Abstract
T lymphocytes rely on several metabolic processes to produce the high amounts of energy and metabolites needed to drive clonal expansion and the development of effector functions. However, many of these pathways result in the production of reactive oxygen species (ROS), which have canonically been thought of as cytotoxic agents due to their ability to damage DNA and other subcellular structures. Interestingly, ROS has recently emerged as a critical second messenger for T cell receptor signaling and T cell activation, but the sensitivity of different T cell subsets to ROS varies. Therefore, the tight regulation of ROS production by cellular antioxidant pathways is critical to maintaining proper signal transduction without compromising the integrity of the cell. This review intends to detail the common metabolic sources of intracellular ROS and the mechanisms by which ROS contributes to the development of T cell-mediated immunity. The regulation of ROS levels by the glutathione pathway and the Nrf2-Keap1-Cul3 trimeric complex will be discussed. Finally, T cell-mediated autoimmune diseases exacerbated by defects in ROS regulation will be further examined in order to identify potential therapeutic interventions for these disorders.
Keywords: Antioxidants, Metabolism, Autoimmunity
INTRODUCTION
Reactive oxygen species (ROS) are a class of highly unstable, oxygen-containing molecules including superoxide, hydrogen peroxide (H2O2), and hydroxyl radicals (1). These molecules have the ability to scavenge electrons from fully reduced compounds in order to increase their own stability. Under normal conditions, endogenous ROS levels are tightly controlled by various antioxidant systems within the cell. However, as these reactive species collect electrons from various sources, they form even more free radicals, resulting in oxidative stress (1). During oxidative stress, ROS can damage DNA, proteins, and lipid-based membranes within the cell. The mutations caused by high levels of cellular ROS have been linked to the development of various cancers and disease states (1).
Despite the danger posed by high levels of these compounds, several studies have implicated moderate levels of ROS as critical mediators of intracellular signaling pathways in various cell types (2,3,4,5). Because ROS levels are known to increase after T cell activation, a large body of research has emerged implicating ROS as important second messengers in T cell receptor (TCR) signaling. In this review, the role of metabolically-produced ROS in T cell-mediated immunity and its regulation by cellular antioxidant mechanisms will be discussed. Additionally, the development of T cell-driven autoimmunity as a consequence of dysregulated ROS production and signaling will be covered in greater detail.
GENERATION OF ROS BY CELLULAR METABOLIC PROCESSES IN T CELLS
Because the energetic requirements of T cells at each stage vary greatly, metabolic flux plays a key role in T lymphocyte maintenance and function. The metabolic pathways utilized by T cells are essential to produce the metabolites and adenosine triphosphate (ATP) necessary for proliferation and the development of effector functions. However, many of these processes generate ROS as a byproduct, and mitochondrial oxidative metabolism has long been known to be the main producer of ROS within the cell. In this section, the generation of ROS in T cells by oxidative phosphorylation (OXPHOS) and the tricarboxylic acid (TCA) cycle will be covered.
OXPHOS as a major source of cellular ROS
After T cell activation, signaling through the TCR stimulates the efflux of calcium ions from the endoplasmic reticulum (6). This calcium then enters the mitochondria, the major site of OXPHOS, and acts upon several enzymes involved in the TCA cycle, increasing their function and leading to the increased production of the redox coenzymes NADH and reduced flavin adenine dinucleotide (FADH2). These coenzymes are the main drivers of the electron transport chain. During this process, NADH transfers electrons to complex I and FADH2 transfers electrons to complex II, both of which are located within the mitochondrial membrane (7). From complex I, the electrons are shuttled to complex III and complex IV via ubiquinone and cytochrome C, respectively. As electrons pass through each complex, protons are pumped across the mitochondrial membrane and into the intermembrane space, creating a proton gradient that provides the energy to drive ATP synthase (7). The electrons are finally transferred to O2, creating H2O as a byproduct of the reaction.
Despite the high efficiency of this process, evidence has shown that electrons can leak through the complexes involved in the electron transport chain to partially reduce O2, resulting in the production of superoxide radicals. Superoxide can then be converted to H2O2 by the superoxide dismutases, SOD1 and SOD2. The majority of this ROS production is believed to occur at complex III, as various inhibitors of complex III have been shown to reduce superoxide production (8,9). Additionally, work done by various groups has highlighted the semiquinone anion as the source of ROS at complex III, as this intermediate is formed when ubiquinone is reduced during the transfer of electrons to complex III (9,10). Because the formation of semiquinone does not depend on an enzyme, this mechanism is capable of producing more superoxide radicals in cells with faster metabolic rates, such as proliferating T cells (11).
Conversely, complex I plays only a minor role in the production of mitochondrial derived ROS, producing about 50% less ROS than complex III (12). Despite this, complex I has still been shown to contribute to cellular ROS levels, as the introduction of excess NADH to submitochondrial particles has been shown to lead to increased superoxide production by complex I (13,14). Although research has implicated the N2 iron-sulfur cluster as the source of superoxide production at this site, the exact identity of the ROS-generating agent within complex I remains unknown (14).
TCA cycle enzymes as drivers for ROS production
Although ROS generation via OXPHOS has been well studied over time, recent work has revealed that the TCA cycle also contributes to cellular ROS levels. The TCA cycle functions mainly to convert the metabolic intermediate acetyl-CoA into various electron carrying cofactors and some ATP. This process requires the action of several enzymes and intermediate proteins. One such enzyme is pyruvate dehydrogenase (PDH), which is responsible for converting pyruvate to acetyl-CoA. During this process, PDH has been shown to produce high levels of H2O2 in human, murine, and rat skeletal muscle cells when nutrients are readily available for cellular consumption (15). Additionally, PDH and α-ketoglutarate dehydrogenase, another TCA cycle enzyme, have been shown to generate both superoxide and H2O2 in murine neural mitochondria (16). These findings contradict the idea that the electron transport chain is the sole producer of mitochondrial ROS (mROS), but future studies are needed to further characterize additional TCA enzymes that may be involved in mROS production. Additionally, whether the mROS generated by these TCA enzymes play a role in T cell-mediated immunity remains to be tested.
IMPORTANCE OF ROS IN T CELL-MEDIATED IMMUNITY
Although naïve T cells rely mainly on OXPHOS to sustain homeostatic levels of ATP and other metabolites necessary for their maintenance, T cells undergo a metabolic switch from predominantly OXPHOS to aerobic glycolysis following activation (17). Because of this, glycolysis has long been thought to be the main metabolic pathway necessary for complete T cell activation. However, several recent studies have shown that ROS derived from OXPHOS plays an essential role in TCR signaling post-activation (18,19). ROS have also been shown to be extremely important in mediating multiple facets of T lymphocyte-mediated immunity downstream of TCR signaling including T cell proliferation, effector function, and death.
Role of ROS in mediating T cell proliferation
When a naïve T cell binds its cognate antigen, the TCR becomes crosslinked, resulting in the activation of a variety of signaling cascades and transcription factors that stimulate rapid T cell proliferation in response to infection. A major class of signaling proteins important for this process is the mitogen-activated protein kinase (MAPK) family. The MAPK pathway is known to be activated early after the TCR binds antigen, leading to the activation of several cellular transcription factors important for promoting T cell growth and survival (20). Several studies have shown that ROS signaling may be one mechanism by which the MAPK pathway is controlled in activated T cells. Research has revealed that both superoxide and H2O2 are produced as early as 2–4 minutes after TCR crosslinking, and that the rapid generation of H2O2 is MAPK signaling dependent (21). Additionally, the activation of MAPK ERK1/2 has been shown to rely upon H2O2 generated via TCR signaling (22,23).
In addition to its role in stimulating the MAPK pathway, ROS has also been implicated in other aspects of TCR signaling and subsequent proliferation. As previously mentioned, TCR stimulation results in the influx of calcium from the extracellular environment as well as the release of calcium from intracellular calcium stores. This released calcium acts upon calcineurin in the cytoplasm which, in turn, activates NFAT. Activated NFAT enters the nucleus and induces the transcription of genes necessary for IL-2 production. IL-2 is essential for CD4 and CD8 T cell proliferation and cytokine production. Interestingly, calcium signaling-induced ROS generation has also been shown to play a role in IL-2 production in activated T cells. A recent study has shown that defects in mitochondrial complex III lead to decreased IL-2 production, as ROS targets the calcium-dependent calcineurin-NFAT signaling pathway to modulate levels of active NFAT in the nucleus (19).
The metabolic switch to aerobic glycolysis has also been implicated in ROS-mediated IL-2 production in T cells, as TCR signaling controls the upregulation of the glycolytic enzyme ADP-dependent glucokinase (ADPGK) (18). ADPGK mediates the production of ROS at complex I by stimulating the reduction of ubiquinone by GDP2, a mitochondrial resident enzyme important in shuttling NADH produced during glycolysis into the mitochondria (18). This complex I-derived ROS then stimulates NF-κB, another transcription factor responsible for the production of IL-2 (18). ROS control of NF-κB target gene transcription has also been attributed to the action of SOD2, which converts superoxide to H2O2 (24). This H2O2 can travel across the mitochondrial membrane into the cytoplasm, where it acts on NF-κB and stimulates the production of IL-2 and other pro-proliferative genes. Taken together, these results indicate that ROS can serve as important second messengers within T cells to modulate cell proliferation and clonal expansion in response to an invading pathogen or a tumor. However, further research is necessary to determine the exact targets and functions of ROS within the TCR signaling cascade.
Although moderate levels of ROS are necessary to promote T cell signaling, high levels of ROS can be detrimental to T cell survival. Therefore, regulation of ROS levels via cellular antioxidant mechanisms such as the glutathione (GSH) pathway and the Nrf2-Keap1-Cul3 trimeric complex are critical for ensuring proper T cell activation and proliferation. The roles of these antioxidant systems in T cell biology will be discussed in greater detail later in this review.
ROS controls the differentiation and function of various T cell subsets
In addition to promoting naïve T cell proliferation, ROS are also known to be extremely important in modulating the differentiation and effector functions of various T cell subsets. For example, high environmental levels of ROS have been shown to be beneficial to the development of T helper (Th) 2 cells, increasing IL-4 production and generating a long-lived Th2-skewed immune phenotype (25). Furthermore, recent work has shown that high cellular ROS increases both IL-4 and IL-2 production in T cells, promoting the longevity of Th2-mediated immune responses (26).
In contrast, reduced levels of ROS have been shown to promote both Th1 and Th17 cell differentiation. Several studies have shown that the use of antioxidants to inhibit both exogenous and endogenous sources of ROS increase interferon (IFN)-γ production in cultured T cells, skewing the immune response to a more Th1 phenotype (23,25). Similarly, treatment with antioxidants has been shown to induce Th17 cell differentiation and lead to the progression of experimental autoimmune encephalomyelitis, the experimental model for multiple sclerosis (MS) (27). Recent research has also shown that treatment with pro-oxidants can prevent the production of IL-17 and IFN-γ from pathogenic Th17 and Th1 cells, respectively (28). As such, targeting the redox state of T lymphocytes may be a novel therapeutic strategy for treating T cell-driven autoimmune diseases.
ROS have also been shown to be important in controlling the differentiation and function of innate-like T lymphocytes such as invariant natural killer T (iNKT) cells. iNKT cells contain a semi-invariant TCR that recognizes lipid antigens presented by CD1d, and these cells are capable of rapidly producing cytokines upon activation. Research has shown that high ROS levels generated by NADPH oxidases is critical for the inflammatory function of peripheral iNKT cells, skewing the iNKT cell response toward NKT1 and NKT17 (29). However, treatment with antioxidants causes these cells to produce IL-4 and shift toward an anti-inflammatory NKT2 phenotype (29). Although it is clear that high ROS is important for the differentiation of iNKT cell subsets, why these cells produce such high homeostatic levels of ROS and how they maintain cellular redox balance remains unknown.
ROS signaling mediates T cell apoptosis following activation
Although moderate levels of ROS can support T cell proliferation and differentiation, uncontrolled proliferation of T cells can lead to autoimmunity. Several mechanisms exist to prevent the over-activation of T cells, but studies have shown that the extrinsic apoptosis pathway is critical for the clearance of excess effector T cells during the resolution of an immune response (30). During the extrinsic apoptosis pathway, the binding of the cell death receptor Fas to its ligand (FasL) results in the induction of programmed cell death (31). Recent work has shown that endogenous superoxide can upregulate the production of FasL in T cells, demonstrating that ROS plays a key role in mediating immune resolution (22). However, further research into the mechanism by which ROS regulates the extrinsic apoptosis pathway is necessary to better understand how ROS controls T cell clearance after infection.
REGULATION OF ROS THROUGH CELLULAR ANTIOXIDANT MECHANISMS
Despite having positive effects on T cell activation and function, prolonged ROS signaling can lead to T cell hyporesponsiveness (32). Thus, maintaining the balance of ROS and antioxidant proteins within the cell is critical for preserving the integrity of T cell-mediated immunity. T lymphocytes utilize several antioxidant systems in order to maintain their intracellular redox balance. The major antioxidant pathways present within T cells will be discussed here in further detail.
GSH
The GSH pathway is known to be the major antioxidant mechanism utilized by T cells (32). GSH is a tripeptide consisting of glutamine, glycine, and cysteine. GSH gains its antioxidant activity from the thiol group within cysteine, as thiol groups are potent reducing agents that can freely scavenge electrons from ROS molecules (33). After cytosolic GSH encounters and reduces some type of intracellular ROS, GSH is converted into its oxidized form glutathione disulfide (GSSG), which can be converted back to active GSH by GSH reductase (33). The intracellular GSH:GSSG ratio can also be used to measure oxidative stress, as GSH is present at higher levels than GSSG under normal conditions. As such, stressed cells will contain more GSSG than GSH, since more GSH is required to quench the rising amounts of ROS.
Intracellular GSH levels have also been shown to be extremely important in mediating T cell proliferation after activation. Several studies have shown that the pharmacological or genetic knockdown of GSH prevents T cell proliferation after stimulation with antigen (34,35,36). This inhibition of T cell proliferation can be abrogated by treatment with various antioxidants or with exogenous GSH itself, highlighting the direct role of GSH in mediating activation-induced proliferation (34,37). Research has also shown that GSH exerts its effects on proliferation through modulation of the IL-2 pathway, a cytokine that is required for prolonged T cell survival and growth. Disruptions in the GSH pathway have been shown to reduce IL-2 production by T cells, and the subsequent defect in proliferation can be reversed via treatment with exogenous IL-2 (32,34,38). Therefore, maintaining proper GSH levels is critical for regulating clonal expansion.
The ability of GSH to influence IL-2 production highlights the role of GSH in controlling T cell survival. In fact, GSH has been implicated in conferring apoptotic resistance to T cells. Research has shown that high levels of cytosolic GSH protect against induction of apoptosis in T cells (39). Additionally, several studies have demonstrated that apoptotic cells actively pump GSH out of the cell, a process that is essential to ensuring the successful completion of the apoptosis process (40,41). If GSH efflux is blocked at any point, apoptosis can be successfully reversed (41). These studies implicate GSH levels as essential mediators of cell death processes in T cells.
In addition to mediating T cell growth and survival, GSH has also been shown to influence T cell metabolism and effector function. Research has shown that cells with defects in the GSH pathway fail to undergo metabolic reprogramming necessary for rapid proliferation following activation (36). T cells deficient in GSH also show improper activation of the nutrient sensor mTOR and defects in both NFAT and Myc signaling (36). These abnormal changes in T cell metabolism severely impact the downstream effector function of these cells. Several studies have shown that defects in the GSH pathway lead to a decreased ability to produce inflammatory cytokines such as IFN-γ, tumor necrosis factor (TNF)-α, and IL-17 (32,36). Consequently, GSH-deficient T cells fail to mount proper inflammatory immune responses against infection (36). Taken together, the GSH antioxidant pathway is essential for ensuring proper T cell proliferation, survival, and effector function.
Nrf2-Keap1-Cul3 trimeric complex
The Nrf2-Keap1-Cul3 trimeric complex is another major contributor to the maintenance of cellular redox states in mammalian cells. The transcription factor Nrf2 lends this complex its antioxidant function, as Nrf2 has been shown to be a major activator of antioxidant response element (ARE)-containing genes both in vitro and in vivo (42,43,44). Following oxidative stress, Nrf2 translocates from the cytoplasm into the nucleus, where it forms a heterodimer with nuclear Maf proteins (45). The Nrf2 heterodimers then bind to the ARE, leading to the recruitment of other factors responsible for the activation of antioxidant response genes (46).
Because some level of ROS is necessary for cellular activation and function, the Nrf2-regulated antioxidant genes cannot be constitutively active. Therefore, nuclear translocation of Nrf2 is tightly regulated by Keap1, which has been shown to bind to the amino terminus of Nrf2 (47). Keap1 is also structurally homologous to the Drosophila protein Kelch, which functions as an actin-binding protein in Drosophila cells (47). As such, the longstanding belief was that Keap1 prevented Nrf2 translocation to the nucleus by sequestering it in the cytoplasm, thereby preventing the antioxidant genes from being expressed.
Nevertheless, continued research has since disproven this theory by revealing that Keap1 instead acts as an adaptor protein for the binding of the E3 ubiquitin ligase Cullin 3 (Cul3) to cytosolic Nrf2 (45). Cul3 has also been shown to catalyze the ubiquitination of Nrf2, subsequently targeting Nrf2 for degradation by the proteasome (48,49). In this way, Keap1 and Cul3 act in concert to mediate the degradation of Nrf2 in the cytoplasm when homeostatic levels of ROS are present. During times of oxidative stress, the structure of Keap1 is modified in such a way that it can no longer bind Nrf2, allowing Nrf2 to enter the nucleus and activate the antioxidant response (46).
Although this pathway has been well characterized in various cell types, regulation of antioxidant genes by the Nrf2-Keap1-Cul3 trimeric complex has only recently begun to be explored in T cells. Several studies have revealed a role for Nrf2 in maintaining various aspects of T cell-mediated immunity. For one, Nrf2 has been shown to be important in T cell activation. Research has shown that induction of Nrf2 in both Jurkat cells and human primary CD4 T cells leads to decreased expression of the early activation markers CD25 and CD69 as well as decreased production of IL-2 (50,51). Increased expression of Nrf2 also decreased the DNA binding-capability of NF-κB, a transcription factor important in T cell activation (50,51). A recent study has also reported that Keap1 deficiency and subsequent systemic activation of Nrf2 in scurfy mice leads to decreased effector T cell activation as measured by CD25, CD44, and CD69 expression (52). Systemic Nrf2 activation and T cell specific Nrf2 activation also led to decreased IFN-γ production by effector Th1 and CD8 T cells in the scurfy model (52). In all, these data show that increased Nrf2 expression limits T cell activation. Thus, modulation of Nrf2 in a clinical setting may lead to novel therapeutic strategies for patients having T cell-mediated inflammatory diseases.
Beyond its ability to impact T cell activation, studies have also revealed that Nrf2 mediates Th cell differentiation. Induction of Nrf2 by in vitro treatment with Nrf2 activators has been shown to lead to decreased IFN-γ production and increased IL-4, IL-5, and IL-13 production (50,53). Additionally, Nrf2 activation promotes the ability of GATA-3 to bind DNA while simultaneously suppressing T-bet from binding DNA (53). Nrf2 has also been shown to play some role in the development of Th17 cells. A recent study revealed that deficiency in Nrf2 increased Th17 differentiation both in vitro and in a murine model of lupus nephritis, promoting the early onset of disease (54). Contrastingly, T cell-specific overexpression of Nrf2 has been shown to lead to increased T regulatory cell development (55). Taken together, these findings indicate that Nrf2 prevents the differentiation of inflammatory Th cell subsets and skews the immune response towards more anti-inflammatory phenotypes.
Although progress has been made in elucidating the role of Nrf2 in T cells, further work is necessary to determine the effects of Nrf2 activation on other aspects of T cell biology such as proliferation and maintenance. Additionally, little is known about the functions of Keap1 and Cul3 in controlling T cell biology. Therefore, continued research into the effects of the dysregulation of this complex in T cells is highly warranted as many T cell-mediated autoimmune diseases are driven by underlying imbalances in antioxidant response pathways.
DYSREGULATION OF ANTIOXIDANT PATHWAYS IN T CELL-DRIVEN DISEASES
As previously mentioned, several prevalent human diseases are characterized by improper control of ROS in peripheral tissues, resulting in damaged tissue and pathology. The following sections describe some of the major T cell-mediated diseases that are characterized by improper ROS regulation.
Cancer
Although tumorigenesis is not solely T cell-mediated, T lymphocytes play a key role in anti-tumor immunity and surveillance. Because the high amounts of ROS secreted by cancerous cells are immunosuppressive, cellular antioxidant levels have been shown to be of critical importance in maintaining the anti-tumor function of T cells within oxidative tumor microenvironments. Research has shown that central memory T cells having higher cytosolic GSH, surface thiol, and intracellular antioxidant levels can persist for longer in a highly immunosuppressive microenvironment and better control tumor growth than effector memory T cells, which exhibit lower cytoplasmic antioxidant levels (56). Additionally, studies have shown that treatment of ex vivo expanded tumor infiltrating lymphocytes (TILs) with antioxidants prevents these T cells from undergoing apoptosis following adoptive transfer into patients (57). Treatment with antioxidants has also been shown to improve the persistence and anti-tumor cytotoxicity of TILs, ultimately leading to prolonged survival of patients receiving these TILs (57). With immunotherapy at the forefront of current cancer treatment methods, the ability to improve the efficacy of adoptively transferred T cells simply through antioxidant treatment is an attractive therapeutic option for cancer patients.
Several studies have also linked the Nrf2-Keap1-Cul3 complex to the development of numerous human cancers. The ability of this complex to mediate tumorigenesis lies in the properties of many known Nrf2 target genes. Several metabolic enzymes including glucose-6-phosphate dehydrogenase, transaldolase 1, and malic enzyme 1 (among others) have been shown to be activated by Nrf2 (58). These enzymes play a key role in mediating cellular glucose metabolism as well as the synthesis of metabolites such as NADPH and nucleotides (59). As such, Nrf2 has been implicated as a key regulator of metabolic reprogramming that is required for cells to undergo rapid proliferation. Nrf2 has also been shown to increase the expression of many cytoprotective factors. This includes the upregulation of drug resistance genes like the multidrug resistance protein family, which encode several drug efflux pumps responsible for removing toxins from the cell (60).
Consistent with its ability to support cell proliferation and survival, Nrf2 dysregulation has been reported in several human cancers. Mutations in both Keap1 and Nrf2 that prevent binding of Keap1 to Nrf2 have been reported in lung, ovarian, and hepatic tumors (61,62,63). These mutations are believed to be advantageous to cancerous cells by supporting their rapid proliferation and conferring resistance to traditional chemotherapeutic drugs. However, the dysregulation of the Nrf2-Keap1-Cul3 antioxidant system has never been studied in peripheral T cell lymphoma (PTCL), despite the high chemotherapeutic resistance of relapsed cases of PTCL (64). Additionally, how the dysregulation of this complex within TILs affects their ability to produce cytokines and mediate killing of cancerous cells remains poorly understood. Therefore, more research is needed to determine whether this complex could be targeted as a potential therapeutic mechanism in T cell lymphomas.
Acute kidney injury (AKI)
AKI is defined by a rapid decline in kidney function that can lead to outcomes ranging in severity from minute tissue injury to complete renal failure (65). AKI is often characterized by an increase in oxidative stress within the renal microenvironment as well as dysfunction of the renal epithelium and the infiltration of immune cells into the damaged organ (55). Research has shown that T cells play a role in mediating the progression of AKI severity, but the mechanisms by which these infiltrating T cells do so is not fully understood (66).
A recent study has shown that T cell specific deletion of Keap1 and subsequent upregulation of Nrf2 leads to decreased inflammatory cytokine production by CD4 T cells present in renal tissue during experimentally-induced AKI (55). Additionally, deletion of Keap1 led to an increase in T regulatory cells present in the damaged kidney (55). Therefore, disruption of the Nrf2-Keap1-Cul3 complex in CD4 T cells may skew the T cell response to a more anti-inflammatory and immunosuppressive phenotype, preventing the development of severe AKI. However, more work is necessary to elucidate how this complex mediates normal T cell function in order to properly target these molecules in disease treatment.
MS
MS is a neurodegenerative disorder characterized by the influx of inflammatory CD4 T cells and macrophages into the central nervous system (CNS). Here, autoreactive CD4 T cells recognize myelin-like peptides presented by antigen presenting cells in the periphery (67). These T cells then differentiate into memory cells that can migrate into the CNS and react against myelin-coated neurons, resulting in progressive demyelination of the brain and spinal cord. This loss of myelin leads to the development of symptoms which include loss of coordination, muscle weakness, and even permanent neurological damage. As such, there is a great need for the development of effective treatments for MS. Because the T cells that mediate this disease secrete high levels of Th1 and Th17 cytokines, treatment with pro-oxidants may reduce the severity of MS symptoms, as the administration of such compounds has been previously shown to prevent the production of these inflammatory cytokines (23,25). More research into this possibility is warranted, as there is currently no cure for MS.
Additionally, recent research investigating the modulation of the Nrf2-mediated antioxidant pathway in T cells shows promise as a new treatment method for MS. Research has shown that Nrf2 activation prevents CD4 T cell infiltration into the CNS, thereby ameliorating autoimmune inflammation in these sites (67,68). Several studies have also revealed that various plant-based compounds like triterpenoids, 3H-1,2-dithiole-3-thione (D3T), and cannabidiol function as potent activators of Nrf2 in T cells, resulting in a host of downstream anti-inflammatory effects. For example, treatment with these compounds has been shown to decrease inflammatory cytokine production by pathogenic Th1 and Th17 cells in both the CNS and the periphery (68,69,70). D3T has also been shown to be capable of suppressing both Th1 and Th17 differentiation, while triterpenoids have been implicated in preventing the differentiation of pathogenic memory T cell subtypes (68,70). Deficiency of Nrf2 has also been implicated in the development of MS-associated pathologies such as optic neuritis, a condition in which the optic nerve becomes inflamed (71). As reported in a study examining the role of ROS in optic neuritis development, the systemic loss of Nrf2 resulted in increased Th1 polarization and subsequent inflammatory T cell infiltration into the optic nerve, leading to increased damage and vision loss (71). Therefore, targeting the Nrf2-Keap1-Cul3 complex through treatment with natural compounds may lead to a therapeutic breakthrough for MS patients.
Dimethyl fumarate (DMF) has recently been approved by the Food and Drug Administration for the treatment of MS. Research has shown that treatment with this compound results in the decreased expression of integrins on memory CD4 T cells, preventing the trafficking of myelin-specific memory cells into the CNS of MS patients (72). The same study also showed that DMF prevents the differentiation of naïve CD4 T cells into effector Th1 cells in vitro (72). Although the previously cited research involving D3T had similar effects on CD4 T cell differentiation, whether DMF exerts its functions on T cells by increasing Nrf2 expression in the pathogenic subsets that mediate MS remains to be tested. Despite the current gaps in knowledge, further research into the role of antioxidation in T cells may not only lead to improved patient outcomes but may also inform the development of novel therapeutics for MS.
CONCLUSION
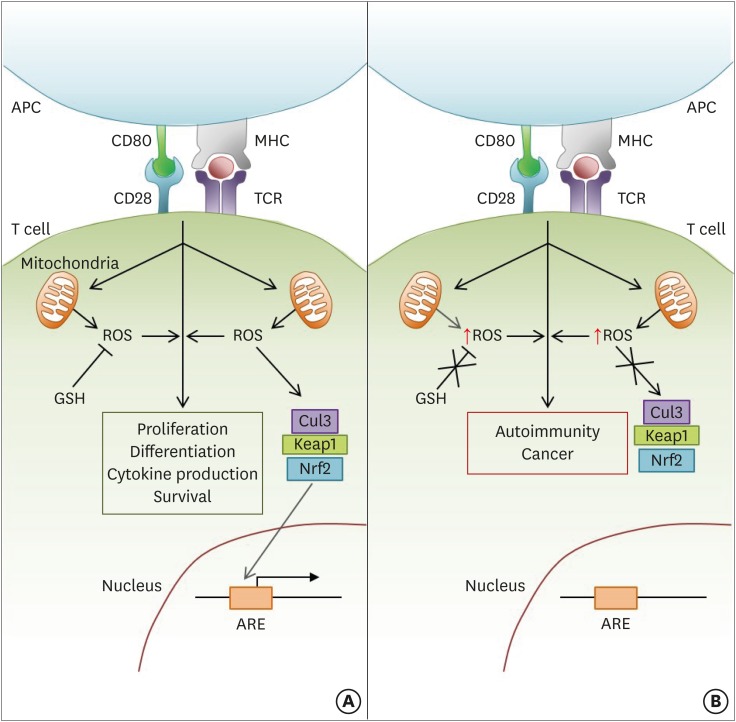
Although previously thought to exert mainly damaging effects on cells, recent studies have shown that ROS are important for regulating the development and effector functions of various T lymphocyte subsets. The majority of this ROS is generated by the electron transport chain within the mitochondria, but new data has indicated that various steps in the TCA cycle can also contribute to the production of these oxidative species. These ROS mediate several aspects of T cell-mediated immunity downstream of TCR signaling including proliferation, differentiation, the development of effector functions, and survival. The role of ROS in each of these processes is depicted in Fig. 1.
Figure 1.
ROS modulate several aspects of T cell-mediated immunity downstream of TCR stimulation. (A) Following activation, signaling through the TCR stimulates the mitochondria to produce ROS, which in turn promote continued TCR signaling. The level of activation-induced ROS within the cell also critically affects the downstream functions of T cell proliferation, differentiation, and survival. Therefore, the modulation of ROS levels by cellular antioxidant pathways (e.g., GSH; Nrf2-Keap1-Cul3 trimeric complex) is crucial in maintaining proper T cell-mediated immunity. GSH regulates ROS levels by directly reducing free radicals encountered in the cytoplasm. In contrast, the Nrf2-Keap1-Cul3 trimeric complex disassociates upon sensing high levels of cellular ROS, allowing Nrf2 to enter the nucleus and activate the ARE-containing genes. (B) If cellular antioxidant pathways are dysregulated or mutated, cellular ROS levels will not be properly controlled. Therefore, high levels of ROS after T cell activation can lead to the development of several T cell-mediated autoimmune diseases and cancer.
APC, antigen presenting cell; MHC, major histocompatibility complex.
Although moderate levels of ROS are necessary for the proper regulation of multiple facets of T cell biology, high levels of ROS can be damaging to T cells. Therefore, the tight control of ROS by cellular antioxidant mechanisms is critical for the maintenance of effective T cell-mediated immunity (Fig. 1A). In mammalian cells, intracellular levels of ROS have been shown to be regulated largely through the Nrf2-Keap1-Cul3 trimeric complex. Although the effects of the disruption of this complex on T cells has not been well studied, dysregulated versions of this pathway have been implicated in tumorigenesis and T cell-driven autoimmune diseases like AKI and MS (Fig. 1B). Therefore, understanding how defects in this pathway affect healthy T cells may lead to novel treatments for autoimmunity and cancer, increasing the quality of life for patients suffering from diseases having otherwise grim prognoses.
ACKNOWLEDGEMENTS
We thank Drs. Kalyani Pyaram and Ajay Kumar for their careful consideration of and aid in revising this manuscript. This work was supported in part by National Institutes of Health Grants AI121156 (to C.H.C.) and T32 AI007413 (E.L.Y.).
Abbreviations
- AKI
acute kidney injury
- ARE
antioxidant response element
- ATP
adenosine triphosphate
- CNS
central nervous system
- D3T
3H-1,2-dithiole-3-thione
- DMF
dimethyl fumarate
- GSH
glutathione
- GSSG
glutathione disulfide
- IFN
interferon
- iNKT
invariant natural killer T
- MAPK
mitogen-activated protein kinase
- mROS
mitochondrial ROS
- MS
multiple sclerosis
- OXPHOS
oxidative phosphorylation
- PDH
pyruvate dehydrogenase
- ROS
reactive oxygen species
- TCR
T cell receptor
- TCA cycle
tricarboxylic acid cycle
- Th
T helper
- TIL
tumor infiltrating lymphocyte
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
Author Contributions: Funding acquisition: Yarosz EL, Chang CH; Writing - original draft: Yarosz EL; Writing - review & editing: Yarosz EL, Chang CH.
References
- 1.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J Exp Med. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byun HO, Kim HY, Lim JJ, Seo YH, Yoon G. Mitochondrial dysfunction by complex II inhibition delays overall cell cycle progression via reactive oxygen species production. J Cell Biochem. 2008;104:1747–1759. doi: 10.1002/jcb.21741. [DOI] [PubMed] [Google Scholar]
- 4.Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, Chandel NS. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011;14:537–544. doi: 10.1016/j.cmet.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imboden JB, Stobo JD. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985;161:446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy AN, Fiskum G, Beal MF. Mitochondria in neurodegeneration: bioenergetic function in cell life and death. J Cereb Blood Flow Metab. 1999;19:231–245. doi: 10.1097/00004647-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Loschen G, Azzi A, Flohe L. Mitochondrial H2O2 formation: relationship with energy conservation. FEBS Lett. 1973;33:84–87. doi: 10.1016/0014-5793(73)80165-6. [DOI] [PubMed] [Google Scholar]
- 9.Turrens JF, Freeman BA, Levitt JG, Crapo JD. The effect of hyperoxia on superoxide production by lung submitochondrial particles. Arch Biochem Biophys. 1982;217:401–410. doi: 10.1016/0003-9861(82)90518-5. [DOI] [PubMed] [Google Scholar]
- 10.Nohl H, Gille L, Schonheit K, Liu Y. Conditions allowing redox-cycling ubisemiquinone in mitochondria to establish a direct redox couple with molecular oxygen. Free Radic Biol Med. 1996;20:207–213. doi: 10.1016/0891-5849(95)02038-1. [DOI] [PubMed] [Google Scholar]
- 11.Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem. 2015;30:11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenaz G. The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life. 2001;52:159–164. doi: 10.1080/15216540152845957. [DOI] [PubMed] [Google Scholar]
- 14.Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 15.Fisher-Wellman KH, Gilliam LA, Lin CT, Cathey BL, Lark DS, Neufer PD. Mitochondrial glutathione depletion reveals a novel role for the pyruvate dehydrogenase complex as a key H2O2-emitting source under conditions of nutrient overload. Free Radic Biol Med. 2013;65:1201–1208. doi: 10.1016/j.freeradbiomed.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci. 2004;24:7779–7788. doi: 10.1523/JNEUROSCI.1899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamiński MM, Sauer SW, Kamiński M, Opp S, Ruppert T, Grigaravičius P, Grudnik P, Gröne HJ, Krammer PH, Gulow K. T cell activation is driven by an ADP-dependent glucokinase linking enhanced glycolysis with mitochondrial reactive oxygen species generation. Cell Reports. 2012;2:1300–1315. doi: 10.1016/j.celrep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu L, Yu X, Akatsuka Y, Cooper JA, Anasetti C. Role of mitogen-activated protein kinases in activation-induced apoptosis of T cells. Immunology. 1999;97:26–35. doi: 10.1046/j.1365-2567.1999.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon J, Devadas S, Williams MS. T cell receptor-stimulated generation of hydrogen peroxide inhibits MEK-ERK activation and lck serine phosphorylation. Free Radic Biol Med. 2003;35:406–417. doi: 10.1016/s0891-5849(03)00318-6. [DOI] [PubMed] [Google Scholar]
- 22.Devadas S, Zaritskaya L, Rhee SG, Oberley L, Williams MS. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J Exp Med. 2002;195:59–70. doi: 10.1084/jem.20010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5:818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 24.Kamiński MM, Röth D, Sass S, Sauer SW, Krammer PH, Gülow K. Manganese superoxide dismutase: a regulator of T cell activation-induced oxidative signaling and cell death. Biochim Biophys Acta. 2012;1823:1041–1052. doi: 10.1016/j.bbamcr.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Frossi B, De Carli M, Piemonte M, Pucillo C. Oxidative microenvironment exerts an opposite regulatory effect on cytokine production by Th1 and Th2 cells. Mol Immunol. 2008;45:58–64. doi: 10.1016/j.molimm.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Kaminski MM, Sauer SW, Klemke CD, Suss D, Okun JG, Krammer PH, Gulow K. Mitochondrial reactive oxygen species control T cell activation by regulating IL-2 and IL-4 expression: mechanism of ciprofloxacin-mediated immunosuppression. J Immunol. 2010;184:4827–4841. doi: 10.4049/jimmunol.0901662. [DOI] [PubMed] [Google Scholar]
- 27.Fu G, Xu Q, Qiu Y, Jin X, Xu T, Dong S, Wang J, Ke Y, Hu H, Cao X, et al. Suppression of Th17 cell differentiation by misshapen/NIK-related kinase MINK1. J Exp Med. 2017;214:1453–1469. doi: 10.1084/jem.20161120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abimannan T, Peroumal D, Parida JR, Barik PK, Padhan P, Devadas S. Oxidative stress modulates the cytokine response of differentiated Th17 and Th1 cells. Free Radic Biol Med. 2016;99:352–363. doi: 10.1016/j.freeradbiomed.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 29.Kim YH, Kumar A, Chang CH, Pyaram K. Reactive oxygen species regulate the inflammatory function of NKT cells through promyelocytic leukemia zinc finger. J Immunol. 2017;199:3478–3487. doi: 10.4049/jimmunol.1700567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell JH, Rush B, Weaver C, Wang R. Mature T cells of autoimmune lpr/lpr mice have a defect in antigen-stimulated suicide. Proc Natl Acad Sci USA. 1993;90:4409–4413. doi: 10.1073/pnas.90.10.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waring P, Mullbacher A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol Cell Biol. 1999;77:312–317. doi: 10.1046/j.1440-1711.1999.00837.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee DH, Son DJ, Park MH, Yoon DY, Han SB, Hong JT. Glutathione peroxidase 1 deficiency attenuates concanavalin A-induced hepatic injury by modulation of T-cell activation. Cell Death Dis. 2016;7:e2208. doi: 10.1038/cddis.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett SJ, Griffiths HR. Regulation of T-cell functions by oxidative stress. In: Alcaraz MJ, Gualillo O, Sánchez-Pernaute O, editors. Studies on Arthritis and Joint Disorders. New York, NY: Humana Press; 2013. pp. 33–48. [Google Scholar]
- 34.Checker R, Sharma D, Sandur SK, Subrahmanyam G, Krishnan S, Poduval TB, Sainis KB. Plumbagin inhibits proliferative and inflammatory responses of T cells independent of ROS generation but by modulating intracellular thiols. J Cell Biochem. 2010;110:1082–1093. doi: 10.1002/jcb.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamilos DL, Zelarney P, Mascali JJ. Lymphocyte proliferation in glutathione-depleted lymphocytes: direct relationship between glutathione availability and the proliferative response. Immunopharmacology. 1989;18:223–235. doi: 10.1016/0162-3109(89)90020-9. [DOI] [PubMed] [Google Scholar]
- 36.Mak TW, Grusdat M, Duncan GS, Dostert C, Nonnenmacher Y, Cox M, Binsfeld C, Hao Z, Brustle A, Itsumi M, et al. Glutathione primes T cell metabolism for inflammation. Immunity. 2017;46:675–689. doi: 10.1016/j.immuni.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 37.Suthanthiran M, Anderson ME, Sharma VK, Meister A. Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens. Proc Natl Acad Sci USA. 1990;87:3343–3347. doi: 10.1073/pnas.87.9.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hadzic T, Li L, Cheng N, Walsh SA, Spitz DR, Knudson CM. The role of low molecular weight thiols in T lymphocyte proliferation and IL-2 secretion. J Immunol. 2005;175:7965–7972. doi: 10.4049/jimmunol.175.12.7965. [DOI] [PubMed] [Google Scholar]
- 39.Friesen C, Kiess Y, Debatin KM. A critical role of glutathione in determining apoptosis sensitivity and resistance in leukemia cells. Cell Death Differ. 2004;11(Suppl 1):S73–S85. doi: 10.1038/sj.cdd.4401431. [DOI] [PubMed] [Google Scholar]
- 40.Franco R, Cidlowski JA. SLCO/OATP-like transport of glutathione in FasL-induced apoptosis: glutathione efflux is coupled to an organic anion exchange and is necessary for the progression of the execution phase of apoptosis. J Biol Chem. 2006;281:29542–29557. doi: 10.1074/jbc.M602500200. [DOI] [PubMed] [Google Scholar]
- 41.Ghibelli L, Fanelli C, Rotilio G, Lafavia E, Coppola S, Colussi C, Civitareale P, Ciriolo MR. Rescue of cells from apoptosis by inhibition of active GSH extrusion. FASEB J. 1998;12:479–486. doi: 10.1096/fasebj.12.6.479. [DOI] [PubMed] [Google Scholar]
- 42.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Kern JT, Walker JR, Johnson JA, Schultz PG, Luesch H. A genomic screen for activators of the antioxidant response element. Proc Natl Acad Sci USA. 2007;104:5205–5210. doi: 10.1073/pnas.0700898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci USA. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cullinan SB, Gordan JD, Jin J, Harper JW, Diehl JA. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol Cell Biol. 2004;24:8477–8486. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furukawa M, Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Mol Cell Biol. 2005;25:162–171. doi: 10.1128/MCB.25.1.162-171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turley AE, Zagorski JW, Rockwell CE. The Nrf2 activator tBHQ inhibits T cell activation of primary human CD4 T cells. Cytokine. 2015;71:289–295. doi: 10.1016/j.cyto.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zagorski JW, Turley AE, Dover HE, VanDenBerg KR, Compton JR, Rockwell CE. The Nrf2 activator, tBHQ, differentially affects early events following stimulation of Jurkat cells. Toxicol Sci. 2013;136:63–71. doi: 10.1093/toxsci/kft172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki T, Murakami S, Biswal SS, Sakaguchi S, Harigae H, Yamamoto M, Motohashi H. Systemic activation of NRF2 alleviates lethal autoimmune inflammation in scurfy mice. Mol Cell Biol. 2017;37:e00063–e17. doi: 10.1128/MCB.00063-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rockwell CE, Zhang M, Fields PE, Klaassen CD. Th2 skewing by activation of Nrf2 in CD4(+) T cells. J Immunol. 2012;188:1630–1637. doi: 10.4049/jimmunol.1101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao M, Chen H, Ding Q, Xu X, Yu B, Huang Z. Nuclear factor erythroid 2-related factor 2 deficiency exacerbates lupus nephritis in B6/lpr mice by regulating Th17 cell function. Sci Rep. 2016;6:38619. doi: 10.1038/srep38619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noel S, Martina MN, Bandapalle S, Racusen LC, Potteti HR, Hamad AR, Reddy SP, Rabb H. T lymphocyte-specific activation of Nrf2 protects from AKI. J Am Soc Nephrol. 2015;26:2989–3000. doi: 10.1681/ASN.2014100978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kesarwani P, Thyagarajan K, Chatterjee S, Palanisamy V, Mehrotra S. Anti-oxidant capacity and anti-tumor T cell function: a direct correlation. OncoImmunology. 2015;4:e985942. doi: 10.4161/2162402X.2014.985942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scheffel MJ, Scurti G, Simms P, Garrett-Mayer E, Mehrotra S, Nishimura MI, Voelkel-Johnson C. Efficacy of adoptive T-cell therapy is improved by treatment with the antioxidant N-acetyl cysteine, which limits activation-induced T-cell death. Cancer Res. 2016;76:6006–6016. doi: 10.1158/0008-5472.CAN-16-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, Yamamoto M, Motohashi H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 59.Chen HY, Chen RH. Cullin 3 ubiquitin ligases in cancer biology: functions and therapeutic implications. Front Oncol. 2016;6:113. doi: 10.3389/fonc.2016.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rushworth SA, Macewan DJ. The role of nrf2 and cytoprotection in regulating chemotherapy resistance of human leukemia cells. Cancers (Basel) 2011;3:1605–1621. doi: 10.3390/cancers3021605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Konstantinopoulos PA, Spentzos D, Fountzilas E, Francoeur N, Sanisetty S, Grammatikos AP, Hecht JL, Cannistra SA. Keap1 mutations and Nrf2 pathway activation in epithelial ovarian cancer. Cancer Res. 2011;71:5081–5089. doi: 10.1158/0008-5472.CAN-10-4668. [DOI] [PubMed] [Google Scholar]
- 62.Ohta T, Iijima K, Miyamoto M, Nakahara I, Tanaka H, Ohtsuji M, Suzuki T, Kobayashi A, Yokota J, Sakiyama T, et al. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68:1303–1309. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- 63.Yoo NJ, Kim HR, Kim YR, An CH, Lee SH. Somatic mutations of the KEAP1 gene in common solid cancers. Histopathology. 2012;60:943–952. doi: 10.1111/j.1365-2559.2012.04178.x. [DOI] [PubMed] [Google Scholar]
- 64.Broccoli A, Argnani L, Zinzani PL. Peripheral T-cell lymphomas: Focusing on novel agents in relapsed and refractory disease. Cancer Treat Rev. 2017;60:120–129. doi: 10.1016/j.ctrv.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10:193–207. doi: 10.1038/nrneph.2013.282. [DOI] [PubMed] [Google Scholar]
- 66.Rabb H, Daniels F, O'Donnell M, Haq M, Saba SR, Keane W, Tang WW. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2000;279:F525–F531. doi: 10.1152/ajprenal.2000.279.3.F525. [DOI] [PubMed] [Google Scholar]
- 67.Johnson DA, Amirahmadi S, Ward C, Fabry Z, Johnson JA. The absence of the pro-antioxidant transcription factor Nrf2 exacerbates experimental autoimmune encephalomyelitis. Toxicol Sci. 2010;114:237–246. doi: 10.1093/toxsci/kfp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuo PC, Brown DA, Scofield BA, Yu IC, Chang FL, Wang PY, Yen JH. 3H-1,2-dithiole-3-thione as a novel therapeutic agent for the treatment of experimental autoimmune encephalomyelitis. Brain Behav Immun. 2016;57:173–186. doi: 10.1016/j.bbi.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 69.Kozela E, Juknat A, Gao F, Kaushansky N, Coppola G, Vogel Z. Pathways and gene networks mediating the regulatory effects of cannabidiol, a nonpsychoactive cannabinoid, in autoimmune T cells. J Neuroinflammation. 2016;13:136. doi: 10.1186/s12974-016-0603-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pareek TK, Belkadi A, Kesavapany S, Zaremba A, Loh SL, Bai L, Cohen ML, Meyer C, Liby KT, Miller RH, et al. Triterpenoid modulation of IL-17 and Nrf-2 expression ameliorates neuroinflammation and promotes remyelination in autoimmune encephalomyelitis. Sci Rep. 2011;1:201. doi: 10.1038/srep00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Larabee CM, Desai S, Agasing A, Georgescu C, Wren JD, Axtell RC, Plafker SM. Loss of Nrf2 exacerbates the visual deficits and optic neuritis elicited by experimental autoimmune encephalomyelitis. Mol Vis. 2016;22:1503–1513. [PMC free article] [PubMed] [Google Scholar]
- 72.Breuer J, Herich S, Schneider-Hohendorf T, Chasan AI, Wettschureck N, Gross CC, Loser K, Zarbock A, Roth J, Klotz L, et al. Dual action by fumaric acid esters synergistically reduces adhesion to human endothelium. Mult Scler. doi: 10.1177/1352458517735189. [DOI] [PubMed] [Google Scholar]