Abstract
Background
Gestational disorders such as intrauterine growth restriction (IUGR) and pre-eclampsia (PE) are main causes of poor perinatal outcomes worldwide. Both diseases are related with impaired materno-fetal nutrient transfer, but the crucial transport mechanisms underlying IUGR and PE are not fully elucidated. In this study, we aimed to identify membrane transporters highly associated with transplacental nutrient deficiencies in IUGR/PE.
Results
In silico analyses on the identification of differentially expressed nutrient transporters were conducted using seven eligible microarray datasets (from Gene Expression Omnibus), encompassing control and IUGR/PE placental samples. Thereby 46 out of 434 genes were identified as potentially interesting targets. They are involved in the fetal provision with amino acids, carbohydrates, lipids, vitamins and microelements. Targets of interest were clustered into a substrate-specific interaction network by using Search Tool for the Retrieval of Interacting Genes. The subsequent wet-lab validation was performed using quantitative RT-PCR on placentas from clinically well-characterized IUGR/PE patients (IUGR, n = 8; PE, n = 5; PE+IUGR, n = 10) and controls (term, n = 13; preterm, n = 7), followed by 2D-hierarchical heatmap generation. Statistical evaluation using Kruskal-Wallis tests was then applied to detect significantly different expression patterns, while scatter plot analysis indicated which transporters were predominantly influenced by IUGR or PE, or equally affected by both diseases. Identified by both methods, three overlapping targets, SLC7A7, SLC38A5 (amino acid transporters), and ABCA1 (cholesterol transporter), were further investigated at the protein level by western blotting. Protein analyses in total placental tissue lysates and membrane fractions isolated from disease and control placentas indicated an altered functional activity of those three nutrient transporters in IUGR/PE.
Conclusions
Combining bioinformatic analysis, molecular biological experiments and mathematical diagramming, this study has demonstrated systematic alterations of nutrient transporter expressions in IUGR/PE. Among 46 initially targeted transporters, three significantly regulated genes were further investigated based on the severity and the disease specificity for IUGR and PE. Confirmed by mRNA and protein expression, the amino acid transporters SLC7A7 and SLC38A5 showed marked differences between controls and IUGR/PE and were regulated by both diseases. In contrast, ABCA1 may play an exclusive role in the development of PE.
Electronic supplementary material
The online version of this article (10.1186/s12864-018-4518-z) contains supplementary material, which is available to authorized users.
Keywords: Bioinformatics, Membrane transporters, Placenta, Pre-eclampsia, Intrauterine growth restriction
Background
Worldwide, more than one third of child deaths can be attributed to maternal and fetal undernutrition [1]. Along the course of gestation, the fetus receives organic and inorganic nutrients from the mother, such as amino acids, carbohydrates, lipids, vitamins and minerals, for adequate development and growth. To avoid direct materno-fetal blood contact, the placenta has distinctive anatomical barriers for regulating the substrate exchange [2, 3]. Various molecules originating from maternal blood enter the fetal circulation after crossing successively the apical microvillous plasma membrane (MVM), the cytoplasm, and the basal plasma membrane (BM) of the trophoblast layer [3, 4]. On the other side, the fetus eliminates waste products via efflux processes at the BM into the maternal veins. This highlights the crucial role of substrate specific membrane transporter proteins at these different locations. Indeed, for several membrane transporter proteins (e.g. Ca2+ -ATPase, ferroportin, system A amino acid transporters) an asymmetric distribution between MVM and BM has been reported. Although this asymmetry is a prerequisite for net transport across the placenta via transporter proteins, the mechanisms of protein trafficking that result in distribution to either MVM or BM in the polarized syncytiotrophoblast are largely unknown. It is evident, however, that any reduction of the quantity, density, and activity of membrane transporters at the placental microvilli could impact the rates of the nutrient supply, which may subsequently compromise the fetal development. It is well recognized that gestational diseases such as intrauterine growth restriction (IUGR) and pre-eclampsia (PE), which complicate a significant proportion of pregnancies leading to poor perinatal outcome, are closely related to impaired placental nutrient transfer [5, 6].
Transfer of amino acids across the placenta is mediated by substrate selective amino acid transport systems. So far, more than 15 different systems have been identified in the human placenta, which include Na+-dependent systems A, N, ASC, β, XAG−, XC−, GLY and Na+-independent systems L, y+, y+L, T, bo,+ [5, 7, 8]. Reduced activity of amino acid transporters e.g. system A in IUGR and PE has been reported [9–11], which might be due to reduced sodium-potassium ATPase (Na+/K+ATPase) activity [12] and a competitive inhibition by physiologically elevated maternal plasma homocysteine [13, 14]. The transplacental transport of glucose mainly occurs along a concentration gradient facilitated by the glucose transporter (GLUT) family, while lipids are mostly packed into circulating protein particles by virtue of active membrane transporters [15]. Current knowledge in this field revealed an increased concentration gradient from maternal to fetal sides in IUGR and PE to achieve higher glucose and lipid transfer [16, 17]. These significant elevations have also been considered as a risk factor for those gestational disorders [18, 19], which may be explained by an altered expression of membrane transporter [20, 21].
The fetal provision with vitamins and dietary elements is required, since they are essential to regulate the fetal metabolism as well as extra- and intra-cellular osmolality. Global studies under the auspices of the World Health Organization in 2008 proposed that deficiencies in micronutrients such as thiamine (Vitamin B1), riboflavin (Vitamin B2), folate (Vitamin B9), cobalamin (Vitamin B12), and ascorbic acid (Vitamin C), are highly prevalent and occur concurrently among pregnant women [1]. It has been reported that the elevated serum homocysteine levels in IUGR and PE were accompanied by decreasing serum levels of the aforementioned class B vitamins. The dietary treatment of pregnant women with diverse vitamin B supplements improved fetal weight [22–26]. However, data regarding the transplacental transport of vitamin B are scarce. In this context SLC19 family members were found to transport folate (SLC19A1) and thiamine (SLC19A2 and SLC19A3) [27]. Interestingly, in IUGR placentas, an increase in mRNA level of SLC19A1 and SLC19A2 has been reported [28], which was interpreted as a compensatory effect. Besides, deficiencies in serum calcium, magnesium and zinc might greatly contribute to the pathogenesis of PE, since it was suggested that the dietary supplementation of these elements can reduce the severity of PE [29–31]. Although calcium is probably the most studied element among all ions, publications investigating the role of transporters or exchangers in placental calcium homeostasis are negligible (< 20). It was found that the expression of Ca2+-selective receptor channels and Na+/Ca2+ exchangers were significantly increased in hypertensive placentae compared to normotensive tissues [32].
The studies mentioned above and numerous others have suggested that an altered transplacental nutrient transport might elicit IUGR/PE [5, 6, 33]. However, the detailed underlying mechanisms involving membrane transporters are still not fully elucidated. Therefore, we conducted the current study to gain a comprehensive knowledge on the importance of placental transporter proteins majorly involved in the fetal provision with amino acids, carbohydrates and derivatives, lipids, vitamins, and microelements. We hypothesized that the expression of placental membrane transporters was modified by IUGR/PE. To identify the key nutrient transporters in placenta, we have used a multifaceted approach including bioinformatic analysis, molecular biology, and mathematical diagramming (Fig. 1).
Fig. 1.
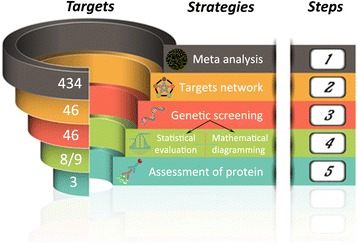
Strategy to target nutrient transporters in intrauterine growth restriction and pre-eclampsia using multiple approaches. The funnel like structure of the different analytical approaches represents the decreasing number of targets which overlap in every step of the analyses. Originating from 434 genes in meta-analysis of microarray data (1), 46 differentially expressed candidate genes were selected and clustered by STRING network (2). Gene expression screening of an in house placental tissue collection in the laboratory (3) was analysed by statistical evaluation and mathematical diagramming (4). The resulting 3 overlapping transporters were finally subjected to the biochemical analyses of protein translation and membrane localization
Methods
Identification of target transporters by microarray processing
Publicly available data was obtained at http://www.ncbi.nlm.nih.gov/geo through the accession numbers GSE 25861, 24129, 12216, 25906, 14722, 35574, 24818. Robust multi-array averaging (RMA) and quantile normalization were used to quantify gene expression. Significant differences were identified applying a Bayesian approach using the limma package (R 3.1, Bioconductor 2.7). We analysed gene expression data of solute carriers (SLCs), ATP-binding cassette transporters (ABCs) and transient receptor potential channels (TRPs). Any SLC, ABC and TRP family member, which i) was represented in more than 3 studies, ii) was listed in top 12 up- or down-regulated genes, and iii) has an annotated a biological significance in placenta, was added to the candidate list.
Visualization of biomolecular interaction network
To better understand the network of the identified nutrient transporters from microarray data, we applied the identified targets of interest to Search Tool for the Retrieval of Interacting Genes (STRING) 9.1 at http://string-db.org with a focus on the direct (physical) and indirect (functional) targets’ connections. In the STRING tool, we restricted the prediction to human placenta with medium protein-protein interactions (associated with a confidence score larger than 0.4), and clustered by the Markov cluster algorithm (MCL).
Placental sample collection
The study was approved by the local ethic institutional review board of the Canton of Bern with an informed consent obtained from each participant prior to the caesarean section. Pregnant women were under obstetrical care at the Department of Obstetrics and Gynecology, University Hospital (Bern, Switzerland). They were assigned to five groups: term controls, preterm, IUGR, PE, and PE combined with IUGR (PE + IUGR). Term controls consisted of the pregnancies without pathologies and terminated at term (≥ 37 gestational weeks) by elective primary caesarean section upon patients’ request or due to breech presentation. The preterm group was defined as the babies born prior to 37 weeks of pregnancy, but without further abnormal maternal or fetal symptoms. According to the definition from the International Society for the Study of Hypertension in Pregnancy, patients with the dramatic increase of systolic/diastolic blood pressure (> 140/90 mmHg) and urinary protein content in 24 h (> 0.30 g) were classified in PE related groups. When the fetal birth weight was below the 10th percentile for babies of the same gestational age, the baby was also referred to as small for gestational age due to the IUGR effects. As it is a severe complication when PE occurs concomitantly with IUGR, we also included and characterized patients suffering from PE combined with IUGR (PE + IUGR).
Placental tissue collection was performed and fully validated as previously described [21, 34]. Immediately after caesarean section, chorionic tissue was dissected from a standardized central location, and snap-frozen in liquid nitrogen after a thorough wash with Dulbecco’s phosphate buffered saline (Gibco). The collected tissue specimens were stored at -80 °C until further analysis.
Quantitative RT-PCR
Total RNA was isolated from placental tissue with a modified protocol, using a Trizol reagent kit (Invitrogen) [34]. Approx. 50 mg frozen placental tissues were homogenized in 1 ml cold Trizol on ice with a Polytron homogenizer (Kinematica AG), followed by phenol/chloroform extraction and precipitation by isopropanol, and final washing with 75% ethanol. After re-suspension in RNase free water, the extracted RNA was evaluated qualitatively (assessment of RNA integrity) by using Agilent 2100 Bioanalyzer (Agilent Technologies) and quantitatively (determination of RNA concentration) with NanoDrop 1000 (Thermo Scientific). All samples used in this study fulfilled the fixed selection criteria which were: RNA integration number (RIN) > 6, spectrophotometric measurements at 260/280 > 2.0 and 260/230 > 1.8, and the yield of RNA > 5 ng/μl.
Two microgram RNA was reverse transcribed into cDNA using GoScript™ Reverse Transcriptase System (Promega). Quantitative RT-PCR reactions were then performed in 10 μl volume with 1 μl cDNA template, 0.3 μM specific primers (listed in Additional file 1) and GoTaq® qPCR Master Mix (Promega) on the ViiA™ 7 Real-Time PCR System (Applied Biosystems). Based on the results of the transporter mRNA expression, the heatmap was generated using centred correlation (Cluster 3.0) and average linkage clustering analysis for similarity measurement and clustering (TreeView at http://diyhpl.us/~bryan/irc/protocol-online/protocolcache/EisenSoftware.htm). The consecutive statistical analysis and mathematical diagramming served for the identification of significant and disease-specific targets, respectively.
Total protein and membrane fraction extraction
To extract total protein from placenta, tissue samples were homogenized in ice-cold hypotonic buffer (10 mM Tris-HCl, 10 mM NaCl, 1.5 mM MgCl2 and 0.1% Triton X-100, pH 7.4) supplemented with protease inhibitor cocktail (Sigma), and centrifuged at 1000 × g for 10 min at 4 °C. The supernatants were collected in fresh tubes and stored at -80 °C.
Membrane fractions from placenta tissues were extracted using membrane, nuclear and cytoplasmic protein extraction kit according to the manufacturer’s protocol (Bio Basic Inc.). The whole extraction procedure was carried out on ice in the presence of protease inhibitor, phosphatase inhibitor, dithiothreitol and phenylmethylsulfonyl fluoride (PMSF). The “identity” of the extracted cellular membrane fractions was validated by detecting the corresponding expression of the plasma membrane marker Novus Biologicals, the nuclear marker histone H3 (Novus Biologicals) and the cytoskeleton marker β-actin (Sigma).
The protein content of total lysates and membrane fractions was quantified by bicinchoninic acid assay (Pierce), using bovine serum albumin as standard.
Western blotting
90 μg of the total tissue lysate or 30 μg of membrane fraction was mixed with Laemmli sample buffer and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (Bio-Rad) using 10% acrylamide gels. The immobilized bands were then semi-dry transferred to nitrocellulose membranes (GE Healthcare). Blots were blocked with 5% non-fat milk in Tris Buffered Saline with 0.1% Tween-20. The following antibodies were used: rabbit anti-ABCA1 (Novus Biologicals), rabbit anti-SLC7A7 (Novus Biologicals), and rabbit anti-SLC38A5 (Aviva Systems Biology), as well as mouse anti-β-actin or mouse anti-Na+/K+ ATPase as internal controls. Primary antibodies were first incubated overnight at 4 °C followed by 4 times washing with Tris-buffered saline supplemented with 0.1% Tween 20 (TBST), and then incubation with DyLight 680 or 800 fluorescence conjugated secondary antibodies (Thermo Scientific). The immunoreactive bands were visualized with the Odyssey® Sa Infrared Imaging System (LI-COR) to obtain relative densitometry values.
Statistical analysis and mathematical diagramming
The comparative threshold cycle (Ct) method was applied to analyze relative gene expression by fold change = 2−∆(∆Ct), where ∆Ct = Cttarget gene - Ctreference gene and ∆(∆Ct) = ∆(∆Cttest - ∆Ctcontrol) [35].
Data were statistically evaluated using GraphPad Prism 6 Software. Kruskal-Wallis analysis followed by a Dunn’s multiple comparison test, were used to analyse statistical differences of the clinical records, the mRNA and protein expression data between the term controls and preterm, IUGR, PE and PE + IUGR groups. A p value of less than 0.05 was considered statistically significant.
In addition, scatter plotting was applied to estimate the influence of gestational diseases on transporter expression. Each point depicts a nutrient transporter, whose x-axis and y-axis represents the average log 2-fold changes (FC) in the IUGR group and PE group, respectively. To avoid possible bias (individual variation, experimental error, etc.), we selected only those targets, whose expression has been modulated with an absolute FC > 1.14 (i.e. > 114% or < 86%) over the expression in the term controls [36]. Subsequently, the ratio of y to x was considered as a rank variable. When the ratio is close to 0, it means that the predominant effect on transporter mRNA expression is caused by PE. Conversely, when it approaches infinity, the altered transporter is primarily influenced by IUGR. An intermediate state (i.e. a ratio equal to 1 or -1), indicates an equal (1) or an equal but reversed (-1) impact on the transporter expression level by IUGR and PE.
Results
Microarray processing
Six placental gene expression studies focusing on IUGR/PE plus one project investigating umbilical cords associated with low birth weight, were included in this meta-analysis (Table 1). These studies represented in total 258 samples, including 129 controls (term and preterm samples), 67 IUGR-related specimens and 62 PE placentas.
Table 1.
Microarray databank information
| Citation | Data sources | Publication date | Platform | Sample origin | Sample size | Probes | ||
|---|---|---|---|---|---|---|---|---|
| Control | IUGR | PE | ||||||
| Dunk et al. [52] | GSE25861 | 2012-04-01 | Affymetrix | Isolated PIMEC | 3 (p) | 6a | 54,675 | |
| Nishizawa et al. [53] | GSE24129 | 2010-09-15 | Affymetrix | Placenta | 8 (m) | 8 | 8 | 32,321 |
| Sitras et al. [54] | GSE12216 | 2008-08-01 | Applied Biosystems | Placenta | 8 (m) | 8a | 32,878 | |
| Tsai et al. [55] | GSE25906 | 2010-12-10 | BeadArray | Placenta | 37 (m) | 23 | 48,701 | |
| Winn et al. [56] | GSE14722 | 2009-02-06 | Affymetrix | Placenta | 11 (p) | 12 | 42,928 | |
| Guo et al. [57] | GSE35574 | 2012-02-07 | Illumina | Placenta | 40 (m) | 27 | 19 | 48,701 |
| GSE24818 | 2011-10-21 | Agilent | Umbilical cord | 22 | 18 | 37,903 | ||
Data used for meta-analysis was obtained by analysing the Gene Expression Omnibus (GEO) at http://www.ncbi.nlm.nih.gov/geo
(p) preterm controls (m) mixture of preterm and term controls
Abbreviation: PIMEC Fetoplacental microvascular endothelial cell
aIUGR is defined as birth weight < 5th percentile
Based on the selection criteria for potential transporter candidates described in the section Methods, 46 of the 434 genes were chosen for consecutive analysis. These genes encode amino acids, carbohydrates and derivatives, lipids, vitamins, microelements, and other transport channels (Table 2). Among the selected targets, 32 genes exhibited increased expression levels in pathological pregnancies compared to controls, while 14 genes showed the opposite trend.
Table 2.
Selected nutrient transporters according to their regulational signature and physiological significance
| Substrate class | Predominant (predicted) substrate | Gene name | Accession number | Protein name | Log (FC) | Representative references |
|---|---|---|---|---|---|---|
| Amino acids | System L, y+L, xc-and asc [58, 59] | SLC3A2 | NM002394 | 4F2hc | − 0.42~ 0.28 | [60–63] |
| Cationic L- amino acids | SLC7A1 | NM003045 | CAT1 | − 0.52~ 0.89 | [64–67] | |
| Large neutral L-amino acids | SLC7A5 | NM003486 | LAT1 | −0.38~ 0.46 | [62, 63, 66, 68–71] | |
| Cationic amino acids, large neutral L-amino acids | SLC7A6 | NM003983 | y+LAT2 | −0.23~ 0.39 | [69, 72] | |
| SLC7A7 | NM001126105 | y+LAT1 | − 0.44~ 0.89 | [72] | ||
| Neutral L – amino acids | SLC7A8 | NM012244 | LAT2 | −0.12~ 0.25 | [73] | |
| Cystine (anionic form), L-glutamate | SLC7A11 | NM014331 | xCT | −0.10~ 0.53 | – | |
| Gln, Ala, Asn, Cys, His, Ser | SLC38A1 | NM030674 | SNAT1 | −0.28~ 1.24 | [68, 74, 75] | |
| Ala, Asn, Cys, Gln, Gly, His, Met, Pro, Ser | SLC38A2 | NM018976 | SNAT2 | − 0.11~ 0.11 | [50, 68, 75–78] | |
| Gln, Asn, His, Ser | SLC38A5 | NM033518 | SNAT5 | 0.01~ 0.21 | [49] | |
| Polyamines, amino acids [79] | SLC12A8 | NM024628 | CCC9 | −0.45~ 1.09 | – | |
| Vitamins | Thiamine [27] | SLC19A2 | NM006996 | THTR1 | 0.28~ 0.66 | [80] |
| SLC19A3 | NM025243 | THTR2 | −1.17~ − 0.12 | [80, 81] | ||
| Folate | SLC19A1 | NM194255 | RFC | −0.10~ 0.84 | [82–85] | |
| SLC46A1 | NM080669 | PCFT | − 0.15~ 0.05 | [82, 86] | ||
| Biotin, pantothenic acid [87] | SLC5A6 | NM021095 | SMVT | 0.01~ 0.04 | [88, 89] | |
| Cobalamin [90] | LMBRD1 | NM018368 | LMBRD1 | −0.26~ 0.22 | – | |
| (Pyridoxine) [91] | SLC22A15 | NM018420 | FLIPT1 | −0.14~ 0.10 | – | |
| L-ascorbic acid [92, 93] | SLC23A1 | NM005847 | SVCT1 | 0.01~ 0.10 | [94, 95] | |
| SLC23A2 | NM005116 | SVCT2 | −0.38~ 0.43 | [95–97] | ||
| SLC23A3 | NM144712 | SVCT3 | − 0.21~ 0.33 | – | ||
| Microelements and ions | Zn2+ [98, 99] | SLC30A1 | NM021194 | ZNT1 | −0.02~ 0.34 | [100–102] |
| SLC30A2 | NM032513 | ZNT2 | − 0.06~ 0.17 | [100, 101, 103] | ||
| SLC30A4 | NM013309 | ZNT4 | −0.21~ 0.05 | [100, 101] | ||
| SLC39A1 | NM014437 | ZIP1 | − 0.13~ 0.04 | [100] | ||
| SLC39A8 | NM022154 | ZIP8 | 0.00~ 0.95 | [104] | ||
| Ca2+ | TRPV6 | NM018646 | TRPV6 | 0.02~ 0.43 | [32, 105–107] | |
| e.g. Cl−,(COO)22− [108] | SLC4A1 | NM000342 | AE1 | −0.48~ 0.53 | [109, 110] | |
| e.g. SO42− [111] | SLC26A2 | NM000112 | DTDST | −0.78~ 0.74 | [43, 112] | |
| SLC26A6 | NM134426 | Pat-1 | − 0.09~ 0.47 | [112] | ||
| e.g. Na+, H+ [113, 114] | SLC9A1 | NM003047 | NHE1 | −0.34-0.00 | [110, 115–117] | |
| SLC9B2 | NM178833 | NHA2 | − 0.35~ − 0.34 | – | ||
| e.g. I− [111, 118] | SLC5A5 | NM000453 | NIS | −0.12~ 0.08 | [119–122] | |
| SLC26A4 | NM000441 | PDS | 0.00~ 0.04 | [121, 123, 124] | ||
| e.g. Fe2+, Zn2+ [125] | SLC11A2 | NM000617 | DMT1 | −0.07~ 0.67 | [126–128] | |
| Lipids | Cholesterol, phospholipids [129] | ABCA1 | NM005502 | ABCA1 | −0.19~ 0.71 | [21, 130, 132] |
| ABCG1 | NM207629 | ABCG1 | − 0.20~ 0.33 | [130, 131] | ||
| Drugs, toxins [133] | ABCG2 | NM004827 | ABCG2 | −0.70~ 0.48 | [134–136] | |
| e.g. flavonoids [137] | SLC47A1 | NM018242 | MATE1 | −0.03~ 0.33 | [138, 139] | |
| LCFA, VLCFA [140] | SLC27A2 | NM003645 | FATP2 | −0.58~ 1.37 | [141, 142] | |
| SLC27A4 | NM005094 | FATP4 | − 0.04~ 0.05 | [142–144] | ||
| Carbohydrates (+ metabolic derivatives) | Glucose [145] | SLC2A1 | NM006156 | GLUT1 | 0.12~ 0.66 | [20, 40, 146] |
| Lactate [147] | SLC16A1 | NM003051 | MCT1 | −0.36~ 1.35 | [148–150] | |
| SLC16A3 | NM004207 | MCT4 | − 0.12~ 0.48 | [148–151] | ||
| SLC16A4 | NM004696 | MCT5 | − 0.50~ 0.47 | [149] | ||
| Neurotransmitter | Norepinephrine [152] | SLC6A2 | NM001043 | NET | −0.31~ 0.54 | [153–155] |
Forty six entries were ordered alphabetically by gene name; pivotal information of targets including a representative example of accession number, and protein name, substrate (class), and Log (FC) value from meta-analysis is listed
Abbreviations: SLC solute carrier, LCFA long chain fatty acid, VLCFA very long chain fatty acid
Integration of interaction between selected candidates
As one of the largest database for protein interactions, STRING aggregates the most available information (e.g. neighbourhood, gene fusion, co-occurrence, co-expression, experiments, databases, text mining and homology) derived from genomic context, high throughput experiments, co-expression and previous knowledge. Using the medium confidence score and clustered by MCL, 46 signature nutrient transporters were mapped into an interaction network (Fig. 2). The nodes visualize the proteins with known structure, and the solid lines represent the predicted functional associations with dashed inter-cluster edges. On grouping the network, it summarized the predicted associations for a particular group of proteins, for instance, amino acids associated transporters (blue cluster), vitamins associated transporters (red cluster), microelements and ions associated transporters (yellow cluster). The STRING view depicted that the selected targets were clearly clustered and strongly interconnected based on their substrate specificity, which reflected a high degree of functional association and a potential interplay among the nutrient transporters.
Fig. 2.
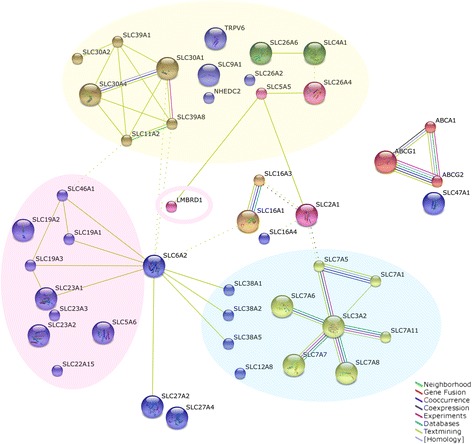
Functional interaction networks of proteins encoded by the potential candidate targets. Forty six transporters selected from microarray analysis were clustered by Search Tool for the Retrieval of Interacting Genes (STRING). The protein-protein interaction in human placenta was performed using customized settings, including medium confidence of 0.4, Markov cluster algorithm (MCL) and 8 criteria for linkage (neighbourhood, gene fusion, co-occurrence, co-expression, experiments, databases, text mining and homology). The red, yellow and blue clusters highlight the substrate-specific components, which are vitamin transporters, microelements and ion transporters, and amino acid transporters, respectively. The available 3D protein structural information is embedded in the node, and the dashed lines represent the inter-cluster edges
Identification of altered transporter gene expression in IUGR/PE
Characterization of patients
To validate the gene expression on a well characterized, clinically annotated tissue set, we have performed quantitative RT-PCR measurements on a number of accredited representative samples. Table 3 shows the demographic and clinical characteristics of the studied population. There were no major differences in the age, BMI, gravidity and parity numbers between the patients. The gestational age clearly isolated the term pregnancies from the rest, which was directly related with the placental weight and the birth weight.
Table 3.
Demographics and clinical characteristics of the studied population
| Term | Preterm | IUGR | PE + IUGR | PE | p-value | |
|---|---|---|---|---|---|---|
| Age, y | 33.2 | 34.0 | 35.3 | 32.0 | 28.7 | 0.41, ns |
| [24.5-41.6] | [29.1-40.7] | [20.9-38.2] | [27.3-40.2] | [23.4-33.3] | ||
| Body mass index (BMI), kg/m2 | 21.3 | 22.8 | 24.6 | 23.3 | 24.1 | 0.32, ns |
| [18.6-32.8] | [21.5-28.9] | [20.8-35.5] | [19.8-26.0] | [19.8-31.9] | ||
| Gravidity | 2.5 | 2.5 | 2.0 | 2.0 | 1.2 | 0.09, ns |
| [2.0-5.0] | [1.0-6.0] | [1.0-3.0] | [1.0-4.0] | [1.0-2.0] | ||
| Parity | 2.0 | 2.0 | 2.0 | 2.0 | 1.0 | 0.06, ns |
| [1.0-4.0] | [1.0-3.0] | [1.0-3.0] | [1.0-3.0] | [1.0-2.0] | ||
| Gestational age, weeks | 38.8 | 33.8** | 35.9* | 29.7*** | 33.7* | < 0.001 |
| [38.3-39.9] | [26.1-34.6] | [21.7-39.3] | [25.3-36.3] | [25.4-36.0] | ||
| Placental weight, g | 547.0 | 393.5 | 324.0* | 300.0** | 365.1 | 0.003 |
| [398.0-1035.0] | [250.0-810.0] | [126.0-487.0] | [132.0-481.0] | [203.0-632.0] | ||
| Birth weight, g | 3340.0 | 2127.5* | 1850.0** | 1170.0*** | 1973.5 * | < 0.001 |
| [2950.0-3695.0] | [745.0-2510.0] | [210.0-2695.0] | [520.0-2125.0] | [605.0-2830.0] | ||
| Systolic blood pressure, mmHg | 109.5 | 116.5 | 126.0 | 170.0*** | 164.4** | < 0.001 |
| [90.0-125.0] | [100.0-146.0] | [109.0-156.0] | [150.0-200.0] | [147.0-213.0] | ||
| Diastolic blood pressure, mmHg | 64.0 | 73.5 | 75.0 | 100.0*** | 98.7*** | < 0.001 |
| [50.0-83.0] | [67.0-91.0] | [63.0-94.0] | [95.0-124.0] | [93.0-104.0] | ||
| Proteinuria, g/24 h | < 0.15 | < 0.15 | < 0.15 | 0.88 | 2.8 | |
| [0.32-2.54] | [0.5-6.0] |
Statistical analysis was performed using Kruskal-Wallis with Dunn’s multiple comparisons test. Data are presented as median with range in brackets; ns, *, **, *** represent a p-value > 0.05, < 0.05, < 0.01, and < 0.001, respectively, of the comparison between the tested group and the term control. Details on patient selection and exclusion criteria are as described in Results (section “Identification of the altered transporter gene expression in IUGR/PE”).
Abbreviations: Term, healthy pregnancies at term; Preterm, uncomplicated preterm labour; IUGR, intrauterine growth restriction; PE, pre-eclampsia; PE + IUGR, PE combined with IUGR
In addition, as a PE related biomarker, placental leptin mRNA levels have been measured. A significantly increased expression of leptin has been shown in PE and PE + IUGR compared to both term and preterm control placentae, but not in isolated IUGR (data not shown).
Selection of reference genes
Prior to evaluating the nutrient transporter regulation in IUGR/PE, we first screened the mRNA abundance of commonly used reference genes: β2-microglobulin, mitochondrial ribosomal protein L19, ubiquitin (UBQ), and tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide (YWHAZ). We found no differences between controls and the herein tested gestational diseases (data not shown). In accordance with our previous work [34], the mean value of UBQ and YWHAZ was used for quantitative RT-PCR normalization of target nutrient transporters.
Gene expression analysis
We evaluated the expression of the selected 46 targets in 43 well-characterized placenta samples using unsupervised hierarchical clustering. The heatmap shown in Fig. 3 illustrates an overview of the expression profiles of these transcripts based on the quantitative RT-PCR results. The generated expression profile data confirmed that the 43 well-characterized placenta samples used in this study highly reproduced the results obtained from microarray data of 258 samples all over the world.
Fig. 3.
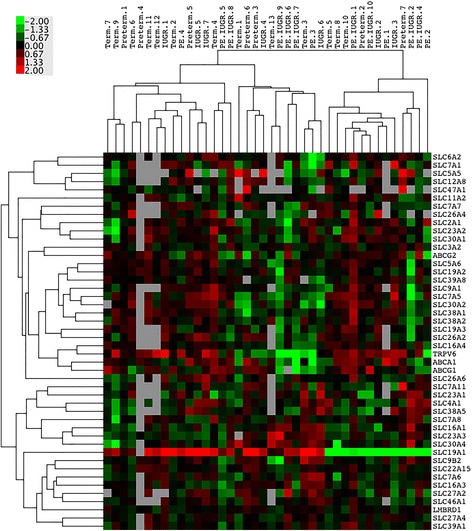
Nutrient transporter mRNA expression profiles of placenta tissues. Heatmap generated from quantitative RT-PCR data from an in-house placental tissue collection, hierarchically clustering the gene expression values of 46 transporters in selected gestational conditions. The clinical status of individual patients, e.g. term (n = 13), preterm (n = 7), intrauterine growth restriction (IUGR, n = 8), pre-eclampsia (PE, n = 5), PE combined with IUGR (PE + IUGR, n = 10), is indicated on the top, and gene names are listed on the right. Up-regulated expressions are marked in red, down-regulations are coloured in green; black reflects no difference in expression levels
Based on Kruskal-Wallis test, eight candidate transporters were identified showing significant changes on mRNA expression among gestational disorders: the amino acid transporters SLC7A7 (Fig. 4a), SLC38A2 (Fig. 4b), SLC38A5 (Fig. 4c), the glucose transporter SLC2A1 (Fig. 4d), the cholesterol transporter ABCA1 (Fig. 4e), the vitamin B transporters SLC19A3 (Fig. 4f) and SLC22A15 (Fig. 4g), as well as the sulphate transporter SLC26A2 (Fig. 4h). In order to directly compare the trend of gene regulation in IUGR or PE with the microarray analysis, we have presented the gene expression as the log (FC) to term controls. Almost all trends of alteration of transporters by IUGR/PE were comparable to those derived from microarray data, except the effects on ABCA1 and SLC38A2 by PE. The latter might be due to different criteria on PE sample selection, which result in greater variations between microarrays.
Fig. 4.
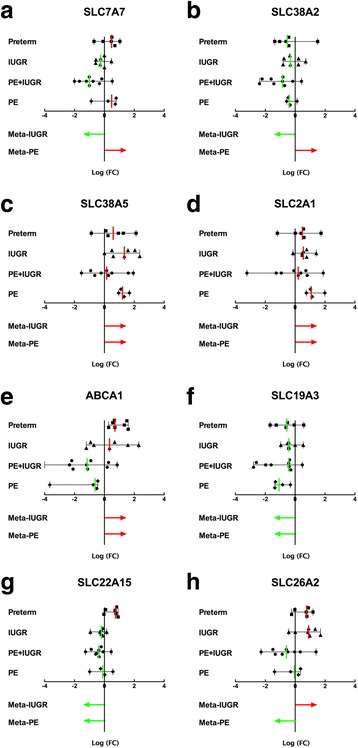
Identification of transporters with a significantly aberrant expression pattern in gestational diseases. The expression patterns of nutrient transporters in placental tissues obtained from controls and the gestational diseases described in Fig. 3 were screened by quantitative RT–PCR. Based on Kruskal-Wallis test (p < 0.05), eight candidate transporters showed significantly altered mRNA abundance in patients, i.e. SLC7A7 (a), SLC38A2 (b), SLC38A5 (c), SLC2A1 (d), ABCA1 (e), SLC19A3 (f), SLC22A15 (g) and SLC26A2 (h). Log 2-fold change (FC) values of gene expressions are shown as scatter plots with lines drawn at median ± range. Each symbol represents the gene expression of one individual. In the same figure, the differential expression trends of transporters in IUGR or PE from the meta-analysis (see Fig. 1) are illustrated by arrows. Red and green colours depict up-regulation and down-regulation, respectively
In addition, mathematical diagramming was applied to assess whether IUGR or PE is the major modulator of the determined alteration in gene expression. In the scatter plot shown in Fig. 5, each point represents the average expression level of one nutrient transporter. The log (FC)IUGR and log (FC)PE of independent transporters obtained from quantitative RT-PCR analysis, were defined as paired X-Y values. The absolute value on the X-axis or Y-axis reflects the severity of the gene expression changes by IUGR or PE, respectively. By ranking the absolute X or Y value, the top 3 genes dominantly influenced by IUGR were SLC23A1, SLC26A2, and SLC7A11 (yellow squares), while SLC16A4, ABCA1, and ABCG2 (blue squares) were regulated mainly by PE. On the contrary, transporters such as SLC7A7, SLC38A5 and SLC7A8 (red squares), were equally affected by IUGR and PE since they locate on the y = x or y = −x line.
Fig. 5.
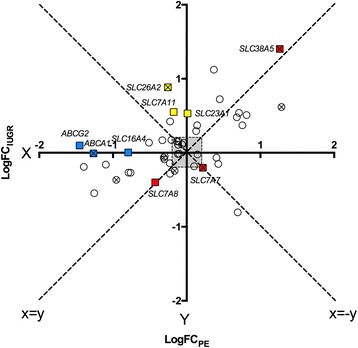
Scatter plot on the influence of IUGR/ PE on transporter expression. Each point represents one nutrient transporter, whose X-axis is defined by its log (FC) expression value in the IUGR group, and the Y-axis is presented by its log (FC) expression value in PE. 0.2 was defined as error threshold. The top 3 genes dominantly influenced by the pathology of IUGR (SLC16A4, ABCA1, ABCG2) or PE (SLC23A1, SLC7A11, SLC26A2), are marked with blue and yellow squares, respectively. Red squares (SLC7A7, SLC7A8, SLC38A5) indicate the transporters that receive comparable effects from IUGR and PE. The points with cross mark depict the significantly regulated transporters by a Kruskal-Wallis test. Abbreviations: log (FC): log 2 fold change
Taking into account the statistical analysis and the mathematical diagramming, the overlapping targets, namely ABCA1, SLC7A7, SLC38A5, and SLC26A2, were further subjected to protein quantification. However, due to the lack of a specific, validated antibody, the evaluation of SLC26A2 protein could not be further pursued.
Protein levels of the key membrane transporters
Using immunoblotting technique, we complemented the quantitative RT-PCR data with corresponding protein expression levels. As shown in Fig. 6, the total protein content of ABCA1 was significantly decreased by PE. In contrast, SLC7A7 transporter showed significant up-regulation in placentae associated with PE, while down-regulation in IUGR placentae was found. SLC38A5 expression in placenta tissue was only slightly elevated in IUGR and PE. The loss of significance may due to the limited sample size. Generally, the changes found on protein level for the three transporters ABCA1, SLC7A7 and SLC38A5, corresponded well with the transcriptional results.
Fig. 6.
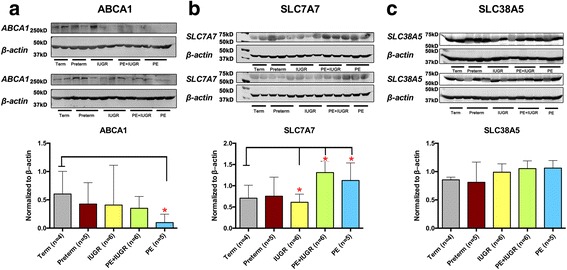
Protein expression of the membrane transporters ABCA1, SLC7A7 and SLC38A5 in total tissue lysates. ABCA1 (a; 250 kD), SLC7A7 (b; 56 kD), and SLC38A5 (c; 51 kD) protein expressions in total lysates were quantified from control and diseased placentas. Western blotting (upper panels) was performed with 90 μg total lysates from term (n = 4), preterm (n = 5), IUGR (n = 6), PE + IUGR (n = 6), and PE (n = 5) (upper panels). After overnight exposure, an immunoreactive signal was observed in all lanes at the expected size. Relative protein expression levels from western blotting results were then calculated as the densitometric ratio of target versus β-actin (43 kD; lower panels). Data are expressed as median ± range. Significance at p < 0.05 was assessed by a Kruskal-Wallis test, followed by Dunn’s multiple comparison
As the target transporters modulate the fluxes of substrates through the plasma membrane to meet fetal requirements, we also isolated plasma membrane protein fractions from the placentae, and confirmed their high purity by enrichment of the marker protein Na+/K+ ATPase (data not shown). The latter was further used to normalize the transporter protein expression detected by Western blotting.
We have previously reported that the protein expression of the lipid transporter ABCA1 in extracted plasma membranes was significantly reduced only in PE placentae [21]. These findings were confirmed in the present study (data not shown). Interestingly, the opposite regulations for SLC7A7 protein between IUGR and PE-associated disease types that we detected in tissue lysates (Fig.6) were generally also reflected in extracted plasma membrane preparations (Fig. 7). Normalization to β-actin and Na+/K+ ATPase revealed a trend to decreased expression of SLC7A7 in IUGR tissues, and confirmed a significantly increased expression in PE tissues (Fig. 7a). SLC38A5 was not significantly altered in membrane proteins obtained from diseased and healthy (control) placentas (Fig. 7b). However, marked increases in IUGR and PE compared with the preterm group were found.
Fig. 7.
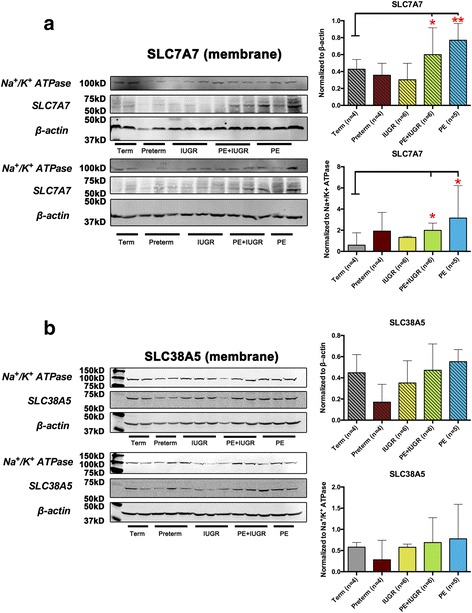
Protein expression of the amino acid transporters SLC7A7 and SLC38A5 in enriched plasma membrane fractions. Representative Western blots of SLC7A7 (a; 56 kD) and SLC38A5 (b; 51 kD) protein expression from isolated placental membrane fractions. Western blotting was performed with 30 μg membrane proteins from term (n = 4), preterm (n = 5), IUGR (n = 6), PE + IUGR (n = 6), and PE (n = 5) (Left panels). Both β-actin (43 kD) and Na+/K+ ATPase (112 kD) were used as loading controls. Relative protein expression levels from western blotting results were then calculated as the densitometric ratio of targeted transporter versus β-actin (43 kD; upper right panel) and Na+/K+ ATPase (112 kD; lower right panel). Data are expressed as median ± range. Based on Kruskal-Wallis test followed by Dunn’s multiple comparison, *, ** denote p < 0.05 and < 0.01, respectively
Discussion
Fetal growth is largely dependent on the availability of nutrients transported into the fetal circulation via the placenta. The transport of macro- and micronutrients across the placental barrier relies on the presence and activity of substrate specific membrane transporters. The altered expression of these transporters is thought to be implicated in diverse mechanisms of the fetal development. The current study focused on studying to what extent and which specific transporters are impaired by IUGR or PE. To this end, we screened the expression profiles of nutrient transporters whose substrates are amino acids, carbohydrates, lipids, vitamins and microelements in diseased and control placentas by analysing 7 publicly available GEO microarray datasets. The selected targets have been further validated on in-house well-characterized placenta tissues. A systematic alteration in mRNA expression of diverse transporters were identified in IUGR/PE. Among the 46 nutrient transporters, 8 were significantly regulated by those gestational disorders. In particular, the amino acid transporters SLC7A7 and SLC38A5 were strongly influenced by both IUGR and PE. The mRNA and protein expression of the cholesterol transporter ABCA1, by contrast, was only regulated under PE condition.
Microarray gene expression data provided a global overview on the nutrient transporter expression. However, the weakness of these data was that they were derived from studies using not exactly the same criteria for the clinical recruitment of patients. In this study, we have systematically reviewed our on-site collected samples in the following ways. First, the applied clinical and diagnostic data for each individual did meet the strictly defined clinical criteria for classification as IUGR or PE (Table 3). Second, the higher gene expression of leptin occurring only in the PE and PE + IUGR groups served as a placenta-derived marker of PE [37, 38]. Third, we have compared the regulated mRNA expressions that were generated on our 43 in-house samples with results from the cohort of character-matched 258 patients in seven microarray projects. It showed that only 26% of the targets exhibited inconsistent pathological effects between our samples and the global tissue sets. Based on aforementioned evidences, we considered the in-house tissue samples applied in this study as adequate to reflect patterns occurring in a wider target population.
We further analysed the impact of IUGR/PE on the mRNA expression levels of the selected 46 transporters. Overall, our quantitative RT-PCR measurements revealed a differential expression pattern between control and diseased placentae, including 8 significantly regulated candidates, which might be critical for the fetal malnutrition associated with IUGR/PE. Within this list, SLC2A1 (GLUT1) has been repeatedly reported as significantly reduced at the mRNA level by PE [39, 40]. The studies in animals, such as rat and sheep, have shown that placental GLUT1 expression is significantly decreased in IUGR [41, 42], which is consistent with our findings. We also detected a mRNA down-regulation of the thiamine transporter SLC19A3 and the putative pyridoxine transporter SLC22A15 in IUGR and PE. To our knowledge, this is the first report demonstrating a reduction of placental SLC19A3 and SLC22A15 in IUGR and PE. Currently, there is a lack of specific clinical data on vitamin B1 or B6 deficiency during human pregnancy, except for B vitamin complex supplements. The detected reduction of SLC19A3 and SLC22A15 mRNA in this study may raise our attention to serum thiamine and pyridoxine alterations during pregnancy, which could interrupt the structural and functional development of cerebella and hippocampus, respectively. Information on the sulfate transporter SLC26A2 in placenta is very scarce. Only Simmons et al. recently reported an abundant mRNA and CTB-specific expression of SLC26A2 in human placenta [43]. In the present study, we identified an up-regulation of SLC26A2 mRNA in IUGR and a down-regulation in PE, based on our in silico screening and the in house gene quantitation. Those opposite but significant changes between IUGR and PE may indicate a different role of SLC26A2 in these two diseases which should be further investigated.
The cholesterol transporter ABCA1, as well as the amino acid transporters SLC7A7 and SLC38A5 were consecutively chosen as the main targets for further investigation, as they popped up both in the statistical analysis and the mathematical diagramming procedure. For ABCA1, we have confirmed the previous finding of an exclusively decreased ABCA1 expression in PE [21]. The impaired ABCA1 function resulted in compromised cholesterol and phospholipid efflux, hence a concomitant accumulation of lipids was observed in PE placentas [44]. The amino acid transporter SLC7A7 encodes the y+LAT-1 protein, which is known to be located at the MVM and BM [7]. It forms a heterodimer complex with h4F2hc (encoded by SLC3A2), mediating the Na+-independent cationic amino acids or large neutral L-amino acids transport [45]. Our finding regarding the decrease of SLC7A7 mRNA and protein in IUGR is in agreement with a previous study using Slc7a7-/- mice [46], which caused fetal growth retardation by downregulating insulin-like growth factor 1. In addition, we found an increased SLC7A7 expression in diseases associated with PE compared with gestational age-matched controls. As lysine, arginine and ornithine are substrates for SLC7A7, high SLC7A7 expression could cause reduced maternal lysine, arginine and ornithine levels. During pregnancy this may evoke similar symptoms as reported for lysinuric protein intolerance, which has been associated with serious gestational complications such as increased risk of developing gestational anaemia and PE [47]. SLC38A5, also known as SNAT5 protein, belongs to the Na+-coupled neutral amino acid transporter system N. It mediates the cotransport of glycine, asparagine, alanine, serine, glutamine and histidine with sodium [48]. Prior to the current study, SLC38A5 has only been proven to localize at the MVM and to participate in placental glutamine homeostasis [49]. Our work reports for the first time that SLC38A5 is differentially regulated (increased) at mRNA level in the clinically highly relevant pregnancy diseases IUGR and PE. The trend towards an elevated expression as compared to the control groups was also confirmed on protein level, but, supposedly due to the lower sample size and higher variation in the Western blotting experiments, the comparison between the groups in the ANOVA was not statistically different. In comparison, another sodium-coupled neural amino acid transporter, SLC38A2, which is localized at both the MVM and BM [50], was shown to be significantly decreased in IUGR and PE in the mRNA screening (Fig. 4). The differentially altered expression patterns of SLC38A5 and SLC38A2 in IUGR and PE (up- versus down-regulation) might not only result from their differing cellular localizations but also from their distinct transport mechanisms. SLC38A2, as a member of system A, is a pH sensitive uniporter. However, SLC38A5 exhibits marked inhibition at low extracellular pH, because it mediates also the coupled countertransport of H+ with the substrate (e.g. glutamine) [51]. Therefore, the uptake or efflux of glutamine via SLC38A5 may strongly depend on the cellular metabolic status, which could be modified in gestational disorders such as IUGR and PE.
Conclusions
The current study reports a novel analytic strategy including bioinformatic analysis of publicly available microarray datasets, followed by molecular biological and biochemical quantification of mRNA and protein expression in biological tissue samples combined with mathematical diagramming. This approach was successfully applied, firstly to provide an overview on placental membrane transporters providing the fetus with optimal nutrients and microelements for adequate fetal growth, and secondly, to explore the significance of altered membrane transporter expressions in the clinically important pregnancy diseases IUGR and PE. A systematic change in nutrient transporter expression was detected, and 8 membrane transporters were found to be significantly regulated in IUGR/PE. Particularly, the amino acid transporters SLC7A7 and SLC38A5, were influenced by both IUGR and PE. In the present study, we demonstrated for the first time an enhanced mRNA expression of SLC38A5 in IUGR and PE placentae and the same trend on protein level. These findings are the basis for future investigations which should examine function of the selected transporters at different regions in placenta and elucidate the precise mechanisms regulating the observed changes in IUGR and/or PE.
Additional file
Gene specific primer sequences used for quantitative RT- PCR. It presents a complete list of the primers designed for the selected candidate and control genes in this study. (DOCX 21 kb)
Acknowledgements
The authors would like to express their gratitude to all patients, nurses and midwives who participated in this study. We thank Mr. Michael Lüthi for the technical support, Prof. Matthias A. Hediger, Dr. Alexandre Simonin, Dr. Sara El-Gebali and Dr.Cedric Simillion for fruitful discussions.
Funding
This work was supported by the Swiss National Science Foundation (SNSF) through the National Center of Competence in Research (NCCR) TransCure, University of Bern, Switzerland, and the Swiss National Science Foundation (Grant No. 310030_149958, CA).
Availability of data and materials
The datasets analysed during the current study are mined from publicly available in the NCBI GEO repository under the following accession number:
GSE 25861 [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE25861]
GSE 24129 [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE24129]
GSE 12216 [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE12216]
GSE 25906 [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE25906]
GSE 14722 [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE14722]
GSE 35574 [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE35574]
GSE 24818 [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE24818]
Other data supporting the conclusions of this article are not publicly available due to privacy concerns, but are available from the corresponding author on request.
Abbreviations
- ABC
ATP-binding cassette
- BM
Basal plasma membrane
- Ct
Cycle threshold
- GEO
Gene expression omnibus
- GLUT
Glucose transporter
- IUGR
Intrauterine growth restriction
- log (FC)
Log 2 fold change
- MCL
Markov cluster algorithm
- MVM
Microvillous plasma membrane
- Na+/K+ ATPase
Sodium potassium ATPase
- PE + IUGR
PE combined with IUGR
- PE
Pre-eclampsia
- PIMEC
Fetoplacental microvascular endothelial cell
- PMSF
Phenylmethylsulfonyl fluoride
- RIN
RNA integrity number
- RMA
Robust multi-array averaging
- SLC
Solute carrier
- STRING
Search tool for the retrieval of interacting genes
- TRP
Transient receptor potential
- UBQ
Ubiquitin
- YWHAZ
Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide
Authors’ contributions
ECO, XH and CA conceived and designed the study. PA and XH performed the bioinformatic analysis. MUB and DVS performed the clinical assessment of the participants and were involved in the interpretation of data. XH performed the for quantitative RT-PCR experiments, and wrote the manuscript. LH and XH evaluated the protein expressions. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study protocol was approved by the local ethic committees of the Canton of Bern, Switzerland (178/03). Written informed consent was obtained from each participant for this research prior to the caesarean section. A copy of the consent form is available for review by the Editor of this journal.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12864-018-4518-z) contains supplementary material, which is available to authorized users.
Contributor Information
Xiao Huang, Email: xiao.huang@ibmm.unibe.ch.
Pascale Anderle, Email: pascale.anderle@sitem-insel.ch.
Lu Hostettler, Email: lu.hostettler@students.unibe.ch.
Marc U. Baumann, Email: marc.baumann@insel.ch
Daniel V. Surbek, Email: daniel.surbek@insel.ch
Edgar C. Ontsouka, Email: corneille.ontsouka@ibmm.unibe.ch
Christiane Albrecht, Email: christiane.albrecht@ibmm.unibe.ch.
References
- 1.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J, Maternal, Child Undernutrition Study G Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 2.Wu G, Imhoff-Kunsch B, Girard AW. Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr Perinat Epidemiol. 2012;26(Suppl 1):4–26. doi: 10.1111/j.1365-3016.2012.01291.x. [DOI] [PubMed] [Google Scholar]
- 3.Pathology of the human placenta. http://www.springer.com/de/book/9783642239403
- 4.Manual of Pathology of the Human Placenta. https://link.springer.com/book/10.1007%2F978-1-4419-7494-5
- 5.Brett KE, Ferraro ZM, Yockell-Lelievre J, Gruslin A, Adamo KB. Maternal-fetal nutrient transport in pregnancy pathologies: the role of the placenta. Int J Mol Sci. 2014;15(9):16153–16185. doi: 10.3390/ijms150916153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desforges M, Sibley CP. Placental nutrient supply and fetal growth. Int J Dev Biol. 2010;54(2-3):377–390. doi: 10.1387/ijdb.082765md. [DOI] [PubMed] [Google Scholar]
- 7.Jansson T. Amino acid transporters in the human placenta. Pediatr Res. 2001;49(2):141–147. doi: 10.1203/00006450-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990;70(1):43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- 9.Mahendran D, Donnai P, Glazier JD, D'Souza SW, Boyd RD, Sibley CP. Amino acid (system a) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res. 1993;34(5):661–665. doi: 10.1203/00006450-199311000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Sibley CP, Turner MA, Cetin I, Ayuk P, Boyd CA, D'Souza SW, Glazier JD, Greenwood SL, Jansson T, Powell T. Placental phenotypes of intrauterine growth. Pediatr Res. 2005;58(5):827–832. doi: 10.1203/01.PDR.0000181381.82856.23. [DOI] [PubMed] [Google Scholar]
- 11.Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, Mahendran D, Marconi AM, Pardi G, Sibley CP. Association between the activity of the system a amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res. 1997;42(4):514–519. doi: 10.1203/00006450-199710000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Johansson M, Karlsson L, Wennergren M, Jansson T, Powell TL. Activity and protein expression of Na+/K+ ATPase are reduced in microvillous syncytiotrophoblast plasma membranes isolated from pregnancies complicated by intrauterine growth restriction. J Clin Endocrinol Metab. 2003;88(6):2831–2837. doi: 10.1210/jc.2002-021926. [DOI] [PubMed] [Google Scholar]
- 13.Tsitsiou E, Sibley CP, D'Souza SW, Catanescu O, Jacobsen DW, Glazier JD. Homocysteine transport by systems L, a and y+L across the microvillous plasma membrane of human placenta. J Physiol. 2009;587(Pt 16):4001–4013. doi: 10.1113/jphysiol.2009.173393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsitsiou E, Sibley CP, D'Souza SW, Catanescu O, Jacobsen DW, Glazier JD. Homocysteine is transported by the microvillous plasma membrane of human placenta. J Inherit Metab Dis. 2011;34(1):57–65. doi: 10.1007/s10545-010-9141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aye IL, Singh AT, Keelan JA. Transport of lipids by ABC proteins: interactions and implications for cellular toxicity, viability and function. Chem Biol Interact. 2009;180(3):327–339. doi: 10.1016/j.cbi.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Marconi AM, Paolini C, Buscaglia M, Zerbe G, Battaglia FC, Pardi G. The impact of gestational age and fetal growth on the maternal-fetal glucose concentration difference. Obstet Gynecol. 1996;87(6):937–942. doi: 10.1016/0029-7844(96)00048-8. [DOI] [PubMed] [Google Scholar]
- 17.Arkwright PD, Rademacher TW, Dwek RA, Redman CW. Pre-eclampsia is associated with an increase in trophoblast glycogen content and glycogen synthase activity, similar to that found in hydatidiform moles. J Clin Invest. 1993;91(6):2744–2753. doi: 10.1172/JCI116515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thadhani R, Stampfer MJ, Hunter DJ, Manson JE, Solomon CG, Curhan GC. High body mass index and hypercholesterolemia: risk of hypertensive disorders of pregnancy. Obstet Gynecol. 1999;94(4):543–550. doi: 10.1016/s0029-7844(99)00400-7. [DOI] [PubMed] [Google Scholar]
- 19.Enquobahrie DA, Williams MA, Butler CL, Frederick IO, Miller RS, Luthy DA. Maternal plasma lipid concentrations in early pregnancy and risk of preeclampsia. Am J Hypertens. 2004;17(7):574–581. doi: 10.1016/j.amjhyper.2004.03.666. [DOI] [PubMed] [Google Scholar]
- 20.Dubova E, Pavlov K, Kulikova G, Shchegolev A, Sukhikh GT. Glucose transporters expression in the placental terminal villi of preeclampsia and intrauterine growth retardation complicated pregnancies. Health. 2013;5:100–104. [Google Scholar]
- 21.Baumann M, Korner M, Huang X, Wenger F, Surbek D, Albrecht C. Placental ABCA1 and ABCG1 expression in gestational disease: pre-eclampsia affects ABCA1 levels in syncytiotrophoblasts. Placenta. 2013;34(11):1079–1086. doi: 10.1016/j.placenta.2013.06.309. [DOI] [PubMed] [Google Scholar]
- 22.Gadhok AK, Sinha M, Khunteta R, Vardey SK, Upadhyaya C, Sharma TK, Jha M. Serum homocysteine level and its association with folic acid and vitamin B12 in the third trimester of pregnancies complicated with intrauterine growth restriction. Clin Lab. 2011;57(11-12):933–938. [PubMed] [Google Scholar]
- 23.Furness D, Fenech M, Dekker G, Khong TY, Roberts C, Hague W. Folate, vitamin B12, vitamin B6 and homocysteine: impact on pregnancy outcome. Matern Child Nutr. 2013;9(2):155–166. doi: 10.1111/j.1740-8709.2011.00364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cattaneo M. Homocysteine and the risk of intrauterine growth retardation. Clin Chem. 2003;49(9):1432–1433. doi: 10.1373/49.9.1432. [DOI] [PubMed] [Google Scholar]
- 25.Leeda M, Riyazi N, de Vries JI, Jakobs C, van Geijn HP, Dekker GA. Effects of folic acid and vitamin B6 supplementation on women with hyperhomocysteinemia and a history of preeclampsia or fetal growth restriction. Am J Obstet Gynecol. 1998;179(1):135–139. doi: 10.1016/s0002-9378(98)70263-7. [DOI] [PubMed] [Google Scholar]
- 26.Wacker J, Fruhauf J, Schulz M, Chiwora FM, Volz J, Becker K. Riboflavin deficiency and preeclampsia. Obstet Gynecol. 2000;96(1):38–44. doi: 10.1016/s0029-7844(00)00847-4. [DOI] [PubMed] [Google Scholar]
- 27.Zhao R, Goldman ID. Folate and thiamine transporters mediated by facilitative carriers (SLC19A1-3 and SLC46A1) and folate receptors. Mol Asp Med. 2013;34(2-3):373–385. doi: 10.1016/j.mam.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keating E, Goncalves P, Costa F, Campos I, Pinho MJ, Azevedo I, Martel F. Comparison of the transport characteristics of bioactive substances in IUGR and normal placentas. Pediatr Res. 2009;66(5):495–500. doi: 10.1203/PDR.0b013e3181b9b4a3. [DOI] [PubMed] [Google Scholar]
- 29.Jain S, Sharma P, Kulshreshtha S, Mohan G, Singh S. The role of calcium, magnesium, and zinc in pre-eclampsia. Biol Trace Elem Res. 2010;133(2):162–170. doi: 10.1007/s12011-009-8423-9. [DOI] [PubMed] [Google Scholar]
- 30.Kumru S, Aydin S, Simsek M, Sahin K, Yaman M, Ay G. Comparison of serum copper, zinc, calcium, and magnesium levels in preeclamptic and healthy pregnant women. Biol Trace Elem Res. 2003;94(2):105–112. doi: 10.1385/BTER:94:2:105. [DOI] [PubMed] [Google Scholar]
- 31.Vafaei H, Dalili M, Hashemi SA. Serum concentration of calcium, magnesium and zinc in normotensive versus preeclampsia pregnant women: a descriptive study in women of Kerman province of Iran. Iran J Reprod Med. 2015;13(1):23–26. [PMC free article] [PubMed] [Google Scholar]
- 32.Yang H, Kim TH, An BS, Choi KC, Lee HH, Kim JM, Jeung EB. Differential expression of calcium transport channels in placenta primary cells and tissues derived from preeclamptic placenta. Mol Cell Endocrinol. 2013;367(1-2):21–30. doi: 10.1016/j.mce.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Bell AW, Ehrhardt RA. Regulation of placental nutrient transport and implications for fetal growth. Nutr Res Rev. 2002;15(2):211–230. doi: 10.1079/NRR200239. [DOI] [PubMed] [Google Scholar]
- 34.Huang X, Baumann M, Nikitina L, Wenger F, Surbek D, Korner M, Albrecht C. RNA degradation differentially affects quantitative mRNA measurements of endogenous reference genes in human placenta. Placenta. 2013;34(7):544–547. doi: 10.1016/j.placenta.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Yang W, Rosenstiel PC, Schulenburg H. ABSSeq: a new RNA-Seq analysis method based on modelling absolute expression differences. BMC Genomics. 2016;17:541. doi: 10.1186/s12864-016-2848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mise H, Sagawa N, Matsumoto T, Yura S, Nanno H, Itoh H, Mori T, Masuzaki H, Hosoda K, Ogawa Y, et al. Augmented placental production of leptin in preeclampsia: possible involvement of placental hypoxia. J Clin Endocrinol Metab. 1998;83(9):3225–3229. doi: 10.1210/jcem.83.9.5117. [DOI] [PubMed] [Google Scholar]
- 38.Laivuori H, Gallaher MJ, Collura L, Crombleholme WR, Markovic N, Rajakumar A, Hubel CA, Roberts JM, Powers RW. Relationships between maternal plasma leptin, placental leptin mRNA and protein in normal pregnancy, pre-eclampsia and intrauterine growth restriction without pre-eclampsia. Mol Hum Reprod. 2006;12(9):551–556. doi: 10.1093/molehr/gal064. [DOI] [PubMed] [Google Scholar]
- 39.Hayashi M, Sakata M, Takeda T, Yamamoto T, Okamoto Y, Sawada K, Kimura A, Minekawa R, Tahara M, Tasaka K, et al. Induction of glucose transporter 1 expression through hypoxia-inducible factor 1alpha under hypoxic conditions in trophoblast-derived cells. J Endocrinol. 2004;183(1):145–154. doi: 10.1677/joe.1.05599. [DOI] [PubMed] [Google Scholar]
- 40.Baumann MU, Zamudio S, Illsley NP. Hypoxic upregulation of glucose transporters in BeWo choriocarcinoma cells is mediated by hypoxia-inducible factor-1. Am J Physiol Cell Physiol. 2007;293(1):C477–C485. doi: 10.1152/ajpcell.00075.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das UG, Sadiq HF, Soares MJ, Hay WW, Jr, Devaskar SU. Time-dependent physiological regulation of rodent and ovine placental glucose transporter (GLUT-1) protein. Am J Phys. 1998;274(2 Pt 2):R339–R347. doi: 10.1152/ajpregu.1998.274.2.R339. [DOI] [PubMed] [Google Scholar]
- 42.Langdown ML, Sugden MC. Enhanced placental GLUT1 and GLUT3 expression in dexamethasone-induced fetal growth retardation. Mol Cell Endocrinol. 2001;185(1-2):109–117. doi: 10.1016/s0303-7207(01)00629-3. [DOI] [PubMed] [Google Scholar]
- 43.Simmons DG, Rakoczy J, Jefferis J, Lourie R, McIntyre HD, Dawson PA. Human placental sulfate transporter mRNA profiling from term pregnancies identifies abundant SLC13A4 in syncytiotrophoblasts and SLC26A2 in cytotrophoblasts. Placenta. 2013;34(4):381–384. doi: 10.1016/j.placenta.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 44.Huang X, Jain A, Baumann M, Korner M, Surbek D, Butikofer P, Albrecht C. Increased placental phospholipid levels in pre-eclamptic pregnancies. Int J Mol Sci. 2013;14(2):3487–3499. doi: 10.3390/ijms14023487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 2004;447(5):532–542. doi: 10.1007/s00424-003-1086-z. [DOI] [PubMed] [Google Scholar]
- 46.Sperandeo MP, Annunziata P, Bozzato A, Piccolo P, Maiuri L, D'Armiento M, Ballabio A, Corso G, Andria G, Borsani G, et al. Slc7a7 disruption causes fetal growth retardation by downregulating Igf1 in the mouse model of lysinuric protein intolerance. Am J Physiol Cell Physiology. 2007;293(1):C191–C198. doi: 10.1152/ajpcell.00583.2006. [DOI] [PubMed] [Google Scholar]
- 47.Mikolajek-Bedner W, Torbe A, Kwiatkowski S, Michalczyk M, Gizewska M, Rokicki D, Rzepka R, Konstanty-Kurkiewicz V, Domanski M, Czajka R. Pregnancy delivery and puerperium in a patient with lysinuric protein intolerance--a case report. Ginekol Pol. 2013;84(7):654–656. doi: 10.17772/gp/1621. [DOI] [PubMed] [Google Scholar]
- 48.Broer S. The SLC38 family of sodium-amino acid co-transporters. Pflugers Arch. 2014;466(1):155–172. doi: 10.1007/s00424-013-1393-y. [DOI] [PubMed] [Google Scholar]
- 49.Day PE, Cleal JK, Lofthouse EM, Goss V, Koster G, Postle A, Jackson JM, Hanson MA, Jackson AA, Lewis RM. Partitioning of glutamine synthesised by the isolated perfused human placenta between the maternal and fetal circulations. Placenta. 2013;34(12):1223–1231. doi: 10.1016/j.placenta.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mando C, Tabano S, Pileri P, Colapietro P, Marino MA, Avagliano L, Doi P, Bulfamante G, Miozzo M, Cetin I. SNAT2 expression and regulation in human growth-restricted placentas. Pediatr Res. 2013;74(2):104–110. doi: 10.1038/pr.2013.83. [DOI] [PubMed] [Google Scholar]
- 51.Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (system N/a) transporters of the SLC38 gene family. Pflugers Arch. 2004;447(5):784–795. doi: 10.1007/s00424-003-1117-9. [DOI] [PubMed] [Google Scholar]
- 52.Dunk CE, Roggensack AM, Cox B, Perkins JE, Asenius F, Keating S, Weksberg R, Kingdom JC, Adamson SL. A distinct microvascular endothelial gene expression profile in severe IUGR placentas. Placenta. 2012;33(4):285–293. doi: 10.1016/j.placenta.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 53.Nishizawa H, Ota S, Suzuki M, Kato T, Sekiya T, Kurahashi H, Udagawa Y. Comparative gene expression profiling of placentas from patients with severe pre-eclampsia and unexplained fetal growth restriction. Reprod Biol Endocrinol. 2011;9:107. doi: 10.1186/1477-7827-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sitras V, Paulssen R, Leirvik J, Vartun A, Acharya G. Placental gene expression profile in intrauterine growth restriction due to placental insufficiency. Reprod Sci. 2009;16(7):701–711. doi: 10.1177/1933719109334256. [DOI] [PubMed] [Google Scholar]
- 55.Tsai S, Hardison NE, James AH, Motsinger-Reif AA, Bischoff SR, Thames BH, Piedrahita JA. Transcriptional profiling of human placentas from pregnancies complicated by preeclampsia reveals disregulation of sialic acid acetylesterase and immune signalling pathways. Placenta. 2011;32(2):175–182. doi: 10.1016/j.placenta.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winn VD, Gormley M, Paquet AC, Kjaer-Sorensen K, Kramer A, Rumer KK, Haimov-Kochman R, Yeh RF, Overgaard MT, Varki A, et al. Severe preeclampsia-related changes in gene expression at the maternal-fetal interface include sialic acid-binding immunoglobulin-like lectin-6 and pappalysin-2. Endocrinology. 2009;150(1):452–462. doi: 10.1210/en.2008-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo L, Tsai SQ, Hardison NE, James AH, Motsinger-Reif AA, Thames B, Stone EA, Deng C, Piedrahita JA. Differentially expressed microRNAs and affected biological pathways revealed by modulated modularity clustering (MMC) analysis of human preeclamptic and IUGR placentas. Placenta. 2013;34(7):599–605. doi: 10.1016/j.placenta.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fotiadis D, Kanai Y, Palacin M. The SLC3 and SLC7 families of amino acid transporters. Mol Asp Med. 2013;34(2-3):139–158. doi: 10.1016/j.mam.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Schioth HB, Roshanbin S, Hagglund MG, Fredriksson R. Evolutionary origin of amino acid transporter families SLC32, SLC36 and SLC38 and physiological, pathological and therapeutic aspects. Mol Asp Med. 2013;34(2-3):571–585. doi: 10.1016/j.mam.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 60.Novak DA, Matthews JC, Beveridge MJ, Yao SY, Young J, Kilberg MS. Demonstration of system y+L activity on the basal plasma membrane surface of rat placenta and developmentally regulated expression of 4F2HC mRNA. Placenta. 1997;18(8):643–648. doi: 10.1016/s0143-4004(97)90005-9. [DOI] [PubMed] [Google Scholar]
- 61.Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273(37):23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- 62.Aiko Y, Askew DJ, Aramaki S, Myoga M, Tomonaga C, Hachisuga T, Suga R, Kawamoto T, Tsuji M, Shibata E. Differential levels of amino acid transporters system L and ASCT2, and the mTOR protein in placenta of preeclampsia and IUGR. BMC Pregnancy Childbirth. 2014;14:181. doi: 10.1186/1471-2393-14-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okamoto Y, Sakata M, Ogura K, Yamamoto T, Yamaguchi M, Tasaka K, Kurachi H, Tsurudome M, Murata Y. Expression and regulation of 4F2hc and hLAT1 in human trophoblasts. Am J Physiol Cell Physiol. 2002;282(1):C196–C204. doi: 10.1152/ajpcell.2002.282.1.C196. [DOI] [PubMed] [Google Scholar]
- 64.Ayuk PT, Sibley CP, Donnai P, D'Souza S, Glazier JD. Development and polarization of cationic amino acid transporters and regulators in the human placenta. Am J Physiol Cell Physiol. 2000;278(6):C1162–C1171. doi: 10.1152/ajpcell.2000.278.6.C1162. [DOI] [PubMed] [Google Scholar]
- 65.Speake PF, Glazier JD, Ayuk PT, Reade M, Sibley CP, D'Souza SW. L-Arginine transport across the basal plasma membrane of the syncytiotrophoblast of the human placenta from normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2003;88(9):4287–4292. doi: 10.1210/jc.2003-030067. [DOI] [PubMed] [Google Scholar]
- 66.Noris M, Todeschini M, Cassis P, Pasta F, Cappellini A, Bonazzola S, Macconi D, Maucci R, Porrati F, Benigni A, et al. L-arginine depletion in preeclampsia orients nitric oxide synthase toward oxidant species. Hypertension. 2004;43(3):614–622. doi: 10.1161/01.HYP.0000116220.39793.c9. [DOI] [PubMed] [Google Scholar]
- 67.Guzman-Gutierrez E, Westermeier F, Salomon C, Gonzalez M, Pardo F, Leiva A, Sobrevia L. Insulin-increased L-arginine transport requires a(2A) adenosine receptors activation in human umbilical vein endothelium. PLoS One. 2012;7(7):e41705. doi: 10.1371/journal.pone.0041705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jones H, Crombleholme T, Habli M. Regulation of amino acid transporters by adenoviral-mediated human insulin-like growth factor-1 in a mouse model of placental insufficiency in vivo and the human trophoblast line BeWo in vitro. Placenta. 2014;35(2):132–138. doi: 10.1016/j.placenta.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Araujo JR, Correia-Branco A, Ramalho C, Goncalves P, Pinho MJ, Keating E, Martel F. L-methionine placental uptake: characterization and modulation in gestational diabetes mellitus. Reprod Sci. 2013;20(12):1492–1507. doi: 10.1177/1933719113488442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loubiere LS, Vasilopoulou E, Bulmer JN, Taylor PM, Stieger B, Verrey F, McCabe CJ, Franklyn JA, Kilby MD, Chan SY. Expression of thyroid hormone transporters in the human placenta and changes associated with intrauterine growth restriction. Placenta. 2010;31(4):295–304. doi: 10.1016/j.placenta.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 71.Ritchie JW, Taylor PM. Role of the system L permease LAT1 in amino acid and iodothyronine transport in placenta. Biochem J. 2001;356(Pt 3):719–725. doi: 10.1042/0264-6021:3560719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dye JF, Vause S, Johnston T, Clark P, Firth JA, D'Souza SW, Sibley CP, Glazier JD. Characterization of cationic amino acid transporters and expression of endothelial nitric oxide synthase in human placental microvascular endothelial cells. FASEB J. 2004;18(1):125–127. doi: 10.1096/fj.02-0916fje. [DOI] [PubMed] [Google Scholar]
- 73.Lewis RM, Glazier J, Greenwood SL, Bennett EJ, Godfrey KM, Jackson AA, Sibley CP, Cameron IT, Hanson MA. L-serine uptake by human placental microvillous membrane vesicles. Placenta. 2007;28(5-6):445–452. doi: 10.1016/j.placenta.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 74.Desforges M, Greenwood SL, Glazier JD, Westwood M, Sibley CP. The contribution of SNAT1 to system a amino acid transporter activity in human placental trophoblast. Biochem Biophys Res Commun. 2010;398(1):130–134. doi: 10.1016/j.bbrc.2010.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nelson DM, Smith SD, Furesz TC, Sadovsky Y, Ganapathy V, Parvin CA, Smith CH. Hypoxia reduces expression and function of system a amino acid transporters in cultured term human trophoblasts. Am J Physiol Cell Physiol. 2003;284(2):C310–C315. doi: 10.1152/ajpcell.00253.2002. [DOI] [PubMed] [Google Scholar]
- 76.Novak D, Quiggle F, Haafiz A. Impact of forskolin and amino acid depletion upon system a activity and SNAT expression in BeWo cells. Biochimie. 2006;88(1):39–44. doi: 10.1016/j.biochi.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 77.Jones HN, Ashworth CJ, Page KR, McArdle HJ. Expression and adaptive regulation of amino acid transport system a in a placental cell line under amino acid restriction. Reproduction. 2006;131(5):951–960. doi: 10.1530/rep.1.00808. [DOI] [PubMed] [Google Scholar]
- 78.Jones HN, Jansson T, Powell TL. IL-6 stimulates system a amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. Am J Physiol Cell Physiol. 2009;297(5):C1228–C1235. doi: 10.1152/ajpcell.00195.2009. [DOI] [PubMed] [Google Scholar]
- 79.Daigle ND, Carpentier GA, Frenette-Cotton R, Simard MG, Lefoll MH, Noel M, Caron L, Noel J, Isenring P. Molecular characterization of a human cation-Cl- cotransporter (SLC12A8A, CCC9A) that promotes polyamine and amino acid transport. J Cell Physiol. 2009;220(3):680–689. doi: 10.1002/jcp.21814. [DOI] [PubMed] [Google Scholar]
- 80.Keating E, Lemos C, Azevedo I, Martel F. Characteristics of thiamine uptake by the BeWo human trophoblast cell line. J Biochem Mol Biol. 2006;39(4):383–393. doi: 10.5483/bmbrep.2006.39.4.383. [DOI] [PubMed] [Google Scholar]
- 81.Eudy JD, Spiegelstein O, Barber RC, Wlodarczyk BJ, Talbot J, Finnell RH. Identification and characterization of the human and mouse SLC19A3 gene: a novel member of the reduced folate family of micronutrient transporter genes. Mol Genet Metab. 2000;71(4):581–590. doi: 10.1006/mgme.2000.3112. [DOI] [PubMed] [Google Scholar]
- 82.Solanky N, Requena Jimenez A, D'Souza SW, Sibley CP, Glazier JD. Expression of folate transporters in human placenta and implications for homocysteine metabolism. Placenta. 2010;31(2):134–143. doi: 10.1016/j.placenta.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 83.Bisseling TM, Steegers EA, van den Heuvel JJ, Siero HL, van de Water FM, Walker AJ, Steegers-Theunissen RP, Smits P, Russel FG. Placental folate transport and binding are not impaired in pregnancies complicated by fetal growth restriction. Placenta. 2004;25(6):588–593. doi: 10.1016/j.placenta.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 84.Keating E, Lemos C, Azevedo I, Martel F. Comparison of folic acid uptake characteristics by human placental choriocarcinoma cells at acidic and physiological pH. Can J Physiol Pharmacol. 2006;84(2):247–255. doi: 10.1139/y05-129. [DOI] [PubMed] [Google Scholar]
- 85.Keating E, Goncalves P, Lemos C, Costa F, Campos I, Smith SB, Bridges CC, Martel F. Progesterone inhibits folic acid transport in human trophoblasts. J Membr Biol. 2007;216(2-3):143–152. doi: 10.1007/s00232-007-9057-5. [DOI] [PubMed] [Google Scholar]
- 86.Yasuda S, Hasui S, Kobayashi M, Itagaki S, Hirano T, Iseki K. The mechanism of carrier-mediated transport of folates in BeWo cells: the involvement of heme carrier protein 1 in placental folate transport. Biosci Biotechnol Biochem. 2008;72(2):329–334. doi: 10.1271/bbb.70347. [DOI] [PubMed] [Google Scholar]
- 87.de Carvalho FD, Quick M. Surprising substrate versatility in SLC5A6: Na+−coupled I- transport by the human Na+/multivitamin transporter (hSMVT) J Biol Chem. 2011;286(1):131–137. doi: 10.1074/jbc.M110.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Crisp SE, Griffin JB, White BR, Toombs CF, Camporeale G, Said HM, Zempleni J. Biotin supply affects rates of cell proliferation, biotinylation of carboxylases and histones, and expression of the gene encoding the sodium-dependent multivitamin transporter in JAr choriocarcinoma cells. Eur J Nutr. 2004;43(1):23–31. doi: 10.1007/s00394-004-0435-9. [DOI] [PubMed] [Google Scholar]
- 89.Wang H, Huang W, Fei YJ, Xia H, Yang-Feng TL, Leibach FH, Devoe LD, Ganapathy V, Prasad PD. Human placental Na+−dependent multivitamin transporter. Cloning, functional expression, gene structure, and chromosomal localization. J Biol Chem. 1999;274(21):14875–14883. doi: 10.1074/jbc.274.21.14875. [DOI] [PubMed] [Google Scholar]
- 90.Rutsch F, Gailus S, Suormala T, Fowler B. LMBRD1: the gene for the cblF defect of vitamin B(1)(2) metabolism. J Inherit Metab Dis. 2011;34(1):121–126. doi: 10.1007/s10545-010-9083-9. [DOI] [PubMed] [Google Scholar]
- 91.Hediger MA, Clemencon B, Burrier RE, Bruford EA. The ABCs of membrane transporters in health and disease (SLC series): introduction. Mol Asp Med. 2013;34(2-3):95–107. doi: 10.1016/j.mam.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Burzle M, Suzuki Y, Ackermann D, Miyazaki H, Maeda N, Clemencon B, Burrier R, Hediger MA. The sodium-dependent ascorbic acid transporter family SLC23. Mol Asp Med. 2013;34(2-3):436–454. doi: 10.1016/j.mam.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 93.Takanaga H, Mackenzie B, Hediger MA. Sodium-dependent ascorbic acid transporter family SLC23. Pflugers Arch. 2004;447(5):677–682. doi: 10.1007/s00424-003-1104-1. [DOI] [PubMed] [Google Scholar]
- 94.Sotiriou S, Gispert S, Cheng J, Wang Y, Chen A, Hoogstraten-Miller S, Miller GF, Kwon O, Levine M, Guttentag SH, et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med. 2002;8(5):514–517. doi: 10.1038/0502-514. [DOI] [PubMed] [Google Scholar]
- 95.Chen L, Zhu H, Pan Y, Tang C, Watanabe M, Ruan H, Wang Y, Wang J, Yao HY, Iguchi T, et al. Ascorbic acid uptaken by sodium-dependent vitamin C transporter 2 induces betahCG expression through Sp1 and TFAP2A transcription factors in human choriocarcinoma cells. J Clin Endocrinol Metab. 2012;97(9):E1667–E1676. doi: 10.1210/jc.2012-1753. [DOI] [PubMed] [Google Scholar]
- 96.Biondi C, Pavan B, Dalpiaz A, Medici S, Lunghi L, Vesce F. Expression and characterization of vitamin C transporter in the human trophoblast cell line HTR-8/SVneo: effect of steroids, flavonoids and NSAIDs. Mol Hum Reprod. 2007;13(1):77–83. doi: 10.1093/molehr/gal092. [DOI] [PubMed] [Google Scholar]
- 97.Harrison FE, Dawes SM, Meredith ME, Babaev VR, Li L, May JM. Low vitamin C and increased oxidative stress and cell death in mice that lack the sodium-dependent vitamin C transporter SVCT2. Free Radic Biol Med. 2010;49(5):821–829. doi: 10.1016/j.freeradbiomed.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang L, Tepaamorndech S. The SLC30 family of zinc transporters - a review of current understanding of their biological and pathophysiological roles. Mol Asp Med. 2013;34(2-3):548–560. doi: 10.1016/j.mam.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 99.Jeong J, Eide DJ. The SLC39 family of zinc transporters. Mol Asp Med. 2013;34(2-3):612–619. doi: 10.1016/j.mam.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Asano N, Kondoh M, Ebihara C, Fujii M, Nakanishi T, Utoguchi N, Enomoto S, Tanaka K, Watanabe Y. Induction of zinc transporters by forskolin in human trophoblast BeWo cells. Reprod Toxicol. 2006;21(3):285–291. doi: 10.1016/j.reprotox.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 101.Asano N, Kondoh M, Ebihara C, Fujii M, Nakanishi T, Soares MJ, Nakashima E, Tanaka K, Sato M, Watanabe Y. Expression profiles of zinc transporters in rodent placental models. Toxicol Lett. 2004;154(1-2):45–53. doi: 10.1016/j.toxlet.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 102.Andrews GK, Wang H, Dey SK, Palmiter RD. Mouse zinc transporter 1 gene provides an essential function during early embryonic development. Genesis. 2004;40(2):74–81. doi: 10.1002/gene.20067. [DOI] [PubMed] [Google Scholar]
- 103.Seo YA, Lopez V, Kelleher SL. A histidine-rich motif mediates mitochondrial localization of ZnT2 to modulate mitochondrial function. Am J Physiol Cell Physiol. 2011;300(6):C1479–C1489. doi: 10.1152/ajpcell.00420.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Galvez-Peralta M, He L, Jorge-Nebert LF, Wang B, Miller ML, Eppert BL, Afton S, Nebert DW. ZIP8 zinc transporter: indispensable role for both multiple-organ organogenesis and hematopoiesis in utero. PLoS One. 2012;7(5):e36055. doi: 10.1371/journal.pone.0036055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hache S, Takser L, LeBellego F, Weiler H, Leduc L, Forest JC, Giguere Y, Masse A, Barbeau B, Lafond J. Alteration of calcium homeostasis in primary preeclamptic syncytiotrophoblasts: effect on calcium exchange in placenta. J Cell Mol Med. 2011;15(3):654–667. doi: 10.1111/j.1582-4934.2010.01039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stumpf T, Zhang Q, Hirnet D, Lewandrowski U, Sickmann A, Wissenbach U, Dorr J, Lohr C, Deitmer JW, Fecher-Trost C. The human TRPV6 channel protein is associated with cyclophilin B in human placenta. J Biol Chem. 2008;283(26):18086–18098. doi: 10.1074/jbc.M801821200. [DOI] [PubMed] [Google Scholar]
- 107.Bernucci L, Henriquez M, Diaz P, Riquelme G. Diverse calcium channel types are present in the human placental syncytiotrophoblast basal membrane. Placenta. 2006;27(11-12):1082–1095. doi: 10.1016/j.placenta.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 108.Romero MF, Chen AP, Parker MD, Boron WF. The SLC4 family of bicarbonate (HCO(3)(−)) transporters. Mol Asp Med. 2013;34(2-3):159–182. doi: 10.1016/j.mam.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Powell TL, Lundquist C, Doughty IM, Glazier JD, Jansson T. Mechanisms of chloride transport across the syncytiotrophoblast basal membrane in the human placenta. Placenta. 1998;19(4):315–321. doi: 10.1016/s0143-4004(98)90064-9. [DOI] [PubMed] [Google Scholar]
- 110.Lacey HA, Nolan T, Greenwood SL, Glazier JD, Sibley CP. Gestational profile of Na+/H+ exchanger and Cl-/HCO3- anion exchanger mRNA expression in placenta using real-time QPCR. Placenta. 2005;26(1):93–98. doi: 10.1016/j.placenta.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 111.Alper SL, Sharma AK. The SLC26 gene family of anion transporters and channels. Mol Asp Med. 2013;34(2-3):494–515. doi: 10.1016/j.mam.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dawson PA, Rakoczy J, Simmons DG. Placental, renal, and ileal sulfate transporter gene expression in mouse gestation. Biol Reprod. 2012;87(2):43. doi: 10.1095/biolreprod.111.098749. [DOI] [PubMed] [Google Scholar]
- 113.Kondapalli KC, Kallay LM, Muszelik M, Rao R. Unconventional chemiosmotic coupling of NHA2, a mammalian Na+/H+ antiporter, to a plasma membrane H+ gradient. J Biol Chem. 2012;287(43):36239–36250. doi: 10.1074/jbc.M112.403550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Donowitz M, Ming Tse C, Fuster D. SLC9/NHE gene family, a plasma membrane and organellar family of Na(+)/H(+) exchangers. Mol Asp Med. 2013;34(2-3):236–251. doi: 10.1016/j.mam.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Speake PF, Mynett KJ, Glazier JD, Greenwood SL, Sibley CP. Activity and expression of Na+/H+ exchanger isoforms in the syncytiotrophoblast of the human placenta. Pflugers Arch. 2005;450(2):123–130. doi: 10.1007/s00424-005-1382-x. [DOI] [PubMed] [Google Scholar]
- 116.Johansson M, Glazier JD, Sibley CP, Jansson T, Powell TL. Activity and protein expression of the Na+/H+ exchanger is reduced in syncytiotrophoblast microvillous plasma membranes isolated from preterm intrauterine growth restriction pregnancies. J Clin Endocrinol Metab. 2002;87(12):5686–5694. doi: 10.1210/jc.2002-020214. [DOI] [PubMed] [Google Scholar]
- 117.Hughes JL, Doughty IM, Glazier JD, Powell TL, Jansson T, D'Souza SW, Sibley CP. Activity and expression of the Na(+)/H(+) exchanger in the microvillous plasma membrane of the syncytiotrophoblast in relation to gestation and small for gestational age birth. Pediatr Res. 2000;48(5):652–659. doi: 10.1203/00006450-200011000-00017. [DOI] [PubMed] [Google Scholar]
- 118.Wright EM. Glucose transport families SLC5 and SLC50. Mol Asp Med. 2013;34(2-3):183–196. doi: 10.1016/j.mam.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 119.Mitchell AM, Manley SW, Morris JC, Powell KA, Bergert ER, Mortimer RH. Sodium iodide symporter (NIS) gene expression in human placenta. Placenta. 2001;22(2-3):256–258. doi: 10.1053/plac.2000.0609. [DOI] [PubMed] [Google Scholar]
- 120.Akturk M, Oruc AS, Danisman N, Erkek S, Buyukkagnici U, Unlu E, Tazebay UH. Na+/I- symporter and type 3 iodothyronine deiodinase gene expression in amniotic membrane and placenta and its relationship to maternal thyroid hormones. Biol Trace Elem Res. 2013;154(3):338–344. doi: 10.1007/s12011-013-9748-y. [DOI] [PubMed] [Google Scholar]
- 121.Burns R, O'Herlihy C, Smyth PP. Regulation of iodide uptake in placental primary cultures. Eur Thyroid J. 2013;2(4):243–251. doi: 10.1159/000356847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Di Cosmo C, Fanelli G, Tonacchera M, Ferrarini E, Dimida A, Agretti P, De Marco G, Vitti P, Pinchera A, Bevilacqua G, et al. The sodium-iodide symporter expression in placental tissue at different gestational age: an immunohistochemical study. Clin Endocrinol. 2006;65(4):544–548. doi: 10.1111/j.1365-2265.2006.02577.x. [DOI] [PubMed] [Google Scholar]
- 123.Karatas A, Erdem H, Albayrak M, Oktay M, Ozlu T, Cakmak B, Keskin F, Donmez ME. Alterations in placental pendrin expression in pre-eclampsia. J Matern Fetal Neonatal Med. 2014;27(7):687–690. doi: 10.3109/14767058.2013.833600. [DOI] [PubMed] [Google Scholar]
- 124.Suzuki K, Royaux IE, Everett LA, Mori-Aoki A, Suzuki S, Nakamura K, Sakai T, Katoh R, Toda S, Green ED, et al. Expression of PDS/Pds, the Pendred syndrome gene, in endometrium. J Clin Endocrinol Metab. 2002;87(2):938. doi: 10.1210/jcem.87.2.8390. [DOI] [PubMed] [Google Scholar]
- 125.Montalbetti N, Simonin A, Kovacs G, Hediger MA. Mammalian iron transporters: families SLC11 and SLC40. Mol Asp Med. 2013;34(2-3):270–287. doi: 10.1016/j.mam.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 126.Gruper Y, Bar J, Bacharach E, Ehrlich R. Transferrin receptor co-localizes and interacts with the hemochromatosis factor (HFE) and the divalent metal transporter-1 (DMT1) in trophoblast cells. J Cell Physiol. 2005;204(3):901–912. doi: 10.1002/jcp.20349. [DOI] [PubMed] [Google Scholar]
- 127.Li YQ, Bai B, Cao XX, Zhang YH, Yan H, Zheng QQ, Zhuang GH. Divalent metal transporter 1 expression and regulation in human placenta. Biol Trace Elem Res. 2012;146(1):6–12. doi: 10.1007/s12011-011-9214-7. [DOI] [PubMed] [Google Scholar]
- 128.Gambling L, Danzeisen R, Fosset C, Andersen HS, Dunford S, Srai SK, MCArdle HJ. Iron and copper interactions in development and the effect on pregnancy outcome. J Nutr. 2003;133(5 Suppl 1):1554S–1556S. doi: 10.1093/jn/133.5.1554S. [DOI] [PubMed] [Google Scholar]
- 129.Quazi F, Molday RS. Lipid transport by mammalian ABC proteins. Essays Biochem. 2011;50(1):265–290. doi: 10.1042/bse0500265. [DOI] [PubMed] [Google Scholar]
- 130.Stefulj J, Panzenboeck U, Becker T, Hirschmugl B, Schweinzer C, Lang I, Marsche G, Sadjak A, Lang U, Desoye G, et al. Human endothelial cells of the placental barrier efficiently deliver cholesterol to the fetal circulation via ABCA1 and ABCG1. Circ Res. 2009;104(5):600–608. doi: 10.1161/CIRCRESAHA.108.185066. [DOI] [PubMed] [Google Scholar]
- 131.Aye IL, Waddell BJ, Mark PJ, Keelan JA. Placental ABCA1 and ABCG1 transporters efflux cholesterol and protect trophoblasts from oxysterol induced toxicity. Biochim Biophys Acta. 2010;1801(9):1013–1024. doi: 10.1016/j.bbalip.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 132.Nikitina L, Wenger F, Baumann M, Surbek D, Korner M, Albrecht C. Expression and localization pattern of ABCA1 in diverse human placental primary cells and tissues. Placenta. 2011;32(6):420–430. doi: 10.1016/j.placenta.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 133.Krishnamurthy P, Schuetz JD. Role of ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol. 2006;46:381–410. doi: 10.1146/annurev.pharmtox.46.120604.141238. [DOI] [PubMed] [Google Scholar]
- 134.Evseenko DA, Murthi P, Paxton JW, Reid G, Emerald BS, Mohankumar KM, Lobie PE, Brennecke SP, Kalionis B, Keelan JA. The ABC transporter BCRP/ABCG2 is a placental survival factor, and its expression is reduced in idiopathic human fetal growth restriction. FASEB J. 2007;21(13):3592–3605. doi: 10.1096/fj.07-8688com. [DOI] [PubMed] [Google Scholar]
- 135.Yeboah D, Kalabis GM, Sun M, Ou RC, Matthews SG, Gibb W. Expression and localisation of breast cancer resistance protein (BCRP) in human fetal membranes and decidua and the influence of labour at term. Reprod Fertil Dev. 2008;20(2):328–334. doi: 10.1071/rd07133. [DOI] [PubMed] [Google Scholar]
- 136.Yeboah D, Sun M, Kingdom J, Baczyk D, Lye SJ, Matthews SG, Gibb W. Expression of breast cancer resistance protein (BCRP/ABCG2) in human placenta throughout gestation and at term before and after labor. Can J Physiol Pharmacol. 2006;84(12):1251–1258. doi: 10.1139/y06-078. [DOI] [PubMed] [Google Scholar]
- 137.Lee JH, Lee JE, Kim Y, Lee H, Jun HJ, Lee SJ. Multidrug and toxic compound extrusion protein-1 (MATE1/SLC47A1) is a novel flavonoid transporter. J Agric Food Chem. 2014;62(40):9690–9698. doi: 10.1021/jf500916d. [DOI] [PubMed] [Google Scholar]
- 138.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24(7):1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- 139.Ahmadimoghaddam D, Zemankova L, Nachtigal P, Dolezelova E, Neumanova Z, Cerveny L, Ceckova M, Kacerovsky M, Micuda S, Staud F. Organic cation transporter 3 (OCT3/SLC22A3) and multidrug and toxin extrusion 1 (MATE1/SLC47A1) transporter in the placenta and fetal tissues: expression profile and fetus protective role at different stages of gestation. Biol Reprod. 2013;88(3):55. doi: 10.1095/biolreprod.112.105064. [DOI] [PubMed] [Google Scholar]
- 140.Anderson CM, Stahl A. SLC27 fatty acid transport proteins. Mol Asp Med. 2013;34(2-3):516–528. doi: 10.1016/j.mam.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jadoon A, Cunningham P, McDermott LC. Regulation of fatty acid binding proteins by hypoxia inducible factors 1alpha and 2alpha in the placenta: relevance to pre-eclampsia. Prostaglandins Leukot Essent Fat Acids. 2015;93:25–29. doi: 10.1016/j.plefa.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 142.Schaiff WT, Bildirici I, Cheong M, Chern PL, Nelson DM, Sadovsky Y. Peroxisome proliferator-activated receptor-gamma and retinoid X receptor signaling regulate fatty acid uptake by primary human placental trophoblasts. J Clin Endocrinol Metab. 2005;90(7):4267–4275. doi: 10.1210/jc.2004-2265. [DOI] [PubMed] [Google Scholar]
- 143.Lager S, Jansson N, Olsson AL, Wennergren M, Jansson T, Powell TL. Effect of IL-6 and TNF-alpha on fatty acid uptake in cultured human primary trophoblast cells. Placenta. 2011;32(2):121–127. doi: 10.1016/j.placenta.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 144.Dube E, Gravel A, Martin C, Desparois G, Moussa I, Ethier-Chiasson M, Forest JC, Giguere Y, Masse A, Lafond J. Modulation of fatty acid transport and metabolism by maternal obesity in the human full-term placenta. Biol Reprod. 2012;87(1):14. doi: 10.1095/biolreprod.111.098095. [DOI] [PubMed] [Google Scholar]
- 145.Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Asp Med. 2013;34(2-3):121–138. doi: 10.1016/j.mam.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jansson T, Wennergren M, Illsley NP. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab. 1993;77(6):1554–1562. doi: 10.1210/jcem.77.6.8263141. [DOI] [PubMed] [Google Scholar]
- 147.Halestrap AP. The SLC16 gene family - structure, role and regulation in health and disease. Mol Asp Med. 2013;34(2-3):337–349. doi: 10.1016/j.mam.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 148.Settle P, Mynett K, Speake P, Champion E, Doughty IM, Sibley CP, D'Souza SW, Glazier J. Polarized lactate transporter activity and expression in the syncytiotrophoblast of the term human placenta. Placenta. 2004;25(6):496–504. doi: 10.1016/j.placenta.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 149.Price NT, Jackson VN, Halestrap AP. Cloning and sequencing of four new mammalian monocarboxylate transporter (MCT) homologues confirms the existence of a transporter family with an ancient past. Biochem J. 1998;329(Pt 2):321–328. doi: 10.1042/bj3290321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Settle P, Sibley CP, Doughty IM, Johnston T, Glazier JD, Powell TL, Jansson T, D'Souza SW. Placental lactate transporter activity and expression in intrauterine growth restriction. J Soc Gynecol Investig. 2006;13(5):357–363. doi: 10.1016/j.jsgi.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 151.Kay HH, Zhu S, Tsoi S. Hypoxia and lactate production in trophoblast cells. Placenta. 2007;28(8-9):854–860. doi: 10.1016/j.placenta.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 152.Pramod AB, Foster J, Carvelli L, Henry LK. SLC6 transporters: structure, function, regulation, disease association and therapeutics. Mol Asp Med. 2013;34(2-3):197–219. doi: 10.1016/j.mam.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bottalico B, Larsson I, Brodszki J, Hernandez-Andrade E, Casslen B, Marsal K, Hansson SR. Norepinephrine transporter (NET), serotonin transporter (SERT), vesicular monoamine transporter (VMAT2) and organic cation transporters (OCT1, 2 and EMT) in human placenta from pre-eclamptic and normotensive pregnancies. Placenta. 2004;25(6):518–529. doi: 10.1016/j.placenta.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 154.Bzoskie L, Yen J, Tseng YT, Blount L, Kashiwai K, Padbury JF. Human placental norepinephrine transporter mRNA: expression and correlation with fetal condition at birth. Placenta. 1997;18(2-3):205–210. doi: 10.1016/s0143-4004(97)90094-1. [DOI] [PubMed] [Google Scholar]
- 155.Ramamoorthy S, Prasad PD, Kulanthaivel P, Leibach FH, Blakely RD, Ganapathy V. Expression of a cocaine-sensitive norepinephrine transporter in the human placental syncytiotrophoblast. Biochemistry. 1993;32(5):1346–1353. doi: 10.1021/bi00056a021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene specific primer sequences used for quantitative RT- PCR. It presents a complete list of the primers designed for the selected candidate and control genes in this study. (DOCX 21 kb)
Data Availability Statement
The datasets analysed during the current study are mined from publicly available in the NCBI GEO repository under the following accession number:
GSE 25861 [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE25861]
GSE 24129 [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE24129]
GSE 12216 [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE12216]
GSE 25906 [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE25906]
GSE 14722 [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE14722]
GSE 35574 [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE35574]
GSE 24818 [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE24818]
Other data supporting the conclusions of this article are not publicly available due to privacy concerns, but are available from the corresponding author on request.


