Abstract
Chemokine (C-X3-C motif) ligand 1 (CX3CL1, also known as fractalkine) and its receptor chemokine (C-X3-C motif) receptor 1 (CX3CR1) are widely expressed in immune cells and non-immune cells throughout organisms. However, their expression is mostly cell type-specific in each tissue. CX3CR1 expression can be found in monocytes, macrophages, dendritic cells, T cells, and natural killer (NK) cells. Interaction between CX3CL1 and CX3CR1 can mediate chemotaxis of immune cells according to concentration gradient of ligands. CX3CR1 expressing immune cells have a main role in either pro-inflammatory or anti-inflammatory response depending on environmental condition. In a given tissue such as bone marrow, brain, lung, liver, gut, and cancer, CX3CR1 expressing cells can maintain tissue homeostasis. Under pathologic conditions, however, CX3CR1 expressing cells can play a critical role in disease pathogenesis. Here, we discuss recent progresses of CX3CL1/CX3CR1 in major tissues and their relationships with human diseases.
Keywords: CX3CR1, CX3CL1, Tissue specificity, Monocytes, Macrophages
CHEMOKINE (C-X3-C MOTIF) LIGAND 1 (CX3CL1) AND CHEMOKINE (C-X3-C MOTIF) RECEPTOR 1 (CX3CR1)
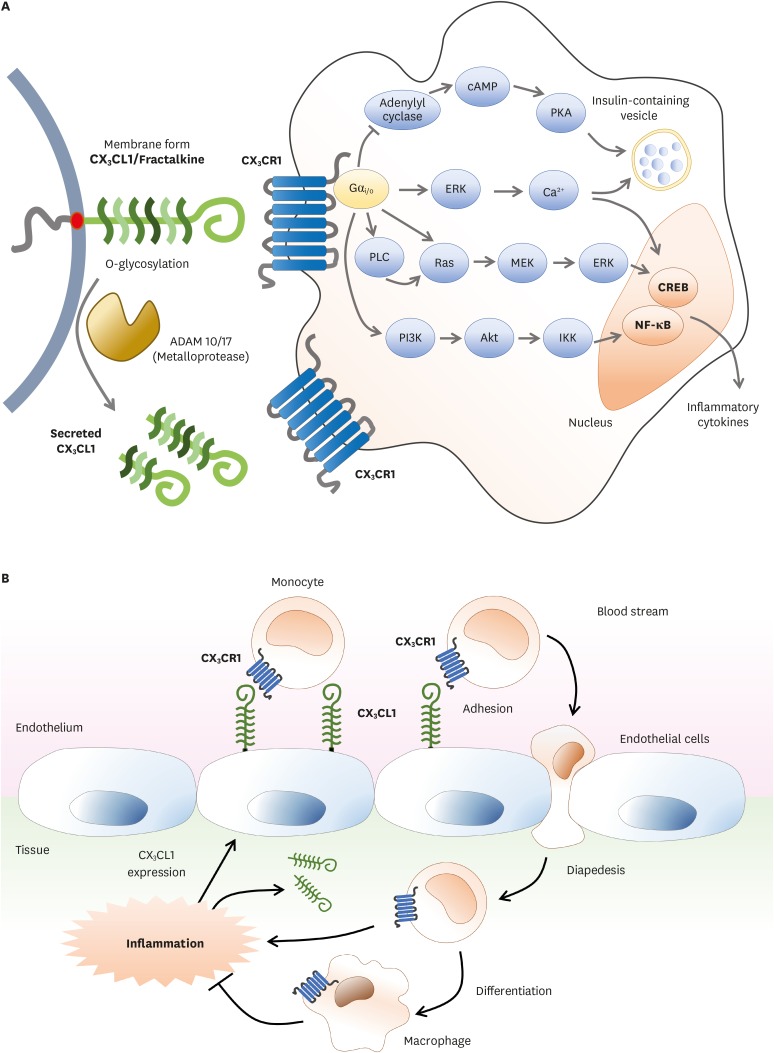
Immune cells including monocytes in our body are circulating through blood flow or lymph vessels. When inflammation occurs, these immune cells need to be recruited into inflamed tissue to alleviate the inflamed condition. At this moment, immune cells can move to the inflamed site through chemotaxis by chemokines and their receptors expressed by cells. CX3CR1 is a member of seven-transmembrane G-protein coupled receptor (GPCR). It is a receptor for its sole ligand, CX3CL1, also known as fractalkine or neurotactin (Fig. 1A) (1). CX3CR1 and its function in immune cells have been extensively studied for over 20 years. CX3CL1 is a transmembrane protein with an extended and highly glycosylated mucin-like stem (2). Proteolytic cleavage by metalloprotease ADAM10 generates various soluble forms of CX3CL1 (3). Under inflammation, CX3CL1 shedding is promoted by ADAM17 (4). Immune cells that express CX3CR1 include monocyte, macrophage, microglia, T helper (Th) 1, CD8+ T effector/memory cell, NK cell, γδ T cell, and dendritic cell (DC). Both CX3CL1 and CX3CR1 are expressed throughout the body. However, their expression is highly cell type-specific depending on organs and tissues. For instance, CX3CR1 in the brain is mostly expressed on microglia. In gut and blood, CX3CR1 expression is restricted to macrophages and monocytes, respectively (5,6). The expression of ligand CX3CL1 has been found in neurons, intestinal epithelium, and inflamed endothelium. The major role of CX3CR1 in immune cells is to recognize and enter inflamed tissue according to CX3CL1 gradient to initiate innate immune system (Fig. 1B) (7). This step is also important for continuing immune response through type 1 adaptive immunity. Thus, interaction between CX3CL1 and CX3CR1 is one initial step in host defense for monocyte crawling or “patrolling” in the lumen of blood vessels.
Figure 1.
CX3CR1-CX3CL1 signaling pathway and migration process of immune cells via their interactions. (A) CX3CL1/fractalkine, a transmembrane protein with O-glycosylated mucin-like stem, is expressed on the surface of immune cells and non-immune cells. Soluble forms of CX3CL1 can be made after proteolytic cleavage by metalloprotease ADAM 10/17. Both membrane bound and soluble forms of CX3CL1 can bind to CX3CR1. CX3CR1 is seven-transmembrane G-protein coupled receptor. The complex of CX3CL1-CX3CR1 can activate NF-κB or CREB signaling pathway which promotes the secretion of inflammatory cytokines. (B) CX3CL1 is expressed on the surface of endothelial cells near inflamed tissues. CX3CL1 induces chemotaxis to promote recruitment of CX3CR1 expressing immune cells. Monocytes flowing through blood stream will encounter and recognize CX3CL1 on the endothelium near inflamed tissues. As CX3CL1-CX3CR1 complex is formed, monocytes are ready to enter inflamed tissues and then enter diapedesis between junctions of endothelial cells. In this process, monocytes will undergo maturation and differentiate into macrophages. Mature macrophages can relieve inflamed conditions.
cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; PLC, phospholipase C; MEK, methyl ethyl ketone; PI3K, phosphoinositide 3-kinase; IKK, IκB kinase; CREB, cAMP response element binding protein.
CX3CR1 expressing immune cells play significant roles in pathological diseases at specific tissues in human. This implies that CX3CR1 expressing immune cells have tissue-specific roles. In the brain, CX3CL1/CX3CR1 signaling can modulate the production of cytokines by microglia cells (8). It has been reported that CX3CL1/CX3CR1 signaling is associated with Alzheimer's disease (9). In the liver, CX3CR1 is expressed in monocytes, CD8+ T cells, and natural killer (NK) cells. Besides immune cells, it is highly expressed in regenerated epithelial cells in bile duct-like structures as well as inflammatory sites (2). It has been verified that CX3CR1 is mainly expressed on lamina propria macrophage in the gut and on circulating monocyte in blood (5). In this review, we will focus on tissue-specific role of CX3CR1 expressing immune cells and their relationships with human diseases.
BONE MARROW AND IMMUNE SYSTEM
Level of CX3CR1 expressed on monocytes is increased with maturation in bone marrow. It is inversely correlated with Ly6C marker and CCR2 in the blood (10). CX3CR1 can reduce the motility of Ly6Chigh monocytes in the bone marrow, thereby controlling their release. CX3CR1-CX3CL1 axis plays a role in the differentiation of both osteoblasts and osteoclasts (11). In patients with idiopathic thrombocytopenic purpura, CD8+ T cells inhibit megakaryocyte apoptosis, leading to impaired platelet production (12). CX3CR1 expression in CD8+ T cells have been increased in bone marrow. This might be related to T cell recruitment from peripheral blood (13). Recruited CX3CR1+CD8+ T cells can inhibit megakaryocyte apoptosis and reduce low ploidy megakaryocytes. In human immunodeficiency virus-infected patients, expression of protease-activated receptor 1 (PAR-1) is increased in CX3CR1+CD8+ T cells which can promote inflammatory response (14). In addition, CX3CR1+CD8+ T cells can migrate to endothelial cells by CX3CR1 signaling. This might be associated with cardiovascular diseases (15).
It has been reported that CX3CR1+ B cells are increased in people with food allergies (16). CX3CR1+ B cells have transforming growth factor (TGF)-β and integrin αvβ6 that can suppress CD4+ T cell activity. Neonatal regulatory B lymphocytes (nBreg cells) can react with protein F of respiratory syncytial virus to upregulate CX3CR1 which can then react with glycoprotein G, resulting in viral infection (16). These interactions could make nBreg cells induce IL-10 production, resulting in weakened cytokine production of Th1 cells (17).
CX3CR1 can mediate the maintenance of killer cell lectin-like receptor subfamily G member 1 (KLRG1)+ NK cells into bone marrow (18). KLRG1 receptors are expressed in the late stage of T cell and NK cell differentiation. CX3CR1+KLRG1+ NK cells are localized in sinusoid of bone marrow while CX3CR1-deficient NK cells are predominantly found in the parenchyma (18). CX3CR1+KLRG1+ NK cells are accumulated in the bone marrow during poly(I:C)-induced hepatitis inflammation (19). CX3CR1 expression on NK cells mediates their migration to the central nervous system (CNS) from the periphery (20).
In the sera and synovial fluids of patients with rheumatoid arthritis (RA), concentration of CX3CL1 is higher compared with other types of arthritis (21). CX3CL1 can be expressed by synovial tissue macrophages, DC, fibroblast-like synoviocytes as well as by vessel endothelial cells. Further, inflammatory cytokines such as tumor necrosis factor (TNF)-α or interferon (IFN)-γ upregulates the expression of membrane-bound CX3CL1 as well as the release of soluble CX3CL1 via ADAM17 (22). Increased CX3CL1 expression contributes to the infiltration of inflammatory cells expressing CX3CR1 into affected joints including CD14+ or CD16+ macrophages, DCs, and T cells (23). Blockade of CX3CL1 by a monoclonal antibody significantly reduces synovial inflammation and joint bone loss in the murine collagen-induced arthritis (24). Consistent with this, CX3CR1-deficient mice demonstrated decreased inflammation compared to the changes seen in wild-type mice (25). These results suggested that inhibition of CX3CL1-CX3CR1 axis could be one of possible targets for RA. A clinical trial of monoclonal antibody against CX3CL1 in patients with RA has demonstrated promising efficacy in patients with active RA who were intolerant of harsh treatment with methotrexate or TNF inhibitors (26).
BLOOD VESSEL SYSTEM AND ATHEROSCLEROSIS
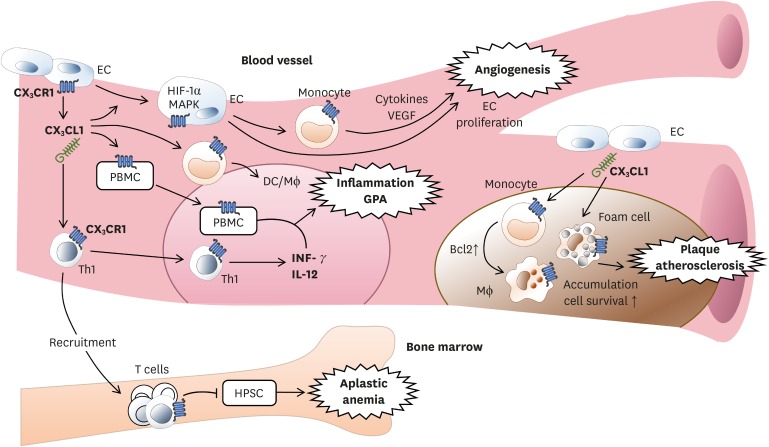
CX3CR1 is expressed in vascular endothelial cells and monocytes/macrophages (Fig. 2). When CX3CR1-expressing cells are activated by CX3CL1, hypoxia-inducible factor (HIF)-1α and mitogen-activated protein kinase (MAPK) are increased in endothelial cells. The production of vascular endothelial growth factor (VEGF) is also increased, thereby promoting cellular proliferation and formation of neo-vessel (27). Deficient CX3CR1 signals can reduce binding of monocyte with injured endothelium, resulting in decreased adherence (28). In limb ischemia, human CD14+ monocyte, one of pro-angiogenic monocytes, is a CX3CR1-expressing cell involved in the formation of extracellular matrix and vascular remodeling by producing cytokines and growth factors involved in angiogenesis (29). Although most blood monocytes express CX3CR1, its expression is significantly greater in CD14+ CD16+ cells (5). CD14+ CD16+ cells can move along blood vessel walls and then differentiate into macrophages or DCs to control inflammation. CX3CR1 expression on CD4+ and CD8+ T cells can be upregulated by CX3CL1 produced by endothelial cells. CX3CL1 and several cytokines can bring CX3CR1+ T cells into blood vessels (30).
Figure 2.
CX3CL1 from endothelial cell affects CX3CR1-expressing cells within blood vessels. For angiogenesis, monocytes expressing CX3CR1 promoted by CX3CL1 can secret cytokines and VEGF. This can lead to formation of extracellular matrix and vascular remodeling for angiogenesis. For inflammation, activation of CX3CR1 which is expressed in PBMCs causes leukocytes to migrate to the site of inflammation. CX3CR1-expressing Th1 also stimulates inflammation by secretion of IFN-γ and IL-12. For plaque, when CX3CL1 binds to CX3CR1 on monocyte, macrophage, and foam cells, these cells will accumulate in blood vessels and promote cell survival via expressing anti-apoptotic Bcl2 within cells. CX3CR1 is required for atherosclerosis. For aplastic anemia, autoreactive T cells expressing CX3CR1 are recruited into bone marrow by CX3CL1 to destroy HPSCs, resulting in aplastic anemia.
VEGF, vascular endothelial growth factor; PBMC, peripheral blood mononuclear cell; HPSC, hematopoietic stem cell; EC, endothelial cell; HIF, hypoxia-inducible factor; MAPK, mitogen-activated protein kinase; GPA, granulomatosis with polyangiitis.
In atherosclerosis, monocyte is a crucial cell type involved in the development, maintenance, and resolution of atherosclerosis (31). Plaque formation, vascular accumulation, and activation of monocytes and foamy macrophages are initiated in the early development stage of atherosclerosis followed by plaque rupture, thrombosis progress, and chronic problems (32). CX3CR1 signal in monocyte can enhance the expression of anti-apoptotic factor B-cell lymphoma 2 (Bcl2) which is required for monocyte homeostasis and arteriosclerosis by promoting cell survival (33). In granulomatosis with polyangitis (GPA) previously known as Wegener's granulomatosis (WG), CX3CR1 expressed in peripheral blood mononuclear cells can promote inflammation by promoting migration of leukocytes into inflammatory lesion (34). In addition, Th1 cells expressing CX3CR1 are increased, thereby increasing Th1-related cytokines IFN-γ and IL-12 and promoting inflammation (35). In patients with acquired aplastic anemia, expression levels of CX3CL1 and the number of T cells expressing CX3CR1 are increased. They can mediate the recruitment of T cells into bone marrow and destroy hematopoietic stem cells (30).
BRAIN AND NEURODEGENERATIVE DISEASES
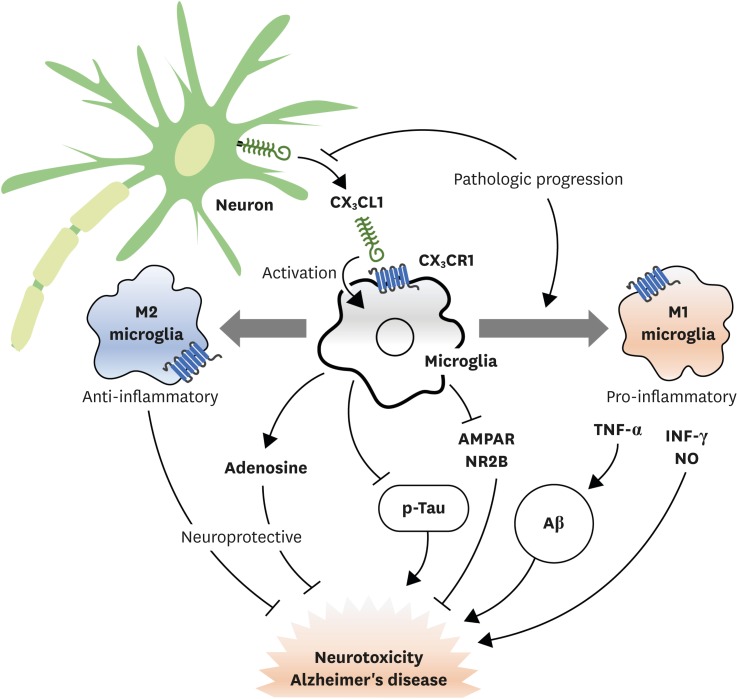
In the brain, microglia and astrocytes express CX3CR1 in steady states. However, expression of CX3CR1 is increased by inflammatory stimulations (Fig. 3) (36). Microglia are originated from primitive myeloid progenitor during embryogenesis which begins with early hematopoiesis, unlike other brain cells (37). Microglia is present in the CNS before neurons migrate to functional stage during development. Microglia can interact with neurons in adult brain. This is controlled by CX3CL1/CX3CR1 signaling (9). In brain tissues, CX3CL1 is mostly expressed in neuron while microglia express CX3CR1, the unique receptor of CX3CL1. In microglia, CX3CR1 is involved in intracellular signaling pathways such as phospholipase C (PLC), PI3K, and ERK by recruiting transcription factors such as NF-κB and cyclic adenosine monophosphate response element binding protein (CREB) (38). Microglia also play an important role in health and disease through CX3CL1/CX3CR1 signaling since neuro-inflammation caused by hyperactivity of microglia is associated with neurodegenerative diseases such as Alzheimer's disease. In rat, administration of recombinant CX3CL1 can result in reduction of neurodegenerative disease (39). In hepatic encephalopathy using azoxymethane treated mice model, injection of soluble CX3CL1 has resulted in the activation of microglia with decreased expression of IL-6 and TNF-α, thus alleviating hepatic encephalopathy (40).
Figure 3.
Neuroprotective and neurotoxic roles of microglia by CX3CL1/CX3CR1 signaling. Microglia are activated by interacting with CX3CL1 released from neuron through CX3CR1. Activated microglia can play a neuroprotective role by stimulating the release of adenosine which can inhibit tau phosphorylation and Aβ clearance by phagocytosis. They can also change the activation state to M2-type which can induce anti-inflammatory cytokines. Activated microglia can also lead to neurotoxicity by inhibiting function of synaptic AMPAR and NMDA receptor (NR2B subunit). They can change the activation state to M1-type which promote pro-inflammatory cytokines and Aβ production. As Alzheimer's disease progresses, CX3CL1 expression is decreased while M1-type microglia are increased, resulting in enhanced neurotoxicity of microglia.
AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor; NMDA, N-methyl-D-aspartate.
Alzheimer's disease might be caused by amyloid β (Aβ)-burdened neuron due to chronic inflammation and neurotoxicity (41). In Aβ administration, CX3CR1 signaling is increased. Blocking CX3CR1 in microglia has resulted in reduced neurotoxicity by inhibiting the production of lactate dehydrogenase and cytotoxicity to hippocampal neurons (42), although this remains controversial. M2-type microglia can promote Aβ plaque reduction by increasing phagocytic activity (43). However, microglia can also increase tau propagation, another mechanism of Alzheimer's disease (44). When a mild decrease in early CX3CL1/CX3CR1 signaling occurs, phagocytosis of microglia increases and Aβ deposition clearance increases. However, severe reduction of CX3CL1/CX3CR1 signaling can result in abnormal regulation of microglia which may cause neuronal damage (45). In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mice model for Parkinson's disease, CX3CL1 from neuron can inhibit the overexpression of pro-inflammatory molecules such as nitric oxide (NO) synthase, IL-1β, TNF-α, and IL-6 as well as α-synuclein, a possible causal factor for neurodegeneration (46). Brain ischemia causes inflammation and activates microglia and macrophages, thus having both neuroprotective and harmful effects on the lesion (47). In a middle cerebral artery occlusion (MCAO) mice model for brain ischemia, microglia and macrophages have adverse effects on neural recovery by inducing inflammatory responses using CX3CR1 signaling in ischemic state (48). In addition, the number of CX3CR1+ bone marrow-derived mesenchymal stem cells (BMSCs) is increased. CX3CL1 seems to migrate BMSCs to ischemic brain lesions by activating Jak2-Stat5α-ERK1/2 pathway of CX3CR1+ BMSCs for neurogenesis (49). However, inhibiting CX3CR1 expression by siRNA can decrease TNF-α and IL-1β cytokine production in a different ischemic animal model (50). In spinal cord injury, microglia can migrate to the injury epicenter by CX3CR1 signaling while CX3CR1 increases inflammatory signaling of microglia (51). Blocking CX3CR1 signaling can improve recovery from injury and decrease lesion pathology (52).
CX3CR1 has a protective effect on autoimmune inflammation. CX3CR1 deficiency has resulted in increased production of IFN-γ and IL-17 followed by experimental autoimmune encephalomyelitis (EAE)-induced demyelination and nerve damage in cerebella and spinal cord (53).
LUNG AND ASTHMA
Allergic asthma is a complex inflammatory disease characterized by various degrees of airflow obstruction, airway hypersensitivity, and inflammation, resulting in infiltration of mast cells, lymphocytes, and eosinophils (54). The amount of CX3CL1 is known to be increased in asthmatic patients compared to that in healthy individuals. It can induce mast cell chemotaxis and increase CX3CR1 function in Th2 cells (55). CX3CR1 in Th2 cells plays an important role in asthma (56). CX3CR1 deficient mice have shown reduced airway hyper-responsiveness, eosinophilia, and cytokine secretion. These results suggest that CX3CL1/CX3CR1 has potential to be used as a target to treat allergic asthma. CX3CL1/CX3CR1 interaction plays an important role in the pathophysiology of chronic obstructive pulmonary disease (COPD), a chronic disease in which mucus exudates accumulate in a narrow airway with destruction of lung parenchymal cells, resulting in enlargement of airspace (57). In COPD model, CX3CL1 expression is increased (58). The number of macrophages and T lymphocytes expressing CX3CR1 is also increased in lung parenchyma (59).
In pulmonary infections, the role of CX3CR1 remains unclear. CX3CR1 deficient mice infected with Vaccinia virus have shown increased viral loads but decreased T cell responses, suggesting that CX3CR1 plays an important role in the protection of DCs against virus infection (60). One of major DC subtypes in the lung is CD11bhigh CD103− DCs which express CX3CR1. CD11bhigh CD103− DCs are differentiated from Ly6ClowCCR2low monocytes by CX3CR1 dependent mechanism. The population of DCs has been found to be significantly decreased in CX3CR1 deficient mice (61). In some bacterial infections such as those caused by Mycobacterium tuberculosis and Francisella tularensis, deficiency of CX3CR1 does not significantly affect host survival (62). CX3CR1 deficient mice have shown an increase of monocytes and neutrophils in lungs compared to WT mice in later stages of pulmonary tularemia. Similar outcomes are also observed in organ burden and survival period (62). The unexpected increase of infiltrates in lungs of CX3CR1 deficient mice might be caused by interactions between CX3CL1 and other molecules similar to CX3CR1 since expression levels of CX3CL1 are increased in CX3CR1 deficient mice (63).
Pulmonary arterial hypertension (PAH) is caused by obstruction of small pulmonary artery for a long-time due to dysfunction and proliferation of endothelial and vascular smooth muscle cells (64). Several studies have shown that inflammation contributes to the development and progression of PAH which might involve CX3CL1/CX3CR1 interaction (65). Inhibiting CX3CR1 using drug or genetic modification has resulted in changes in monocyte recruitment, macrophage phenotype ratio, and inhibition of pulmonary-artery smooth muscle cell proliferation (65). CX3CL1/CX3CR1 interaction can be associated with development of hypoxic-induced pulmonary hypertension. Under hypoxic condition, M2 macrophage predominantly proliferates. M2 macrophages can stimulate cytokine secretion and induce pulmonary vascular remodeling (66). CX3CR1 deficiency has resulted in reduced M2 predominant proliferation in hypoxic challenge and balance with M1 macrophages (67).
LIVER
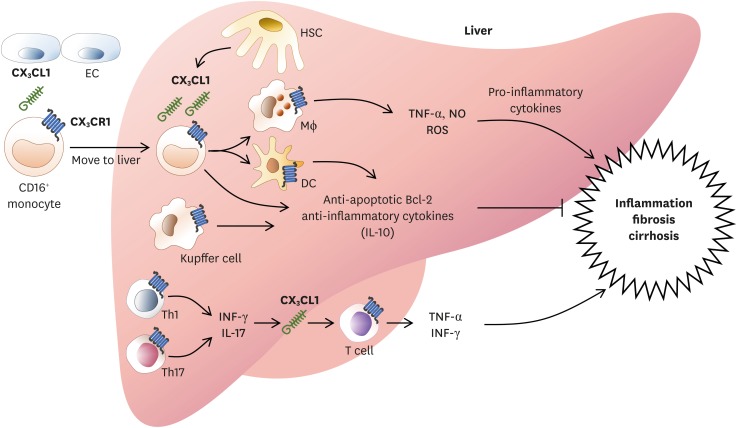
In the liver, M2 macrophage and myeloid DC are important for regulation of inflammation. These cells are derived from CD16+ monocytes expressing CX3CR1 (Fig. 4) (68). CX3CR1 is also expressed in Kupffer cells (also known as stellate macrophages) which are specialized macrophages located in the liver (69). CX3CR1 is also expressed in intraepithelial T cells in bile duct and lymphocytes, monocytes, and NK cells in portal tract. Most cells expressing CX3CR1 are CD3+ T cells. They have more CD8+ T cells than CD4+ T cells (70).
Figure 4.
CX3CL1/CX3CR1 signaling in liver inflammation. In the liver, HSCs and ECs both express CX3CL1. CX3CR1 is also expressed in macrophages derived from CD16+ monocytes, myeloid DCs, Kupffer cells, and T cells. These cells can secrete NO and ROS as well as pro-inflammatory cytokines such as TNF-α, IFN-γ, IL-1β, and IL-6 to mediate inflammatory response of the liver and restore hepatocytes. Regeneration of liver epithelium results in promotion of fibrosis and cirrhosis. Upon CX3CL1 signals, CX3CR1-expressing Kupffer cells and hepatic DCs can produce anti-inflammatory cytokines such as IL-10 to reduce liver inflammation. CX3CL1/CX3CR1 signals can enhance cell survival via inducing anti-apoptotic Bcl2, thus decreasing liver inflammation and tissue damages.
HSC, hepatic stellate cell; EC, endothelial cell; NO, nitric oxide; ROS, reactive oxygen species.
CX3CL1/CX3CR1 is upregulated in chronic inflammatory conditions such as viral hepatitis (71). In acute hepatic damage, CX3CR1-expressing Kupffer cells, liver infiltrating lymphocytes, biliary epithelial cells in the portal tract, and hepatic stellate cells (HSCs) all contribute to necrosis and inflammation. CX3CR1 expressing cells are also involved in the regulation of liver fibrosis (72). CX3CR1 mediates essential survival signal for hepatic monocyte-derived macrophages by activating anti-apoptotic Bcl2 expression (73). CX3CR1 limits liver fibrosis in vivo by controlling differentiation and survival of intrahepatic monocytes. CX3CL1 treatment can induce the expression of IL-10 and arginase-1 in Kupffer cells through CX3CR1, which in turn can suppress HSC activation (74). CX3CL1-CX3CR1 interaction inhibits inflammatory properties in Kupffer cells/macrophages, resulting in decreased liver inflammation and fibrosis (75). CX3CL1/CX3CR1 axis can promote IL-10-mediated anti-inflammatory actions of hepatic DCs (75). Primary biliary cirrhosis is an autoimmune injury caused by chronic inflammation of Th1/Th17 (76). Th1/Th17 can secrete IFN-γ or IL-17 which then upregulates CX3CL1. Correlation between primary biliary cirrhosis and CX3CL1 expression is significantly proportional (77). CX3CL1 is a chemokine and cell adhesion molecule that can attract cells expressing CX3CR1. Therefore, T cells expressing CX3CR1 can transmigrate into inflamed tissue and produce inflammatory cytokines such as TNF-α and IFN-γ.
GUT AND IMMUNE TOLERANCE
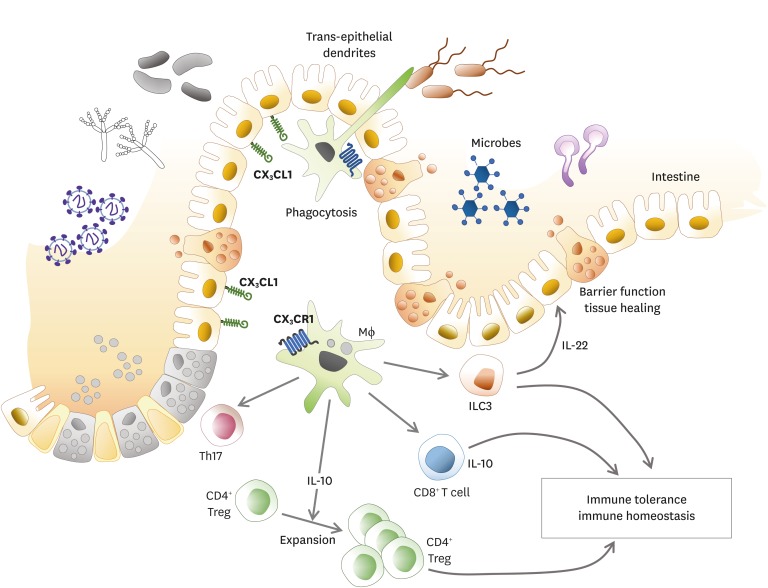
In the gut, 2 major phenotypic populations of mucosal mononuclear phagocytes have now been proposed: conventional DC and macrophages. Most macrophages and some DC subsets express CX3CR1. SIRPα+CD11b+CD103+ or CD103− DC subsets express low levels of CX3CR1 depending on Zbtb46 and Flt3L for development and differentiation (78). They can migrate to intestinal draining lymph node depending on CCR7. They also present soluble antigen to naïve CD4+ T cells. In mice, lamina propria macrophages express classical macrophages markers such as CD11b, CD64, MERTK, and F4/80 as well as high levels of MHC II and CX3CR1 (79). In resting mucosa, the role of lamina propria CX3CR1+ macrophage is to pass captured antigen via trans-epithelial dendrites or phagocytosis onto DC for transport to mesenteric lymph node (MLN) to prime immune responses like lamina propria DC (Fig. 5) (6). These transepithelial dendrites can cross junctions between epithelial cells and participate in the clearance of entero-invasive pathogens through CX3CR1 dependent process, thereby regulating immune tolerance or inflammation to commensal and pathogenic bacteria (80). CX3CR1-deficient animals have shown impaired Listeria clearance and higher susceptibility to Salmonella infection (80). Deletion of CX3CR1 or CX3CL1 has resulted in a specific and significant reduction in lamina propria macrophages with decreased translocation of bacteria to MLNs and their ability to take up pathogens. These findings demonstrate that CX3CR1 is a specific marker for lamina propria macrophages and a critical component in maintaining lamina propria macrophage homeostasis (81). However, it has also been reported that CX3CR1 deficient mice have normal numbers of intestinal macrophages (82).
Figure 5.
Role of CX3CR1 expressing immune cells in the gut. Lamina propria macrophages and DC subsets are major CX3CR1 expressing immune cells in the intestine. CX3CR1+ macrophages can extend trans-epithelial dendrites to capture antigens in the intestinal lumen. These captured antigens can be ingested and directly or indirectly presented to T cells. CX3CR1+ macrophages can maintain immune homeostasis in the intestine. CD4+ Tregs are expanded to maintain immune tolerance through IL-10 secreted by CX3CR1+ macrophages. CX3CR1+ macrophages can also prime naive CD8+ T cell via cross-presentation. CX3CR1+ macrophages can stimulate ILC3s to secret IL-22 for sustained barrier function and tissue healing. CX3CR1+ macrophages can induce microbiota specific Th17 cells in the gut.
Treg, regulatory T cell; ILC, innate lymphoid cell.
CD11b+CD14+CX3CR1+ lamina propria phagocytes derived from Ly6Chi but not Ly6Clo monocytes have shown to be involved in massive local DC proliferation in the colonic mucosa under inflammation condition (83). Monocyte-derived CX3CR1+ phagocytes can interfere with restoration of epithelial integrity by secreting TNF-α (84). Consistent with this, CX3CR1 deficiency is associated with reduced release of IL-6 and TNF-α as well as reduced inducible NO synthase production. Intestinal microbiota can influence local accumulation of CX3CR1+ phagocytes because the number of CX3CR1+ cells is reduced in germ-free mouse (85).
CX3CR1+ macrophages produce immunoregulatory cytokines such as IL-10 which can maintain macrophage inertia in an autocrine manner. It can also facilitate terminal differentiation and maintenance of Foxp3+ regulatory T cell (Treg) within the lamina propria (86). At steady state, antigens sampling by CX3CR1+ phagocytes can induce the differentiation of CD8+ T cells expressing IL-10 which can inhibit inflammatory CD4+ T cell activation (87). Monocyte-derived CX3CR1+ macrophages are possibly a subset of macrophages that can prime segmented filamentous bacteria (SFB)-specific T cells and direct Th17 cell differentiation (88). Lack of CX3CR1 expression is associated with significantly altered intestinal microbiota composition which is linked to impaired intestinal barrier. CX3CR1 is a gatekeeper for intestinal barrier integrity to limit steatohepatitis by maintaining intestinal homeostasis in mice (89).
Inflammatory bowel disease (IBD) refers to chronic inflammatory disorders affecting the gastrointestinal tract. There are 2 main clinical forms of IBD: Crohn's disease that affects any part of the gastrointestinal tract and ulcerative colitis (UC) whose pathology is restricted to the colonic mucosa (90). CX3CL1/CX3CR1 axis also plays important roles in IBD. In patients with Crohn's disease, there is a significant increase of CX3CL1 transcription in inflamed lesions compared to that in non-inflamed colonic mucosa (91). CX3CR1-deficient and CX3CL1-deficient mice are relatively protected from dextran sulfate sodium (DSS)-induced acute colitis (92). However, intestinal CX3CR1highCD11b+CD11c+ Mreg cell subset can directly inhibit T-cell proliferation and, thereby preventing T-cell-dependent intestinal inflammation (93). CX3CR1 deficiency can enhance Th17 responses and exacerbate acute intestinal inflammation (94). Microbiota-dependent crosstalk between CX3CR1+ macrophages and innate lymphoid cell (ILC) 3 can promote intestinal homeostasis by establishing IL-22 production (95). CX3CR1high and CX3CR1int cells possess distinct functions by suppressing and activating T cells. CX3CR1 expression on peripheral CD4+ T cells is significantly upregulated in both UC and Crohn's disease patients. CX3CR1+CD4+ T cells have dual functions as Th1 effector and cytotoxic T cells (91).
CANCER
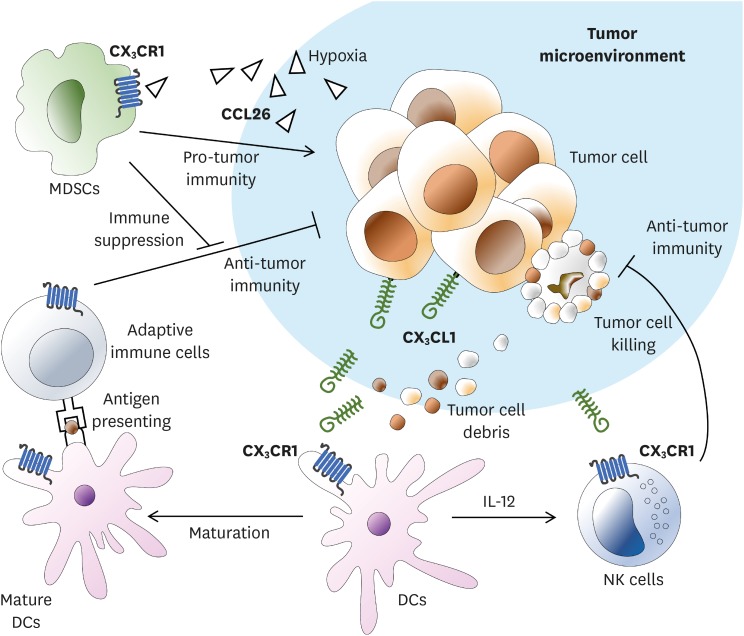
Tumor can be attacked by a variety of immune cells such as CD8+ T cell, B cell, NK cell, and macrophage involving CX3CL1 (96). CX3CL1 expression on the surface of a tumor cell can facilitate antitumor immunity by inducing infiltration of T cells, NK cells, and DCs into tumor tissues (Fig. 6) (97,98). CX3CL1-expressing tumor can be more efficiently eliminated than non-expressing tumor. CX3CL1 induces T-cell-dependent antitumor immunity through chemoattraction and activation of DCs (97). Clinical results have shown that patients with CX3CL1-expressing tumors have better prognosis than those with non-CX3CL1-expressing tumors (99). On the other hand, CX3CL1 can enhance the cytotoxicity of NK cells. NK cells pretreated with CX3CL1 have shown more potent cytotoxicity whereas those pretreated with anti-CX3CL1 or anti-CX3CR1 antibodies have shown attenuated cytotoxicity (100). CX3CR1 also plays an important role in antitumor activity in tumor cells that do not express CX3CL1 on the cell surface (101). Lung tumor mass of B16F10 melanoma without expressing CX3CL1 is increased in CX3CR1 deficient mice compared to that of normal mice. In addition, CX3CR1 deficient NK cells have shown decreased secretion of IFN-γ but increased IL-6 secretion (101). On the other hand, in some carcinomas, CX3CL1/CX3CR1 interaction does not affect antitumor activity. In OV-HM ovarian carcinoma which expresses CX3CL1, CX3CL1 attracts immune cells without invading the tumor (102). They only stay in surrounding blood vessels without exerting antitumor activity.
Figure 6.
The role of CX3CR1 expressing immune cells in tumor microenvironment. NK cells or DCs expressing CX3CR1 are attracted by CX3CL1 expressed on tumor cell surface or soluble CX3CL1. NK cells can invade tumor mass and cause tumor cell lysis. DCs will undergo maturation following uptake of lysed tumor cell debris. Mature DCs will increase IL-12 expression. Increased expression of IL-12 can further enhance NK cell cytotoxicity, leading to increased NK-cell mediated cell lysis. Adaptive immune cells are activated by antigen presentation of mature DCs and initiates antitumor immunity specific to cancer cells. In human hepatocellular carcinoma cells, the expression level of CCL26 is increased in hypoxia state. CCL26 can interact with CX3CR1 expressed on the surface of MDSCs, thus allowing MDSCs to access tumor microenvironment. As a result, MDSCs can regulate anti-tumor immunity in tumor microenvironment.
Myeloid-derived suppressor cell (MDSC) is an immature-myeloid cell that has immune regulating effect (103). Similar to other myeloid cells, MDSCs express CX3CR1 on their surface (104). In a human hepatocellular carcinoma model, migration of MDSC into the hypoxia region is mediated through CCL26/CX3CR1. Increased HIFs under hypoxia condition can increase the expression of CCL26 on the surface of cancer cells and promote the recruitment of MDSCs expressing CX3CR1 (105). Taken together, these results indicate that CX3CR1-mediated antitumor effect varies for each carcinoma.
CONCLUSION
Among different classes of chemokines, fractalkine/CX3CL1 with unique functional and structural characteristics can participate in either inflammation or anti-inflammation. Tissue-specific CX3CL1/CX3CR1 axis contributes to progression of various diseases in a given tissue. However, therapeutics targeting CX3CL1 or CX3CR1 have not been developed yet for clinical use. Emerging role of CX3CL1/CX3CR1 axis provides evidence that it might be used as a potential therapeutic target to control cardiovascular diseases, allergic asthma, neurodegenerative diseases, cancers, and other diseases related to vascular inflammation.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT and future Planning (NRF-2017R1A2B4002419) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number HI15C1980).
Abbreviations
- Aβ
amyloid β
- Bcl2
B-cell lymphoma 2
- BMSC
bone marrow-derived mesenchymal stem cell
- CD
cluster of differentiation
- CX3CL1
chemokine (C-X3-C motif) ligand 1
- CX3CR1
chemokine (C-X3-C motif) receptor 1
- DC
dendritic cell
- IBD
inflammatory bowel disease
- IFN
interferon
- KLRG1
killer cell lectin-like receptor subfamily G member 1
- MDSC
myeloid-derived suppressor cell
- NK
natural killer
- RA
rheumatoid arthritis
- Th
T helper
- TNF
tumor necrosis factor
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
Author Contributions: Conceptualization: Lee M, Lee Y, Song J, Lee J, Chang SY; Visualization: Lee Y, Chang SY; Writing - original draft: Lee M, Lee Y, Song J, Lee J, Chang SY; Writing - review & editing: Lee M, Chang SY
References
- 1.Kim KW, Vallon-Eberhard A, Zigmond E, Farache J, Shezen E, Shakhar G, Ludwig A, Lira SA, Jung S. In vivo structure/function and expression analysis of the CX3C chemokine fractalkine. Blood. 2011;118:e156–e167. doi: 10.1182/blood-2011-04-348946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 3.Hundhausen C, Misztela D, Berkhout TA, Broadway N, Saftig P, Reiss K, Hartmann D, Fahrenholz F, Postina R, Matthews V, et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood. 2003;102:1186–1195. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
- 4.Garton KJ, Gough PJ, Blobel CP, Murphy G, Greaves DR, Dempsey PJ, Raines EW. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1) J Biol Chem. 2001;276:37993–38001. doi: 10.1074/jbc.M106434200. [DOI] [PubMed] [Google Scholar]
- 5.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 6.Bain CC, Mowat AM. Intestinal macrophages - specialised adaptation to a unique environment. Eur J Immunol. 2011;41:2494–2498. doi: 10.1002/eji.201141714. [DOI] [PubMed] [Google Scholar]
- 7.Hamon P, Loyher PL, Baudesson de Chanville C, Licata F, Combadière C, Boissonnas A. CX3CR1-dependent endothelial margination modulates Ly6Chigh monocyte systemic deployment upon inflammation in mice. Blood. 2017;129:1296–1307. doi: 10.1182/blood-2016-08-732164. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Condello C, Schain A, Harb R, Grutzendler J. CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-β phagocytosis. J Neurosci. 2010;30:17091–17101. doi: 10.1523/JNEUROSCI.4403-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuhrmann M, Bittner T, Jung CK, Burgold S, Page RM, Mitteregger G, Haass C, LaFerla FM, Kretzschmar H, Herms J. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer's disease. Nat Neurosci. 2010;13:411–413. doi: 10.1038/nn.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacquelin S, Licata F, Dorgham K, Hermand P, Poupel L, Guyon E, Deterre P, Hume DA, Combadière C, Boissonnas A. CX3CR1 reduces Ly6Chigh-monocyte motility within and release from the bone marrow after chemotherapy in mice. Blood. 2013;122:674–683. doi: 10.1182/blood-2013-01-480749. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino A, Ueha S, Hanada S, Imai T, Ito M, Yamamoto K, Matsushima K, Yamaguchi A, Iimura T. Roles of chemokine receptor CX3CR1 in maintaining murine bone homeostasis through the regulation of both osteoblasts and osteoclasts. J Cell Sci. 2013;126:1032–1045. doi: 10.1242/jcs.113910. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Wang L, Zhao C, Li L, Peng J, Hou M. CD8+ T cells suppress autologous megakaryocyte apoptosis in idiopathic thrombocytopenic purpura. Br J Haematol. 2007;139:605–611. doi: 10.1111/j.1365-2141.2007.06737.x. [DOI] [PubMed] [Google Scholar]
- 13.Olsson B, Ridell B, Carlsson L, Jacobsson S, Wadenvik H. Recruitment of T cells into bone marrow of ITP patients possibly due to elevated expression of VLA-4 and CX3CR1. Blood. 2008;112:1078–1084. doi: 10.1182/blood-2008-02-139402. [DOI] [PubMed] [Google Scholar]
- 14.Hurley A, Smith M, Karpova T, Hasley RB, Belkina N, Shaw S, Balenga N, Druey KM, Nickel E, Packard B, et al. Enhanced effector function of CD8(+) T cells from healthy controls and HIV-infected patients occurs through thrombin activation of protease-activated receptor 1. J Infect Dis. 2013;207:638–650. doi: 10.1093/infdis/jis730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apostolakis S, Spandidos D. Chemokines and atherosclerosis: focus on the CX3CL1/CX3CR1 pathway. Acta Pharmacol Sin. 2013;34:1251–1256. doi: 10.1038/aps.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Z. CX3CR1(+) B cells show immune suppressor properties. J Biol Chem. 2014;289:22630–22635. doi: 10.1074/jbc.M114.569459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhivaki D, Lemoine S, Lim A, Morva A, Vidalain PO, Schandene L, Casartelli N, Rameix-Welti MA, Hervé PL, Dériaud E, et al. Respiratory syncytial virus infects regulatory B cells in human neonates via chemokine receptor CX3CR1 and promotes lung disease severity. Immunity. 2017;46:301–314. doi: 10.1016/j.immuni.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sciumè G, De Angelis G, Benigni G, Ponzetta A, Morrone S, Santoni A, Bernardini G. CX3CR1 expression defines 2 KLRG1+ mouse NK-cell subsets with distinct functional properties and positioning in the bone marrow. Blood. 2011;117:4467–4475. doi: 10.1182/blood-2010-07-297101. [DOI] [PubMed] [Google Scholar]
- 19.Ponzetta A, Sciumè G, Benigni G, Antonangeli F, Morrone S, Santoni A, Bernardini G. CX3CR1 regulates the maintenance of KLRG1+ NK cells into the bone marrow by promoting their entry into circulation. J Immunol. 2013;191:5684–5694. doi: 10.4049/jimmunol.1300090. [DOI] [PubMed] [Google Scholar]
- 20.Hamann I, Unterwalder N, Cardona AE, Meisel C, Zipp F, Ransohoff RM, Infante-Duarte C. Analyses of phenotypic and functional characteristics of CX3CR1-expressing natural killer cells. Immunology. 2011;133:62–73. doi: 10.1111/j.1365-2567.2011.03409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yano R, Yamamura M, Sunahori K, Takasugi K, Yamana J, Kawashima M, Makino H. Recruitment of CD16+ monocytes into synovial tissues is mediated by fractalkine and CX3CR1 in rheumatoid arthritis patients. Acta Med Okayama. 2007;61:89–98. doi: 10.18926/AMO/32882. [DOI] [PubMed] [Google Scholar]
- 22.Sawai H, Park YW, Roberson J, Imai T, Goronzy JJ, Weyand CM. T cell costimulation by fractalkine-expressing synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2005;52:1392–1401. doi: 10.1002/art.21140. [DOI] [PubMed] [Google Scholar]
- 23.Blaschke S, Koziolek M, Schwarz A, Benöhr P, Middel P, Schwarz G, Hummel KM, Müller GA. Proinflammatory role of fractalkine (CX3CL1) in rheumatoid arthritis. J Rheumatol. 2003;30:1918–1927. [PubMed] [Google Scholar]
- 24.Nanki T, Urasaki Y, Imai T, Nishimura M, Muramoto K, Kubota T, Miyasaka N. Inhibition of fractalkine ameliorates murine collagen-induced arthritis. J Immunol. 2004;173:7010–7016. doi: 10.4049/jimmunol.173.11.7010. [DOI] [PubMed] [Google Scholar]
- 25.Tarrant TK, Liu P, Rampersad RR, Esserman D, Rothlein LR, Timoshchenko RG, McGinnis MW, Fitzhugh DJ, Patel DD, Fong AM. Decreased Th17 and antigen-specific humoral responses in CX3 CR1-deficient mice in the collagen-induced arthritis model. Arthritis Rheum. 2012;64:1379–1387. doi: 10.1002/art.34320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka Y, Takeuchi T, Umehara H, Nanki T, Akama H, Yasuda N, Tago F, Kawakubo M, Hojo S, Kawano T, et al. Safety and efficacy of E6011, an anti-fractalkine monoclonal antibody, in a first-in-patient phase 1/2 study in rheumatoid arthritis. Arthritis Rheumatol. 2015;67:3960–3962. [Google Scholar]
- 27.Ryu J, Lee CW, Hong KH, Shin JA, Lim SH, Park CS, Shim J, Nam KB, Choi KJ, Kim YH, et al. Activation of fractalkine/CX3CR1 by vascular endothelial cells induces angiogenesis through VEGF-A/KDR and reverses hindlimb ischaemia. Cardiovasc Res. 2008;78:333–340. doi: 10.1093/cvr/cvm067. [DOI] [PubMed] [Google Scholar]
- 28.Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation. 1994;90:775–778. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- 29.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren J, Hou XY, Ma SH, Zhang FK, Zhen JH, Sun L, Sun YX, Hao YL, Cheng YF, Hou M, et al. Elevated expression of CX3C chemokine receptor 1 mediates recruitment of T cells into bone marrow of patients with acquired aplastic anaemia. J Intern Med. 2014;276:512–524. doi: 10.1111/joim.12218. [DOI] [PubMed] [Google Scholar]
- 31.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landsman L, Bar-On L, Zernecke A, Kim KW, Krauthgamer R, Shagdarsuren E, Lira SA, Weissman IL, Weber C, Jung S. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood. 2009;113:963–972. doi: 10.1182/blood-2008-07-170787. [DOI] [PubMed] [Google Scholar]
- 34.Bjerkeli V, Damås JK, Fevang B, Holter JC, Aukrust P, Frøland SS. Increased expression of fractalkine (CX3CL1) and its receptor, CX3CR1, in Wegener's granulomatosis--possible role in vascular inflammation. Rheumatology (Oxford) 2007;46:1422–1427. doi: 10.1093/rheumatology/kem168. [DOI] [PubMed] [Google Scholar]
- 35.Csernok E, Trabandt A, Müller A, Wang GC, Moosig F, Paulsen J, Schnabel A, Gross WL. Cytokine profiles in Wegener's granulomatosis: predominance of type 1 (Th1) in the granulomatous inflammation. Arthritis Rheum. 1999;42:742–750. doi: 10.1002/1529-0131(199904)42:4<742::AID-ANR18>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 36.Croitoru-Lamoury J, Guillemin GJ, Boussin FD, Mognetti B, Gigout LI, Chéret A, Vaslin B, Le Grand R, Brew BJ, Dormont D. Expression of chemokines and their receptors in human and simian astrocytes: evidence for a central role of TNF alpha and IFN gamma in CXCR4 and CCR5 modulation. Glia. 2003;41:354–370. doi: 10.1002/glia.10181. [DOI] [PubMed] [Google Scholar]
- 37.Sellner S, Paricio-Montesinos R, Spieß A, Masuch A, Erny D, Harsan LA, Elverfeldt DV, Schwabenland M, Biber K, Staszewski O, et al. Microglial CX3CR1 promotes adult neurogenesis by inhibiting Sirt 1/p65 signaling independent of CX3CL1. Acta Neuropathol Commun. 2016;4:102. doi: 10.1186/s40478-016-0374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheridan GK, Murphy KJ. Neuron-glia crosstalk in health and disease: fractalkine and CX3CR1 take centre stage. Open Biol. 2013;3:130181. doi: 10.1098/rsob.130181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, et al. Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2011;32:2030–2044. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMillin M, Grant S, Frampton G, Andry S, Brown A, DeMorrow S. Fractalkine suppression during hepatic encephalopathy promotes neuroinflammation in mice. J Neuroinflammation. 2016;13:198. doi: 10.1186/s12974-016-0674-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 42.Dworzak J, Renvoisé B, Habchi J, Yates EV, Combadière C, Knowles TP, Dobson CM, Blackstone C, Paulsen O, Murphy PM. Neuronal Cx3cr1 deficiency protects against amyloid β-induced neurotoxicity. PLoS One. 2015;10:e0127730. doi: 10.1371/journal.pone.0127730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cherry JD, Olschowka JA, O'Banion MK. Arginase 1+ microglia reduce Aβ plaque deposition during IL-1β-dependent neuroinflammation. J Neuroinflammation. 2015;12:203. doi: 10.1186/s12974-015-0411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, Wolozin B, Butovsky O, Kügler S, Ikezu T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci. 2015;18:1584–1593. doi: 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cardona SM, Mendiola AS, Yang YC, Adkins SL, Torres V, Cardona AE. Disruption of fractalkine signaling leads to microglial activation and neuronal damage in the diabetic retina. ASN Neuro. doi: 10.1177/1759091415608204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nash KR, Moran P, Finneran DJ, Hudson C, Robinson J, Morgan D, Bickford PC. Fractalkine over expression suppresses α-synuclein-mediated neurodegeneration. Mol Ther. 2015;23:17–23. doi: 10.1038/mt.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang Z, Gan Y, Liu Q, Yin JX, Liu Q, Shi J, Shi FD. CX3CR1 deficiency suppresses activation and neurotoxicity of microglia/macrophage in experimental ischemic stroke. J Neuroinflammation. 2014;11:26. doi: 10.1186/1742-2094-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Zheng J, Zhou Z, Zhou H, Wang Y, Gong Z, Zhu J. Fractalkine promotes chemotaxis of bone marrow-derived mesenchymal stem cells towards ischemic brain lesions through Jak2 signaling and cytoskeletal reorganization. FEBS J. 2015;282:891–903. doi: 10.1111/febs.13187. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, Wu XM, Luo QQ, Huang S, Yang QW, Wang FX, Ke Y, Qian ZM. CX3CL1/CX3CR1-mediated microglia activation plays a detrimental role in ischemic mice brain via p38MAPK/PKC pathway. J Cereb Blood Flow Metab. 2015;35:1623–1631. doi: 10.1038/jcbfm.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hansen CN, Fisher LC, Deibert RJ, Jakeman LB, Zhang H, Noble-Haeusslein L, White S, Basso DM. Elevated MMP-9 in the lumbar cord early after thoracic spinal cord injury impedes motor relearning in mice. J Neurosci. 2013;33:13101–13111. doi: 10.1523/JNEUROSCI.1576-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donnelly DJ, Longbrake EE, Shawler TM, Kigerl KA, Lai W, Tovar CA, Ransohoff RM, Popovich PG. Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+ macrophages. J Neurosci. 2011;31:9910–9922. doi: 10.1523/JNEUROSCI.2114-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia JA, Pino PA, Mizutani M, Cardona SM, Charo IF, Ransohoff RM, Forsthuber TG, Cardona AE. Regulation of adaptive immunity by the fractalkine receptor during autoimmune inflammation. J Immunol. 2013;191:1063–1072. doi: 10.4049/jimmunol.1300040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8:218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 55.Rimaniol AC, Till SJ, Garcia G, Capel F, Godot V, Balabanian K, Durand-Gasselin I, Varga EM, Simonneau G, Emilie D, et al. The CX3C chemokine fractalkine in allergic asthma and rhinitis. J Allergy Clin Immunol. 2003;112:1139–1146. doi: 10.1016/j.jaci.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 56.Mionnet C, Buatois V, Kanda A, Milcent V, Fleury S, Lair D, Langelot M, Lacoeuille Y, Hessel E, Coffman R, et al. CX3CR1 is required for airway inflammation by promoting T helper cell survival and maintenance in inflamed lung. Nat Med. 2010;16:1305–1312. doi: 10.1038/nm.2253. [DOI] [PubMed] [Google Scholar]
- 57.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 58.Ning W, Li CJ, Kaminski N, Feghali-Bostwick CA, Alber SM, Di YP, Otterbein SL, Song R, Hayashi S, Zhou Z, et al. Comprehensive gene expression profiles reveal pathways related to the pathogenesis of chronic obstructive pulmonary disease. Proc Natl Acad Sci USA. 2004;101:14895–14900. doi: 10.1073/pnas.0401168101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McComb JG, Ranganathan M, Liu XH, Pilewski JM, Ray P, Watkins SC, Choi AM, Lee JS. CX3CL1 up-regulation is associated with recruitment of CX3CR1+ mononuclear phagocytes and T lymphocytes in the lungs during cigarette smoke-induced emphysema. Am J Pathol. 2008;173:949–961. doi: 10.2353/ajpath.2008.071034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonduelle O, Duffy D, Verrier B, Combadière C, Combadière B. Cutting edge: Protective effect of CX3CR1+ dendritic cells in a vaccinia virus pulmonary infection model. J Immunol. 2012;188:952–956. doi: 10.4049/jimmunol.1004164. [DOI] [PubMed] [Google Scholar]
- 61.Jakubzick C, Tacke F, Ginhoux F, Wagers AJ, van Rooijen N, Mack M, Merad M, Randolph GJ. Blood monocyte subsets differentially give rise to CD103+ and CD103- pulmonary dendritic cell populations. J Immunol. 2008;180:3019–3027. doi: 10.4049/jimmunol.180.5.3019. [DOI] [PubMed] [Google Scholar]
- 62.Hall JD, Kurtz SL, Rigel NW, Gunn BM, Taft-Benz S, Morrison JP, Fong AM, Patel DD, Braunstein M, Kawula TH. The impact of chemokine receptor CX3CR1 deficiency during respiratory infections with Mycobacterium tuberculosis or Francisella tularensis. Clin Exp Immunol. 2009;156:278–284. doi: 10.1111/j.1365-2249.2009.03882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cardona AE, Sasse ME, Liu L, Cardona SM, Mizutani M, Savarin C, Hu T, Ransohoff RM. Scavenging roles of chemokine receptors: chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues. Blood. 2008;112:256–263. doi: 10.1182/blood-2007-10-118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Archer S, Rich S. Primary pulmonary hypertension: a vascular biology and translational research “Work in progress”. Circulation. 2000;102:2781–2791. doi: 10.1161/01.cir.102.22.2781. [DOI] [PubMed] [Google Scholar]
- 65.Balabanian K, Foussat A, Dorfmüller P, Durand-Gasselin I, Capel F, Bouchet-Delbos L, Portier A, Marfaing-Koka A, Krzysiek R, Rimaniol AC, et al. CX(3)C chemokine fractalkine in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165:1419–1425. doi: 10.1164/rccm.2106007. [DOI] [PubMed] [Google Scholar]
- 66.Amsellem V, Abid S, Poupel L, Parpaleix A, Rodero M, Gary-Bobo G, Latiri M, Dubois-Rande JL, Lipskaia L, Combadiere C, et al. Roles for the CX3CL1/CX3CR1 and CCL2/CCR2 chemokine systems in hypoxic pulmonary hypertension. Am J Respir Cell Mol Biol. 2017;56:597–608. doi: 10.1165/rcmb.2016-0201OC. [DOI] [PubMed] [Google Scholar]
- 67.Dorfmüller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J. 2003;22:358–363. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- 68.Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, Gabuzda D. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med. 2003;197:1701–1707. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 70.Sasaki M, Miyakoshi M, Sato Y, Nakanuma Y. Chemokine-chemokine receptor CCL2-CCR2 and CX3CL1-CX3CR1 axis may play a role in the aggravated inflammation in primary biliary cirrhosis. Dig Dis Sci. 2014;59:358–364. doi: 10.1007/s10620-013-2920-6. [DOI] [PubMed] [Google Scholar]
- 71.Efsen E, Grappone C, DeFranco RM, Milani S, Romanelli RG, Bonacchi A, Caligiuri A, Failli P, Annunziato F, Pagliai G, et al. Up-regulated expression of fractalkine and its receptor CX3CR1 during liver injury in humans. J Hepatol. 2002;37:39–47. doi: 10.1016/s0168-8278(02)00065-x. [DOI] [PubMed] [Google Scholar]
- 72.Wasmuth HE, Zaldivar MM, Berres ML, Werth A, Scholten D, Hillebrandt S, Tacke F, Schmitz P, Dahl E, Wiederholt T, et al. The fractalkine receptor CX3CR1 is involved in liver fibrosis due to chronic hepatitis C infection. J Hepatol. 2008;48:208–215. doi: 10.1016/j.jhep.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 73.Karlmark KR, Zimmermann HW, Roderburg C, Gassler N, Wasmuth HE, Luedde T, Trautwein C, Tacke F. The fractalkine receptor CX3CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology. 2010;52:1769–1782. doi: 10.1002/hep.23894. [DOI] [PubMed] [Google Scholar]
- 74.Aoyama T, Inokuchi S, Brenner DA, Seki E. CX3CL1-CX3CR1 interaction prevents carbon tetrachloride-induced liver inflammation and fibrosis in mice. Hepatology. 2010;52:1390–1400. doi: 10.1002/hep.23795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sutti S, Heymann F, Bruzzì S, Peusquens J, Trautwein C, Albano E, Tacke F. CX3CR1 modulates the anti-inflammatory activity of hepatic dendritic cells in response to acute liver injury. Clin Sci (Lond) 2017;131:2289–2301. doi: 10.1042/CS20171025. [DOI] [PubMed] [Google Scholar]
- 76.Harada K, Van de Water J, Leung PS, Coppel RL, Ansari A, Nakanuma Y, Gershwin ME. In situ nucleic acid hybridization of cytokines in primary biliary cirrhosis: predominance of the Th1 subset. Hepatology. 1997;25:791–796. doi: 10.1002/hep.510250402. [DOI] [PubMed] [Google Scholar]
- 77.Shimoda S, Harada K, Niiro H, Taketomi A, Maehara Y, Tsuneyama K, Kikuchi K, Nakanuma Y, Mackay IR, Gershwin ME, et al. CX3CL1 (fractalkine): a signpost for biliary inflammation in primary biliary cirrhosis. Hepatology. 2010;51:567–575. doi: 10.1002/hep.23318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Joeris T, Müller-Luda K, Agace WW, Mowat AM. Diversity and functions of intestinal mononuclear phagocytes. Mucosal Immunol. 2017;10:845–864. doi: 10.1038/mi.2017.22. [DOI] [PubMed] [Google Scholar]
- 79.Gross M, Salame TM, Jung S. Guardians of the gut - murine intestinal macrophages and dendritic cells. Front Immunol. 2015;6:254. doi: 10.3389/fimmu.2015.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 81.Ferretti E, Pistoia V, Corcione A. Role of fractalkine/CX3CL1 and its receptor in the pathogenesis of inflammatory and malignant diseases with emphasis on B cell malignancies. Mediators Inflamm. 2014;2014:480941. doi: 10.1155/2014/480941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6:498–510. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med. 2012;209:139–155. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, Mack M, Shpigel N, Boneca IG, Murphy KM, et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37:1076–1090. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 85.Bain CC, Bravo-Blas A, Scott CL, Perdiguero EG, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, Mowat AM. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15:929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Müller W, Sparwasser T, Förster R, Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 87.Chang SY, Song JH, Guleng B, Cotoner CA, Arihiro S, Zhao Y, Chiang HS, O'Keeffe M, Liao G, Karp CL, et al. Circulatory antigen processing by mucosal dendritic cells controls CD8(+) T cell activation. Immunity. 2013;38:153–165. doi: 10.1016/j.immuni.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Panea C, Farkas AM, Goto Y, Abdollahi-Roodsaz S, Lee C, Koscsó B, Gowda K, Hohl TM, Bogunovic M, Ivanov II. Intestinal monocyte-derived macrophages control commensal-specific th17 responses. Cell Reports. 2015;12:1314–1324. doi: 10.1016/j.celrep.2015.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schneider KM, Bieghs V, Heymann F, Hu W, Dreymueller D, Liao L, Frissen M, Ludwig A, Gassler N, Pabst O, et al. CX3CR1 is a gatekeeper for intestinal barrier integrity in mice: Limiting steatohepatitis by maintaining intestinal homeostasis. Hepatology. 2015;62:1405–1416. doi: 10.1002/hep.27982. [DOI] [PubMed] [Google Scholar]
- 90.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kobayashi T, Okamoto S, Iwakami Y, Nakazawa A, Hisamatsu T, Chinen H, Kamada N, Imai T, Goto H, Hibi T. Exclusive increase of CX3CR1+CD28-CD4+ T cells in inflammatory bowel disease and their recruitment as intraepithelial lymphocytes. Inflamm Bowel Dis. 2007;13:837–846. doi: 10.1002/ibd.20113. [DOI] [PubMed] [Google Scholar]
- 92.Kostadinova FI, Baba T, Ishida Y, Kondo T, Popivanova BK, Mukaida N. Crucial involvement of the CX3CR1-CX3CL1 axis in dextran sulfate sodium-mediated acute colitis in mice. J Leukoc Biol. 2010;88:133–143. doi: 10.1189/jlb.1109768. [DOI] [PubMed] [Google Scholar]
- 93.Kayama H, Ueda Y, Sawa Y, Jeon SG, Ma JS, Okumura R, Kubo A, Ishii M, Okazaki T, Murakami M, et al. Intestinal CX3C chemokine receptor 1(high) (CX3CR1(high)) myeloid cells prevent T-cell-dependent colitis. Proc Natl Acad Sci USA. 2012;109:5010–5015. doi: 10.1073/pnas.1114931109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Medina-Contreras O, Geem D, Laur O, Williams IR, Lira SA, Nusrat A, Parkos CA, Denning TL. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J Clin Invest. 2011;121:4787–4795. doi: 10.1172/JCI59150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xin H, Kikuchi T, Andarini S, Ohkouchi S, Suzuki T, Nukiwa T. Huqun, Hagiwara K, Honjo T, Saijo Y. Antitumor immune response by CX3CL1 fractalkine gene transfer depends on both NK and T cells. Eur J Immunol. 2005;35:1371–1380. doi: 10.1002/eji.200526042. [DOI] [PubMed] [Google Scholar]
- 97.Guo J, Zhang M, Wang B, Yuan Z, Guo Z, Chen T, Yu Y, Qin Z, Cao X. Fractalkine transgene induces T-cell-dependent antitumor immunity through chemoattraction and activation of dendritic cells. Int J Cancer. 2003;103:212–220. doi: 10.1002/ijc.10816. [DOI] [PubMed] [Google Scholar]
- 98.Lavergne E, Combadière B, Bonduelle O, Iga M, Gao JL, Maho M, Boissonnas A, Murphy PM, Debré P, Combadière C. Fractalkine mediates natural killer-dependent antitumor responses in vivo . Cancer Res. 2003;63:7468–7474. [PubMed] [Google Scholar]
- 99.Park MH, Lee JS, Yoon JH. High expression of CX3CL1 by tumor cells correlates with a good prognosis and increased tumor-infiltrating CD8+ T cells, natural killer cells, and dendritic cells in breast carcinoma. J Surg Oncol. 2012;106:386–392. doi: 10.1002/jso.23095. [DOI] [PubMed] [Google Scholar]
- 100.Zhang X, Wei H, Wang H, Tian Z. Involvement of interaction between Fractalkine and CX3CR1 in cytotoxicity of natural killer cells against tumor cells. Oncol Rep. 2006;15:485–488. [PubMed] [Google Scholar]
- 101.Yu YR, Fong AM, Combadiere C, Gao JL, Murphy PM, Patel DD. Defective antitumor responses in CX3CR1-deficient mice. Int J Cancer. 2007;121:316–322. doi: 10.1002/ijc.22660. [DOI] [PubMed] [Google Scholar]
- 102.Gao JQ, Tsuda Y, Katayama K, Nakayama T, Hatanaka Y, Tani Y, Mizuguchi H, Hayakawa T, Yoshie O, Tsutsumi Y, et al. Antitumor effect by interleukin-11 receptor alpha-locus chemokine/CCL27, introduced into tumor cells through a recombinant adenovirus vector. Cancer Res. 2003;63:4420–4425. [PubMed] [Google Scholar]
- 103.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kittang AO, Kordasti S, Sand KE, Costantini B, Kramer AM, Perezabellan P, Seidl T, Rye KP, Hagen KM, Kulasekararaj A, et al. Expansion of myeloid derived suppressor cells correlates with number of T regulatory cells and disease progression in myelodysplastic syndrome. OncoImmunology. 2015;5:e1062208. doi: 10.1080/2162402X.2015.1062208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chiu DK, Xu IM, Lai RK, Tse AP, Wei LL, Koh HY, Li LL, Lee D, Lo RC, Wong CM, et al. Hypoxia induces myeloid-derived suppressor cell recruitment to hepatocellular carcinoma through chemokine (C-C motif) ligand 26. Hepatology. 2016;64:797–813. doi: 10.1002/hep.28655. [DOI] [PubMed] [Google Scholar]