Abstract
Purpose: Spontaneous pneumothorax (PNTX) is a common disease frequently operated at specialized thoracic surgery units. Videothoracoscopic surgery (VATS) has become the standard for treatment and recurrence prevention. While there is broad consensus regarding indications and techniques of PNTX surgery, postoperative risks and consecutive patient behavioral advice have not been sufficiently elucidated.
Methods: Single-center cohort analysis of 641 patients operated for primary PNTX by VATS over 10 years. Putatively recurrence-prone lifestyle activities (smoking status, flying habits, and scuba diving) and actual occurrence of recurrences were correlated.
Results: Follow-up rate was 46% (279/607 patients). Mean time interval between primary operation and follow-up was 61 (range: 5–177) months. In 10 patients (3.6%), a PNTX recurrence was observed. Regarding postoperative risk behavior reported at follow-up, 28% of patients were active smokers (15 ± 7 cigarettes/day), 59% traveled by plane repeatedly, and only two patients did scuba diving (0.7%). Low body-mass-index was associated with an increase in PNTX recurrence, whereas smoking, flying, and scuba diving could not be identified as risk factors.
Conclusion: In our study, none of the supposed “classic” lifestyle-associated risk factors for PNTX recurrence after VATS proved to be a significant threat. Postoperative patient behavior might not be constrained by overcautious medical advice.
Keywords: long-term follow-up, pneumothorax, recurrence, risk factors, VATS
Introduction
Pneumothorax (PNTX) is a common disease frequently treated at primary care hospitals and specialized thoracic surgery units alike. It may arise in adolescents with healthy lung tissue (primary PNTX) or in elder patients with underlying lung disease (secondary PNTX). Depending on gender, the incidence is estimated between 1.2 and 6 in females and 18–28 in males per 100,000/year. Without specific surgical therapy, recurrence rates of primary PNTX were reported between 4% and 17%, depending on the applied interventional method.1–3) Videothoracoscopic surgery (VATS) including pulmonary wedge resection and parietal pleurectomy has become the standard of care for PNTX treatment and recurrence prevention.4) While there is broad consensus regarding indications and techniques of PNTX surgery, ambiguity exists regarding essential medical advice for risk avoidance in operated patients to minimize recurrence rates.
Materials and Methods
Study design: We present a single-center cohort study at a specialized thoracic surgery unit. Consecutive patients operated for primary PNTX between January 1994 and December 2005 were included in the analysis. Patients with double-sided primary PNTX during the inclusion period were not considered for further work-up. Data were retrieved from patient records. For follow-up, patients were sent a questionnaire and contacted by telephone. The study protocol was assessed and approved by the Institutional Review Board of the Eberhard-KarlsUniversity Tuebingen (vote 280/2010A).
Surgical decision and patient education: The treatment policy for PNTX consisted of chest tube insertion for all primary PNTX events. In case of prolonged air leaks or persisting PNTX or recurrent PNTX, VATS surgery was performed. Standard operative technique during the study period consisted of three-port VAT access using 5 and 10 mm ports, total parietal pleurectomy, and wedge resection of the lung apex. At hospital discharge, patients were educated about the supposed increased risk of PNTX recurrence associated with continued smoking, air travel, and scuba diving.
Follow-up procedures: For follow-up, patients were sent a questionnaire querying postoperative PNTX recurrences, previously delineated sequelae following VATS surgery, that is, long-term physical and anxietyinduced limitations, and putatively recurrence-prone risk behavior. The latter included current smoking (specified by number of cigarettes per day), scuba diving, and flying habits. If patients had not returned the questionnaire within 4 weeks, they were contacted by telephone to obtain the required information.
Statistical analysis: The association between selfreported patient characteristics (age, height, gender, and body mass index), smoking habits, and risk behavior and the recurrence of PNTX was analyzed. To test for differences in disease characteristics, risk behavior, and postoperative sequelae between patients with and without PNTX recurrence, the Mann–Whitney-U test or Kruskal–Wallis test were used as appropriate for continuous variables. Conversely, the χ2-method or Fisher’s exact test were implemented for categorical variables. A p-value of <0.05 was considered to be statistically significant; all calculations were two-tailed. Statistical analyses were performed using the SPSS Statistics 22 software package (SPSS Inc., Chicago, IL, USA).
Results
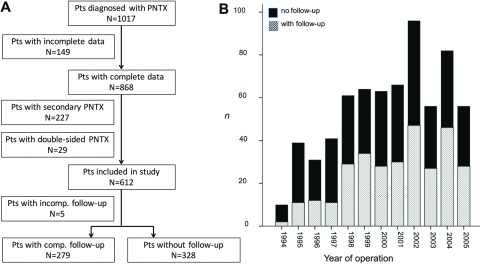
Patient cohort: Within the study period, 1017 patients were hospitalized for PNTX. Of these, 149 patient records were incomplete and therefore excluded from analysis (Fig. 1). Another 227 patients were excluded due to confirmed secondary PNTX. Furthermore, 29 patients with double-sided PNTX during their respective disease course were excluded from analysis to prevent retrospective data analysis issues, leaving 612 patients. Of these, 284 patients (47%) were available for followup, which was consistently completed in 279 patients. Our subgroup analysis revealed no relevant differences between the patients with and without completed followup. Interestingly, the postoperative hospital stay was shorter in the former subgroup (p = 0.02). Mean followup time was 61 months with a range of 5–177 months. VATS surgery was performed in 68 patients for the first occurrence of PNTX, 142 patients with the first recurrence, and 73 patients with more frequent recurrences (2nd recurrence: n = 52; 3rd recurrence: n = 16; 4th recurrence: n = 5) (Table 1).
Fig. 1. Composition of study cohort. (A) Flow chart showing reasons for patient exclusion from study. (B) Distribution of patients with and without follow-up over study period. Pts: patients; PNTX: pneumothorax.
Table 1. Comparison of subgroups with and without follow-up.
| All pts | Pts without follow-up | Pts with follow-up | p | |
|---|---|---|---|---|
| N | 607 | 328 | 279 | |
| Demographic characteristics | ||||
| Age, y, mean (SD) | 37.4 (16.1) | 37.7 (16.9) | 37.2 (15.2) | 0.93 |
| Female sex, n (%) | 162 (26.7) | 76 (23.2) | 86 (30.8) | 0.10 |
| Disease characteristics | ||||
| First manifestation PNTX , n (%) | 168 (27.7) | 106 (32.3) | 62 (22.2) | 0.02* |
| Number of recurrences of recurrent PNTX, mean (SD) | 1.4 (0.7) | 1.3 (0.6) | 1.5 (0.8) | 0.07 |
| In-hospital treatment results | ||||
| Perioperative complications, n (%) | 55 (9.1) | 30 (9.1) | 25 (9.0) | >0.99 |
| Postoperative hospital stay, d, mean (SD) | 11.9 (7.6) | 12.9 (8.9) | 10.1 (5.3) | 0.02* |
*Statistically significant. PNTX: pneumothorax; Pts: patients; y: years; d: days; SD: standard deviation
Long-term results: In all, 10 patients (3.6%) in the follow-up cohort sustained an ipsilateral PNTX recurrence following surgery after 1434 ± 1081 days (Table 2). Events occurred spontaneously in seven patients and were mostly treated conservatively (Table 3). Concerning afflictions attributed to previous VATS surgery, 49 patients (18%) declared limitations in their workaday life in terms of subjective inability to perform as before the event, associated with a permanent fear of PNTX recurrence in 33 individuals (12%).
Table 2. Long-term results and postoperative risk behavior in follow-up group.
| All pts with follow-up | Pts with PNTX recurrence | Pts without PNTX recurrence | p | |
|---|---|---|---|---|
| n | 279 | 10 | 269 | |
| Age, y, mean (SD) | 36.7 (15.2) | 31.1 (18.8) | 36.9 (15.1) | 0.34 |
| Female sex, n (%) | 86 (30.8) | 4 (40.0) | 82 (30.5) | 0.50 |
| BMI, mean (SD) | 23.4 (4.2) | 20.5 (2.4) | 23.5 (4.2) | 0.03* |
| Disease characteristics | ||||
| First manifestation PNTX, n (%) | 62 (22.2) | 1 (10.0) | 61 (22.7) | 0.47 |
| Number of recurrences of recurrent PNTX, mean (SD) | 1.7 (0.9) | 1.7 (0.9) | 1.5 (0.8) | 0.52 |
| In-hospital treatment results | ||||
| Perioperative complications, n (%) | 25 (9.0) | 1 (10.0) | 24 (8.9) | >0.99 |
| Postoperative hospital stay, d, mean (SD) | 10.7 (5.3) | 9.2 (3.3) | 10.7 (5.3) | 0.81 |
| Risk behaviors | ||||
| Smokers, n (%) | 78 (28.0) | 4 (40.0) | 74 (27.5) | 0.47 |
| No of cigarettes/d if smoker, mean (SD) | 15.3 (7.4) | 11.3 (7.5) | 15.5 (7.4) | 0.72 |
| Air travels n (%) | 166 (59.5) | 5 (50.0) | 161 (59.9) | 0.53 |
| Scuba diving n (%) | 2 (0.7) | 0 | 2 (0.7) | >0.99 |
| Follow-up results | ||||
| Postoperative sequelae | ||||
| Recurrence n (%) | 10 (3.6) | 10 (100) | 0 | - |
| Chest wall pain n (%) | 130 (46.6) | 6 (60.0) | 124 (46.1) | 0.52 |
| Dyspnea n (%) | 85 (30.5) | 5 (50.0) | 80 (29.7) | 0.17 |
| Workaday restrictions n (%) | 49 (17.6) | 3 (30.0) | 46 (17.1) | 0.39 |
*Statistically significant. PNTX: pneumothorax; Pts: patients; BMI: body mass index; SD: standard deviation
Table 3. PNTX recurrences after surgery.
| Cause of recurrence | Event-free interval (d) | Treatment |
|---|---|---|
| Spontaneous | 1660 | Conservative |
| Spontaneous | 690 | Conservative |
| Spontaneous | 22 | Conservative |
| Exercise-triggered | 2670 | Conservative |
| Spontaneous | 1882 | Re-operation |
| Secondary abdominal surgery | 329 | Conservative |
| Spontaneous | 2612 | Re-operation |
| Spontaneous | 163 | Conservative |
| Spontaneous | 2838 | Chest drain insertion |
| Common cold strain | 1470 | Conservative |
PNTX: pneumothorax
Moreover, 30% of patients reported episodes of dyspnea (daily: n = 22 (8%); weekly: n = 22 (8%); monthly: n = 42 (15%)), in part deriving from permanent (n = 41, 15%) or recurrent (weekly: n = 26; monthly: n = 65) chest pain (n = 132, 47% of patients).
Postoperative risk behavior: Of the 279 patients available for follow-up, 28% (n = 78) were active smokers with a mean consumption of 15 ± 7 cigarettes per day. Questioned whether their history of PNTX restrained patients from air travels or scuba diving, 59% of patients (n = 166) declared that they repeatedly traveled by plane, but only two patients (0.7%) had started leisure scuba diving. Recurrence of PNTX was associated with body mass index (BMI) <21 kg/m2, whereas none of the aforementioned putative risk factors proved to be of any statistical significance in triggering a relapse (Table 2).
Discussion
Primary PNTX is a common disease entity in a young and healthy patient cohort with high recurrence rates when not treated adequately.1–3) Several guidelines and consensus statements about the treatment of primary PNTX exist and VATS procedures have become the mainstay of therapy and prevention of recurrences.5) While long-term results regarding recurrence prevention by VATS for PNTX therapy have been reported extensively in the literature, no data are available concerning recurrence-prone risk behavior of operated patients. Therefore, we conducted a cohort analysis of patients operated for primary PNTX at our institution, primarily addressing this open issue.
We analyzed the records of all patients operated for primary PNTX over a total time period of 11 years. Although mean follow-up time was more than 5 years, only 46% of our operated patients could be retrieved for follow-up, probably reflecting the difficulty of properly following these patients: They are usually young, thus relocate frequently, and do not see the need for surgical control once they have been treated. The different lengths in postoperative hospital stay of patients with and without completed follow-up may be interpreted as selection bias favoring a feedback of patients with a more “smooth” postoperative recovery. However, our analysis of perioperative complications defined as persistent PNTX, persistent air leak, soft tissue emphysema, pulmonary re-expansion edema, bleeding, wound infection, pleural empyema, phrenic nerve palsy, and Horner syndrome did not indicate a difference between both groups (Table 1).
Our postoperative PNTX recurrence rate of 3.6% equates previous findings of other groups (Table 4).4,6–21) However, concerning long-term sequelae in terms of workaday restrictions caused by pain and anxiety attributed to surgery, the presented data have to be interpreted with caution. Especially in the light of (i) strong interindividual estimation differences as to the degree of impairment and (ii) the VATS technique applied at the investigated timeframe (three-port approach), a somewhat higher percentage (45% of VATS patients) can be anticipated. In contrast, new uniportal approaches may be associated with significantly fewer and less untoward consecutive symptoms22–24) and eventually may offer economic advantages.25)
Table 4. Long-term follow-up studies in patients operated for primary PNTX.
| First author | Publication year | Study period (months) | Patient number (number of Ops) | Follow-up rate (%) | Follow-up period (months) | Recurrence rate (%) | Complication rate (%) |
|---|---|---|---|---|---|---|---|
| Chen6) | 2012 | 2006–2009 (40) | 369 (160) | N/A | 26 | 3.8 | 6.25 |
| Shaikhrezai7) | 2011 | 1992–2008 (192) | 569 (644) | N/A | 73 | 2 | 12 |
| Rena8) | 2008 | 2001–2004 (42) | 208 (220) | 80.5 | 46 (24–66) | 5.4 | N/A |
| Marcheix9) | 2007 | 1995–2004 (112) | 250 | 73.6 | 34 | 1.1 | N/A |
| Cardillo10) | 2006 | 1995–2004 (112) | 861 | 93.4 | 52.5 (3–108) | 1.7 | 3.36 |
| Chen11) | 2003 | 2001–2002 (12) | 63 | 100 | 8 (2–14) | 3.6 | 7.9 |
| Lang-Lazdunski4) | 2003 | 1991–1997 (78) | 182 | 92 | 93 (57–134) | 3 | 27.4 |
| Passlick12) | 1998 | 1992–1995 (45) | 99 | N/A | 29 | 4.8 | 7 |
| Mouroux13) | 1996 | 1991–1994 (42) | 97 (100) | 100 | 30 (7–49) | 3 | 10 |
| Körner14) | 1996 | 1974–1992 (216) | 120 (132) | 97 | 84 (6–229) | 9.8 | 9.8 |
| Bertrand15) | 1996 | 1991–1994 (38) | 163 | 91.5 | 24.5 (5–42) | 3.7 | 5.5 |
| Naunheim16) | 1995 | 1991–1993 (35) | 113 (121) | 89 | 13 (1–34) | 4.1 | 8 |
| Donahue17) | 1993 | 1965–1985 (240) | 83 | 100 | 109 (60–300) | 9.6 | 16.8 |
| Thomas18) | 1993 | 1988–1978 (36) | 107 | 91.6 | 27 (3–51) | 0 | 14 |
| Thevenet19) | 1992 | 1970–1989 (228) | 278 (325) | 74.8 | 84 | 1 | 5.5 |
| Weeden20) | 1983 | 1972–1982 (156) | 233 (241) | 97 | 56 (5–137) | 0.4 | 24 |
| Deslauriers21) | 1980 | 1962–1978 (192) | 362 (409) | 86 | 54 (12–192) | 0.6 | 8 |
PNTX: pneumothorax; Ops: operations
Regarding relapse association with previously supposed “risky bearings,” we were surprised to find no published indication for any specific postoperative cautious advice to be given to PNTX patients.
In our analysis, active cigarette smoking does not predispose operated patients to new PNTX episodes (Table 2). This finding seems to contradict previous clinical observations of other groups, that cigarette smoking is associated with PNTX recurrence following surgery26,27) although the literature is less than unequivocal regarding this aspect.28) Nevertheless, our observation could be explained by our study being underpowered to detect small, yet significant differences considering there are as few as 87 smokers included in our analysis. The same certainly holds true for scuba diving (only two patients).
As regards flying by plane, barometric variations present during takeoff and landing phases of air travels are generally believed to represent potential hazards for patients that were operated for PNTX. So far, this assumption has not been confirmed in clinical studies. Therefore, we were interested (i) whether our patients operated for PNTX followed our recommendation to avoid air travels and (ii) whether we could find evidence that air travels were associated with increased recurrence rates for PNTX. In our study population, 58% of patients travelled by plane on a regular basis. This finding, however, did not translate into increased proportions of recurrence for PNTX (Table 2), even taking into account, that the prevalence of individuals traveling by plane is higher in our patient cohort than in the average German population.29) Therefore, our data do not support the assumption, that air travels represent a relevant risk factor for PNTX recurrences following VAT surgery.
Conclusion
Neither air travel and scuba diving nor smoking could be identified as independent risk factors for PNTX recurrence after VATS and thus “classic” medical behavioral restriction advice might not be justified from the current point of view. The shortcoming of recommendations from this and other retrospective single-center analyses should be solidified in future prospective, randomized, multicenter clinical trials such as the ongoing WOPP study (“Wedge Resection or Parietal Pleurectomy for the Treatment of Recurrent Pneumothorax”; NCT01855464).
Disclosure Statement
Volker Steger, Ulrike Sostheim, Marcus Leistner, and Thorsten Walles have no conflicts of interest.
References
- 1).Brown SGA, Ball EL, Macdonald SPJ, et al. Spontaneous pneumothorax; a multicentre retrospective analysis of emergency treatment, complications and outcomes. Intern Med J 2014; 44: 450-7. [DOI] [PubMed] [Google Scholar]
- 2).Massongo M, Leroy S, Scherpereel A, et al. Outpatient management of primary spontaneous pneumothorax: a prospective study. Eur Respir J 2014; 43: 582-90. [DOI] [PubMed] [Google Scholar]
- 3).Parlak M, Uil SM, van den Berg JW. A prospective, randomised trial of pneumothorax therapy: manual aspiration versus conventional chest tube drainage. Respir Med 2012; 106: 1600-5. [DOI] [PubMed] [Google Scholar]
- 4).Lang-Lazdunski L, Chapuis O, Bonnet PM, et al. Videothoracoscopic bleb excision and pleural abrasion for the treatment of primary spontaneous pneumothorax: long-term results. Ann Thorac Surg 2003; 75: 960-5. [DOI] [PubMed] [Google Scholar]
- 5).Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001; 119: 590-602. [DOI] [PubMed] [Google Scholar]
- 6).Chen JS, Hsu HH, Huang PM, et al. Thoracoscopic pleurodesis for primary spontaneous pneumothorax with high recurrence risk: a prospective randomized trial. Ann Surg 2012; 255: 440-5. [DOI] [PubMed] [Google Scholar]
- 7).Shaikhrezai K, Thompson AI, Parkin C, et al. Video-assisted thoracoscopic surgery management of spontaneous pneumothorax–long-term results. Eur J Cardiothorac Surg 2011; 40: 120-3. [DOI] [PubMed] [Google Scholar]
- 8).Rena O, Massera F, Papalia E, et al. Surgical pleurodesis for Vanderschueren’s stage III primary spontaneous pneumothorax. Eur Respir J 2008; 31: 837-41. [DOI] [PubMed] [Google Scholar]
- 9).Marcheix B, Brouchet L, Renaud C, et al. Videothoracoscopic silver nitrate pleurodesis for primary spontaneous pneumothorax: an alternative to pleurectomy and pleural abrasion? Eur J Cardiothorax Surg 2007; 31: 1106-9. [DOI] [PubMed] [Google Scholar]
- 10).Cardillo G, Carleo F, Giunti R, et al. Videothoracoscopic talc poudrage in primary spontaneous pneumothorax: a single-institution experience in 861 cases. J Thorac Cardiovasc Surg 2006; 131: 322-8. [DOI] [PubMed] [Google Scholar]
- 11).Chen JS, Hsu HH, Kuo SW, et al. Needlescopic versus conventional video-assisted thoracic surgery for primary spontaneous pneumothorax: a comparative study. Ann Thorac Surg 2003; 75: 1080-5. [DOI] [PubMed] [Google Scholar]
- 12).Passlick B, Born C, Häussinger K, et al. Efficiency of video-assisted thoracic surgery for primary and secondary spontaneous pneumothorax. Ann Thorac Surg 1998; 65: 324-7. [DOI] [PubMed] [Google Scholar]
- 13).Mouroux J, Elkaïm D, Padovani B, et al. Video-assisted thoracoscopic treatment of spontaneous pneumothorax: technique and results of one hundred cases. J Thorac Cardiovasc Surg 1996; 112: 385-91. [DOI] [PubMed] [Google Scholar]
- 14).Körner H, Andersen KS, Stangeland L, et al. Surgical treatment of spontaneous pneumothorax by wedge resection without pleurodesis or pleurectomy. Eur J Cardiothorac Surg 1996; 10: 656-9. [DOI] [PubMed] [Google Scholar]
- 15).Bertrand PC, Regnard JF, Spaggiari L, et al. Immediate and long-term results after surgical treatment of primary spontaneous pneumothorax by VATS. Ann Thorac Surg 1996; 61: 1641-5. [DOI] [PubMed] [Google Scholar]
- 16).Naunheim KS, Mack MJ, Hazelrigg SR, et al. Safety and efficacy of video-assisted thoracic surgical techniques for the treatment of spontaneous pneumothorax. J Thorac Cardiovasc Surg 1995; 109: 1198-203; discussion 1203-1194. [DOI] [PubMed] [Google Scholar]
- 17).Donahue DM, Wright CD, Viale G, et al. Resection of pulmonary blebs and pleurodesis for spontaneous pneumothorax. Chest 1993; 104: 1767-9. [DOI] [PubMed] [Google Scholar]
- 18).Thomas P, Le Mee F, Le Hors H, et al. [Results of surgical treatment of persistent or recurrent pneumothorax]. Ann Chir 1993; 47: 136-40. (in French) [PubMed] [Google Scholar]
- 19).Thévenet F, Gamondès JP, Bodzongo D, et al. [Spontaneous and recurrent pneumothorax. Surgical treatment. Apropos of 278 cases]. Ann Chir 1992; 46: 165-9. (in French) [PubMed] [Google Scholar]
- 20).Weeden D, Smith GH. Surgical experience in the management of spontaneous pneumothorax, 1972-82. Thorax 1983; 38: 737-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Deslauriers J, Beaulieu M, Després JP, et al. Transaxillary pleurectomy for treatment of spontaneous pneumothorax. Ann Thorac Surg 1980; 30: 569-74. [DOI] [PubMed] [Google Scholar]
- 22).Jutley RS, Khalil MW, Rocco G. Uniportal vs standard three-port VATS technique for spontaneous pneumothorax: comparison of post-operative pain and residual paraesthesia. Eur J Cardiothorac Surg 2005; 28: 43-6. [DOI] [PubMed] [Google Scholar]
- 23).Yang HC, Cho S, Jheon S. Single-incision thoracoscopic surgery for primary spontaneous pneumothorax using the SILS port compared with conventional three-port surgery. Surg Endosc 2013; 27: 139-45. [DOI] [PubMed] [Google Scholar]
- 24).Chen PR, Chen CK, Lin YS, et al. Single-inclusion thoracoscopic surgery for primary spontaneous pneumothorac. J Cardiothorac Surg 2011; 6: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Salati M, Brunelli A, Xiumè F, et al. Uniportal video-assisted thoracic surgery for primary spontaneous pneumothorax: clinical and economic analysis in comparison to the traditional approach. Interact Cardiovasc Thorac Surg 2008; 7: 63-6. [DOI] [PubMed] [Google Scholar]
- 26).Cheng YL, Huang TW, Lin CK, et al. The impact of smoking in primary spontaneous pneumothorax. J Thorac Cardiovasc Surg 2009; 138: 192-5. [DOI] [PubMed] [Google Scholar]
- 27).Janssen JP, Schramel FM, Sutedja TG, et al. Videothoracoscopic appearance of first and recurrent pneumothorax. Chest 1995; 108: 330-4. [DOI] [PubMed] [Google Scholar]
- 28).Uramoto H, Shimokawa H, Tanaka F. What factors predict recurrence of a spontaneous pneumothorax? J Cardiothorac Surg 2012; 7: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Jansen U, Delbeck J, Koska T. Herausforderungen nachhaltiger Verkehrspolitik - Welche Rolle spielt Verkehrsverlagerung? Protokoll der Arbeitsgruppen 2007. In: Wuppertal Institute for Climate, Environment and energy. URL: http://wupperinst.org/info/details/wi/a/s/ad/436. Accessed on 2017 July 31. (in German)