Abstract
Background
Attention-Deficit/Hyperactivity Disorder (ADHD) is thought to stem from aberrancies in large-scale cognitive control networks. However, the exact nature of aberrant brain circuit dynamics involving these control networks is poorly understood. Using a saliency-based triple-network model of cognitive control, we test the hypothesis that dynamic cross-network interactions among the salience (SN), central executive (CEN), and default mode (DMN) networks are dysregulated in children with ADHD, and investigate how these dysregulations contribute to inattention.
Methods
Using fMRI data from 140 children with ADHD and typically developing (TD) children from two cohorts (Primary cohort = 80; Replication Cohort = 60) in a case-control design, we examined both time-averaged and dynamic time-varying cross-network interactions in each cohort separately.
Results
Time-averaged measures of SN-centered cross-network interactions were significantly lower in children with ADHD compared to TD children and were correlated with severity of inattention symptoms. Children with ADHD displayed more variable dynamic cross-network-interaction patterns, including less persistent brain states, significantly shorter mean lifetimes of brain states, and intermittently weaker cross-network interactions. Importantly, dynamic time-varying measures of cross-network interactions were more strongly correlated with inattention symptoms than time-averaged measures of functional connectivity. Crucially, we replicated these findings in the two independent cohorts of children with ADHD and TD children
Conclusions
Aberrancies in time-varying engagement of the SN with the CEN and DMN are a robust and clinically-relevant neurobiological signature of childhood ADHD symptoms. The triple-network neurocognitive model provides a novel, replicable and parsimonious dynamical systems neuroscience framework for characterizing childhood ADHD and inattention.
Keywords: salience network, cognitive control, functional connectivity, human, inattention, dynamic brain state
Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD) is a neurodevelopmental disorder with global prevalence rates of over 5% in children and adolescents (1–3). The primary behavioral features of ADHD are inattention, hyperactivity, and impulsivity, which have profound effects on children’s cognitive, affective, and social development (4–9). Neurobiologically, ADHD is now increasingly viewed as a disorder stemming from disturbances in large-scale brain networks (10–17). However, the precise nature of these disturbances is not well understood as extant studies of functional brain connectivity in ADHD have produced conflicting findings with some studies reporting hyper- and hypo-connectivity relative to neurotypical controls and others reporting null findings, often between the same brain regions (10, 16, 18–23), likely due to weak theoretical models, inadequate quantitative approaches, and variation in protocols and measures across data collection sites. Crucially, little is known about brain network dynamics in ADHD, as previous studies have assumed functional interactions between brain networks to be stationary. Here, we overcome limitations of previous work by using a principled systems neuroscience approach (24) to characterize aberrancies in dynamic time-varying interactions among key cognitive control networks and investigate how such altered dynamic functional circuits contribute to core cognitive deficits associated with childhood ADHD, in two independent cohorts.
Our analysis of the neurobiological basis of childhood ADHD is based on a triple-network model involving three large-scale brain networks that play important and distinct roles in higher-order cognition (25–29): the salience (SN), central executive (CEN), and default mode (DMN) networks. The SN, which is anchored in anterior insula (AI) and anterior cingulate cortex (ACC), plays a crucial role in identifying biologically and cognitively salient events necessary for guiding attention and goal-directed behaviors (28). The CEN, which is anchored in dorsolateral prefrontal cortex (DLPFC) and posterior parietal cortex (PPC), is important for the active maintenance and manipulation of information in working memory (30–32). The DMN, which is anchored in posterior cingulate cortex (PCC) and medial prefrontal cortex (MPFC), plays an important role in self-referential mental processes (33). Notably, the SN, CEN, and DMN are often co-activated or deactivated during attentionally-demanding tasks (34–37), suggesting that these networks function in concert to support attention and cognition. In particular, the triple-network model posits a central role for the SN in initiating switching between the CEN and DMN, a process essential for attention and flexible cognitive control (38–41).
A systematic investigation using theoretically-informed neurocognitive models has the potential to significantly advance a principled understanding of the neurobiology of childhood ADHD. Meta-analyses of task-based fMRI studies have consistently pointed to aberrant activation of SN, CEN and DMN regions in individuals with ADHD compared with neurotypical individuals (42, 43). Previous studies have pointed to abnormal time-averaged functional connectivity within the DMN, and between the DMN and cingulo-opercular and occipital regions in children and adolescents with ADHD (10, 16, 20) (19) and their associations with cognitive control deficits and clinical symptoms. However, no studies to date have investigated dynamic time-varying interactions among large-scale brain networks in children with ADHD, and a comprehensive understanding of dysfunctional interactions among SN, CEN and DMN and their relation to clinical symptoms is still missing. Based on growing evidence that attention and cognitive control in adults relies on dynamic cross network interactions (44–47), we investigated both time-averaged as well as dynamic time-varying cross-network interactions among SN, CEN, and DMN. The specific goals of our study were therefore to investigate whether (1) time-averaged cross-network interactions between SN-CEN and SN-DMN would be weaker in children with ADHD compared to TD children, (2) weak time-averaged cross-network interactions would predict inattention, (3) children with ADHD would show considerably more temporal variability in dynamic cross-network interactions, and (4) variability of dynamic cross-network interactions would predict inattention symptoms.
We used data from two independent cohorts of 140 children with ADHD and age-, gender-, handedness-, IQ- and motion-matched typically developing (TD) children. A SN-centered network interaction index (NII) (48) was used to assess the integrity of cross-network interactions and the extent to which the SN is temporally integrated with the CEN while simultaneously dissociated from the DMN (28, 49). SN-centered cross-network interaction was defined as the difference between the strength of SN interactions with the CEN and DMN (48). Both time-averaged cross-network interactions measured across the entire time series, and time-varying interaction measured using 40 second long moving windows centered were computed at each time point (45, 50, 51). Time-averaged NII measures were first compared between the two groups, and their relation to inattention was examined. Next, we examined time-varying cross-network interactions among the three networks. To identify distinct brain states associated with time-varying cross-network interactions, we applied a group-wise temporal clustering on the time-dependent functional correlation matrices. The optimal number of clusters was determined based on maximal silhouette value, which measures validity (specifically, how well the resulting clusters are separated), across multiple iterations of the clustering procedures (52). Each temporal cluster characterizes a dynamic brain state. Dynamic features associated with these brain states, including mean lifetime (how long a state lasts before switching to another state) and dynamic NII measures, were then compared between the two groups. We predicted that the number of brain states would be greater and their mean lifetime would be shorter in ADHD, compared to TD children, indicative of more volatile and variable cross-network interactions across time in affected children. We further predicted that greater variability of dynamic NII would predict inattention.
Methods and Materials
Dataset access and participant selection
Behavioral and brain imaging data acquired by researchers at New York University (Primary cohort) and Peking University (Replication cohort), and made available through the ADHD-200 consortium (53) were used in this study (Table 1; Supplementary Materials).
Table 1.
Descriptive statistics for the ADHD and TD groups across the two cohorts. Within each cohort, the two groups were matched on age, gender, handedness, IQ and head motion during fMRI.
| Primary cohort | Replication cohort | |||||
|---|---|---|---|---|---|---|
| TD (N=40) | AD (N=40) | p | TD (N=30) | AD (N=30) | p | |
| Age | 11.9(3.2) | 11.7(2.9) | 0.66 | 12.3(1.5) | 12.6(2.1) | 0.47 |
|
Gender (female/male) |
17/23 | 13/27 | 0.36 | 0/30 | 0/30 | 1.00 |
| Handedness scores | 0.6(0.3) | 0.6(0.3) | 0.84 | 0.9(0.2) | 1(0) | 0.33 |
| IQ | 111(15) | 107(14) | 0.26 | 113(10) | 113(9) | 1.00 |
| Inattention | 45(5) | 71(9) | 0.00 | 15(3) | 28(4) | 0.00 |
| Hyperactivity/Impulsivity | 46(5) | 66(12) | 0.00 | 12(4) | 23(7) | 0.00 |
| Head Motion | ||||||
| Range | ||||||
| X (mm) | 0.307(0.194) | 0.324(0.203) | 0.71 | 0.271(0.161) | 0.241(0.135) | 0.44 |
| Y (mm) | 0.545(0.392) | 0.676(0.5) | 0.19 | 0.596(0.301) | 0.563(0.333) | 0.69 |
| Z (mm) | 0.555(0.309) | 0.667(0.346) | 0.13 | 0.884(0.498) | 0.922(0.458) | 0.76 |
| Pitch (deg) | 0.012(0.007) | 0.014(0.009) | 0.27 | 0.013(0.007) | 0.015(0.008) | 0.56 |
| Roll (deg) | 0.006(0.003) | 0.006(0.004) | 0.43 | 0.007(0.004) | 0.008(0.005) | 0.74 |
| Yaw (deg) | 0.007(0.003) | 0.007(0.002) | 0.65 | 0.006(0.005) | 0.006(0.003) | 0.72 |
| RMS | ||||||
| X (mm) | 0.093(0.064) | 0.091(0.092) | 0.92 | 0.097(0.082) | 0.093(0.079) | 0.86 |
| Y (mm) | 0.217(0.178) | 0.235(0.192) | 0.66 | 0.257(0.149) | 0.198(0.124) | 0.10 |
| Z (mm) | 0.196(0.137) | 0.247(0.198) | 0.19 | 0.341(0.222) | 0.324(0.225) | 0.77 |
| Pitch (deg) | 0.005(0.003) | 0.005(0.003) | 0.64 | 0.005(0.004) | 0.005(0.004) | 0.56 |
| Roll (deg) | 0.002(0.001) | 0.002(0.002) | 0.65 | 0.002(0.002) | 0.003(0.002) | 0.34 |
| Yaw (deg) | 0.003(0.002) | 0.003(0.002) | 0.46 | 0.003(0.003) | 0.002(0.002) | 0.57 |
| Scan to Scan motion (mm) | 0.08(0.036) | 0.092(0.04) | 0.16 | 0.082(0.033) | 0.093(0.039) | 0.25 |
The Conners’ Parent Rating Scale-Revised, Long Version and the ADHD Rating Scale were used as dimensional measures of ADHD symptoms in the Primary and Replication cohorts, respectively. Note that Inattention and hyperactivity/impulsivity scores are raw scores provided by ADHD200 Consortium. Handedness scores: 1, right-handed; 0, left-handed.
Medication status
We conducted an additional analysis comparing medication naïve and non-naïve individuals with ADHD and demonstrated that our findings were not confounded by medication status (Supplementary Materials).
fMRI preprocessing & analysis
A standard preprocessing procedure was implemented using SPM8, including slice-timing correction, realignment, normalization, spatial smoothing (6 mm smoothing kernel), regression of nuisance variables (24 motion parameters, white matter and cerebrospinal fluid signals) and bandpass filtering (0.008 Hz < f < 0.1 Hz).
Preprocessed data from the ADHD and TD samples were concatenated and entered into a group independent component analysis (ICA) to identify large-scale networks in the combined population for each cohort separately (MELODIC, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MELODIC). The number of components was set to 30. Determining the number of components using an unsupervised learning algorithm like ICA remains an unresolved challenge. Our choice was therefore based on common practices in the field (48, 54). Four components (SN, left and right CEN and DMN) corresponding to the previously described triple-network model (28) were determined based on a widely-used visual inspection procedure (54, 55).
Time-averaged cross-network interaction
We computed a network interaction index (NII) (48) to assess cross-network interactions among the three networks based on the hypothesized role of the SN in switching interactions with the CEN and DMN (28, 49). NII has the advantage of capturing interactions simultaneously among all three networks. Specifically, NII was computed as the difference in correlation between SN and CEN time series and correlation between SN and DMN. The rationale here is that SN and CEN are typically co-activated during cognitively demanding tasks, while SN and DMN are typically anti-correlated (49, 56). NII thus captures the extent to which SN can temporally integrate itself with CEN and dissociate itself from DMN. We computed NII for each participant and compared the NII values between ADHD and TD groups in each cohort (see Supplementary Methods for details).
Relation of time-averaged NII measures to clinical symptoms
The relation between time-averaged NII and individual clinical scores was investigated using Pearson’s correlation and its significance was examined using a permutation testing procedure because of non-normal distribution of clinical scores in our samples (Supplementary Materials).
Dynamic time-varying cross-network interactions
Time-varying cross-network interaction was measured using a dynamic functional connectivity approach (50). We estimated dynamic functional interactions between brain regions using a temporal sliding window approach with a rectangle shape and a window length of 40 seconds (20 TRs) and a sliding step of 2 seconds (1 TR) (45, 50). A sliding window with an exponentially decaying shape and a window length of 40 seconds was also used to test the robustness of our findings and results are reported in the Supplementary Materials. Within each time window, we computed the z-transformed Pearson correlation between the ICA time-series taken pairwise. This resulted in a time-series of correlation matrices (T × C); here T is the number of time windows and C is number of pairwise interactions among SN, CEN, DMN at each time point. To identify distinct group-specific states associated with dynamic functional connectivity, we applied a group-wise k-mean clustering on the time-series of correlation matrices in each group separately with the number of clusters (k) ranging from 2 to 20. Twenty-five different initializations were used to reduce the chance of local minima. Clustering performance was estimated using the silhouette method and the optimal number of clusters was determined based on maximal silhouette across all the iterations (Supplementary Figure 2) (52). Because our goal was to investigate whether dynamic temporal properties, including the number of states and their mean lifetimes, differed between the two groups we allowed the number of clusters to differ between the ADHD and control groups, instead of keeping them exactly the same (50). Statistical significance of differences in the number of clusters between the two groups was evaluated using a permutation testing procedure. In each permutation, group labels were randomly shuffled, group-wise k-mean clustering were conducted and a group difference in the optimal number of clusters was computed. Group differences in the optimal number of cluster from 500 permutations were used to construct the empirical null distribution from which a p value was obtained. Robustness of our findings was tested using different window lengths. To quantify dwelling time of dynamic brain states, we computed mean lifetime of each brain state for each participant, based on the average time spent continuously in that state. Two sample t-tests were conducted to evaluate the difference in mean lifetime between brain states in TD and children with ADHD. Brain state-specific NII was used to characterize cross-network interaction in each dynamic brain state. We computed NII for each sliding window and averaged NIIs for the windows corresponding to the same dynamic brain state. Two sample t-tests were used to test the difference in NIIs of brain states in TD and children with ADHD.
Relation of dynamic time-varying NII measures to clinical symptoms
We first computed the variability (measured by standard deviations) of time-varying NIIs across all the dynamic brain states for each participant and examined the difference between the variability of time-varying NIIs between the two groups using two sample t-tests. We then examined the relation between variability of time-varying NIIs and clinical symptoms using the same procedures as described above.
Results
Time-averaged cross-network interaction
NII, a cross-network coupling measure, was used to investigate interactions among the four ICA-identified brain networks (Figure 1). NII values were normally distributed in each dataset (Supplementary Materials). Notably, SN-centered NII was significantly lower in the ADHD group than in the TD group in the Primary cohort (p<0.005, Cohen’s d=0.64) and Replication cohort (p<0.05, Cohen’s d=0.55) (Figure 2A). Additional analyses further confirmed significantly lower NII values in ADHD than TD after controlling for movement and other parameters including age, gender, handedness and IQ in the Primary cohort (p=0.005) and Replication cohort (p=0.03) (Supplementary Table 1).
Figure 1. Salience, Central Executive and Default Mode Networks in the Primary and Replication cohorts.
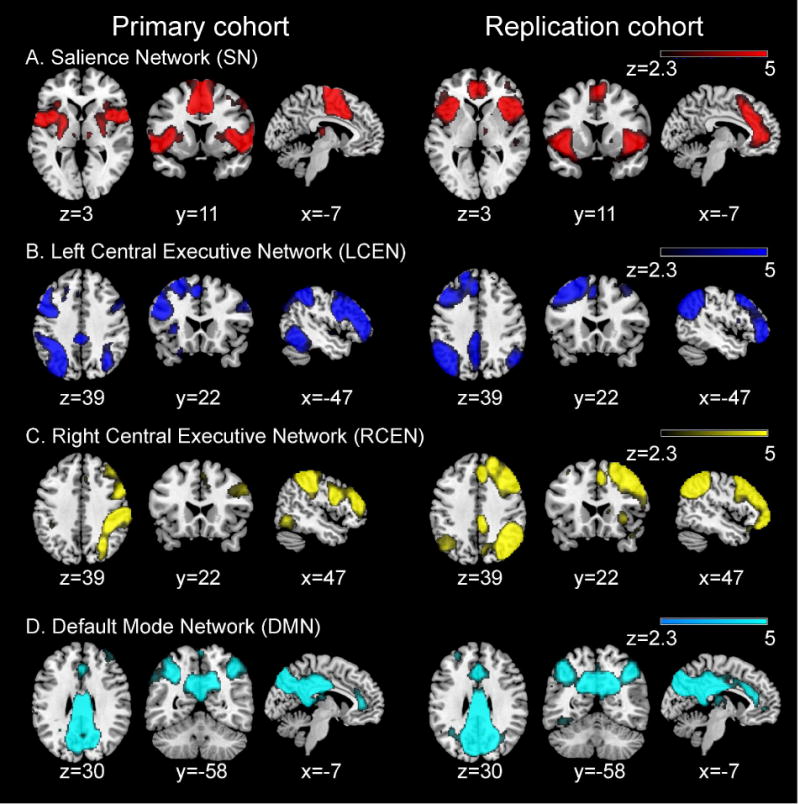
(a) Salience network (SN). (b) Left Central Executive network (LCEN). (c) Right Central Executive network (RCEN). (d) Default Mode network (DMN). Group-level independent component analysis was used to identify these networks in data from each cohort. Maps are displayed at z>2.3 (p<0.01, corrected).
Figure 2. Time-averaged cross-network interactions between SN, CEN and DMN in children with ADHD and TD children, and relation to ADHD symptoms.
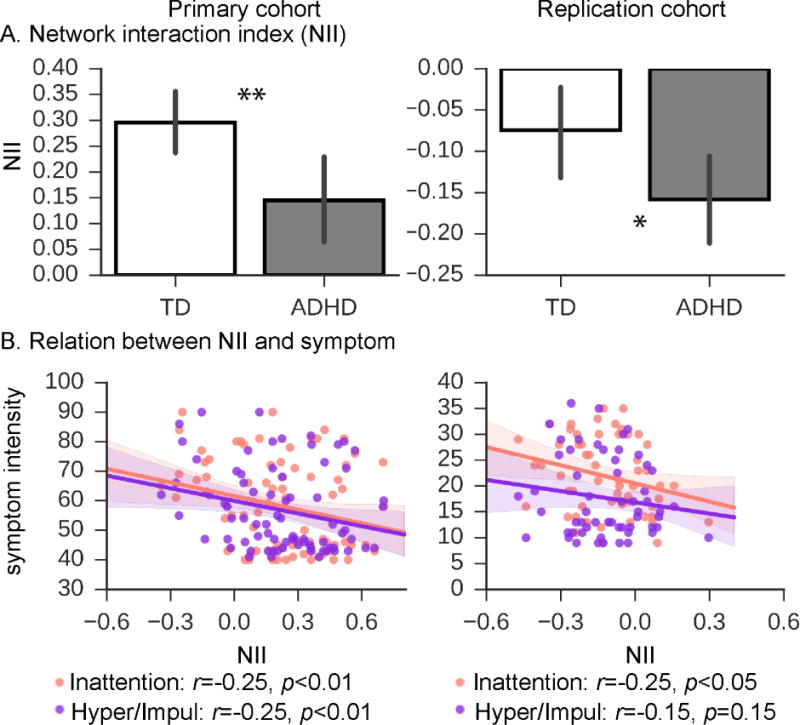
(A) Cross-network interaction, assessed using a SN-centered network interaction index (NII) (see Methods), was significantly lower in ADHD than in TD children for each cohort. (B) SN-centered NII was strongly negatively correlated with the inattention symptoms of ADHD in both cohorts. SN-centered NII was correlated with hyperactivity/impulsivity in data from the Primary cohort. The Conners’ Parent Rating Scale-Revised, Long Version and the ADHD Rating Scale were used as dimensional measures of ADHD symptoms in the Primary and Replication cohorts, respectively. *, p<0.05; **, p<0.01.
We then examined the specificity of the above effects by testing two alternate models involving parallel constructs with a CEN-centered network and a DMN-centered network. Neither index was consistently different between groups across the two cohorts (Supplementary Materials).
Next, we investigated whether functional connectivity between any two of SN, CEN, and DMN was different between the ADHD and TD groups. Pairwise correlations did not differ between the groups in the two cohorts (all ps>0.05) (Supplementary Figure 3).
Time-averaged cross-network interaction in relation to inattention
We found that individual inattention scores were negatively correlated with SN-centered NII in the Primary cohort (r=−0.25, p<0.01) and Replication cohort (r=−0.25, p<0.05), despite the use of different questionnaires at each cohort (Figure 2B). Multiple linear regression further demonstrated that SN-centered NII outperformed other measures, including scan-to-scan head motion, age and IQ, in predicting inattention scores in both cohorts (Table 2). The relation between SN-centered NII and hyperactivity/impulsivity scores was significant in the Primary cohort (r=−0.25, p<0.01) but not in Replication cohort (p=0.15), suggesting that the relation to inattention is a more replicable finding.
Table 2.
Multiple linear regression revealed that SN-centered interactions with CEN and DMN, as assessed using Network Interaction Index (NII), were the most robust predictor of inattention symptoms in children with ADHD.
| Inattention | Hyperactivity/Impulsivity | |||
|---|---|---|---|---|
|
| ||||
| Beta | P | Beta | P | |
| Primary cohort | ||||
|
| ||||
| NII | −14.54 | 0.04* | −14.81 | 0.03* |
| Motion | 34.38 | 0.45 | 34.98 | 0.39 |
| Age | −0.19 | 0.75 | −0.16 | 0.76 |
| IQ | 0 | 0.86 | 0.01 | 0.15 |
|
| ||||
| Replication cohort | ||||
|
| ||||
| NII | −12.72 | 0.04* | −8.55 | 0.2 |
| Motion | 26.15 | 0.35 | 31.29 | 0.3 |
| Age | −0.34 | 0.56 | −0.58 | 0.35 |
| IQ | −0.02 | 0.86 | −0.01 | 0.94 |
p<0.05
Dynamic time-varying cross-network interactions
Analysis of dynamic functional interactions among SN, CEN and DMN revealed two states (temporal clusters) in the TD and five in ADHD group in both cohorts (Figure 3A), reflecting variation in cross-network interactions across time in both groups. Permutation analysis revealed significantly more states in the ADHD than TD groups in the Primary cohort (p<0.05) and Replication cohort (p=0.002).
Figure 3. Dynamic time-varying cross-network interactions between the SN, CEN, and DMN in children with ADHD and TD children.
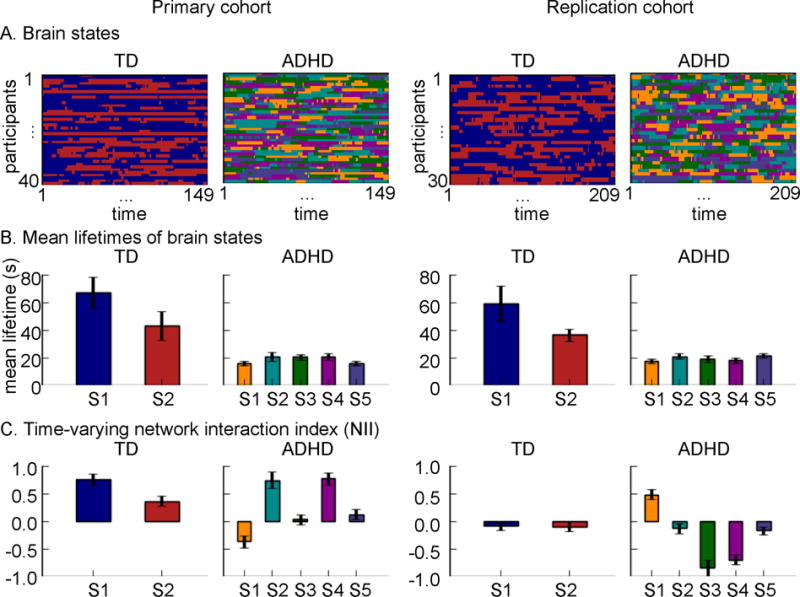
(A) In both the Primary and Replication cohorts, children with ADHD showed five states, significantly higher than the two states in TD children. Color codes distinct states in each participant. (B) Mean lifetimes of dynamic brain states were shorter in children with ADHD, compared to TD children in both cohorts. (C) NII of dynamic brain states shows intermittently weaker, and more variable, SN-centered cross-network interaction in children with ADHD compared to TD children in both cohorts.
Next, we compared mean lifetime of dynamic brain states between the two groups. In the Primary cohort, the mean lifetime of state 1 in the TD group was significantly longer than the mean lifetime of any of the five states in ADHD group (ps<0.001). The mean lifetime of state 2 in the TD group was significantly longer than the mean lifetime of state 1 and 5 in the ADHD group (ps<0.05). In the Replication cohort, the mean lifetime of states 1 and 2 in TD group was significantly longer than the mean lifetime of any of the five states in the ADHD group (ps<0.05) (Figure 3B). Bonferroni correction was used for multiple comparisons. These results demonstrate that, compared to TD, children with ADHD show less persistent and more volatile brain states. Note that states are defined independently in the Primary and Replication cohorts.
We then compared NII of dynamic brain states between the two groups. In the Primary cohort, NII of states 1 and 3 in the ADHD group was significantly lower than NII of any of the states in the TD group (ps<0.05). In the Replication cohort, the NII of states 3 and 4 in the ADHD group was significantly lower than NII of all states in the TD group (ps<0.05) and NII of state 1 in the ADHD group was significantly higher than NII of states in the TD group (ps<0.05) (Figure 3C). Bonferroni correction was used for multiple comparisons. These results demonstrate an intermittent lack of integration of the SN with the CEN and decoupling of the SN from the DMN in children with ADHD, and that cross-network interactions are more variable in children with ADHD than TD children.
Variability of dynamic time-varying cross-network interactions and its relation to inattention
Compared to TD children, children with ADHD showed greater variability in dynamic NII strength across states in both cohorts (ps<0.001) (Figure 4A). Additional analyses further confirmed lower NII values in ADHD than TD after controlling for confounds (Supplementary Table 2). Notably, we found that individual inattention scores were positively correlated with variability of time-varying NII measures in the Primary (r=0.39, p=0.002) and Replication (r=0.65, p=0.002) cohorts, despite the use of different clinical questionnaires at each cohort (Figure 4B). Effect sizes (R2) were much higher for time-varying interactions (Primary: 0.15; Replication: 0.42) as compared to time-averaged interactions (Primary: 0.06; Replication: 0.06). Individual hyperactivity/impulsivity scores were also positively correlated with variability of time-varying NII measures in the Primary and Replication cohorts (p=0.002) (Figure 4B). Multiple linear regression further demonstrated that variability of dynamic time-varying NII outperformed other variables in predicting clinical symptom scores (Table 3).
Figure 4. Variability of dynamic cross-network interactions between the SN, CEN and DMN in children with ADHD and TD children, and relation to ADHD symptoms.
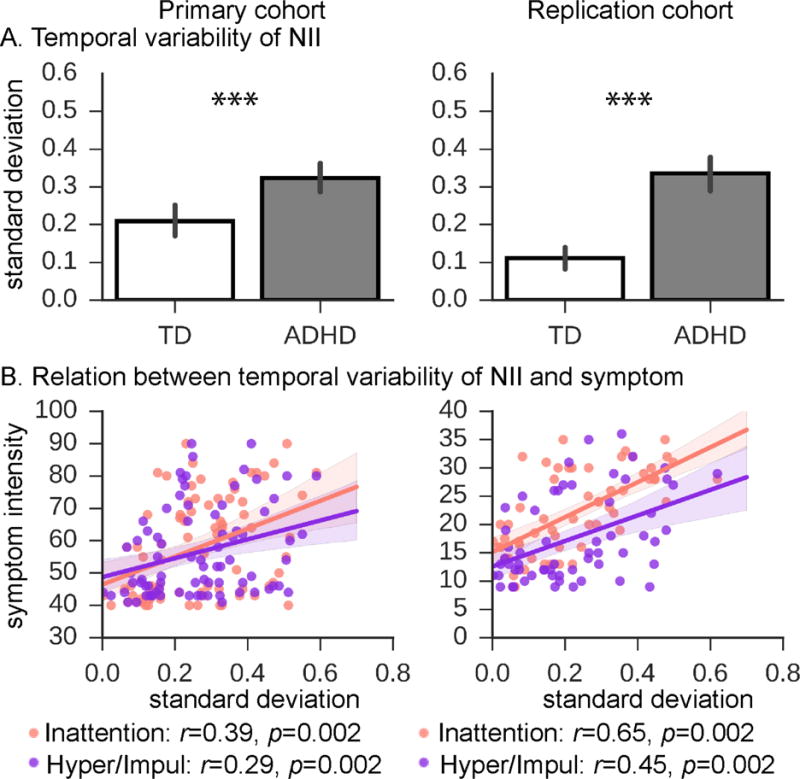
(A) Temporal variability of dynamic cross-network interaction, assessed using standard deviation of dynamic NIIs across states, was significantly higher in ADHD, compared to TD children, in both cohorts. (B) Temporal variability of dynamic NIIs was strongly positively correlated with inattention and hyperactivity/impulsivity symptoms of ADHD in both cohorts. The Conners’ Parent Rating Scale-Revised, Long Version and the ADHD Rating Scale were used as dimensional measures of ADHD symptoms in the Primary and Replication cohorts, respectively. ***, p<0.001.
Table 3.
Multiple linear regression revealed that variability of dynamic time-varying NII were the most robust predictor of inattention symptoms in children with ADHD.
| Inattention | Hyperactivity/Impulsivity | |||
|---|---|---|---|---|
|
| ||||
| Beta | P | Beta | P | |
| Primary cohort | ||||
|
| ||||
| NII | 45.4 | 0.001 | 29.82 | 0.007 |
| Motion | 69.26 | 0.1 | 65.41 | 0.1 |
| Age | 0.05 | 0.93 | −0.02 | 0.96 |
| IQ | −0.01 | 0.49 | 0.007 | 0.34 |
|
| ||||
| Replication cohort | ||||
|
| ||||
| NII | 30.86 | 0.001 | 22.64 | 0.001 |
| Motion | 7.26 | 0.74 | 17.01 | 0.52 |
| Age | −0.34 | 0.43 | −0.86 | 0.1 |
| IQ | −0.02 | 0.78 | −0.01 | 0.94 |
Robustness of findings with respect to temporal windows
The aforementioned findings were replicated using window lengths of 60 and 80 seconds (ps<0.05) as well as exponentially-decaying sliding window, in both cohorts (Supplementary Materials).
Discussion
Our findings support the triple-network model, which posits that the integrity and mutual interactions of SN, CEN, and DMN play a crucial role in cognition (28) and that dysregulation in cross-network interactions can lead to deficits in attention, cognitive control, and other goal-directed and adaptive behaviors (24, 28). In agreement with this hypothesis, we found that children with ADHD had weaker time-averaged cross-network interactions among the SN, CEN, and DMN, and that the degree of these brain aberrations were related to severity of inattention symptoms. Analysis of dynamic functional connectivity further revealed that cross-network interactions vary considerably across time and that these networks exhibit variable, and weaker, dynamic cross-network interactions in ADHD, compared to TD children. Furthermore, variability of dynamic time-varying cross-network interaction was strongly related with severity of inattention symptoms. Crucially, we replicated our findings in two independent cohorts consisting of 140 children from distinct geographical sites, demonstrating the robustness of our findings.
Aberrant SN-centered time-averaged cross-network interactions in children with ADHD
The first key finding of our study is that the SN-centered cross-network interactions were weaker in children with ADHD relative to their TD peers. Previous studies using seed-based functional connectivity have reported abnormal functional connectivity between DMN and cingulo-opercular and occipital regions in children with ADHD (10, 16), suggesting atypical interaction between DMN and other brain networks underlying dysfunction in ADHD. However, temporal interactions among the SN, CEN and DMN have not been examined, thereby limiting our understanding of impairments in neurocognitive control systems involved in attention. The current study was designed to specifically test a triple network model of cognitive control and test the hypothesis that SN interactions with the CEN and DMN are impaired in children with ADHD (24, 28). We found evidence that SN-centered interactions with the CEN and DMN were impaired in ADHD, and this finding was observed in both the Primary and Replication cohorts. Further, control analyses using CEN- and DMN-centered cross network interactions did not show such differences. Moreover, single pairwise correlation between the networks were not different between the ADHD from the TD groups in either cohort. Thus, our findings demonstrate that the NII has the advantage of capturing aberrant interactions simultaneously among all three networks, and quantifies the extent to which the SN is temporally integrated with the CEN and dissociated from the DMN.
The SN has been shown to play a crucial role in switching between the CEN and the DMN to facilitate access to task-relevant attentional resources (28, 40). Specifically, the AI node of the SN is thought to be involved in detecting salient events and signaling the fronto-parietal CEN to recruit cognitive resources essential for attentionally demanding cognitive tasks (28, 29, 34, 57). This is in line with previous evidence of aberrant attention-related responses in the AI and the ACC in ADHD (58, 59). Our findings suggest that aberrant interactions of the SN with the CEN and DMN may contribute to the atypical activations on a wide range of cognitive tasks in ADHD (9, 60–67).
Aberrant and more variable time-varying cross-network interactions in children with ADHD
The second key finding of our study relates to the temporal characteristics of cross-network interactions in children with ADHD. In the two independent cohorts, we revealed more than one dynamic brain state in each group and greater number of dynamic brain states in ADHD, compared to TD groups. Notably, the mean lifetime of dynamic brain states was shorter in the ADHD, compared to TD, group suggesting that cross-network interactions are not only highly-variable but also highly volatile in children with ADHD. Additionally, dynamic cross-network interactions in ADHD were characterized by intermittent lack of integration of the SN with CEN and decoupling of the SN from DMN, and the strength of these dynamic interactions were more variable in children with ADHD. These results provide further insights into the temporally variable nature of aberrant network interactions underlying ADHD. These findings are particularly noteworthy in the context of intra-individual response variability and transient fluctuations in task performance that are a hallmark of ADHD (68–70). Moreover, a prominent neurocognitive model of ADHD suggests that abnormal fluctuation of brain states may underlie attentional lapses and atypical goal-directed behaviors in affected individuals (71). Our demonstration that children with ADHD have (a) more variable and short-lived brain states than TD children, and (b) greater temporal variability in state-specific cross-network interactions provides experimental evidence in support of this model.
Aberrant SN-centered cross-network interactions are related to attention deficits
The third important finding our study is that SN-centered cross-network interactions were significantly correlated with inattention symptoms. Importantly, this finding was observed with both time-averaged and time-varying measures of cross-network interactions, and replicated across two independent cohorts. Notably, we did not find differences in brain-inattention symptom relations between ADHD and TD groups and the strongest relations emerged with the combined group of participants. It should also be noted that inattention scores were continuous across groups. Importantly, our methodology is consistent with the research domain criteria (RDoC) framework, which emphasizes the use of measures that capture the entire range of clinically-relevant behavioral measures from typical to atypical (72). Consistent with our hypothesis that abnormal fluctuations in brain states may underlie attention difficulties, we found that greater temporal variability in state-specific cross-network interactions was associated with increased inattention symptoms in children. Crucially, dynamic measures of temporal variability in cross-network interactions were more strongly correlated with inattention symptoms than time-averaged measures of cross-network interactions. We postulate that inattention symptoms in children with ADHD are related to their difficulty in engaging task-relevant brain states while disengaging from task-irrelevant brain states, arising from weak dynamic modulation of cross-network interactions among the SN, CEN and DMN.
Reproducibility
Reproducibility is particularly important for clinical neuroimaging studies (73). Leveraging datasets shared by the ADHD-200 consortium (53), we demonstrate replicable neurobiological signatures of childhood ADHD. Despite differences in geographical location, scanner, acquisition protocols, and sample size, we replicated five key findings across the two cohorts: (i) weaker SN-centered cross-network interactions in ADHD compared to TD children, (ii) correlation between strength of SN-centered network interactions and severity of inattention symptoms, (iii) more variable and volatile time-varying network interactions in ADHD compared to TD children, (iv) correlation between variability of network interactions across time and severity of inattention symptoms, and (v) measures of network dynamics outperformed measures of static network interactions in predicting a core clinical symptom of ADHD.
Limitations
While the case-control design using here is optimal for minimizing the impact of confounds such as extensive head motion and low IQ, the extent to which findings can be generalized to low functioning individuals remains unknown. Puberty is another potential confound whose effects could not be examined in our study as these measures were not available in the ADHD-200 cohorts. Because the primary goal of the present work was to test a theory-based model of circuit deficits in children with ADHD, the present study has focused on network interactions among cognitive control systems involving the SN, CEN and DMN. Their interconnectivity with other brain systems, such as the basal ganglia and reward pathways implicated in ADHD remain to be investigated (74–76). Further work is also needed to investigate how the circuit deficits identified in this study influence stimulus processing during attention and cognitive control tasks in children with ADHD, and how their developmental maturation is altered with respect to their typically developing peers (77).
Conclusion
Our study demonstrates a robust neurobiological signature of ADHD using a theoretically-informed systems neuroscience model and suggests that dysregulation of cross-network interactions is a key feature of the disorder. Crucially, the replication of the study findings across two independent cohorts further suggests that the triple-network model of SN-centered deficits in dynamic functional interactions encompassing CEN and DMN provides a novel and parsimonious framework for investigating attention and cognitive deficits in ADHD.
Supplementary Material
Acknowledgments
This research was supported by an NIH Career Development award to W.C. (MH105625), NARSAD Young Investigator award to K.S., and grants from the NIH BRAINS initiative (NS086085 and EB022907) and the Stanford Child Health Research Institute. The Primary data cohort was acquired at New York University with grant support from the NIH (MH083246), Autism Speaks, The Stavros Niarchos Foundation, The Leon Levy Foundation and an endowment provided by Phyllis Green and Randolph Cōwen. The Replication data cohort was acquired at Peking University with grant support from The Commonwealth Sciences Foundation, Ministry of Health, China (200802073), The National Foundation, Ministry of Science and Technology, China (2007BAI17B03), The National Natural Sciences Foundation, China (30970802), The Funds for International Cooperation of the National Natural Science Foundation of China (81020108022), The National Natural Science Foundation of China (8100059), and Open Research Fund of the State Key Laboratory of Cognitive Neuroscience and Learning. We thank Dr. Aarthi Padmanabhan for useful suggestions. Last, but not the least, we would like to thank the ADHD-200 consortium for openly sharing the data, without which this work would not have been possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Faraone SV, Biederman J. What is the prevalence of adult ADHD? Results of a population screen of 966 adults. Journal of attention disorders. 2005;9:384–391. doi: 10.1177/1087054705281478. [DOI] [PubMed] [Google Scholar]
- 2.Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition? World psychiatry : official journal of the World Psychiatric Association. 2003;2:104–113. [PMC free article] [PubMed] [Google Scholar]
- 3.Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. The American journal of psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 4.Alessandri SM. Attention, play, and social behavior in ADHD preschoolers. Journal of abnormal child psychology. 1992;20:289–302. doi: 10.1007/BF00916693. [DOI] [PubMed] [Google Scholar]
- 5.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 6.Biederman J. Impact of comorbidity in adults with attention-deficit/hyperactivity disorder. The Journal of clinical psychiatry. 2004;65(Suppl 3):3–7. [PubMed] [Google Scholar]
- 7.Nigg JT, Stavro G, Ettenhofer M, Hambrick DZ, Miller T, Henderson JM. Executive functions and ADHD in adults: evidence for selective effects on ADHD symptom domains. Journal of abnormal psychology. 2005;114:706–717. doi: 10.1037/0021-843X.114.3.706. [DOI] [PubMed] [Google Scholar]
- 8.Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of child psychology and psychiatry, and allied disciplines. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- 9.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Barber AD, Jacobson LA, Wexler JL, Nebel MB, Caffo BS, Pekar JJ, et al. Connectivity supporting attention in children with attention deficit hyperactivity disorder. NeuroImage Clinical. 2015;7:68–81. doi: 10.1016/j.nicl.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends in cognitive sciences. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elton A, Alcauter S, Gao W. Network connectivity abnormality profile supports a categorical-dimensional hybrid model of ADHD. Human brain mapping. 2014;35:4531–4543. doi: 10.1002/hbm.22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Human brain mapping. 2010;31:904–916. doi: 10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makris N, Biederman J, Monuteaux MC, Seidman LJ. Towards conceptualizing a neural systems-based anatomy of attention-deficit/hyperactivity disorder. Developmental neuroscience. 2009;31:36–49. doi: 10.1159/000207492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Posner J, Park C, Wang Z. Connecting the dots: a review of resting connectivity MRI studies in attention-deficit/hyperactivity disorder. Neuropsychology review. 2014;24:3–15. doi: 10.1007/s11065-014-9251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sripada C, Kessler D, Fang Y, Welsh RC, Prem Kumar K, Angstadt M. Disrupted network architecture of the resting brain in attention-deficit/hyperactivity disorder. Human brain mapping. 2014;35:4693–4705. doi: 10.1002/hbm.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sripada CS, Kessler D, Angstadt M. Lag in maturation of the brain’s intrinsic functional architecture in attention-deficit/hyperactivity disorder. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:14259–14264. doi: 10.1073/pnas.1407787111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao X, Cao Q, Long X, Sun L, Sui M, Zhu C, et al. Abnormal resting-state functional connectivity patterns of the putamen in medication-naive children with attention deficit hyperactivity disorder. Brain research. 2009;1303:195–206. doi: 10.1016/j.brainres.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 19.Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fair DA, Posner J, Nagel BJ, Bathula D, Dias TG, Mills KL, et al. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;68:1084–1091. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoekzema E, Carmona S, Ramos-Quiroga JA, Richarte Fernandez V, Bosch R, Soliva JC, et al. An independent components and functional connectivity analysis of resting state fMRI data points to neural network dysregulation in adult ADHD. Human brain mapping. 2013;35:1261–1272. doi: 10.1002/hbm.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy H, Skokauskas N, Mulligan A, Donohoe G, Mullins D, Kelly J, et al. Attention network hypoconnectivity with default and affective network hyperconnectivity in adults diagnosed with attention-deficit/hyperactivity disorder in childhood. JAMA psychiatry. 2013;70:1329–1337. doi: 10.1001/jamapsychiatry.2013.2174. [DOI] [PubMed] [Google Scholar]
- 23.Tian L, Jiang T, Wang Y, Zang Y, He Y, Liang M, et al. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neuroscience letters. 2006;400:39–43. doi: 10.1016/j.neulet.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in cognitive sciences. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 26.Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human brain mapping. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cognitive, affective & behavioral neuroscience. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 33.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai W, Ryali S, Chen T, Li CS, Menon V. Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: evidence from intrinsic and task-related functional parcellation, connectivity, and response profile analyses across multiple datasets. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:14652–14667. doi: 10.1523/JNEUROSCI.3048-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83:238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai W, Chen T, Ryali S, Kochalka J, Li CS, Menon V. Causal Interactions Within a Frontal-Cingulate-Parietal Network During Cognitive Control: Convergent Evidence from a Multisite-Multitask Investigation. Cerebral cortex. 2015 doi: 10.1093/cercor/bhv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen T, Michels L, Supekar K, Kochalka J, Ryali S, Menon V. Role of the anterior insular cortex in integrative causal signaling during multisensory auditory-visual attention. The European journal of neuroscience. 2015;41:264–274. doi: 10.1111/ejn.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Supekar K, Menon V. Developmental maturation of dynamic causal control signals in higher-order cognition: a neurocognitive network model. PLoS computational biology. 2012;8:e1002374. doi: 10.1371/journal.pcbi.1002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. The American journal of psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. Journal of child psychology and psychiatry, and allied disciplines. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 44.Braun U, Schafer A, Walter H, Erk S, Romanczuk-Seiferth N, Haddad L, et al. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:11678–11683. doi: 10.1073/pnas.1422487112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen T, Cai W, Ryali S, Supekar K, Menon V. Distinct Global Brain Dynamics and Spatiotemporal Organization of the Salience Network. PLoS biology. 2016;14:e1002469. doi: 10.1371/journal.pbio.1002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spadone S, Della Penna S, Sestieri C, Betti V, Tosoni A, Perrucci MG, et al. Dynamic reorganization of human resting-state networks during visuospatial attention. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:8112–8117. doi: 10.1073/pnas.1415439112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vatansever D, Menon DK, Manktelow AE, Sahakian BJ, Stamatakis EA. Default Mode Dynamics for Global Functional Integration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:15254–15262. doi: 10.1523/JNEUROSCI.2135-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA. Large-Scale Brain Network Coupling Predicts Acute Nicotine Abstinence Effects on Craving and Cognitive Function. JAMA psychiatry. 2014;71:523–530. doi: 10.1001/jamapsychiatry.2013.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Menon V. Large-scale functional brain organization. In: Toga AW, editor. In Brain Mapping: An Encyclopedic Reference. Academic Press; Elsevier; 2015. pp. 449–459. [Google Scholar]
- 50.Rashid B, Arbabshirani MR, Damaraju E, Cetin MS, Miller R, Pearlson GD, et al. Classification of schizophrenia and bipolar patients using static and dynamic resting-state fMRI brain connectivity. NeuroImage. 2016;134:645–657. doi: 10.1016/j.neuroimage.2016.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zalesky A, Fornito A, Cocchi L, Gollo LL, Breakspear M. Time-resolved resting-state brain networks. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:10341–10346. doi: 10.1073/pnas.1400181111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bellec P, Rosa-Neto P, Lyttelton OC, Benali H, Evans AC. Multi-level bootstrap analysis of stable clusters in resting-state fMRI. NeuroImage. 2010;51:1126–1139. doi: 10.1016/j.neuroimage.2010.02.082. [DOI] [PubMed] [Google Scholar]
- 53.Consortium HD. The ADHD-200 Consortium: A Model to Advance the Translational Potential of Neuroimaging in Clinical Neuroscience. Frontiers in systems neuroscience. 2012;6:62. doi: 10.3389/fnsys.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C, et al. Salience network-based classification and prediction of symptom severity in children with autism. JAMA psychiatry. 2013;70:869–879. doi: 10.1001/jamapsychiatry.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calhoun VD, Adali T, McGinty VB, Pekar JJ, Watson TD, Pearlson GD. fMRI activation in a visual-perception task: network of areas detected using the general linear model and independent components analysis. NeuroImage. 2001;14:1080–1088. doi: 10.1006/nimg.2001.0921. [DOI] [PubMed] [Google Scholar]
- 56.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai W, Chen T, Ide JS, Li CR, Menon V. Dissociable Fronto-Operculum-Insula Control Signals for Anticipation and Detection of Inhibitory Sensory Cue. Cerebral cortex. 2016 doi: 10.1093/cercor/bhw219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orinstein AJ, Stevens MC. Brain activity in predominantly-inattentive subtype attention-deficit/hyperactivity disorder during an auditory oddball attention task. Psychiatry research. 2014;223:121–128. doi: 10.1016/j.pscychresns.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tamm L, Menon V, Ringel J, Reiss AL. Event-related FMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:1430–1440. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- 60.Alderson RM, Kasper LJ, Hudec KL, Patros CH. Attention-deficit/hyperactivity disorder (ADHD) and working memory in adults: a meta-analytic review. Neuropsychology. 2013;27:287–302. doi: 10.1037/a0032371. [DOI] [PubMed] [Google Scholar]
- 61.Alderson RM, Rapport MD, Kofler MJ. Attention-deficit/hyperactivity disorder and behavioral inhibition: a meta-analytic review of the stop-signal paradigm. Journal of abnormal child psychology. 2007;35:745–758. doi: 10.1007/s10802-007-9131-6. [DOI] [PubMed] [Google Scholar]
- 62.Boonstra AM, Oosterlaan J, Sergeant JA, Buitelaar JK. Executive functioning in adult ADHD: a meta-analytic review. Psychological medicine. 2005;35:1097–1108. doi: 10.1017/s003329170500499x. [DOI] [PubMed] [Google Scholar]
- 63.Homack S, Riccio CA. A meta-analysis of the sensitivity and specificity of the Stroop Color and Word Test with children. Archives of clinical neuropsychology: the official journal of the National Academy of Neuropsychologists. 2004;19:725–743. doi: 10.1016/j.acn.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 64.Kasper LJ, Alderson RM, Hudec KL. Moderators of working memory deficits in children with attention-deficit/hyperactivity disorder (ADHD): a meta-analytic review. Clinical psychology review. 2012;32:605–617. doi: 10.1016/j.cpr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 65.Lansbergen MM, Kenemans JL, van Engeland H. Stroop interference and attention-deficit/hyperactivity disorder: a review and meta-analysis. Neuropsychology. 2007;21:251–262. doi: 10.1037/0894-4105.21.2.251. [DOI] [PubMed] [Google Scholar]
- 66.Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? Journal of abnormal psychology. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- 67.Metin B, Roeyers H, Wiersema JR, van der Meere J, Sonuga-Barke E. A meta-analytic study of event rate effects on Go/No-Go performance in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;72:990–996. doi: 10.1016/j.biopsych.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 68.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nature reviews Neuroscience. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 69.Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biol Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 70.Kuntsi J, Klein C. Intraindividual variability in ADHD and its implications for research of causal links. Current topics in behavioral neurosciences. 2012;9:67–91. doi: 10.1007/7854_2011_145. [DOI] [PubMed] [Google Scholar]
- 71.Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neuroscience and biobehavioral reviews. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 72.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. The American journal of psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 73.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nature reviews Neuroscience. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 74.Cha J, Fekete T, Siciliano F, Biezonski D, Greenhill L, Pliszka SR, et al. Neural Correlates of Aggression in Medication-Naive Children with ADHD: Multivariate Analysis of Morphometry and Tractography. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40:1717–1725. doi: 10.1038/npp.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Costa Dias TG, Iyer SP, Carpenter SD, Cary RP, Wilson VB, Mitchell SH, et al. Characterizing heterogeneity in children with and without ADHD based on reward system connectivity. Developmental cognitive neuroscience. 2015;11:155–174. doi: 10.1016/j.dcn.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oldehinkel M, Beckmann CF, Pruim RH, van Oort ES, Franke B, Hartman CA, et al. Attention-Deficit/Hyperactivity Disorder symptoms coincide with altered striatal connectivity. Biological psychiatry Cognitive neuroscience and neuroimaging. 2016;1:353–363. doi: 10.1016/j.bpsc.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kessler D, Angstadt M, Sripada C. Growth Charting of Brain Connectivity Networks and the Identification of Attention Impairment in Youth. JAMA psychiatry. 2016;73:481–489. doi: 10.1001/jamapsychiatry.2016.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


