Abstract
Purpose: To analyze the role of surgery in patients with Masaoka stage IVa thymoma treated with multimodality therapy.
Methods: Of 191 patients undergoing surgery for thymoma in our department between January 2002 and December 2015, 39 (20.4%) had Masaoka stage IVa. Histopathological tumor type, myasthenic status of the Osserman–Genkins score, Masaoka stage at the first surgery, neoadjuvant treatment, number and type of surgeries, and survival rates were recorded.
Results: Thymoma B2 was the most common histopathological tumor type (n = 16, 41%). Twenty-six (66.7%) patients underwent primary surgeries for Masaoka stage IVa thymoma, whereas nine (23.1%) underwent secondary surgeries and four (10.3%) underwent tertiary surgeries for pleural or pericardial recurrences. Median survival was 132 ± 25 (82–181; 95% confidence interval [CI]) months. Overall 3-, 5-, and 10-year survival rates were 93%, 93%, and 56%, respectively.
Conclusion: Surgical treatment should be considered as a completion modality to oncological therapy and has the potential to provide long-term survival of Masaoka stage IVa in patients with thymoma. The type of surgery should be determined based on the invasiveness of the lesion.
Keywords: thymoma, surgery, Masaoka stage IVa
Introduction
Thymomas are the most common tumors of the anterior mediastinum, comprising more than half of anterior mediastinal tumors.1) The histological classification of thymomas is determined by the World Health Organization (WHO), and the Masaoka staging system describes anatomic invasion into the mediastinal structures, neighboring organs, or pleural cavity.2,3) Stage IVa thymoma is defined as a tumor with microscopically confirmed nodules separate from the primary tumor involving the visceral or parietal pleural surfaces or the pericardial or epicardial surfaces. The prognosis is associated with WHO histology, Masaoka stage, and complete surgical resection of the tumor.4–6) Surgery alone is the gold standard treatment for early stage thymomas.4–6) However, Masaoka stage IVa tumors are often not completely resectable, and the proper treatment for these patients remains unclear. Multimodality strategies combining chemotherapy (CT), radiotherapy (RT), and surgery have been proposed; however, the type of surgery remains controversial. Pleuropneumonectomy (PP), radical pleurectomy/decortication (P/D), and local excision (LE) of pleural, pericardial, and diaphragmatic implants are possible surgical options.7–9)
Materials and Methods
Patient characteristics
Of 191 patients undergoing surgery for thymoma in our department between January 2002 and December 2015, 39 (20.4%) had Masaoka stage IVa. Informed consent was obtained from all patients, and the study was approved by our institutional review board. Age, sex, histopathological tumor type, myasthenic status of the Ossermann–Genkins score, Masaoka stage at the first surgery, neoadjuvant treatment, number and type of surgeries, preferred incision, complications, perioperative mortality, length of postoperative hospital stay, adjuvant treatment, and survival rates were recorded. A retrospective analysis was performed based on a prospectively collected database.
Indications
In all, 26 (66.6%) patients had Masaoka stage IVa thymoma at first admission to our department and underwent primary surgeries, whereas nine (23.1%) underwent secondary surgeries and four (10.3%) underwent tertiary surgeries for pleural or pericardial recurrences. All patients were evaluated preoperatively by the thoracic oncology board for multimodality treatment. Patients with generalized myasthenia gravis (MG) were examined in the neurology and anesthesiology departments and underwent surgery only with the approval of these departments. Patients with poor generalized myasthenic conditions were recommended for intravenous immunoglobulin (IVIG) treatment prior to surgery.
Operative technique
Anesthetic management. An experienced anesthetist preoperatively assessed all patients for MG and thoracic surgery. If needed, patients with MG received IVIG treatment the week prior to surgery, and all patients received the usual dose of pyridostigmine and steroids on the day of surgery. Electrocardiogram, blood pressure, pulse oximetry (Primus; Dräger, Lübeck, Germany), and neuromuscular function (TOF-Watch S monitor; Organon, Dublin, Ireland) were monitored in the operating room. Double lumen tubes (Mallinckrodt Medical, Athlone, Ireland) (35, 37, or 39 Fr) were used for lung isolation.
Surgical technique. In this cohort, patients underwent three types of surgeries: 1) PP, 2) P/D, and 3) LE of pleural, pericardial, and diaphragmatic implants. PP procedures were performed with posterolateral thoracotomy, as with the mesothelioma surgery described by Sugarbaker et al.10) Double-level thoracotomy with a single incision was necessary in most cases. Indications for PP included the need for pneumonectomy as well as disseminated pleural and diaphragm metastases. In addition to total median sternotomy, posterolateral thoracotomy, thoracoscopy, and sternothoracotomy were used in the other two procedures. In all primary surgeries, radical thymothymectomy was performed initially, with resection of the mediastinal pleura, pericardium, lung, or any associated vascular structure. Mediastinal lymph node dissection was performed in all surgeries. Pleuropericardial and diaphragmatic thymoma spreads were resected with PP, P/D, or LE in secondary and tertiary surgeries. Pericardial defects were reconstructed with polypropylene or polytetrafluoroethylene (PTFE) patches and diaphragmatic defects with PTFE patches. Three thoracic drains (28 F) were placed for P/D; the other surgeries required single or double drains.
Postoperative treatment
Patients underwent platinum-based CT, 45–60 Gy RT, or chemoradiotherapy (CT + RT), based on their oncological status and performance.
Statistical analyses
Data were presented as the mean ± standard error of the mean. Patient survival was calculated by Kaplan–Meier analysis with the log-rank test to determine statistically significant differences between groups. The median survival time was presented as the median ± standard error with 95% confidence interval (CI). Statistical analyses were performed using SPSS 22.0 software for Windows (SPSS Inc., Chicago, IL). A P value less than 0.05 was considered statistically significant.
Results
The average age was 41.8 ± 12 years (range, 17–70 years), and 22 (56%) patients were male. Thymoma B2 was the most common histopathological tumor type (n = 16, 41%). In all, 18 (46.2%) patients had MG. Patient characteristics and preoperative data are listed in Table 1.
Table 1. Demographics and outcomes of the 39 Masaoka stage IVa patients.
| Sex, n (%) | Male: 22 (56.4) |
|---|---|
| Female: 17 (43.6) | |
| Age, mean ± SD (range, years) | 41.8 ± 12 (17–70) |
| Thymoma histological classification, n (%) | B1: 8 (20.5) |
| B2: 16 (41) | |
| B3: 6 (15.4) | |
| C: 9 (23.1) | |
| Myasthenia gravis presence, n (%) | Positive: 18 (46.2) patients |
| Negative: 21 (53.8) patients | |
| Modified Osserman–Genkins score, n (%) | Class 2: 6 (15.4) patients |
| Class 3: 9 (23.1) patients | |
| Class 4: 3 (7.7) patients | |
| Neoadjuvant treatment, n (%) | 25 (64.1) patients |
| Number of the operation, n (%) | Primary: 26 (66.6) |
| Secondary: 9 (23.1) | |
| Tertiary: 4 (10.3) | |
| Operation type, n (%) | Pleuropneumonectomy: 3 (7.7) |
| Radical pleurectomy/decortication: 19 (48.7) | |
| Local excision of the pleural/pericardial/ diaphragmatic implants: 17 (43.6) |
|
| Preferred incision, n (%) | Sternotomy: 18 (46.2) |
| Thoracotomy: 13 (33.3) | |
| (four single level, nine double level) | |
| Sternotomy + thoracoscopy: 4 (10.3) | |
| Sternotomy + thoracotomy: 4 (10.3) | |
| Complications, n (%) | 12 (30.8) patients |
| Hemorrhage: 1 (2.6) | |
| Wound infection: 3 (7.7) | |
| Pneumonia: 1 (2.6) | |
| Cardiac arrhythmia: 2 (5.1) | |
| Bronchopleural fistula: 1 (2.6) | |
| Chylothorax: 4 (10.3) | |
| Perioperative mortality, n (%) | 1 (2.6) |
| PP: 1/3 (33%) | |
| P/D: 0 | |
| LE: 0 | |
| Mean length of hospital stay (mean ± SD [range, days]) | 12.0 ± 1.5 (4–61) |
| PP: 18 ± 8.7 (13–28) | |
| P/D: 13.7 ± 12.5 (6–61) | |
| LE: 9.2 ± 3.3 (4–18) | |
| Adjuvant treatment, n (%) | 35 (89.7) patients |
| CT: 13 (33.3) | |
| RT: 8 (20.5) | |
| CT-RT: 14 (35.9) | |
PP: pleuropneumonectomy; P/D: radical pleurectomy/decortication; LE: local excision of the pleural/pericardial/diaphragmatic implants; CT: chemotherapy; RT: radiotherapy; CT + RT: chemo-radiotherapy; SD: standard deviation
In all, 26 patients underwent primary surgeries for Masaoka stage IVa thymoma, whereas nine underwent secondary and four underwent tertiary surgeries for pleural or pericardial recurrences. Three patients underwent PP, 19 underwent radical P/D, and 17 underwent LE of pleural, pericardial, and diaphragmatic implants. Double-level thoracotomy with a single incision was necessary for two PP, nine P/D, and two LE cases.
In total, 25 (64.1%) patients underwent neoadjuvant treatment before surgery; 18 of these underwent primary surgeries.
The morbidity and mortality rates were 30.8% and 2.6%, respectively. The only death occurred because of inoperable disease in a patient who underwent CT + RT (56 Gy). As a complication of treatment, he developed sepsis and a destroyed lung. For salvage surgery, he underwent left pleural–pericardial–pneumonectomy, which resulted in complete bronchial dehiscence, and he died from sepsis on postoperative day 6. Mean hospital stay was 12.0 ± 1.5 (4–61) days. In all, 35 (89.7%) patients underwent postoperative adjuvant treatment: 24 after primary surgeries, eight after secondary surgeries, and three after tertiary surgeries.
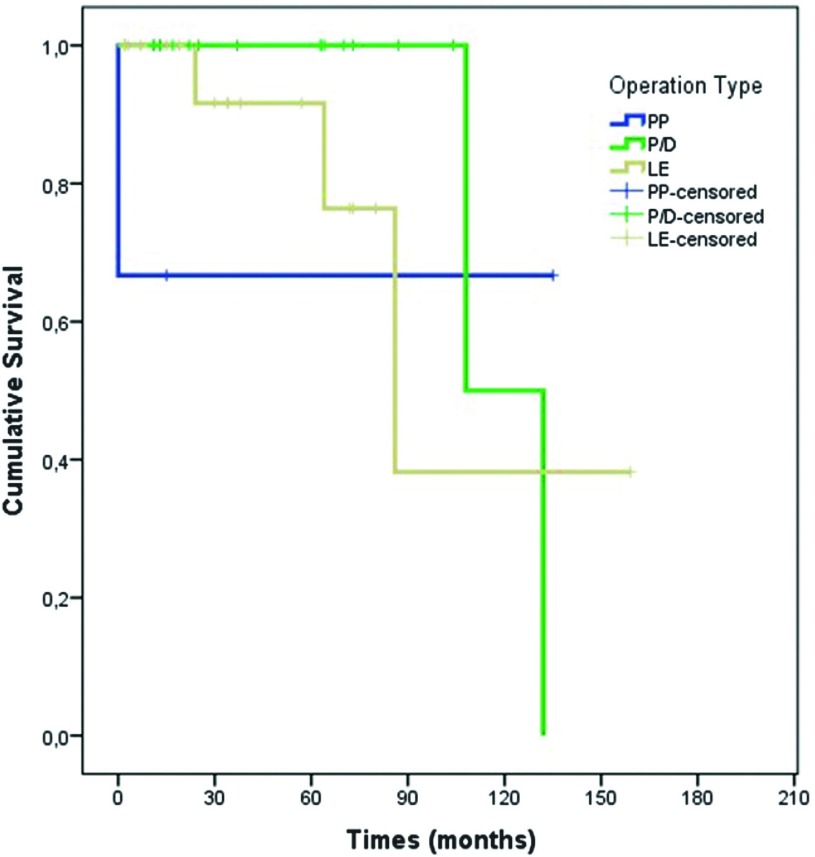
Median survival of all patients was 132 ± 25 (82–181; 95% CI) months. Median survival for secondary surgeries was 108 months, whereas it was 86 months for tertiary surgeries (Fig. 1). Overall, 3-, 5-, and 10-year survival rates were 93%, 93%, and 56%, respectively. The 3-, 5-, and 10-year survival rates were 90%, 90%, and 72% for primary surgeries; 100%, 100%, and 0% for secondary surgeries; and 100%, 100%, and 50% for tertiary surgeries, respectively.
Fig. 1. Survival graphic: surgery type. PP: pleuropneumonectomy; P/D: radical pleurectomy/decortication; LE: local excision of the pleural/pericardial/diaphragmatic implants.
No statistically significant differences were found in survival analysis in terms of sex, histological tumor subtype, MG presence, Ossermann–Genkins score, neoadjuvant treatment, number of surgeries, surgery type, preferred incision, or complications (P >0.05). The patients who underwent adjuvant CT + RT had better survival rates than those who underwent no adjuvant treatment or only CT or only RT; however, this difference was not statistically significant (P = 0.08) (Table 2).
Table 2. Survival analyze.
| N = | 3 years | 5 years | 10 years | Univariate analysis P value | |
|---|---|---|---|---|---|
| Sex | 0.93 | ||||
| Male | 22 | 87% | 87% | 58% | |
| Female | 17 | 100% | 100% | 45% | |
| Histological subtype | 0.11 | ||||
| B1 | 8 | 100% | 100% | 75% | |
| B2 | 16 | 94% | 94% | 94% | |
| B3 | 6 | 100% | 100% | 100% | |
| C | 9 | 75% | 75% | - | |
| MG presence | 0.98 | ||||
| Yes | 18 | 100% | 100% | 37% | |
| No | 21 | 86% | 86% | 71% | |
| Osserman | 0.74 | ||||
| 1 | 21 | 86% | 86% | 71% | |
| 2 | 6 | 100% | 100% | 0% | |
| 3 | 9 | 100% | 100% | - | |
| 4 | 3 | 100% | 100% | 50% | |
| Neoadjuvant | 0.80 | ||||
| Yes | 25 | 88% | 88% | 51% | |
| No | 14 | 100% | 100% | 67% | |
| Number of the operation | 0.81 | ||||
| Primary | 26 | 90% | 90% | 72% | |
| Secondary | 9 | 100% | 100% | - | |
| Tertiary | 4 | 100% | 100% | 50% | |
| Operation type | 0.77 | ||||
| PP | 3 | 67% | 67% | 67% | |
| P/D | 19 | 100% | 100% | 50% | |
| LE | 17 | 92% | 92% | 38% | |
| Preferred incision | 0.27 | ||||
| Sternotomy | 18 | 93% | 93% | 70% | |
| Thoracotomy | 13 | 92% | 92% | - | |
| Sternotomy + thoracoscopy | 4 | 100% | 100% | - | |
| Sternotomy + thoracotomy | 4 | 100% | 50% | - | |
| Complications | 0.78 | ||||
| Yes | 12 | 92% | 92% | 46% | |
| No | 27 | 94% | 94% | 62% | |
| Adjuvant | 0.08 | ||||
| None | 4 | 75% | 75% | - | |
| CT | 13 | 100% | 100% | 40% | |
| RT | 8 | 83% | 83% | 83% | |
| CT-RT | 14 | 100% | 100% | 100% | |
PP: pleuropneumonectomy; P/D: radical pleurectomy/decortication; LE: local excision of the pleural/pericardial/diaphragmatic implants; CT: chemotherapy; RT: radiotherapy; CT + RT: chemo-radiotherapy
Eight patients had a Masaoka stage ≤ III tumor and 31 had stage IVa (de novo IVa) at the primary surgery. Table 3 shows the comparison of patients with Masaoka stage IVa and stage ≤ III at the primary surgery who had secondary or tertiary surgeries for recurrences.
Table 3. Comparison of de novo Masaoka stage IVa and recurrences after primary surgery.
| Median age | Sex | MG | Neo | Adjuvant | Masaoka stage | Histology | Median time to recurrence (months) | Median time to re-recurrence (months) | Number of operation | 3-years survival (%) | 5-years survival (%) | 10-years survival (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage at primary operation ≤ III n: 8 |
37.4 ± 11.2 [22–58] |
5 ♂ 2 ♀ | 7 | 5 | None: 2 CT: 3 RT: 0 CT-RT: 3 |
II: 6 III: 2 |
B1: 4 B2: 2 B3: 1 C: 1 |
51.4 ± 39.1 | 38.7 ± 26.0 | 5 secondary, 3 tertiary |
100 | 100 | 38 |
| Stage at primary operation = IVa (de novo IVa) n: 31 |
42.9 ± 12.0 [17–70] |
20 ♂ 11 ♀ |
11 | 20 | None: 2 CT: 10 RT: 8 CT-RT: 11 |
IVa: 31 | B1: 4 B2: 14 B3: 5 C: 8 |
35.7 ± 24.3 | 30.0 ± 0 | 26 primary 4 secondary, 1 tertiary |
91 | 91 | 80 |
MG: myasthenia gravis; CT-RT: chemotherapy and radiotherapy; CT: chemotherapy; RT: radiotherapy
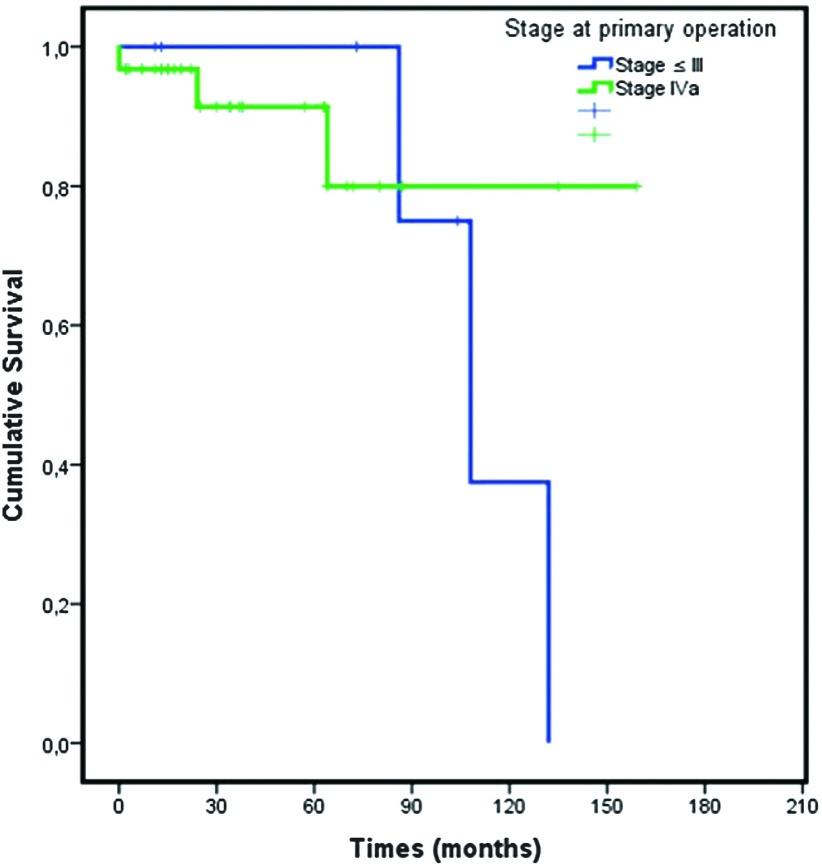
Mean survival for patients with Masaoka stage ≤ III tumor at the primary surgery was 111.5 ± 11.9 months and 135.7 ± 12.8 months for patients with de novo IVa (P = 0.53) (Fig. 2). The 3-, 5-, and 10-year survival rates of patients with Masaoka stage ≤III at the primary surgery were 100%, 100%, and 38%, respectively, and those of patients with de novo IVa were 91%, 91%, and 80%, respectively (Table 3).
Fig. 2. Survival graphic: stage at primary surgery.
Discussion
A mean age of 41.8 years, male predominance, and B2 as the most common histological subtype are consistent with the literature.7,8) We obtained an MG positivity rate of 46%, which is higher than the expected percentage (35%),1) possibly because of genetic heterogeneity of the disease.
Like most investigators, we prefer to perform surgery after two to four cycles of platinum-based induction CT.9) Surgeries were generally performed within 6 weeks after the completion of neoadjuvant treatment.
Sternotomy is the gold standard incision for primary thymothymectomy surgeries, but thoracotomy is always necessary for PP or P/D procedures.7–9) Contradictory to the literature, chylothorax was the most common complication in our study and was possibly caused by aggressive lymphadenectomy. Bronchopleural fistula (BPF), cardiac arrhythmia, hemorrhage, and empyema are the more common complications presented in the literature.7,8) The median hospital stay in our study was 12 days, which is shorter than that reported in the literature; a mean hospital stay of 15 days was reported by Reid et al.8) PP is always associated with a higher mortality rate. Fabre et al.7) reported a postoperative mortality rate of 29.4% for PP, whereas ours was 33.3%. Postpneumonectomy, BPF, and acute respiratory distress syndrome are common fatal complications in these patients. Although the incidences of complications and mortality rates are decreasing, candidates for PP should be chosen carefully.7) Criteria include young age (<60 years old), good functional status, and no comorbidities, so the benefit of surgery is worth the risk.7) The only patient who died in our study was operated on after CT and RT; he had a destroyed and septic left lung due to radical oncological treatment and was in poor condition. The 5-year survival rate for PP has been reported to be over 75% in three large series, whereas it was 67% in ours.11–13)
Radical P/D and LE surgeries have been performed for years; Huang12) reported 14 patients, Ishikawa13) seven patients, and Lucchi14) 20 patients after pleurectomy of stage IVa tumor with zero mortality, acceptable morbidity, and satisfactory overall survival results. The presented 5- and 10-year survival rates of these patients were over 40% and 25%, respectively.12–14) Those patients who were inoperable and underwent CT + RT were reported to have a 70% overall response rate and a 5-year survival of 53%, which is comparable to reported incomplete surgery results.15) In another study that included 30 patients with stage IV or locally progressive recurrent tumor, CT + RT achieved a 50% response rate and a 5-year survival of 32%.16) As seen in the literature, survival without surgery for Masaoka stage IVa tumor is poor. Our survival rates seem to be superior to others; however, unresectability or the poor general condition of patients who were not referred to surgery should be considered.
Patients with de novo IVa tumors had a tendency to show a shorter time to recurrence, re-recurrence, and worse 3- and 5-year survival rates than those with Masaoka stage ≤ III tumors at the first surgery; however, this difference was not statistically significant (P >0.05).
The question of which surgery should be done, PP, P/D, or LE, cannot be answered definitively. Recurrence localization, patients’ medical condition, and surgeons’ expertise are the deciding factors. Heated perfusion of intrapleural CT has been performed after radical P/D with relatively good results; this may also be considered.17) We conclude that all three types of resections and re-resections are treatment options for Masaoka stage IV thymomas, to achieve local control and maintain good long-term survival.
In our experience, the magnitude of resection is strongly related to tumor invasiveness. We performed PP in patients who could not be treated otherwise. We prefer to perform P/D in patients whose resection can be completed with pleural and diaphragmatic–pericardial resections with lung preservation. We believe a lung-preservation strategy may be superior to PP in terms of postoperative and long-term morbidity. Our long-term results did not show any significant survival advantage of PP; however, our other two patients are recurrence free and healthy. LE may be an option for those with limited pleural metastases. In these patients, P/D may be an alternative after CT in case of recurrence in the ipsilateral or contralateral hemithoracic cavity.
Conclusion
This report demonstrated that surgical treatment should be considered as a completion modality to oncological therapy and has the potential to provide long-term survival of Masaoka stage IVa in patients with thymoma. Invasiveness of the lesion should determine the type of surgery required in these patients. We speculate that PP may be excessive for a technically suitable P/D or LE candidate.
Disclosure Statement
We have no financial or potential conflicts of interest.
References
- 1).Detterbeck FC, Parsons AM. Thymic tumors. Ann Thorac Surg 2004; 77: 1860-9. [DOI] [PubMed] [Google Scholar]
- 2).Rosai J, Sobin L. Histological classification of tumours of the thymus. In: Rosai J, Sobin L. eds.; World Health Organization International Histological Classification of Tumours. Histological Typing of Tumours of the Thymus; Heidelberg, Springer, 1999; pp 1-16. [Google Scholar]
- 3).Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981; 48: 2485-92. [DOI] [PubMed] [Google Scholar]
- 4).Venuta F, Anile M, Diso D, et al. Thymoma and thymic carcinoma. Eur J Cardiothorac Surg 2010; 37: 13-25. [DOI] [PubMed] [Google Scholar]
- 5).Ried M, Guth H, Potzger T, et al. Surgical resection of thymoma still represents the first choice of treatment. Thorac Cardiovasc Surg 2012; 60: 145-9. [DOI] [PubMed] [Google Scholar]
- 6).Margaritora S, Cesario A, Cusumano G, et al. Thirty-five-year follow-up analysis of clinical and pathologic outcomes of thymoma surgery. Ann Thorac Surg 2010; 89: 245-52; discussion 252. [DOI] [PubMed] [Google Scholar]
- 7).Fabre D, Fadel E, Mussot S, et al. Long-term outcome of pleuropneumonectomy for Masaoka stage IVa thymoma. Eur J Cardiothorac Surg 2011; 39: e133-8. [DOI] [PubMed] [Google Scholar]
- 8).Ried M, Potzger T, Sziklavari Z, et al. Extended surgical resections of advanced thymoma Masaoka stages III and IVa facilitate outcome. Thorac Cardiovasc Surg 2014; 62: 161-8. [DOI] [PubMed] [Google Scholar]
- 9).Wright CD. Stage IVA thymoma: patterns of spread and surgical management. Thorac Surg Clin 2011; 21: 93-7, vii. [DOI] [PubMed] [Google Scholar]
- 10).Sugarbaker DJ, Mentzer SJ, Strauss G. Extrapleural pneumonectomy in the treatment of malignant pleural mesothelioma. Ann Thorac Surg 1992; 54: 941-6. [DOI] [PubMed] [Google Scholar]
- 11).Wright CD. Pleuropneumonectomy for the treatment of Masaoka stage IVA thymoma. Ann Thorac Surg 2006; 82: 1234-9. [DOI] [PubMed] [Google Scholar]
- 12).Huang J, Rizk NP, Travis WD, et al. Feasibility of multimodality therapy including extended resections in stage IVA thymoma. J Thorac Cardiovasc Surg 2007; 134: 1477-83; discussion 1483-4. [DOI] [PubMed] [Google Scholar]
- 13).Ishikawa Y, Matsuguma H, Nakahara R, et al. Multimodality therapy for patients with invasive thymoma disseminated into the pleural cavity: the potential role of extrapleural pneumonectomy. Ann Thorac Surg 2009; 88: 952-7. [DOI] [PubMed] [Google Scholar]
- 14).Lucchi M, Davini F, Ricciardi R, et al. Management of pleural recurrence after curative resection of thymoma. J Thorac Cardiovasc Surg 2009; 137: 1185-9. [DOI] [PubMed] [Google Scholar]
- 15).Loehrer PJ, Chen M, Kim K, et al. Cisplatin, doxorubicin, and cyclophosphamide plus thoracic radiation therapy for limited-stage unresectable thymoma: an intergroup trial. J Clin Oncol 1997; 15: 3093-9. [DOI] [PubMed] [Google Scholar]
- 16).Loehrer PJ, Sr, Kim K, Aisner SC, et al. Cisplatin plus doxorubicin plus cyclophosphamide in metastatic or recurrent thymoma: final results of an intergroup trial. The Eastern Cooperative Oncology Group, Southwest Oncology Group, and Southeastern Cancer Study Group. J Clin Oncol 1994; 12: 1164-8. [DOI] [PubMed] [Google Scholar]
- 17).Yellin A, Simansky DA, Ben-Avi R, et al. Resection and heated pleural chemoperfusion in patients with thymic epithelial malignant disease and pleural spread: a single-institution experience. J Thorac Cardiovasc Surg 2013; 145: 83-7; discussion 87-9. [DOI] [PubMed] [Google Scholar]