Since its discovery in 1974 by Martin Fleischmann et al., surface-enhanced Raman scattering (SERS) has become a powerful sensing and imaging technique in biomedicine.1,2 SERS is based on the physical phenomenon of plasmon resonance whereby the inelastic scattering of photons can be significantly enhanced during the adsorption of molecules on metallic surfaces. A widely accepted mechanism for this effect is attributed to the excitation of localized surface plasmons on metal surfaces, resulting in concentrated electromagnetic optical fields and a greater increase in the electric field interactions with surrounding analyte molecules.2,3 Due to the influence of these localized surface plasmon “hot spots”, the close proximity of an analyte to metallic surfaces at nanometer length scales significantly intensifies the analyte’s Raman scattering. With enhancement factors of up to 10 orders of magnitude, SERS-based techniques are posed to achieve sensitivities significantly higher than conventional fluorescence microscopy.
While the SERS technique offers unprecedented possibilities in biomedicine, its translation into the clinics has been largely impeded because of poor reproducibility of SERS-active structures. Dana Dlott and co-workers4 report that less than 1% of Raman-active dye molecules on a silver-coated nanosphere have enhancement factors on the order of 107, while they are significantly lower for the rest. This poor distribution of SERS enhancement factors is the result of an inability to control particle–dye interactions with precision. Thus, the main challenge for improving SERS techniques lies in amalgamating dye and nanostructures effectively to manufacture reproducible SERS-active “hot spots” to develop bioprobes with a narrow distribution of high enhancement factors, moving the field a step closer to the adoption of SERS-based probes in clinical applications.
Writing in ACS Central Science,5 Jwa-Min Nam and co-workers now report a facile synthesis to fabricate dealloyed intra-nanogap particles (DIPs) with an impressive yield of ∼95%. More importantly, they introduce a neat method to enable the generation of nanometer-sized intragaps by selective silver-etching and interdiffusion of silver atoms. Recognizing the significance of intra-nanogap formation in SERS enhancement in their early work,6 the group takes one step further by applying these SERS-active probes for biosensing and imaging applications (Figure 1).
Figure 1.
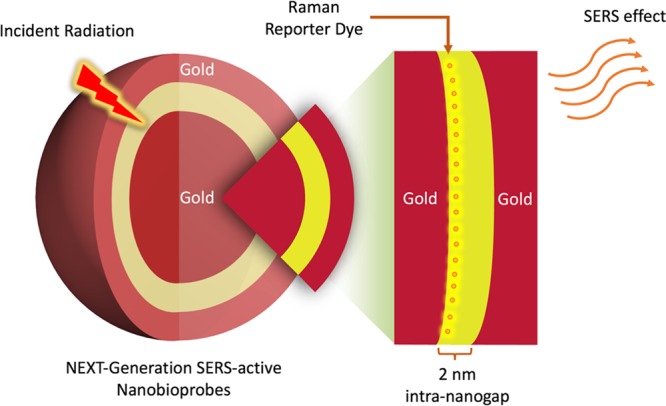
Schematic showing the SERS effect of an intra-nanogap inside a gold nanoparticle on Raman reporter molecules that are conjugated to the nanogap interface.
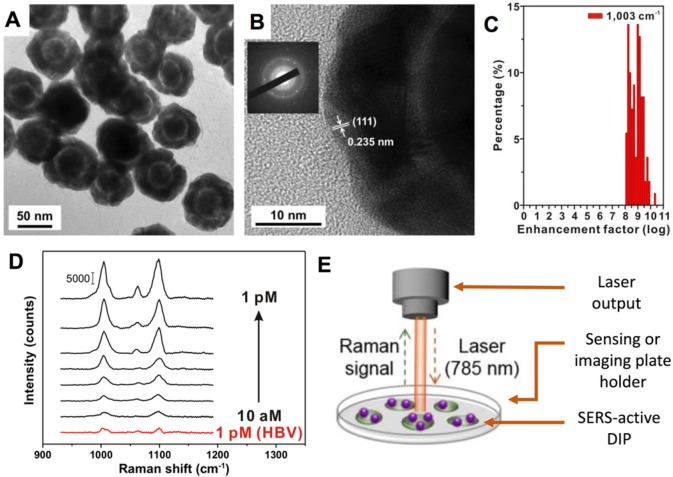
Using an Au nanoparticle, modified with Raman reporter molecules, as a seed particle, the growths of silver and gold shells are conducted simultaneously. Because of the strong affinity between silver and the pyrrolidone group, the silver shells grows preferentially at a faster rate than that of the gold, enabling the creation of an intermediate silver and outer gold layer. Subsequent selective silver-etching via ferric nitrate allows an elegant intra-nanogap of ∼2 nm to be formed (Figure 2A,B). With the construction of these nanogaps, localized surface plasmon “hot spots” are created. What is remarkable is that all DIPs under study display a consistent SERS enhancement factor on the order of ∼108 with a narrow distribution (Figure 2C). This approach nicely overcomes SERS’s common limitations of poor enhancement factor distributions and low reproducibility in plasmon “hot spot” environments for effective interactions with Raman reporter molecules.
Figure 2.
Dealloyed intra-nanogap particles (DIPs) for SERS biosensing and bioimaging applications. (A) Transmission electron microscopic image of SERS-active DIPs. (B) High-magnification electron microscopic imaging of a typical DIP. (C) SERS enhancement factors obtained with DIPs. (D) SERS intensity profiles obtained for DNA sensing at different concentrations using DIPs. (E) Sketch of the experimental setup for DIP-based molecular sensing and cellular imaging. Reproduced with permission from ref (5). Copyright 2018 American Chemical Society.
Another exciting achievement is the validation of such intra-nanogap gold structures for DNA biological sensing7 and targeted imaging.8 Nam and colleagues have shown the feasibility of applying these DIPs for ultrasensitive DNA detection (Figure 2D). To test the approach, the team built a sandwich nanostructure by assembling DNA-modified DIPs with magnetic microspheres using established DNA gold conjugation techniques.9 This design offers a limit of DNA detection of as low as 10 aM. In a parallel experiment, the research team tested the use of peptide-modified DIPs for integrin targeting and imaging in cells (Figure 2E). Due to the high sensitivities enabled by SERS, the implementation of a low power laser with a short exposure time is adequate, thereby reducing potential physiological damage to biological samples while maintaining high signal response for bioimaging.
A caveat in this work might lie in how to control the dealloying process to effectively etch the silver intermediate layer to form nanogaps. A simulation is conducted based on the assumption that metal residues exist. An ineffective etching of the silver layer might result in toxicity issues known for Ag ions.10 It would also be interesting to understand the toxicity of these DIPs in in vitro and in vivo models. Resolving this potential pitfall would tremendously accelerate the integration of SERS probes with medical devices and facilitate their commercialization for medical uses.
Despite its tremendous potential, SERS is still in its infancy for clinical applications. Moving forward, the holy grails in the field are developing SERS to guided surgery and therapy, in addition to ultrasensitive multiplexed rapid detection of disease markers with minimal sample preparations.11 In addition, SERS, with its capability for single-molecule sensing, could also tremendously benefit fundamental research in cellular biology to elucidate metabolite mechanisms with super resolution imaging.12 Recent advancements in biophotonics, spectroscopy, and microscopy have improved the infrastructures for SERS, paving the way for better integration of SERS with current state-of-the-art equipment.13,14 To complement this growth, reliable SERS-active probes must be innovated. The systematic redox approach presented by Nam and co-workers has allowed for fine-tuning of gap structures with nanometer precision and provided a valuable thought process for creating new synthetic strategies. This work may spark deliberate efforts in the design of more complex and reliable SERS-active intra-nanogap probes. To conclude, this is an exciting era for SERS with new opportunities ranging from materials design and chemical synthesis to clinical diagnostics, imaging, and medical therapy.
References
- Fleischmann M.; Hendra P. J.; McQuillan A. J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. 10.1016/0009-2614(74)85388-1. [DOI] [Google Scholar]
- Laing S.; Jamieson L. E.; Faulds K.; Graham D. Surface-enhanced Raman spectroscopy for in vivo biosensing. Nat. Rev. Chem. 2017, 1, 0060. 10.1038/s41570-017-0060. [DOI] [Google Scholar]
- Qian X. M.; Nie S. M. Single-molecule and single-nanoparticle SERS: from fundamental mechanisms to biomedical applications. Chem. Soc. Rev. 2008, 37, 912–920. 10.1039/b708839f. [DOI] [PubMed] [Google Scholar]
- Fang Y.; Seong N.-H.; Dlott D. D. Measurement of the Distribution of Site Enhancements in Surface-Enhanced Raman Scattering. Science 2008, 321, 388. 10.1126/science.1159499. [DOI] [PubMed] [Google Scholar]
- Kim M.; Ko S. M.; Kim J.-M.; Son J.; Lee C.; Rhim W.-K.; Nam J.-M. Dealloyed Intra-Nanogap Particles with Highly Robust, Quantifiable Surface-Enhanced Raman Scattering Signals for Biosensing and Bioimaging Applications. ACS Cent. Sci. 2018, 10.1021/acscentsci.7b00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D.-K.; Jeon K.-S.; Hwang J.-H.; Kim H.; Kwon S.; Suh Y. D.; Nam J.-M. Highly uniform and reproducible surface-enhanced Raman scattering from DNA-tailorable nanoparticles with 1-nm interior gap. Nat. Nanotechnol. 2011, 6, 452. 10.1038/nnano.2011.79. [DOI] [PubMed] [Google Scholar]
- Xie X.; Xu W.; Liu X. Improving Colorimetric Assays through Protein Enzyme-Assisted Gold Nanoparticle Amplification. Acc. Chem. Res. 2012, 45, 1511–1520. 10.1021/ar300044j. [DOI] [PubMed] [Google Scholar]
- Chourpa I.; Lei F. H.; Dubois P.; Manfait M.; Sockalingum G. D. Intracellular applications of analytical SERS spectroscopy and multispectral imaging. Chem. Soc. Rev. 2008, 37, 993–1000. 10.1039/b714732p. [DOI] [PubMed] [Google Scholar]
- Mirkin C. A.; Letsinger R. L.; Mucic R. C.; Storhoff J. J. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 1996, 382, 607. 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- Kittler S.; Greulich C.; Diendorf J.; Köller M.; Epple M. Toxicity of Silver Nanoparticles Increases during Storage Because of Slow Dissolution under Release of Silver Ions. Chem. Mater. 2010, 22, 4548–4554. 10.1021/cm100023p. [DOI] [Google Scholar]
- Laing S.; Gracie K.; Faulds K. Multiplex in vitro detection using SERS. Chem. Soc. Rev. 2016, 45, 1901–1918. 10.1039/C5CS00644A. [DOI] [PubMed] [Google Scholar]
- Willets K. A. Super-resolution imaging of SERS hot spots. Chem. Soc. Rev. 2014, 43, 3854–3864. 10.1039/C3CS60334B. [DOI] [PubMed] [Google Scholar]
- Cialla-May D.; Zheng X. S.; Weber K.; Popp J. Recent progress in surface-enhanced Raman spectroscopy for biological and biomedical applications: from cells to clinics. Chem. Soc. Rev. 2017, 46, 3945–3961. 10.1039/C7CS00172J. [DOI] [PubMed] [Google Scholar]
- Palonpon A. F.; Ando J.; Yamakoshi H.; Dodo K.; Sodeoka M.; Kawata S.; Fujita K. Raman and SERS microscopy for molecular imaging of live cells. Nat. Protoc. 2013, 8, 677. 10.1038/nprot.2013.030. [DOI] [PubMed] [Google Scholar]