Abstract
Emerging evidence demonstrates that the microbiota plays an essential role in shaping the development and function of host immune responses. A variety of environmental stimuli, including foods and commensals, are recognized by the host through the epithelium, acting as a physical barrier. Two allergic diseases, atopic dermatitis and food allergy, are closely linked to the microbiota, because inflammatory responses occur on the epidermal border. The microbiota generates metabolites such as short-chain fatty acids and poly-γ-glutamic acid (γPGA), which can modulate host immune responses. Here, we review how microbial metabolites can regulate allergic immune responses. Furthermore, we focus on the effect of γPGA on allergic T helper (Th) 2 responses and its therapeutic application.
Keywords: Microbial metabolites; Dermatitis, atopic; Food allergy; Poly-γ-glutamic acid; iNKT cells
INTRODUCTION
Allergy is a major health concern, especially in industrialized countries. Currently, there is a global increase in patients with atopic dermatitis (AD) and food allergy (FA). Recent studies (1,2) showed that skin sensitization of food antigens has a remarkable influence on the occurrence of FA in high-risk infants, suggesting a strong link between the skin and gut immune responses. One feature common to the skin and gut is that these are the first organs to confront various microorganisms that invade the human body. These physical barriers not only provide protection against harmful pathogens such as bacteria and viruses, but also supply niches for the microbiota. Coexisting microbes maintain homeostasis within the host; however, at the same time, they tend to change themselves depending on the environment (such as changes in food consumption). Emerging evidence showed that microorganisms generate a variety of metabolites with abilities of regulating the host immune responses. Some microbial metabolites are beneficial to the host, while in some cases, they trigger detrimental immune responses in the host, eventually developing a pathogenic status. In this review, we focus on allergic immune diseases, especially AD and FA, and discuss how microbial metabolites can affect these allergic conditions.
IMMUNE EFFECTORS IN AD AND FA
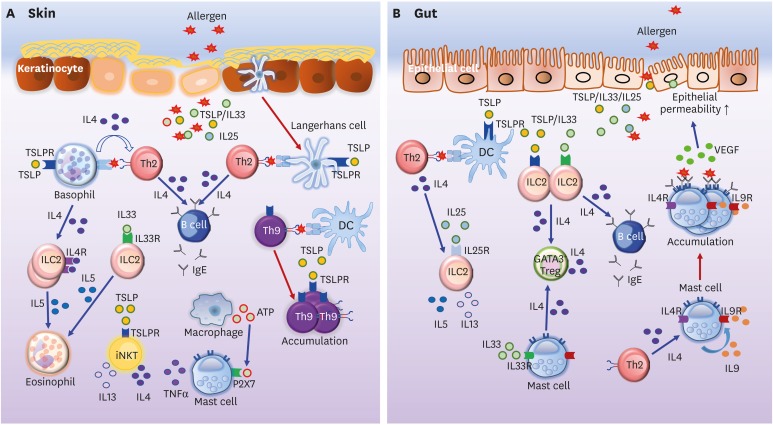
In the lesional skin associated with AD, pro-T helper (Th) 2 cytokines (e.g., thymic stromal lymphopoietin [TSLP], IL25, and IL33) are mainly produced by epidermal keratinocytes and fibroblasts. Moreover, these cytokines have central roles in initiating skin inflammation, by promoting the production of Th2-type cytokines such as IL4, IL5, and IL13 (3). In particular, the skin-specific expression of IL33 increases the proportion of group 2 innate lymphoid cells (ILC2s), which produce IL5, and the infiltration of eosinophils and mast cells into the skin, consequently resulting in spontaneous dermatitis (4). TSLP, IL25, and IL33 are considered essential initiators of inflammatory events in the skin.
Strong skin-driven Th2 polarization is an essential process to augment the production of IgE by B cells. Upon exposure to allergens, Langerhans cells (LCs) initiate epicutaneous sensitization with protein antigens via TSLP signaling. Consequently, increased Th2-type immune responses by TSLP-activated LCs cause an acceleration of allergic skin dermatitis in mice (5). Basophils have also been identified as specialized antigen-presenting cells, which are capable of inducing the polarization of Th2 cells (6).
Upon epicutaneous sensitization, basophil-derived IL4 is necessary for the differentiation of skin Th2 cells; induction of antigen-specific Th2 differentiation by these cells are restricted to peptide antigens, because basophils cannot take up and process protein antigens (6). ILC2s express IL4 receptor (IL4R) α on the cell surfaces, and proliferate in response to basophil-derived IL4 secretion during cutaneous inflammation, indicating that basophils are required for the accumulation of ILC2 in the inflamed skin associated with AD in both humans and mice (7). In cutaneous inflammation, dermal ILC2s secrete a high concentration of IL5 and recruit eosinophils, consequently leading to spontaneous dermatitis (8). Invariant natural killer T (iNKT) cells are innate-like T cells that recognize glycolipid antigens via the major histocompatibility complex (MHC) class I-like protein, CD1d. iNKT cells rapidly produce Th2-type cytokines (IL4 and IL13), as well as Th1-type cytokines (tumor necrosis factor [TNF] α and interferon [IFN] γ); thus, iNKT cells play an important role in affecting the pathogenesis of allergic diseases (9). Besides, Lee et al. (10) reported that iNKT cells could be subdivided into 3 groups: iNKT1 for IFNγ, iNKT2 for IL4, and iNKT17 for IL17 production. iNKT2-derived IL4 contributed to the Th2 dominance and increased serum IgE levels (10,11). Moreover, iNKT cells activated by TSLP secrete high amounts of IL4 and IL13, but not IFNγ, in patients with severe AD; they might play an essential role in the innate allergic immune response in AD (12). In AD, the pro-Th2 cytokine-driven immune cascade leads to initial activation of skin-resident innate immune cells, followed by the promotion of adaptive Th2 cell responses (Fig. 1A).
Figure 1.
Allergic immune responses in the skin and gut. (A) Basophils and LCs activated by lesional skin-derived TSLP induce the Th2 differentiation of allergen-specific CD4+ T cells. Th2 cells stimulate B cells to switch, and to thus produce IgE. Basophil-derived IL4 can induce IL5 production, promoting the accumulation of eosinophils in the skin. Besides, IL5 produced by IL33-activated ILC2s recruits eosinophils into the skin. Th9 cells induced by DCs accumulate under TSLP stimulation in the skin. TSLP also promotes iNKT cells to secrete IL4 and IL13. Furthermore, macrophage-derived ATP causes the release of TNFα by mast cells via P2X7 signaling, consequently resulting in skin inflammation. (B) TSLP triggers DCs to induce naive CD4+ T cell differentiation into Th2 cells. Th2 cells and IL25 stimulate ILC2s to secrete IL5 and IL13. IL4 produced by IL33-activated ILC2s and mast cells also induces GATA3+ Treg differentiation in the gut. IL4 secreted by IL33-triggered ILC2s increases IgE production by B cells in the intestine. Mast cells stimulated by Th2-derived IL4 produce IL9, which further induces the accumulation of mast cells in the intestine, in an autocrine fashion. IL9-producing mast cells increase gut permeability via the expression of VEGF. Note that blue arrows indicate induction or stimulation while red arrows represent migration or proliferation.
ATP, adenosine triphosphate; TSLPR, TSLP receptor.
On the other hand, food allergens increase the epithelial barrier dysfunction and induce intestinal FA. The production of pro-Th2 cytokines by gut epithelial cells is also associated with the severity of FA. TSLP produced by gut epithelial cells elicits FA by elevating the secretion of IL4, IL5, and IL10 by mesenteric lymph node lymphocytes (13). In addition to TSLP, intestinal IL25 signaling also promotes the development of IgE-mediated experimental FA by triggering IL13 production from ILC2s (14). Regulatory T cells (Tregs) can suppress ILC2 expansion and activation during FA (15). Furthermore, Tregs directly inhibit FcεR1-dependent mast cell degranulation through OX40-OX40L interaction (16). In humans, the lack of Foxp3+ T cells by a mutation in the Foxp3 gene is related to severe FA and elevated IgE levels (17). Children who are naturally tolerant to egg or peanut possess markedly increased number of Tregs (18). These data suggest that allergen-specific Tregs play a central role in an oral tolerance induction, which is essential for the suppression of FA. The re-programming of Tregs into Th2-like cells via IL4R signaling leads to the failure of Treg-mediated oral tolerance and increases susceptibility to FA (15). Moreover, IL4 secretion by ILC2s and mast cells contribute to allergic inflammation in the gut by reducing allergen-specific Tregs (19). Thus, these results indicate that the proportion and phenotypic conversion of Tregs into Th2-like cells are strongly linked with the induction of IL4 production. Activation of ILC2s and mast cells in the gut results in IL4-mediated Treg reduction, consequently leading to the induction of FA (Fig. 1B).
ROLES OF THE MICROBIOTA AND ITS METABOLITES IN ALLERGIC IMMUNE RESPONSES
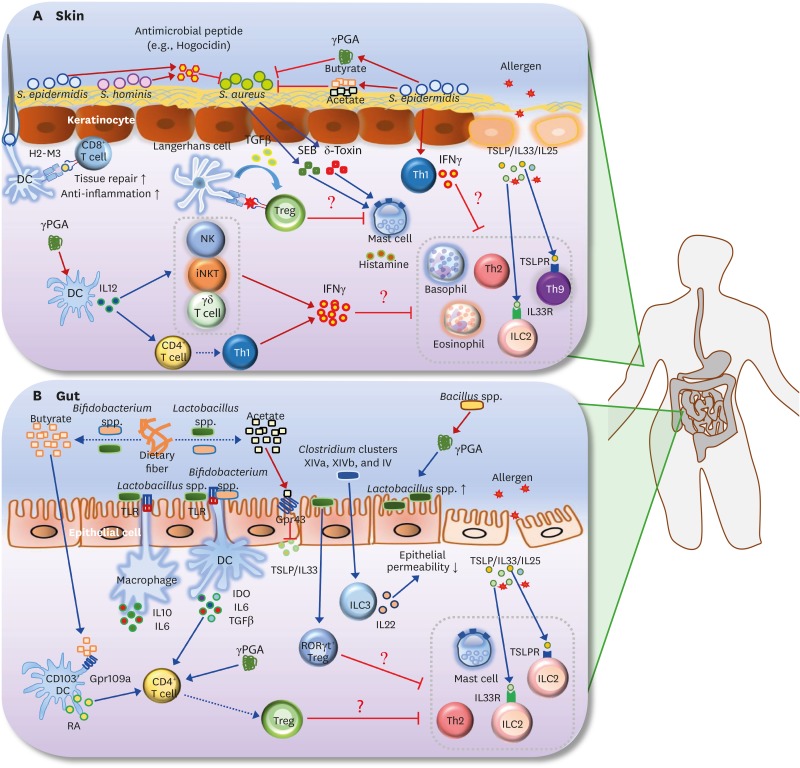
Skin commensal bacteria including Staphylococcus, Corynebacterium, and Propionibacterium spp., reside mostly on the superficial layers of the epidermis (20). Patients with AD have higher colonization density levels of Staphylococcus aureus in the skin than healthy individuals, and the lesional skin of patients with AD is more frequently colonized with S. aureus than the non-lesional skin of the same patients (21). A recent study showed that S. aureus is predominant in patients with the more severe form of the disease, while Staphylococcus epidermidis is predominant in patients with less severe symptoms (22). These data indicate that changes in the ratio of S. aureus and S. epidermidis are strongly correlated with the onset of AD. When compared with non-AD subjects, antimicrobials produced by the skin commensal bacteria are rare in AD subjects. These antimicrobials act selectively against S. aureus, but not Staphylococcus hominis and S. epidermidis, implying that the distinct responsiveness to antimicrobials is correlated with colonization by S. aureus (23). Moreover, S. epidermidis secretes poly-γ-glutamic acid (γPGA) to facilitate its growth and survival in the human host. Importantly, γPGA efficiently shelters S. epidermidis from critical components of the innate host defense, namely antimicrobial peptides and neutrophil phagocytosis (24). Due to the antimicrobial activity of γPGA, magnetite nanoparticles coated with γPGA have an antibacterial activity against S. aureus (25). Collectively, these data suggest that bacterial interference between S. aureus and S. epidermidis is related to AD progression, possibly due to their different responses to the antimicrobial activity of compounds produced by skin commensal bacteria (Fig. 2A).
Figure 2.
Roles of the microbiota and its metabolites in AD and FA. (A) S. aureus is known to be the main pathogen that induces AD. SEB and δ-toxin, secreted by S. aureus, induce degranulation of mast cells in the skin. Survival of S. aureus in the skin is selectively inhibited by antimicrobial peptide (e.g., hogocidin) derived from commensal bacteria including S. epidermidis and S. hominis. S. epidermidis-derived γPGA suppresses survival of S. aureus. S. epidermidis also produces SCFAs (butyrate and acetate), which suppress the colonization of S. aureus in the skin. In addition, H2-M3-restricted commensal-specific CD8+ T cells, induced by S. epidermidis-stimulated DCs, contribute to both anti-inflammatory and tissue repair functions. The skin Treg population can be increased by the resting LCs via TGFβ; these Tregs may be responsible for the suppression of mast cell degranulation. Skin-resident commensal S. epidermidis increases IFNγ production by dermal T cells. In addition, γPGA from the skin commensal bacteria can activate DCs to induce differentiation of Th1 and activation of IFNγ-producing cells such as NK, iNKT, and γδ T cells. Consequently, IFNγ derived from S. epidermidis-activated Th1 cells, γPGA-induced Th1, and IFNγ-producing cells may lead to the suppression of skin allergic effector cells (e.g., ILC2, basophils, eosinophil, Th2 cells, and Th9 cells). (B) Butyrate and acetate are produced from dietary fiber by commensal bacteria including Lactobacillus spp. and Bifidobacterium spp. Acetate suppresses TSLP and IL33 via epithelial GPR43 signaling, and butyrate triggers CD103+ DCs to produce retinoic acid via GPR109a signaling. Lactobacillus spp. stimulates macrophages to produce IL10 and IL6 in a TLR2/TLR6-dependent manner. Moreover, commensal bacteria including Lactobacillus spp. and Bifidobacterium spp. induce differentiation of naive CD4+ T cells into Foxp3+ Tregs via IDO, IL10, and TGFβ. γPGA from gut commensal bacteria can directly induce the generation of adaptive Foxp3+ Tregs from naive CD4+ T cells. Gut extracellular γPGA, derived from Bacillus spp., induces compositional change of microbiota, such as increase of Lactobacillus spp. Non-toxin-producing Clostridium spp., including Clostridium clusters XIVa, XIVb, and IV decrease intestinal permeability via increased IL22 production by ILC3s. Lactobacillus spp. (e.g., Lactobacillus casei) promotes RORγt+ Treg differentiation in the gut. Thus, skin Tregs, induced by commensal microbiota and its metabolites (γPGA and SCFAs), can participate in the inhibition of gut allergic effector cells (e.g., ILC2, mast cells, and Th2 cells). Note that blue arrows indicate induction or stimulation, red arrows represent secretion, and red flat lines indicate inhibition. Moreover, dotted arrows indicate decomposition or differentiation.
SEB, S. aureus exotoxin B; TSLPR, TSLP receptor.
S. epidermidis-stimulated dendritic cells (DCs) can induce commensal-specific CD8+ T cells in a non-classical MHC class I (H2-M3)-restricted manner, which leads to the expression of both anti-inflammatory and tissue-repair genes, thereby contributing to the skin-wound healing (26). Lipoteichoic acid (LTA) from S. epidermidis inhibits both inflammatory cytokine release from keratinocytes and inflammation triggered by injury, through a Toll-like receptor (TLR) 2-dependent mechanism (27). In contrast, cross-communication between skin microbiota and the TLR2-dependent production of P2X7 ligands, such as the extracellular production of adenosine triphosphate from inflammatory monocytes and macrophages, increases the activation of mast cells, thereby resulting in retinoid dermatitis (28). Exotoxins such as δ-Toxin and S. aureus exotoxin B, which are secondary metabolites secreted by bacteria, induce mast cell degranulation, resulting in skin allergies (29,30). Short-chain fatty acids (SCFAs) produced by Propionibacterium acnes inhibit histone deacetylase activity in keratinocytes, and suppress the immune tolerance of the epidermis to TLR ligands (31). However, the production of SCFAs (acetic, butyric, and propionic acid) by S. epidermidis displays beneficial effects, such as the inhibition of methicillin-resistant S. aureus colonization in skin wounds of mice (32). Therefore, the skin microbiota and its metabolic products interact with the host immune system to affect the development of AD (Fig. 2A).
Microbiota analysis in a well-characterized cohort of infants with FA shows that infants with IgE-mediated FA have increased numbers of Clostridium sensustricto and Anaerobacter, and decreased numbers of Bacteroides and Clostridium XVIII in feces. In particular, it is interesting that Clostridium sensustricto numbers are positively correlated with serum-specific IgE (33). In mice, a change in the microbial composition is also associated with the induction of FA (34). Clostridium clusters XIVa, XIVb, and IV have a beneficial effect on the function of the intestinal barrier, unlike the toxin-producing species such as Clostridium difficile, Clostridium botulinum, Clostridium tetani, and Clostridium perfringens. In particular, these beneficial Clostridium species induced IL22 production by RORγt+ ILC3s, resulting in increased levels of IL22, which regulates intestinal epithelial permeability and expression of antimicrobial peptides (e.g., Reg3b), and is thus, protective against FA (35). Protection against FA, under a high-fiber diet, is related to vitamin A metabolism and the products of dietary fiber fermentation by the gut microbiota (e.g., SCFAs) (36). Previous studies support the fact that the composition of the gut microbiota and its metabolites regulates intestinal immune responses to food allergens (Fig. 2B).
IMMUNOLOGICAL LINK BETWEEN AD AND FA
Individual candidates for AD with mutations in the gene encoding the skin barrier protein filaggrin (FLG) have elevated transepidermal water loss at birth and 2 months after birth (37). Importantly, individuals with FLG mutations present with a significant risk factor for IgE-mediated peanut allergy (2). Another study also showed that the incidence of IgE-mediated FA is significantly higher in children with moderate-severe AD, than in healthy children (38). Recently, a genome-wide association study showed that the risks of FA are involved with genes related to immunological regulation and epithelial barrier function (1). In mice, it has been shown that epidermal skin exposure to protein allergens breaks oral tolerance to peanuts, and selectively drives Th2-type immune responses (39). These data indicate that AD with epithelial barrier dysfunction is associated with the pathogenesis of FA. However, it is not yet clear whether the skin and gut are directly connected to IgE-mediated allergic responses. TSLP is considered to be one of the most crucial factors combining the involvement of the skin and gut in allergies. TSLP is mainly expressed in the skin, lung, and gut epithelial cells. Previous studies have suggested that TSLP expression by epithelial cells is a potent triggering factor not only for the pathogenesis of AD, but also for the progression from AD to asthma and FA (40,41,42). Furthermore, the TSLP-basophil axis has been proved to be necessary for the epicutaneous induction of gastrointestinal FA (43). Emerging pieces of evidence have shown that the Th9 and IL9 cytokine play central roles in the connection between AD and FA. IL9 is a common effector cytokine in the pathogenesis of both AD (44,45) and FA (46). It has been previously demonstrated that the frequency of Th9, Th2, and Th17 cells, but not Th1 cells, is significantly higher in peripheral blood mononuclear cells (PBMCs) from infants with AD, than in PBMCs from non-AD controls (38). Atopic infants also display substantially higher serum IL9 and TSLP levels than non-atopic patients (47). The innate cellular sources of IL9 are the mucosal mast cells in AD patients that concomitantly developed FA, and the induction of IL9-producing mast cells is a central step to acquire the susceptibility to IgE-mediated FA (46,48). Besides, gut-homing peanut-activated memory Th9 cells are significantly related to peanut allergies in children (49). T cell-derived IL9 mediates mast cell accumulation and activation in lung tissues in allergic inflammation (50). IL9, in synergy with stem cell factor, increases the development of human mast cells (51). Based on the above reports, it has been suggested that the accumulation of mast cells is induced via both autocrine and paracrine IL9 signals. IL9 stimulation causes the secretion of vascular endothelial growth factor (VEGF), but neither degranulation nor the release of proinflammatory cytokines in human mast cells (45). VEGF-mediated increase of vascular permeability in nasal mucosa occurs via a different pathway, and is stronger than its histamine-mediated counterpart (51). Furthermore, it has been demonstrated that mast cells modulate vascular permeability by the regulation of the VEGF pathway (52). Overall, the VEGF produced by IL9-activated mast cells appears to be involved with an increase in gut vascular permeability in FA.
Th1/Th2 REGULATION OF ALLERGIC DISEASES
Dysregulation of the IL12/IFNγ Th1 pathway is associated with a shift from balanced Th1/Th2 immune responses towards Th2 dominance in AD (53). Low IL12p40 gene expression and unresponsiveness to IL12 are correlated with the development of AD, suggesting that the administration of IL12 is one of the therapeutic strategies to treat AD (54). In humans, it has been reported that the impaired ability to produce IL12 in monocytes from patients with AD is involved with abnormal Th2 development in this disease condition (55). Adoptive transfer of CpG-stimulated DCs producing high levels of IL12 is enough to reduce both clinical symptoms and IL4 expression in AD model NC/Nga mice (56). Moreover, administration of poly (I:C) inhibits the development of AD in NC/Nga mice through the maintenance of the Th1/Th2 balance (57). It has been shown that IFNγ therapy is effective for reducing the pathogenesis of AD in mice (58) and humans (59). For example, in humans, oral tolerance induction by subcutaneous injection of IFNγ is effective for treating IgE-mediated FA (60). Another study suggests that effective therapy for FA using IFNγ appears to result from desensitization to food allergens (61). Tolerogenic effects of IFNγ on FA are partly related to the induction of allergen-specific IL10-producing regulatory B cells (Br1) (62). Although IFNγ has been used as an effective treatment for AD during the last few decades, the exact underlying mechanism of IFNγ as an AD inhibitor is not yet defined.
Previous studies have shown that IFNγ treatment elicits the apoptotic death of mast cells via a suppressor of cytokine signaling-1/signal transducer and activator of transcription (STAT) 1-dependent mechanism (63). IFNγ/TNFα stimulation promoted not only Fas/FasL-mediated apoptosis in human blood eosinophils, but also the differentiation of myeloid progenitors to eosinophils (64,65). Furthermore, an increase in IFNγ levels via STAT1 activation suppresses IL4 production by basophils (66). Both IFNγ and IFNβ act directly on ILC2s, decreasing their proliferation and consequently reducing the production of Th2-type cytokines, including IL4, IL5, IL9, and IL13 (67,68). An increase in IFNγ represses the activation of ILC2s and their function as Th2-type cytokine producers in response to IL33, implying that it acts as a counter-regulator against IL33 (69,70). It has been known that the Th9 and IL9 cytokine contribute to the pathogenesis of AD (44). IFNγ inhibits the differentiation of Th9 directly as well as indirectly, via the production of DC-derived IL27 (Fig. 2) (71).
EFFECT OF THE MICROBIOTA AND ITS METABOLITES ON Th1/Th2 BALANCE
Microbial metabolites can influence immune responses. It was reported that feeding 10-hydroxy-cis-12-octadecenoic acid (HYA), a new gut microbial metabolite of linoleic acid, to NC/Nga mice decreased the plasma IgE levels and skin infiltration of mast cells, with a concomitant decrease in the clinical dermatitis score (72). This treatment could change the microbial composition in mice, suggesting that alterations in the intestinal microbiota might be associated with the antiallergic effect of HYA.
High-fiber feeding increases the production of SCFAs, mainly acetate and butyrate, by commensal bacteria including Lactobacillus spp. and Bifidobacterium spp. In particular, acetate diminishes the epithelial output of pro-Th2 cytokines including TSLP and IL33, via epithelial GPR43 signaling. Additionally, butyrate triggers CD103+ DCs to produce retinoic acid via GPR109a signaling in the presence of vitamin A, consequently resulting in the protection against FA through increased Treg differentiation by DC-derived retinoic acid (36). Topical treatment of sodium butyrate suppresses hapten-induced skin inflammation via the induction of skin Tregs, suggesting that SCFAs produced by commensal skin bacteria exert suppressive effects on skin inflammation (73). Gut microbes may produce metabolites other than SCFAs, for example, γPGA during the fermentation of soybeans. γPGA is present predominantly in Bacillus subtilis. Oral administration of γPGA significantly increased natural killer (NK) cell-mediated antitumor activity, which was dependent on DC and TLR4 signaling (74). Furthermore, DCs activated by γPGA induced Th1, rather than Th2 cell differentiation, in naive CD4+ T cells (75). In addition to γPGA-stimulated Th1 cells, IFNγ can be produced by NK receptor-expressing DCs soon after γPGA treatment (76). Favoring of Th1 development by the γPGA/DC/IL12 axis suppressed the progression of Th2-mediated allergic asthma (77). Moreover, oral administration of γPGA actively prevents the development of AD-like symptoms in NC/Nga mice via the induction of Th1 responses, and γPGA-treated mice show increased IFNγ production by NK and γδ T cells compared to the control group (78).
IFNγ-producing cells are essential in controlling Th2-type innate immune cells (e.g., ILC2, basophils, mast cells, and eosinophils). Vα14-Jα18-expressing iNKT cells are innate-like T cells with regulatory functions to produce a variety of cytokines such as IFNγ very quickly upon stimulation, which meets the criteria as an immune regulator. iNKT cells activated by TSLP secrete high amounts of IL4 and IL13, but not IFNγ in patients with severe AD; these might play an essential role in the innate allergic immune response in AD (12). Moreover, iNKT2-derived IL4 contributed to Th2 dominance, and increased serum IgE levels (10,11), and iNKT cell-derived IL33 led to the activation of ILC2s (68). In contrast, iNKT cell-derived IFNγ suppresses IL5 and IL13 production by ILC2s (68), and also exerts inhibitory effects on both IL4 production and survival of basophils (79). It has been reported that the proportion of circulating NK cells and γδ T cells, and their IFNγ production was significantly reduced in AD patients (80). Collectively, it can be said that in AD and FA, Th1-type immune cells (NK cells, iNKT cells, and γδ T cells) have an antagonistic effect to Th2 response. In addition, B. subtilis-derived γPGA promotes Th1 differentiation through both DC-derived IL12p40 and NK cell-derived IFNγ production, in a TLR4-dependent manner (74). Recently, our group demonstrated that the repeated injection of γPGA reduced the abundance of basophils and their production of IL4 in mice. Furthermore, the γPGA-mediated depletion of basophils was dependent on the TLR4/DC/IL12 axis (79). Notably, the suppressive effect of γPGA on Th2 immune responses was mainly attributed to iNKT cells that produced IFNγ (Fig. 2) (79).
Expansion of memory Th1 cells by Th1-deriving adjuvant CpG is protective against invasive S. aureus infection (81). Skin-resident commensal S. epidermidis increases IFNγ production by dermal T cells in an IL1- and MyD88-dependent manner (82). Additionally, oral treatment with the probiotic strain Lactobacillus rhamnosus prevents the pathogenesis of AD by the induction of local IFNγ production in mice (83). In humans, the improvement of the condition in very young children with severe AD by probiotic treatment is associated with a significant increase in IFNγ production (84). The commensal microflora promotes gut-resident T lymphocytes to produce IFNγ, and consequently, these interactions induce IL7 production by intestinal epithelial cells (IECs) (85). Furthermore, DCs activated by γPGA induce the differentiation of Th1 cells, rather than Th2 cells, through the TLR4/IL12 axis (75). Oral treatment with γPGA efficiently decreases the levels of serum IgE and Th2 cytokines, consequently attenuating the clinical symptoms of AD via the Th1/Th2 balance (78). The regulation of Th1/Th2 balance by treatment with commensal bacteria and microbial metabolites could be a useful therapeutic strategy for treating AD and FA.
EFFECT OF THE MICROBIOTA AND ITS METABOLITES ON Treg/Th2 BALANCE
In healthy skin, resting epidermal LCs selectively give rise to the activation and proliferation of skin-resident Tregs, and their functions are dependent on antigen-presenting molecules (MHC II), co-stimulatory molecules (CD80 and CD86), and cytokines (IL2 and IL15) (86). Microbial colonization plays a vital role in regulating and fine-tuning the immune system throughout the lifespan of an individual (87). Altering the composition of the skin commensal microbiota in the neonatal period limits the migration of Tregs into skin, which mediates tolerance to bacterial antigens, resulting in skin inflammation (88). The presence of Escherichia coli is associated with a higher risk of developing eczema, and that of C. difficile, with increasing severity of AD, implying that the composition of gut microbiota is highly correlated with the development of skin inflammation (89). Supplementation with L. rhamnosus reduces the cumulative prevalence of eczema in humans (90). Moreover, a previous study showed that Lactobacillus probiotics are useful for the prevention, rather than treatment, of AD (52). Recently, Lactobacillus plantarum was also found to reduce the clinical index in children with AD, and its effect is associated with an increase in Treg population (91). In mice, a probiotic mixture including bifidobacteria and lactic acid bacteria attenuated AD via the generation of Tregs (92,93). Treg induction by probiotics is mediated by suppressive molecules such as IL10, transforming growth factor (TGF) β, indoleamine 2,3-dioxygenase (IDO), and cyclooxygenase 2 (Cox2) secreted from DCs (92). Although previous studies reveal that probiotics are useful for the therapy of AD and FA, the mechanisms by which probiotics induce regulatory effects are yet to be fully elucidated. Recent studies have demonstrated that immune regulatory pathways induced by lactic acid bacteria are dependent on TLR2/TLR6 (94); these bacteria induce Tregs through the suppression of mammalian target of rapamycin (mTOR) signaling, resulting in the amelioration of allergic responses (95). Recently, it has been revealed that RORγt+ Tregs, characterized as a new subpopulation of Tregs, can suppress Th2 responses and lead to an increase in IgE production (96,97). Oral administration of the probiotic strain Lactobacillus casei induces the expansion of intestine and systemic RORγt+ Tregs (98). Thus, it is of interest to see whether probiotics cause the expansion of Tregs in the skin. Previous studies provide evidence supporting the claim that oral treatment with probiotics enhances the abundance of skin Tregs, and consequently alleviates skin inflammation, implying that oral probiotics affect the Treg population in the skin (99). Long-term oral treatment with γPGA was previously shown to be significantly effective in treating AD. However, how the change in commensal bacterial composition is linked to the therapeutic effect of γPGA-based oral treatments in AD remains unclear. Jin et al. (100) recently demonstrated that oral administration of γPGA increased the abundance of Lactobacillus spp., while reducing the abundance of Clostridium spp. in the murine gut, suggesting that alteration of the gut microbial composition by γPGA treatment could affect the onset of AD through an increase in Treg population. Moreover, γPGA directly induces differentiation of naive CD4+ T cells into Foxp3+ Tregs, implying that oral administration of γPGA can enhance tolerance to food antigens by the regulation of Treg expansion (101). Collectively, the regulation of Treg/Th2 balance by treatment with commensal bacteria and microbial metabolites could be a promising therapeutic strategy against AD and FA (Fig. 2).
CONCLUSION
This article reviews the immune mechanisms of IgE-mediated allergic responses in the skin and gut, and the roles of the microbiota and its metabolites in the functioning of the host immune system. In particular, allergic immune responses can be categorized into 3 phases for therapeutic targeting. The early phase is initiated by the epithelia-derived pro-allergic cytokines IL33, TSLP, and IL25 upon exposure to allergens. Then, Th2-type innate immune cells (e.g., ILC2, basophils, mast cells, and eosinophils) are activated in response to pro-allergic cytokines, and these cells become the main cellular source of early IL4, which are responsible for inducing the differentiation of Th2 cells. Lastly, allergen-specific Th2 immune responses result in the generation of allergen-specific Th2 memory cells.
Moreover, microbes and their metabolites can act as immune modulators and ultimately play a critical role in modulating host immune responses. In the early phase, these metabolites effectively regulate the secretion of pro-allergic cytokines (e.g., TSLP and IL33) by epithelial cells by stimulating G-coupled protein receptors. Additionally, these can antagonize the release of IL4 by Th2-type innate immune cells by activating NK lineage cells (NK and iNKT cells) to produce IFNγ. Finally, these molecules can induce immune tolerance against allergens via allergen-specific Th1 and Treg induction.
Interestingly, it has been reported that glycolipid antigens with the agonistic ability to iNKT cells exist in intestinal commensal bacteria, and these antigens can regulate inflammatory responses in the liver, where iNKT cells are predominant (102). Although the roles of intestinal microbiota and its metabolites on iNKT cells, in the setting of AD and FA, are not yet well-understood, it is notable that γPGA, a B. subtilis-derived metabolite, can affect the activation of iNKT cells in an indirect way. In addition, it will be interesting to investigate whether any alteration in gut microbiota composition occurs upon γPGA treatment. Moreover, since the interaction between iNKT and Treg cells is quite well known (9), it will be of interest to further investigate how newly identified microbial metabolites can regulate the development of AD and FA, possibly via regulatory immune cells such as iNKT cells.
ACKNOWLEDGEMENTS
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1D1A1A09919293).
Abbreviations
- AD
atopic dermatitis
- DC
dendritic cell
- FA
food allergy
- IFN
interferon
- ILC2
group 2 innate lymphoid cell
- iNKT
invariant natural killer T
- LC
langerhans cell
- MHC
major histocompatibility complex
- NK
natural killer
- SCFA
short-chain fatty acid
- TGF
transforming growth factor
- Th
T helper
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- Treg
regulatory T cell
- TSLP
thymic stromal lymphopoietin
- VEGF
vascular endothelial growth factor
- γPGA
poly-γ-glutamic acid
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
Author Contributions: Conceptualization: Park HJ, Lee SW, Hong S; Data curation: Park HJ; Funding acquisition: Hong S; Writing - original draft: Park HJ, Lee SW, Hong S; Writing - review & editing: Lee SW, Hong S.
References
- 1.Marenholz I, Grosche S, Kalb B, Ruschendorf F, Blumchen K, Schlags R, Harandi N, Price M, Hansen G, Seidenberg J, et al. Genome-wide association study identifies the SERPINB gene cluster as a susceptibility locus for food allergy. Nat Commun. 2017;8:1056. doi: 10.1038/s41467-017-01220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Ginkel CD, Flokstra-de Blok BM, Kollen BJ, Kukler J, Koppelman GH, Dubois AE. Loss-of-function variants of the filaggrin gene are associated with clinical reactivity to foods. Allergy. 2015;70:461–464. doi: 10.1111/all.12569. [DOI] [PubMed] [Google Scholar]
- 3.Brandt EB, Sivaprasad U. Th2 cytokines and atopic dermatitis. J Clin Cell Immunol. 2011;2:110. doi: 10.4172/2155-9899.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imai Y, Yasuda K, Sakaguchi Y, Haneda T, Mizutani H, Yoshimoto T, Nakanishi K, Yamanishi K. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci USA. 2013;110:13921–13926. doi: 10.1073/pnas.1307321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seneschal J, Clark RA, Gehad A, Baecher-Allan CM, Kupper TS. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity. 2012;36:873–884. doi: 10.1016/j.immuni.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otsuka A, Nakajima S, Kubo M, Egawa G, Honda T, Kitoh A, Nomura T, Hanakawa S, Sagita Moniaga C, Kim B, et al. Basophils are required for the induction of Th2 immunity to haptens and peptide antigens. Nat Commun. 2013;4:1739. doi: 10.1038/ncomms2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim BS, Wang K, Siracusa MC, Saenz SA, Brestoff JR, Monticelli LA, Noti M, Tait Wojno ED, Fung TC, Kubo M, et al. Basophils promote innate lymphoid cell responses in inflamed skin. J Immunol. 2014;193:3717–3725. doi: 10.4049/jimmunol.1401307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, Mitchell AJ, Tay SS, Jain R, Forbes-Blom E, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.La Cava A, Van Kaer L, Shi FD. CD4+CD25+ Tregs and NKT cells: regulators regulating regulators. Trends Immunol. 2006;27:322–327. doi: 10.1016/j.it.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14:1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon DI, Lee YJ. Lineage differentiation program of invariant natural killer t cells. Immune Netw. 2017;17:365–377. doi: 10.4110/in.2017.17.6.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu WH, Park CO, Oh SH, Kim HJ, Kwon YS, Bae BG, Noh JY, Lee KH. Thymic stromal lymphopoietin-activated invariant natural killer T cells trigger an innate allergic immune response in atopic dermatitis. J Allergy Clin Immunol. 2010;126:290–299. 299.e1–299.e4. doi: 10.1016/j.jaci.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Frossard CP, Zimmerli SC, Rincon Garriz JM, Eigenmann PA. Food allergy in mice is modulated through the thymic stromal lymphopoietin pathway. Clin Transl Allergy. 2016;6:2. doi: 10.1186/s13601-016-0090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JB, Chen CY, Liu B, Mugge L, Angkasekwinai P, Facchinetti V, Dong C, Liu YJ, Rothenberg ME, Hogan SP, et al. IL-25 and CD4(+) TH2 cells enhance type 2 innate lymphoid cell-derived IL-13 production, which promotes IgE-mediated experimental food allergy. J Allergy Clin Immunol. 2016;137:1216–1225.e5. doi: 10.1016/j.jaci.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noval Rivas M, Burton OT, Wise P, Charbonnier LM, Georgiev P, Oettgen HC, Rachid R, Chatila TA. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity. 2015;42:512–523. doi: 10.1016/j.immuni.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, Viola A, Odom S, Rivera J, Colombo MP, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torgerson TR, Linane A, Moes N, Anover S, Mateo V, Rieux-Laucat F, Hermine O, Vijay S, Gambineri E, Cerf-Bensussan N, et al. Severe food allergy as a variant of IPEX syndrome caused by a deletion in a noncoding region of the FOXP3 gene. Gastroenterology. 2007;132:1705–1717. doi: 10.1053/j.gastro.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 18.Qamar N, Fishbein AB, Erickson KA, Cai M, Szychlinski C, Bryce PJ, Schleimer RP, Fuleihan RL, Singh AM. Naturally occurring tolerance acquisition to foods in previously allergic children is characterized by antigen specificity and associated with increased subsets of regulatory T cells. Clin Exp Allergy. 2015;45:1663–1672. doi: 10.1111/cea.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noval Rivas M, Burton OT, Oettgen HC, Chatila T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J Allergy Clin Immunol. 2016;138:801–811.e9. doi: 10.1016/j.jaci.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9:244–253. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong JQ, Lin L, Lin T, Hao F, Zeng FQ, Bi ZG, Yi D, Zhao B. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double-blind multicentre randomized controlled trial. Br J Dermatol. 2006;155:680–687. doi: 10.1111/j.1365-2133.2006.07410.x. [DOI] [PubMed] [Google Scholar]
- 22.Byrd AL, Deming C, Cassidy SK, Harrison OJ, Ng WI, Conlan S, Belkaid Y, Segre JA, Kong HH. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci Transl Med. 2017;9:eaal4651. doi: 10.1126/scitranslmed.aal4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, Shafiq F, Kotol PF, Bouslimani A, Melnik AV, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 2017;9:eaah4680. doi: 10.1126/scitranslmed.aah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kocianova S, Vuong C, Yao Y, Voyich JM, Fischer ER, DeLeo FR, Otto M. Key role of poly-gamma-DL-glutamic acid in immune evasion and virulence of Staphylococcus epidermidis . J Clin Invest. 2005;115:688–694. doi: 10.1172/JCI23523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inbaraj BS, Kao TH, Tsai TY, Chiu CP, Kumar R, Chen BH. The synthesis and characterization of poly(gamma-glutamic acid)-coated magnetite nanoparticles and their effects on antibacterial activity and cytotoxicity. Nanotechnology. 2011;22:075101. doi: 10.1088/0957-4484/22/7/075101. [DOI] [PubMed] [Google Scholar]
- 26.Linehan JL, Harrison OJ, Han SJ, Byrd AL, Vujkovic-Cvijin I, Villarino AV, Sen SK, Shaik J, Smelkinson M, Tamoutounour S, et al. Non-classical immunity controls microbiota impact on skin immunity and tissue repair. Cell. 2018;172:784–796.e18. doi: 10.1016/j.cell.2017.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, Wu ZR, Hooper LV, Schmidt RR, von Aulock S, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med. 2009;15:1377–1382. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurashima Y, Amiya T, Fujisawa K, Shibata N, Suzuki Y, Kogure Y, Hashimoto E, Otsuka A, Kabashima K, Sato S, et al. The enzyme Cyp26b1 mediates inhibition of mast cell activation by fibroblasts to maintain skin-barrier homeostasis. Immunity. 2014;40:530–541. doi: 10.1016/j.immuni.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Munoz-Planillo R, Hasegawa M, Villaruz AE, Cheung GY, McGavin MJ, Travers JB, et al. Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature. 2013;503:397–401. doi: 10.1038/nature12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ando T, Matsumoto K, Namiranian S, Yamashita H, Glatthorn H, Kimura M, Dolan BR, Lee JJ, Galli SJ, Kawakami Y, et al. Mast cells are required for full expression of allergen/SEB-induced skin inflammation. J Invest Dermatol. 2013;133:2695–2705. doi: 10.1038/jid.2013.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanford JA, Zhang LJ, Williams MR, Gangoiti JA, Huang CM, Gallo RL. Inhibition of HDAC8 and HDAC9 by microbial short-chain fatty acids breaks immune tolerance of the epidermis to TLR ligands. Sci Immunol. 2016;1:eaah4609. doi: 10.1126/sciimmunol.aah4609. [DOI] [PubMed] [Google Scholar]
- 32.Kao MS, Huang S, Chang WL, Hsieh MF, Huang CJ, Gallo RL, Huang CM. Microbiome precision editing: Using PEG as a selective fermentation initiator against methicillin-resistant Staphylococcus aureus . Biotechnol J. 2017;12:1600399. doi: 10.1002/biot.201600399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ling Z, Li Z, Liu X, Cheng Y, Luo Y, Tong X, Yuan L, Wang Y, Sun J, Li L, et al. Altered fecal microbiota composition associated with food allergy in infants. Appl Environ Microbiol. 2014;80:2546–2554. doi: 10.1128/AEM.00003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diesner SC, Bergmayr C, Pfitzner B, Assmann V, Krishnamurthy D, Starkl P, Endesfelder D, Rothballer M, Welzl G, Rattei T, et al. A distinct microbiota composition is associated with protection from food allergy in an oral mouse immunization model. Clin Immunol. 2016;173:10–18. doi: 10.1016/j.clim.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK, Tjota MY, Seo GY, Cao S, Theriault BR, et al. Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci USA. 2014;111:13145–13150. doi: 10.1073/pnas.1412008111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan J, McKenzie C, Vuillermin PJ, Goverse G, Vinuesa CG, Mebius RE, Macia L, Mackay CR. Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Reports. 2016;15:2809–2824. doi: 10.1016/j.celrep.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 37.Kelleher M, Dunn-Galvin A, Hourihane JO, Murray D, Campbell LE, McLean WH, Irvine AD. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol. 2015;135:930–935.e1. doi: 10.1016/j.jaci.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BA, Sampson HA. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101:E8. doi: 10.1542/peds.101.3.e8. [DOI] [PubMed] [Google Scholar]
- 39.Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Epicutaneous exposure to peanut protein prevents oral tolerance and enhances allergic sensitization. Clin Exp Allergy. 2005;35:757–766. doi: 10.1111/j.1365-2222.2005.02260.x. [DOI] [PubMed] [Google Scholar]
- 40.Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM, Bautista DM. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han H, Xu W, Headley MB, Jessup HK, Lee KS, Omori M, Comeau MR, Marshak-Rothstein A, Ziegler SF. Thymic stromal lymphopoietin (TSLP)-mediated dermal inflammation aggravates experimental asthma. Mucosal Immunol. 2012;5:342–351. doi: 10.1038/mi.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han H, Thelen TD, Comeau MR, Ziegler SF. Thymic stromal lymphopoietin-mediated epicutaneous inflammation promotes acute diarrhea and anaphylaxis. J Clin Invest. 2014;124:5442–5452. doi: 10.1172/JCI77798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noti M, Kim BS, Siracusa MC, Rak GD, Kubo M, Moghaddam AE, Sattentau QA, Comeau MR, Spergel JM, Artis D. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis. J Allergy Clin Immunol. 2014;133:1390–1399. 1399.e1–1399.e6. doi: 10.1016/j.jaci.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma L, Xue HB, Guan XH, Shu CM, Zhang JH, Yu J. Possible pathogenic role of T helper type 9 cells and interleukin (IL)-9 in atopic dermatitis. Clin Exp Immunol. 2014;175:25–31. doi: 10.1111/cei.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sismanopoulos N, Delivanis DA, Alysandratos KD, Angelidou A, Vasiadi M, Therianou A, Theoharides TC. IL-9 induces VEGF secretion from human mast cells and IL-9/IL-9 receptor genes are overexpressed in atopic dermatitis. PLoS One. 2012;7:e33271. doi: 10.1371/journal.pone.0033271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen CY, Lee JB, Liu B, Ohta S, Wang PY, Kartashov AV, Mugge L, Abonia JP, Barski A, Izuhara K, et al. Induction of interleukin-9-producing mucosal mast cells promotes susceptibility to IgE-mediated experimental food allergy. Immunity. 2015;43:788–802. doi: 10.1016/j.immuni.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao W, Zhang Y, Jabeen R, Nguyen ET, Wilkes DS, Tepper RS, Kaplan MH, Zhou B. Interleukin-9 is required for allergic airway inflammation mediated by the cytokine TSLP. Immunity. 2013;38:360–372. doi: 10.1016/j.immuni.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JB. Regulation of IgE-mediated food allergy by IL-9 producing mucosal mast cells and type 2 innate lymphoid cells. Immune Netw. 2016;16:211–218. doi: 10.4110/in.2016.16.4.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brough HA, Cousins DJ, Munteanu A, Wong YF, Sudra A, Makinson K, Stephens AC, Arno M, Ciortuz L, Lack G, et al. IL-9 is a key component of memory TH cell peanut-specific responses from children with peanut allergy. J Allergy Clin Immunol. 2014;134:1329–1338.e10. doi: 10.1016/j.jaci.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 50.Sehra S, Yao W, Nguyen ET, Glosson-Byers NL, Akhtar N, Zhou B, Kaplan MH. TH9 cells are required for tissue mast cell accumulation during allergic inflammation. J Allergy Clin Immunol. 2015;136:433–440.e1. doi: 10.1016/j.jaci.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsune S, Ohori J, Yoshifuku K, Kurono Y. Effect of vascular endothelial growth factor on nasal vascular permeability. Laryngoscope. 2010;120:844–848. doi: 10.1002/lary.20586. [DOI] [PubMed] [Google Scholar]
- 52.Lee J, Seto D, Bielory L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol. 2008;121:116–121.e11. doi: 10.1016/j.jaci.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi N, Akahoshi M, Matsuda A, Ebe K, Inomata N, Obara K, Hirota T, Nakashima K, Shimizu M, Tamari M, et al. Association of the IL12RB1 promoter polymorphisms with increased risk of atopic dermatitis and other allergic phenotypes. Hum Mol Genet. 2005;14:3149–3159. doi: 10.1093/hmg/ddi347. [DOI] [PubMed] [Google Scholar]
- 54.Namkung JH, Lee JE, Kim E, Kim S, Shin ES, Cho EY, Yang JM. Association of single nucleotide polymorphisms in the IL-12 (IL-12A and B) and IL-12 receptor (IL-12Rbeta1 and beta2) genes and gene-gene interactions with atopic dermatitis in Koreans. J Dermatol Sci. 2010;57:199–206. doi: 10.1016/j.jdermsci.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Itazawa T, Adachi Y, Okabe Y, Hamamichi M, Adachi YS, Toyoda M, Morohashi M, Miyawaki T. Developmental changes in interleukin-12-producing ability by monocytes and their relevance to allergic diseases. Clin Exp Allergy. 2003;33:525–530. doi: 10.1046/j.1365-2222.2003.01608.x. [DOI] [PubMed] [Google Scholar]
- 56.Park ST, Kim KE, Na K, Kim Y, Kim TY. Effect of dendritic cells treated with CpG ODN on atopic dermatitis of Nc/Nga mice. J Biochem Mol Biol. 2007;40:486–493. doi: 10.5483/bmbrep.2007.40.4.486. [DOI] [PubMed] [Google Scholar]
- 57.Kim CH, Park CD, Lee AY. Administration of poly(I:C) improved dermatophagoides farinae-induced atopic dermatitis-like skin lesions in NC/Nga mice by the regulation of Th1/Th2 balance. Vaccine. 2012;30:2405–2410. doi: 10.1016/j.vaccine.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 58.Hattori K, Nishikawa M, Watcharanurak K, Ikoma A, Kabashima K, Toyota H, Takahashi Y, Takahashi R, Watanabe Y, Takakura Y. Sustained exogenous expression of therapeutic levels of IFN-gamma ameliorates atopic dermatitis in NC/Nga mice via Th1 polarization. J Immunol. 2010;184:2729–2735. doi: 10.4049/jimmunol.0900215. [DOI] [PubMed] [Google Scholar]
- 59.Panahi Y, Davoudi SM, Madanchi N, Abolhasani E. Recombinant human interferon gamma (Gamma Immunex) in treatment of atopic dermatitis. Clin Exp Med. 2012;12:241–245. doi: 10.1007/s10238-011-0164-3. [DOI] [PubMed] [Google Scholar]
- 60.Lee JH, Noh G, Noh J, Lee S, Choi WS, Kim HS, Lee K, Choi S, Jin H, Cho S. Clinical characteristics of oral tolerance induction of IgE-mediated and non-IgE-mediated food allergy using interferon gamma. Allergy Asthma Proc. 2010;31:e39–e47. doi: 10.2500/aap.2010.31.3345. [DOI] [PubMed] [Google Scholar]
- 61.Noh G, Jang EH. Dual specific oral tolerance induction using interferon gamma for IgE-mediated anaphylactic food allergy and the dissociation of local skin allergy and systemic oral allergy: tolerance or desensitization? J Investig Allergol Clin Immunol. 2014;24:87–97. [PubMed] [Google Scholar]
- 62.Noh J, Noh G, Lee SJ, Lee JH, Kim A, Kim HS, Choi WS. Tolerogenic effects of interferon-gamma with induction of allergen-specific interleukin-10-producing regulatory B cell (Br1) changes in non-IgE-mediated food allergy. Cell Immunol. 2012;273:140–149. doi: 10.1016/j.cellimm.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 63.Mann-Chandler MN, Kashyap M, Wright HV, Norozian F, Barnstein BO, Gingras S, Parganas E, Ryan JJ. IFN-gamma induces apoptosis in developing mast cells. J Immunol. 2005;175:3000–3005. doi: 10.4049/jimmunol.175.5.3000. [DOI] [PubMed] [Google Scholar]
- 64.Luttmann W, Dauer E, Schmidt S, Marx O, Hossfeld M, Matthys H, Virchow JC., Jr Effects of interferon-gamma and tumour necrosis factor-alpha on CD95/Fas ligand-mediated apoptosis in human blood eosinophils. Scand J Immunol. 2000;51:54–59. doi: 10.1046/j.1365-3083.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 65.de Bruin AM, Buitenhuis M, van der Sluijs KF, van Gisbergen KP, Boon L, Nolte MA. Eosinophil differentiation in the bone marrow is inhibited by T cell-derived IFN-gamma. Blood. 2010;116:2559–2569. doi: 10.1182/blood-2009-12-261339. [DOI] [PubMed] [Google Scholar]
- 66.Moore ML, Newcomb DC, Parekh VV, Van Kaer L, Collins RD, Zhou W, Goleniewska K, Chi MH, Mitchell D, Boyce JA, et al. STAT1 negatively regulates lung basophil IL-4 expression induced by respiratory syncytial virus infection. J Immunol. 2009;183:2016–2026. doi: 10.4049/jimmunol.0803167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duerr CU, McCarthy CD, Mindt BC, Rubio M, Meli AP, Pothlichet J, Eva MM, Gauchat JF, Qureshi ST, Mazer BD, et al. Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat Immunol. 2016;17:65–75. doi: 10.1038/ni.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kudo F, Ikutani M, Seki Y, Otsubo T, Kawamura YI, Dohi T, Oshima K, Hattori M, Nakae S, Takatsu K, et al. Interferon-gamma constrains cytokine production of group 2 innate lymphoid cells. Immunology. 2016;147:21–29. doi: 10.1111/imm.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Molofsky AB, Van Gool F, Liang HE, Van Dyken SJ, Nussbaum JC, Lee J, Bluestone JA, Locksley RM. Interleukin-33 and interferon-gamma counter-regulate group 2 innate lymphoid cell activation during immune perturbation. Immunity. 2015;43:161–174. doi: 10.1016/j.immuni.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han M, Hong JY, Jaipalli S, Rajput C, Lei J, Hinde JL, Chen Q, Hershenson NM, Bentley JK, Hershenson MB. IFN-gamma blocks development of an asthma phenotype in rhinovirus-infected baby mice by inhibiting type 2 innate lymphoid cells. Am J Respir Cell Mol Biol. 2017;56:242–251. doi: 10.1165/rcmb.2016-0056OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murugaiyan G, Beynon V, Pires Da Cunha A, Joller N, Weiner HL. IFN-gamma limits Th9-mediated autoimmune inflammation through dendritic cell modulation of IL-27. J Immunol. 2012;189:5277–5283. doi: 10.4049/jimmunol.1200808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaikiri H, Miyamoto J, Kawakami T, Park SB, Kitamura N, Kishino S, Yonejima Y, Hisa K, Watanabe J, Ogita T, et al. Supplemental feeding of a gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, alleviates spontaneous atopic dermatitis and modulates intestinal microbiota in NC/nga mice. Int J Food Sci Nutr. 2017;68:941–951. doi: 10.1080/09637486.2017.1318116. [DOI] [PubMed] [Google Scholar]
- 73.Schwarz A, Bruhs A, Schwarz T. The short-chain fatty acid sodium butyrate functions as a regulator of the skin immune system. J Invest Dermatol. 2017;137:855–864. doi: 10.1016/j.jid.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 74.Lee TY, Kim YH, Yoon SW, Choi JC, Yang JM, Kim CJ, Schiller JT, Sung MH, Poo H. Oral administration of poly-gamma-glutamate induces TLR4- and dendritic cell-dependent antitumor effect. Cancer Immunol Immunother. 2009;58:1781–1794. doi: 10.1007/s00262-009-0689-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim S, Yang JY, Lee K, Oh KH, Gi M, Kim JM, Paik DJ, Hong S, Youn J. Bacillus subtilis-specific poly-gamma-glutamic acid regulates development pathways of naive CD4(+) T cells through antigen-presenting cell-dependent and -independent mechanisms. Int Immunol. 2009;21:977–990. doi: 10.1093/intimm/dxp065. [DOI] [PubMed] [Google Scholar]
- 76.Lee SW, Park HJ, Park SH, Kim N, Hong S. Immunomodulatory effect of poly-gamma-glutamic acid derived from Bacillus subtilis on natural killer dendritic cells. Biochem Biophys Res Commun. 2014;443:413–421. doi: 10.1016/j.bbrc.2013.11.097. [DOI] [PubMed] [Google Scholar]
- 77.Lee K, Kim SH, Yoon HJ, Paik DJ, Kim JM, Youn J. Bacillus-derived poly-gamma-glutamic acid attenuates allergic airway inflammation through a Toll-like receptor-4-dependent pathway in a murine model of asthma. Clin Exp Allergy. 2011;41:1143–1156. doi: 10.1111/j.1365-2222.2011.03792.x. [DOI] [PubMed] [Google Scholar]
- 78.Lee SW, Park HJ, Park SH, Hong S. Oral administration of poly-gamma-glutamic acid prevents the development of atopic dermatitis in NC/Nga mice. Exp Dermatol. 2013;22:561–563. doi: 10.1111/exd.12198. [DOI] [PubMed] [Google Scholar]
- 79.Park HJ, Lee SW, Park SH, Hong S. iNKT cells are responsible for the apoptotic reduction of basophils that mediate Th2 immune responses elicited by papain in mice following gammaPGA stimulation. PLoS One. 2016;11:e0152189. doi: 10.1371/journal.pone.0152189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Katsuta M, Takigawa Y, Kimishima M, Inaoka M, Takahashi R, Shiohara T. NK cells and gamma delta+ T cells are phenotypically and functionally defective due to preferential apoptosis in patients with atopic dermatitis. J Immunol. 2006;176:7736–7744. doi: 10.4049/jimmunol.176.12.7736. [DOI] [PubMed] [Google Scholar]
- 81.Brown AF, Murphy AG, Lalor SJ, Leech JM, O'Keeffe KM, Mac Aogain M, O'Halloran DP, Lacey KA, Tavakol M, Hearnden CH, et al. Memory Th1 cells are protective in invasive Staphylococcus aureus infection. PLoS Pathog. 2015;11:e1005226. doi: 10.1371/journal.ppat.1005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tanaka A, Jung K, Benyacoub J, Prioult G, Okamoto N, Ohmori K, Blum S, Mercenier A, Matsuda H. Oral supplementation with Lactobacillus rhamnosus CGMCC 1.3724 prevents development of atopic dermatitis in NC/NgaTnd mice possibly by modulating local production of IFN-gamma. Exp Dermatol. 2009;18:1022–1027. doi: 10.1111/j.1600-0625.2009.00895.x. [DOI] [PubMed] [Google Scholar]
- 84.Prescott SL, Dunstan JA, Hale J, Breckler L, Lehmann H, Weston S, Richmond P. Clinical effects of probiotics are associated with increased interferon-gamma responses in very young children with atopic dermatitis. Clin Exp Allergy. 2005;35:1557–1564. doi: 10.1111/j.1365-2222.2005.02376.x. [DOI] [PubMed] [Google Scholar]
- 85.Shalapour S, Deiser K, Sercan O, Tuckermann J, Minnich K, Willimsky G, Blankenstein T, Hammerling GJ, Arnold B, Schuler T. Commensal microflora and interferon-gamma promote steady-state interleukin-7 production in vivo . Eur J Immunol. 2010;40:2391–2400. doi: 10.1002/eji.201040441. [DOI] [PubMed] [Google Scholar]
- 86.Nakajima S, Igyártó BZ, Honda T, Egawa G, Otsuka A, Hara-Chikuma M, Watanabe N, Ziegler SF, Tomura M, Inaba K, et al. Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling. J Allergy Clin Immunol. 2012;129:1048–1055.e6. doi: 10.1016/j.jaci.2012.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martin R, Nauta AJ, Ben Amor K, Knippels LM, Knol J, Garssen J. Early life: gut microbiota and immune development in infancy. Benef Microbes. 2010;1:367–382. doi: 10.3920/BM2010.0027. [DOI] [PubMed] [Google Scholar]
- 88.Scharschmidt TC, Vasquez KS, Truong HA, Gearty SV, Pauli ML, Nosbaum A, Gratz IK, Otto M, Moon JJ, Liese J, et al. A wave of regulatory t cells into neonatal skin mediates tolerance to commensal microbes. Immunity. 2015;43:1011–1021. doi: 10.1016/j.immuni.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, Adams H, van Ree R, Stobberingh EE. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007;56:661–667. doi: 10.1136/gut.2006.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wickens K, Black PN, Stanley TV, Mitchell E, Fitzharris P, Tannock GW, Purdie G, Crane J. A differential effect of 2 probiotics in the prevention of eczema and atopy: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2008;122:788–794. doi: 10.1016/j.jaci.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 91.Prakoeswa CR, Herwanto N, Prameswari R, Astari L, Sawitri S, Hidayati AN, Indramaya DM, Kusumowidagdo ER, Surono IS. Lactobacillus plantarum IS-10506 supplementation reduced SCORAD in children with atopic dermatitis. Benef Microbes. 2017;8:833–840. doi: 10.3920/BM2017.0011. [DOI] [PubMed] [Google Scholar]
- 92.Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, Nam JH, Rhee JH, Hwang KC, Im SH. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci USA. 2010;107:2159–2164. doi: 10.1073/pnas.0904055107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shin JH, Chung MJ, Seo JG. A multistrain probiotic formulation attenuates skin symptoms of atopic dermatitis in a mouse model through the generation of CD4(+)Foxp3(+) T cells. Food Nutr Res. 2016;60:32550. doi: 10.3402/fnr.v60.32550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ren C, Zhang Q, de Haan BJ, Zhang H, Faas MM, de Vos P. Identification of TLR2/TLR6 signalling lactic acid bacteria for supporting immune regulation. Sci Rep. 2016;6:34561. doi: 10.1038/srep34561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fu L, Peng J, Zhao S, Zhang Y, Su X, Wang Y. Lactic acid bacteria-specific induction of CD4(+)Foxp3(+)T cells ameliorates shrimp tropomyosin-induced allergic response in mice via suppression of mTOR signaling. Sci Rep. 2017;7:1987. doi: 10.1038/s41598-017-02260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kluger MA, Nosko A, Ramcke T, Goerke B, Meyer MC, Wegscheid C, Luig M, Tiegs G, Stahl RA, Steinmetz OM. RORgammat expression in Tregs promotes systemic lupus erythematosus via IL-17 secretion, alteration of Treg phenotype and suppression of Th2 responses. Clin Exp Immunol. 2017;188:63–78. doi: 10.1111/cei.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jung MK, Kwak JE, Shin EC. IL-17A-producing Foxp3(+) regulatory T cells and human diseases. Immune Netw. 2017;17:276–286. doi: 10.4110/in.2017.17.5.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cortes-Perez NG, Lozano-Ojalvo D, Maiga MA, Hazebrouck S, Adel-Patient K. Intragastric administration of Lactobacillus casei BL23 induces regulatory FoxP3+RORgammat+ T cells subset in mice. Benef Microbes. 2017;8:433–438. doi: 10.3920/BM2016.0174. [DOI] [PubMed] [Google Scholar]
- 99.Hacini-Rachinel F, Gheit H, Le Luduec JB, Dif F, Nancey S, Kaiserlian D. Oral probiotic control skin inflammation by acting on both effector and regulatory T cells. PLoS One. 2009;4:e4903. doi: 10.1371/journal.pone.0004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jin HE, Choi JC, Lim YT, Sung MH. Prebiotic effects of poly-gamma-glutamate on bacterial flora in murine gut. J Microbiol Biotechnol. 2017;27:412–415. doi: 10.4014/jmb.1611.11023. [DOI] [PubMed] [Google Scholar]
- 101.Lee K, Hwang S, Paik DJ, Kim WK, Kim JM, Youn J. Bacillus-derived poly-gamma-glutamic acid reciprocally regulates the differentiation of T helper 17 and regulatory T cells and attenuates experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2012;170:66–76. doi: 10.1111/j.1365-2249.2012.04637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wei Y, Zeng B, Chen J, Cui G, Lu C, Wu W, Yang J, Wei H, Xue R, Bai L, et al. Enterogenous bacterial glycolipids are required for the generation of natural killer T cells mediated liver injury. Sci Rep. 2016;6:36365. doi: 10.1038/srep36365. [DOI] [PMC free article] [PubMed] [Google Scholar]