonym. Khuskia H.J. Huds., Trans. Brit. Mycol. Soc. 46: 358. 1963.
Type species. Khuskia oryzae H.J. Huds., Trans. Brit. Mycol. Soc. 46: 358. 1963.
Classification — Apiosporaceae, Xylariales, Sordariomycetes.
Colonies on PDA at first white with small, shiny black conidia easily visible under a low-power dissecting microscope due to its large size, later becoming brown or black when sporulation is abundant. Mycelia immersed or partly superficial. Stroma absent. Hyphopodia absent. Setae rarely observed. Conidiophores micronematous or semi-macronematous, branched, flexuous, hyaline to brown, smooth, usually reduced to conidiogenous cells. Conidiogenous cells monoblastic, discrete, solitary, determinate, subspherical, doliiform, ampulliform, subcylindrical to clavate, hyaline. Conidia solitary, acrogenous, with an equatorial hyaline line or a germ slit in some species, simple, spherical or broadly ellipsoidal or pyriform, compressed dorsiventrally, black, shiny, smooth, aseptate, rarely with a violent discharge mechanism. Ascomata perithecial, formed in clusters of 1–7, uniseriate or in irregular rows, subepidermal, erumpent, globose obovoid, with papillate ostioles; surrounded by blackened host tissue. Asci short-stalked, unitunicate, clavate, with eight biseriate ascospores. Paraphyses thin-walled, septate. Ascospores hyaline, granular, curved, inequilateral, tapering towards base with rounded ends, initially aseptate, at times with a single transverse septum.
Notes — The conidiophores of most species of Nigrospora are reduced to conidiogenous cells, each of which normally produces a single conidium. The conidia of Nigrospora are deeply pigmented, with germ slits present in some species. Mason (1927, 1933) revised the taxonomy of Nigrospora, and pointed out that numerous species apparently differ only in spore size, and so far traditional classification has been mainly based on conidial dimensions. In this study, morphological characters were re-evaluated and combined DNA sequence data were analysed to investigate the phylogenetic relationships of Nigrospora species. Furthermore, additional distinguishable characters were employed for distinguishing species, such as conidiogenous cell dimensions, and the presence/absence of vesicles and setae. Sterile cells are often observed in Nigrospora species (Mason 1927, Minter 1985). They are similar to conidia in being deeply pigmented, but are much larger than conidia in dimensions. In addition, sterile cells are formed directly from the hyphae, rather than borne from the conidiogenous cells. Setae are also deeply pigmented and borne from the hyphae, but differ from sterile cells in being longer and narrower, and 1–2-septate.
Nigrospora arundinacea (Cooke & Massee) Potl., Microbiologia, Moscow 21: 224. 1952 — Fig. 3
Fig. 3.

Nigrospora arundinacea (from holotype K(M) 203264). a–c. Overview of the type specimen; d. conidia on Arundo conspicua; e–f. conidiogenous cells; g. conidia. — Scale bars = 10 μm.
Type. England, from Arundo conspicua, 1887, Cooke & Massee (holotype K(M) 203264).
Basionym. Hadrotrichum arundinaceum Cooke & Massee, Grevillea 16, no.77: 11. 1887.
Hyphae dark brown, smooth, branched, septate. Conidiophores usually reduced to conidiogenous cells. Conidiogenous cells monoblastic, discrete, solitary, determinate, pale brown, smooth, subglobose or ampulliform. Conidia globose or subglobose, solitary, black, shiny, smooth, aseptate, 17–21 μm diam (av. = 19.24 ± 0.83).
Notes — The conidial size of N. arundinacea was described as 30 μm diam (Cooke 1887). However, we did not find any conidia matching these dimensions on the type specimen loaned from K. Conidia of N. arundinacea were globose or subglobose, 17–21 μm diam and resembled those of N. sphaerica. We failed to find sufficiently distinguishable morphological characters between the two species solely based on the morphology of their type specimens. DNA extraction from the type specimen of N. arundinacea from K was not permitted, thus the relationship between N. arundinacea and N. sphaerica remains unsolved pending further collections and typification.
Nigrospora aurantiaca Mei Wang & L. Cai, sp. nov. — MycoBank MB820730; Fig. 4
Fig. 4.
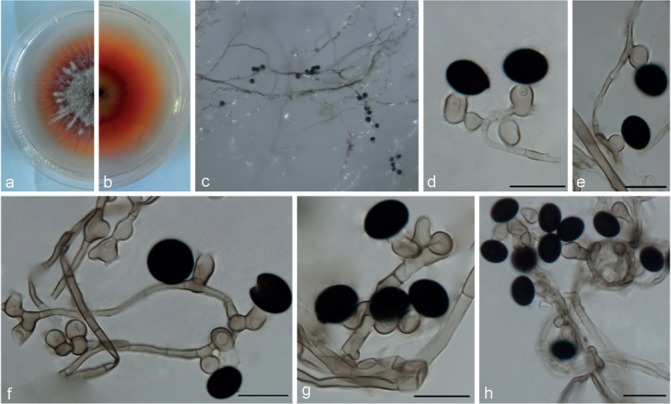
Nigrospora aurantiaca (from ex-type strain CGMCC3.18130). a–b. Upper surface and reverse overview of culture 5 d after inoculation on PDA medium; c. colony on SNA; d–h. conidiogenous cells giving rise to conidia. — Scale bars = 10 μm.
Etymology. Named after the orange colony colour on PDA.
Type. China, Jiangxi Province, Jiangxi Agricultural University, on Nelumbo sp., 21 Sept. 2015, M.F. Hu (HMAS 247065 holotype, ex-type living culture CGMCC3.18130 = LC7302 = JAUCC0677).
Hyphae pale brown, smooth, branched, septate, 1.5–5 μm diam. Conidiophores reduced to conidiogenous cells. Conidiogenous cells dispersed on hyphae, pale brown, monoblastic, discrete, solitary, determinate, doliiform, ovoid or ampulliform, 7.5–13 × 6–8.5 μm (av. = 9.76 ± 1.34 × 7.06 ± 0.56). Conidia solitary, mostly ellipsoidal, black, shiny, smooth, 12–16.5 × 9–15.5 μm (av. = 14.82 ± 0.79 × 11.78 ± 1.07).
Culture characteristics — On PDA, colonies flat, edge entire, floccose at the centre with grey aerial mycelia, initially orange, becoming black with age in the centre. Colonies reaching 9 cm diam after 7 d at 25 °C. On SNA, colonies flat, spreading, with abundant aerial mycelia, surface dirty white to greyish and reverse light pink with olivaceous grey patches.
Additional specimen examined. China, Hainan Province, Chengmai, on leaves of Musa paradisiaca, 25 Dec. 2015, F.J. Liu, living culture LC7034 = WM268.
Notes — Two strains representing N. aurantiaca clustered in a well-supported clade (Fig. 2), and closely related to N. vesicularis (99 % identity in ITS; 89 % in TEF1-α; 96 % in TUB2), N. lacticolonia (99 % in ITS; 87 % in TEF1-α; 93 % in TUB2) and N. osmanthi (99 % in ITS; 88 % in TEF1-α; 94 % in TUB2). Nigrospora aurantiaca differs from N. vesicularis in the absence of vesicles surrounding the septum between its conidiogenous cells and conidia, from N. lacticolonia in the colour of the culture (initially orange, becoming black in N. aurantiaca vs remaining creamy white in N. lacticolonia), from N. osmanthi in the larger conidiogenous cells (av. = 9.76 ± 1.34 × 7.06 ± 0.56 in N. aurantiaca vs av. = 8.02 ± 1.5 × 6.04 ± 1.16 in N. osmanthi). In addition, N. aurantiaca is a morphologically distinct species of Nigrospora that produces orange pigment in culture.
Nigrospora bambusae Mei Wang & L. Cai, sp. nov. — MycoBank MB820800; Fig. 5
Fig. 5.

Nigrospora bambusae (from ex-type strain LC7114). a–b. Upper surface and reverse overview of culture 5 d after inoculation on PDA medium; c. colony on SNA; d–f. conidiogenous cells giving rise to conidia; g. conidia. — Scale bars: d–g = 10 μm.
Etymology. Named after the host from which all strains were isolated, bamboo.
Type. China, Guangdong Province, on bamboo leaves, 10 July 2016, D.W. Xiao (HMAS 246696 holotype, ex-type living culture CGMCC3.18327 = LC7114).
Hyphae smooth, hyaline to pale brown, branched, septate, 2.5–7 μm diam. Conidiophores reduced to conidiogenous cells. Conidiogenous cells aggregated in clusters on hyphae, pale brown, globose to subglobose to ampulliform, 5.5–12.5 × 3–9.5 μm (av. = 7.85 ± 1.41 × 6.27 ± 1.31). Conidia solitary, globose or subglobose, black, shiny, smooth, aseptate, 13.5–17.5 × 10–17 μm (av. = 15.99 ± 0.94 × 14.23 ± 1.84).
Culture characteristics — On PDA, colonies floccose, edge entire, initially white, becoming grey to black with age, reaching 9 cm diam after 7 d at 25 °C, reverse smoke-grey with black patches. On SNA, colonies flat, with some mycelia immersed, surface olivaceous grey and reverse olivaceous grey with black patches due to sporulation.
Additional specimens examined. China, Jiangxi Province, on bamboo leaves, 19 July 2016, J.E. Huang, living culture, LC7244 = WM478; ibid., living culture LC7245 = WM479.
Notes — Three strains representing N. bambusae clustered in a well-supported clade and related to N. rubi (99 % identity in ITS; 93 % in TEF1-α; 94 % in TUB2). Nigrospora bambusae differs from N. rubi (Fig. 17) in producing slightly larger conidia (13.5–17.5 × 10–17 μm vs 11.5–16.5 μm). In addition, N. bambusae sporulates easier than N. rubi (5 d vs 1 mo on SNA). Nigrospora bambusae occurs on bamboo (Poaceae) while N. rubi occurs on Rubus sp. (Rosaceae).
Fig. 17.
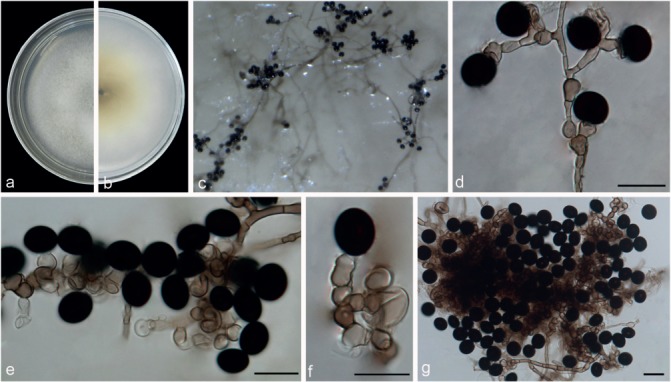
Nigrospora rubi (from ex-type strain LC2698). a–b. Upper surface and reverse overview of culture 5 d after inoculation on PDA medium; c. colony on SNA; d–f. conidiogenous cells giving rise to conidia; g. conidia. — Scale bars: d–f = 10 μm; g = 20 μm.
Nigrospora camelliae-sinensis Mei Wang & L. Cai, sp. nov. — MycoBank MB820731; Fig. 6
Fig. 6.

Nigrospora camelliae-sinensis (from ex-type strain CGMCC3.18125). a–b. Upper surface and reverse overview of culture 4 d after inoculation on PDA medium; c. colony on SNA; d–g. conidiophores and conidiogenous cells giving rise to conidia; h. conidia. — Scale bars = 10 μm.
Etymology. Named after the epithet of Camellia sinensis, the host from which most strains were collected in this study.
Type. China, Guangxi Province, on Camellia sinensis, Sept. 2013, T.W. Hou (HMAS 247068 holotype, ex-type living culture CGMCC3.18125 = LC3500).
Hyphae smooth, hyaline, branched, septate, 1.5–4 μm diam. Conidiophores mostly reduced to conidiogenous cells and aggregated in clusters on hyphae. Conidiogenous cells hyaline to pale brown, globose to ampulliform or clavate (ear-shaped), sometimes appearing as the bulge directly from the mycelia without septa, 6–11 × 4.5–8.5 μm (av. = 7.85 ± 1.43 × 5.95 ± 0.78). Conidia solitary, spherical or slightly ellipsoidal, black, shiny, smooth, aseptate, spherical, 13–18 μm diam (av. = 15.57 ± 1.19), ellipsoidal, 12–18 × 9–14.5 μm (av. = 14.24 ± 1.43 × 10.84 ± 1.21).
Culture characteristics — On PDA, colonies flat, edge entire. Colonies initially white, becoming grey due to sporulation, reaching 9 cm diam in 8 d at 25 °C. On SNA, colonies flat, growing slowly, spreading, mycelia partially immersed, surface white to greyish and reverse grey olivaceous without patches, sporulating profusely.
Additional specimens examined. China, Guangxi Province, Guilin, on Camellia sinensis, Sept. 2013, T.W. Hou, living culture LC3496; Hainan Province, on the leaf of Musa paradisiaca, 21 Sept. 2015, F.J. Liu, living culture LC6984 = WM218; ibid., LC6989 = WM223; ibid., LC6992 = WM226; ibid., LC7018 = WM252; ibid., LC7044 = WM278; Jiangxi Province, on Camellia sinensis, 24 Apr. 2013, F. Liu, living culture LC3287; on Castanopsis sp., 6 Sept. 2013, N. Zhou, living culture LC2710; ibid., LC4460; Yunnan Province, on Camellia sinensis, 21 April 2015, F. Liu, living culture LC6304 = LF1311.
Notes — Five strains representing N. camelliae-sinensis clustered in a well-supported clade (Fig. 2), sister to N. pyriformis (99 % identity in ITS; 99 % identity in TUB2; 96 % identity in TEF1-α). Morphologically, N. camelliae-sinensis differs from N. pyriformis (Fig. 16) in its smaller conidiogenous cells (6–11 × 4.5–8.5 μm vs 7.5–26 × 3.5–8.5 μm) and conidial shape. Nigrospora camelliae-sinensis is comparable to N. lacticolonia (Fig. 11) in conidial size, but its conidiogenous cells are scattered rather than aggregated as in N. lacticolonia.
Fig. 16.
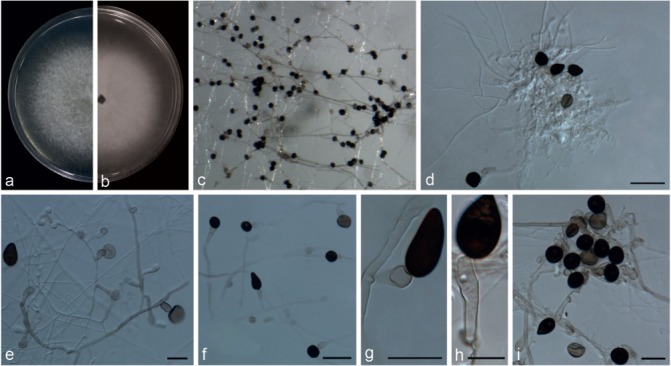
Nigrospora pyriformis (from ex-type strain CGMCC3.18122). a–b. Upper surface and reverse overview of culture 5 d after inoculation on PDA medium; c. colony on SNA; d–f. conidiophores and conidiogenous cells giving rise to conidia; g–i. conidia. — Scale bars: d–f, i = 20 μm; g–h = 10 μm.
Fig. 11.
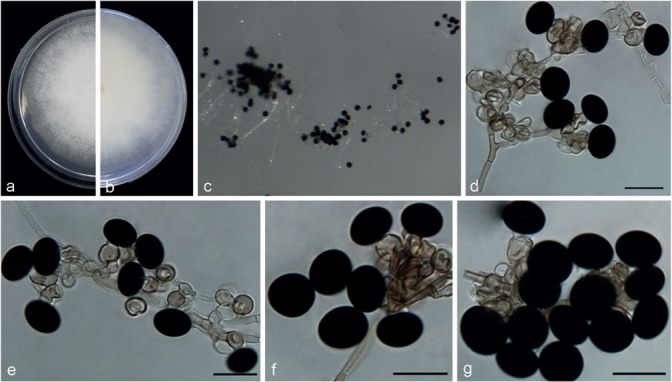
Nigrospora lacticolonia (from ex-type strain CGMCC3.18123). a–b. Upper surface and reverse overview of culture 6 d after inoculation on PDA medium; c. colony on SNA; d–f. conidiogenous cells giving rise to conidia; g. conidia. — Scale bars = 10 μm.
Nigrospora chinensis Mei Wang & L. Cai, sp. nov. — MycoBank MB820732; Fig. 7
Fig. 7.
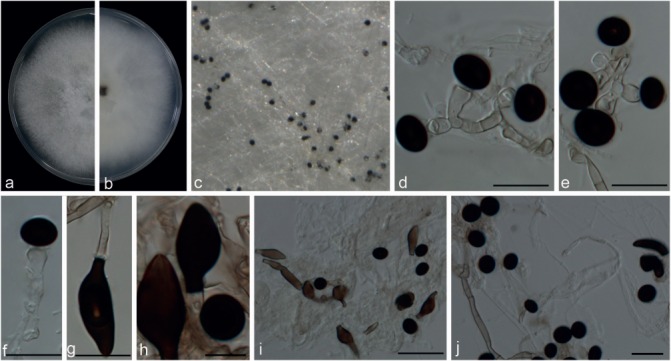
Nigrospora chinensis (from ex-type strain CGMCC3.18127). a–b. Upper surface and reverse overview of culture 5 d after inoculation on PDA medium culture; c. colony on SNA; d–f. conidiogenous cells giving rise to conidia; g–h. sterile cells; i–j. conidia. — Scale bars: d–h = 10 μm; i–j = 20 μm.
Etymology. Named after the country where this species was first collected, China.
Type. China, Jiangxi Province, on Machilus breviflora, 5 Sept. 2013, Y.H. Gao (HMAS 247069 holotype, ex-type living culture CGMCC3.18127 = LC4575).
Hyphae hyaline, smooth, branched, septate, 2–5 μm diam. Conidiophores reduced to conidiogenous cells. Conidiogenous cells monoblastic, discrete, solitary, determinate, ampulliform, or subspherical, hyaline, 5–9.5 × 4–7 μm (av. = 7.59 ± 1.29 × 5.7 ± 0.72). Sterile cells terminal on hyphae, pale to dark brown, elongated ellipsoidal to clavate, 23–40.5 × 5.5–12.5 μm, or somewhat curved or irregularly angled or lobed. Conidia solitary, globose or subglobose, black, shiny, smooth, aseptate, 10–14 μm diam (av. = 12.19 ± 1.07); ellipsoidal, 10–14.5 × 7.5–11 μm (av. = 11.78 ± 0.75 × 9.18 ± 0.61).
Culture characteristics — On PDA, colonies floccose, undulate. Colonies growing quickly, initially white, becoming black with age, reaching 9 cm diam in 6 d at 25 °C. On SNA, with sparse aerial mycelia, surface dirty white to greyish, and reverse iron-grey with dark patches but sporulating poorly.
Additional specimens examined. China, Guangxi Province, on Camellia sinensis, 7 Sept. 2013, Y. Zhang, living culture LC3441; ibid., living culture LC3493; Hainan Province, on Musa paradisiaca, 25 Dec. 2015, F.J. Liu, living culture LC 6972 = WM206; Jiangxi Province, on Lindera aggregate, 6 Sept. 2013, N. Zhou, living culture LC2696; on Camellia sinensis, 24 Apr. 2013, F. Liu, living culture LC3085; ibid., living culture LC3175; ibid., living culture LC3275; ibid., living culture LC3286; ibid., living culture LC3293; ibid., living culture LC3400; on Aucuba japonica, 5 Sept. 2013, Y.H. Gao, living culture LC4364; on Castanopsis sp., 5 Sept. 2013, Y.H. Gao, living culture LC4433; on Itea sp., 5 Sept. 2013, Y.H. Gao, living culture LC4565; on Machilus duthiei, 5 Sept. 2013, Y.H. Gao, living culture LC4593; on Osmanthus sp., 5 Sept. 2013, Y.H. Gao, living culture LC4619; on Quercus sp., 5 Sept. 2013, Y.H. Gao, living culture LC4660; on Smilax ocreata, 5 Sept. 2013, Y.H. Gao, living culture LC4673; Yunnan Province, on Camellia sinensis, 19 Apr. 2015, F. Liu, living culture LC6631 = LF1276; Tibet, 14 June 2015, Q. Chen, living culture LC6851 = WM085.
Notes — Strains of N. chinensis constitutes a distinct clade on concatenated gene trees with a high support value and basal to all other Nigrospora species (Fig. 2). Morphologically it is similar to N. gallarum, reported from dead larvae of Lipara lucens from France (Mason 1927). However, N. chinensis differs from N. gallarum in producing longer sterile cells (23–40.5 μm vs max. 18 μm).
Nigrospora gorlenkoana Novobr., Novosti Sist. Nizsh. Rast. 9: 180. 1972 — Fig. 8
Fig. 8.
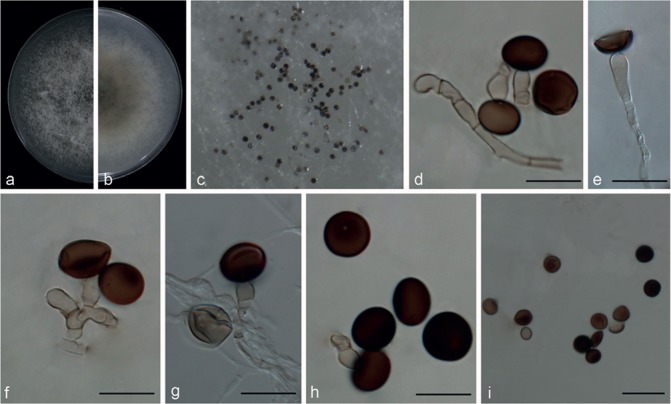
Nigrospora gorlenkoana (from ex-isotype strain CBS 480.73). a–b. Upper surface and reverse overview of culture 6 d after inoculation on PDA medium; c. colony on SNA; d–g. conidiogenous cells giving rise to conidia; h–i. conidia. — Scale bars: d–h = 10 μm; i = 20 μm.
Type. Kazakhstan, Alma-Ata region, from Vitis vinifera, leaf and fruit, 1972, T.I. Novobranova (isotype CBS H-7430, ex-isotype living culture CBS 480.73 = ATCC 24718 = IMI 174726 = VKMF-1761).
Hyphae smooth, hyaline, branched, septate, 1.5–4.5 μm diam. Conidiophores micronematous or semi-macronematous, flexuous or straight, pale brown, smooth, 2–6 μm thick. Conidiogenous cells pale brown, monoblastic, discrete, solitary, determinate, doliiform to ampulliform, 7–13.5 × 4–9 μm (av. = 10.09 ± 1.94 × 5.98 ± 1.11). Conidia sparse, solitary, globose or subglobose, pale brown to black, discrete on aerial mycelia, 11.5–17 μm diam (av. = 14.79 ± 1.21), shiny, smooth, with equatorial slit.
Culture characteristics — On PDA, colonies flat, woolly, spreading, initially white, becoming greyish with age. Colonies reaching 9 cm in 6 d at 25 °C. On SNA, colonies flat, with sparse mycelia and growing poorly, reverse with no patches, sporulating poorly.
Notes — Nigrospora gorlenkoana is currently listed as a synonym of N. oryzae in MycoBank. However, we could not find any literature in support of this synonymy. Our multi-locus molecular phylogeny herein also depicts that these two species cannot be considered as conspecific. These two species are also morphologically distinct. The conidiophores of N. gorlenkoana are discrete, solitary, rather than aggregated in clusters as in N. oryzae, and the conidial colour of N. gorlenkoana is paler brown than that of N. oryzae. Equatorial slits are present in some conidia of N. gorlenkoana, but absent in N. oryzae. However, the affinities of the ex-type of N. gorlenkoana are still unresolved as it is an independent taxon and its relationships to N. hainanensis as well as N. musae lack support (Fig. 2).
Nigrospora guilinensis Mei Wang & L. Cai, sp. nov. — MycoBank MB820733; Fig. 9
Fig. 9.
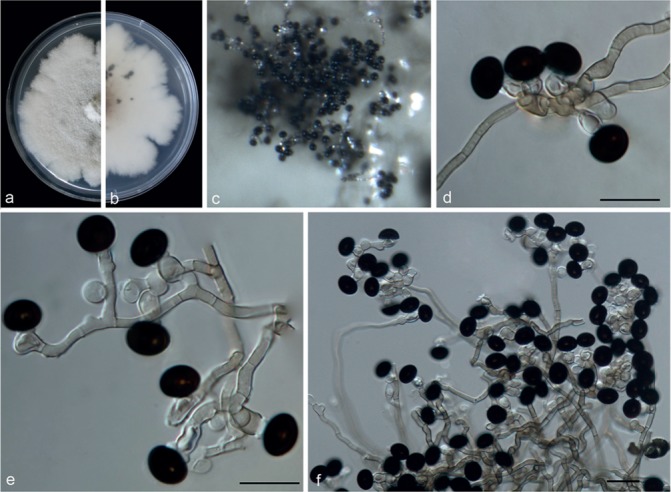
Nigrospora guilinensis (from ex-type strain CGMCC3.18124). a–b. Upper surface and reverse overview of culture 9 d after inoculation on PDA medium; c. colony on SNA; d–e. conidiogenous cells giving rise to conidia; f. conidia. — Scale bars: d–e = 10 μm; f = 20 μm.
Etymology. Referring to the location where the holotype was collected, Guilin.
Type. China, Guangxi Province, on Camellia sinensis, 7 Sept. 2013, T.W. Hou (HMAS 247072 holotype, ex-type living culture CGMCC3.18124 = LC3481).
Hyphae smooth, hyaline to pale brown, branched, septate, 1.5–4 μm diam. Conidiophores usually reduced to conidiogenous cells, aggregated in clusters on hyphae. Conidiogenous cells monoblastic, determinate, hyaline, smooth, doliiform to clavate to ampulliform, in clusters on aerial mycelia, 6–11 × 4–7.5 μm (av. = 8.73 ± 1.33 × 6.01 ± 0.64). Conidia solitary, black, shiny, smooth, aseptate, spherical, 11.5–15 μm diam (av. = 12.91 ± 0.7), ellipsoidal, 10.5–14 × 8–12 μm (av. = 12.46 ± 0.62 × 9.69 ± 0.71).
Culture characteristics — On PDA, colonies woolly, cottony, margin irregular. Colonies growing slowly, dirty white to greyish and producing red pigment after 3 wk. Colonies reaching 9 cm after 14 d at 25 °C. Reverse dirty white to light pink with black patches due to pigment. On SNA, colonies flat, growing slowly, mycelia partially immersed in the medium, surface dirty white to grey and reverse pale brown with black patches.
Additional specimen examined. China, Jiangxi Province, on the stem of Nelumbo sp., 21 Sept. 2015, M.F. Hu, culture LC7301 = JAUCC0673.
Notes — Two strains representing N. guilinensis clustered in a well-supported clade (Fig. 2), which appeared closely related to N. vesicularis (98 % identity in ITS; 84 % in TEF1-α; 92 % in TUB2), N. aurantiaca (98 % in ITS; 83 % in TEF1-α; 91 % in TUB2), N. osmanthi (98 % in ITS; 86 % in TEF1-α; 91% in TUB2) and N. lacticolonia (98 % in ITS; 84 % in TEF1-α; 91 % in TUB2). Nigrospora guilinensis is morphologically distinct from these four species. It differs from N. vesicularis (Fig. 20) in the absence of a vesicle, from N. aurantiaca (Fig. 4) in producing different pigment in culture (red pigment in N. guilinensis vs orange pigment in N. aurantiaca ), from N. osmanthi (Fig. 15) in the arrangement of conidiogenous cells (aggregated in clusters in N. guilinensis vs scattered in N. osmanthi) and from N. lacticolonia (Fig. 11) in the smaller ellipsoidal conidia (10.5–14 × 8–12 μm vs 13.5–17.5 × 10.5–13.5 μm).
Fig. 20.
Nigrospora vesicularis (from ex-type strain CGMCC3.18128). a–b. Upper surface and reverse overview of culture 5 d after inoculation on PDA medium; c. colony on SNA; d–h. conidiogenous cells giving rise to conidia; i. conidia. — Scale bars = 10 μm.
Fig. 15.
Nigrospora osmanthi (from ex-type strain CGMCC3.18126). a–b. Upper surface and reverse overview of culture 8 d after inoculation on PDA medium; c. colony on SNA; d–g. conidiogenous cells giving rise to conidia; h. conidia. — Scale bars = 10 μm.
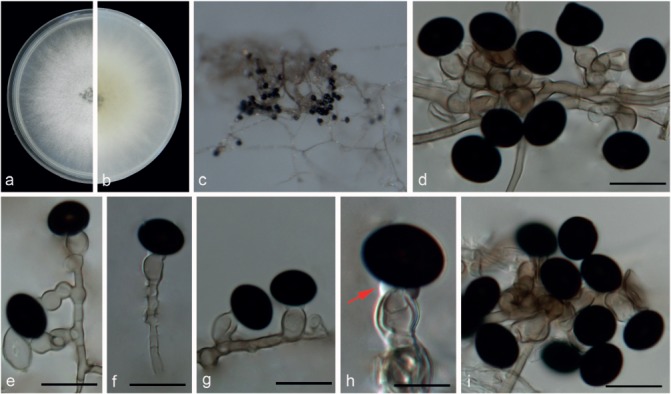
Nigrospora hainanensis Mei Wang & L. Cai, sp. nov. — MycoBank MB820734; Fig. 10
Fig. 10.
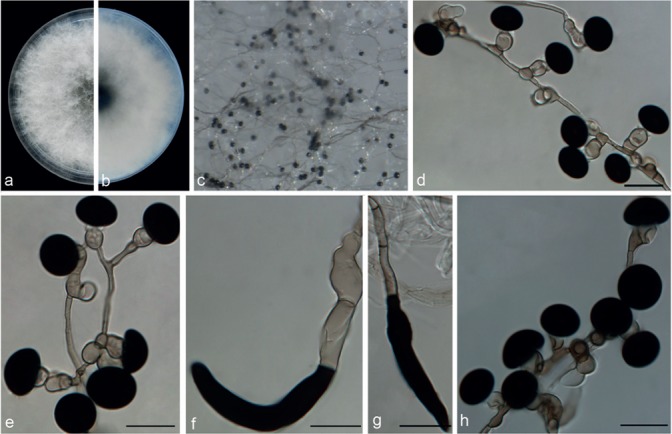
Nigrospora hainanensis (from ex-type strain CGMCC3.18129). a–b. Upper surface and reverse overview of culture 5 d after inoculation on PDA medium; c. colony on SNA; d–e. conidiogenous cells giving rise to conidia; f–g. setae; h. conidia. — Scale bars = 10 μm.
Etymology. Named after the province in China where the type was collected, Hainan.
Type. China, Hainan Province, on a leaf of Musa paradisiaca, 21 Sept. 2015, F.J. Liu (HMAS 247064 holotype, ex-type living culture CGMCC3.18129 = LC7030).
Hyphae smooth, hyaline to pale brown, branched, septate, 2–6 μm diam. Conidiophores usually reduced to conidiogenous cells, which are dispersed on hyphae. Conidiogenous cells monoblastic, discrete, solitary, determinate, hyaline, smooth, globose or ampulliform, 6.5–12.5 × 4.5–9.5 μm (av. = 8.89 ± 1.28 × 6.85 ± 0.94). Conidia sphaerical or ellipsoidal, solitary, black, shiny, smooth, aseptate, spherical, 12.5–17.5 μm diam (av. = 15.39 ± 1.04), ellipsoidal 13.5–19 × 9–16.5 μm (av. = 15.98 ± 0.98 × 12.11 ± 1.25). Setae straight to irregularly curved, black, smooth, subcylindrical, tapering in apical cell to subobtuse or obtuse apex, base truncate, up to 60 μm long, 5–12 μm diam.
Culture characteristics — On PDA, colonies floccose, margin circular, growing rapidly, initially white, becoming black with age, reaching 9 cm diam in 5 d at 25 °C. On SNA, colonies spreading, not flat, mycelia partially immersed in the medium, cottony, surface grey to black and reverse with dark grey patches at the edge and black in the middle due to abundant sporulation.
Additional specimens examined. China, Hainan Province, on the leaf of Musa paradisiaca, 21 Sept. 2015, F.J. Liu, living culture, LC6979 = WM213; ibid., living culture LC7031 = WM265; ibid., living culture LC7042 = WM276.
Notes — All strains of N. hainanensis were isolated from Musa paradisiaca from Hainan, China, and they clustered in a well-supported clade (Fig. 2), appearing closely related to N. gorlenkoana (99 % identity in ITS; 84 % in TEF1-α; 95 % in TUB2). These two species could be morphologically differentiated from each other based on conidial colour, which is darker in N. hainanensis. Morphologically, N. hainanensis also resembles N. guilinensis (Fig. 9), but differs in the arrangement of conidiogenous cells (dispersed on aerial mycelia, discrete, solitary, unbranched in N. hainanensis vs clustered on aerial mycelia, or forming black sporodochial conidiomata in N. guilinensis) and setae (present in N. hainanensis vs absent in N. guilinensis). Another two Nigrospora species, i.e., N. musae and N. canescens have also been reported from Musa spp. Nigrospora hainanensis differs in producing smaller conidia (spherical, 12.5–17.5 μm diam in N. hainanensis vs globose or subglobose, 15–19.5 μm in N. musae, and subglobose, 19 × 17 μm in N. canescens) and the presence of setae.
Nigrospora lacticolonia Mei Wang & L. Cai, sp. nov. — MycoBank MB820735; Fig. 11
Etymology. Named after the creamy white colony colour on PDA and SNA.
Type. China, Jiangxi Province, on Camellia sinensis, 24 Apr. 2013, F. Liu (HMAS 247070 holotype, ex-type living culture CGMCC 3.18123 = LC3324).
Hyphae smooth, hyaline, branched, septate, 1.5–4 μm diam. Conidiophores reduced to conidiogenous cells. Conidiogenous cells aggregated in clusters on hyphae, pale brown, finely verruculose, globose to clavate to doliiform, 6.5–11.5 × 5.5–9 μm (av. = 8.29 ± 1.11 × 6.82 ± 0.73). Conidia solitary, spherical or slightly ellipsoidal, black, shiny, smooth, aseptate, spherical 11.5–16.5 μm diam (av. = 14.36 ± 1.04), ellipsoidal 13.5–17.5 × 10.5–13.5 μm (av. = 15.21 ± 0.75 × 11.72 ± 0.66).
Culture characteristics — On PDA, colonies floccose, entire edge, surface and reverse creamy white, with dark brown patches in the reverse, reaching 9 cm diam in 6 d at 25 °C. On SNA, colonies flat, surface and reverse remains white and black patches in the reverse, with moderate aerial mycelia, growing very quickly, but sporulating after 2 wk.
Additional specimen examined. China, Hainan Province, on Musa paradisiaca, 25 Dec. 2015, F.J. Liu, living culture LC7009 = WM243.
Notes — Two strains representing N. lacticolonia clustered in a well-supported clade which is closely related to N. osmanthi (100 % identity in ITS; 91 % in TEF1-α; 98 % in TUB2), but they could be distinguished from one another based on the morphology of their conidiogenous cells (Fig. 11, 17).
Nigrospora musae McLennan & Hoëtte, Aust. Inst. Sci. Industr. Res. Bull. 75: 15. 1933 — Fig. 12
Fig. 12.
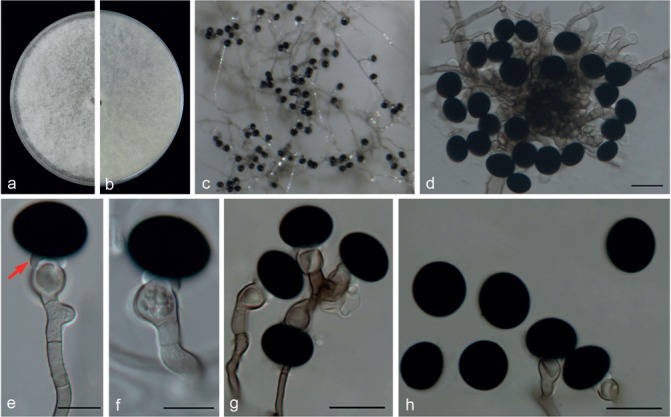
Nigrospora musae (from ex-type strain CBS 319.34). a–b. Upper surface and reverse overview of culture 7 d after inoculation on PDA medium; c. colony on SNA; d. conidiophores; e–g. conidiogenous cells giving rise to conidia; h. conidia. — Scale bars: d = 20 μm; e–h = 10 μm.
Type. Australia, from the fruit of Musa sapientum, 1933, E. McLennan (ex-type culture CBS 319.34 = MUCL 8368).
Hyphae pale brown, smooth, branched, septate, 2–6 μm diam. Conidiophores aggregated in black sporodochia, micronematous or semi-macronematous, flexuous or straight, pale brown, smooth, much branched, 3.5–8 μm diam, some conidiophores reduced to conidiogenous cells. Conidiogenous cells aggregated, pale brown, monoblastic, subglobose to ampulliform, 6.5–14 × 6–9 μm (av. = 9.16 ± 1.49 × 7.45 ± 0.74); hyaline vesicles (Fig. 12, arrowed) delimiting the conidia from conidiogenous cells. Conidia sparse, solitary, globose or subglobose, black, shiny, smooth, 15–19.5 (mostly 16–18 μm) (av. = 17.01 ± 0.84).
Culture characteristics — On PDA, colonies woolly, margin circular. Colonies initially white, becoming dark grey with age, most mycelia immersed, and the reverse were olive-citrine, reaching 9 cm diam in 7 d at 25 °C. On SNA, colonies flat, the aerial mycelia growing sparsely, most mycelia immersed, reverse olivaceous grey with black patches, with abundantly sporulation.
Additional specimen examined. China, Guizhou Province, on Camellia sinensis, 21 July 2014, Z.F. Zhang, living culture, LC6385 = LF1013.
Notes — Nigrospora musae was originally described from fruit of Musa sapientum in Australia (McLennan & Hoëtte 1933), but the ex-type strain (CBS 319.34) appeared to be sterile. We therefore re-described it based on a freshly collected strain LC6385 (similarity: 99 % identity in ITS; 99 % in TEF1-α; 99 % in TUB2) from Camellia sinensis. The description of N. musae was emended in this study, adding the presence of hyaline vesicles. Nigrospora canescens, originally reported from leaves of Musa sapientum (McLennan & Hoëtte 1933), was never isolated from the fruits and was endophytic in banana. Moreover, N. canescens sporulates more quickly and abundantly than N. musae in culture.
Nigrospora oryzae (Berk. & Broome) Petch, J. Indian Bot. Soc. 4: 24. 1924 — Fig. 13, 14
Fig. 13.
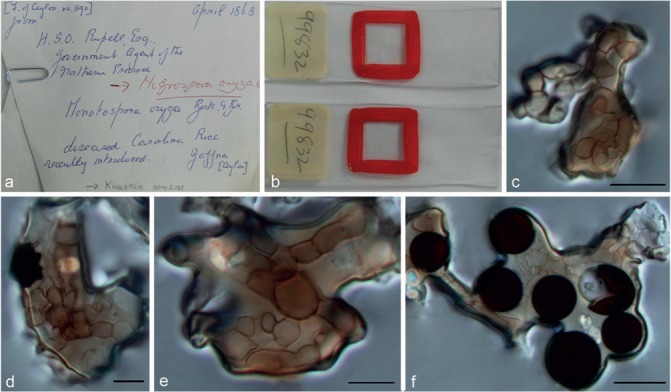
Nigrospora oryzae (from slide of holotype K(M) 99832). a–b. Overview of the type specimen; c–e. conidiophores; f. conidia. — Scale bars = 10 μm.
Fig. 14.
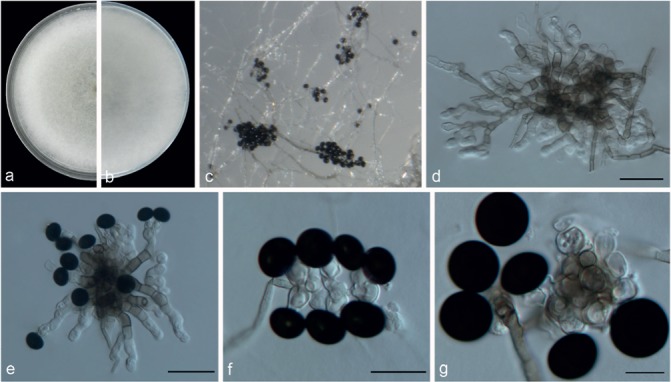
Nigrospora oryzae (LC7293). a–b. Upper surface and reverse overview of culture 7 d after inoculation on PDA medium; c. colony on SNA; d. conidiophores; e–f. conidiogenous cells giving rise to conidia; g. conidia. — Scale bars: d–e = 20 μm; f–g = 10 μm.
Basionym. Monotospora oryzae Berk. & Broome, J. Linn. Soc., Bot. 14: 99. 1873.
Synonym. Khuskia oryzae H.J. Huds., Trans. Brit. Mycol. Soc. 46: 358. 1963.
Type. Sri Lanka, Jaffra, H.S.O. Russell, Esq. Government Agent of the Northern Provinces, from rice leaves, 1873, Berk. & Broome (IMI 99832, slide of holotype).
Hyphae branched, septate, smooth, hyaline, 2–6 μm diam, becoming brown closer to the conidiogenous region. Conidiophores aggregated in black sporodochia, micronematous or semi-macronematous, multiseptate, extensively branched, flexuous or straight, pale brown, smooth, 3–7 μm diam; sometimes reduced to conidiogenous cells. Conidiogenous cells aggregating in clusters on hyphae, monoblastic, determinate, ampulliform or subspherical, hyaline, 4–13 × 3–8.5 μm (av. = 8.26 ± 1.03 × 6.45 ± 0.76). Conidia formed abundantly, solitary, globose or subglobose, black, shiny, smooth, aseptate, 12.5–16 (mostly 12–14) μm diam (av. = 14.26 ± 0.79, n = 50). Perithecia formed in clusters of 1–7, uniseriate or in irregular rows, up to 2 mm long, subepidermal, erumpent, globose, obovoid, up to 250 μm diam, with papillate ostioles; perithecial clusters surrounded by a blackened area of host tissue. Asci 8-spored, biseriate, short-stalked, unitunicate, clavate, 55–75 × 8.5–12 μm. Paraphyses thin-walled, septate. Ascospores hyaline, granular, curved, inequilateral, 16–21 × 5–7 μm, tapering to the base with rounded ends, initially aseptate but on discharge from the ascus and on germination ascospores may develop a single transverse septum.
Culture characteristics — On PDA, colonies woolly, floccose, margin circular, growing rapidly, white to grey to black with age, reaching 9 cm diam in 5 d at 25 °C. On SNA, colonies flat, with abundant aerial mycelia, surface and reverse dark brown to black without patches, sporulating quickly and abundantly.
Additional specimens examined. China, Fujiang Province, on Pittosporum illicioides, 8 Nov. 2012, Q. Chen, living culture LC4961; Hubei Province, on Citrus reticulate, 25 Sept. 2015, X. Zhou, living culture LC 6893 = WM127; on Oryza sativa, 25 Sept. 2015, X. Zhou, living culture LC 6923 = WM157; ibid., living culture LC 6955 = WM189; ibid., living culture LC 6957 = WM191; ibid., living culture LC 6759 = HBN3-18; ibid., living culture LC 6760 = HBN4-11; ibid., living culture LC 6763 = HBN4-23; ibid., living culture LC 6766 = JXN1-4; Jiangxi Province, on Nelumbo sp., 21 Sept. 2014, M.F. Hu, living culture, LC6029; ibid., living culture LC7299 = JAUCC0669; ibid., living culture LC7300 = JAUCC0672; ibid., living culture LC7305 = JAUCC0708; ibid., living culture LC7306 = JAUCC0709; ibid., living culture LC7308 = JAUCC0713; ibid., living culture LC7309 = JAUCC0757; ibid., living culture LC7310 = JAUCC0758; ibid., living culture LC7311 = JAUCC0767; 15 Sept. 2014, X.X. Zhan, living culture LC7293 = JAUCC0004; ibid., living culture LC7297 = JAUCC00027; on Rhododendron sp., 5 Sept. 2013, Y.H. Gao, living culture, LC4260; on Osmanthus sp., 5 Sept. 2013, Y.H. Gao, living culture, LC4679; ibid., living culture LC2689; on Cephalotaxus sinensis, 5 Sept. 2013, Y.H. Gao, living culture LC4273; on Rhododendron sp., 5 Sept. 2013, Y.H. Gao, living culture LC4275; on submerged wood, 21 Sept. 2014, M.F. Hu, living culture LC5964; ibid., living culture LC5982; ibid., living culture LC5999; on Neolitsea sp., 6 Sept. 2013, N. Zhou, living culture LC2693; on Rubus reflexus, 6 Sept. 2013, N. Zhou, living culture LC2695; on Hamamelis mollis, 3 Sept. 2013, N. Zhou, living culture LC2699; on Rubus sp., 2 Sept. 2013, N. Zhou, living culture LC2702; on Rhododendron sp., 2 Sept. 2013, N. Zhou, living culture LC2704; ibid., living culture LC2706; ibid., living culture LC2707; ibid., living culture LC2708; ibid., living culture LC2709; on Castanopsis sp., 6 Sept. 2013, N. Zhou, living culture LC2712; on Ternstroemia sp., 3 Sept. 2013, N. Zhou, living culture LC2749; on Osmanthus sp., 4 Sept. 2013, N. Zhou, living culture LC2752; on Symplocos zizyphoides, 2 Sept. 2013, N. Zhou, living culture LC3690; on Daphniphyllum macropodum, 5 Sept. 2013, Y.H. Gao, living culture LC4294; ibid., living culture LC4295; on Daphniphyllum oldhamii, 5 Sept. 2013, Y.H. Gao, living culture LC4320, on Camellia sp., living culture LC4327; ibid., living culture LC4345; ibid., living culture LC4680; Qinghai Province, on Pentactina rupicola, 2 Sept. 2013, Q. Chen, living culture LC5181; Sichuan Province, on Tutcheria microcarpa, 5 Oct. 2012, D.M. Hu, living culture LC2972, on Cleyera japonica, 5 Oct. 2012, F. Liu, living culture LC2991.
Notes — The type of N. oryzae is preserved in K as two slides, and only partial morphological characters could be observed (Fig. 13), e.g., branched conidiophores, black and globose/subglobose conidia, 12.5–16 μm diam. Although we could not obtain sequences from the type specimen or culture for comparisons, all strains from the original host (rice) isolated in this study clustered in one single clade, sister to N. zimmermanii (Fig. 2), and their morphological characteristics were in good accordance with N. oryzae. Therefore, we regard this clade as N. oryzae.
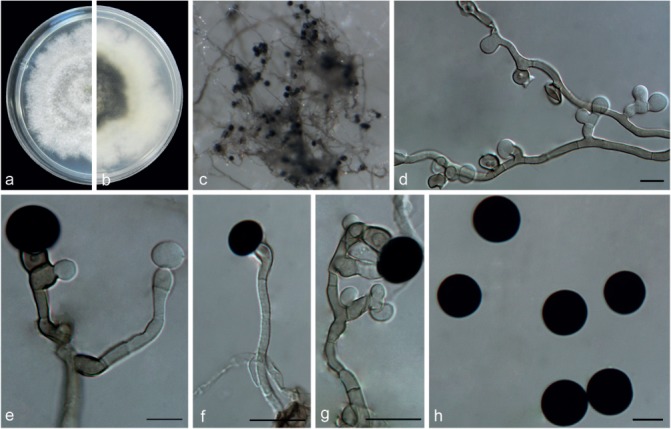
Nigrospora osmanthi Mei Wang & L. Cai, sp. nov. — MycoBank MB820736; Fig. 15
Etymology. Named after the host genus from which the holotype was collected, Osmanthus.
Type. China, Jiangxi Province, on Osmanthus sp., 5 Sept. 2013, Y.H. Gao (HMAS 247066 holotype, ex-type living culture CGMCC3.18126 = LC4350).
Hyphae branched, septate, hyaline to pale brown, 2.5–4.5 μm diam. Conidiophores mostly reduced to conidiogenous cells. Conidiogenous cells monoblastic, discrete, solitary, determinate, at first hyaline, subspherical, then turning to pale brown, ampulliform to cylindrical, 5.5–12 × 4–8.5 μm (av. = 8.02 ± 1.5 × 6.04 ± 1.16). Conidia solitary, globose or subglobose, black, shiny, smooth, sometimes formed directly from the mycelia, aseptate, 13.5–16.5 μm diam (av. = 14.87 ± 0.63).
Culture characteristics — On PDA, colonies flat, floccose, lobate. Colonies growing slowly, initially white, becoming black with age, reaching 9 cm diam in 10 d at 25 °C. On SNA, colonies flat, surface greyish to grey olivaceous with dark grey patches and reverse dark brown with black patches, mycelia sparse on the surface with delayed sporulation.
Additional specimen examined. China, Jiangxi Province, on Hedera nepalensis, 5 Sept. 2013, Y.H. Gao, living culture LC4487.
Notes — Two strains representing N. osmanthi clustered in a well-supported clade which is closely related to N. lacticolonia (Fig. 2), but they could be distinguished from one another based on the morphology of their conidiogenous cells (Fig. 11, 17). Morphologically, N. osmanthi also resembles N. oryzae in its conidial size, but differs in its conidiophores that are reduced to conidiogenous cells in N. osmanthi, but branched and clustered in N. oryzae. Nigrospora osmanthi differs from another morphologically similar species, N. gallarum, by the absence of sterile cells.
Nigrospora pyriformis Mei Wang & L. Cai, sp. nov. — MycoBank MB820737; Fig. 16
Etymology. Named after the presence of pyriform conidia.
Type. China, Jiangxi Province, Citrus reticulata, 11 Mar. 2012, X.M. Tan (HMAS 247067 holotype, ex-type culture CGMCC3.18122 = LC2045).
Hyphae smooth, hyaline, branched, septate, 2–6 μm diam. Conidiophores reduced to conidiogenous cells. Conidiogenous cells monoblastic, discrete, solitary, determinate, ampulliform or subcylindrical, pale brown, 7.5–26 × 3.5–8.5 μm (av. = 13.38 ± 4.81 × 6.31 ± 1.46). Conidia initially pale brown, become black with age, dimorphic, globose to subglobose, black, shiny, smooth, aseptate, 12.5–16.5 μm diam (av. = 15.41 ± 0.77); or pyriform, black, shiny, smooth, aseptate, 17.5–27.5 × 10–18.5 μm (av. = 19.97 ± 4.95 × 11.77 ± 2.53).
Culture characteristics — On PDA, colonies woolly, floccose, margin circular. Colonies initially white, becoming black with age, reaching 9 cm diam in 7 d at 25 °C. On SNA, colonies flat, spreading, with moderate aerial mycelia, reverse black due to sporulation.
Additional specimens examined. China, Hainan Province, on leaves of Musa paradisiaca, 21 Sept. 2015, F.J. Liu, living culture LC6985 = WM219; ibid., LC6988 = WM222; Jiangxi Province, on Camellia sinensis, 24 Apr. 2013, F. Liu, living culture LC3099; ibid., LC3292; on Lindera aggregata, 6 Sept. 2013, N. Zhou, living culture LC2688; on Rubus reflexus, 6 Sept. 2013, N. Zhou, living culture LC2694; on Castanopsis sp., 5 Sept. 2013, Y.H. Gao, living culture LC4669; on Rosa sp., 2 Sept. 2013, N. Zhou, living culture LC2690.
Notes — Five strains representing N. pyriformis clustered in a well-supported clade (Fig. 2), and appeared as a sister clade to N. camelliae-sinensis (99 % identity in ITS; 96 % in TEF1-α; 99 % in TUB2). Morphologically, N. pyriformis is unique in Nigrospora by producing pyriform conidia.
Nigrospora rubi Mei Wang & L. Cai, sp. nov. — MycoBank MB820801; Fig. 17
Etymology. Named after the host genus on which the holotype was collected, Rubus.
Type. China, Jiangxi Province, on Rubus sp., 6 Sept. 2013, N. Zhou (HMAS 246699 holotype, ex-type living culture CGMCC3.18326 = LC2698).
Hyphae smooth, hyaline, branched, septate, 2–6 μm diam. Conidiophores reduced to conidiogenous cells. Conidiogenous cells aggregated in clusters on hyphae, pale brown, subglobose to ampulliform to lageniform, 6.5–14 × 5–9 μm (av. = 9.94 ± 1.71 × 7.16 ± 0.8). Conidia solitary, spherical or subglobose, black, shiny, smooth, aseptate, (11.5–)13–15(–16.5) μm diam (av. = 14.23 ± 0.97).
Culture characteristics — On PDA, colonies floccose, entire edge, initially white, becoming black with age, reaching 9 cm diam in 6 d at 25 °C, reverse smoke-grey in patches. On SNA, colonies flat, with moderate aerial mycelia, surface dirty white, growing very quickly, but with delayed sporulation. Surface and reverse pale luteous to sienna with greyish patches.
Notes — See notes of N. bambusae.
Nigrospora sphaerica (Sacc.) E.W. Mason, Trans. Brit. Mycol. Soc. 12: 158. 1927 — Fig. 18, 19
Fig. 18.
Nigrospora sphaerica (from slide of holotype K(M) 103253). a–b. Overview of the type specimen; c. conidia. — Scale bars = 10 μm.
Fig. 19.

Nigrospora sphaerica (from strain LC2840). a–b. Upper surface and reverse overview of culture 6 d after inoculation on PDA medium; c. colony on SNA; d–g. conidiogenous cells giving rise to conidia; h–i. conidia. — Scale bars: d, h–i = 20 μm; e–g = 10 μm.
Type. USA, Newfield, N.J., from Zea mays, 1822, P.A. Saccardo (slide of holotype, IMI 103253).
Basionym. Trichosporum sphaericum Sacc., Michelia 2 (no. 8): 579. 1882.
Hyphae smooth, hyaline, branched, septate, 3–8 μm diam. Conidiophores micronematous or semi-macronematous, multiseptate, extensively branched, flexuous or straight, hyaline to pale brown, smooth, 4–8 μm thick; hyaline vesicles (Fig. 19, arrowed) usually surrounding the septum to delimit the conidia and their conidiogenous cells. Conidiogenous cells pale brown, monoblastic, determinate, subspherical, 6–12 μm diam (av. = 7.97 ± 0.99). Conidia are formed abundantly, solitary, globose or subglobose, black, shiny, smooth, aseptate, 16–21 (mostly 16–19) μm diam (av. = 18.22 ± 1.0).
Culture characteristics — On PDA, colonies floccose, margin circular. Colonies initially white, becoming black with age, reaching 9 cm diam in 6 d at 25 °C. On SNA, colonies flat, spreading, with abundant aerial mycelia, surface greyish and reverse olivaceous grey without patches, sporulating profusely.
Additional specimens examined. China, Hainan Province, on Musa paradisiacal, 24 Dec. 2015, F.J. Liu, living culture LC7026 = WM260; ibid., living culture LC6969 = WM 203; ibid., living culture LC6996 = WM 230; ibid., living culture LC 6998 = WM 232; Jiangxi Province, on Nelumbo sp., 25 Feb. 2014, X.X. Zhan, living culture LC7294 = JAUCC0005; ibid., living culture LC7295 = JAUCC0006; ibid., living culture LC7296 = JAUCC0007; ibid., living culture LC7312 = JAUCC0009; ibid., living culture LC7298 = JAUCC00030; on Nelumbo sp., 21 Sept. 2015, M.F. Hu, living culture JAUCC0705; ibid., living culture LC7304 = JAUCC0706; on submerged wood, 24 Aug. 2014, X.T. Wu, living culture LC5944, ibid., living culture LC5966; ibid., living culture LC5901; ibid., living culture LC5932; on Rosa sp., 2 Sept. 2013, N. Zhou, living culture LC2705; on Camellia sinensis, 7 Sept. 2013, Y. Zhang, living culture LC 3420; ibid., living culture LC3477; on Rhododendron sp., 5 Sept. 2014, Y.H. Gao, living culture LC4174; ibid., living culture LC4263; ibid., living culture LC4264; ibid., living culture LC4274; ibid., living culture LC4278; ibid., living culture LC4291; ibid., living culture LC4303; ibid., living culture LC4307; ibid., living culture LC4372; on Daphniphyllum macropodum, 5 Sept. 2013, Y.H. Gao, living culture LC4293; on Deutzia sp., 5 Sept. 2013, Y.H. Gao, living culture LC4241; unknown host, 5 Sept. 2013, Y.H. Gao, living culture LC4447; Sichuang Province, on Cleyera japonica, 5 Oct. 2012, F. Liu, living culture LC2958; on Camellia sp., 5 Oct. 2013, F. Liu, living culture LC2983; Yunnan Province, on Camellia sinensis, 16 Apr. 2015, F. Liu, living culture LC6294 = LF1301; on Harpullia longipetala, 15 Sept. 2011, F. Liu, living culture LC2839; ibid., living culture LC2840.
Notes — We examined the type specimen of N. sphaerica from K, and the conidia were revealed to be globose or subglobose, 16–19(–21) μm diam. The conidial size of all strains in the clade of N. sphaerica (Fig. 2) are consistent with that of the type specimen, and the vesicle structures presented in all of these strains. Although no sequence data were available from the type specimen for comparison, we concluded that these strains represented N. sphaerica. In addition, the ITS sequence of N. sphaerica isolate QY-6 (KP731976) causing leaf blight on Camellia sinensis, also clustered in this clade (Liu et al. 2015). However, this isolate was not added into the multi-locus phylogenetic analysis in this study as all loci could not be successfully amplified.
Nigrospora vesicularis Mei Wang & L. Cai, sp. nov. — MycoBank MB820738; Fig. 20
Etymology. Vesicularis, referring to the vesicle, a structure surrounding the septum, delimiting the conidium from its conidiogenous cell.
Type. China, Hainan province, on Musa paradisiaca, 25 Dec. 2015, F.J. Liu (HMAS 247071 holotype, ex-type living culture CGMCC 3.18128 = LC7010 = WM244).
Hyphae smooth, hyaline, branched, septate, 1.5–4 μm diam. Conidiophores reduced to conidiogenous cells. Conidiogenous cells monoblastic, discrete, solitary, determinate, scattered, hyaline to pale brown, smooth, doliiform to ampulliform, 7–12.5 × 6–9 μm (av. = 9.13 ± 1.12 × 7.04 ± 0.75). Hyaline vesicles (Fig. 20, arrowed) usually surrounding the septum to delimit the conidia from their conidiogenous cells. Conidia sparse, solitary, globose, subglobose, black, shiny, smooth, aseptate, 12.5–16.5 μm diam (av. = 14.8 ± 0.76, n = 50); or ellipsoidal, 12.5–16.5 × 9–15 μm (av. = 14.7 ± 0.7 × 11.63 ± 1.09).
Culture characteristics — On PDA, colonies floccose, entire edge. Colony surface white to greyish and pale luteous reverse, reaching 9 cm diam in 6 d at 25 °C. On SNA, colonies flat, surface dirty white and reverse dirty white to greyish without patches, with abundant aerial mycelia, but with delayed and sparse sporulation.
Additional specimen examined. Thailand, Chiang Rai, endophyte of unknown host plant, s.d., D.S. Manamgoda, living culture LC0322.
Notes — Two strains representing N. vesicularis clustered in a well-supported clade, and appeared closely related to N. aurantiaca (99 % identity in ITS; 89 % in TEF1-α; 96 % in TUB2), N. lacticolonia (99 % in ITS; 87 % in TEF1-α; 93 % in TUB2) and N. osmanthi (99 % in ITS; 88 % in TEF1-α; 93 % in TUB2). Nigrospora vesicularis differs from N. aurantiaca, N. lacticolonia and N. osmanthi by the presence of vesicles surrounding the septum between its conidiogenous cells and conidia. In addition, conidia of N. vesicularis (globose, 12.5–16.5 μm; ellipsoidal, 12.5–16.5 × 9–15 μm) are much smaller than those of other Nigrospora species which produce vesicles, e.g., N. panici (25–30 × 22–25 μm), N. sphaerica (16–21 μm diam) and N. musae (15–19.5 μm diam).
Nigrospora zimmermanii Crous, sp. nov. — MycoBank MB820739; Fig. 21
Fig. 21.

Nigrospora zimmermanii (from ex-type strain CBS 290.62). a. Sporulating colony on MEA medium; b–g. conidiogenous cells giving rise to conidia on SNA. — Scale bars = 10 μm.
Etymology. Named for Albrecht Wilhelm Phillip Zimmerman (1860–1931), who introduced the genus Nigrospora.
Type. Ecuador, Ingenio Valdez, on leaf of Saccharum officinarum, 1962, J.L. Bezerra (CBS H-23018 holotype, ex-type living culture CBS 290.62 = IMI 129007).
Hyphae hyaline, smooth, branched, septate, 2–3.5 μm diam. Conidiophores solitary or aggregated in sporodochia, subcylindrical, hyaline to pale brown, smooth, 0–2-septate, branched or not, with terminal conidiogenous cells, 10–50 × 3–7 μm. Conidiogenous cells monoblastic, discrete, determinate, smooth, hyaline to pale brown, ampulliform, 10–20 × 5–7 μm. Conidia solitary, spherical or ellipsoid, dark brown, granular, smooth, (11–)14–16(–18) × (14–)15–16(–18) μm (av. = 15 × 15.5).
Culture characteristics — Colonies on PDA floccose, margin circular, regular, reaching 9 cm diam in 7 d at 25 °C, surface and reverse olivaceous grey. On SNA, spreading, flat, with immersed mycelia and sparse aerial hyphae.
Additional specimens examined. Brazil, Salvador, Bahia, on leaf of Saccharum officinarum, Oct. 1969, C. Ram, CBS H-15168, living culture CBS 984.69 = DSM 3392. – Unknown location, C. van Overeem, living culture CBS 167.26.
Notes — Three strains representing N. zimmermanii clustered in a well-supported clade, and closely to N. oryzae (97 % identity in ITS; 82 % in TEF1-α; 89 % in TUB2). Nigrospora zimmermanii differs from N. oryzae by its larger conidiogenous cells (10–20 × 5–7 μm vs 4.0–13.0 × 3.0–8.5 μm) and different shaped, larger conidia ((11–)14–16(–18) × (14–)15–16(–18) μm vs 12–14(–16) μm diam).
SPECIES EXCLUDED FROM NIGROSPORA
Arthrinium vietnamensis (Hol.-Jech.), Mei Wang & L. Cai, comb. nov. — MycoBank MB820740; Fig. 22
Fig. 22.
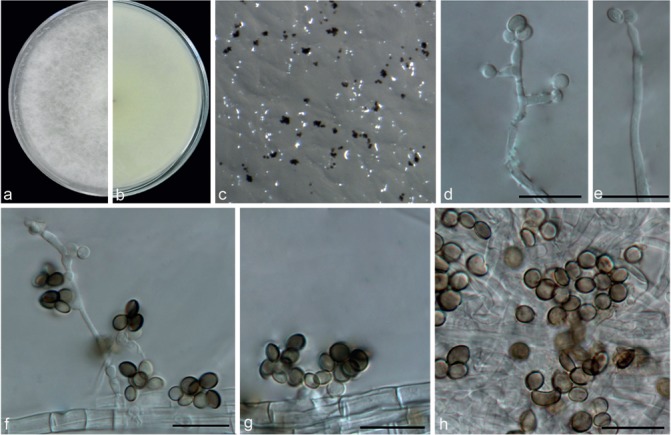
Arthrinium vietnamensis (from ex-type strain IMI 99670). a–b. Upper surface and reverse overview of culture 6 d after inoculation on PDA medium; c. colony on SNA; d–g. conidiogenous cells giving rise to conidia; h. globose conidia in surface view. — Scale bars = 10 μm.
Basionym. Nigrospora vietnamensis Hol.-Jech., Česká Mykol. 17: 19. 1963.
Synonym. Arthrinium malaysianum Crous in Crous &J.Z. Groenew., IMA Fungus 144: 2013.
Descriptions — See Jechová (1963) and Crous et al. (2013).
Specimen examined. Vietnam, on decayed fruit of Citrus sinensis, 1960, deposited in CABI in 1963, V. Jechova, ex-type living culture IMI 99670.
Notes — Arthrinium is morphologically similar to the genus Nigrospora in many aspects, such as the deeply pigmented conidia with a germ slit, presence of setae, abnormal conidia and violent spore discharge mechanism (Minter 1985, Webster 1952, 1966). The most peculiar difference between these two genera is that only one conidium is produced on each conidiogenous cell in Nigrospora, while the conidia of Arthrinium are usually produced in clusters, and two or more conidia are produced on each conidiogenous cell (Minter 1985, Crous et al. 2013).
The ex-type strain of N. vietnamensis (IMI 99670) produces aggregated, brown and globose conidia, about 5–6 μm diam in surface view, 3–4 μm diam in side view, and the conidia are much smaller and paler-coloured than that of other species of Nigrospora. In the LSU tree (Fig. 1), strain IMI 99670 is nested within the Arthrinium clade, and cluster together with A. malaysianum. Other analyses based on ITS phylogeny (results not shown) also demonstrated that the ex-type strain of N. vietnamensis and A. malaysianum are conspecific. Morphological data available herein also support that these two species should be conspecific. Therefore, a new combination Arthrinium vietnamensis is proposed because Nigrospora vietnamensis is the older name.
DISCUSSION
In this study Nigrospora was confirmed as belonging to the family Apiosporaceae (Xylariales, Sordariomycetes). Based on the newly accepted unitary nomenclature (Hawksworth et al. 2011), the sexual morph, Khusia, is accepted as synonym of Nigrospora. In previous studies, species of Nigrospora were primarily delimited via a comparison of morphological characters, especially conidial dimensions (Mason 1927, 1933). However, as we have shown here, conidial sizes frequently overlap among morphologically similar, but phylogenetically distinct species of Nigrospora. For instance, conidia of N. musae (15–)16–18(–19.5 μm) and N. sphaerica 16–19(–21) μm are similar, but the two species are phylogenetically distinct (Fig. 2). Overlapping morphologies are commonly observed among Nigrospora species, such as N. osmanthi and N. camelliaesinensis, as well as N. lacticolonia and N. vesicularis, resulting in ambiguity in the traditional taxonomic treatments of this genus. The phylogenetic investigations among Nigrospora species in this study significantly stabilise the taxonomy of the genus, as well as provide a classification framework for future species discovery. It also underlines the fact that in future studies species of Nigrospora would best be distinguished based on a combination of morphological and molecular data, rather than one without the other.
This study contributed to an increase in the number of known species in Nigrospora from 15 to 27, with the descriptions of five previously known species (i.e., N. arundinacea, N. gorlenkoana, N. musae, N. oryzae and N. sphaerica) emended with additional characters (conidiogenous cells, sterile cells and the presence of vesicles and setae) through careful examination of type specimens or fresh collections. New species were characterised employing morphological and molecular characters, as well as information of host associations and ecological distributions. Another two distinct clades (Fig. 2) representing two distinct phylogenetic species are not named and described because they remained sterile in culture in spite of all attempts to induce sporulation.
Type specimens of a few known species in Nigrospora have not been available for molecular study, which to some extent impeded the full resolution of species relationships. For example, the conidial dimensions of N. gossypii (12–13.6 μm diam) was inseparable from that of N. oryzae (12.5–16 μm diam). Jaczewski (1929), however, treated them as distinct species based on the fact that the latter had only been recorded on monocotyledons, and was not known from Russia and Central Asia. The type of the genus, N. panici, was reported from Panicum amphibium from Java (Zimmerman 1902) and its holotype has been lost. Unfortunately, to date we have been unable to find a suitable specimen to neotypify this species. Nevertheless, Nigrospora (= Khusia) has been shown to be a monophyletic genus in the Apiosporaceae.
Overall the data presented here revealed that, for the most part, species of Nigrospora do not display evidence of host or geographical limitation (Palmateer et al. 2003, Wu et al. 2014, Eken et al. 2016). Comparing the heatmap (Fig. 23) with the phylogeny (Fig. 1, 2), it is noteworthy that the top three most ubiquitous Nigrospora species (i.e., N. sphaerica, N. oryzae, N. chinensis), all belong to early divergent species in the genus (Fig. 2). On the other hand, species hitherto only known from a single host genus include N. arundinacea, N. bambusae, N. canescens, N. gorlenkoana, N. gossypii, N. hainanensis, N. javanica, N. padwickii and N. rubi (Fig. 23). Among these, N. bambusae, N. gorlenkoana, N. hainanensis and N. rubi have available DNA sequences and thus have been analysed for their phylogenetic relationships. Interestingly, these four species clustered in the upper part of the tree (Fig. 2), which unambiguously belong to the recently evolved taxa in the genus. This is a strong indication that the general evolutionary trend in Nigrospora is from species with a wide to a narrow host range. The latter generally refers to species that are considered to be plant pathogens, and that are more important to agriculture and forestry management.
Fig. 23.
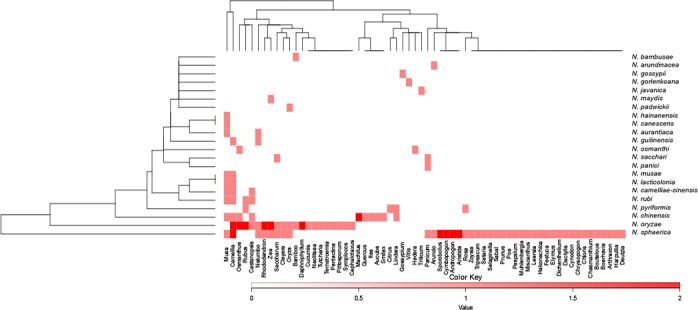
Heat-map showing the fungal distribution on host (genus level).
Acknowledgments
We thank Qian Chen, Yu Zhang, Yahui Gao, Nan Zhou, Jiarui Jiang, Xin Zhou, Xiaoling Zhang and Dianming Hu for their help in data analysis and providing strains. We kindly acknowledge the curator of the Kew herbarium for providing access to type specimens. This work was financially supported by the NSFC 31400017, and Key Research and Development Program of Guangxi Province (2016AB07288). Mei Wang acknowledges the CAS grant QYZDB-SSW-SMC044 for supporting her post graduate studentship. Mieke Starink and Ewald Groenewald are sincerely thanked for providing access to DNA sequence data of CBS strains.
REFERENCES
- Barnett HL, Hunter BB. 1998. Illustrated genera of imperfect fungi. APS Press, Minnesota. [Google Scholar]
- Berkeley MJ, Broome CE. 1873. Enumeration of the fungi of Ceylon. Part II. Botanical Journal of the Linnean Society 14: 29–141. [Google Scholar]
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Chen Z, Dong Z, Wen J, et al. 2016. A new sesquiterpene from the endophytic fungus Nigrospora sphaerica. Records of Natural Products 10: 307–310. [Google Scholar]
- Cooke MC. 1887. New British fungi. Grevillea 16: 7–11. [Google Scholar]
- Crous PW, Braun U, Hunter GC, et al. 2013. Phylogenetic lineages in Pseudocercospora. Studies in Mycology 75: 37–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, et al. 2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Crous PW, Groenewald JZ. 2013. A phylogenetic re-evaluation of Arthrinium. IMA Fungus 4: 133–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hoog GS, Guarro J, Gene J, et al. 2000. Atlas of clinical fungi: 708–711. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands: and Universitat Rovira i Virgili. Reus, Spain. [Google Scholar]
- Eken C, Spanbayev A, Tulegenova Z, et al. 2016. First report of Nigrospora oryzae on wheat in Kazakhstan. Plant Disease 100: 861. [Google Scholar]
- Fan YM, Huang WM, Li W, et al. 2009. Onychomycosis caused by Nigrospora sphaerica in an immunocompetent man. Archives of Dermatology 145: 611–612. [DOI] [PubMed] [Google Scholar]
- Farr DF, Rossman AY. 2017. Fungal databases, U.S. National Fungus Collections, ARS, USDA. https://nt.ars-grin.gov/fungaldatabases/. [Retreived 12 Feb. 2017.]
- Fukushima T, Tanaka M, Gohbara M, et al. 1998. Phytotoxicity of three lactones from Nigrospora sacchari. Phytochemistry 48: 625–630. [Google Scholar]
- Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo LD, Hyde KD, Liew ECY. 2000. Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New Phytologist 147: 617–630. [DOI] [PubMed] [Google Scholar]
- Hawksworth DL, Crous PW, Redhead SA, et al. 2011. The Amsterdam declaration on fungal nomenclature. IMA Fungus 2: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson HJ. 1963. The perfect state of Nigrospora oryzae. Transactions of the British Mycological Society 46: 355–360. [Google Scholar]
- Ibrahim D, Lee CC, Yenn TW, et al. 2015. Effect of the extract of endophytic fungus, Nigrospora sphaerica CL-OP 30, against the growth of methicillin-resistant Staphylococcus aureus (MRSA) and Klebsiella pneumonia cells. Tropical Journal of Pharmaceutical Research 14: 2091–2097. [Google Scholar]
- Jaczewski AA. 1929. Some diseases of cotton fibres. Review of Applied Mycology 9: 159–167. [Google Scholar]
- Jechová V. 1963. New species of the genus Nigrospora causing rots of southern fruits. Nigrospora maydis (Garov.) Jeehova and N. vietnamensis Jechova. Česká Mykologie 17: 12–20. [Google Scholar]
- Jones DR, Stover RH. 2000. Fungal diseases of banana fruit. In: Jones DR. (ed), Diseases of banana, abacá and enset: 173–211. CABI publishing, Wallingford, UK. [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AAH, Karuppayil SM. 2012. Fungal pollution of indoor environments and its management. Saudi Journal of Biological Sciences 19: 405–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindo AJ, Subramanian A, Suresh K. 2014. Nigrospora sphaerica causing corneal ulcer in an immunocompetent woman: a case report. International Journal of Case Reports and Images (IJCRI) 5: 675–679. [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, et al. 2008. Dictionary of the Fungi 10th edn. CABI Bioscience, UK. [Google Scholar]
- Lee S, Groenewald JZ, Crous PW. 2004. Phylogenetic reassessment of the coelomycete genus Harknessia and its teleomorph Wuestneia (Diaporthales), and the introduction of Apoharknessia gen. nov. Studies in Mycology 50: 235–252. [Google Scholar]
- Liu YJ, Tang Q, Fang L. 2015. First report of Nigrospora sphaerica causing leaf blight on Camellia sinensis in China. Plant Disease 100: 221. [Google Scholar]
- Mason EW. 1927. On species of the genus Nigrospora Zimmermann recorded on monocotyledons. Transactions of the British Mycological Society 12: 152–165. [Google Scholar]
- Mason EW. 1933. Annotated account of fungi received at the Imperial Mycological Institute (Fascicle 3, Special part.): 102–114.
- Mason-Gamer RJ, Kellogg EA. 1996. Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Gramineae). Systematic Biology 45: 524–545. [Google Scholar]
- McLennan EI, Hoëtte S. 1933. Nigrospora musae n. sp. and its connexion with “squirter” disease in bananas. Council for Scientific and Industrial Research 75: 1–36. [Google Scholar]
- Meepagala KM, Becnel JJ, Estep AS. 2015. Phomalactone as the active constituent against mosquitoes from Nigrospora spherica. Agricultural Sciences 6: 1195. [Google Scholar]
- Minter DW. 1985. A re-appraisal of the relationships between Arthrinium and other hyphomycetes. Proceedings: Plant Sciences 94: 281–308. [Google Scholar]
- Nirenberg HI. 1976. Untersuchungen uber die morphologische und biologische differenzierung in der Fusarium-sektion Liseola. Mitteilungen der Biologischen Bundesanstalt für Land- und Forstwirtschaft 169: 1–117. [Google Scholar]
- Novobranova TI. 1972. Species novae fungorum imperfectorum e regione Alma-Ataensi. Novosti Sistematiki Nizshikh Rastenii 9: 180. [Google Scholar]
- O’Donnell K, Kistler HC, Cigelnik E, et al. 1998. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences 95: 2044–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm BT. 1918. Eenige ziekten, waargenomen aan de tarwe op Java. Drukkerij Ruygrok & Company. [Google Scholar]
- Palmateer AJ, McLean KS, Van Santen E, et al. 2003. Occurrence of Nigrospora lint rot caused by Nigrospora oryzae on cotton in Alabama. Plant Disease 87: 873. [DOI] [PubMed] [Google Scholar]
- Posada D. 2008. jModelTest: phylogenetic model averaging. Molecular Biology and Evolution 25: 1253–1256. [DOI] [PubMed] [Google Scholar]
- R Development Core Team 2015. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: Available at http://www.R-project.org/. [Google Scholar]
- Rayner RW. 1970. A mycological colour chart. Commonwealth Mycological Institute, Kew, Surrey. [Google Scholar]
- Ronquist F, Teslenko M, Van der Mark P, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha M, Bhattacharya K. 2015. Aeromycoflora over rice field and their allergenic effect on farmers of N24 Parganas, West Bengal, India. European Respiratory Journal 46: 4101. [Google Scholar]
- Santo-Pietro KA. 2006. Microbial volatile organic compounds (MVOC’s). Available at http://www.emlab.com.
- Sharma P, Meena PD, Chauhan JS. 2013. First report of Nigrospora oryzae (Berk. & Broome) Petch causing stem blight on Brassica juncea in India. Journal of Phytopathology 161: 439–441. [Google Scholar]
- Stamatakis A, Alachiotis N. 2010. Time and memory efficient likelihood-based tree searches on phylogenomic alignments with missing data. Bioinformatics 26: 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, et al. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalavaipandian A, Ramesh V, Bagyalakshmi, et al. 2011. Diversity of fungal endophytes in medicinal plants of Courtallam hills, Western Ghats, India. Mycosphere 2: 575–582. [Google Scholar]
- Uzor PF, Ebrahim W, Osadebe PO, et al. 2015. Metabolites from Combretum dolichopetalum and its associated endophytic fungus Nigrospora oryzae – evidence for a metabolic partnership. Fitoterapia 105: 147–150. [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster J. 1952. Spore projection in the hyphomycete Nigrospora sphaerica. New Phytologist 51: 229–235. [Google Scholar]
- Webster J. 1966. Spore projection in Epicoccum and Arthrinium. Transactions of the British Mycological Society 49: 339–343. [Google Scholar]
- White TJ, Bruns T, Lee S, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications 18: 315–322. [Google Scholar]
- Wu JB, Zhang CL, Mao PP, et al. 2014. First report of leaf spot caused by Nigrospora oryzae on Dendrobium candidum in China. Plant Disease 98: 996. [DOI] [PubMed] [Google Scholar]
- Wu PC, Tsai JC, Li FC, et al. 2004. Increased levels of ambient fungal spores in Taiwan are associated with dust events from China. Atmospheric Environment 38: 4879–4886. [Google Scholar]
- Wu SH, Chen YW, Shao SC, et al. 2009. Two new solanapyrone analogues from the endophytic fungus Nigrospora sp. YB-141 of Azadirachta indica. Chemistry & Biodiversity 6: 79–85. [DOI] [PubMed] [Google Scholar]
- Zhang K, Su YY, Cai L. 2013. An optimized protocol of single spore isolation for fungi. Cryptogamie, Mycologie 34: 349–356. [Google Scholar]
- Zhaxybayeva O, Gogarten JP. 2002. Bootstrap, Bayesian probability and maximum likelihood mapping: exploring new tools for comparative genome analyses. BMC Genomics 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A. 1902. Ueber einige an tropischen Kulturpflanzen beobachtete Pilze III. Zentralblatt für Bakteriologie, Parasitenkunde 8: 216–221. [Google Scholar]


