Abstract
Mutations in the AT-rich interactive domain 1A gene, which encodes a subunit of the Switch/Sucrose nonfermentable chromatin remodeling complex, can result in loss of protein expression and are associated with different cancers. Here, we used immunohistochemistry to investigate the significance of AT-rich interactive domain 1A loss in 73 pancreatic ductal adenocarcinoma cases with paired paracancerous normal pancreatic tissues. The relationship between levels of the AT-rich interactive domain 1A protein product, BAF250a, and clinicopathological parameters in the 73 pancreatic cancer specimens was also analyzed. We found that the expression of AT-rich interactive domain 1A in normal pancreatic tissue was higher than that in tumor tissue. Loss of AT-rich interactive domain 1A expression in pancreatic tumors was associated with tumor differentiation (P = .002) and tumor stage (P = .048). Meanwhile, BAF250a protein levels were not related to lymph node metastasis, distant metastasis, sex, or age and were not associated with survival. Transfection of the pancreatic cancer cell lines AsPC-1 and PANC-1 with small-interfering RNA specific for AT-rich interactive domain 1A resulted in elevated messenger RNA and protein expression levels of B-cell lymphoma-2 (Bcl-2), CyclinD1, and Kirsten rat sarcoma viral oncogene (KRAS). The AT-rich interactive domain 1A expression level in the cells was increased following microRNA-31 (miR-31) inhibitor transfection. Our data provide additional evidence that AT-rich interactive domain 1A might function as a tumor suppressor gene in pancreatic carcinogenesis.
Keywords: ARID1A, SWI/SNF complex, pancreatic ductal cancer, miR-31, KRAS
Introduction
The Switch/Sucrose nonfermentable (SWI/SNF) subunit AT-rich interactive domain 1A (ARID1A) gene was recently postulated to act as a tumor suppressor of cancer and a driver gene in carcinogenesis. A recent study using second-generation whole-genome sequencing revealed a high prevalence of ARID1A mutations in a variety of tumors.1-4 The ARID1A is located in the chromosome 1p36 region, which is frequently deleted in human tumors. Thus, ARID1A has emerged as a tumor suppressor in which frequent somatic mutations occur in several different human cancers. In whole-exome analyses of 22 malignant mixed Müllerian tumors, Jones et al identified genetic alterations in ARID1A and ARID1B, which are both involved in chromatin remodeling.5 These findings highlight the importance of chromatin remodeling dysregulation in carcinosarcoma tumorigenesis and suggest new avenues for personalized therapies. Meanwhile, Wang et al performed exome sequencing of 22 gastric cancer (GC) samples and also found frequent mutations in genes involved in chromatin modification.6 Through exome sequencing of 32 intrahepatic cholangiocarcinomas, Jiao et al discovered frequent inactivating mutations in multiple chromatin-remodeling genes, including BRCA1-associated protein 1 (BAP1), ARID1A, and polybromo 1 (PBRM1).2 The ARID1A was also found to act as a tumor suppressor gene in the pathogenesis of Barrett esophagus.7
The ARID1A gene encodes the BAF250a protein, which is an important component of the multi-protein SWI/SNF chromatin-remodeling complex. Most ARID1A somatic mutations are frame-shift or nonsense mutations that contribute to messenger RNA (mRNA) decay and loss of protein expression.8 Most ARID1A mutations resulting in a truncated protein are prone to rapid degradation.9 Mao et al discovered that the percentage of BAF250a protein loss increased from complex atypical hyperplasia to endometrioid carcinoma.10 These findings highlight the importance of ARID1A dysregulation in uterine endometrioid carcinoma tumorigenesis. The ARID1A mutation plays an important role in the process. The ARID1A is a member of SWI/SNF factors. If cancer cells had BAF250a protein loss, they were deficient in DNA repair and vulnerable to DNA damage.11
In pancreatic ductal adenocarcinoma, there are 4 major mutated genes (the so-called genetic mountains for PDAC), such as KRAS, TP53, SMAD4, and CDKN2A, but there are other important genes.12,13 Jones et al identified ARID1A mutations in 2% to 8% of tumors of the pancreas, breast, brain medulloblastomas, prostate, and lung.14 Mutations of ARID1A in PDAC have been recognized as a late event in PDAC carcinogenesis, since they are very rare in precursor lesions such as pancreatic intraepithelial neoplasms.15 Despite these findings, the relationship between ARID1A expression and clinicopathological features of PDAC is unclear. Pancreatic cancer is the fourth leading cause of cancer-related deaths in the United States,16 and PDAC accounts for over 90% of reported cases of pancreatic cancer. Treatment of pancreatic cancer continues to present a significant clinical challenge. Gene changes often affect protein expression levels and can ultimately affect cell function. As such, a better understanding of how the expression status of ARID1A affects pancreatic cancer disease progression could provide a foundation for the development of improved diagnostic and treatment strategies for PDAC.
Materials and Methods
Immunohistochemistry and Microscopic Analysis
This study was approved by the Peking Union Medical College Hospital (PUMCH) Ethics Committee for the Protection of Human Subjects (Peking, China). Informed consent was obtained from all participants prior to participating in the study and the inclusion of their data in the analysis. And the consent was written. Tissues were collected from patients undergoing PDAC surgery at PUMCH, Chinese Academy of Medical Sciences from April 2008 to October 2012. A total of 73 formalin-fixed, paraffin-embedded pancreatic adenocarcinoma samples with paired paracancerous normal pancreatic tissues were available. Immunohistochemical staining was performed on 3-μm-thick paraffin sections using anti-ARID1A (ab176395, clone: BAF250a, Abcam, Cambridge, MA, USA, 1:400) antibodies, with appropriate positive and negative controls according to the manufacturer’s standardized staining protocols. The ARID1A gene product BAF250a is a nuclear protein that is expressed to varying degrees in most human cells. As nuclear expression of BAF250a is expected to occur in lymphocytes, endothelial cells, and stromal cells, these cells served as internal positive controls. Slides were treated with conventional dewaxing and hydration, followed by washing with 10 mM Phosphate Buffered Saline (PBS). Antigen retrieval was achieved by boiling the samples for 3 minutes. The samples were incubated in 3% H2O2 for 10 minutes to block endogenous peroxidase. Nonspecific binding was blocked by incubating the tissue sections with diluted normal goat serum for 60 minutes. The ARID1A antibody was applied overnight at 4°C followed by incubation with a secondary antibody coupled to a horseradish peroxidase enzyme in Streptomyces egg white element working liquid, DAB coloration, double staining, and then sealing.
Cell Lines
Two human pancreatic adenocarcinoma cell lines (AsPC-1, PANC-1) were obtained from ATCC (Manassas, Virginia). All cell lines were cultured in the appropriate medium (Mediatech, Manassas, Virginia) containing 10% fetal bovine serum (Thermo Scientific, Waltham, Massachusetts) and maintained at 37°C and 5% CO2.
BAF250a Knockdown in Pancreatic Cancer Cell Lines AsPC-1 and PANC-1 Using Small-Interfering RNA Specific for ARID1A
Small-interfering RNA (siRNA) knockdown assay forward (5′-GCCCUGAACAAUAACCUCATT-3′) and reverse (5′-UGAGGUUAUUGUUCAGGGCTT-3′) oligonucleotides for ARID1A were purchased from Jima Shanghai, Shanghai, China. The oligonucleotides were annealed following the manufacturer’s protocol to generate double-stranded siRNAs at a final concentration of 20 μM. Cells (2 × 105) were cultured in 6-well plastic plates in 1 mL Dulbecco’s Modified Eagle Medium (DMEM) without serum and transfected at ∼40% confluence by adding 4 μL Oligofectamine (Invitrogen, San Diego, CA, USA) and 10 μL of 20 μM stock siRNAs. Cells were incubated at 37°C for 4 hours in a CO2 incubator followed by the addition of growth medium containing 3× the normal concentration of serum. Cells were maintained in culture before reverse transcription and quantitative real-time (PCR) and Western blotting. Three independent replicates were performed for each experiment.
miR-31 Treatment of AsPC-1 Pancreatic Cancer Cells
miR-31 inhibitor (5′-AGCUAUGCCAGCAUCUUGCCU-3′) was purchased from Jima Shanghai. Cells (2 × 105) were cultured in 6-well plastic plates in 1 mL DMEM without serum and transfected at ∼40% confluence by adding 4 μL Oligofectamine (Invitrogen) and 10 μL of 20 μM stock miR-31 inhibitor. Cells were incubated at 37°C for 4 hours in a CO2 incubator, followed by the addition of growth medium containing 2× the normal concentration of serum. Cells were maintained in culture for an additional 48 hours before Western blotting. Three independent replicates were performed for each experiment.
RNA Isolation and Quantitative Real-Time PCR Analysis
Total RNA was isolated from AsPC-1 and PANC-1 cells using TRIzol reagent (Thermo Fisher Scientific, Cat. No. 15596-018) as recommended by the manufacturer. Total RNA was reverse transcribed using SuperScript III First-Strand Synthesis System for RT-PCR (Thermo Fisher Scientific, Waltham, MA, USA, Cat. No. 18080-051). The forward primer for ARID1A was CCTGAAGAACTCGAACGGGAA (5′ to 3′) and the reverse primer was TCCGCCATGTTGTTGGTGG (5′ to 3′). For KRAS, the forward primer was GACTCTGAAGATGTACCTATGGTCCTA (5′ to 3′) and the reverse primer was CATCATCAACACCCTGTCTTGTC (5′ to 3′). For E-cadherin, the forward primer was CGAGAGCTACACGTTCACGG (5′ to 3′) and the reverse primer was GGGTGTCGAGGGAAAAATAGG. For CyclinD1, the forward primer was GCTGCGAAGTGGAAACCATC and the reverse primer was CCTCCTTCTGCACACATTTGAA. For Bcl-2, the forward primer was GGTGGGGTCATGTGTGTGG and the reverse primer was CGGTTCAGGTACTCAGTCATCC. For Smad-3, the forward primer was TGGACGCAGGTTCTCCAAAC and the reverse primer was CCGGCTCGCAGTAGGTAAC. GAPDH was used for standardization with the forward primer GCACCGTCAAGGCTGAGAAC and the reverse primer GCCTTCTCCATGGTGGTGAA. After optimization of primer concentrations, 2.0 μL complementary DNA (cDNA) was amplified using Maxima SYBR Green/ROX qPCR master mix (Thermo Fisher Scientific, Pittsburgh, Pennsylvania) and an ABI StepOnePlus thermal cycler (Applied Biosystems, Life Technologies) using standard settings. Relative quantification (RQ) levels were generated by SDS v2.0 software (ABI) using the Ct method with GAPDH as the endogenous control and day 0 as the calibrator sample. Three replicates were performed.
Protein Extraction and Western Blot Analysis
Cultured cells were washed with 1× Dulbecco's Phosphate Buffered Saline (DPBS) buffer, digested with 0.25% trypsin, and pelleted by centrifugation at 800 rpm for 5 minutes. Cells were then lysed on ice in Radio Immunoprecipitation Assay (RIPA) buffer by vortexing every 5 minutes for 1 hour. The extracts were collected by centrifugation at 10 000 rpm for 30 minutes at 4°C followed by transfer of the supernatant to a new tube. All protein concentrations were determined using a BCA Protein Assay Kit (Kangrun, Beijing, China). Proteins (50-100 μg/lane) were loaded on a 10% Sodium dodecyl sulfate (SDS) polyacrylamide gel, transferred to an Immun-Blot PVDF Membrane (Bio-Rad, Hercules, California, USA), blocked using 1× Tris-Buffered Saline and Tween 20 (TBST) containing 5% nonfat powdered milk, and incubation of the membrane with the primary antibody solution overnight at 4°C. The antibodies used were polyclonal rabbit antihuman ARID1A (1:400; ab176395, Abcam), bcl-2 (1:200, ab32124, Abcam), and anti-KRAS (1:200, ab55391, Abcam). The membrane was then washed with 1× TBST and incubated with the secondary antibody for 1 hour at room temperature. Protein bands were visualized on X-ray film using an enhanced chemiluminescence substrate. Mouse antihuman β-actin served as an internal control. For the Western blotting assay, 3 independent replicates/each group were performed for each experiment.
Scoring and Statistical Analysis
The interpretation criteria referred to the criteria of Samartzis et al.17,18 The percentage of positive cells was scored as: 0 (0%); 1+ (≤10%); ++ (11%-50%); +++ (51%-80%); and ++++ (>80%). The staining intensity was defined as: 0* (negative), 1* (weak), 2* (moderate), and 3* (strong). For the immunoreactive score, the percentage of positive cells and staining intensity was multiplied to yield a value between 0 and 12. SPSS software (version 19.0; SPSS, Chicago, Illinois) was used for statistical evaluations. Generally, P values <.05 were considered as significant. The statistical significance of the association between BAF250a expression and clinicopathological parameters was assessed by a χ2 test for trends, and a Fisher exact test or a Spearman r test (bivariate correlation analysis) where appropriate. Kaplan-Meier survival curves were generated for overall survival. Comparisons between survival curves were performed using log-rank tests. Cox regression analyses were used to assess relationships between clinicopathologic factors.
Results
Clinical Description of Patients
The 73 patients with pancreatic cancer ranged in age from 33 to 80 (mean: 66) years. The stage distribution was: stage I n = 17 (IA, n = 4; IB, n = 13), stage II n = 41 (IIA, n = 15; IIB, n = 26), stage III n = 6, and stage IV n = 9.
Association Between Distinct Immunohistochemistry Expression Pattern of the ARID1A Protein Product BAF250a and Survival in Pancreatic Adenocarcinoma
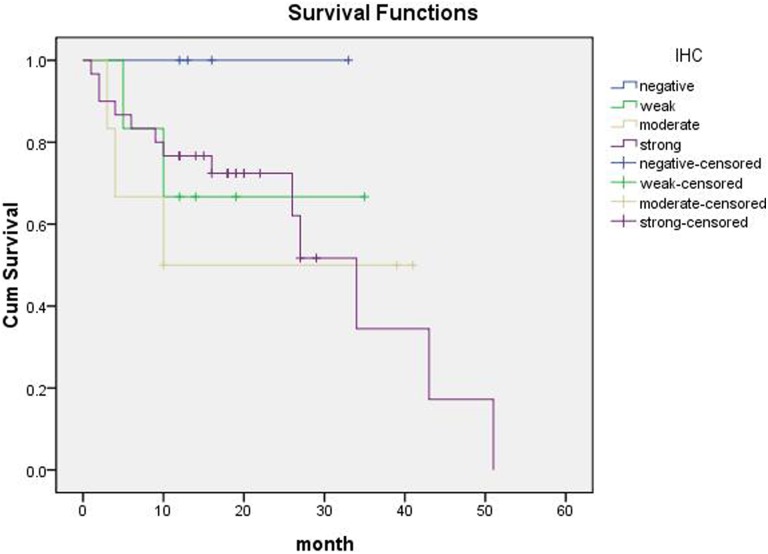
Of our study participants, 7% of cases had complete loss of BAF250a protein expression. Loss of expression of the ARID1A protein product BAF250a was associated with poor pathological differentiation (P = .002) and late Tumor Node Metastasis (TNM) stage (P = .048; Table 1). There were no statistically significant associations between BAF250a expression and sex, age, primary tumor size, and lymph node metastasis (P > .05; Table 1). The expression level of BAF250a was associated with tumor differentiation (P < .05), wherein the pancreatic cancers with a lower degree of differentiation also had lower BAF250a protein expression (Table 1). Among the poorly differentiated pancreatic ductal adenocarcinoma cases, 5 patients were negative for BAF250a protein staining, but there were no negative cases in patients with highly differentiated and moderately differentiated ductal adenocarcinoma. Staining of the fiber cell nucleus of the internal control was positive, and BAF250a staining is often completely lost in poorly differentiated pancreatic cancer (Figure 1A-D). In pancreatic cancers with high and medium differentiation, BAF250a staining was often strongly positive or moderately positive (Figure 2A-D). Meanwhile, some cases appeared to have loss of BAF250a protein expression, but this absence was a focal rather than general loss (Figure 3A-B). In contrast, in normal pancreas tissue, BAF250a expression was strongly positive and higher than that seen for tumor tissue (P < .05, Figure 3C-D). Of the 46 cases that were available for follow-up, there was no statistical difference between patients with BAF250a+ and BAF250− tumors in terms of overall survival (P = .602, Figure 4).
Table 1.
Association of BAF250a (ARID1A) Expression With Clinicopathological Features in Pancreatic Ductal Cancer.
| Variables | All Cases | BAF250a Expression | P Value | |||
|---|---|---|---|---|---|---|
| Negative | Weak | Moderate | Strong | |||
| Sex | ||||||
| Male | 39 | 2 | 8 | 4 | 25 | .052 |
| Female | 34 | 3 | 6 | 8 | 17 | |
| Age, years | ||||||
| ≤60 | 33 | 4 | 6 | 3 | 20 | .239 |
| >60 | 40 | 1 | 8 | 9 | 22 | |
| Primary tumor | ||||||
| T1 | 3 | 0 | 0 | 0 | 3 | .942 |
| T2 | 15 | 1 | 3 | 5 | 6 | |
| T3 | 46 | 3 | 8 | 6 | 29 | |
| T4 | 9 | 1 | 3 | 1 | 4 | |
| Differentiation | ||||||
| High | 15 | 0 | 2 | 3 | 10 | .002 |
| Moderate | 43 | 0 | 8 | 7 | 28 | |
| Poor | 15 | 5 | 4 | 2 | 4 | |
| TNM stage | ||||||
| IA | 4 | 0 | 1 | 0 | 3 | .048 |
| IB | 13 | 0 | 3 | 5 | 5 | |
| IIA | 15 | 2 | 2 | 3 | 8 | |
| IIB | 26 | 2 | 5 | 3 | 16 | |
| III | 6 | 1 | 1 | 0 | 4 | |
| IV | 9 | 0 | 2 | 1 | 6 | |
| Lymph node metastasis | ||||||
| Yes | 39 | 3 | 9 | 4 | 23 | .593 |
| No | 34 | 2 | 5 | 8 | 19 | |
| Distant metastasis | ||||||
| Yes | 9 | 0 | 2 | 1 | 6 | .424 |
| No | 64 | 5 | 12 | 11 | 36 | |
Abbreviation: ARID1A, AT-rich interactive domain 1A. A P-value <0.05 was considered to indicate statistical significance.
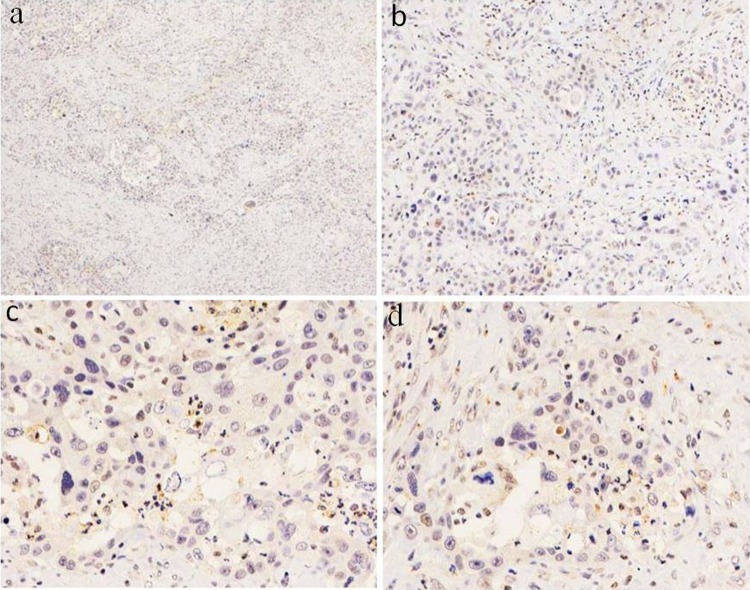
Figure 1.
Complete loss of ARID1A/BAF250a protein expression in poorly differentiated PDAC. A, BAF250a staining was completely negative in this case, in that the nucleus has blue staining; original magnification ×40. B, Negative immunohistochemical staining of BAF250a in poorly differentiated PDAC; original magnification ×100. C-D, Poorly differentiated tumor cells had negative staining. Lymphocytes, endothelial cells, and stromal cells with nuclear BAF250a expression served as internal positive controls; original magnification ×400. ARID1A indicates AT-rich interactive domain 1A. PDAC, pancreatic ductal adenocarcinoma.
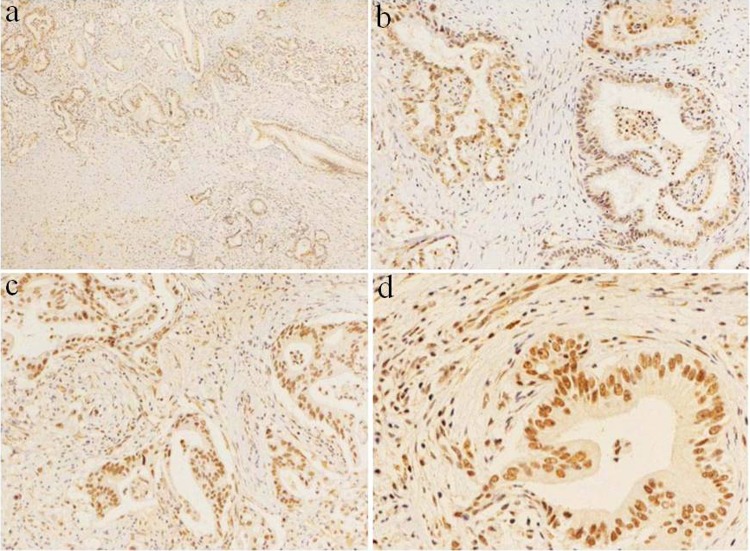
Figure 2.
ARID1A/BAF250a was often strongly positive or moderately positive in PDAC of high and medium differentiation. A, Immunohistochemical staining for BAF250a, and there was no completely negative staining of BAF250a in PDAC with high and medium differentiation; original magnification ×40. B-C, Immunohistochemical staining for BAF250a; original magnification ×200. D, Immunohistochemical staining for BAF250a; original magnification ×400. Nuclear staining was strongly positive and positive staining of stromal cells served as an internal positive control. ARID1A indicates AT-rich interactive domain 1A. PDAC, pancreatic ductal adenocarcinoma.
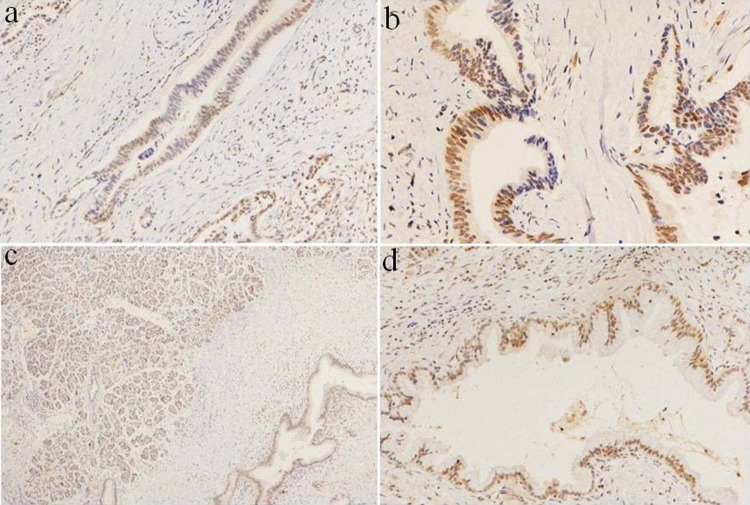
Figure 3.
Local loss of ARID1A/BAF250a expression in moderately differentiated PDAC. A-B, Part of the nuclear staining was negative, indicating partial loss of expression; original magnification ×200. C, BAF250a expression in normal pancreas tissue, with positive nuclear staining; original magnification ×40. D, BAF250a expression in normal pancreas tissue with positive nuclear staining. There was no protein loss in normal pancreas BAF250a; original magnification ×100. ARID1A indicates AT-rich interactive domain 1A. PDAC, pancreatic ductal adenocarcinoma.
Figure 4.
Survival curves of patients with different ARID1A/BAF250a staining scores (expression level). Patients with BAF250a+ and BAF250− tumors displayed no statistically significant differences in overall survival (P = .602). ARID1A indicates AT-rich interactive domain 1A.
siRNA-Mediated ARID1A Knockdown in Pancreatic Cell Lines Affects the Expression of Genes Related to Tumor Cell Infiltration, Metastasis, Apoptosis, and Proliferation
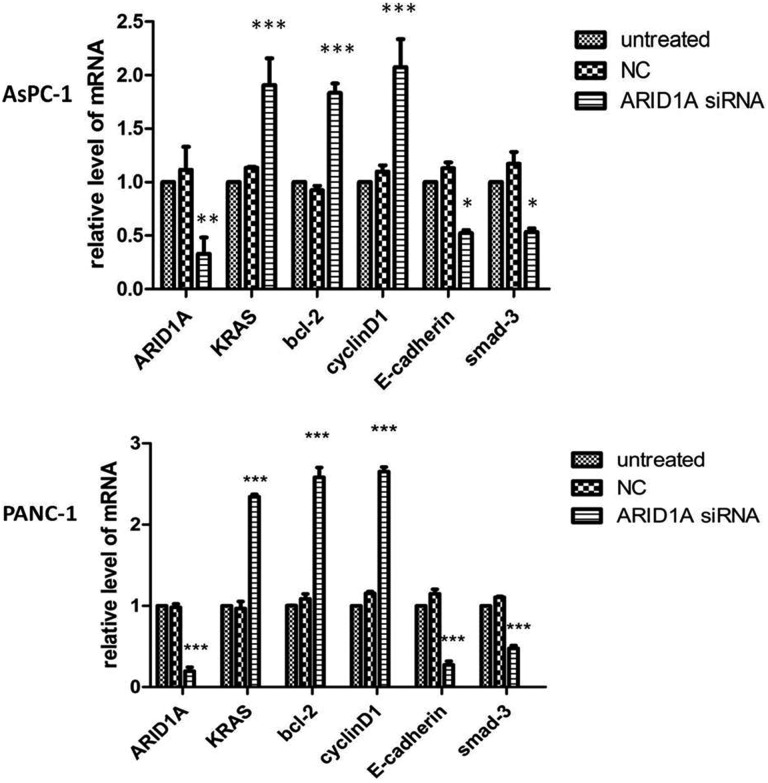
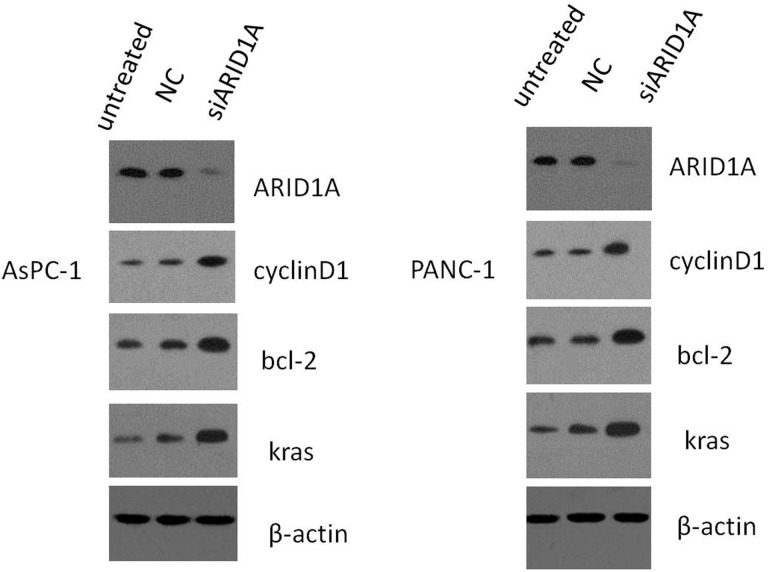
Next, we examined the effects of siRNA-mediated knockdown of ARID1A expression in the pancreatic cancer cell lines AsPC-1 and PANC-1. Real-time quantitative fluorescence PCR confirmed that the siRNA-treated cells had reduced levels of ARID1A mRNA compared to untreated control cells. The siRNA-treated cells had elevated mRNA expression of Bcl-2, CyclinD1, and KRAS relative to control cells, whereas E-cadherin and mothers against decapentaplegic homolog 3 (SMAD-3) mRNA levels were reduced (Figure 5). Protein expression of ARID1A, KRAS, Bcl-2, and CyclinD1 was similarly increased in the presence of ARID1A gene knockdown (Figure 6).
Figure 5.
Effects on gene expression following transfection of pancreatic cancer cell lines AsPC-1 and PANC-1 with ARID1A siRNA. Compared to the untreated group and negative control group, Bcl-2, CyclinD1, and KRAS mRNA levels of the ARID1A siRNA group were elevated. ARID1A, E-cadherin, and SMAD-3 mRNA levels were reduced. ARID1A indicates AT-rich interactive domain 1A; siRNA, small-interfering RNA. KRAS, kirsten rat sarcoma viral oncogene; Bcl-2, B-cell lymphoma-2.
Figure 6.
Effects on protein expression following transfection of pancreatic cancer cell lines AsPC-1 and PANC-1 with ARID1A siRNA. Compared to the untreated group and negative control group, ARID1A gene knockdown in AsPC-1 and PANC-1 cells increased protein expression of KRAS, Bcl-2, and CyclinD1, and ARID1A protein expression decreased. ARID1A indicates AT-rich interactive domain 1A; siRNA, small-interfering RNA. KRAS, kirsten rat sarcoma viral oncogene; Bcl-2, B-cell lymphoma-2.
miR-31 Affects ARID1A Expression in Pancreatic Cell Lines
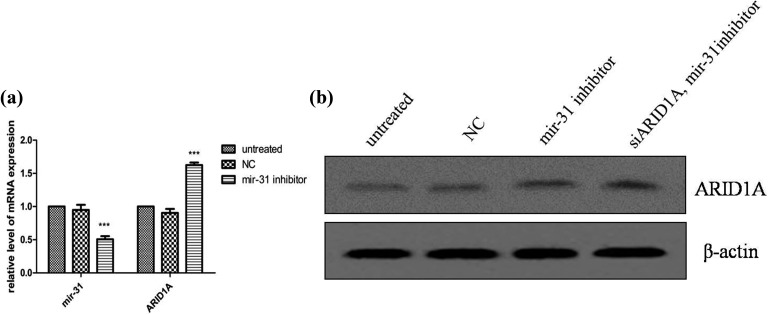
When cells were treated with ARID1A siRNA, ARID1A protein expression decreased in ARID1A knockdown cells (Figure 6). Then we added mir-31 inhibitors into these cells; downregulation of ARID1A induced by ARID1A knockdown is reversed by mir-31 inhibition. The level of ARID1A mRNA and BAF250a protein was upregulated in AsPC-1 cell, which was transfected with miR-31 inhibitor (Figure 7).
Figure 7.
ARID1A messenger RNA (mRNA) and protein expression after transfection of pancreatic cancer cell line AsPC-1 with miR-31 inhibitor. A, Transfection of the pancreatic cancer cell AsPC-1 with miR-31 inhibitor increased ARID1A mRNA levels. B, Downregulation of ARID1A induced by ARID1A knockdown is reversed by miR-31 inhibition. ARID1A indicates AT-rich interactive domain 1A. miR-31, microRNA-31.
Discussion
Several earlier studies implicated ARID1A as a tumor suppressor gene that is frequently mutated in a variety of cancers.19,20 Here, we showed that the expression of the ARID1A gene product BAF250a was indeed lost in some pancreatic cancer cases. In our study participants who had pancreatic cancer with high and medium differentiation, immunohistochemistry showed that BAF250a expression was strongly or moderately positive. Moreover, BAF250a was strongly expressed in normal pancreas tissue. In contrast, samples from those cases with poorly differentiated pancreatic cancer typically showed negative or only weakly positive BAF250a staining. Of our study participants, 7% of cases had complete loss of BAF250a protein expression.
BAF250a expression levels were related to tumor differentiation and TNM stage in our study, wherein cases with a lower degree of differentiation and higher TNM stage also had lower BAF250a protein expression levels. Several studies found that BAF250a could serve as a new prognostic marker in patients with clear cell renal cell carcinoma (CCRCC) in that decreased protein expression was associated with higher nuclear grade and higher pTNM stage.21 The patients with CCRCC who had low levels of ARID1A protein expression also had significantly shorter cancer-specific and progression-free survival times. Furthermore, Cox regression analysis showed that ARID1A expression was an independent prognostic factor for progression-free survival.21 In breast cancer, ARID1A mRNA or BAF250a nuclear protein expression is associated with more aggressive breast cancer phenotypes, such as high tumor grade. Moreover, ARID1A expression loss becomes more prevalent during the later stages of breast tumor progression.22 Together, these findings suggest that ARID1A may be a candidate tumor suppressor gene in cancer. Similar correlations between lost or lowered ARID1A expression and high-grade tumor, higher T stage, and poorer overall survival were seen for small intestinal carcinoma.23 However, another study found that the loss of ARID1A expression is uncommon and is not associated with oncologic outcome, but instead may be related to less invasive clinicopathologic features in colorectal cancer and GC.24
Indeed, several studies focusing on other cancers have obtained inconsistent results concerning the prognostic significance of changes in ARID1A expression. In gastric carcinomas, changes in ARID1A expression were associated with a trend toward better prognosis, whereas cases with loss of ARID1A expression had poor survival.25 Another study found no significant difference in clinicopathologic features or patient survival between endometrial carcinoma cases that were positive or negative for ARID1A expression.26 Sequencing analysis of neuroblastoma cases using several approaches showed chromosomal deletions and sequence alterations of ARID1A and ARID1B that were associated with early treatment failure and decreased survival.27
For survival analysis of our study participants, 46 PDAC cases were available for follow-up and the P value for these patients was .602 between ARID1A-positive and ARID1A-negative expression, indicating that there was no statistical difference between the groups in our experiment, although this outcome could be attributed to our small sample size. However, a recent study concerning pancreatic cancer and ARID1A expression did show that lower BAF250a protein levels are associated with overall survival.28 Thus, future studies involving larger sample sizes are needed to examine the relationship between ARID1A expression and prognosis.
In this study, siRNA-mediated knockdown of ARID1A gene expression in AsPC-1 and PANC-1 cells reduced mRNA levels of ARID1A and SMAD-3, as well as E-cadherin, which is related to tumor invasion and metastasis. Meanwhile, KRAS, Bcl-2, and CyclinD1 mRNA and protein levels increased in the presence of ARID1A knockdown. KRAS oncogene point mutations occur in more than 95% of patients with pancreatic cancer. It is the most important proto-oncogene in pancreatic cancer. The KRAS protein can activate various downstream effector molecules which influence proliferation and differentiation.29 Earlier studies that analyzed ARID1A expression in endometrial cancers in the context of PI3K-Akt pathway proteins, TP53 status, and microsatellite instability suggested that there is crosstalk between ARID1A and the PI3K/Akt pathways.30 In our experiment, knockdown of ARID1A gene affected the gene expression level of KRAS, which is the most important proto-oncogene in pancreatic cancer. Oncogenic KRAS promotes pancreatic tumorigenesis through the activation of multiple downstream pathways, including phosphatidylinositol-3-kinase. This means KRAS has a certain relationship with chromatin remodeling complexes. Based on these earlier findings, the effect of ARID1A on PI3K/Akt signaling in pancreatic cancer requires further investigation. Bcl-2 and CyclinD1 are related to cell apoptosis and proliferation.31 As shown above, ARID1A expression knockdown downregulated E-cadherin. E-cadherin overexpression is known to inhibit cancer cell migration and invasion. In GC cells lines, restoration of ARID1A expression significantly inhibited cell proliferation and colony formation.32
Abnormal expression of miR-31 is associated with various malignancies. Upregulation of miR-31 expression is seen in colorectal, hepatocellular, and lung cancer, but bladder, breast, gastric, ovarian, and prostate cancer have downregulated miR-31 expression.33,34 miR-31 plays an evident role in regulating cell migration and invasion and thereby the ability of cancer cells to metastasize. Cervical cancer cell lines showed miR-31 upregulation, and in cervical cancer tissue samples, high levels of miR-31 were associated with higher FIGO stage, node metastasis, vascular involvement, and deep stromal invasion. miR-31 could also serve as an independent prognostic factor for cervical cancer, as patients with high expression of miR-31 also had poorer overall survival than patients with low expression. Downregulation of miR-31 impaired cell proliferation, colony formation, and cell migration and invasion in vitro and inhibited xenograft tumor growth in vivo.35
miR-31 expression levels were very low in normal pancreas tissue, but 10 pancreatic cell lines had very high expression, including AsPC-1. The ARID1A may be a potential target for miR-31 through bioinformatics algorithms.36 In our study, transfection of AsPC-1 cell with miR-31 inhibitor increased ARID1A mRNA and protein levels. This study verified ARID1A is related to miR-31, which was further confirmed by the inverse expression of miR-31 and ARID1A. The ARID1A was also verified as a direct target of miR-31 in cervical cancer and head and neck squamous cell carcinoma.35,37
Although additional studies involving larger patient populations are needed to understand the prognostic value of ARID1A expression, this study shows that changes in ARID1A are an important factor in tumorigenesis. Does ARID1A siRNA knockdown enhance Epithelial-Mesenchymal transition (EMT) and increase the invasiveness? We will evaluate the issue in vitro in the future experiments. The ARID1A is associated with KRAS and is regulated by noncoding RNA. In hepatocellular carcinoma, the interaction between ARID1A repressed cell proliferation and migration through lncRNA associated with microvascular invasion (lncRNA MVIH).38 Zhou et al found that lncRNA can predict patients’ survival in many cancers.39-45 The relationship between ARID1A and lncRNAs in pancreatic cancer can be clarified in further studies. The SWI/SNF chromatin remodeling complex plays an important role in the development and progression of pancreatic cancer.
Acknowledgments
The authors gratefully acknowledge the support from the National Nature Science Foundations of China (No. 8172334, No. 81341070, No. 81472326).
Abbreviations
- ARID1A
AT-rich interactive domain 1A
- CCRCC
clear cell renal cell carcinoma
- GC
gastric cancer
- mRNA
messenger RNA
- PUMCH
Peking Union Medical College Hospital
- siRNA
small-interfering RNA
- SWI/SNF
Switch/Sucrose nonfermentable.
Authors’ Note: L.Z. contributed to conception and design of the study, contributed to acquisition of data, contributed to analysis and interpretation of data, and drafted the article. C.W., S.Y., C.J., and J.Y. contributed to acquisition of data. J.C. and Z.L. contributed to conception and design of the study. J.C. contributed to final approval of the article. All authors reviewed the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Nature Science Foundations of China (No. 8172334, No. 81341070, No. 81472326), and the CAMS Science and Technology Innovation Program Fund for Medical Sciences and health (2016-I2M-1-001).
References
- 1. Huang J, Deng Q, Wang Q, et al. Exome sequencing of hepatitis B virus-associated hepatocellular carcinoma. Nat Genet. 2012;44(10):1117–1121. [DOI] [PubMed] [Google Scholar]
- 2. Jiao Y, Pawlik TM, Anders RA, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45(12):1470–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Le Gallo M, O’Hara AJ, Rudd ML, et al. Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nat Genet. 2012;44(12):1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Helming KC, Wang X, Wilson BG, et al. ARID1B is a specific vulnerability in ARID1A-mutant cancers. Nat Med. 2014;20(3):251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones S, Stransky N, McCord CL, et al. Genomic analyses of gynaecologic carcinosarcomas reveal frequent mutations in chromatin remodelling genes. Nat Commun. 2014;5:5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang K, Kan J, Yuen ST, et al. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet. 2011;43(12):1219–1223. [DOI] [PubMed] [Google Scholar]
- 7. Streppel MM, Lata S, DelaBastide M, et al. Next-generation sequencing of endoscopic biopsies identifies ARID1A as a tumor-suppressor gene in Barrett’s esophagus. Oncogene. 2014;33(3):347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guan B, Gao M, Wu CH, et al. Functional analysis of in-frame indel ARID1A mutations reveals new regulatory mechanisms of its tumor suppressor functions. Neoplasia. 2012;14(10):986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luchini C, Veronese N, Solmi M, et al. Prognostic role and implications of mutation status of tumor suppressor gene ARID1A in cancer: a systematic review and meta-analysis. Oncotarget. 2015;6(36):39088–39097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mao TL, Ardighieri L, Ayhan A, et al. Loss of ARID1A expression correlates with stages of tumor progression in uterine endometrioid carcinoma. Am J Surg Pathol. 2013;37(9):1342–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Watanabe R, Ui A, Kanno S, et al. SWI/SNF factors required for cellular resistance to DNA damage include ARID1A and ARID1B and show interdependent protein stability. Cancer Res. 2014;74(9):2465–2475. [DOI] [PubMed] [Google Scholar]
- 12. Numata M, Morinaga S, Watanabe T, et al. The clinical significance of SWI/SNF complex in pancreatic cancer. Int J Oncol. 2013;42(2):403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(7540):495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jones S, Li M, Parsons DW, et al. Somatic mutations in the chromatin remodeling gene ARID1A occur in several tumor types. Hum Mutat. 2012;33(1):100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hosoda W, Chianchiano P, Griffin JF, et al. Genetic analyses of isolated high-grade pancreatic intraepithelial neoplasia (HG-PanIN) reveal paucity of alterations in TP53 and SMAD4. J Pathol. 2017;242(1):16–23. doi:10.1002/path.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22(44):9694–9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Samartzis EP, Samartzis N, Noske A, et al. Loss of ARID1A/BAF250a-expression in endometriosis: a biomarker for risk of carcinogenic transformation? Mod Pathol. 2012;25(6):885–892. [DOI] [PubMed] [Google Scholar]
- 18. Weichert W, Röske A, Gekeler V, et al. Association of patterns of class I histone deacetylase expression with patient prognosis in gastric cancer: a retrospective analysis. Lancet Oncol. 2008;9(2):139–148 [DOI] [PubMed] [Google Scholar]
- 19. Wiegand KC, Lee AF, Al-Agha OM, et al. Loss of BAF250a (ARID1A) is frequent in high-grade endometrial carcinomas. J Pathol. 2011;224(3):328–333. [DOI] [PubMed] [Google Scholar]
- 20. Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363(16):1532–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park JH, Lee C, Suh JH, et al. Decreased ARID1A expression correlates with poor prognosis of clear cell renal cell carcinoma. Hum Pathol. 2015;46(3):454–460. [DOI] [PubMed] [Google Scholar]
- 22. Mamo A, Cavallone L, Tuzmen S, et al. An integrated genomic approach identifies ARID1A as a candidate tumor-suppressor gene in breast cancer. Oncogene. 2012;31(16):2090–2100. [DOI] [PubMed] [Google Scholar]
- 23. Kim MJ, Gu MJ, Chang HK, et al. Loss of ARID1A expression is associated with poor prognosis in small intestinal carcinoma. Histopathology. 2015;66(4):508–516. [DOI] [PubMed] [Google Scholar]
- 24. Lee SY, Kim DW, Lee HS, et al. Loss of AT-rich interactive domain 1A expression in gastrointestinal malignancies. Oncology. 2015;88(4):234–240. [DOI] [PubMed] [Google Scholar]
- 25. Inada R, Sekine S, Taniguchi H, et al. ARID1A expression in gastric adenocarcinoma: clinicopathological significance and correlation with DNA mismatch repair status. World J Gastroenterol. 2015;21(7):2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rahman M, Nakayama K, Rahman MT, et al. Clinicopathologic analysis of loss of AT-rich interactive domain 1A expression in endometrial cancer. Hum Pathol. 2013;44(1):103–109. [DOI] [PubMed] [Google Scholar]
- 27. Sausen M, Leary RJ, Jones S, et al. Integrated genomic analyses identify ARID1A and ARID1B alterations in the childhood cancer neuroblastoma. Nat Genet. 2013;45(1):12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Witkiewicz AK, McMillan EA, Balaji U, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun. 2015;6:6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morris JP, 4th, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 2010;10(10):683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang HN, Lin MC, Huang WC, et al. Loss of ARID1A expression and its relationship with PI3K-Akt pathway alterations and ZNF217 amplification in ovarian clear cell carcinoma. Mod Pathol. 2014;27(7):983–990. [DOI] [PubMed] [Google Scholar]
- 31. Zhang Y, Xu X, Zhang M, et al. ARID1A is downregulated in non-small cell lung cancer and regulates cell proliferation and apoptosis. Tumour Biol. 2014;35(6):5701–5707. [DOI] [PubMed] [Google Scholar]
- 32. Yan HB, Wang XF, Zhang Q, et al. Reduced expression of the chromatin remodeling gene ARID1A enhances gastric cancer cell migration and invasion via downregulation of E-cadherin transcription. Carcinogenesis. 2014;35(4):867–876. [DOI] [PubMed] [Google Scholar]
- 33. Caramés C, Cristobal I, Moreno V, et al. MicroRNA-31 emerges as a predictive biomarker of pathological response and outcome in locally advanced rectal cancer. Int J Mol Sci. 2016;17(6):pii: E878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luo LJ, Yang F, Ding JJ, et al. MiR-31 inhibits migration and invasion by targeting SATB2 in triple negative breast cancer. Gene. 2016;594(1):47–58. [DOI] [PubMed] [Google Scholar]
- 35. Wang N, Zhou Y, Zheng L, et al. MiR-31 is an independent prognostic factor and functions as an oncomir in cervical cancer via targeting ARID1A. Gynecol Oncol. 2014;134(1):129–137. [DOI] [PubMed] [Google Scholar]
- 36. Laurila EM, Sandström S, Rantanen LM, et al. Both inhibition and enhanced expression of miR-31 lead to reduced migration and invasion of pancreatic cancer cells. Genes Chromosomes Cancer. 2012;51(6):557–568. [DOI] [PubMed] [Google Scholar]
- 37. Lu WC, Liu CJ, Tu HF, et al. miR-31 targets ARID1A and enhances the oncogenicity and stemness of head and neck squamous cell carcinoma. Oncotarget. 2016;7(35):57254–57267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheng S, Wang L, Deng CH, et al. ARID1A represses hepatocellular carcinoma cell proliferation and migration through lncRNA MVIH. Biochem Biophys Res Commun. 2017;491(1):178–182. [DOI] [PubMed] [Google Scholar]
- 39. Zhou M, Zhang Z, Zhao H, et al. An immune-related six-lncRNA signature to improve prognosis prediction of glioblastoma multiforme. Mol Neurobiol. 2017. doi:10.1007/s12035-017-0572-9. [DOI] [PubMed] [Google Scholar]
- 40. Zhou M, Zhao H, Xu W, et al. Discovery and validation of immune-associated long non-coding RNA biomarkers associated with clinically molecular subtype and prognosis in diffuse large B cell lymphoma. Mol Cancer. 2017;16(1):16 doi:10.1186/s12943-017-0580-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou M, Xu W, Yue X, et al. Relapse-related long non-coding RNA signature to improve prognosis prediction of lung adenocarcinoma. Oncotarget. 2016;7(20):29720–29738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhou M, Wang X, Shi H, et al. Characterization of long non-coding RNA-associated ceRNA network to reveal potential prognostic lncRNA biomarkers in human ovarian cancer. Oncotarget. 2016;7(11):12598–12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou M, Sun Y, Sun Y, et al. Comprehensive analysis of lncRNA expression profiles reveals a novel lncRNA signature to discriminate nonequivalent outcomes in patients with ovarian cancer. Oncotarget. 2016;7(22):32433–32448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhou M, Zhao H, Wang Z, et al. Identification and validation of potential prognostic lncRNA biomarkers for predicting survival in patients with multiple myeloma. J Exp Clin Cancer Res. 2015;34:102 doi:10.1186/s13046-015-0219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou M, Guo M, He D, et al. A potential signature of eight long non-coding RNAs predicts survival in patients with non-small cell lung cancer. J Transl Med. 2015;13:231 doi:10.1186/s12967-015-0556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]