Abstract
Lymphocytes, such as T cells, B cells, and innate lymphoid cells (ILCs), play central roles in regulating immune responses. Retinoic acids (RAs) are vitamin A metabolites, produced and metabolized by certain tissue cells and myeloid cells in a tissue-specific manner. It has been established that RAs induce gut-homing receptors on T cells, B cells, and ILCs. A mounting body of evidence indicates that RAs exert far-reaching effects on functional differentiation and fate of these lymphocytes. For example, RAs promote effector T cell maintenance, generation of induced gut-homing regulatory and effector T cell subsets, antibody production by B cells, and functional maturation of ILCs. Key functions of RAs in regulating major groups of innate and adaptive lymphocytes are highlighted in this article.
Keywords: Retinoic acid, T-cells, B-cells, NK cells, Innate lymphoid cells
INTRODUCTION
Lymphocytes play central roles in regulating immune responses. They are divided into innate and adaptive lymphocytes. Natural killer (NK) cells and innate lymphoid cells (ILCs) are innate lymphocytes, whereas T cells and B cells are adaptive lymphocytes that express antigen-specific receptors. Decades of research led to discoveries of many lymphocyte populations and their subsets. Among lymphocytes, cluster of differentiation (CD) 4+ αβ T cells include naïve, memory, and effector cells. Memory and effector T cells include polarized T cells such as T helper (Th) 1, Th2, Th9, T follicular helper (Tfh), and Th17 cells. Additionally, regulatory T cells (Tregs) that rein in immune responses are included in this group. These T cells are mainly composed of thymus-derived naïve and memory type FoxP3+ cells, and peripherally induced FoxP3+ T cells and IL-10-producing type 1 Tregs. CD8+ αβ T cells are also classified into naïve, memory, and effector cell subsets. There are also atypical T cells such as natural killer T (NKT) cells, γδ T cells, intestinal epithelial lymphocytes (IELs), and mucosal associated invariant T (MAIT) cells (1). T cells sense antigens through their T cell receptors (TCRs) and produce immune regulatory cytokines and other effector molecules that activate or suppress each other and other cells. Depending on migratory behavior, T cell subsets can be also called circulating central memory, non-lymphoid tissue-homing effector memory, or tissue-resident effector T cells (2,3). On the other hand, the B lymphocyte group includes conventional follicular B cells (also called B2) and B1 cells (B1a and B1b), which produce antibodies to protect the host from pathogens (4,5). The lymphocyte domain has been recently expanded by the discovery of ILCs (6). Like T cells, ILCs are highly heterogenous, including NK, ILC1, ILC2, and ILC3 subsets (7). These cells largely function like CD4 helper (ILC1/2/3) and CD8 cytotoxic (NK) T cells in terms of their effector functions but do not have antigen receptors. ILCs are primarily activated by tissue-derived cytokines produced during infection and inflammation by tissue cells and myeloid cells (8). However, recent studies indicate that they are regulated by other tissue-derived factors beyond cytokines. One class of such factors includes retinoic acids (RAs).
RAs, such as all-trans retinoic acid (ATRA) and 9-cis-RA, are vitamin A metabolites. There are many different vitamin A metabolites beyond the 2 major biologically active RAs because of many different isomerases and oxidases that modify or break down RAs. Please note that most of the functions of RAs described in this article are for ATRA and 9-cis-RA. They are essential for development and function of many organs, including limb development, body symmetry formation, spermatogenesis, and central nervous system functions including circadian rhythm regulation (9,10,11). Importantly, recent studies established essential roles of RAs in development and maintenance of the immune system (12,13,14). RAs regulate functional maturation and specialization of innate and adaptive lymphocytes. RAs also regulate contraction of activated effector T cells in the gut (15). Our current understanding of the roles of RAs in regulating innate and adaptive lymphocytes are reviewed here.
THE BASICS OF RA: TISSUE-DISTRIBUTION AND RECEPTORS
RA distribution in the body is highly regulated by retinol availability and tissue/cell-specific expression of RA-synthesizing and catabolizing enzymes. During embryo development, RA gradients serve as major morphogens to regulate organ development (16,17). ATRA is produced by cells that express alcohol dehydrogenases (ADHs) and retinal dehydrogenases (RALDH1–3) that metabolize retinol. Retinol level is high in the small intestinal tissue environment because dietary retinol from the gut lumen is mainly absorbed here. Absorbed retinol is carried by plasma retinol-binding protein (RBP) in the blood circulation. Blood retinol is taken up by cells in stimulated by retinoic acid 6 (STRA6)-dependent and independent manners. STRA6 functions as a membrane receptor to bind and take over retinol from RBP (18). Retinol is stored in the liver and fat and released into the blood circulation (12,13,14). RALDHs are expressed by epithelial cells, stromal cells, macrophages, dendritic cells (DCs), and hematopoietic progenitor cells in many tissues (19,20,21,22). In the small intestine, epithelial cells express RALDH1; stromal cells express RALDH1, RALDH2, and RALDH3; DCs mainly express RALDH2 (23). The RA-degrading enzymes, CYP26A1 and CYP26A2, are widely expressed in the body, including testis, brain, lung, pancreas, uterus, and skin (24). Expression of these enzymes are induced by RAs because the genes for these enzymes harbor retinoic acid response elements (RAREs) (25). Increased expression of the RA-degrading enzymes provides a negative feedback signal to prevent build-up of RAs, which can be toxic. RA levels are typically maintained at up to 2 nM in the blood circulation (26). Compared to RA levels in the intestine and mesenteric lymph node (MLN), this is a relatively low level but can exert certain regulatory effects on lymphoid tissues and other systemic organs. The concentrations of RAs in the intestine and MLN are expected to be greater (5–10 nM or higher), which is high enough to induce gut-homing receptors such as CCR9 and α4β7 and to exert other regulatory functions (27).
RAs primarily function through retinoic acid receptors (RARα, β, γ), which are nuclear receptors that bind RAREs in many RA-responsive genes. Peroxisome proliferator-activated receptor (PPAR) β/δ serves as a minor RA receptor (28). Because many genes have RAREs, RAs have diverse functions throughout the body. While RAs promote RARα binding to DNA, RARs can bind DNA and regulate gene expression even when RA concentrations are limited. RAR functions are not limited to DNA-binding-dependent transcriptional activities because certain functions of RARs are mediated by fast-acting non-genomic signaling, which occurs in the cytoplasm. For example, phosphatidylinositol-3-kinase and extracellular signal-regulated kinases (ERKs) are activated by RA-liganded RARs within a few minutes following RA treatment (29,30). Moreover, RA receptors interact with other DNA-binding proteins to exert RARE-independent transcriptional activities. The RA-RAR axis regulates many transcriptional factors and certain micro-RNAs (miRNAs) (31), and, therefore, many of the RA-induced genes are indirectly regulated downstream of the genes that are directly regulated by RAs.
RA POTENTIATES EFFECTOR FUNCTIONS OF VARIOUS T CELL POPULATIONS
Effects of RA on T cells are either direct through T cells themselves or indirect through other cell types such as DCs and tissue cells (32). Early studies found that RAs regulate thymocyte apoptosis in vitro (33,34). Also, it has been observed that RAs promote Th2 but suppress Th1 responses (35,36,37,38). Other studies found no clear impact of RAs on Th1 and Th2 cells in vitro and in vivo (39). Recently, it was observed that Th1 responses including ERK activation, tyrosine phosphorylation, and interferon (IFN) γ production were decreased in vitamin A- or RARα-deficiency during infection by the intracellular pathogen Toxoplasma Gondii (40). Similarly, increased Th17, but decreased Th1, responses were observed in T cells expressing a dominant negative form of retinoic acid receptor (dnRAR) α (41), which suggests that RA signaling supports Th1 responses in general. This indicates that during active immune responses, RAs potentiate Th1 cell responses (Fig. 1). While the mechanism for this regulation remains unclear, this effect is potentially mediated by direct and indirect effects of RARα binding and epigenetic regulation such as DNA methylation and histone acetylation on regulatory regions of master regulatory genes such as Tbx21 that codes for the T-bet protein.
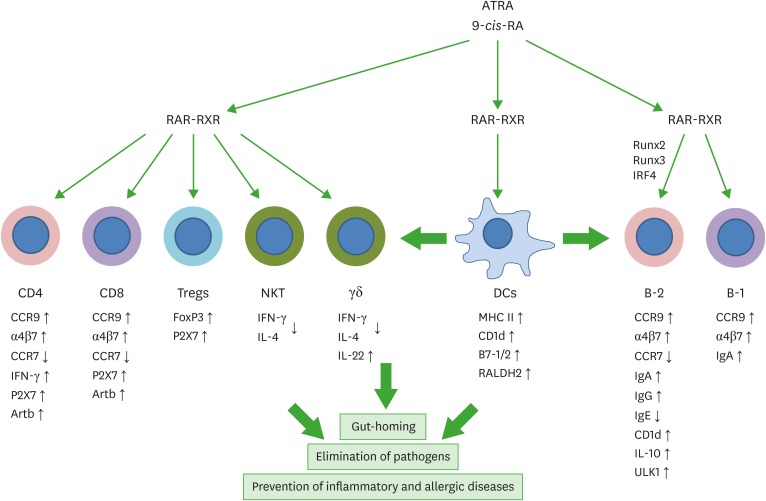
Figure 1.
Regulation of T cells and B cells by RAs. T cells and B cells express RARs and are major targets of RA regulation. RAs and their receptors appear to regulate T and B cells through genomic and non-genomic functions. RAs affect the effector function, gut-homing receptor expression, and apoptosis of CD4+ T cells. In the intestine, RAs promote gut-homing effector T cells (Th1 and Th17) and Tregs. Regulation of T cells by RAs occurs at the time of antigen priming, and DCs express RA-producing RALDH2. Moreover, RAs induce co-stimulatory receptors and RALDH2 in DCs. Therefore, DCs and other antigen presenting cells play central roles in regulating T cells by activating lymphocytes and producing RAs. RAs also induce the expression of P2X7 and Art2b on T cells to induce apoptosis caused by inflammatory mediators such as NAD and ATP, which are typically leaked out of dying cells in inflammatory conditions. This function of RAs induces effector T cell contraction in the intestines. RAs also induce gut-homing IgA-producing B-1 and B-2 cells in gut-associated lymphoid tissues. However, RAs function to suppress IgE production. RAs promote IL-10-producing regulatory T and B cells. The arrows indicate either positive (↑) or negative (↓) effect of RAs.
RXR, retinoid X receptor; ULK1, UNC51-like kinase-1; IRF4, interferon regulatory factor 4.
In addition, RAs induce peripheral or in vitro-generated FoxP3+ Tregs (pTregs and iTregs, respectively) (42,43,44,45) (Fig. 1). RAs enhance transforming growth factor (TGF)-β1-induced iTreg generation but suppress Th17 cell responses in vitro. A question of importance is if the reciprocal function of RAs on Treg versus Th17 cells would be also observed in vivo. Unexpectedly, pTreg generation is not defective in vitamin A deficiency. Rather, Treg numbers were increased in vitamin A deficiency (39). Based on the suppressive effect of RAs on Th17 induction, it was expected that Th17 cell generation would be increased in vitamin A- or RARα signaling-deficiency in dnRARα mice. However, Th17 cell numbers are rather decreased in these conditions, particularly in the intestines (39,41). Thus, RAs are not necessarily Treg-inducing factors only in vivo. Rather, RAs have the potential to induce both Tregs and effector T cells, including Th1 and Th17 cells, depending on environmental conditions shaped by specific types of pathogens, tissues, and inflammatory responses. We reason that the universal function of RAs in boosting lymphocytes may be mediated, in part, by their potent epigenetic regulatory functions in general gene expression (46).
In accounting for the effect of RAs on lymphocytes in vivo, we need to consider the function of RAs in regulating lymphocyte migration (47). RAs induce CCR9 and α4β7 expression on CD4+ and CD8+ T cells (47,48), and, therefore, affect numbers of effector T cells in the intestine versus other tissues such as secondary lymphoid tissues (Fig. 1). This explains why gut-homing T cells are generated in gut-associated lymphoid tissues such as MLN and Peyer's patches (PPs) in response to the inductive effects of RAs produced by DCs, stromal cells, and epithelial cells (23,48,49,50). RAs and RARs induce the expression of integrin α4α4 by binding to regulatory regions of the gene, whereas the expression of integrin β7β7 is primarily induced by TGF-β1 (51). This cooperative relationship between RAs and TGF-β1 in expressing integrin α4β7 along with FoxP3 provides a key mechanism for generating gut-homing Tregs (45).
Two of the most highly induced genes by RAs in activated T cells are P2x7 and Art2b. P2X7 is a purinergic receptor activated by adenosine triphosphate (ATP) and NAD (52,53). While ATP can activate P2X7 by itself, activation of P2X7 by NAD requires the ADP ribosylation activity of Art2b. Art2b expression is largely limited to T cells, suggesting that NAD activation of P2X7 specifically targets T cells, whereas ATP can activate P2X7 on many cell types. P2X7 activation induces calcium signaling, inflammasome activation, and cell death depending on cell type (52,53). P2X7 is expressed by most memory and effector T cells, including TCR αβ and γδ T cells in the intraepithelial and lamina propria compartments of the small intestine (15). While lower than the T cells in the small intestine, large intestinal T cells also highly express P2X7. T cells in other tissues either express P2X7 at lower levels or do not express. In the intestines, antigen priming of T cells drives P2X7 expression in the presence of RAs. The P2x7 gene has RAR binding sites to turn on gene expression in response to antigen priming and RAs (15). P2X7 is particularly highly expressed by Th1 and Th17 cells among intestinal CD4+ T cells (15). During active immune responses to infection by a mouse enteric pathogen, Citrobacter rodentium, the numbers of Th1 and Th17 cells were abnormally increased in the intestine of P2X7-deficient mice, compared to those in wild type animals. This indicates that the induction of P2X7 expression by RAs is a novel tolerogenic mechanism to prevent excessive build-up of inflammatory T cells in the intestine (Fig. 1). To support this, repeated NAD injection decreased inflammatory T cells and suppressed colitis (15).
CD1d is a major histocompatibility complex (MHC) I-like molecule and presents lipids to activate NKT cells. RAs induce CD1d expression on antigen-presenting cells such as B cells, monocytes, and DCs (20,54). Also, the expression of CD1d and CD1c on human B cells is induced by RAs (55). Activation of PPARβ/δ by RAs is responsible for this induction. Another rather contradictory function is that RAs suppress the production of IFN-γ and IL-4 by NKT cells and, therefore, can decrease NKT-dependent inflammatory activities (56,57). This appears to be mediated through regulation of phosphatase 2A and ERKs. In a manner similar to NKT cells, γδ T cells provide fast T cell responses during infection or injury. RAs enhance IL-22 production by γδ T cells, stimulated with inflammatory cytokines such as IL-1β, IL-18, and IL-23 (58). By increasing IL-22 production, RAs decrease inflammatory responses induced by dextran sulfate sodium (DSS) or C. rodentium infection. IL-22 strengthens the gut barrier function and decreases bacterial invasion. RARs directly bind the promoter of the Il22 gene for its expression.
RA SELECTIVELY BOOSTS ANTIBODY RESPONSES
Retinol and its metabolites RAs have been identified decades ago as co-stimulators of B cell proliferation (59). RAs drive bone marrow lymphoid progenitors into B cells in the periphery, a phenomenon associated with elevated expression of key transcription factors, such as early B-cell factor 1 (EBF1) and Pax-5, which are required for B lymphopoiesis (60). RAs induce interferon regulatory factor 4 (IRF4) expression and drive plasma B cell generation (61,62,63). This results in expression of activation-induced deaminase, Blimp-1, and CD138/syndecan-1 in response to B cell activation. The positive role of RAs is supported by the observation that vitamin A-deficient mice are defective in T-dependent IgG responses (64,65). It was later found that vitamin A-deficient mice are particularly more deficient in IgA production (66). RAs induce IgA-class switch and gut-homing receptor expression in B cells, generating plasma B cells that migrate to the intestines and possibly to other mucosal tissues as well (67). RAs boost the effects of cytokine such as TGF-β1, IL-5, and IL-6 in inducing IgA expression in B cells. RA-producing DCs, isolated from mucosal lymphoid tissues such as MLN and PPs, are highly efficient in inducing IgA-class switch in B cells (66). Also, follicular dendritic cells (FDCs), stimulated with bacterial products and RAs, express B-cell helping factors such as CXCL13, B cell-activating factor receptor and TGF-β1, creating a condition conducive for B cell differentiation into IgA producers (68). At molecular level, certain transcription factors, such as Runx2 and Runx3, are required for RA-induced IgA class switch (69).
While RAs induce IgA, they suppress IgE and certain IgG isotypes including IgG1 in mice (70,71) (Fig. 1). RAs also induce IL-10 production in B cells, generating regulatory B cells (72). The most likely sources of RAs to affect B cells are epithelial cells in the respiratory tract that express RALDH1, and DCs that express RALDH2 (73). Autophagy is important for B cell production of antibodies (74,75), and this process is promoted by RAs. In this regard, RAs induce UNC51-like kinase-1 (ULK1), a signaling molecule required to induce autophagy (76). ULK1 knock-down abolished RA-induced IgG production. RA-deficiency or blocking RAR signaling by dnRAR expression alters the gut microbiota (77). This was expected because 30%–50% gut commensal bacteria in the colon are coated with IgA and therefore controlled by IgA (78). RAs also induce IgA production and gut-homing receptor expression in B-1 cells (79,80). Blocking RAR signaling by dnRAR expression led to defective development of marginal zone and B-1 B cells and decreased T-independent antibody responses (81). The B cell modulatory function is shared by ATRA and 9-cis-RA (82). Thus, RAs promote IgA and IgG but suppress IgE production, which can enhance immunity to fight bacterial and viral pathogens but decrease allergic responses (Fig. 1).
RA POTENTIATES THE ACTIVITIES OF ILCS
Innate lymphocytes include NK cells and ILCs. RAs suppress the human NK cell cytotoxicity activated by IFN-α (83). NK cell activity, including cytotoxicity, is regulated by various inhibitory and activating receptors. Also, antibody-dependent cell cytotoxicity (ADCC) is a major mechanism to direct NK cytotoxicity toward virus-infected or tumor cells that are coated with antibodies (84). RAs increase the expression of killer inhibitory receptor (KIR) CD158b, an NK inhibitory receptor (85). RAs also suppress NF-κB activity and the expression of granzyme B and NKp46 (86). As a mechanism for the RA-induced suppression of NK cell cytotoxicity, RAs induce the expression of miR-23a, which negatively regulates cathepsin C and subsequent granzyme B activation (87). RAs also up-regulate MHC class I chain-related protein A and B (MICA and MICB) on tumor cells (88), which function as the ligands for NK cell-activating receptor NKG2D. Thus, RAs have the potential to bidirectionally modulate NK cell cytotoxicity (Fig. 2).
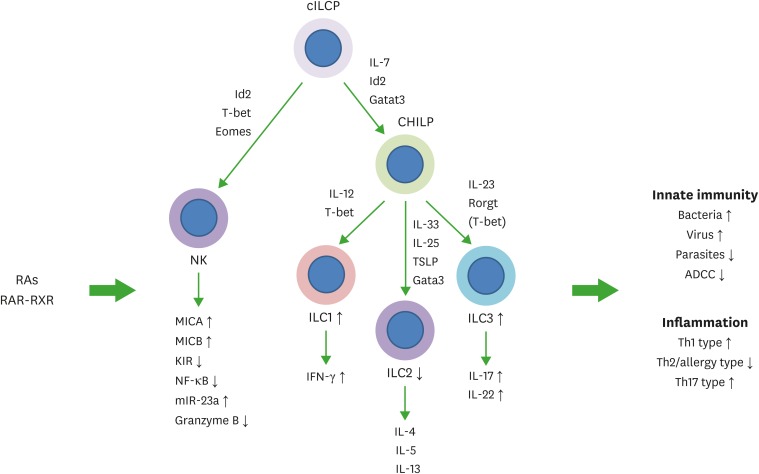
Figure 2.
Regulation of NK cells and ILCs by RAs. Common ILC progenitors (cILCP or also called αLP) and common helper-like ILC progenitors generate ILCs. Generation and functional maturation of innate lymphocytes are primarily regulated by cytokines and transcription factors. In addition, RAs regulate these NK cells and ILCs. RAs bidirectionally regulate NK cell functions. They induce MICA and MICB to indirectly activate NK cells but can directly suppress NK cell activation as well. While the overall effect of RAs on NK cells during various types of immune responses needs to be established, it appears that RAs function to support NK cell activity but restrain their activity in certain conditions. In general, RAs promote ILC1 and ILC3 activity, whereas they suppress ILC2 generation from ILC progenitors from the bone marrow or embryonic hematopoietic organs. RAs promote IL-22 production by ILC3 to strengthen the barrier immunity, and support IFN-γ production by ILC1. These ILC-boosting functions of RAs were observed on human ILCs as well. However, there appear to be species-specific differences in the regulation. Overall, RAs are important regulators of ILCs. The arrows indicate either positive (↑) or negative (↓) effect of RAs.
ADCC, antibody-dependent cell cytotoxicity; KIR, killer inhibitory receptor; MICA, major histocompatibility complex class I chain-related protein A; MICB, major histocompatibility complex class I chain-related protein B; RXR, retinoid X receptor; TSLP, thymic stromal lymphopoietin.
Lymphoid tissue inducer (LTi) cells belong to the ILC3 group and play an essential role in the development of secondary lymphoid tissues. Maternally derived RAs are required for fetal LTi cell development during embryo development (89). RAs up-regulate the transcription factor RORγt for fetal LTi development (Fig. 2). Thus, maternal vitamin A supports lymphoid tissue development in the fetus by increasing LTi cells (89). Similarly, RA-RAR signaling is required for intestinal ILC3 and LTi cells in adult animals (90). ILC3 numbers are decreased in vitamin A-deficient mice, and RAs induce IL-22 expression by ILC3 in the intestine. Thus, RAs promote intestinal barrier immunity by promoting ILC3 responses.
RAs also play critical roles in inducing gut-homing receptors on ILC3 and ILC1. Vitamin A-deficient mice are deficient in ILC3 and ILC1 in the intestines. RAs induce CCR9 and α4β7 expression on ILC3 and ILC1 to generate gut-homing ILCs. This imprinting appears to occur in MLN. Therefore, ILC3 and ILC1 undergo a trafficking receptor switch very similar to that of conventional T cells (91). In contrast, the expression of CCR9 and α4β7 by ILC2 is not regulated by RAs. ILC2 constitutively express these gut-homing receptors in most peripheral tissues. It appears that ILC2 acquire the gut-homing receptors at or before the ILC2 progenitors stage in the bone marrow rather than in the periphery (91). It has been reported that RAs promote ILC3-dependent immunity against C. rodentium but suppress ILC2-dependent immunity against certain types of parasites (92). In human ILCs, RAs also induce the expression of integrin α4β7 in vitro. In this study on human ILCs, RAs promoted IFN-γ expression in ILC1 and ILC3, whereas RAs induced IL-5 and IL-13 expression in ILC2 in an in vitro setting (93). While further studies are required, it is apparent that RAs generate gut-homing ILCs and boost ILC functions.
CONCLUDING REMARKS
Vitamin A and RAs exert essential regulatory effects on both innate and adaptive lymphocytes. It is apparent that RAs regulate almost all lymphocyte populations identified so far. The effects of RAs on ILCs and γδ T cells potentiate innate immunity and barrier functions to prevent and fight infections. The effects of RAs on NK cells are mixed, potentially suppressing and enhancing NK activities. This is important to limit unwanted inflammatory responses due to uncontrolled NK cell activity. The effects of RAs on T cells are also mixed, enhancing the activities of both regulatory and effector T cell populations. This function is particularly apparent in the intestines. RAs boost systemic IgG responses and mucosal IgA responses. However, RAs suppress IgE production and this function together with their function to suppress ILC2 can be important in regulating type 2 immune response-mediated allergic responses. Vitamin A has been called the anti-infective vitamin. The recent research has provided enough evidence for this role. Additionally, a mounting body of information indicates that vitamin A metabolites function to prevent inflammatory diseases. This function can be mediated by inducing regulatory cells or effectively clearing pathogens to prevent chronic inflammatory responses. Overall, RAs bidirectionally regulate the functions of most lymphocytes to protect the host from pathogens and inflammation.
ACKNOWLEDGEMENTS
The authors thank current and past members of Kim Laboratory at Purdue and University of Michigan for their input and assistance in preparing this article. This study was supported, in part, from grants from NIH (1R01AI121302, R01AI080769) to Chang H. Kim.
Abbreviations
- ATP
adenosine triphosphate
- ATRA
all-trans retinoic acid
- CD
cluster of differentiation
- DC
dendritic cell
- dnRAR
dominant negative form of retinoic acid receptor
- ERK
extracellular signal-regulated kinase
- IFN
interferon
- ILC
innate lymphoid cell
- LTi
lymphoid tissue inducer
- MHC
major histocompatibility complex
- MLN
mesenteric lymph node
- NK
natural killer
- NKT
natural killer T
- RA
retinoic acid
- RALDH
retinal dehydrogenases
- RAR
retinoic acid receptor
- RARE
retinoic acid response element
- TGF
transforming growth factor
- Th
T helper
- Treg
regulatory T cell
Footnotes
Conflict of Interest: The author declares no potential conflicts of interest.
References
- 1.Fan X, Rudensky AY. Hallmarks of tissue-resident lymphocytes. Cell. 2016;164:1198–1211. doi: 10.1016/j.cell.2016.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13:309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 3.Carbone FR. Tissue-resident memory T cells and fixed immune surveillance in nonlymphoid organs. J Immunol. 2015;195:17–22. doi: 10.4049/jimmunol.1500515. [DOI] [PubMed] [Google Scholar]
- 4.Hardy RR, Hayakawa K. Perspectives on fetal derived CD5+ B1 B cells. Eur J Immunol. 2015;45:2978–2984. doi: 10.1002/eji.201445146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark MR, Mandal M, Ochiai K, Singh H. Orchestrating B cell lymphopoiesis through interplay of IL-7 receptor and pre-B cell receptor signalling. Nat Rev Immunol. 2014;14:69–80. doi: 10.1038/nri3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells--how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 7.Tait Wojno ED, Artis D. Emerging concepts and future challenges in innate lymphoid cell biology. J Exp Med. 2016;213:2229–2248. doi: 10.1084/jem.20160525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinette ML, Colonna M. Immune modules shared by innate lymphoid cells and T cells. J Allergy Clin Immunol. 2016;138:1243–1251. doi: 10.1016/j.jaci.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ransom J, Morgan PJ, McCaffery PJ, Stoney PN. The rhythm of retinoids in the brain. J Neurochem. 2014;129:366–376. doi: 10.1111/jnc.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raverdeau M, Gely-Pernot A, Féret B, Dennefeld C, Benoit G, Davidson I, Chambon P, Mark M, Ghyselinck NB. Retinoic acid induces Sertoli cell paracrine signals for spermatogonia differentiation but cell autonomously drives spermatocyte meiosis. Proc Natl Acad Sci USA. 2012;109:16582–16587. doi: 10.1073/pnas.1214936109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tulachan SS, Doi R, Kawaguchi Y, Tsuji S, Nakajima S, Masui T, Koizumi M, Toyoda E, Mori T, Ito D, et al. All-trans retinoic acid induces differentiation of ducts and endocrine cells by mesenchymal/epithelial interactions in embryonic pancreas. Diabetes. 2003;52:76–84. doi: 10.2337/diabetes.52.1.76. [DOI] [PubMed] [Google Scholar]
- 12.Erkelens MN, Mebius RE. Retinoic acid and immune homeostasis: a balancing act. Trends Immunol. 2017;38:168–180. doi: 10.1016/j.it.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Raverdeau M, Mills KH. Modulation of T cell and innate immune responses by retinoic acid. J Immunol. 2014;192:2953–2958. doi: 10.4049/jimmunol.1303245. [DOI] [PubMed] [Google Scholar]
- 14.Kim CH. Host and microbial factors in regulation of T cells in the intestine. Front Immunol. 2013;4:141. doi: 10.3389/fimmu.2013.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto-Hill S, Friesen L, Kim M, Kim CH. Contraction of intestinal effector T cells by retinoic acid-induced purinergic receptor P2X7. Mucosal Immunol. 2017;10:912–923. doi: 10.1038/mi.2016.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham TJ, Duester G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat Rev Mol Cell Biol. 2015;16:110–123. doi: 10.1038/nrm3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vermot J, Gallego Llamas J, Fraulob V, Niederreither K, Chambon P, Dollé P. Retinoic acid controls the bilateral symmetry of somite formation in the mouse embryo. Science. 2005;308:563–566. doi: 10.1126/science.1108363. [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 19.Goverse G, Molenaar R, Macia L, Tan J, Erkelens MN, Konijn T, Knippenberg M, Cook EC, Hanekamp D, Veldhoen M, et al. Diet-derived short chain fatty acids stimulate intestinal epithelial cells to induce mucosal tolerogenic dendritic cells. J Immunol. 2017;198:2172–2181. doi: 10.4049/jimmunol.1600165. [DOI] [PubMed] [Google Scholar]
- 20.Szatmari I, Pap A, Rühl R, Ma JX, Illarionov PA, Besra GS, Rajnavolgyi E, Dezso B, Nagy L. PPARgamma controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J Exp Med. 2006;203:2351–2362. doi: 10.1084/jem.20060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broadhurst MJ, Leung JM, Lim KC, Girgis NM, Gundra UM, Fallon PG, Premenko-Lanier M, McKerrow JH, McCune JM, Loke P. Upregulation of retinal dehydrogenase 2 in alternatively activated macrophages during retinoid-dependent type-2 immunity to helminth infection in mice. PLoS Pathog. 2012;8:e1002883. doi: 10.1371/journal.ppat.1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hess DA, Wirthlin L, Craft TP, Herrbrich PE, Hohm SA, Lahey R, Eades WC, Creer MH, Nolta JA. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107:2162–2169. doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vicente-Suarez I, Larange A, Reardon C, Matho M, Feau S, Chodaczek G, Park Y, Obata Y, Gold R, Wang-Zhu Y, et al. Unique lamina propria stromal cells imprint the functional phenotype of mucosal dendritic cells. Mucosal Immunol. 2015;8:141–151. doi: 10.1038/mi.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Topletz AR, Thatcher JE, Zelter A, Lutz JD, Tay S, Nelson WL, Isoherranen N. Comparison of the function and expression of CYP26A1 and CYP26B1, the two retinoic acid hydroxylases. Biochem Pharmacol. 2012;83:149–163. doi: 10.1016/j.bcp.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto Y, Zolfaghari R, Ross AC. Regulation of CYP26 (cytochrome P450RAI) mRNA expression and retinoic acid metabolism by retinoids and dietary vitamin A in liver of mice and rats. FASEB J. 2000;14:2119–2127. doi: 10.1096/fj.00-0061com. [DOI] [PubMed] [Google Scholar]
- 26.Moulas AN, Gerogianni IC, Papadopoulos D, Gourgoulianis KI. Serum retinoic acid, retinol and retinyl palmitate levels in patients with lung cancer. Respirology. 2006;11:169–174. doi: 10.1111/j.1440-1843.2006.00833.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang C, Kang SG. HogenEsch H, Love PE, Kim CH. Retinoic acid determines the precise tissue tropism of inflammatory Th17 cells in the intestine. J Immunol. 2010;184:5519–5526. doi: 10.4049/jimmunol.0903942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw N, Elholm M, Noy N. Retinoic acid is a high affinity selective ligand for the peroxisome proliferator-activated receptor beta/delta. J Biol Chem. 2003;278:41589–41592. doi: 10.1074/jbc.C300368200. [DOI] [PubMed] [Google Scholar]
- 29.Huo L, Cui D, Yang X, Gao Z, Trier K, Zeng J. All-trans retinoic acid modulates mitogen-activated protein kinase pathway activation in human scleral fibroblasts through retinoic acid receptor beta. Mol Vis. 2013;19:1795–1803. [PMC free article] [PubMed] [Google Scholar]
- 30.Masiá S, Alvarez S, de Lera AR, Barettino D. Rapid, nongenomic actions of retinoic acid on phosphatidylinositol-3-kinase signaling pathway mediated by the retinoic acid receptor. Mol Endocrinol. 2007;21:2391–2402. doi: 10.1210/me.2007-0062. [DOI] [PubMed] [Google Scholar]
- 31.Wu H, Zhao J, Fu B, Yin S, Song C, Zhang J, Zhao S, Zhang Y. Retinoic acid-induced upregulation of miR-219 promotes the differentiation of embryonic stem cells into neural cells. Cell Death Dis. 2017;8:e2953. doi: 10.1038/cddis.2017.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiss I, Rühl R, Szegezdi E, Fritzsche B, Tóth B, Pongrácz J, Perlmann T, Fésüs L, Szondy Z. Retinoid receptor-activating ligands are produced within the mouse thymus during postnatal development. Eur J Immunol. 2008;38:147–155. doi: 10.1002/eji.200737342. [DOI] [PubMed] [Google Scholar]
- 33.Szondy Z, Lecoeur H, Fesus L, Gougeon ML. All-trans retinoic acid inhibition of anti-CD3-induced T cell apoptosis in human immunodeficiency virus infection mostly concerns CD4 T lymphocytes and is mediated via regulation of CD95 ligand expression. J Infect Dis. 1998;178:1288–1298. doi: 10.1086/314446. [DOI] [PubMed] [Google Scholar]
- 34.Szondy Z, Reichert U, Bernardon JM, Michel S, Tóth R, Karászi E, Fésüs L. Inhibition of activation-induced apoptosis of thymocytes by all-trans- and 9-cis-retinoic acid is mediated via retinoic acid receptor alpha. Biochem J. 1998;331:767–774. doi: 10.1042/bj3310767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cantorna MT, Nashold FE, Hayes CE. Vitamin A deficiency results in a priming environment conducive for Th1 cell development. Eur J Immunol. 1995;25:1673–1679. doi: 10.1002/eji.1830250629. [DOI] [PubMed] [Google Scholar]
- 36.Hoag KA, Nashold FE, Goverman J, Hayes CE. Retinoic acid enhances the T helper 2 cell development that is essential for robust antibody responses through its action on antigen-presenting cells. J Nutr. 2002;132:3736–3739. doi: 10.1093/jn/132.12.3736. [DOI] [PubMed] [Google Scholar]
- 37.Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int Immunol. 2003;15:1017–1025. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- 38.Stephensen CB, Jiang X, Freytag T. Vitamin A deficiency increases the in vivo development of IL-10-positive Th2 cells and decreases development of Th1 cells in mice. J Nutr. 2004;134:2660–2666. doi: 10.1093/jn/134.10.2660. [DOI] [PubMed] [Google Scholar]
- 39.Kang SG, Wang C, Matsumoto S, Kim CH. High and low vitamin A therapies induce distinct FoxP3+ T-cell subsets and effectively control intestinal inflammation. Gastroenterology. 2009;137:1391–1402. 1402.e1–1402.e6. doi: 10.1053/j.gastro.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, et al. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34:435–447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown CC, Esterhazy D, Sarde A, London M, Pullabhatla V, Osma-Garcia I, Al-Bader R, Ortiz C, Elgueta R, Arno M, et al. Retinoic acid is essential for Th1 cell lineage stability and prevents transition to a Th17 cell program. Immunity. 2015;42:499–511. doi: 10.1016/j.immuni.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, O'Shea JJ. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179:3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 46.Urvalek A, Laursen KB, Gudas LJ. The roles of retinoic acid and retinoic acid receptors in inducing epigenetic changes. Subcell Biochem. 2014;70:129–149. doi: 10.1007/978-94-017-9050-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 48.Edele F, Molenaar R, Gütle D, Dudda JC, Jakob T, Homey B, Mebius R, Hornef M, Martin SF. Cutting edge: instructive role of peripheral tissue cells in the imprinting of T cell homing receptor patterns. J Immunol. 2008;181:3745–3749. doi: 10.4049/jimmunol.181.6.3745. [DOI] [PubMed] [Google Scholar]
- 49.Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Márquez G, Agace W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med. 2003;198:963–969. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 51.Kang SG, Park J, Cho JY, Ulrich B, Kim CH. Complementary roles of retinoic acid and TGF-β1 in coordinated expression of mucosal integrins by T cells. Mucosal Immunol. 2011;4:66–82. doi: 10.1038/mi.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bartlett R, Stokes L, Sluyter R. The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol Rev. 2014;66:638–675. doi: 10.1124/pr.113.008003. [DOI] [PubMed] [Google Scholar]
- 53.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 Receptor in Infection and Inflammation. Immunity. 2017;47:15–31. doi: 10.1016/j.immuni.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 54.Chen Q, Ross AC. Retinoic acid regulates CD1d gene expression at the transcriptional level in human and rodent monocytic cells. Exp Biol Med (Maywood) 2007;232:488–494. [PMC free article] [PubMed] [Google Scholar]
- 55.Allan LL, Stax AM, Zheng DJ, Chung BK, Kozak FK, Tan R, van den Elzen P. CD1d and CD1c expression in human B cells is regulated by activation and retinoic acid receptor signaling. J Immunol. 2011;186:5261–5272. doi: 10.4049/jimmunol.1003615. [DOI] [PubMed] [Google Scholar]
- 56.Chang HK, Hou WS. Retinoic acid modulates interferon-γ production by hepatic natural killer T cells via phosphatase 2A and the extracellular signal-regulated kinase pathway. J Interferon Cytokine Res. 2015;35:200–212. doi: 10.1089/jir.2014.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee KA, Song YC, Kim GY, Choi G, Lee YS, Lee JM, Kang CY. Retinoic acid alleviates Con A-induced hepatitis and differentially regulates effector production in NKT cells. Eur J Immunol. 2012;42:1685–1694. doi: 10.1002/eji.201142322. [DOI] [PubMed] [Google Scholar]
- 58.Mielke LA, Jones SA, Raverdeau M, Higgs R, Stefanska A, Groom JR, Misiak A, Dungan LS, Sutton CE, Streubel G, et al. Retinoic acid expression associates with enhanced IL-22 production by γδ T cells and innate lymphoid cells and attenuation of intestinal inflammation. J Exp Med. 2013;210:1117–1124. doi: 10.1084/jem.20121588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buck J, Ritter G, Dannecker L, Katta V, Cohen SL, Chait BT, Hämmerling U. Retinol is essential for growth of activated human B cells. J Exp Med. 1990;171:1613–1624. doi: 10.1084/jem.171.5.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen X, Esplin BL, Garrett KP, Welner RS, Webb CF, Kincade PW. Retinoids accelerate B lineage lymphoid differentiation. J Immunol. 2008;180:138–145. doi: 10.4049/jimmunol.180.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morikawa K, Nonaka M. All-trans-retinoic acid accelerates the differentiation of human B lymphocytes maturing into plasma cells. Int Immunopharmacol. 2005;5:1830–1838. doi: 10.1016/j.intimp.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 62.Chen Q, Ross AC. Retinoic acid promotes mouse splenic B cell surface IgG expression and maturation stimulated by CD40 and IL-4. Cell Immunol. 2007;249:37–45. doi: 10.1016/j.cellimm.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Indrevær RL, Moskaug JO, Paur I, Bøhn SK, Jørgensen SF, Blomhoff R, Aukrust P, Fevang B, Blomhoff HK. IRF4 is a critical gene in retinoic acid-mediated plasma cell formation and is deregulated in common variable immunodeficiency-derived B cells. J Immunol. 2015;195:2601–2611. doi: 10.4049/jimmunol.1500250. [DOI] [PubMed] [Google Scholar]
- 64.Chun TY, Carman JA, Hayes CE. Retinoid repletion of vitamin A-deficient mice restores IgG responses. J Nutr. 1992;122:1062–1069. doi: 10.1093/jn/122.5.1062. [DOI] [PubMed] [Google Scholar]
- 65.DeCicco KL, Youngdahl JD, Ross AC. All-trans-retinoic acid and polyriboinosinic: polyribocytidylic acid in combination potentiate specific antibody production and cell-mediated immunity. Immunology. 2001;104:341–348. doi: 10.1046/j.1365-2567.2001.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 67.Hammerschmidt SI, Friedrichsen M, Boelter J, Lyszkiewicz M, Kremmer E, Pabst O, Förster R. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J Clin Invest. 2011;121:3051–3061. doi: 10.1172/JCI44262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suzuki K, Maruya M, Kawamoto S, Sitnik K, Kitamura H, Agace WW, Fagarasan S. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity. 2010;33:71–83. doi: 10.1016/j.immuni.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 69.Watanabe K, Sugai M, Nambu Y, Osato M, Hayashi T, Kawaguchi M, Komori T, Ito Y, Shimizu A. Requirement for Runx proteins in IgA class switching acting downstream of TGF-beta 1 and retinoic acid signaling. J Immunol. 2010;184:2785–2792. doi: 10.4049/jimmunol.0901823. [DOI] [PubMed] [Google Scholar]
- 70.Scheffel F, Heine G, Henz BM, Worm M. Retinoic acid inhibits CD40 plus IL-4 mediated IgE production through alterations of sCD23, sCD54 and IL-6 production. Inflamm Res. 2005;54:113–118. doi: 10.1007/s00011-004-1331-8. [DOI] [PubMed] [Google Scholar]
- 71.Tokuyama H, Tokuyama Y. The regulatory effects of all-trans-retinoic acid on isotype switching: retinoic acid induces IgA switch rearrangement in cooperation with IL-5 and inhibits IgG1 switching. Cell Immunol. 1999;192:41–47. doi: 10.1006/cimm.1998.1438. [DOI] [PubMed] [Google Scholar]
- 72.Eriksen AB, Berge T, Gustavsen MW, Leikfoss IS, Bos SD, Spurkland A, Harbo HF, Blomhoff HK. Retinoic acid enhances the levels of IL-10 in TLR-stimulated B cells from patients with relapsing-remitting multiple sclerosis. J Neuroimmunol. 2015;278:11–18. doi: 10.1016/j.jneuroim.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 73.Rudraraju R, Jones BG, Surman SL, Sealy RE, Thomas PG, Hurwitz JL. Respiratory tract epithelial cells express retinaldehyde dehydrogenase ALDH1A and enhance IgA production by stimulated B cells in the presence of vitamin A. PLoS One. 2014;9:e86554. doi: 10.1371/journal.pone.0086554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Conway KL, Kuballa P, Khor B, Zhang M, Shi HN, Virgin HW, Xavier RJ. ATG5 regulates plasma cell differentiation. Autophagy. 2013;9:528–537. doi: 10.4161/auto.23484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pengo N, Scolari M, Oliva L, Milan E, Mainoldi F, Raimondi A, Fagioli C, Merlini A, Mariani E, Pasqualetto E, et al. Plasma cells require autophagy for sustainable immunoglobulin production. Nat Immunol. 2013;14:298–305. doi: 10.1038/ni.2524. [DOI] [PubMed] [Google Scholar]
- 76.Eriksen AB, Torgersen ML, Holm KL, Abrahamsen G, Spurkland A, Moskaug JO, Simonsen A, Blomhoff HK. Retinoic acid-induced IgG production in TLR-activated human primary B cells involves ULK1-mediated autophagy. Autophagy. 2015;11:460–471. doi: 10.1080/15548627.2015.1009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pantazi E, Marks E, Stolarczyk E, Lycke N, Noelle RJ, Elgueta R. Cutting edge: retinoic acid signaling in B cells is essential for oral immunization and microflora composition. J Immunol. 2015;195:1368–1371. doi: 10.4049/jimmunol.1500989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan PH, Hu J, Peter I, Zhang W, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158:1000–1010. doi: 10.1016/j.cell.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kang SH, Jin BR, Kim HJ, Seo GY, Jang YS, Kim SJ, An SJ, Park SR, Kim WS, Kim PH. Lactoferrin combined with retinoic acid stimulates B1 cells to express IgA isotype and gut-homing molecules. Immune Netw. 2015;15:37–43. doi: 10.4110/in.2015.15.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roy B, Brennecke AM, Agarwal S, Krey M, Düber S, Weiss S. An intrinsic propensity of murine peritoneal B1b cells to switch to IgA in presence of TGF-β and retinoic acid. PLoS One. 2013;8:e82121. doi: 10.1371/journal.pone.0082121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marks E, Ortiz C, Pantazi E, Bailey CS, Lord GM, Waldschmidt TJ, Noelle RJ, Elgueta R. Retinoic acid signaling in B cells is required for the generation of an effective T-independent immune response. Front Immunol. 2016;7:643. doi: 10.3389/fimmu.2016.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heine G, Hollstein T, Treptow S, Radbruch A, Worm M. 9-cis retinoic acid modulates the type I allergic immune response. J Allergy Clin Immunol. doi: 10.1016/j.jaci.2017.03.046. [DOI] [PubMed] [Google Scholar]
- 83.Abb J, Abb H, Deinhardt F. Effect of retinoic acid on the spontaneous and interferon-induced activity of human natural killer cells. Int J Cancer. 1982;30:307–310. doi: 10.1002/ijc.2910300309. [DOI] [PubMed] [Google Scholar]
- 84.Eremin O, Ashby J, Rhodes J. Inhibition of antibody-dependent cellular cytotoxicity and natural cytotoxicity by retinoic acid. Int Arch Allergy Appl Immunol. 1984;75:2–7. doi: 10.1159/000233581. [DOI] [PubMed] [Google Scholar]
- 85.Konjevic G, Mirjacic-Martinovic K, Vuletic A, Babovic N. In vitro increased natural killer cell activity of metastatic melanoma patients with interferon-α alone as opposed to its combination with 13-cis retinoic acid is associated with modulation of NKG2D and CD161 activating receptor expression. J BUON. 2012;17:761–769. [PubMed] [Google Scholar]
- 86.Li A, He M, Wang H, Qiao B, Chen P, Gu H, Zhang M, He S. All-trans retinoic acid negatively regulates cytotoxic activities of nature killer cell line 92. Biochem Biophys Res Commun. 2007;352:42–47. doi: 10.1016/j.bbrc.2006.10.132. [DOI] [PubMed] [Google Scholar]
- 87.Sanchez-Martínez D, Krzywinska E, Rathore MG, Saumet A, Cornillon A, Lopez-Royuela N, Martínez-Lostao L, Ramirez-Labrada A, Lu ZY, Rossi JF, et al. All-trans retinoic acid (ATRA) induces miR-23a expression, decreases CTSC expression and granzyme B activity leading to impaired NK cell cytotoxicity. Int J Biochem Cell Biol. 2014;49:42–52. doi: 10.1016/j.biocel.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 88.Jinushi M, Takehara T, Tatsumi T, Kanto T, Groh V, Spies T, Kimura R, Miyagi T, Mochizuki K, Sasaki Y, et al. Expression and role of MICA and MICB in human hepatocellular carcinomas and their regulation by retinoic acid. Int J Cancer. 2003;104:354–361. doi: 10.1002/ijc.10966. [DOI] [PubMed] [Google Scholar]
- 89.van de Pavert SA, Ferreira M, Domingues RG, Ribeiro H, Molenaar R, Moreira-Santos L, Almeida FF, Ibiza S, Barbosa I, Goverse G, et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508:123–127. doi: 10.1038/nature13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goverse G, Labao-Almeida C, Ferreira M, Molenaar R, Wahlen S, Konijn T, Koning J, Veiga-Fernandes H, Mebius RE. Vitamin A controls the presence of RORγ+ innate lymphoid cells and lymphoid tissue in the small intestine. J Immunol. 2016;196:5148–5155. doi: 10.4049/jimmunol.1501106. [DOI] [PubMed] [Google Scholar]
- 91.Kim MH, Taparowsky EJ, Kim CH. Retinoic acid differentially regulates the migration of innate lymphoid cell subsets to the gut. Immunity. 2015;43:107–119. doi: 10.1016/j.immuni.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, Nutman TB, Urban JF, Jr, Wang J, Ramalingam TR, et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432–437. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ruiter B, Patil SU, Shreffler WG. Vitamins A and D have antagonistic effects on expression of effector cytokines and gut-homing integrin in human innate lymphoid cells. Clin Exp Allergy. 2015;45:1214–1225. doi: 10.1111/cea.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]