Abstract
Background
Recommendations regarding cancer screening vary from country to country, and may also vary within countries depending on the organization making the recommendations. The goal of this study was to summarize the cancer screening recommendations from the 21 countries with the highest per capita spending on healthcare.
Main body
Cancer screening guidelines were identified for each country based on a review of the medical literature, internet searches, and contact with key informants in most countries. The highest level recommendation was identified for each country, in the order of national recommendation, cancer society recommendation, or medical specialty society recommendation. Breast cancer screening recommendations were generally consistent across countries, most commonly recommending mammography biennially from ages 50 to 69 or 70 years. In the USA, specialty societies generally offered more intensive screening recommendations. All countries also recommend cervical cancer screening, although there is some heterogeneity regarding the test (cytology or HPV or both) and the age of initiation and screening interval. Most countries recommend colorectal cancer screening using fecal immunochemical (FIT) testing, while only seven countries recommend general or selective screening for prostate cancer, and a similar number explicitly recommend against screening for prostate cancer. Screening for lung and skin cancer is only recommended by a few countries. Greater per capita healthcare expenditures are not associated with greater screening intensity, with the possible exception of prostate cancer.
Conclusions
Guidelines for cancer screening differ between countries, with areas of commonality but also clear differences. Recommendations have important commonalities for well-established cancer screening programs such as breast and cervical cancer, with greater variation between countries regarding prostate, colorectal, lung, and skin cancer screening. Ideally, recommendations should be made by a professionally diverse, independent panel of experts that make evidence-based recommendations regarding screening based on the benefits, harms, and available resources in that country’s context.
Keywords: Cancer screening, Breast cancer, Colorectal cancer, Prostate cancer, Cervical cancer, Overdiagnosis, Healthcare economics, Lung cancer, Skin cancer
Background
Cancer is the second leading cause of death worldwide [1], with 8.8 million cancer deaths in 2015 [1], and over 14 million new cancer cases diagnosed in 2012 [2]. About 30% of cancer deaths occurred in high-income countries [1]. Cancer screening programs have the potential to reduce cancer-specific and possibly all-cause mortality [3, 4]. The United States Preventive Services Task Force (USPSTF) has concluded that there is at least adequate evidence of a net benefit for screening for lung, breast, cervical, and colorectal cancers [5–8], and screening programs for cancer are widespread in other high resource countries [3, 4, 9–11]. For example, of over 31 million women eligible for breast cancer screening in European countries, 79% were invited to screen and 49% were screened [3]. Breast cancer mortality has decreased in the USA from 31.4 to 20.5 deaths per 100,000 women between 1975 and 2014, with similar trends in Europe, an effect attributable to both screening and improved treatment [12].
Cancer screening programs have largely been implemented in high-income countries with greater available resources [9, 13, 14]. However, there is considerable variation in terms of screening methods, starting age, stopping age, and screening interval between countries [3, 4, 9]. For example, the USPSTF recommends that adults aged 50 to 75 years should be screened for colorectal cancer with one of seven tests or combinations of tests (including colonoscopy), [8] while the Canadian Task Force on Preventive Health Care does not recommend colonoscopy as a screening test [15]. In Europe, even after the European Union recommendations in 2003, the implementation of the Council recommendations differs between countries [3]. We hypothesize that countries spending more on healthcare per capita will have more intensive cancer screening recommendations, screening for more cancers over a longer age range, and at a shorter screening interval (Appendix).
In the current report, we will review and compare cancer screening recommendations implemented in 21 high resource countries that in 2015 spent at least $3000 per capita on healthcare. We will review national recommendations where available, or the most relevant other guidelines for that country where national or federal recommendations such as those of the USPSTF do not exist. The cancers addressed will include breast, cervical, colorectal, prostate, skin, and lung cancer. As a point of comparison with US national guidelines from the USPSTF, we include relevant specialty society guidelines from the American College of Radiology, American College of Obstetrics and Gynecology, and others.
Methods
The goal of the study was to compare cancer screening recommendations in countries with comparable levels of healthcare spending, and to try to understand the relationship between healthcare spending and the intensity of screening recommendations. Membership in the Organization for Economic Cooperation and Development (OECD) was chosen as the initial qualification for inclusion in the study to ensure that the countries compared were similar economically. It was then decided that total health expenditure per capita was the most relevant statistic to national cancer screening recommendations. Therefore, the 35 OECD member countries were rank ordered according to total healthcare expenditure per capita using data from 2015. From this ranked list, all countries that spent over USD3000 per capita on health that year were included (n = 21) [16].
The next step was to determine which national organization’s cancer screening guidelines would be chosen as that country’s national recommendations. First, colleagues of the authors in each country of the study were consulted as local content experts. This was supplemented by searches of the internet where necessary. Once the national recommendations were determined for each country, the data were abstracted in tandem by two members of the research team, consulting with the project leader (Dr. Ebell) to resolve any discrepancies.
To create a uniform grading scheme, each screening test for each type of cancer was graded as recommended (which includes both strongly recommended and recommended), recommended selectively, recommended against, or insufficient evidence, corresponding to the categories used by the US Preventive Services Task Force. After analysis of each country’s national screening recommendations, the data were organized into tables. Finally, screening intensity was defined for breast, cervical, and colorectal cancer as the total number of lifetime screening tests recommended for an average risk person.
Results
Information for the organizations making screening recommendations is shown in Table 1. We abstracted the screening’s recommendations from national guideline committee’s websites for 15 out of the 21 selected countries. The other countries’ recommendations are from cancer society’s websites. Notably, for the USA, beside the United Stated Preventive Services Task Force (USPSTF), we additionally abstracted the recommendations from the American Cancer Society (since it is widely used by US physicians) and three specialty society’s websites.
Table 1.
Organizations making screening recommendations, by country
| Country | Organization Name | Type of organization |
|---|---|---|
| United States | United States Preventive Services Task Force | National guideline committee |
| United States | American Cancer Society | Cancer society |
| United States | American College of Obstetrics & Gynecology | Specialty society |
| United States | American College of Radiology | Specialty society |
| United States | American Urological Association | Specialty society |
| Luxembourg | Ministry of Health | National guideline committee |
| Switzerland | League Against Cancer | Cancer society |
| Norway | Cancer Registry of Norway | Cancer society |
| Netherlands | National Institute for Public Health and the Environment | National guideline committee |
| Germany | Federal Joint Committee | National guideline committee |
| Sweden | National Board of Health and Welfare | National guideline committee |
| Ireland | National Screening Service | National guideline committee |
| Austria | Austrian Cancer Aid Society | Cancer society |
| Denmark | National Board of Health | National guideline committee |
| Belgium | Foundation Against Cancer | Cancer society |
| Canada | Canadian Task Force for Preventive Health Care | National guideline committee |
| Australia | Australian Government Department of Health | National guideline committee |
| France | National Cancer Institute | National guideline committee |
| Japan | National Cancer Center | National guideline committee |
| Iceland | Icelandic Cancer Society | Cancer society |
| United Kingdom | United Kingdom National Screening Committee | National guideline committee |
| Finland | Cancer Society of Finland | Cancer society |
| New Zealand | Ministry of Health | National guideline committee |
| Italy | National Screening Observatory | National guideline committee |
| Spain | Cancer Strategy of National Health System | National guideline committee |
Breast cancer screening
The recommendations for breast cancer screening with mammography are presented in Table 2. Overall, the recommendations for breast cancer are quite similar among the 21 selected countries. The most common screening age ranges are from 50 to 69 years old, and most of those countries are European. Screening for breast cancer every 2 years is recommended in most of the countries. Only Japan does not specify the screening interval. The American College of Radiology has the longest age range for screening and is the only guideline in the world recommending an annual screening interval, while the United Kingdom has the longest screening interval (every 3 years).
Table 2.
Recommendations for breast cancer screening with mammography, in order of overall healthcare spending
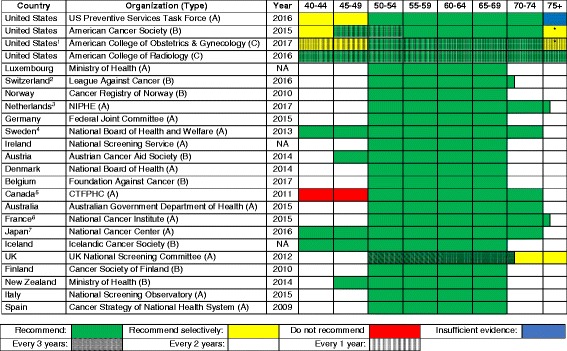
Type of organization: A national guideline committee, B cancer society, C specialty society, D other
1United States American College of Obstetrics and Gynecology: screening interval of 1 or 2 years
2Switzerland: screening age of 50–70 years
3Netherlands: screening age of 50–75 years
4Sweden: screening interval is 18 months from age 40 to 54 years
5Canada: screening interval of 2 or 3 years
6France: screening age of 50–75 years
7Japan: Do not recommend a screening interval
Abbreviations: UK United Kingdom, USA United States of America, NIPHE National Institute for Public Health and the Environment, CTFPHC Canadian Task Force on Preventive Health Care, NA not available (cannot find information)
*Consider the person’s life expectancy when making a decision
Cervical cancer screening
Table 3 describes cervical cancer screening’s recommendations. Luxembourg is the only country with no national recommendation identified for cervical cancer screening. There was some heterogeneity regarding the recommended tests (cytology, HPV, or both), the age to begin screening, and screening intervals. Most countries recommend an age of initiation of screening from 18 to 29 years and a stopping age between 60 and 70 years. Countries most commonly recommend a screening interval of 3 to 5 years. Six out of the 21 countries have adopted HPV testing as a primary test for cervical cancer screening, and cytology is still predominantly recommended with a screening interval of 3 years.
Table 3.
Recommendations for cervical cancer screening, in order of overall healthcare spending
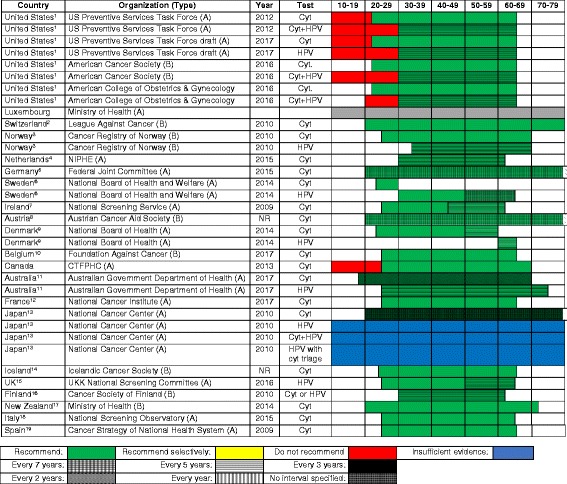
Type of organization: A national guideline committee, B cancer society, C specialty society, D other
Start and stop age coding: wavy border—not specified
Most guidelines recommend that screening cease between ages 65 and 70 years in women with consistently normal screening in the previous decade. Screening is not recommended for women who have had a hysterectomy for benign disease
1USA: screening age of 21–65 years for cytology and 30–65 years for cytology and HPV testing
2Switzerland: start and stop ages are not recommended
3Norway: screening age of and 25–69 years for cytology and 34–69 years for HPV testing
4Netherlands: screening age of 30–60 years
5Germany: stop age is not recommended
6Sweden: screening age of 23–29 years for cytology and 30–64 years for HPV testing
7Ireland: screening age of 25–61 years
8Austria: stop age is not recommended
9Denmark: screening age of 23–59 years for cytology and 60–64 years for HPV testing
10Belgium: screening age of 25–65 years for cytology
11Australia: screening age of 18–69 years for cytology (current recommendation) and 25–75 years for HPV testing (will implement from December 2017)
12France: screening age of 25–65 years
13Japan: stop age and screening interval are not recommended
14Iceland: screening age of 23–65 years
15UK: screening age of 25–64 years
16Finland: screening age of 30–60 years
17New Zealand: screening age of 20–70 years
18Italy: screening age of 25–64 years (some programs have moved into 25–30/35 years with cytology and 30/35–64 years with HPV testing)
19Spain: screening age of 25–65 years
Abbreviations: USPSTF United States Preventive Services Task Force, NIPHE National Institute for Public Health and the Environment, CTFPHC Canadian Task Force on Preventive Health Care, NA not applicable (cannot find information), Cyt. cytology, Cyt + HPV cytology plus HPV co-testing
+Date website with recommendation last updated
Colorectal cancer screening
Table 4 demonstrates the variability in screening recommendations across different countries with respect to both the type and schedule of screening. Even within the USA differences in guidelines exist, as the USPSTF and American Cancer Society (ACS) express no preference concerning the type of test, while the recent joint recommendations from several specialty societies recommend a tiered approach with colonoscopy or fecal immunochemical (FIT) testing offered first [17]. All other countries recommend a test for fecal occult blood, colonoscopy, or either. Regarding fecal occult blood testing, FIT is generally preferred over gFOBT, especially in more recent guidelines. Concerning the use of colonoscopy as a screening method, the only countries outside the USA that recommend it are Switzerland, Germany, and Austria. In some countries, the infrastructure may be lacking in terms of the number of trained gastroenterologists to support this screening method, although cost and acceptability are also important factors. The results summarized in Table 4 reflect no clear association between total healthcare expenditure per capita and colorectal cancer screening recommendations, other than that 4 of 5 countries recommending the shortest screening interval are among the lowest spenders per capita. Notably, only Austria and Japan recommend initiation of screening for average risk persons at age 40.
Table 4.
International colorectal cancer screening recommendations for the general population (persons not at “high-risk”), in order of decreasing total healthcare expenditure per capita
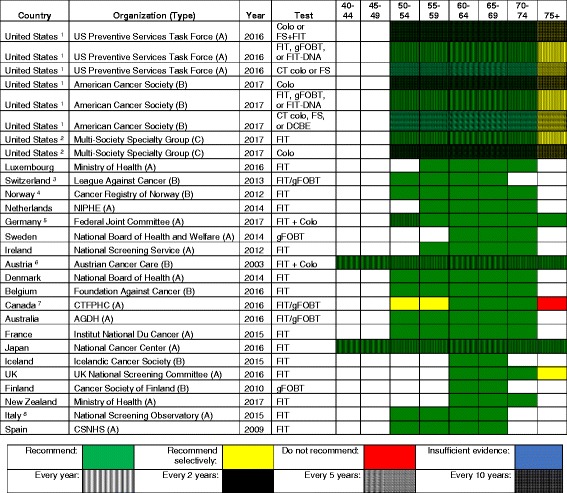
Type of organization: A national guideline committee, B cancer society, C specialty society or societies
1USPSTF and ACS: no preference regarding choice of tests; high sensitivity gFOBT preferred, with recommendation against in-office FIT or gFOBT; FIT-DNA is every 1 or 3 years; FS + FIT is FS every 10 years and FIT annually
2US Multi-Society Guidelines: consider screening between 75 and 85 if no previous screening
3Switzerland: or colonoscopy every 10 years
4Norway: currently being compared to flexible sigmoidoscopy (with screening once every 10 years from age 50 to 74) in a pilot study
5Germany: beginning at age 55, also obtain colonoscopy every 10 years (at a minimum), stopping after 2 colonoscopies
6Austria: beginning at age 50, also obtain colonoscopy every 7–10 years
7Canada: or flexible sigmoidoscopy every 10 years; colonoscopy is not recommended
8Italy: FIT or flexible sigmoidoscopy once at age 58 or 60, and then FIT every 2 years from age 59 to 69
Abbreviations: USA United States of America, Colo colonoscopy, CT colo CT colonography, FIT fecal immunochemical test, gFOBT guaiac fecal occult blood test, NIPHE National Institute for Public Health and the Environment, CTFPHC Canadian Task Force on Preventive Health Care, CSNHS Cancer Strategy of the National Health System, AGDH Australian Government Dept of Health
Prostate cancer screening
Table 5 demonstrates the variability between various countries regarding screening for prostate cancer. Unlike that with the other cancers, the chief disagreement concerning prostate cancer is not between the type and frequency of test but rather whether or not to screen at all. In fact, if one includes the current 2012 USPSTF recommendation, eight countries explicitly recommend against prostate cancer screening. Of the remaining countries that did not recommend against screening, the vast majority did not have an organized national screening program in place, recommended that an individual consult with their physician or did not make a recommendation.
Table 5.
International prostate cancer screening recommendations for the general population (persons not at “high-risk”), in order of decreasing total healthcare expenditure per capita
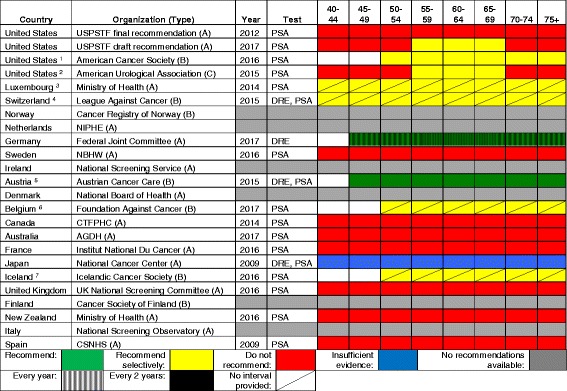
Interval coding: vertical cross-hatch every year, solid every 2 years, diagonal line no interval provided
Type of organization: A national guideline committee, B cancer society, C specialty society
1US American Cancer Society: screening should be done every year if PSA levels are greater than 2.5 ng/mL; stop screening in asymptomatic men with less than 10 years life expectancy; men at increased risk should consider beginning screening at 40 or 45 years
2US American Urological Association: an interval of 2 years or more is preferred. Additionally, baseline PSA levels can be used to individualize rescreening intervals. Screening should be stopped in asymptomatic men whose life expectancy is less than 10 years
3Luxembourg: age to start screening not provided. No organized national guidelines; screening based on individual discussion between patient and physician
4Switzerland: age to start screening not provided. No organized national guidelines; screening based on individual discussion between patient and physician
5Austria: “Screening should be carried out ‘regularly’ from the 45th birthday”
6Belgium: no organized national guidelines; screening based on individual discussion between patient and physician
7Iceland: no organized national guidelines; screening based on individual discussion between patient and physician
Abbreviations: USPSTF United States Preventive Services Task Force, PSA prostate-specific antigen, NIPHE National Institute for Public Health and the Environment, DRE digital rectal examination, NBHW National Board of Health and Welfare, CTFPHC Canadian Task Force on Preventive Health Care, AGDH Australian Government Department of Health, CSNHS Cancer Strategy of the National Health System
Skin cancer screening
Most countries included did not make a recommendation regarding skin cancer screening. Only the USA, Germany, Austria, and France address skin cancer screening. The latter three recommend screening, while the USPSTF has determined that there is insufficient evidence to recommend visual skin examination by a physician. Germany recommends that such examination take place every 2 years beginning at age 35, and France provides seven questions for general practitioners to ask their patients in order to assess risk. Uniquely, Austria recommends self-examination and recommends doing so twice a year (before and after the summer months). None of the countries recommending screening provide an age to stop screening.
Lung cancer screening
Among 21 selected countries, only 5 countries have recommendations regarding lung cancer screening (USA, Canada, Japan, United Kingdom, and Australia). Australia and the United Kingdom recommend against lung cancer screening. Both the USPSTF and the Canadian Task Force recommend low-dose computed tomography (CT) for smokers with at least 30 pack-year smoking history and who smoke or quit smoking less than 15 years. However, the USPSTF recommends screening for the age range 55 to 80 years, while the Canadian Task Force recommends screening for a narrower age range from 55 to 74 years, with a screening interval of 1 to 3 years. In contrast to the USA and Canada, Japan recommends chest radiography to screen for lung cancer starting at age 40 years, but concludes that there is insufficient evidence for low-dose CT. Japan does not recommend a stopping age or screening interval. Several European countries have screening trials underway, so these recommendations may change when those results are available [18, 19].
Association between healthcare expenditures and screening intensity
Figure 1 summarizes the relationship between healthcare expenditures and the intensity of screening, measured as the total lifetime number of screening tests recommended for an average risk person for breast, cervical, and colorectal cancer (the latter based on recommendations for use of the FIT). It shows that there is no clear association between expenditures and screening intensity for these cancers, disproving our initial hypothesis. Regarding prostate cancer, 5 of the top 10 countries in terms of healthcare expenditure recommend selective or routine screening, compared with only 2 of the bottom 10 countries. Finally, the three countries recommending lung cancer screening are ranked 1st, 12th, and 15th in spending, again creating no pattern of higher spending equating to higher intensity of screening.
Fig. 1.
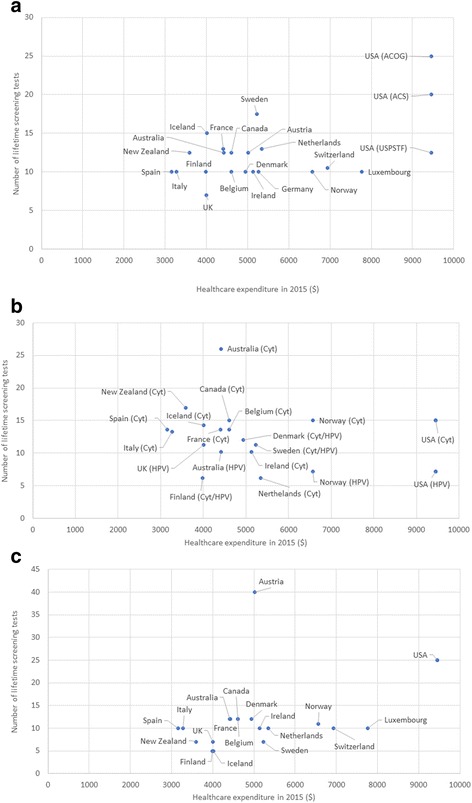
This figure shows the relationship between per capita spending on healthcare with the number of lifetime screening tests recommended for a) breast cancer (mammography), b) cervical cancer (cytology or HPV), and c) colorectal cancer (FIT)
Discussion
We have summarized national screening recommendations for the 21 countries with the highest total healthcare expenditure per capita. These countries were selected because they are likely to have adequate resources for a cancer screening program of some kind; comparison with less well-resourced settings would be unfair and would be less likely to reflect the differences in values, priorities, and the assessment of evidence that we are interested in. As physicians and researchers in the USA, we are especially interested in how our recommendations compare with other countries, and what we can learn from them.
In comparing recommendations, we are not making value judgements or endorsing a single “correct” answer regarding age at initiation, screening interval, or age at cessation of screening. These decisions likely reflect differences between countries and organizations regarding how benefits and harms are valued and balanced at a societal level, the type of evidence considered, the availability of resources and infrastructure for screening (for example, adequate trained medical personnel to perform colonoscopy), whether and how cost is considered, and different standards for assembling, evaluating, and interpreting a complex evidence base. For example, does the body making a recommendation consider only randomized controlled trials with cancer-specific mortality as the outcome, or does it also consider observational data and modeling studies? [20] Does the panel consider outcomes beyond mortality, such as disease progression, cost, stage shifts, or quality of life? Is cost explicitly considered or is the decision made solely on the balance of potential benefits and harms?
Note that in our discussion below, we consider the USPSTF to represent the US national screening recommendation, although recommendations from USA specialty and cancer societies are also presented. Also, when we use the term “more aggressive” or “more conservative” to describe screening recommendations, we refer to recommendations with a broader age range and/or a more frequent interval vs a narrower age range and/or a less frequent interval. Finally, a review of the process used by each country or organization to develop and update guidelines is beyond the scope of this article.
Commonalities
There was considerable homogeneity regarding screening recommendations for breast cancer, cervical cancer, and to some extent, colorectal cancer. A starting age for routine mammography of 50 years was recommended by 16 of 21 countries; all countries but one recommended a biennial interval, and 14 recommended a stopping age of 69 or 70 years. Similarly, most countries recommended cervical cancer screening beginning between 21 and 30 years of age (depending on whether or not testing for human papillomavirus was employed), and most recommended a stopping age between 65 and 70 years. Similarly, most countries recommended that colorectal cancer screening begin at age 50 or 55 years and stop by 75 years. Finally, there is a general consensus against screening for lung cancer and melanoma, with only the USA, Japan, and Canada recommending lung cancer screening and only Germany, Austria, and France recommend some approach to screening for skin cancer.
Differences
The approach to screening for cervical cancer is evolving, with some countries still recommending cytology only, some recommending HPV testing or co-testing, and some giving clinicians the option of choosing the favored approach. Four countries (Iceland, UK, Sweden, and Finland) do not recommend screening for colorectal cancer until age 60, compared to start ages between 40 and 50 years for most other countries. The recommended test for colorectal cancer screening also varies. FIT was most widely recommended, while the USPSTF offered seven different options, and Germany and Austria recommended FIT for younger patients followed by a series of colonoscopies. There is considerable heterogeneity regarding prostate cancer screening: seven countries recommend screening for prostate cancer in some form, while eight explicitly recommend against it. This is likely due to variation in how guideline panels assess the potential benefits and harms.
Variation by country and type of organization
For prostate cancer screening, of the seven countries with a recommendation to screen or screen selectively for prostate cancer, five were from the top half of the selected countries based on per capita health expenditures. Four of the five countries with the shortest interval for colorectal cancer screening (Iceland, UK, Finland, and New Zealand) are among the six lowest spending countries. However, there was no apparent association between per capita health expenditures and the intensity of screening for breast and cervical cancer.
Other than within the USA, there was no clear difference in terms of the intensity of screening recommendations coming from national guideline committees, cancer societies or leagues, and specialty societies. In the USA, the recommendations regarding mammography from the American Cancer Society, American College of Obstetrics and Gynecology, and American College of Radiology were the only ones identified that recommended annual screening and also had longer screening intervals for patients at average risk. The American College of Radiology was the only body that recommended annual mammography starting at age 40 years, with no specified stopping age. On the other hand, there is considerable similarity regarding colorectal cancer screening between the USPSTF, ACS, and specialty society guidelines.
Prostate cancer screening recommendations are now similar between the USPSTF draft recommendation of 2017 and the American Urology Association, and recommendations regarding cervical cancer screening between the USPSTF, ACS, and ACOG are nearly identical. Assuming that they are based on the best available evidence, this kind of “harmonization” between guidelines from different groups within a country sends a clear, unified message to patients and physicians. In the absence of such harmonization, confusion may reign and physicians may do what feels right or what is requested by patients rather than what is supported by the best evidence. Due to medicolegal concerns, some physicians may feel compelled to practice based on the most aggressive set of recommendations or based on patient request. This is especially true in the USA context, where “failure to diagnose” is the most common reason for a malpractice lawsuit [21].
Conclusions
Guidelines for cancer screening differ between countries, with areas of commonality but also clear differences. Per capita healthcare spending among wealthy countries appears to have relatively little impact on recommendations; differences are more likely to stem from variation in how benefit and harm are evaluated, and which evidence is considered. Intellectual and financial conflicts of interest inherent to professional societies may contribute to more aggressive recommendations for breast cancer screening from these groups in the USA. A greater intensity of screening may alter the balance of benefits and harms by increasing the likelihood of direct harms of the screening test, as well as the serious harms associated with overdiagnosis [22]. In some cases, where specialty societies have adopted more rigorous methods, their recommendations have become more conservative, as when the American Urologic Association moved from a consensus to an evidence-based process [23]. Of course, one could also argue that national bodies like the USPSTF or Canadian Task Force are overly conservative since they rely largely on randomized trials and modeling for their recommendations. And, even groups using similarly rigorous methods may reach different conclusions, with USA and Canadian recommendations for colorectal cancer screening a good example.
In conclusion, we encourage the formation of independent panels of experts in each country, modeled after the USPSTF, Canadian Task Force, and the UK National Screening Committee. These panels should make independent, evidence-based recommendations regarding screening that assess the benefits, harms, and available resources in that country’s context. Consistent with IOM recommendations, the panels should include primary care physicians, patients, methodologic experts and relevant subspecialists, recommendations should be regularly updated, and panel members should be free of financial and intellectual conflict of interest to the greatest extent possible [24].
Acknowledgements
Dr. Roland Grad (Magill University, Montreal, Canada) and Dr. Kenneth W. Lin (Georgetown University, Washington, DC) provided feedback and comments on a final draft of the manuscript.
Funding
This project was unfunded.
Availability of data and materials
Not applicable
Appendix
Table 6.
The references of the national recommendations for breast, cervical, colorectal, prostate, skin, and lung cancer screening by country
Authors’ contributions
MHE conceptualized and led the project. TNT and KJR abstracted the data and created the tables, and also wrote the first drafts of the “Introduction,” “Methods,” and “Results” sections. These were edited by MHE, who also reviewed the data abstraction for accuracy and wrote the “Discussion” section. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO . Global Health Estimates 2015: deaths by cause, age, sex, by country and by region, 2000–2015. 2016. [Google Scholar]
- 2.Ferlay J, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer . Cancer screening in the European Union (2017): report on the implementatation of the council recommendation on cancer screening. 2017. [Google Scholar]
- 4.International Agency for Research on Cancer . IARC handbooks of cancer prevention volume 10: cervix cancer screening. Lyon: IARC; 2005. [Google Scholar]
- 5.Siu AL. Screening for breast cancer: U.S. Preventive Services Task Force Recommendation statement. Ann Intern Med. 2016;164(4):279–296. doi: 10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- 6.Moyer VA. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–891. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 7.Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 8.Force, U.S.P.S.T et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(23):2564–2575. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 9.Schreuders EH, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64(10):1637–1649. doi: 10.1136/gutjnl-2014-309086. [DOI] [PubMed] [Google Scholar]
- 10.WHO . WHO Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention. 2013, World Health Organization copyright (c) Geneva: World Health Organization; 2013. Guidelines approved by the guidelines review committee. [Google Scholar]
- 11.WHO . WHO position paper on mammography screening. Geneva: World Health Organization; 2014. [PubMed] [Google Scholar]
- 12.Berry DA, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 13.Lauby-Secretan B, et al. Breast-cancer screening—viewpoint of the IARC Working Group. N Engl J Med. 2015;372(24):2353–2358. doi: 10.1056/NEJMsr1504363. [DOI] [PubMed] [Google Scholar]
- 14.Olson B, et al. Cervical cancer screening programs and guidelines in low- and middle-income countries. Int J Gynaecol Obstet. 2016;134(3):239–246. doi: 10.1016/j.ijgo.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Bacchus CM, et al. Recommendations on screening for colorectal cancer in primary care. CMAJ. 2016;188(5):340–348. doi: 10.1503/cmaj.151125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Development, O.f.E.C.a. Health expenditure and financing. OECD Statistics; Available from: http://stats.oecd.org/Index.aspx?DataSetCode=SHA. Accessed Feb 2018.
- 17.Rex DK, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal cancer. Am J Gastroenterol. 2017;112(7):1016–1030. doi: 10.1038/ajg.2017.174. [DOI] [PubMed] [Google Scholar]
- 18.Field JK, et al. The UK Lung Cancer Screening Trial: a pilot randomised controlled trial of low-dose computed tomography screening for the early detection of lung cancer. Health Technol Assess. 2016;20(40):1–146. doi: 10.3310/hta20400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paci E, et al. Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax. 2017;72(9):825–831. doi: 10.1136/thoraxjnl-2016-209825. [DOI] [PubMed] [Google Scholar]
- 20.Owens DK, et al. Use of decision models in the development of evidence-based clinical preventive services recommendations: methods of the U.S. Preventive Services Task Force. Ann Intern Med. 2016;165(7):501–508. doi: 10.7326/M15-2531. [DOI] [PubMed] [Google Scholar]
- 21.Wallace E, et al. The epidemiology of malpractice claims in primary care: a systematic review. BMJ Open. 2013;3(7):e002929. [DOI] [PMC free article] [PubMed]
- 22.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367(21):1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 23.Lenzer J, et al. Ensuring the integrity of clinical practice guidelines: a tool for protecting patients. BMJ. 2013;347:f5535. doi: 10.1136/bmj.f5535. [DOI] [PubMed] [Google Scholar]
- 24.Institute of Medicine . Clinical practice guidelines we can trust. Washington, D.C.: The National Academies Press; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable


