Abstract
DWARF14 (D14) is a strigolactone receptor that plays a central role in suppression of shoot branching, and hence is a potential target to increase crop productions and biomass. Recently, we reported a fluorescence turn-on probe, Yoshimulactone Green (YLG), which generates a strong fluorescence upon the hydrolysis by D14-type strigolactone receptors. Herein, we applied a YLG-based in vitro assay to a high-throughput chemical screening and identified a novel small molecule DL1 as a potent inhibitor of D14. DL1 competes with endogenous strigolactones, thereby increasing the number of shoot branching in a model plant Arabidopsis as well as in rice. Thus, DL1 is expected to be useful not only as a tool to understand the biological roles of D14 receptors in plant growth and development, but also as a potent agrochemical to improve the crop yield.
Short abstract
We discovered a small molecule DL1 that inhibits D14 strigolactone receptor and hence induces shoot branching in plants. DL1 can be used in plant biology as well as in improving crop yield.
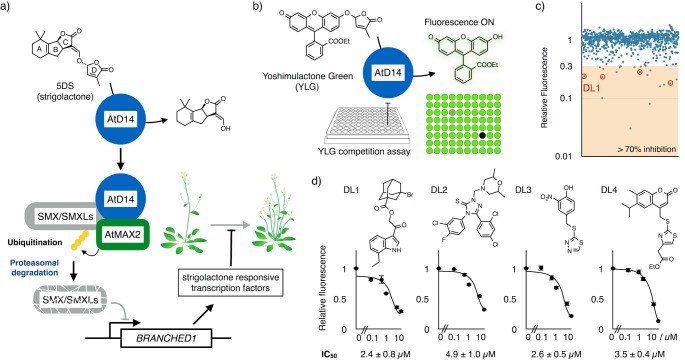
Regulating the number of branches in plants is critical for controlling plant architecture and efficient production of biomass.1 Increased shoot branching has historically been a desirable trait because it is associated with high crop yields.2 Substantial progress has been made in our understanding of the mechanism of how plants increase or suppress shoot branching in response to the environment. Genetic analysis revealed that carotenoid-derived signaling molecules regulate the shoot branching. This key molecule was identified as strigolactone,3,4 which was initially discovered as a germination stimulant of the parasitic plant Striga hermonthica.5 Strigolactone is a sesquiterpene lactone composed of a tricyclic lactone (ABC-ring) and a butenolide (D-ring), which are connected via an enol ether bond (Figure 1a).6 Recent studies revealed that strigolactone also plays a significant role in symbiosis with arbuscular mycorrhizal fungi.7 In Arabidopsis thaliana, DWARF14 (AtD14)(8) and MORE AXILLARY GROWTH2 (AtMAX2)(9) genes are essential in strigolactone-dependent suppression of axillary bud outgrowth. Loss-of-function in these signaling components results in an increased number of shoot branching. D14 encodes a group of α/β-hydrolase fold proteins that bind to and hydrolyze strigolactones into an ABC-ring and D-ring (5-hydroxyl-3-methyl-butenolide) during the perception process.10MAX2 encodes a F-box protein of ubiquitin E3 ligase. Previous studies including crystallographic analysis of strigolactone-induced complex of AtD14 and D3 (MAX2 orthologue in rice) demonstrated that these two proteins interact through strigolactone-dependent conformational change of AtD14.11,12 This complex ubiquitinates specific downstream repressors including SMXL6, 7, and 8 for proteasomal degradation.13−16 As a consequence, transcription factors including BRANCHED1 (BRC1) are upregulated to express a subset of strigolactone inducible genes.17,18 It was revealed that BRC1 is expressed in developing bud, and its downregulation leads to bud outgrowth.19
Figure 1.
Yoshimulactone-based chemical screening of D14 ligands. (a) Strigolactone signaling that suppresses shoot branching. (b) Schematic workflow of high-throughput screening for AtD14-ligand. (c) AtD14-binding activity of 800 compounds examined by YLG competition assay. The fluorescence intensities were measured after the incubation of YLG (1 μM) with AtD14 in the presence of library compounds (10 μM). Vertical axis indicates relative fluorescence normalized by no ligand control. Red circles indicate the four compounds that passed the second screening. (d) Chemical structure of the hit compounds and their inhibitory activity for D14-mediated YLG hydrolysis. Error bar indicates SE (n = 3 biological replicates).
As AtD14 plays a critical role in strigolactone-induced branching suppression, molecules that inhibit D14 should increase the number of branching. A small-molecule approach is beneficial for agriculture because it enables precise control of timing and degree of branch emergence. However, in vivo chemical screening with the branching phenotype is impractical due to long incubation time, growth space, and the amount of small molecules required for the assay. Recently, we reported a fluorogenic molecule, Yoshimulactone Green (YLG),20 whose fluorescence emission is turned on by AtD14-dependent hydrolysis.21 Because YLG shares the binding site of AtD14 with strigolactones, competition assays with YLG allowed us to estimate the binding affinity of natural and synthetic strigolactones to AtD14. Here, we utilized the YLG-based competition assay to high-throughput screening of ligands for AtD14 (Figure 1b).
We screened 800 compounds from an in-house developed chemical library as below. YLG (1 μM) was incubated with recombinant AtD14 (10 μg/mL) in the presence of library compound (10 μM). The difference in fluorescence intensity before and after the incubation is summarized in Figure 1c. In the first screening, 18 compounds exhibited >70% inhibition of AtD14-mediated YLG hydrolysis. The activities of these 18 compounds were retested in a second screening, and four compounds were validated as AtD14 ligands with >70% inhibitory activity at 10 μM (Figure 1d and Figure S1). We estimated the half maximal inhibitory concentration (IC50) value from the dose–response curve of these compounds. Among the four compounds, DL1 displayed the highest inhibitory activity to AtD14 with the IC50 value of 2.4 ± 0.8 μM, which is comparable with the widely used synthetic strigolactone racemic-GR24 (4.9 ± 0.1 μM).21−23 DL1 also inhibited the AtD14-mediated hydrolysis of the natural strigolactone (+)-5-deoxystrigol (5DS) with the IC50 value of 2.2 ± 0.2 μM (Figure S2). The intrinsic fluorescence of AtD14 was reduced by the addition of DL1 in a concentration-dependent manner, which further validates the binding of DL1 to AtD14 (Figure 2a). The dissociation constant (Kd) of DL1 to AtD14 was estimated as 3.4 ± 0.5 μM, whereas an inactive strigolactone analogue, carba-GR24, did not show a significant effect on the fluorescence (Figure 2b).21 The binding of DL1 is specific to AtD14, as we did not observe binding to its closely related homologue, HYPOSENSITIVE TO LIGHT (AtHTL), which is involved in seed germination stimulated by wildfire smoke-derived karrikins (Kd > 250 μM).24,25
Figure 2.
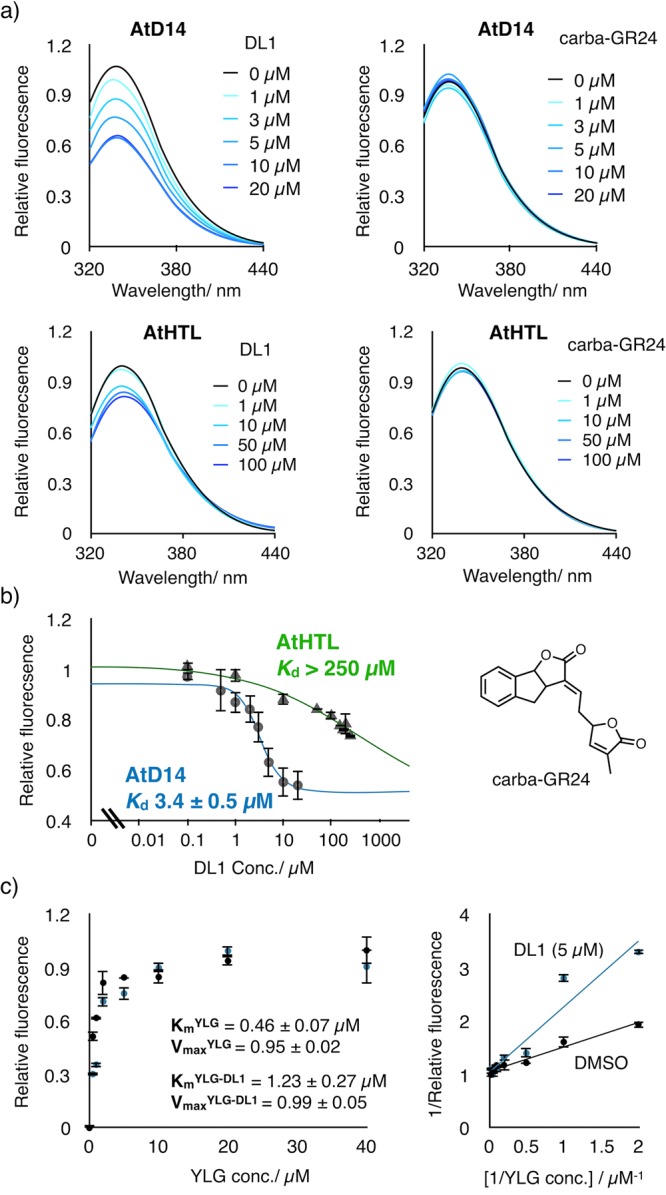
Biochemical analysis for the AtD14 inhibition by DL1. (a) Quenching of AtD14 or AtHTL intrinsic fluorescence by DL1. The intrinsic fluorescence of AtD14 and AtHTL was measured in the presence of DL1 or carba-GR24 at the indicated concentration (ex. 288 nm). (b) The relative fluorescence intensity of AtD14 (blue) and AtHTL (green) at 340 nm was plotted against DL1 concentrations. Error bar indicates SE (n = 3 biological replicates). (c) Michaelis–Menten (left) and Lineweaver–Burk plots (right) for the inhibition of AtD14 by DL1 (5 μM). Km and Vmax values were calculated by using the linear least-square method. Error bar indicates SE (n = 3 biological replicates).
We evaluated inhibitory kinetics of DL1 on AtD14-mediated hydrolysis of strigolactone. No significant change of the maximal velocity (Vmax) was observed in the Lineweaver–Burk plot, indicating that DL1 inhibits the hydrolytic activity of AtD14 in a competitive manner (Figure 2c). Moreover, we did not detect any hydrolytic product of DL1 after the incubation with AtD14 (Figure S2). Therefore, we concluded that DL1 reversibly binds to the active pocket of AtD14 and competitively inhibits its binding to strigolactones.
Next, we tested the activity of DL1 in planta. BRANCHED1 (BRC1), a TCP transcription factor gene, and STH7, a double B-box domain transcription factor, are known as strigolactone-responsive genes.9,17 We investigated whether the (+)-GR24-induced expression of BRC1 and STH7 could be inhibited by DL1. The results of real-time PCR experiments using 10-day-old seedlings of Arabidopsis are shown in Figure 3a. Consistent with previous reports, the expression of BRC1 and STH7 genes were increased by the treatment with (+)-GR24 for 24 h. The (+)-GR24-induced expression of BRC1 and STH7 were suppressed by DL1 (Figure 3a). Moreover, treatment of Arabidopsis with DL1 alone displayed a significant decrease in the expression level of STH7, indicating that the signaling of endogenous strigolactones was inhibited by DL1.
Figure 3.
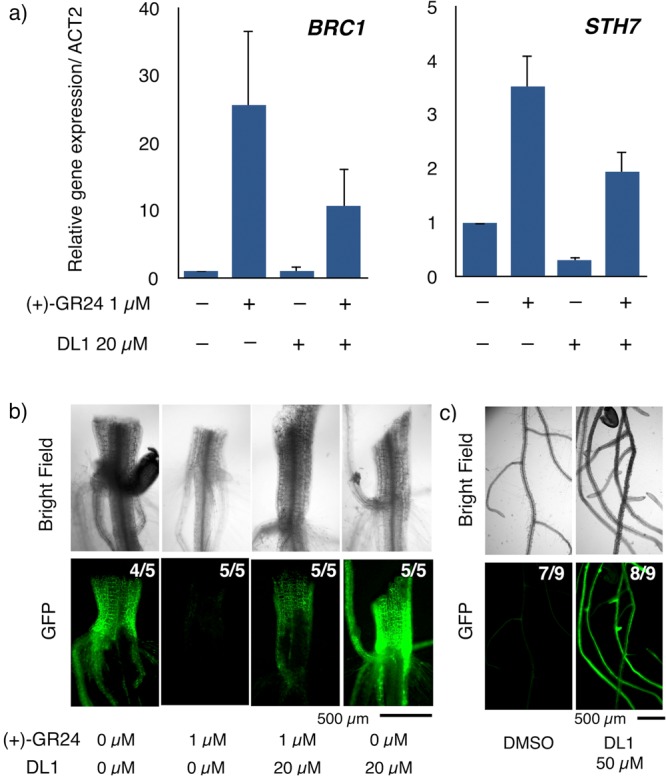
Inhibition of strigolactone signaling by DL1. (a) qRT-PCR analysis for the expression level of strigolactone-responsive genes, BRC1 and STH7. The 10-day-old wild-type Arabidopsis seedlings were treated with 1 μM (+)-GR24 and 20 μM DL1 for 24 h. The relative values are normalized to ACT2. The value means ± SE (n = 3 biological replicates). (b, c) Bright field (top) and fluorescence (bottom) images of the hypocotyl (b) and root (c) in 2-week-old 35S::AtD14-GFP/atd14–1 seedlings treated with 1 μM (+)-GR24 and indicated concentration of DL1 for 24 h. Numerals at the upper right indicate the number of plants showing the figure phenotype per total number of plants examined.
The extent of strigolactone signaling is suppressed by negative feedback regulation of AtD14. After activating downstream signal transduction, AtD14 protein is degraded through a MAX2-dependent proteasomal pathway.26 We investigated whether DL1 inhibits the strigolactone-dependent AtD14 degradation using transgenic Arabidopsis plants expressing AtD14-GFP fusion protein. As reported previously, a striking reduction of GFP signal in hypocotyl was observed by the treatment with (+)-GR24 for 24 h (Figure 3b). The reduction of fluorescence was alleviated by DL1, indicating that DL1 inhibits strigolactone-dependent interaction of AtD14 with MAX2 within plant cell. Furthermore, the treatment of DL1 alone displayed brighter fluorescence than the no-treatment control, indicating that DL1 inhibits basal signaling induced by the endogenous strigolactones. This result was further validated at the root tissue (Figure 3c). Only a faint fluorescence was observed in a control experiment due to the degradation of AtD14-GFP induced by endogenous strigolactones. In marked contrast, DL1-treated plants showed bright fluorescence in the root. These results imply that the DL1 inhibits strigolactone-dependent AtD14 degradation by competing with endogenous strigolactones.
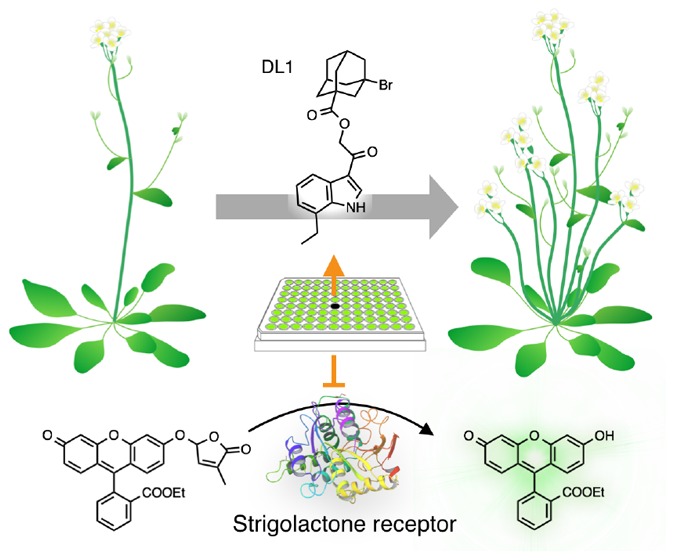
Since DL1 was shown to inhibit the perception of endogenous strigolactones by AtD14, DL1 is expected to work as a shoot-branching enhancer. To investigate the effect of DL1 on shoot branching, Arabidopsis seedlings were grown in the presence of DL1 for one month. As expected, DL1-treated Arabidopsis displayed an increase in the number of branches (Figure 4a,b and Figure S4). In contrast, DL1 did not affect the AtHTL-dependent signaling. Karrikins as well as non-natural stereoisomers of GR24 were previously reported to stimulate seed germination through the binding to AtHTL in Arabidopsis.27 DL1 neither induced nor inhibited the seed germination induced by racemic-GR24, which contains non-natural stereoisomer (Figure S3). Taken together, DL1 is a selective inhibitor of AtD14 and increases the number of shoot branching in a model plant Arabidopsis by competing with endogenous strigolactones.
Figure 4.
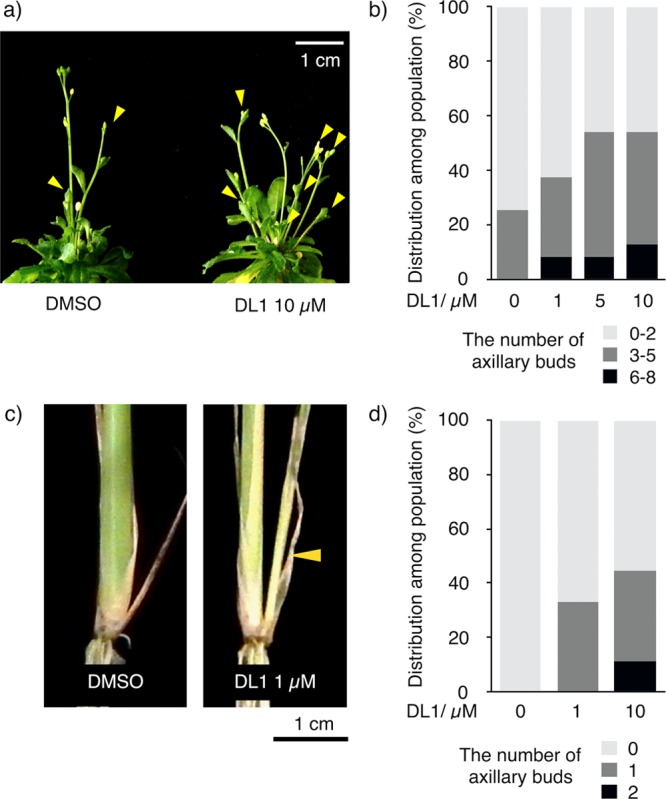
DL1-enhanced shoot branching in Arabidopsis and rice. (a) 30-day-old wild-type Arabidopsis plants treated with DMSO or 10 μM DL1. The yellow arrowheads indicate axillary buds. (b) Distribution diagram of plants with primary rosette branches at least 0.5 cm long (n = 24). (c) 40-day-old rice (nipponbare) treated with DMSO or 1 μM DL1. The yellow arrowhead indicates axillary bud. (d) Distribution diagram of plants with tillering (n = 9).
Crystallographic studies revealed that the binding pocket of rice D14 protein (OsD14) shows high similarity to that in AtD14, indicating that DL1 might inhibit OsD14 as well.28 To test the idea, we exposed rice plants to DL1 for 40 days. The DL1-treated rice displayed an increased tiller number, indicating that DL1 inhibited the endogenous strigolactone signaling in rice as well as in Arabidopsis (Figure 4c,d and Figure S5). Moreover, the 40 days’ exposure with DL1 did not induce apparent toxicity nor growth inhibition on the rice plants as the DL1 treatment did not significantly change shoot dry weight (Figure S6). Our study shows that DL1 can be directly applied to crop species.
In conclusion, we have harnessed the YLG-based competition assay for a high-throughput screening of AtD14 ligands. This screening enabled identification of DL1 as the first AtD14-selective inhibitor that induces shoot branching in Arabidopsis and rice. Because the structure of strigolactone-binding pocket in D14 is conserved in angiosperm species,8 DL1 may induce shoot branching in these plant species. DL1 can be a potent agrochemical to improve the crop yield as well as a tool to understand the biological roles of D14 receptors in plant growth and development. Feasibility studies of DL1 such as soil persistence and human toxicity are now ongoing.
Acknowledgments
We thank Dr. Tsuyoshi Nakagawa for providing pGWB605. This work was funded by a Grant-in-Aid for Scientific Research on Innovative Areas “Chemistry for Multimolecular Crowding Biosystems” (JSPS KAKENHI Grant No. JP17H06350) and PRESTO (Japan Science and Technology Agency, JPMJPR15Q9).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.7b00554.
Synthesis of DL1; NMR spectrum; Supplementary Figures S1–S5; Materials and methods (plant culture and treatment, RT-PCR analysis, protein purification, YLG-based assay, LC-MS based analysis, intrinsic fluorescence assay, seed germination assay (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Wang Y.; Li J. Branching in Rice. Curr. Opin. Plant Biol. 2011, 14, 94–99. 10.1016/j.pbi.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Yaish M. W. F.; Guevara D. R.; El-Kereamy A.; Rothstein S. J.. Axillary Shoot Branching in Plants. In Plant Developmental Biology–Biotechnological Perspectives; Pua E. C., Davey M. R., Ed.; Springer-Verlag: Berlin Heidelberg, 2010; pp 37–52. [Google Scholar]
- Gomez-Roldan V.; Fermas S.; Brewer P. B.; Puech-Pagès V.; Dun E. A.; Pillot J.-P.; Letisse F.; Matusova R.; Danoun S.; Portais J.-C.; et al. Strigolactone Inhibition of Shoot Branching. Nature 2008, 455, 189–194. 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- Umehara M.; Hanada A.; Yoshida S.; Akiyama K.; Arite T.; Takeda-Kamiya N.; Magome H.; Kamiya Y.; Shirasu K.; Yoneyama K.; et al. Inhibition of Shoot Branching by New Terpenoid Plant Hormone. Nature 2008, 455, 195–200. 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- Cook C. E.; Whichard L. P.; Turner B.; Wall M. E.; Egley G. H. Germination of Witchweed (Striga lutea Lour.): Isolation and Properties of a Potent Stimulant. Science 1966, 154, 1189–1190. 10.1126/science.154.3753.1189. [DOI] [PubMed] [Google Scholar]
- Zwanenburg B.; Pospíšil T. Structure and Activity of Strigolactones: New Plant Hormones with a Rich Future. Mol. Mol. Plant 2013, 6, 38–62. 10.1093/mp/sss141. [DOI] [PubMed] [Google Scholar]
- Akiyama K.; Matsuzaki K.; Hayashi H. Plant Sesquiterpenes Induce Hyphal Branching in Arbuscular Mycorrhizal Fungi. Nature 2005, 435, 824–827. 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- Arite T.; Umehara M.; Ishikawa S.; Hanada A.; Maekawa M.; Yamaguchi S.; Kyozuka J. d14, a Strigolactone-Insensitive Mutant of Rice, Shows an Accelerated Outgrowth of Tillers. Plant Cell Physiol. 2009, 50, 1416–1424. 10.1093/pcp/pcp091. [DOI] [PubMed] [Google Scholar]
- Nelson D. C.; Scaffidi A.; Dun E. A.; Waters M. T.; Flematti G. R.; Dixon K. W.; Beveridge C. A.; Ghisalberti E. L.; Smith S. M. F-box Protein MAX2 Has Dual Roles in Karrikin and Strigolactone Signaling in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 8897–8902. 10.1073/pnas.1100987108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamiaux C.; Drummond R. S. M.; Janssen B. J.; Ledger S. E.; Cooney J. M.; Newcomb R. D.; Snowden K. C. DAD2 Is an α/β Hydrolase Likely to Be Involved in the Perception of the Plant Branching Hormone, Strigolactone. Curr. Biol. 2012, 22, 2032–2036. 10.1016/j.cub.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Yao R.; Ming Z.; Yan L.; Li S.; Wang F.; Ma S.; Yu C.; Yang M.; Chen L.; Chen L.; et al. DWARF14 is a Non-canonical Hormone Receptor for Strigolactone. Nature 2016, 536, 469–473. 10.1038/nature19073. [DOI] [PubMed] [Google Scholar]
- de Saint Germain A.; Clavé G.; Badet-Denisot M.-A.; Pillot J.-P.; Cornu D.; Le Caer J.-P.; Burger M.; Pelissier F.; Retailleau P.; Turnbull C.; et al. An Histidine Covalent Receptor and Butenolide Complex Mediates Strigolactone Perception. Nat. Chem. Biol. 2016, 12, 787–794. 10.1038/nchembio.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F.; Lin Q.; Zhu L.; Ren Y.; Zhou K.; Shabek N.; Wu F.; Mao H.; Dong W.; Gan L.; et al. D14-SCFD3-dependent Degradation of D53 Regulates Strigolactone Signalling. Nature 2013, 504, 406–410. 10.1038/nature12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L.; Liu X.; Xiong G.; Liu H.; Chen F.; Wang L.; Meng X.; Liu G.; Yu H.; Yuan Y.; et al. DWARF 53 Acts as a Repressor of Strigolactone Signalling in Rice. Nature 2013, 504, 401–405. 10.1038/nature12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Wang B.; Jiang L.; Liu X.; Li X.; Lu Z.; Meng X.; Wang Y.; Smith S. M.; Li J. Strigolactone Signaling in Arabidopsis Regulates Shoot Development by Targeting D53-Like SMXL Repressor Proteins for Ubiquitination and Degradation. Plant Cell 2015, 27, 3128–3142. 10.1105/tpc.15.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soundappan I.; Bennett T.; Morffy N.; Liang Y.; Stanga J. P.; Abbas A.; Leyser O.; Nelson D. C. SMAX1-LIKE/D53 Family Members Enable Distinct MAX2-Dependent Responses to Strigolactones and Karrikins in Arabidopsis. Plant Cell 2015, 27, 3143–3159. 10.1105/tpc.15.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiguchi K.; Sasaki E.; Shimada Y.; Nagae M.; Ueno K.; Nakano T.; Yoneyama K.; Suzuki Y.; Asami T. Feedback-Regulation of Strigolactone Biosynthetic Genes and Strigolactone-Regulated Genes in Arabidopsis. Biosci., Biotechnol., Biochem. 2009, 73, 2460–2465. 10.1271/bbb.90443. [DOI] [PubMed] [Google Scholar]
- Braun N.; de Saint Germain A.; Pillot J.-P.; Boutet-Mercey S.; Dalmais M.; Antoniadi I.; Li X.; Maia-Grondard A.; Le Signor C.; Bouteiller N.; et al. The Pea TCP Transcription Factor PsBRC1 Acts Downstream of Strigolactones to Control Shoot Branching. Plant Physiol. 2012, 158, 225–238. 10.1104/pp.111.182725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Martinez J. A.; Poza-Carrion C.; Cubas P. Arabidopsis BRANCHED1 Acts as an Integrator of Branching Signals within Axillary Buds. Plant Cell 2007, 19, 458–472. 10.1105/tpc.106.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YLG is commercially available from Tokyo Chemical Industry Co., Ltd. (TCI Product number: E1238).
- Tsuchiya Y.; Yoshimura M.; Sato Y.; Kuwata K.; Toh S.; Holbrook-Smith D.; Zhang H.; McCourt P.; Itami K.; Kinoshita T.; et al. Probing Strigolactone Receptors in Striga hermonthica with Fluorescence. Science 2015, 349, 864–868. 10.1126/science.aab3831. [DOI] [PubMed] [Google Scholar]
- Johnson A. W.; Gowada G.; Hassanali A.; Knox J.; Monaco S.; Razavi Z.; Rosebery G. The Preparation of Synthetic Analogues of Strigol. J. Chem. Soc., Perkin Trans. 1 1981, 1734–1743. 10.1039/p19810001734. [DOI] [Google Scholar]
- Mangnus E. M.; Dommerholt F. J.; de Jong R. L. P.; Zwanenburg B. Improved Synthesis of Strigol Analogue GR24 and Evaluation of the Biological Activity of Its Diastereomers. J. Agric. Food Chem. 1992, 40, 1230–1235. 10.1021/jf00019a031. [DOI] [Google Scholar]
- Waters M. T.; Nelson D. C.; Scaffidi A.; Flematti G. R.; Sun Y. K.; Dixon K. W.; Smith S. M. Specialisation Within the DWARF14 Protein Family Confers Distinct Responses to Karrikins and Strigolactones. Development 2012, 139, 1285–1295. 10.1242/dev.074567. [DOI] [PubMed] [Google Scholar]
- Flematti G. R.; Ghisalberti E. L.; Dixon K. W.; Trengove R. D. A Compound from Smoke That Promotes Seed Germination. Science 2004, 305, 977. 10.1126/science.1099944. [DOI] [PubMed] [Google Scholar]
- Chevalier F.; Nieminen K.; Sanchez-Ferrero J. C.; Rodriguez M. L.; Chagoyen M.; Hardtke C. S.; Cubas P. Strigolactone Promotes Degradation of DWARF14, an α/β Hydrolase Essential for Strigolactone Signaling in. Plant Cell 2014, 26, 1134–1150. 10.1105/tpc.114.122903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi A.; Waters M. T.; Sun Y. K.; Skelton B. W.; Dixon K. W.; Ghisalberti E. L.; Flematti G. R.; Smith S. M. Strigolactone Hormones and Their Stereoisomers Signal Through Two Related Receptor Proteins to Induce Different Physiological Responses in Arabidopsis. Plant Physiol. 2014, 165, 1221–1232. 10.1104/pp.114.240036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagiyama M.; Hirano Y.; Mori T.; Kim S. Y.; Kyozuka J.; Seto Y.; Yamaguchi S.; Hakoshima T. Structures of D14 and D14L in the Strigolactone and Karrikin Signaling Pathways. Genes Cells 2013, 18, 147–160. 10.1111/gtc.12025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.