Abstract
Background
Although impaired myeloid-derived suppressor cells (MDSCs) recently have been studied in immune thrombocytopenia (ITP), another myeloid-derived cell population signified as M2 macrophages has not been investigated properly in ITP patients. In the present study, we intended to determine the features of circulating M2-like macrophages, to examine its relationship with MDSCs, and to explore their prognostic values in ITP.
Methods
Peripheral blood mononuclear cells from healthy controls and primary ITP patients were isolated to test the circulating M2-like macrophages and MDSCs. The circulating M2-like macrophage population defined as CD68+CD163+ and circulating MDSC population as CD11b+CD33+HLA-DR− were determined by flow cytometry. Plasma inflammatory cytokines were measured by multiplex ELISA.
Results
The percentages of MDSCs were found to be expanded in newly diagnosed patients of ITP, especially among those of the complete response (CR) group (p < 0.0001). Positive linear correlation was verified between percentages of M2-like macrophages and MDSCs. The same correlation was also determined in the CR group. After treatment, the percentages of M2-like macrophages and MDSCs were both increased significantly in CR group, while those patients among the PR + NR group manifested a significant numeric decrease of MDSCs but only a moderate decrease in M2-like macrophages. MIP-1α/CCL3 was negatively correlated with M2-like macrophages while MCP-1 possessed a positive correlation with M2-like macrophages, eotaxin-1/CCL11 was negatively correlated with MDSCs and interleukin-1β (IL-1β) was found to be negatively correlated with both M2-like macrophages and MDSCs.
Conclusions
The present findings indicated critical roles of both circulating M2-like macrophages and MDSCs in ITP. The positive correlation between them might be related to inflammatory factors-mediated bidirectional interactions or partially due to their similar background patterns during differentiation. MIP-1α/CCL3, MCP-1, eotaxin-1/CCL11 and IL-1β might play a critical role in the expansion of both M2 macrophages and MDSCs population in ITP patients, which deserves further investigation.
Electronic supplementary material
The online version of this article (10.1186/s12967-018-1424-8) contains supplementary material, which is available to authorized users.
Keywords: Immune thrombocytopenia, M2-like macrophages, MDSCs, Dexamethasone
Background
Immune thrombocytopenia (ITP) is an acquired autoimmune hemorrhagic disorder, characterized by immune-mediated platelet destruction and impaired megakaryocyte maturation [1]. High-dose dexamethasone (HD-DXM), an appropriate first-line therapy for adult patients with ITP [2], has achieved a satisfying initial response rate up to 85% [3]. Clinical investigations for the pathogenesis of ITP have been largely focused on the aberrations related to adaptive immunity including platelet autoantibodies [4], T cell clonality and T helper differentiation [5]. Recently, the role of innate immunity and myeloid-derived immune modulator cells have been recognized in the pathogenesis of ITP [6, 7].
Myeloid-derived suppressor cells (MDSCs), generally recognized as circulating immature myeloid cells with remarkable immunosuppressive properties [8], have been verified to contribute to the inactivation of T-cells and antigen presenting cells during various diseases, particularly in cancer [9, 10]. The role of MDSCs in autoimmune diseases is just starting to be elucidated. Recently, studies have identified the pathogenic role of MDSCs in ITP. Impaired MDSCs have been implicated in the pathogenesis of ITP [6] via direct inhibition of T cell activation or the indirect induction of regulatory T cells (Tregs), which could also act as a prognostic marker for treatment responses in ITP [7]. As another important myeloid-derived cell compartment, macrophages have been implicated to be active in the perturbation of immune tolerance in ITP. The utmost emphasis placed on macrophages is the phagocytosis function, especially the disturbed balance of the activating and inhibitory Fcγ receptor (FcγRs) [11]. Alternatively activated, also known as M2 macrophages, which are predominantly characterized as CD68+ (pan macrophages marker) and CD163+ (M2 specific marker), could dampen the immune response and limit inflammation. It has been argued to be conducive to the progression of neoplasia [12, 13] as well as to successfully inhibit experimental autoimmune encephalomyelitis [14, 15]. However, M2 macrophages have not yet been investigated properly in ITP patients.
In the present study, we intended to determine the features of circulating M2-like macrophages, to examine its relationship with MDSCs, and to evaluate the proinflammatory milieu leading to the expansion of these two cell compartments, thereby providing novel insights for the immune dysregulation as well as prognostic indicators of ITP.
Methods
Patients and controls
Thirty-three patients with newly diagnosed primary ITP with blood platelets count less than 30 × 109/L and required medical intervention were enrolled in the study between September 2015 and May 2017. All patients met the diagnosis criteria of ITP according to an international working group [16], cases with diabetes, hypertension, cardiovascular diseases, pregnancy, active or chronic infection, connective tissue diseases were excluded from this study. Bone marrow aspirate was performed to exclude other diseases resulting in thrombocytopenia [17]. All patients received HD-DXM regimen (40 mg of oral dexamethasone daily for 4 consecutive days). A complete response (CR) was defined as an increase in platelet level to ≥ 100 × 109/L by day 10 after the initiation of treatment. Partial response (PR) was defined as platelet counts (30–100 × 109/L) and no response (NR) was defined as a platelet level still < 30 × 109/L by day 10 after treatment. Other treatments were considered if there was no response to dexamethasone. The control group consisted of 18 healthy volunteers. The present study was approved by institutional review board of Zhongshan Hospital Fudan University in accordance with the Declaration of Helsinki, and written informed consent was obtained from all patients.
Isolation of peripheral blood mononuclear cells
EDTA-anticoagulated venous blood samples were freshly collected from ITP patients before and 10 days after dexamethasone treatment and from healthy volunteers. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density-gradient centrifugation and resuspended in cell freezing medium (10% DMSO in fetal bovine serum) at 5 × 105 cells/mL. The cell suspensions were frozen at − 80 °C and thawed for testing on separate occasions.
Flow cytometry analysis
MDSCs were defined as CD11b+/CD33+/HLA-DR−. Single cell suspensions were surface stained with APC-conjugated anti-human CD11b (ICRF44; BD Biosciences, San Diego, CA), FITC-conjugated anti-human CD33 (HIM3-4; BD Biosciences) and PerCP-Cy5.5-conjugated anti-human HLA-DR (G46-6; BD Biosciences). All samples were prepared according to the manufactures’ instructions. The phenotype of circulating M2-like macrophages was analyzed by intracellular CD68 (Y1/82A; BD Biosciences) and CD163 (GHI/61; BD Biosciences) staining after fixing with 4% paraformaldehyde and permeabilizing by FACS permeabilizing solution (BD PharMingen, San Diego, CA). Flow analysis was performed on a FACS Aria II flow cytometer (BD Biosciences) and data were analyzed using FlowJo 7.6.1 (Tree Star, San Carlos, CA) software.
Plasma inflammation cytokine array
Cytokine profiles were measured by Quantibody Human Inflammation Array 3 (RayBiotech, Norcross, GA) that detected 40 inflammation-associated cytokines simultaneously using plasma samples (10 healthy controls and 23 out of these 33 primary ITP patients) cryopreserved at − 80 °C. The array slides were incubated with thawed plasma samples. Then washed and incubated with a cocktail of biotinylated antibodies according to the protocol provided by the manufacturer. The slides bounded with biotin were then incubated with streptavidin-conjugated Hylite Plus 555 fluor. Relative fluorescent strength was measured by LuxScan 10 K-A Microarray Scanner (CapitalBio Corporation, Beijing, China).
Statistical analysis
All data were expressed as mean ± standard deviation, and were analyzed using the SPSS 13.0 software (SPSS, Chicago, IL). Continuous variables between groups were determined by the one-way ANOVA or Mann–Whitney U test as appropriate. Two-tailed Student’s non-paired t test was applied for evaluating statistically significant differences between two independent groups, and the differences between pre- and post-treatment groups were detected by paired Student’s t test. The correlation between MDSCs and M2-like macrophages was accessed by Pearson’s correlation coefficient. Two-tailed p < 0.05 was considered to be statistically significant (Additional file 1: Figure S1).
Results
Clinical characteristics of primary ITP patients
Among 33 ITP patients in the present study, 22 achieved CR with dexamethasone while 5 patients achieved PR and 6 unresponsive patients. Clinical characteristics were presented in Table 1. The median age of eighteen healthy controls (10 females and 8 males) was 44 years (range 34–53 years) and the median platelet count was 236 × 109/L (range 114–304 × 109/L).
Table 1.
Clinical characteristics of ITP patients
| Characteristics | All patients (N = 33) | |
|---|---|---|
| No. | % | |
| Gender | ||
| Female | 19/33 | 58 |
| Male | 14/33 | 42 |
| Age, years median (range) | 47 (28, 80) | – |
| Platelet counts (×109/L), median (range) | ||
| Before treatment | 9 (2, 29) | – |
| After treatment | 105 (3, 220) | – |
| Response to HD-DXM regimen | ||
| CR | 22/33 | 67 |
| PR | 5/33 | 15 |
| NR | 6/33 | 18 |
Expansion of peripheral MDSCs but not M2-like macrophages in ITP
The percentages of MDSCs and M2-like macrophages were analyzed in healthy controls (HC) and ITP patients. Otherwise, ITP patients were further grouped into CR or PR + NR according to their later treatment responses. MDSCs were found significantly elevated in ITP patients comparing with HC (6.21 ± 2.94% vs. 2.29 ± 0.76%, p < 0.0001, Fig. 1a, c). When considering treatment response, the expansion of MDSCs was especially accentuated in CR group, but less prominent in PR + NR group (6.60 ± 3.08 vs. 2.29 ± 0.76, CR vs. HC, p < 0.0001, and 4.97 ± 2.63 vs. 2.29 ± 0.76, PR + NR vs. HC, p = 0.23, Fig. 1c). No significant difference was observed in circulating M2-like macrophages among ITP patients when compared with HC (Fig. 1b, d). These experiments pointed towards the dramatically elevated percentages of MDSCs in ITP patients, especially among the CR group (Additional file 2: Figure S2).
Fig. 1.
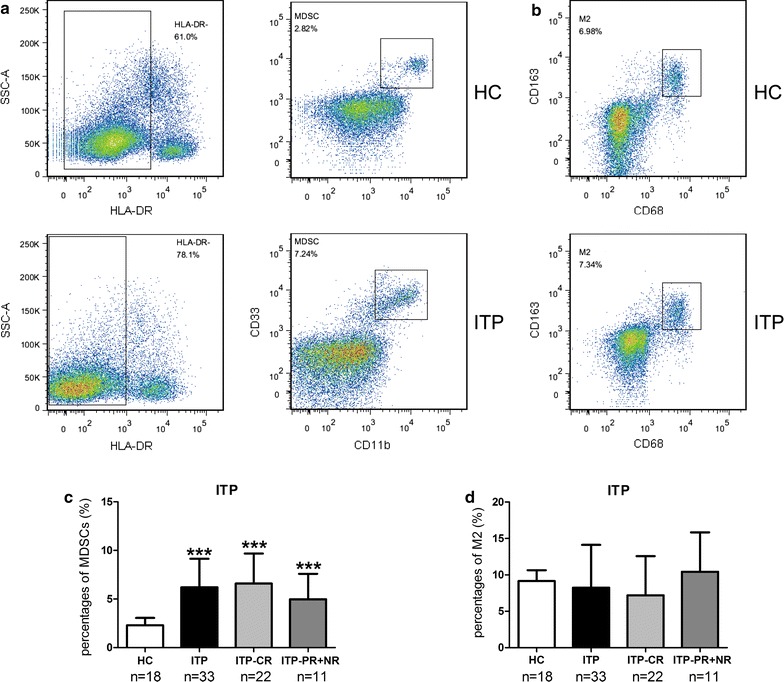
Peripheral MDSCs and M2-like macrophages in newly diagnosed ITP patients (n = 33) before treatment and health control (n = 18). a Representative dot plots of CD11b+CD33+HLA-DR− MDSCs in the PBMCs of HC and ITP patients. b Representative dot plots of CD68+CD163+ M2-like macrophages in the PBMCs of HC and ITP patients. c Peripheral MDSCs were significantly elevated in ITP patients, with no respect of their later treatment response to HD-DXM regimen. d Peripheral M2-like macrophages were not found elevated in ITP patients, with no respect of their later treatment response to HD-DXM regimen. ***p < 0.001, compared to HC in two-tailed Student’s non-paired t test. Bars represented SD
Peripheral M2-like macrophages correlated positively to MDSCs in ITP
No significant correlation between M2-like macrophages and MDSCs was noted among the HC (r = − 0.40, p = 0.10, Fig. 2a), but a significant positive linear correlation was verified in ITP patients (r = 0.42, p = 0.02, Fig. 2b), especially in cases of the CR group (r = 0.59, p = 0.01, Fig. 2c). No correlation was found between M2-like macrophages and MDSCs in PR + NR group (r = 0.33, p = 0.31, Fig. 2d). The positive correlation between peripheral M2-like macrophages and MDSCs was found in ITP patients, but not in HC.
Fig. 2.
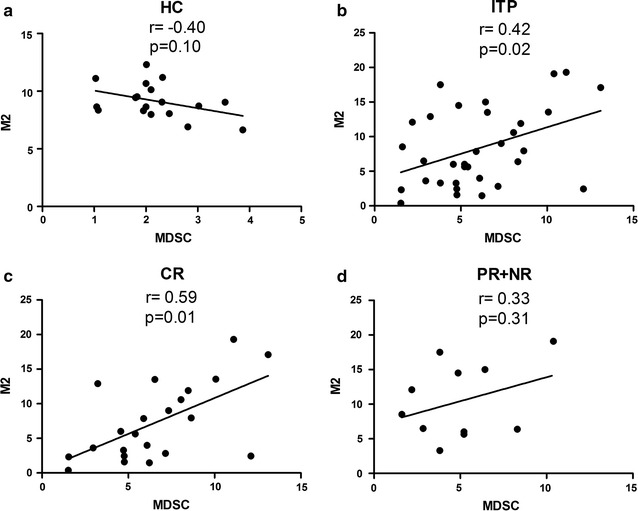
Peripheral MDSCs correlated positively with M2-like macrophages in ITP patients before treatment. Correlation between the percentages of MDSCs and M2-like macrophages in HC (n = 18) (a) and newly diagnosed ITP patients (n = 33) (b), among CR group (n = 22) (c) and among PR + NR group (n = 11) (d). Data analyzed by Pearson’s correlation test
M2-like macrophages and MDSCs expanded after the treatment in CR group
Since all the ITP patients received HD-DXM treatment, we next sought to determine whether it influenced M2-like macrophages or MDSCs. Taking advantages of the matched data, which were taken before and after the treatment of HD-DXM in both CR and PR + NR group, we found the percentage of circulating M2-like macrophages of CR group was significantly elevated after the treatment (7.07 ± 5.35% vs. 10.91 ± 5.39%, p = 0.01, Fig. 3a), and so were the MDSCs (6.60 ± 3.15% vs. 10.22 ± 8.07%, p = 0.02, Fig. 3b). PR + NR patients manifested moderate decrease in M2-like macrophages (10.32 ± 5.47% vs. 8.26 ± 5.08%, p = 0.14, Fig. 3c) but significant decline in circulating MDSCs (4.97 ± 2.63% vs. 2.77 ± 2.07%, p = 0.04, Fig. 3d). In conclusion, M2-like macrophages, along with MDSCs, increased significantly after the treatment of HD-DXM in the CR group, while decreased in the PR + NR group (Additional file 3: Table S1).
Fig. 3.
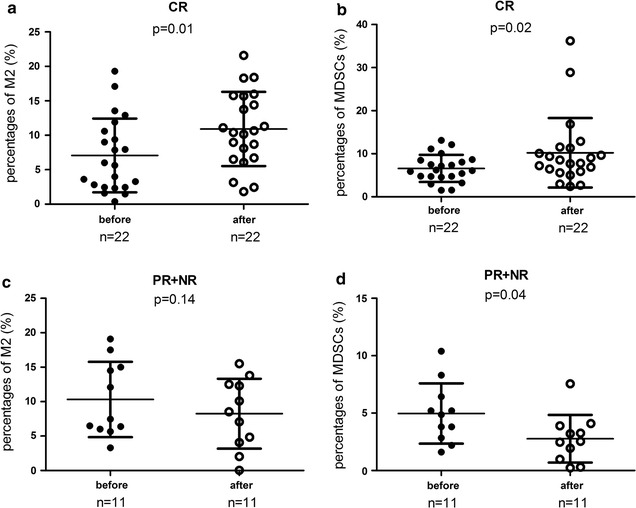
Frequencies of M2-like macrophages and MDSCs increased after treatment among the patients in CR group but decreased in PR + NR group. Differences in M2-like macrophages among the CR group (n = 22) (a) and among PR + NR group (n = 11) (c); changes in the circulating MDSCs among the CR group (b) and among PR + NR group (d) monitored before and after treatment. Data analyzed by paired Student’s t test
Plasma cytokines correlated with M2-like macrophages and MDSCs
To determine the inflammatory environment of myeloid-derived immune modulator cells, forty inflammation-associated cytokines were analyzed. Within the detection panel of cytokines measured, macrophage-inflammatory protein-1α/CC chemokine ligand 3 (MIP-1α/CCL3), monocyte chemoattractant protein-1 (MCP-1), Eotaxin-1/CeC motif chemokine 11 (CCL11) and interleukin-1β (IL-1β) were found to be significantly down-regulated in primary ITP patients (Table 2, Fig. 4a–d). After treatment, plasma levels of MCP-1, Eotaxin-1/CCL11 and IL-1β were markedly augmented in the CR group (p < 0.01, Fig. 4b–d). Correlation analysis between cytokine levels and both myeloid-derived cell concentrations were performed among ITP patients (Fig. 4e). Among all the significantly downregulated cytokines, MIP-1α/CCL3 was found negatively correlated with M2-like macrophages (r = − 0.45, p = 0.03) while MCP-1 manifested a positive correlation with M2-like macrophages (r = 0.46, p = 0.03). Eotaxin-1/CCL11 was negatively correlated with MDSCs (r = − 0.54, p = 0.01). In addition, IL-1β was found to be negatively correlated with both M2-like macrophages (r = − 0.45, p = 0.03) and MDSCs (r = − 0.44, p = 0.04). We proposed that low levels of pro-inflammatory cytokines in ITP could provide a moderate inflammatory environment favoring the recruiting and development of M2-like macrophages and MDSCs.
Table 2.
Plasma cytokines portraits of primary ITP patients (pg/ml)
| Health control (n = 10) | Primary ITP (n = 23) | |
|---|---|---|
| Eotaxin-1/CCL11 | 220.40 (195.40–220.40) | 154.5 (106.80–189.10)*** |
| Eotaxin-2 (×103) | 1.17 (0.84–2.20) | 0.92 (0.49–1.41) |
| G-CSF | 825.40 (562.20–855.90) | 76.70 (37.00–175.90)*** |
| GM-CSF | 80.05 (66.35–97.28) | 11.00 (8.00–16.90)*** |
| I-309 | 83.70 (68.45–134.50) | 33.70 (19.60–55.70)*** |
| ICAM-1 (×103) | 39.40 (16.75–43.33) | 21.87 (6.46–45.08) |
| IFNγ (×103) | 0.79 (0.71–0.94) | 0.16 (0.12–0.20)** |
| IL-1α (×103) | 1.16 (0.94–1.33) | 0.20 (0.11–0.45)*** |
| IL-1β | 65.75 (59.90–79.70) | 37.80 (24.10–47.50)*** |
| IL-1ra (×103) | 1.28 (1.01–1.63) | 0.35 (0.26–0.41)*** |
| IL-2 | 264.90 (187.80–343.20) | 44.90 (33.10–71.60)*** |
| IL-4 | 415.40 (340.80–504.10) | 110.5 (54.7–164.0)*** |
| IL-5 | 185.40 (158.00–243.40) | 42.10 (32.70–51.60)*** |
| IL-6 | 83.90 (59.03–101.40) | 16.50 (7.90–26.80)*** |
| IL-6R (×103) | 17.90 (17.86–18.78) | 17.80 (17.80–18.90) |
| IL-7 | 130.30 (96.93–147.60) | 26.20 (22.10–34.60)*** |
| IL-8 | 99.40 (75.40–121.80) | 11.90 (7.70–19.30)*** |
| IL-10 | 94.45 (63.40–192.20) | 17.10 (11.10–23.40)*** |
| IL-11 (×103) | 1.43 (1.07–1.93) | 0.41 (0.24–0.53)*** |
| IL-12p40 | 15.85 (12.20–26.78) | 13.50 (4.50–18.10) |
| IL-12p70 | 41.85 (28.08–55.05) | 7.30 (4.60–9.80)*** |
| IL-13 | 37.80 (26.48–46.33) | 10.10 (6.10–12.90)*** |
| IL-15 | 57.50 (35.35–71.68) | 27.60 (12.20–36.90)* |
| IL-16 | 124.40 (75.65–171.50) | 62.30 (31.80–106.60)* |
| IL-17A | 29.95 (14.50–41.38) | 4.00 (1.80–6.80)*** |
| MCP-1 (×103) | 0.30 (0.27–0.35) | 0.16 (0.12–0.30)** |
| M-CSF | 20.45 (16.28–28.30) | 8.20 (4.20–14.00)** |
| CXCL9/MIG | 52.55 (39.88–67.83) | 44.60 (25.80–55.00) |
| MIP-1α/CCL3 | 25.10 (20.45–30.30) | 17.30 (10.60–24.30)* |
| MIP-1β | 97.05 (88.08–140.40) | 85.50 (63.50–155.00) |
| MIP-1d (×103) | 1.31 (0.88–2.95) | 1.80 (0.95–2.94) |
| PDGF-BB (×103) | 5.36 (3.87–6.72) | 0.70 (0.34–1.82)*** |
| RANTES (×103) | 14.62 (13.92–15.71) | 8.90 (4.08–14.59)** |
| TIMP-1 (×103) | 8.21 (7.00–10.29) | 16.13 (11.99–19.03)*** |
| TIMP-2 (×103) | 20.46 (13.93–32.76) | 11.92 (7.81–14.82)** |
| TNFα (×103) | 1.06 (0.71–1.80) | 0.24 (0.15–0.37)*** |
| TNFβ (×103) | 0.90 (0.70–1.65) | 0.30 (0.21–0.38)*** |
| TNFRI (×103) | 5.21 (4.11–5.93) | 7.02 (5.40–13.51)* |
| TNFRII (×104) | 1.80 (1.53–2.01) | 2.12 (1.82–3.17)* |
Data are presented as median (interquartile range)
CCL11 CeC motif chemokine 11, G-CSF granulocyte colony-stimulating factor, GM-CSF granulocyte–macrophage colony-stimulating factor, ICAM-1 intercellular adhesion molecule-1, IFNγ interferon-gamma, IL interleukin, MCP-1 monocyte chemoattractant protein-1, M-CSF macrophage colony-stimulating factor, CXCL9 CXC ligand 9, MIP macrophage-inflammatory protein, PDGF-BB platelet-derived growth factor BB, RANTES regulated on activation normal T expressed and secreted chemokines, TIMP tissue inhibitor of metalloproteinases, TNF tumor necrosis factor, TNFR tumor necrosis factor receptor
* p < 0.05; ** p < 0.01; *** p < 0.001, ITP(n = 23) compared with HC(n = 10)
Fig. 4.
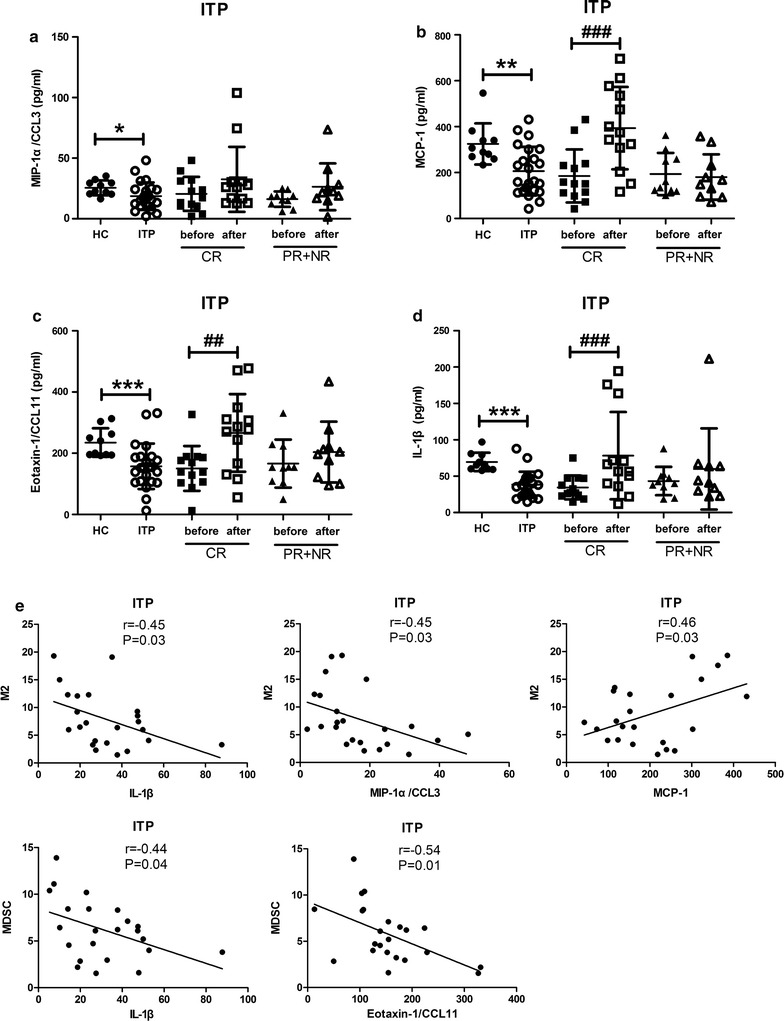
Plasma cytokines correlated with M2-like macrophages and MDSCs. Plasma levels of MIP-1α/CCL3 (a), MCP-1 (b), Eotaxin-1/CCL11 (c) and IL-1β (d) in ITP patients. Correlations were found between MDSCs with plasma levels of IL-1β, MIP-1α/CCL3, and MCP-1; between M2-like macrophages with plasma levels of IL-1β and Eotaxin-1/CCL11 (e). ***p < 0.001, **p < 0.01, *p < 0.05, ITP (n = 23) compared to HC (n = 10) in two-tailed Mann–Whitney U test; ###p < 0.001, ##p < 0.01 after treatment vs. before treatment in corresponding CR group (n = 13) and PR + NR group (n = 10) in two-tailed Mann–Whitney U test
Discussion
ITP is an acquired autoimmune disease and HD-DXM was adopted as one of the first-line therapy. Antiplatelet autoantibodies and T-cell-mediated cytotoxicity accelerate platelet destruction and impair megakaryocyte maturation in ITP [4, 5]. Abnormal regulatory T cells, and regulatory B cells are involved in disabling immune tolerance in ITP [18–21]. Through examining the percentages of M2-macrophages and MDSCs, their relationship, their further changes after treatment and the cytokine profiles in ITP, our study here provided novel insights into the role of myeloid-derived immune modulator cells in ITP.
Identified as immune modulators, M2 macrophages [15] and MDSCs [8] play active roles in the suppression of autoimmunity. MDSCs are uniformly characterized by the co-expression of myeloid-cell lineage differentiation antigen GR1 and CD11b [22, 23] in mice, but cell surface markers defining human MDSCs have not yet to be confirmed [24–26]. CD11b+CD33+HLA-DR− cells sharing multiple MDSCs features were recently identified in ITP patients [6, 7]. M2 macrophages are often identified based on the expression patterns of a set of diverse markers. CD163, a cell surface maker, was stained intracellularly in the present study to show the potential monocyte-to-M2 macrophages polarization. Considering the fact that only monocytes but not activated macrophages exist in the peripheral blood, this cell population was defined as M2-like macrophages.
Monocyte-derived macrophages can polarize into pro-inflammatory M1 or anti-inflammatory M2 phenotype in response to various environment stimuli. The preferred M1 polarization in ITP spleens and impaired immunosuppressive expression of M2 markers are involved in the pathogenesis of ITP [27]. MDSCs could expand under pathological conditions including multiple sclerosis [28], rheumatoid arthritis [29, 30] and autoimmune hepatitis [31]. Recently, studies have shown that circulating MDSCs were reduced in ITP patients [6, 7]. In the present study, accumulation of MDSCs but not the M2-like macrophages was observed in ITP patients. Seemingly the accumulated MDSCs might play a protective role to attenuate autoimmunity, but plasma cytokine assay demonstrated a significant downregulation of IL-10, the most important immunosuppressive cytokine secreted by MDSCs that could skew the macrophages polarization towards tumor associated macrophages (TAM) [32]. Thus, the protective effects of MDSCs accumulation may be functionally invalid, which suggests an immediate compensating response to the proinflammatory environment, instead of adequate immunosuppressive properties.
The decreased levels of MIP-1α/CCL3, MCP-1, eotaxin-1/CCL1, and IL-1β, together with other down-regulated pro-inflammatory cytokines produced a moderate proinflammatory environment in ITP. The chemotactic ability of MIP-1α/CCL3 for M2 is significantly stronger than for M1 in spite of the fact that M1 is produced more [33]. In addition to its pro-inflammatory activities, MIP-1α/CCL3 could negatively regulate the proliferation of hematopoietc stem/progenitor cells in leukemia [34]. This presently defined mechanism is likely applicable to elucidate the negative correlation between MIP-1α/CCL3 and M2-like macrophages. MCP-1, a potent proinflammatory chemokine, recruits monocyte/macrophages to sites of inflammation in a wide variety of pathological conditions [35, 36]. An increase in MCP-1 expression has been found to contribute to the M1 macrophages infiltration and to promote tumor rejection, but low to intermediate levels of MCP-1 could recruit M2 macrophages and trigger a profuse vascular network favoring tumor growth [37, 38]. This biphasic action might indicate that the low to mediate level of MCP-1 in ITP might emerge as a potential marker for the recruitment of M2 macrophages.
Eotaxin-1/CCL11 has been shown to promote chemotaxis of eosinophils and mast cells by binding to chemokine receptor 3 (CCR3), but its effects on MDSCs recruitment remain unknown [39]. Recent study hypothesized that eotaxin-1/CCL11 recruits MDSCs in pancreatic ductal adenocarcinoma but in the end the fact is proved to be contrary to this hypothesis [40]. Our data indicated that eotaxin-1/CCL11 was negatively correlated with MDSCs, suggesting that the downregulated eotaxin-1/CCL11 might emerge as a potential marker for the recruitment of MDSCs. The most intriguing finding of the present study was the negative correlation of IL-1β with both M2-like macrophages and MDSCs. A few studies have predicted a positive association between IL-1β and MDSCs. IL-1β could stimulate the accumulation of MDSCs in cancer [41] and MDSCs is also argued to play a significant pro-inflammatory role by inducing Th17 development in an IL-1β-dependent manner [30, 42]. In addition, IL-1β initiates innate immunity through binding with the IL-1 receptors [43] and has been further proved to be essential in the pathogenesis and progression of autoimmune disease, including experimental autoimmune encephalomyelitis [44], systemic lupus erythematous [45] and ITP [46]. Thus IL-1β may be related to M2-like macrophages and MDSC, which are all critical compartments of innate immunity. Low to intermediate levels of pro-inflammatory cytokines in ITP patients might contribute to the construct of a moderate inflammatory environment which favoring the recruitment of both M2-like macrophages and MDSCs, implicating their integrated roles as myeloid-derived immune modulator cells in ITP.
After HD-DXM treatment, M2-like macrophages increased significantly in the CR group, along with the elevated percentages of MDSCs and the increasing levels of IL-10. While in the PR + NR group, both cells populations decreased and the levels of IL-10 remained unchanged. These phenomena might be related to the glucocorticoid efficacy towards MDSCs and M2-like macrophages, and the improvement of these immune modulators might predict better prognosis and explain why some patients just achieved PR but not CR. Known as the classical M2 macrophages activator, IL-4 and IL-13 were significantly upregulated after treatment in the CR group. We postulated that they might be related with the increasing M2-like macrophages. An increase in circulating M2-like macrophages and MDSCs might predict better response in ITP patients. When compared with HC, no significant difference of M2-like macrophages was found before treatment. However, the CR group revealed significant elevated level of M2-like macrophages after treatment, indicating that this novel cell subpopulation may be a potential predictor of better treatment response. Similarly, the continuous increasing of MDSCs in CR group furnished evidence that it may be a reliable indicator of the effectiveness of ITP treatment, unveiling its potential role in measuring the response to different treatment regimens.
It has been shown that 1–5% of MDSCs could develop into myeloid-cell colonies and that about one-third of this colony could differentiate into macrophages under appropriate conditions [47]. In the present study, a significant positive linear association between circulating M2-macrophages and MDSCs was observed in ITP patients, but not in the healthy controls. More intriguingly, not only before the treatment, but also after the treatment, circulating M2-like macrophages frequencies correlated positively to the MDSCs, especially among the CR group, suggesting that this positive correlation might be beneficial to the remission of ITP patients. These positive correlations might be resulted from bidirectional interactions or partially due to their similar background patterns during differentiation. Many tumor analyses have verified that chronic inflammation could enhance the interaction between MDSCs and M2 macrophages [32]. Proinflammation cytokines might play an important role in this orchestrating interaction. In the present study, IL-6 and other inflammatory factors including IL-1β, INF-γ and TNF-α were found to be significantly elevated after treatment, thus developing an inflammatory environment and in turn strengthening the relationship between M2-like macrophages and MDSCs, and driving IL-10 accumulation. The ability of M2-like macrophages and MDSCs to promote and reduce inflammation, seemingly contradictory at first look, might be crucial for the fine tune of the human inflammatory microenvironment.
Conclusions
The present study focused on the correlative observation of M2-like macrophages and MDSCs in ITP. The percentages of circulating MDSCs expand in newly diagnosed ITP patients, although it might just reflect the quick response to the pathologic environment without any immunosuppressive properties. The increase of circulating M2-like macrophages and MDSCs were found to be correlated with complete response, suggesting that it might predict a better prognosis of ITP. In addition, M2-like macrophages positively correlated with MDSCs in ITP patients. The present study also suggested a novel immune therapeutic approach targeting M2 macrophages and MDSCs in the management of ITP, which deserved further investigation.
Additional files
Additional file 1: Figure S1. The FSC × SSC gate of PBMCs for gating MDSCs (A) and M2-like macrophages (B).
Additional file 2: Figure S2. Populations of MDSCs and M2-like macrophages in matched patient samples in the CR group (A) and the PR + NR group (B) before and after HD-DXM regimen. Pre-DXM before treatment of HD-DXM; post-DXM: after treatment of HD-DXM.
Additional file 3: Table S1. Clinical characteristics of ITP patients.
Authors’ contributions
XS, BW and YC conceived the study; XS, BW, LC, FL and YC performed the literature review, drafted and revised the manuscript; HC and YC contributed to the critical revision of the manuscript; YZ, ZM, CL, YK, LJ and LS performed the experiments, analyzed data. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Jianjun Jin and Bijun Zhu for their technical support.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was in accordance with the ethical standards formulated in the Helsinki Declaration and was approved by the respective local Medical Ethics Committees of Zhongshan Hospital of Fudan University. Written informed consent was obtained from each patient before being included in the study.
Funding
This work was supported by Grants from the National Natural Science Foundation of China (81470282, 81500090, 81600090, 81170473, 81300381).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ITP
immune thrombocytopenia
- MDSC
myeloid-derived suppressor cells
- HD-DXM
high-dose dexamethasone
- CCL11
CeC motif chemokine 11
- MCP-1
monocyte chemoattractant protein-1
- IL-1β
interleukin-1β
- PBMCs
peripheral blood mononuclear cells
- MIP-1α/CCL3
macrophage-inflammatory protein-1α/CC chemokine ligand 3
- HC
health control
- CR
complete response
- PR
partial response
- NR
no response
Footnotes
Xia Shao and Boting Wu contributed equally to this work
Electronic supplementary material
The online version of this article (10.1186/s12967-018-1424-8) contains supplementary material, which is available to authorized users.
Contributor Information
Xia Shao, Email: 16111210011@fudan.edu.cn.
Boting Wu, Email: wu.boting@zs-hospital.sh.cn.
Luya Cheng, Email: cheng.luya@zs-hospital.sh.cn.
Feng Li, Email: li.feng@zs-hospital.sh.cn.
Yanxia Zhan, Email: zhan.yanxia@zs-hospital.sh.cn.
Chanjuan Liu, Email: 15111210015@fudan.edu.cn.
Lili Ji, Email: ji.lili@zs-hospital.sh.cn.
Zhihui Min, Email: min.zhihui@zs-hospital.sh.cn.
Yang Ke, Email: ke.yang@zs-hospital.sh.cn.
Lihua Sun, Email: qpsunlh023@126.com.
Hao Chen, Email: h.chen@fudan.edu.cn.
Yunfeng Cheng, Phone: +86-21-60267312, Email: yfcheng@fudan.edu.cn.
References
- 1.McMillan R. Chronic idiopathic thrombocytopenic purpura. N Engl J Med. 1981;304:1135–1147. doi: 10.1056/NEJM198105073041904. [DOI] [PubMed] [Google Scholar]
- 2.Mazzucconi MG, Fazi P, Bernasconi S, De Rossi G, Leone G, Gugliotta L, Vianelli N, Avvisati G, Rodeghiero F, Amendola A, et al. Therapy with high-dose dexamethasone (HD-DXM) in previously untreated patients affected by idiopathic thrombocytopenic purpura: a GIMEMA experience. Blood. 2007;109:1401–1407. doi: 10.1182/blood-2005-12-015222. [DOI] [PubMed] [Google Scholar]
- 3.Cheng Y, Wong RS, Soo YO, Chui CH, Lau FY, Chan NP, Wong WS, Cheng G. Initial treatment of immune thrombocytopenic purpura with high-dose dexamethasone. N Engl J Med. 2003;349:831–836. doi: 10.1056/NEJMoa030254. [DOI] [PubMed] [Google Scholar]
- 4.Brighton TA, Evans S, Castaldi PA, Chesterman CN, Chong BH. Prospective evaluation of the clinical usefulness of an antigen-specific assay (MAIPA) in idiopathic thrombocytopenic purpura and other immune thrombocytopenias. Blood. 1996;88:194–201. [PubMed] [Google Scholar]
- 5.Audia S, Samson M, Mahevas M, Ferrand C, Trad M, Ciudad M, Gautheron A, Seaphanh F, Leguy V, Berthier S, et al. Preferential splenic CD8(+) T-cell activation in rituximab-nonresponder patients with immune thrombocytopenia. Blood. 2013;122:2477–2486. doi: 10.1182/blood-2013-03-491415. [DOI] [PubMed] [Google Scholar]
- 6.Hou Y, Feng Q, Xu M, Li GS, Liu XN, Sheng Z, Zhou H, Ma J, Wei Y, Sun YX, et al. High-dose dexamethasone corrects impaired myeloid-derived suppressor cell function via Ets1 in immune thrombocytopenia. Blood. 2016;127:1587–1597. doi: 10.1182/blood-2015-10-674531. [DOI] [PubMed] [Google Scholar]
- 7.Zhou J, Zhou Y, Wen J, Sun X, Zhang X. Circulating myeloid-derived suppressor cells predict disease activity and treatment response in patients with immune thrombocytopenia. Braz J Med Biol Res. 2017;50:e5637. doi: 10.1590/1414-431X20165637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heine A, Held S, Schulte-Schrepping J, Wolff J, Klee K, Ulas T, Schmacke NA, Daecke SN, Riethausen K, Schultze JL, Brossart P. Generation and functional characterization of MDSC-like cells. Oncoimmunology. 2017;6:e1295203. doi: 10.1080/2162402X.2017.1295203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol. 2011;11:856–861. doi: 10.1016/j.intimp.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su Z, Ni P, She P, Liu Y, Richard SA, Xu W, Zhu H, Wang J. Bio-HMGB1 from breast cancer contributes to M-MDSC differentiation from bone marrow progenitor cells and facilitates conversion of monocytes into MDSC-like cells. Cancer Immunol Immunother. 2017;66:391–401. doi: 10.1007/s00262-016-1942-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Liu XG, Liu S, Feng Q, Liu XN, Li GS, Sheng Z, Chen P, Liu Y, Wei Y, Dong XY, et al. Thrombopoietin receptor agonists shift the balance of Fcgamma receptors toward inhibitory receptor IIb on monocytes in ITP. Blood. 2016;128:852–861. doi: 10.1182/blood-2016-01-690727. [DOI] [PubMed] [Google Scholar]
- 12.Yeung OW, Lo CM, Ling CC, Qi X, Geng W, Li CX, Ng KT, Forbes SJ, Guan XY, Poon RT, et al. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J Hepatol. 2015;62:607–616. doi: 10.1016/j.jhep.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 13.Partecke LI, Gunther C, Hagemann S, Jacobi C, Merkel M, Sendler M, van Rooijen N, Kading A, Nguyen TD, Lorenz E, et al. Induction of M2-macrophages by tumour cells and tumour growth promotion by M2-macrophages: a quid pro quo in pancreatic cancer. Pancreatology. 2013;13:508–516. doi: 10.1016/j.pan.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Vaknin I, Kunis G, Miller O, Butovsky O, Bukshpan S, Beers DR, Henkel JS, Yoles E, Appel SH, Schwartz M. Excess circulating alternatively activated myeloid (M2) cells accelerate ALS progression while inhibiting experimental autoimmune encephalomyelitis. PLoS ONE. 2011;6:e26921. doi: 10.1371/journal.pone.0026921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denney L, Kok WL, Cole SL, Sanderson S, McMichael AJ, Ho LP. Activation of invariant NKT cells in early phase of experimental autoimmune encephalomyelitis results in differentiation of Ly6Chi inflammatory monocyte to M2 macrophages and improved outcome. J Immunol. 2012;189:551–557. doi: 10.4049/jimmunol.1103608. [DOI] [PubMed] [Google Scholar]
- 16.Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, Chong BH, Cines DB, Gernsheimer TB, Godeau B, Grainger J. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168–186. doi: 10.1182/blood-2009-06-225565. [DOI] [PubMed] [Google Scholar]
- 17.Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, Cines DB, Chong BH, Cooper N, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386–2393. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 18.Li F, Ji L, Wang W, Hua F, Zhan Y, Zou S, Yuan L, Ke Y, Min Z, Song D, et al. Insufficient secretion of IL-10 by Tregs compromised its control on over-activated CD4+ T effector cells in newly diagnosed adult immune thrombocytopenia patients. Immunol Res. 2015;61:269–280. doi: 10.1007/s12026-014-8620-2. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Heck S, Patel V, Levan J, Yu Y, Bussel JB, Yazdanbakhsh K. Defective circulating CD25 regulatory T cells in patients with chronic immune thrombocytopenic purpura. Blood. 2008;112:1325–1328. doi: 10.1182/blood-2008-01-135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hua F, Ji L, Zhan Y, Li F, Zou S, Chen L, Gao S, Li Y, Chen H, Cheng Y. Aberrant frequency of IL-10-producing B cells and its association with Treg/Th17 in adult primary immune thrombocytopenia patients. Biomed Res Int. 2014;2014:571302. doi: 10.1155/2014/571302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Zhong H, Bao W, Boulad N, Evangelista J, Haider MA, Bussel J, Yazdanbakhsh K. Defective regulatory B-cell compartment in patients with immune thrombocytopenia. Blood. 2012;120:3318–3325. doi: 10.1182/blood-2012-05-432575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte–macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 25.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 26.Gordon IO, Freedman RS. Defective antitumor function of monocyte-derived macrophages from epithelial ovarian cancer patients. Clin Cancer Res. 2006;12:1515–1524. doi: 10.1158/1078-0432.CCR-05-2254. [DOI] [PubMed] [Google Scholar]
- 27.Feng Q, Xu M, Yu YY, Hou Y, Mi X, Sun YX, Ma S, Zuo XY, Shao LL, Hou M, et al. High-dose dexamethasone or all-trans-retinoic acid restores the balance of macrophages towards M2 in immune thrombocytopenia. J Thromb Haemost. 2017;15:1845–1858. doi: 10.1111/jth.13767. [DOI] [PubMed] [Google Scholar]
- 28.Moline-Velazquez V, Cuervo H, Vila-Del SV, Ortega MC, Clemente D, de Castro F. Myeloid-derived suppressor cells limit the inflammation by promoting T lymphocyte apoptosis in the spinal cord of a murine model of multiple sclerosis. Brain Pathol. 2011;21:678–691. doi: 10.1111/j.1750-3639.2011.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurko J, Vida A, Glant TT, Scanzello CR, Katz RS, Nair A, Szekanecz Z, Mikecz K. Identification of myeloid-derived suppressor cells in the synovial fluid of patients with rheumatoid arthritis: a pilot study. BMC Musculoskelet Disord. 2014;15:281. doi: 10.1186/1471-2474-15-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, Huang Y, Wang S, Fu R, Guo C, Wang H, Zhao J, Gaskin F, Chen J, Yang N, Fu SM. Myeloid-derived suppressor cells contribute to bone erosion in collagen-induced arthritis by differentiating to osteoclasts. J Autoimmun. 2015;65:82–89. doi: 10.1016/j.jaut.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Natarajan S, Thomson AW. Tolerogenic dendritic cells and myeloid-derived suppressor cells: potential for regulation and therapy of liver auto- and alloimmunity. Immunobiology. 2010;215:698–703. doi: 10.1016/j.imbio.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 33.He Z, Zhang H, Yang C, Zhou Y, Zhou Y, Han G, Xia L, Ouyang W, Zhou F, Zhou Y, Xie C. The interaction between different types of activated RAW 264.7 cells and macrophage inflammatory protein-1 alpha. Radiat Oncol. 2011;6:86. doi: 10.1186/1748-717X-6-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baba T, Mukaida N. Role of macrophage inflammatory protein (MIP)-1alpha/CCL3 in leukemogenesis. Mol Cell Oncol. 2014;1:e29899. doi: 10.4161/mco.29899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitamura T, Qian BZ, Soong D, Cassetta L, Noy R, Sugano G, Kato Y, Li J, Pollard JW. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med. 2015;212:1043–1059. doi: 10.1084/jem.20141836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baeck C, Wehr A, Karlmark KR, Heymann F, Vucur M, Gassler N, Huss S, Klussmann S, Eulberg D, Luedde T, et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut. 2012;61:416–426. doi: 10.1136/gutjnl-2011-300304. [DOI] [PubMed] [Google Scholar]
- 37.Nesbit M, Schaider H, Miller TH, Herlyn M. Low-level monocyte chemoattractant protein-1 stimulation of monocytes leads to tumor formation in nontumorigenic melanoma cells. J Immunol. 2001;166:6483–6490. doi: 10.4049/jimmunol.166.11.6483. [DOI] [PubMed] [Google Scholar]
- 38.Gazzaniga S, Bravo AI, Guglielmotti A, van Rooijen N, Maschi F, Vecchi A, Mantovani A, Mordoh J, Wainstok R. Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. J Investig Dermatol. 2007;127:2031–2041. doi: 10.1038/sj.jid.5700827. [DOI] [PubMed] [Google Scholar]
- 39.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 40.Zambirinis CP, Levie E, Nguy S, Avanzi A, Barilla R, Xu Y, Seifert L, Daley D, Greco SH, Deutsch M, et al. TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. J Exp Med. 2015;212:2077–2094. doi: 10.1084/jem.20142162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Draghiciu O, Lubbers J, Nijman HW, Daemen T. Myeloid derived suppressor cells—an overview of combat strategies to increase immunotherapy efficacy. Oncoimmunology. 2015;4:e954829. doi: 10.4161/21624011.2014.954829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, Boireau W, Simon B, Ryffel B, Connat JL, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19:57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 43.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 44.Lalor SJ, Dungan LS, Sutton CE, Basdeo SA, Fletcher JM, Mills KH. Caspase-1-processed cytokines IL-1beta and IL-18 promote IL-17 production by gammadelta and CD4 T cells that mediate autoimmunity. J Immunol. 2011;186:5738–5748. doi: 10.4049/jimmunol.1003597. [DOI] [PubMed] [Google Scholar]
- 45.Wang JB, Li H, Wang LL, Liang HD, Zhao L, Dong J. Role of IL-1beta, IL-6, IL-8 and IFN-gamma in pathogenesis of central nervous system neuropsychiatric systemic lupus erythematous. Int J Clin Exp Med. 2015;8:16658–16663. [PMC free article] [PubMed] [Google Scholar]
- 46.Wu KH, Peng CT, Li TC, Wan L, Tsai CH, Tsai FJ. Interleukin-1beta exon 5 and interleukin-1 receptor antagonist in children with immune thrombocytopenic purpura. J Pediatr Hematol Oncol. 2007;29:305–308. doi: 10.1097/MPH.0b013e3180590615. [DOI] [PubMed] [Google Scholar]
- 47.Li Q, Pan PY, Gu P, Xu D, Chen SH. Role of immature myeloid Gr-1+ cells in the development of antitumor immunity. Cancer Res. 2004;64:1130–1139. doi: 10.1158/0008-5472.CAN-03-1715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. The FSC × SSC gate of PBMCs for gating MDSCs (A) and M2-like macrophages (B).
Additional file 2: Figure S2. Populations of MDSCs and M2-like macrophages in matched patient samples in the CR group (A) and the PR + NR group (B) before and after HD-DXM regimen. Pre-DXM before treatment of HD-DXM; post-DXM: after treatment of HD-DXM.
Additional file 3: Table S1. Clinical characteristics of ITP patients.
Data Availability Statement
Not applicable.


