Abstract

The distant and selective activation of unreactive C–H and C–C bonds remains one of the biggest challenges in organic chemistry. In recent years, the development of remote functionalization has received growing interest as it allows for the activation of rather challenging C–H and C–C bonds distant from the initiation point by means of a “metal-walk”. A “metal-walk” or “chain-walk” is defined by an iterative series of consecutive 1,2- or 1,3-hydride shifts of a metal complex along a single hydrocarbon chain. With this approach, simple building blocks or mixtures thereof can be transformed into complex scaffolds in a convergent and unified strategy. A variety of catalytic systems have been developed and refined over the past decade ranging from late-transition-metal complexes to more sustainable iron- and cobalt-based systems. As the possibilities of this field are slowly unfolding, this area of research will contribute considerably to provide solutions to yet unmet synthetic challenges.
Short abstract
The transition-metal-catalyzed remote functionalization of olefins is highlighted with a special emphasis on the most recent developments of this emerging area of research.
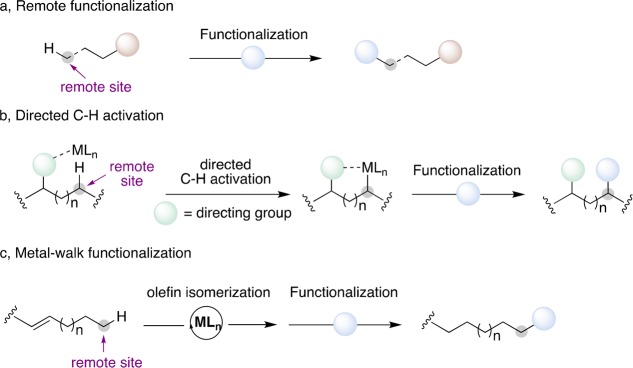
Initiated by the pioneering work of Breslow in the early 1970s for biomimetic transformations1,2 and later used by Schwarz in gas-phase chemistry,3 the concept of remote functionalization consists of an indirect activation of a site distant from the initial functional group (Figure 1a). This distant activation of an “unactivated position” strongly modifies our perception of organic transformations which was mostly concentrated on direct transformation of the chemically most reactive position. For instance, the concept of activation of carbon–hydrogen bonds, coined as C–H activation, has the ability to decorate hydrocarbons of all couleur from simple arenes to highly functionalized natural products.4−7 To achieve control of the selectivity of these transformations, the inherent directionality of the substrate is usually exploited (Figure 1b). This goal is commonly achieved by utilizing directing groups, present or preinstalled, or through the undirected activation of particularly reactive C–H bonds. While the field of directed activation of C–H bonds matured considerably in the past 20 years, the undirected process still poses significant challenges limiting its applicability to C–H bonds “within reach” of the catalyst.8 To overcome these limitations, the field of remote functionalization has spurred renewed interest by organic chemists. This conceptually different approach relies on the ability of transition-metal complexes to undergo rapid olefin isomerization, or “metal-walk”, preceding a final, yet formal, C–H functionalization. “Chain-walking, chain-running or metal-walk” has been defined as a process in which discrete alkyl–metal species undergo an iterative sequence of 1,2- or 1,3-hydride shifts along a single hydrocarbon chain. Thus, the site of functionalization is located at a different position in the molecule allowing the activation of C–H bonds beyond reach in classical C–H activation. An additional inherent advantage of such “metal-walk” is that the chemical outcome is usually independent of the position and geometry of the initial double bond. This seemingly innocent observation opens a yet unexplored field of research, as simple and readily available hydrocarbon precursors can act as powerful scaffolds to build up molecular complexity (Figure 1c).
Figure 1.
“Metal-walk” vs classical modes of activation: (a) general concept of remote functionalization; (b) directed C–H activation at close proximity; (c) metal-walk for remote functionalization.
The “metal-walk” via formal olefin migration along the saturated hydrocarbon chain can proceed through a variety of mechanisms, which are briefly elucidated in the following sections along with representative examples.
Mechanistic Aspects of Olefin Isomerization
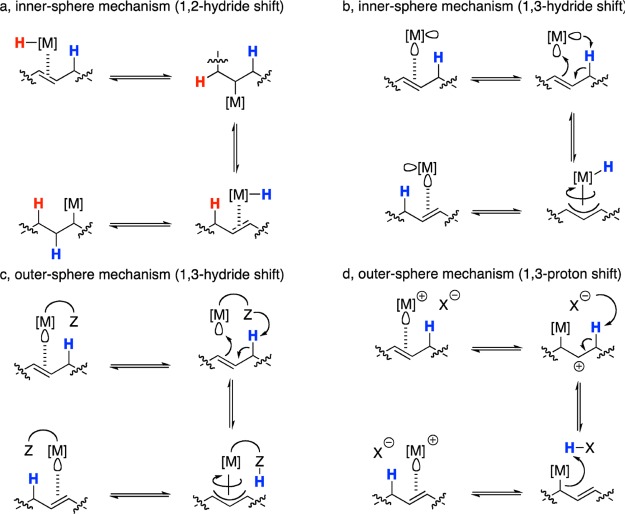
Olefin isomerization can occur via either 1,2- or 1,3-hydride shift mechanism, and both pathways can potentially compete with each other.9,10 In the 1,2-hydride shift mechanism, the metal–hydride complex undergoes migratory insertion into an olefin, commonly referred to as hydrometalation reaction, giving rise to a well-defined alkyl–metal species (Figure 2a). Subsequent β-hydride elimination furnishes the isomerized olefin π-complex, which undergoes rotation and hydrometalation.
Figure 2.
Mechanistic intricacies of olefin isomerization: (a) 1,2-hydride shift mechanism; (b) 1,3-hydride shift; (c) 1,3-hydride shift with a ligand acting as a base; (d) 1,3-proton shift.
Alternatively, olefin isomerization can occur via a 1,3-hydride shift mechanism through an inner- or outer-sphere mechanism. The inner-sphere mechanism requires two vacant orbitals for this process: one for olefin coordination and the second one for the C–H allylic activation to occur (Figure 2b). After formation of the η3-allyl complex, reductive elimination provides the isomerized olefin–metal complex. The vacant filled orbital can also be replaced by a strategically placed base within the ligand sphere of the catalyst (Figure 2c). Upon olefin coordination and allylic activation, this base can abstract the allylic proton to furnish the η3-allyl complex, which undergoes reductive elimination to give the isomerized olefin. Finally, some π-acidic transition metals such as cationic silver and palladium complexes are able to acidify the allylic position upon olefin coordination (Figure 2d). Intermolecular deprotonation subsequently furnishes the η3-allyl complex, which rapidly undergoes protodemetalation resulting in overall olefin migration. Independent of the mechanism, the directionality of the “chain-walking” depends on the terminating event as all steps in the isomerization sequence are in rapid equilibrium. Consequently, a variety of catalytic systems with unique termination steps have been developed and are discussed in the following with representative examples.
Titanium and Zirconium Complexes
The isomerization of branched Grignard reagents to linear compounds via the formation of olefin intermediates under titanium or zirconium catalysis has been reported as early as 1961.11,12 But only in the past two decades have efforts been devoted toward the development of protocols that allowed the extension of these findings for the selective isomerization of olefins.13 Most notably, Schwartz’s reagent (Cp2Zr(H)Cl)14,15 and Negishi reagent (Cp2Zr(C4H8))16 exhibit high activity for the isomerization of olefins and dienes. These reagents are easily accessible from zirconocene dichloride (Cp2ZrCl2) and can be readily prepared in situ. “Chain-walking” of linear olefins occurs under mild conditions whereas hindered substrates typically require higher temperatures. For example, simple fatty alcohols underwent a sequence comprising olefin isomerization, elimination, and electrophilic trapping in the presence of stoichiometric amounts of the Negishi reagent (Figure 3a).17 The in situ formed alkoxyzirconocene species serves as a leaving group to yield the required allylzirconocene. As double bond geometry and position in the starting material are inconsequential of the reaction outcome, a convergent synthesis from isomeric mixtures can be realized. If simple olefins are employed in the presence of Schwartz’s reagent, the resulting terminal alkylzirconium species are suitable reaction partners in asymmetric conjugate additions (Figure 3b).18
Figure 3.
Zr complexes in remote functionalization: (a) zirconium-mediated C–H allylic activation–elimination sequence; (b) migratory hydrozirconation for enantioselectice copper-catalyzed 1,4-addition reaction; (c) zirconium-mediated allylic C–H bond activation followed by a selective ring-opening reaction.
Recently it was found that strategically positioned cyclopropanes offer the possibility to achieve bisfunctionalization of olefins by means of zirconium “chain-walk”, C–C bond cleavage, and electrophilic trapping.19,20 The transiently formed allyl zirconacyclobutane selectively reacts first with carbonyl electrophiles at the allylic position. Subsequent electrophilic activation of the remaining alkylzirconium bond then furnishes the product (Figure 3c). Although currently stoichiometric amounts of zirconium reagent have to be employed, these results might pave the way for the implementation of a catalytic methodology. Furthermore, the development of titanium-based protocols appears desirable under sustainability viewpoints considering the natural abundance of both elements.
Ruthenium, Rhodium, and Iridium Catalysis
In 1974, Wells and co-workers described the isomerization of 1-pentene to 2-pentene under homogeneous ruthenium catalysis.21 Following these initial findings, different research groups investigated this transformation in more detail and eventually discovered that internal olefins undergo this transformation as well. Furthermore, it was also found that α,β-unsaturated carbonyls readily underwent deconjugative isomerization, favoring the formation of enol ethers.22 The isomerization of olefins over a large number of positions has also been achieved utilizing a bifunctional catalyst via the outer-sphere 1,3-hydride shift mechanism. In the presence of this “alkene-zipper” catalyst, olefin isomerizations over more than 30 positions became possible (Figure 4a).23 In combination with an olefin metathesis catalyst (W-1), a tandem olefin isomerization/metathesis sequence has been applied for the synthesis of long-chain olefins (Figure 4b)24 or phenylpropenoids.25
Figure 4.
Ru and Rh “chain-walking” via olefin isomerization: (a) isomerization of alkenyl alcohols with the alkene zipper catalyst; (b) tandem Ru-catalyzed isomerization–W-catalyzed olefin metathesis reactions; (c) tandem Rh-catalyzed isomerization–hydroformylation reaction and functionalization at the terminal position.
With the finding of Wilkinson and Osborn that PPh3 modified rhodium catalysts undergo hydroformylation reactions of terminal olefins,26,27 this field underwent impressive developments in the past 50 years.28 In the past two decades,29 catalytic systems have been developed that favor an isomerization/hydroformylation sequence, even starting from mixtures of olefins, to furnish linear aldehydes and derivatives thereof (Figure 4c).30−32 Furthermore, a rhodium-catalyzed olefin isomerization/Michael addition sequence has been developed based on the Rh/BIPHEPHOS system (Figure 5a).33 Based on earlier findings that cationic iridium complexes in the presence of hydrogen readily promote olefin isomerization,34,35 a protocol for the tandem isomerization/enantioselective allylation was developed.36 Under the same reaction conditions, a tandem hydroboration/isomerization/allylation sequence was demonstrated as well (Figure 5b).
Figure 5.
Rh or Ir “chain-walk”/C–C bond formation: (a) tandem Rh-catalyzed isomerization–conjugate addition reactions; (b) tandem Ir-catalyzed isomerization −allylation reactions.
As can be seen from these examples, the true potential of long-range olefin isomerization can be unleashed when coupled with an ensuing coupling reaction. Furthermore, the catalytic systems employed demonstrate a reasonable chemoselectivity, allowing a variety of tandem reactions to be conducted.
Iron Catalysis
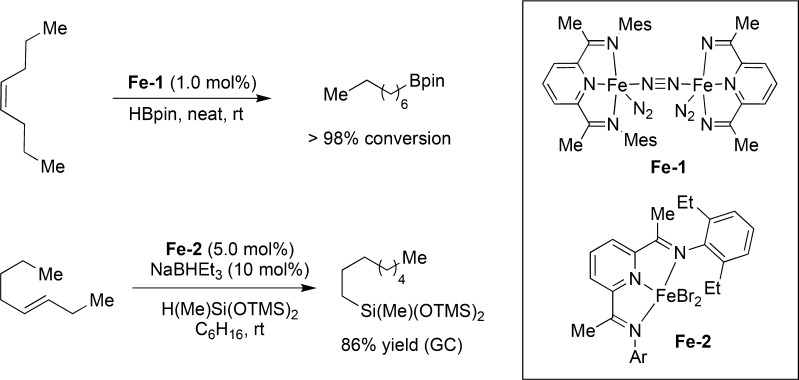
Since its disclosure in 1964,37 the iron-catalyzed olefin isomerization has witnessed renewed interest in the past decade.38,39 With continuing efforts being made toward more sustainable catalytic systems, iron catalysts offer a promising alternative to late transition metals. Although to date only a few examples on “chain-walking” have been reported, this field will likely witness important advancements in the upcoming years. In 2013, a series of mild iron-catalyzed olefin isomerization/hydroborations and hydrosilylations of a variety of internal olefins were reported.40,41 The corresponding iron complexes show similar reactivity although their high sensitivity toward air and moisture render them particularly difficult to handle. Therefore, a protocol for in situ activation of an iron precursor has been developed and applied to a tandem dehydrogenation/isomerization/hydroboration sequence (Figure 6).42
Figure 6.
Fe “chain-walking” hydroboration(hydrosilylation) of internal olefins.
Cobalt Catalysis
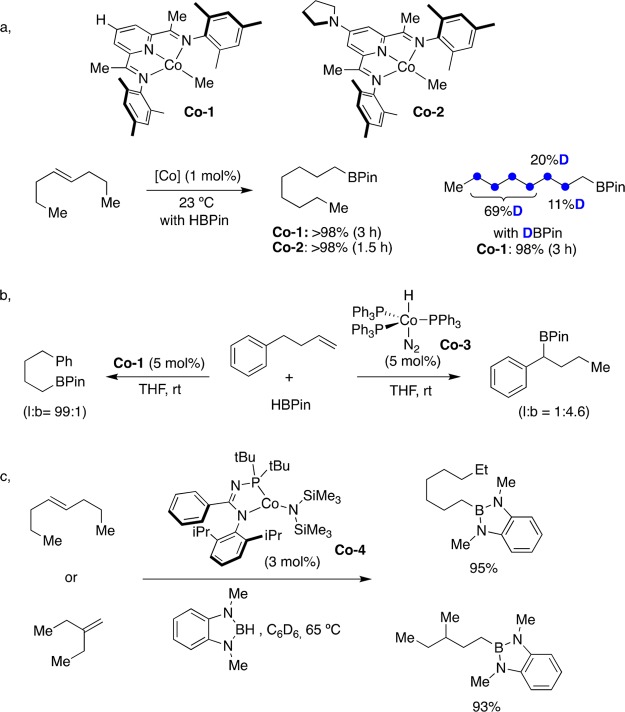
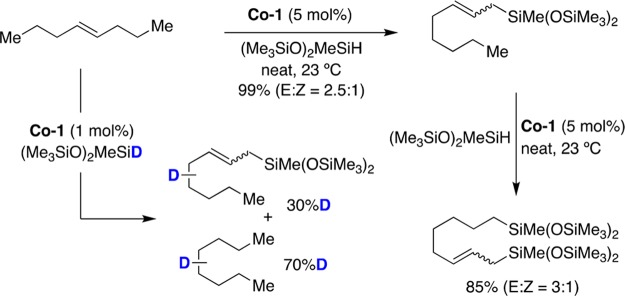
Prompted by Brookhart’s studies on the hydrosilylation of alkenes with cyclopentadienyl cobalt species,43 a Co-catalyzed isomerization/hydroboration of alkenes using pinacolborane (HBPin) with bis(imino)pyridine ligands was reported (Figure 7a).44 The utilization of aliphatic internal olefins resulted in the formation of hydroboration products where the BPin residue was invariably located at the terminal reaction site. The presence of electron-donating amino groups at the para position of the pyridine ring in the ligand culminated in more active catalysts (Co-2), which were able to convert cis- and/or trans-4-octene into 1-octylboronic ester at room temperature with excellent yields and linear selectivity. Labeling experiments showed deuterium incorporation at various positions on the aliphatic chain, suggesting a chain-walking migration via consecutive β-hydride elimination/olefin reinsertion. The reaction was later extended to more challenging hindered tri- and tetrasubstituted alkenes by using redox-active terpyridine and α-diimine ligands.45 Later on, it was univocally shown that site selectivity could be tuned and controlled depending on the ligand backbone.46 Specifically, terminal olefins decorated with a pending arene underwent selective C–B bond-forming reaction at the benzylic position with [(PPh3)3CoH(N2)] (Co-3) whereas the employment of Co-1 supported by nitrogen donors gave rise to linear-selective borylation (Figure 7b). The utilization of N-phosphinoamidinate ligands has been shown to be particularly suited in cobalt-catalyzed hydroboration of unactivated olefins via Co-4 (Figure 7c).47 Unlike other nitrogen donors, the isomerization/hydroboration was not limited to HBpin and could be extended to 1,3-dimethyl-1,3-diaza-2-boracyclopentane or benzo-1,3,2-diazaborolane with equal ease.48 The dichotomy exerted by the boron source was further illustrated by the utilization of 2-ethyl-1-butene as counterpart; while HBPin resulted in a mixture of linear alkylboronic esters, clean C–B bond formation at the less-hindered site was achieved with benzo-1,3,2-diazaborolane.
Figure 7.
Co-catalyzed “chain-walking” hydroboration events: (a) tandem Co-catalyzed isomerization–hydroboration reactions; (b) effect of the Co catalyst on the regioselectivity of the isomerization; (c) Co-catalyzed isomerization–hydroboration reactions with a N-phosphinoamidinate ligand.
Cobalt complexes have shown a superior performance in olefin isomerization/hydrosilylation when compared to precious metals used for similar means,49,50 and intriguing cooperative effects of well-defined Co(I) catalysts supported by β-diketiminate ligands were described.51,52 In 2014, a dehydrogenative silylation of alkenes by using bis(imino)pyridine cobalt complexes was discovered, ending up in allylsilanes with the C–Si bond formation occurring exclusively at the terminal reaction site (Figure 8).53 A sequential site-selective dehydrogenative silylation was within reach, offering an opportunity to promote multiple C–Si bond-forming reactions from simple precursors. Stoichiometric experiments revealed the initial formation of Co–SiR3 active species with concomitant formation of methane upon exposure of the cobalt complex Co-1 to R3SiH. Preferential formation of the allyl- versus vinylsilane was consistent with a preferred β-hydride elimination distal to the bulky tertiary silyl group.
Figure 8.
Co-catalyzed “chain-walking” hydrosilylation.
The involvement of cobalt catalysts in chain-walking scenarios is by no means limited to hydroboration or hydrosilylation reactions. Indeed, C–C bond formations at distal reaction sites could also be achieved via the intermediacy of cobalt hydride species generated by C–H activation54 in the presence of indole scaffolds decorated with imine directing groups followed by a sequence of olefin insertion/β-hydride elimination (Figure 9).55 Subtle changes in the reaction conditions resulted in a regiodivergent scenario with unactivated olefins possessing a pending arene on the side chain, accessing either linear or branched products with excellent site selectivities. Specifically, the utilization of N-heterocyclic carbenes at 60 °C allowed for an olefin isomerization en route to the thermodynamically more stable styrene prior to C–C bond formation at the benzylic position, whereas linear selectivity was observed in the absence of N-heterocyclic carbene with tBuCH2MgBr as promoter. While the role of the ligand still remains unclear, the differences on both tBuCH2MgBr and CyMgBr are likely attributed to the presence of β-hydrogens in the latter, thus setting the basis for the generation of cobalt hydride species. Recently, the generality of cobalt bis(imino)pyridine complexes in chain-walking scenarios was extended by showing the viability to conduct hydroaluminations of internal olefins via an initial isomerization prior to C–Al bond formation.56
Figure 9.
Co-catalyzed “chain-walking” via olefin isomerization/C–C bond formation.
Palladium Catalysis
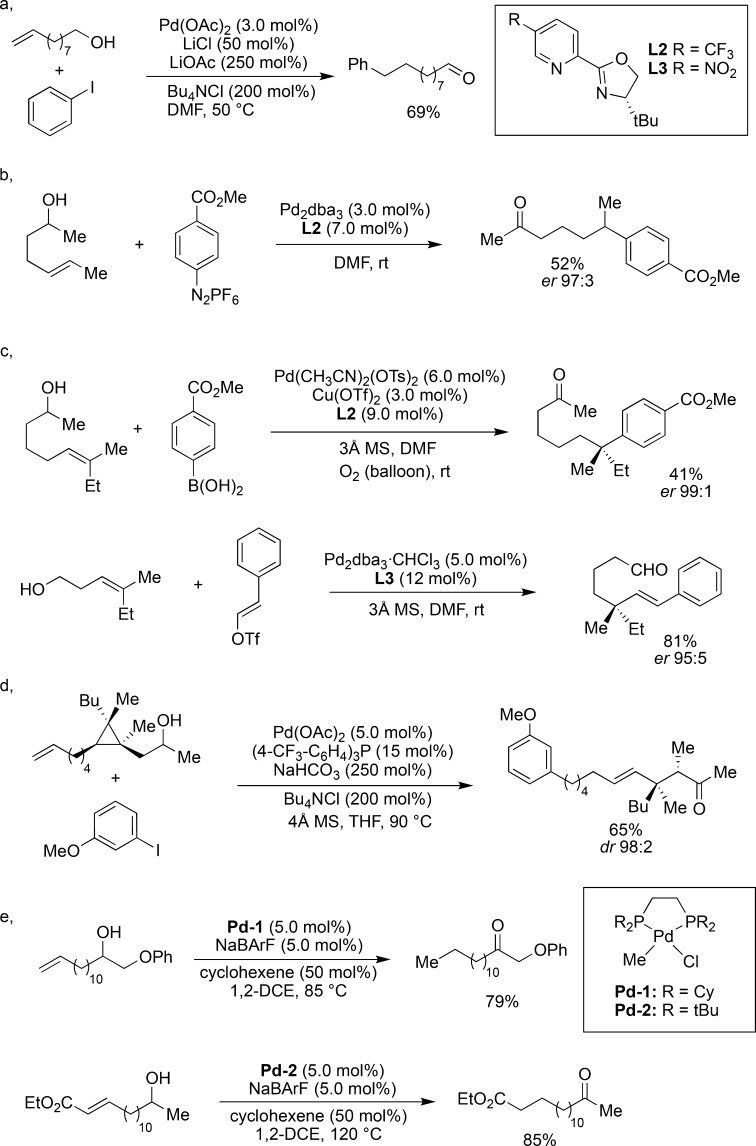
Despite the rapid development of a range of catalytic systems to induce remote functionalization via “chain-walking”, this area of research is largely dominated by palladium-based catalysts. The ready availability of palladium complexes, mild reaction conditions, and high functional group tolerance are key factors for its popularity. Additionally, most palladium (pre)catalysts can easily be handled outside a glovebox, advocating their robustness and user-friendliness. The strong propensity of alkyl–palladium complexes to undergo β-hydride elimination is a well-studied phenomenon in organic chemistry with which every practitioner is familiar, and ligands favoring this elimination came recently into the focus of research. “Chain-walking” or “chain-running” palladium (and nickel catalysts) had already made their way into polymerization chemistry but were largely neglected in organic synthesis. The first reports on palladium-catalyzed Heck reaction combined with a metal-walk on ω-alkenols were disclosed in 1976 providing low yields of a mixture of products under harsh reaction conditions.57−59 These early examples were subsequently extended to the remote functionalization of longer-chain olefinic alcohols to yield the corresponding carbonyls (Figure 10a).60 Later, a protocol for the enantioselective remote functionalization of olefinic alcohols was disclosed utilizing chiral PyrOx ligands under mild conditions61 (Figure 10b). Such a transformation enables the formation of acyclic quaternary stereocenters in high enantioselectivity, a long-standing challenge in organic synthesis, from trisubstituted olefins using different coupling partners such as diazonium salts, boronic acids, or vinyl triflates (Figure 10c).62−71 A similar protocol was found to promote the remote functionalization of olefinic alcohols comprising a cyclopropane in the side chain with concomitant selective ring cleavage (Figure 10d).72 Alternatively, the “chain-walking” can also be initiated by an in situ formed palladium hydride, resulting in an overall long-range redox isomerization of olefinic alcohols.73 Deconjugative redox isomerization starting from α,β-unsaturated carbonyls has been disclosed as well (Figure 10e).74
Figure 10.
Pd-catalyzed “chain-walking” via C–C bond formations terminated by carbonyl formation: (a) Pd-catalyzed isomerization of alkenyl alcohols; (b) enantioselective Pd-catalyzed isomerization of alkenols; (c) enantioselective Heck reaction on trisubstituted alkenols; (d) palladium-catalyzed Heck isomerization as a trigger for selective ring-opening of cyclopropanes; (e) isomerization of unsaturated alcohols promoted by metal–hydride catalysts.
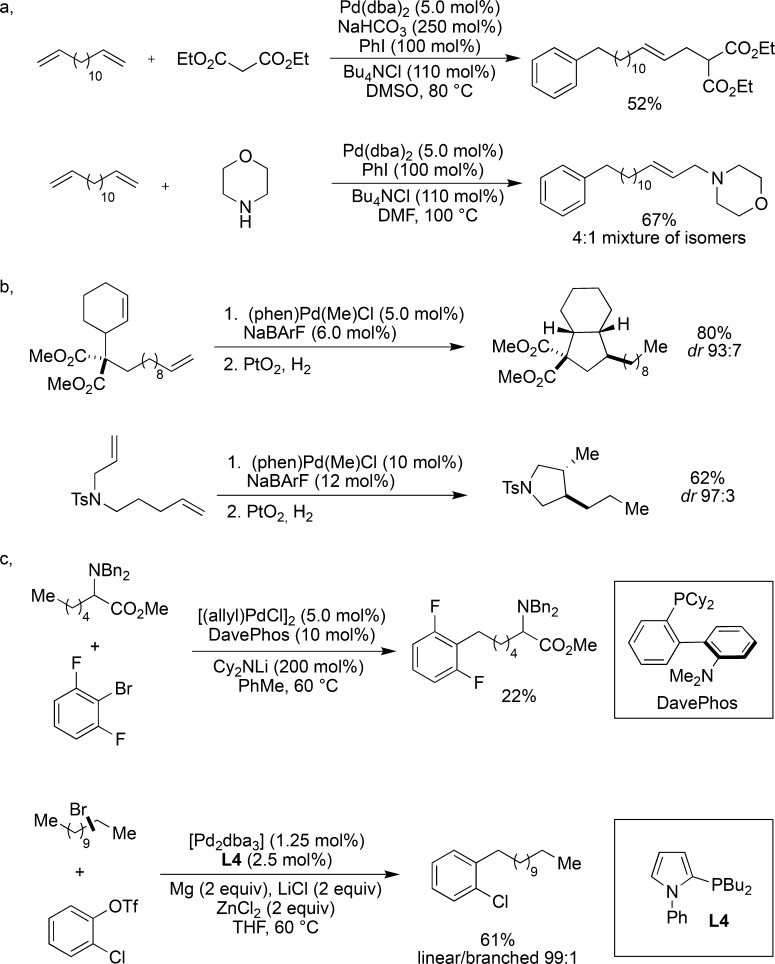
Instead of relying on carbonyl formation as the terminating event, other strategies exploited the increased stability of allylpalladium species opposed to the initial alkylpalladium. Nucleophilic trapping was accomplished with a variety of carbon and heteroatom nucleophiles giving rise to remotely bisfunctionalized products.75−77 This strategy was also utilized in the total syntheses of pyridine alkaloids (Figure 11a).78 If the substrate contains a strategically positioned olefin, an ensuing cyclization of the palladium intermediate gives rise to various cyclopentane scaffolds. Although this catalytic system has been extensively utilized in the cycloisomerizations of 1,6-dienes,79,80 this methodology was only recently extended to a tandem sequence of “chain-walking”/cyclization (Figure 11b).81,82 Contrary to the examples presented above, it has also been shown that the palladium(II) species generated from oxidative insertion can undergo “chain-walking” prior to reductive elimination. This strategy was successfully applied to the remote arylation of amino acid derivatives.83 Later it was demonstrated that a linear selective Negishi-type cross-coupling was also feasible starting from mixtures of branched alkyl bromides (Figure 11c).84
Figure 11.
Pd-catalyzed “chain-walking” via C–C bond formation by nonterminating carbonyl formation: (a) Pd-catalyzed tandem isomerization–nucleophilic addition; (b) palladium-catalyzed tandem isomerization–cyclization reactions; (c) Pd-catalyzed isomerization–functionalization via either an initial C–H activation or oxidative addition reaction.
Nickel Catalysis
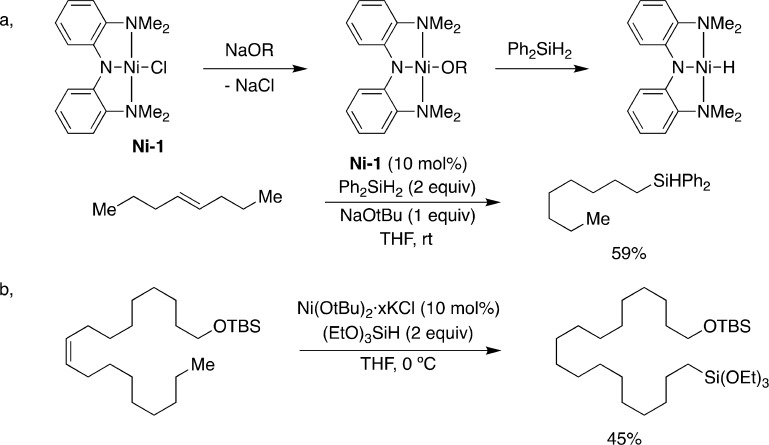
Prompted by the prospective impact of nickel catalysts in the Shell higher olefin process (SHOP) for the homo- and copolymerization of ethylene or α-olefins85,86 as well as the Dupont process for preparing adiponitrile via hydrocyanation of butadiene,87 the past years have witnessed an increasing interest in nickel-catalyzed olefin isomerization as a vehicle for remote functionalization. In 2015, a mild chemoselective alkene isomerization/hydrosilylation catalyzed by nickel pincer complexes was reported in which the putative nickel hydride species were generated upon exposure to both metal alkoxides and silanes (Figure 12a).88,89 A significant improvement was observed with nickel nanoparticles, allowing the scope of these reactions to be extended to tertiary silanes such as (EtO)3SiH (Figure 12b). In line with previous findings with both iron and cobalt catalysis, redox-active α-diimine ligands were found to be competent in nickel-catalyzed hydrosilylation reactions. Labeling studies with (EtO)3SiD and 1-octene resulted in deuterium incorporation at multiple sites throughout the alkyl side chain.90 This seemingly innocent experiment showed the inherent ability of nickel catalysts to promote an olefin isomerization prior to C–Si bond formation, an observation that could further be corroborated by observing linear hydrosilylation with 4-octene as counterpart.
Figure 12.
Ni-catalyzed “chain-walking” hydrosilylations of internal olefins: (a) Ni-pincer complex catalyzed isomerization–silylation reactions; (b) Ni-nanoparticle catalyzed isomerization–silylation reactions.
The first example of a nickel-catalyzed olefin isomerization/hydroarylation of alkenes was disclosed via initial C–H functionalization of heteroarenes containing particularly acidic C–H bonds and N-heterocyclic carbenes as ligand donors (Figure 13a).90 Intriguingly, the presence of AlMe3 inhibited olefin isomerization, obtaining linear-selective hydroarylation products. A related hydroarylation reaction but with electron-deficient arenes was also reported (Figure 13b).91 Although this reaction formally falls into the category of a classical “chain walk”, labeling studies and theoretical calculations left a reasonable doubt about the intermediacy of nickel hydride intermediates, arguing against an initial oxidative addition into the C–H bond. The authors suggested that the origin of the linear selectivity arises from the C–C bond-forming reductive elimination rather than the difference in stabilities of the linear versus branched alkyl metal complexes. More recently, a site-selective linear alkylation of anilides using nickel catalysts and aluminum-based Lewis acids was reported with 2-octene as coupling partner.92
Figure 13.
Ni-catalyzed “chain-walking” hydroarylation initiated by formal C–H activation: (a) Ni-catalyzed olefin isomerization–hydroarylation of alkenes; (b) tandem isomerization–hydroarylation reactions using a N-heterocyclic carbene Ni(0) complex.
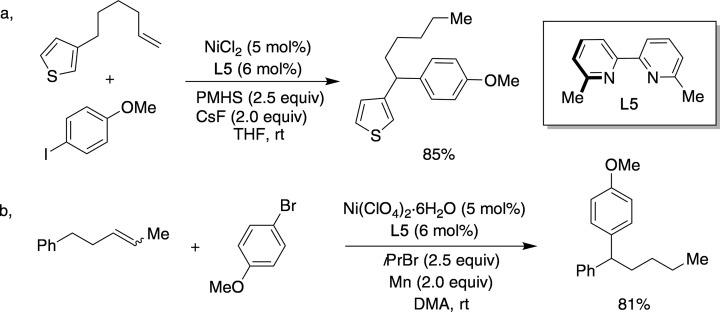
Aryl halides can be readily utilized as counterparts in nickel-catalyzed olefin isomerization followed by site-selective C–C bond formation. In early 2017, a site-selective catalytic hydroarylation of alkenes occurring exclusively at benzylic sp3 C–H sites under a Ni/bipyridine regime was reported, using polymethylhydrosiloxane (PMHS) both as reducing agent and as hydride source (Figure 14a).93 Prompted by a recent report that used light alkyl bromides as hydride sources via β-hydride elimination in nickel-catalyzed hydroamidations of alkynes,94 an alternative hydroarylation technique with comparable yields and selectivities to those observed in their preceding protocol based on PMHS was subsequently reported (Figure 14b).95 Such a protocol could be extended to a formal cross-electrophile remote functionalization by using organic halides in lieu of olefin partners.96
Figure 14.
Ni-catalyzed “chain-walking” hydroarylation with aryl halide counterparts: (a) Ni-catalyzed hydroarylation at benzylic sp3 C–H sites; (b) Ni-catalyzed hydroarylation with aryl bromides.
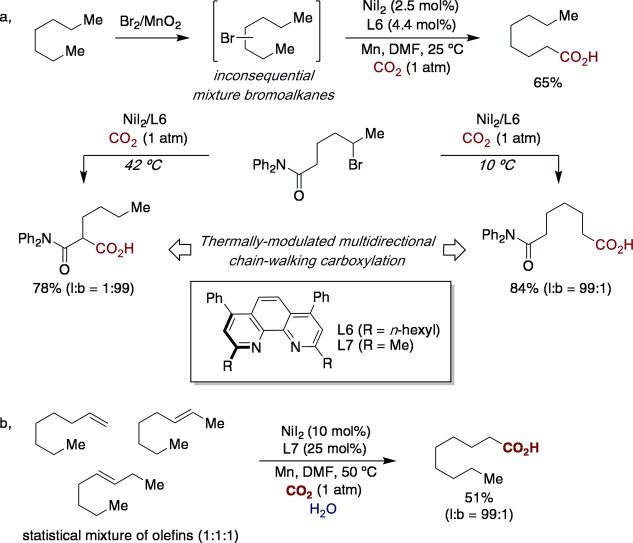
In the past decade, nickel-catalyzed cross-coupling reactions of unactivated alkyl halides have matured into robust and reliable technologies for introducing saturated hydrocarbon side chains into organic backbones.97 These reactions invariably occur at the initial sp3 C–halide bond via formal functional group interconversion, contributing to the perception that a nickel-catalyzed “chain-walk” of unactivated alkyl halides initiated by oxidative addition would be particularly problematic. In 2017, a remote sp3 C–H carboxylation of unactivated alkyl bromides under atmospheric pressure of carbon dioxide (CO2) was designed (Figure 15a).98 Unlike related nickel-catalyzed “chain-walking” hydroarylation events,93,94 this transformation occurred selectively at the primary sp3 C–H bond, even in the presence of arenes on the side chain, thus offering an opportunity to activate less-reactive reactive sites in the presence of a priori more reactive ones. A one-pot bromination/carboxylation sequence could be used as a platform for directly converting alkanes or unrefined mixtures of olefins into fatty acids as single regioisomers. Site selectivity could be tuned and controlled among different sp3 C–H bonds by a subtle thermal modulation in the presence of carbonyl groups on the side chain. Intriguingly, no significant erosion in enantioselectivity was observed in the presence of stereogenic centers, tacitly suggesting that the nickel catalyst remains bound to the olefin intermediate throughout the “chain-walk” along the hydrocarbon side chain.
Figure 15.
Ni-catalyzed “chain-walking” for remote carboxylation of hydrocarbons with CO2: (a) Ni-catalyzed remote sp3 C–H carboxylation of unactivated alkyl bromides; (b) water as an inexpensive hydride source for the remote carboxylation of alkenes.
Subsequently, it has recently been shown that water—typically used as proton source—can be harnessed as a safe, inexpensive hydride source in a remote carboxylation at sp3 C–H sites of unrefined mixtures of olefins under atmospheric pressure of CO2 (Figure 15b).98 Such a method offered an opportunity to formally repurpose three renewable chemical feedstocks (olefins, CO2, and water) while reducing the chemical footprint of the hydride source in “chain-walking” scenarios by using water in lieu of high molecular weight silanes or organometallic reagents.
Summary and Outlook
The functionalization of distal reaction sites has recently gained considerable momentum in the community. This popularity is largely due to the notion that prefunctionalization is not required at the targeted reaction site, thus changing prevailing perceptions in synthetic organic chemistry when building up molecular complexity. Among the various conceivable scenarios, including directing group methodologies, radical-type pathways, or enzymatic protocols, the ability of a metal to cause a dynamic displacement throughout a saturated hydrocarbon chain (“chain-walk”) is particularly appealing. This interest arises from the utilization of simple hydrocarbons as starting precursors, renewable feedstocks of utmost relevance for our chemical industry, and the avoidance of directing groups that are not particularly trivial to modify. The key aspect of “chain-walking” scenarios relies on the utilization of competent organometallic species that trigger a rapid olefin isomerization prior to the subsequent C–C or C–heteroatom bond formation at a previously unfunctionalized reaction site.
The wealth of recent literature on catalytic “chain-walking” reactions suggests that these rather unconventional methodologies represent a powerful alternative to existing protocols for promoting functionalization of distal reaction sites. As for other cross-coupling reactions, the role exerted by the supporting ligand to fine-tune the properties of putative reaction intermediates is rather critical. Indeed, such modulation can result in a tunable and controllable multidirectional “chain-walking”, enabling regiodivergent scenarios via site-selective discrimination among differently substituted remote reaction sites on a hydrocarbon chain. A wide number of daunting challenges remain, however. Among these, the development of enantioselective chain-walking scenarios is still at its infancy, with the available portfolio indicating that the chiral center should be introduced at a reaction site that originally contained an olefin residue. From both a conceptual and practical standpoint, it would be particularly attractive to set the chirality at a previously unfunctionalized reaction site or the desymmetrization of a prochiral substrate through the “chain-walking event”. Likewise, the discovery of new chemical knowledge occurring at the boundaries between classical organometallic catalysis and radical pathways or enzyme catalysis will likely invigorate the field by borrowing the best features associated with these disciplines.
Despite the elegant empirical discoveries realized in “chain-walking” reactions, however, the mechanisms by which many of these reactions operate remain, in most instances, rather speculative and are based on indirect evidence by isotope labeling. This is probably attributed to the in situ formation of short-lived, yet exceptionally sensitive, organometallic entities that render their isolation and characterization a rather challenging task. Undoubtedly, efforts toward this goal will have a significant impact on the field of “chain-walking”, as understanding the mechanistic intricacies at a molecular level will enable the design of conceptually novel technologies not apparent at first sight. In view of the recent meteoric development in “chain-walking” strategies, it is inevitable to predict spectacular progress in the years to come, providing a technological push that will change prevailing dogmas in retrosynthetic analysis.
Acknowledgments
We thank ICIQ, European Research Council under the Seventh Framework Program of the European Community for the Israeli PI (ERC Grant Agreement 338912) and MINECO (CTQ2015-65496-R & Severo Ochoa Accreditation SEV-2013-0319) for financial support. I.M. is holder of the Sir Michael and Lady Sobell Academic Chair. H.S. acknowledges the Max-Planck-Society for a Minerva Fellowship.
Author Contributions
‡ H.S. and F.J.-H. contributed equally to this work.
The authors declare no competing financial interest.
References
- Breslow R. Centenary Lecture. Biomimetic chemistry. Chem. Soc. Rev. 1972, 1 (4), 553. 10.1039/cs9720100553. [DOI] [Google Scholar]
- Breslow R. Biomimetic control of chemical selectivity. Acc. Chem. Res. 1980, 13 (6), 170. 10.1021/ar50150a002. [DOI] [Google Scholar]
- Schwarz H. Remote functionalization of C-H and C-C bonds by ″naked″ transition-metal ions (Cosi Fan Tutte). Acc. Chem. Res. 1989, 22 (8), 282. 10.1021/ar00164a004. [DOI] [Google Scholar]
- Labinger J. A.; Bercaw J. E. Understanding and exploiting C–H bond activation. Nature 2002, 417, 507. 10.1038/417507a. [DOI] [PubMed] [Google Scholar]
- Bergman R. G. C–H activation. Nature 2007, 446, 391. 10.1038/446391a. [DOI] [PubMed] [Google Scholar]
- Gensch T.; Hopkinson M. N.; Glorius F.; Wencel-Delord J. Mild metal-catalyzed C-H activation: examples and concepts. Chem. Soc. Rev. 2016, 45 (10), 2900. 10.1039/C6CS00075D. [DOI] [PubMed] [Google Scholar]
- Davies H. M. L.; Morton D. Collective Approach to Advancing C–H Functionalization. ACS Cent. Sci. 2017, 3 (9), 936. 10.1021/acscentsci.7b00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. F.; Larsen M. A. Undirected, Homogeneous C–H Bond Functionalization: Challenges and Opportunities. ACS Cent. Sci. 2016, 2 (5), 281. 10.1021/acscentsci.6b00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S. Mechanistic Understanding of Transition-Metal-Catalyzed Olefin Isomerization: Metal-Hydride Insertion-Elimination vs. π-Allyl Pathways. Comments Inorg. Chem. 2015, 35 (6), 300. 10.1080/02603594.2015.1059325. [DOI] [Google Scholar]
- Crabtree R. H. In The Organometallic Chemistry of the Transition Metals; John Wiley & Sons, Inc.: New Haven, CT, 2014; p 224. [Google Scholar]
- Finkbeiner H.; Cooper G. Titanium-Catalyzed Isomerization and Olefin-Exchange Reactions of Alkylmagnesium Halides: A Novel Method for Preparation of the Grignard Reagent. J. Org. Chem. 1961, 26 (11), 4779. 10.1021/jo01069a607. [DOI] [Google Scholar]
- Cooper G. D.; Finkbeiner H. L. Titanium-Catalyzed Rearrangement and Olefin-Exchange of Grignard Reagents1. J. Org. Chem. 1962, 27 (5), 1493. 10.1021/jo01052a001. [DOI] [Google Scholar]
- Prosenc M.-H.; Brintzinger H.-H. Zirconium–Alkyl Isomerizations in Zirconocene-Catalyzed Olefin Polymerization: A Density Functional Study. Organometallics 1997, 16 (18), 3889. 10.1021/om970227b. [DOI] [Google Scholar]
- Carr D. B.; Schwartz J. Preparation of organoaluminum compounds by hydrozirconation-transmetalation. J. Am. Chem. Soc. 1979, 101 (13), 3521. 10.1021/ja00507a017. [DOI] [Google Scholar]
- Buchwald S. L.; LaMaire S. J.; Nielsen R. B.; Watson B. T.; King S. M. A Modified Procedure for the Preparation of Cp2Zr(H)Cl (Schwartz’s Reagent). Tetrahedron Lett. 1987, 28 (34), 3895. 10.1016/S0040-4039(00)96413-X. [DOI] [Google Scholar]
- Negishi E.-I.; Takahashi T. Patterns of Stoichiometric and Catalytic Reactions of Organozirconium and Related Complexes of Synthetic Interest. Acc. Chem. Res. 1994, 27 (5), 124. 10.1021/ar00041a002. [DOI] [Google Scholar]
- Marek I.; Chinkov N.; Levin A. A Zirconium Promenade - An Efficient Tool in Organic Synthesis. Synlett 2006, 2006 (04), 0501. 10.1055/s-2006-932483. [DOI] [Google Scholar]
- Mola L.; Sidera M.; Fletcher S. P. Asymmetric Remote C–H Functionalization: Use of Internal Olefins in Tandem Hydrometallation–Isomerization–Asymmetric Conjugate Addition Sequences. Aust. J. Chem. 2015, 68 (3), 401. 10.1071/CH14556. [DOI] [Google Scholar]
- Masarwa A.; Didier D.; Zabrodski T.; Schinkel M.; Ackermann L.; Marek I. Merging allylic carbon-hydrogen and selective carbon-carbon bond activation. Nature 2014, 505 (7482), 199. 10.1038/nature12761. [DOI] [PubMed] [Google Scholar]
- Vasseur A.; Perrin L.; Eisenstein O.; Marek I. Remote functionalization of hydrocarbons with reversibility enhanced stereocontrol. Chem. Sci. 2015, 6 (5), 2770. 10.1039/C5SC00445D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham D.; Webster D. E.; Wells P. B. Homogeneous catalysis of olefin isomerisation. Part V. Pent-1-ene isomerisation catalysed by solutions of RuHCl(PPh3)3 and of RuHCl(CO)(PPh3)3; variation of the isomeric composition of pent-2-ene and its attribution to steric factors. J. Chem. Soc., Dalton Trans. 1974, 14, 1519. 10.1039/dt9740001519. [DOI] [Google Scholar]
- Wakamatsu H.; Nishida M.; Adachi N.; Mori M. Isomerization Reaction of Olefin Using RuClH(CO)(PPh3)3. J. Org. Chem. 2000, 65 (13), 3966. 10.1021/jo9918753. [DOI] [PubMed] [Google Scholar]
- Grotjahn D. B.; Larsen C. R.; Gustafson J. L.; Nair R.; Sharma A. Extensive Isomerization of Alkenes Using a Bifunctional Catalyst: An Alkene Zipper. J. Am. Chem. Soc. 2007, 129 (31), 9592. 10.1021/ja073457i. [DOI] [PubMed] [Google Scholar]
- Dobereiner G. E.; Erdogan G.; Larsen C. R.; Grotjahn D. B.; Schrock R. R. A One-Pot Tandem Olefin Isomerization/Metathesis-Coupling (ISOMET) Reaction. ACS Catal. 2014, 4 (9), 3069. 10.1021/cs500889x. [DOI] [Google Scholar]
- Higman C. S.; de Araujo M. P.; Fogg D. E. Tandem catalysis versus one-pot catalysis: ensuring process orthogonality in the transformation of essential-oil phenylpropenoids into high-value products via olefin isomerization-metathesis. Catal. Sci. Technol. 2016, 6 (7), 2077. 10.1039/C5CY02038G. [DOI] [Google Scholar]
- Osborn J. A.; Wilkinson G.; Young J. F. Mild hydroformylation of olefins using rhodium catalysts. Chem. Commun. 1965, (2), 17. 10.1039/c19650000017. [DOI] [Google Scholar]
- Evans D.; Osborn J. A.; Wilkinson G. Hydroformylation of alkenes by use of rhodium complex catalysts. J. Chem. Soc. A 1968, 0, 3133. 10.1039/j19680003133. [DOI] [Google Scholar]
- Franke R.; Selent D.; Börner A. Applied Hydroformylation. Chem. Rev. 2012, 112 (11), 5675. 10.1021/cr3001803. [DOI] [PubMed] [Google Scholar]
- Vilches-Herrera M.; Domke L.; Börner A. Isomerization–Hydroformylation Tandem Reactions. ACS Catal. 2014, 4 (6), 1706. 10.1021/cs500273d. [DOI] [Google Scholar]
- Behr A.; Obst D.; Schulte C.; Schosser T. Highly selective tandem isomerization–hydroformylation reaction of trans-4-octene to n-nonanal with rhodium-BIPHEPHOS catalysis. J. Mol. Catal. A: Chem. 2003, 206 (1), 179. 10.1016/S1381-1169(03)00461-8. [DOI] [Google Scholar]
- Seayad A.; Ahmed M.; Klein H.; Jackstell R.; Gross T.; Beller M. Internal Olefins to Linear Amines. Science 2002, 297 (5587), 1676. 10.1126/science.1074801. [DOI] [PubMed] [Google Scholar]
- Edwards D. R.; Crudden C. M.; Yam K. One-Pot Carbon Monoxide-Free Hydroformylation of Internal Olefins to Terminal Aldehydes. Adv. Synth. Catal. 2005, 347 (1), 50. 10.1002/adsc.200404228. [DOI] [Google Scholar]
- Ohlmann D. M.; Gooßen L. J.; Dierker M. Regioselective Synthesis of β-Aryl- and β-Amino-Substituted Aliphatic Esters by Rhodium-Catalyzed Tandem Double-Bond Migration/Conjugate Addition. Chem. - Eur. J. 2011, 17 (34), 9508. 10.1002/chem.201100654. [DOI] [PubMed] [Google Scholar]
- Ohmura T.; Yamamoto Y.; Miyaura N. Stereoselective Synthesis of Silyl Enol Ethers via the Iridium-Catalyzed Isomerization of Allyl Silyl Ethers. Organometallics 1999, 18 (3), 413. 10.1021/om980794e. [DOI] [Google Scholar]
- Nelson S. G.; Bungard C. J.; Wang K. Catalyzed Olefin Isomerization Leading to Highly Stereoselective Claisen Rearrangements of Aliphatic Allyl Vinyl Ethers. J. Am. Chem. Soc. 2003, 125 (43), 13000. 10.1021/ja037655v. [DOI] [PubMed] [Google Scholar]
- Miura T.; Nishida Y.; Morimoto M.; Murakami M. Enantioselective Synthesis of Anti Homoallylic Alcohols from Terminal Alkynes and Aldehydes Based on Concomitant Use of a Cationic Iridium Complex and a Chiral Phosphoric Acid. J. Am. Chem. Soc. 2013, 135 (31), 11497. 10.1021/ja405790t. [DOI] [PubMed] [Google Scholar]
- Manuel T. A. The Iron Carbonyl-Catalyzed Isomerization Of Olefins. Trans. N. Y. Acad. Sci. 1964, 26 (4 Series II), 442–445. 10.1111/j.2164-0947.1964.tb03522.x. [DOI] [Google Scholar]
- Sawyer K. R.; Glascoe E. A.; Cahoon J. F.; Schlegel J. P.; Harris C. B. Mechanism for Iron-Catalyzed Alkene Isomerization in Solution. Organometallics 2008, 27 (17), 4370. 10.1021/om800481r. [DOI] [Google Scholar]
- Shang R.; Ilies L.; Nakamura E. Iron-Catalyzed C–H Bond Activation. Chem. Rev. 2017, 117 (13), 9086. 10.1021/acs.chemrev.6b00772. [DOI] [PubMed] [Google Scholar]
- Obligacion J. V.; Chirik P. J. Highly Selective Bis(imino)pyridine Iron-Catalyzed Alkene Hydroboration. Org. Lett. 2013, 15 (11), 2680. 10.1021/ol400990u. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Peng D.; Leng X.; Huang Z. Iron-Catalyzed, Atom-Economical, Chemo- and Regioselective Alkene Hydroboration with Pinacolborane. Angew. Chem., Int. Ed. 2013, 52 (13), 3676. 10.1002/anie.201210347. [DOI] [PubMed] [Google Scholar]
- Jia X.; Huang Z. Conversion of alkanes to linear alkylsilanes using an iridium–iron-catalysed tandem dehydrogenation–isomerization–hydrosilylation. Nat. Chem. 2016, 8, 157. 10.1038/nchem.2417. [DOI] [PubMed] [Google Scholar]
- Brookhart M.; Grant B. E. Mechanism of a cobalt(III)-catalyzed olefin hydrosilation reaction: direct evidence for a silyl migration pathway. J. Am. Chem. Soc. 1993, 115 (6), 2151. 10.1021/ja00059a008. [DOI] [Google Scholar]
- Obligacion J. V.; Chirik P. J. Bis(imino)pyridine Cobalt-Catalyzed Alkene Isomerization–Hydroboration: A Strategy for Remote Hydrofunctionalization with Terminal Selectivity. J. Am. Chem. Soc. 2013, 135 (51), 19107. 10.1021/ja4108148. [DOI] [PubMed] [Google Scholar]
- Palmer W. N.; Diao T.; Pappas I.; Chirik P. J. High-Activity Cobalt Catalysts for Alkene Hydroboration with Electronically Responsive Terpyridine and α-Diimine Ligands. ACS Catal. 2015, 5 (2), 622. 10.1021/cs501639r. [DOI] [Google Scholar]
- Scheuermann M. L.; Johnson E. J.; Chirik P. J. Alkene Isomerization–Hydroboration Promoted by Phosphine-Ligated Cobalt Catalysts. Org. Lett. 2015, 17 (11), 2716. 10.1021/acs.orglett.5b01135. [DOI] [PubMed] [Google Scholar]
- Ruddy A. J.; Sydora O. L.; Small B. L.; Stradiotto M.; Turculet L. (N-Phosphinoamidinate)cobalt-Catalyzed Hydroboration: Alkene Isomerization Affords Terminal Selectivity. Chem. - Eur. J. 2014, 20 (43), 13918. 10.1002/chem.201403945. [DOI] [PubMed] [Google Scholar]
- Ogawa T.; Ruddy A. J.; Sydora O. L.; Stradiotto M.; Turculet L. Cobalt- and Iron-Catalyzed Isomerization–Hydroboration of Branched Alkenes: Terminal Hydroboration with Pinacolborane and 1,3,2-Diazaborolanes. Organometallics 2017, 36 (2), 417. 10.1021/acs.organomet.6b00823. [DOI] [Google Scholar]
- Karstedt B. D., 1973; U.S. Patent 3715334A.
- Speier J. L.; Webster J. A.; Barnes G. H. The Addition of Silicon Hydrides to Olefinic Double Bonds. Part II. The Use of Group VIII Metal Catalysts. J. Am. Chem. Soc. 1957, 79 (4), 974. 10.1021/ja01561a054. [DOI] [Google Scholar]
- Chen C.; Hecht M. B.; Kavara A.; Brennessel W. W.; Mercado B. Q.; Weix D. J.; Holland P. L. Rapid, Regioconvergent, Solvent-Free Alkene Hydrosilylation with a Cobalt Catalyst. J. Am. Chem. Soc. 2015, 137 (41), 13244. 10.1021/jacs.5b08611. [DOI] [PubMed] [Google Scholar]
- Noda D.; Tahara A.; Sunada Y.; Nagashima H. Non-Precious-Metal Catalytic Systems Involving Iron or Cobalt Carboxylates and Alkyl Isocyanides for Hydrosilylation of Alkenes with Hydrosiloxanes. J. Am. Chem. Soc. 2016, 138 (8), 2480. 10.1021/jacs.5b11311. [DOI] [PubMed] [Google Scholar]
- Atienza C. C. H.; Diao T.; Weller K. J.; Nye S. A.; Lewis K. M.; Delis J. G. P.; Boyer J. L.; Roy A. K.; Chirik P. J. Bis(imino)pyridine Cobalt-Catalyzed Dehydrogenative Silylation of Alkenes: Scope, Mechanism, and Origins of Selective Allylsilane Formation. J. Am. Chem. Soc. 2014, 136 (34), 12108. 10.1021/ja5060884. [DOI] [PubMed] [Google Scholar]
- Ilies L.; Chen Q.; Zeng X.; Nakamura E. Cobalt-Catalyzed Chemoselective Insertion of Alkene into the Ortho C–H Bond of Benzamide. J. Am. Chem. Soc. 2011, 133 (14), 5221. 10.1021/ja200645w. [DOI] [PubMed] [Google Scholar]
- Yamakawa T.; Yoshikai N. Alkene Isomerization–Hydroarylation Tandem Catalysis: Indole C2-Alkylation with Aryl-Substituted Alkenes Leading to 1,1-Diarylalkanes. Chem. - Asian J. 2014, 9 (5), 1242. 10.1002/asia.201400135. [DOI] [PubMed] [Google Scholar]
- Weliange N. M.; McGuinness D. S.; Gardiner M. G.; Patel J. Cobalt-bis(imino)pyridine complexes as catalysts for hydroalumination-isomerisation of internal olefins. Dalton Transactions 2016, 45 (26), 10842. 10.1039/C6DT01113F. [DOI] [PubMed] [Google Scholar]
- Chalk A. J.; Magennis S. A. Palladium-catalyzed vinyl substitution reactions. I. New synthesis of 2- and 3-phenyl-substituted allylic alcohols, aldehydes, and ketones from allylic alcohols. J. Org. Chem. 1976, 41 (2), 273. 10.1021/jo00864a018. [DOI] [Google Scholar]
- Chalk A. J.; Magennis S. A. Palladium-catalyzed vinyl substitution reactions. II. Synthesis of aryl substituted allylic alcohols, aldehydes, and ketones from aryl halides and unsaturated alcohols. J. Org. Chem. 1976, 41 (7), 1206. 10.1021/jo00869a026. [DOI] [Google Scholar]
- Melpolder J. B.; Heck R. F. Palladium-catalyzed arylation of allylic alcohols with aryl halides. J. Org. Chem. 1976, 41 (2), 265. 10.1021/jo00864a017. [DOI] [Google Scholar]
- Larock R. C.; Leung W.-Y.; Stolz-Dunn S. Synthesis of aryl-substituted aldehydes and ketones via palladium-catalyzed coupling of aryl halides and non-allylic unsaturated alcohols. Tetrahedron Lett. 1989, 30 (48), 6629. 10.1016/S0040-4039(00)70636-8. [DOI] [Google Scholar]
- Werner E. W.; Mei T.-S.; Burckle A. J.; Sigman M. S. Enantioselective Heck Arylations of Acyclic Alkenyl Alcohols Using a Redox-Relay Strategy. Science 2012, 338 (6113), 1455. 10.1126/science.1229208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei T.-S.; Werner E. W.; Burckle A. J.; Sigman M. S. Enantioselective Redox-Relay Oxidative Heck Arylations of Acyclic Alkenyl Alcohols using Boronic Acids. J. Am. Chem. Soc. 2013, 135 (18), 6830. 10.1021/ja402916z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton M. J.; Xu L.-P.; Norrby P.-O.; Wu Y.-D.; Wiest O.; Sigman M. S. Investigating the Nature of Palladium Chain-Walking in the Enantioselective Redox-Relay Heck Reaction of Alkenyl Alcohols. J. Org. Chem. 2014, 79 (24), 11841. 10.1021/jo501813d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei T.-S.; Patel H. H.; Sigman M. S. Enantioselective construction of remote quaternary stereocentres. Nature 2014, 508, 340. 10.1038/nature13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; Hilton M. J.; Zhang X.; Norrby P.-O.; Wu Y.-D.; Sigman M. S.; Wiest O. Mechanism, Reactivity, and Selectivity in Palladium-Catalyzed Redox-Relay Heck Arylations of Alkenyl Alcohols. J. Am. Chem. Soc. 2014, 136 (5), 1960. 10.1021/ja4109616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel H. H.; Sigman M. S. Palladium-Catalyzed Enantioselective Heck Alkenylation of Acyclic Alkenols Using a Redox-Relay Strategy. J. Am. Chem. Soc. 2015, 137 (10), 3462. 10.1021/ja5130836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.; Santiago C. B.; Crawford J. M.; Sigman M. S. Enantioselective Dehydrogenative Heck Arylations of Trisubstituted Alkenes with Indoles to Construct Quaternary Stereocenters. J. Am. Chem. Soc. 2015, 137 (50), 15668. 10.1021/jacs.5b11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.; Santiago C. B.; Kou L.; Sigman M. S. Alkenyl Carbonyl Derivatives in Enantioselective Redox Relay Heck Reactions: Accessing α,β-Unsaturated Systems. J. Am. Chem. Soc. 2015, 137 (23), 7290. 10.1021/jacs.5b04289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.-M.; Hilton M. J.; Sigman M. S. Palladium-Catalyzed Enantioselective Redox-Relay Heck Arylation of 1,1-Disubstituted Homoallylic Alcohols. J. Am. Chem. Soc. 2016, 138 (36), 11461. 10.1021/jacs.6b06994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel H. H.; Sigman M. S. Enantioselective Palladium-Catalyzed Alkenylation of Trisubstituted Alkenols To Form Allylic Quaternary Centers. J. Am. Chem. Soc. 2016, 138 (43), 14226. 10.1021/jacs.6b09649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.-M.; Nervig C. S.; DeLuca R. J.; Sigman M. S. Palladium-Catalyzed Enantioselective Redox-Relay Heck Alkynylation of Alkenols To Access Propargylic Stereocenters. Angew. Chem., Int. Ed. 2017, 56 (23), 6651. 10.1002/anie.201703089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.; Bruffaerts J.; Vasseur A.; Marek I. A unique Pd-catalysed Heck arylation as a remote trigger for cyclopropane selective ring-opening. Nat. Commun. 2017, 8, 14200. 10.1038/ncomms14200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larionov E.; Lin L.; Guénée L.; Mazet C. Scope and Mechanism in Palladium-Catalyzed Isomerizations of Highly Substituted Allylic, Homoallylic, and Alkenyl Alcohols. J. Am. Chem. Soc. 2014, 136 (48), 16882. 10.1021/ja508736u. [DOI] [PubMed] [Google Scholar]
- Lin L.; Romano C.; Mazet C. Palladium-Catalyzed Long-Range Deconjugative Isomerization of Highly Substituted α,β-Unsaturated Carbonyl Compounds. J. Am. Chem. Soc. 2016, 138 (32), 10344. 10.1021/jacs.6b06390. [DOI] [PubMed] [Google Scholar]
- Larock R. C.; Lu Y. D.; Bain A. C.; Russell C. E. Palladium-catalyzed coupling of aryl iodides, nonconjugated dienes and carbon nucleophiles by palladium migration. J. Org. Chem. 1991, 56 (15), 4589. 10.1021/jo00015a002. [DOI] [Google Scholar]
- Larock R. C.; Wang Y.; Lu Y.; Russell C. A. Synthesis of Aryl-Substituted Allylic Amines via Palladium-Catalyzed Coupling of Aryl Iodides, Nonconjugated Dienes, and Amines. J. Org. Chem. 1994, 59 (26), 8107. 10.1021/jo00105a030. [DOI] [Google Scholar]
- Albéniz A. C.; Espinet P.; Lin Y.-S. Palladium Migration along Linear Carbon Chains: The Detection of η1-η2-Enyl Intermediates and the Study of Their Rearrangement. Organometallics 1997, 16 (19), 4138. 10.1021/om9703907. [DOI] [Google Scholar]
- Wang Y.; Dong X.; Larock R. C. Synthesis of Naturally Occurring Pyridine Alkaloids via Palladium-Catalyzed Coupling/Migration Chemistry. J. Org. Chem. 2003, 68 (8), 3090. 10.1021/jo026716p. [DOI] [PubMed] [Google Scholar]
- Goj L. A.; Widenhoefer R. A. Mechanistic Studies of the Cycloisomerization of Dimethyl Diallylmalonate Catalyzed by a Cationic Palladium Phenanthroline Complex. J. Am. Chem. Soc. 2001, 123 (45), 11133. 10.1021/ja0108685. [DOI] [PubMed] [Google Scholar]
- Kisanga P.; Goj L. A.; Widenhoefer R. A. Cycloisomerization of Functionalized 1,5- and 1,6-Dienes Catalyzed by Cationic Palladium Phenanthroline Complexes. J. Org. Chem. 2001, 66 (2), 635. 10.1021/jo001596b. [DOI] [PubMed] [Google Scholar]
- Kochi T.; Hamasaki T.; Aoyama Y.; Kawasaki J.; Kakiuchi F. Chain-Walking Strategy for Organic Synthesis: Catalytic Cycloisomerization of 1,n-Dienes. J. Am. Chem. Soc. 2012, 134 (40), 16544. 10.1021/ja308377u. [DOI] [PubMed] [Google Scholar]
- Hamasaki T.; Aoyama Y.; Kawasaki J.; Kakiuchi F.; Kochi T. Chain Walking as a Strategy for Carbon-Carbon Bond Formation at Unreactive Sites in Organic Synthesis: Catalytic Cycloisomerization of Various 1,n-Dienes. J. Am. Chem. Soc. 2015, 137 (51), 16163. 10.1021/jacs.5b10804. [DOI] [PubMed] [Google Scholar]
- Aspin S.; Goutierre A. S.; Larini P.; Jazzar R.; Baudoin O. Synthesis of aromatic alpha-aminoesters: palladium-catalyzed long-range arylation of primary C sp 3-H bonds. Angew. Chem., Int. Ed. 2012, 51 (43), 10808. 10.1002/anie.201206237. [DOI] [PubMed] [Google Scholar]
- Dupuy S.; Zhang K.-F.; Goutierre A.-S.; Baudoin O. Terminal-Selective Functionalization of Alkyl Chains by Regioconvergent Cross-Coupling. Angew. Chem., Int. Ed. 2016, 55 (47), 14793. 10.1002/anie.201608535. [DOI] [PubMed] [Google Scholar]
- Möhring V. M.; Fink G. Novel Polymerization of α-Olefins with the Catalyst System Nickel/Aminobis(imino)phosphorane. Angew. Chem., Int. Ed. Engl. 1985, 24 (11), 1001. 10.1002/anie.198510011. [DOI] [Google Scholar]
- Johnson L. K.; Killian C. M.; Brookhart M. New Pd(II)- and Ni(II)-Based Catalysts for Polymerization of Ethylene and.alpha.-Olefins. J. Am. Chem. Soc. 1995, 117 (23), 6414. 10.1021/ja00128a054. [DOI] [Google Scholar]
- van Leeuwen P. W. N. M.Homogeneous Catalysis: Understanding of the Art; Kluwer Academic Publishers: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Buslov I.; Becouse J.; Mazza S.; Montandon-Clerc M.; Hu X. Chemoselective Alkene Hydrosilylation Catalyzed by Nickel Pincer Complexes. Angew. Chem., Int. Ed. 2015, 54 (48), 14523. 10.1002/anie.201507829. [DOI] [PubMed] [Google Scholar]
- Buslov I.; Song F.; Hu X. An Easily Accessed Nickel Nanoparticle Catalyst for Alkene Hydrosilylation with Tertiary Silanes. Angew. Chem., Int. Ed. 2016, 55 (40), 12295. 10.1002/anie.201606832. [DOI] [PubMed] [Google Scholar]
- Lee W.-C.; Wang C.-H.; Lin Y.-H.; Shih W.-C.; Ong T.-G. Tandem Isomerization and C–H Activation: Regioselective Hydroheteroarylation of Allylarenes. Org. Lett. 2013, 15 (20), 5358. 10.1021/ol402644y. [DOI] [PubMed] [Google Scholar]
- Bair J. S.; Schramm Y.; Sergeev A. G.; Clot E.; Eisenstein O.; Hartwig J. F. Linear-Selective Hydroarylation of Unactivated Terminal and Internal Olefins with Trifluoromethyl-Substituted Arenes. J. Am. Chem. Soc. 2014, 136 (38), 13098. 10.1021/ja505579f. [DOI] [PubMed] [Google Scholar]
- Okumura S.; Komine T.; Shigeki E.; Semba K.; Nakao Y. Site-Selective Linear Alkylation of Anilides by Cooperative Nickel/Aluminum Catalysis. Angew. Chem., Int. Ed. 2018, 57, 929–932. 10.1002/anie.201710520. [DOI] [PubMed] [Google Scholar]
- He Y.; Cai Y.; Zhu S. Mild and Regioselective Benzylic C–H Functionalization: Ni-Catalyzed Reductive Arylation of Remote and Proximal Olefins. J. Am. Chem. Soc. 2017, 139 (3), 1061. 10.1021/jacs.6b11962. [DOI] [PubMed] [Google Scholar]
- Wang X.; Nakajima M.; Serrano E.; Martin R. Alkyl Bromides as Mild Hydride Sources in Ni-Catalyzed Hydroamidation of Alkynes with Isocyanates. J. Am. Chem. Soc. 2016, 138 (48), 15531. 10.1021/jacs.6b10351. [DOI] [PubMed] [Google Scholar]
- Chen F.; Chen K.; Zhang Y.; He Y.; Wang Y.-M.; Zhu S. Remote Migratory Cross-Electrophile Coupling and Olefin Hydroarylation Reactions Enabled by in Situ Generation of NiH. J. Am. Chem. Soc. 2017, 139 (39), 13929. 10.1021/jacs.7b08064. [DOI] [PubMed] [Google Scholar]
- Peng L.; Li Y.; Li Y.; Wang W.; Pang H.; Yin G. Ligand-Controlled Nickel-Catalyzed Reductive Relay Cross-Coupling of Alkyl Bromides and Aryl Bromides. ACS Catal. 2018, 8, 310–313. 10.1021/acscatal.7b03388. [DOI] [Google Scholar]
- Juliá-Hernández F.; Moragas T.; Cornella J.; Martin R. Remote carboxylation of halogenated aliphatic hydrocarbons with carbon dioxide. Nature 2017, 545, 84. 10.1038/nature22316. [DOI] [PubMed] [Google Scholar]
- Gaydou M.; Moragas T.; Juliá-Hernández F.; Martin R. Site-Selective Catalytic Carboxylation of Unsaturated Hydrocarbons with CO2 and Water. J. Am. Chem. Soc. 2017, 139 (35), 12161. 10.1021/jacs.7b07637. [DOI] [PubMed] [Google Scholar]