Abstract
Background
The objective of this study was to investigate the clinical course and final outcome in patients afflicted with severe dysphagia following a diagnosis of lateral medullary syndrome (LMS).
Methods
The patients with severe dysphagia after LMS admitted to a rehabilitation unit were included and their respective clinical data were prospectively collected. The criteria of ‘severe dysphagia’ was defined as the condition that showed decreased pharyngeal constriction with no esophageal passage in a videofluoroscopic swallowing study (VFSS) and initially required enteral tube feeding. The data included VFSS findings, types of diet and postural modification, penetration-aspiration scale (PAS) and functional oral intake scale (FOIS).
Results
A total of 11 patients were included and VFSS was performed every 2 weeks after stroke onset. Esophageal passage began to show at an average 34.7 ± 18.3 days, and the patients were able to begin consuming a partial oral diet with postural modification. It was 52.2 ± 21.8 days till they were advanced to a full oral diet. PAS and FOIS were significantly improved over time.
Conclusions
Patients with severe dysphagia after LMS were able to tolerate a partial oral diet at about 5 weeks following onset, and they were advanced to a normal diet after 10 weeks. This clinical course might help in predicting the prognosis, as well as assist in making practical decisions regarding a rehabilitation program.
Keywords: deglutition, dysphagia, lateral medullary syndrome, rehabilitation, stroke
Introduction
Lateral medullary syndrome (LMS) is a commonly occurring type of cerebrovascular accident caused by ischemia in the lateral part of the medulla oblongata. From 51% to 100% of the patients with LMS have been noted to experience some degree of swallowing difficulty, among the other recognized sequelae of LMS.1–3 It is known that dysphagia is more profound and has longer duration in LMS patients than in hemispheric stroke patients.4 Most patients with dysphagia are not serious, and recover quickly.5
Despite good prognosis, there are sometimes very severe cases that show no esophageal passage and require tube feeding for a long time in a clinical situation. There is little information about progression of severe dysphagia over time, with particular attention paid to videofluoroscopic swallowing study (VFSS) findings, dietary and/or postural modifications. Therefore, the objective of this study was to investigate the clinical course and outcome in patients with severe dysphagia after LMS.
Methods
Patients with diagnoses of ‘severe dysphagia after LMS’ admitted to the rehabilitation unit during the period January 2013 through December 2016 were included in this study, and the relevant medical data were prospectively collected. The criteria of ‘severe dysphagia after LMS’ was defined as: (1) acute or subacute LMS patients whose condition was confirmed by CT or MRI scan; (2) decreased pharyngeal constriction and apparent absence of any evidence of esophageal passage in VFSS findings; and (3) those patients who initially required enteral tube feeding. All patients performed VFSS according to the standard protocol6 every almost 2 weeks after onset, and appropriate adjustments were made in terms of their dietary and/or postural modification by two expert physicians. The data included information regarding types of initial diet, initial modified Barthel index (MBI), serial VFSS findings, status of diet and/or postural modification, penetration-aspiration scale (PAS)7 and functional oral intake scale (FOIS).8 Based on these data, we evaluated the change in swallowing function over time using statistical methods (Wilcoxon signed rank test) and tried to identify the time point at which the severe dysphagia patients could commence oral feeding and discontinue dietary and/or postural modifications. This study was approved by the Dongguk University Hospital Institutional Review Board (approval number 2012-1-41) and informed written consent was obtained from every participant.
Results
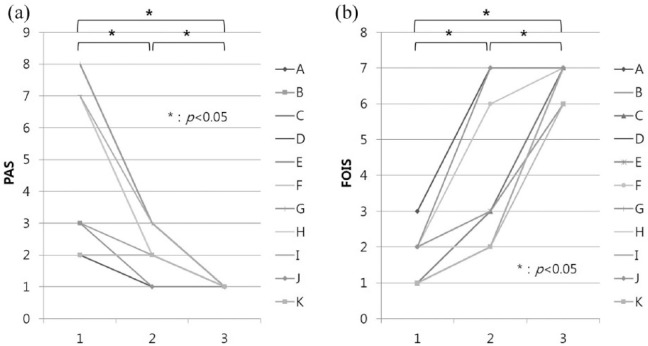
A total of 11 patients (6 men, 5 women; mean age, 59.5 years; range, 38–74 years) were included from among the total 36 isolated LMS dysphagia patients during the study period. The rest of the patients (n = 25, 69.4%) were excluded because they showed apparent esophageal passage in initial VFSS and thus did not require enteral tube feeding. The lesion was on the left side of the brain in four cases, and the right side in seven. The types of initial diet were enteral tube feeding in all cases. Average initial MBI score was 74.8 (Table 1). We provide a summary of the 11 patients with severe dysphagia after LMS in Table 2, considering the VFSS findings and following therapeutic interventions, dietary and/or postural modification. The first VFSS was performed at 13.4 ± 13.2 (mean ± SD) days following onset. Esophageal passage eventually began to show at an average of 34.7 ± 18.3 days, and the patients were able to tolerate a partial oral diet (tube feeding plus oral diet) with postural modification (head rotation). It took 52.2 ± 21.8 days to advance the patients to a full oral diet. After 68.1 ± 25.1 days, postural modification was no longer required in seven cases. Almost all patients ultimately completely recovered from the dysphagia, and were finally allowed a normal diet without any dietary modifications. We have documented this progression timeline in Supplement 1. PAS scores were significantly decreased and FOIS was also increased over time, as expected (Figure 1).
Table 1.
Demographics and clinical characteristics of patients at initial evaluation.
| Patient | Sex | Age (years) | Lesion side | Initial VFSS (days from onset) | Initial diet | NIHSS | Initial MBI score |
|---|---|---|---|---|---|---|---|
| A | Male | 38 | Left | 17 | Tube feeding | 0 | 100 |
| B | Male | 74 | Right | 33 | Tube feeding | 2 | 76 |
| C | Male | 44 | Right | 7 | Tube feeding | 2 | 92 |
| D | Female | 59 | Right | 3 | Tube feeding | 4 | 74 |
| E | Male | 62 | Left | 33 | Tube feeding | 2 | 98 |
| F | Female | 61 | Right | 5 | Tube feeding | 5 | 32 |
| G | Female | 58 | Right | 3 | Tube feeding | 3 | 57 |
| H | Male | 74 | Right | 33 | Tube feeding | 2 | 76 |
| I | Male | 73 | Left | 2 | Tube feeding | 7 | 63 |
| J | Female | 56 | Right | 8 | Tube feeding | 3 | 55 |
| K | Female | 55 | Left | 3 | Tube feeding | 1 | 100 |
MBI, modified Barthel index; NIHSS, National Institute of Health Stroke Scale.
Table 2.
Summary of 11 patients’ VFSS findings and following therapeutic interventions, diet and/or postural modification.
| Patient | VFSS findings | Interventions | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |
| A | PC decreased, EP (–) | EP (+), Aspiration (–), PR (–) | – | Tube feeding, Lt HR + dry swallowing | FOD | – |
| B | PC decreased, EP (–) | EP (+) with Rt HR partial | PR on Rt side | Tube feeding Rt HR + dry swallowing |
POD + Rt HR | FOD + Rt HR |
| C | PC (–), EP (–), Aspiration (+) | PC decreased, EP (+) with Rt HR partial | EP (+) without HR, PR (+) only sticky food | Tube feeding Rt HR + dry swallowing |
POD + Rt HR | FOD |
| D | PC (–), EP (–), Aspiration (+) | PC decreased, EP (+) with Rt HR partial | EP (+) without HR, PR (+) only sticky food | Tube feeding Rt HR + dry swallowing |
POD + Rt HR | FOD |
| E | PC decreased, EP (–), Aspiration (+) | EP (+) with Lt HR | PR on Lt side | Tube feeding Lt HR + dry swallowing |
POD + Lt HR | FOD + Lt HR |
| F | PC decreased, EP (–) | PC decreased, EP (+) with Rt HR partial | EP (+) without HR, Aspiration (–) | Tube feeding Rt HR + dry swallowing |
FOD + Rt HR | FOD |
| G | PC decreased, EP (–), Aspiration (+) | EP (+) with Rt HR partial | EP (+) without HR, PR (+) only sticky food | Tube feeding Rt HR + dry swallowing |
POD + Rt HR | FOD |
| H | PC decreased, EP (–), Aspiration (+) | EP (+) with Rt HR | PR on Rt side | Tube feeding Rt HR + dry swallowing |
POD + Rt HR | FOD + Rt HR |
| I | PC decreased, EP (–), Aspiration (+) | EP (+) with Lt HR | EP (+) without HR, Aspiration (–) | Tube feeding Lt HR + dry swallowing |
POD + Lt HR | FOD |
| J | PC decreased, Lt EP (–) | EP (+) without HR, Aspiration (–) | – | Tube feeding Rt HR + dry swallowing |
FOD | – |
| K | PC decreased, Both side EP (–) | EP (+) with Lt HR partial | PR (+) on Lt side | Tube feeding Lt HR + dry swallowing |
POD + Lt HR | FOD + Lt HR |
EP, esophageal passage; FOD, full oral diet; HR, head rotation; Lt, left; PC, pharyngeal constriction; POD, partial oral diet; PR, pharyngeal residue; Rt, right.
Figure 1.
(a) Changes in the penetration-aspiration scale (PAS) with time. PAS was significantly decreased over time. (b) Changes in the functional oral intake scale (FOIS). FOIS was significantly increased over time.
Discussion
In this study, we have presented the first description of the progression of the clinical course and outcome of severe dysphagia after LMS in relation to VFSS findings, dietary and/or postural modification. Approximately 5 weeks following onset, some esophageal passage could be seen using VFSS, and patients were able to commence oral feeding (although it was necessary that the patients rotate their heads to the weak side and have the viscosity modified dysphagia diet). After 10 weeks, they were advanced to a normal diet without any dietary modifications.
LMS, also called Wallenberg’s syndrome, is usually caused by a vascular event in the area of the posterior inferior cerebellar artery or the vertebral artery.3 This type of stroke is characterized by impairment in pain and temperature sensation and perception involving the ipsilateral face and contralateral body; it can also serve to produce sequelae such as ipsilateral Horner syndrome; dysphagia, dysarthria and dysphonia; nystagmus; vertigo; nausea and vomiting; and ipsilateral limb ataxia. In contrast to the sequelae of hemispheric stroke, dysphagia in LMS involves a reflex swallowing problem in pharyngeal and esophageal phases.
It is known to be related to the neural structures of the swallowing center located in the medulla oblongata [such as the nucleus ambiguous (NA) and nucleus tractus solitarius (NTS)], which can serve to coordinate the pharyngeal and esophageal phases of swallowing.9–11 Several sub-networks are considered to be present, especially in the subnucleus centralis for the esophageal phase of swallowing.12,13 At present, the central pattern generator for swallowing is conceptualized as a serial network of linked neurons within the NTS, with neighboring reticular formation and related sequential excitation along the deglutition pathway.14 This swallowing center is situated on both sides of the brain stem, and are interconnected. Lateral medullary infarction commonly affects the NA and NTS (within the medulla oblongata) unilaterally. Although the lesion is unilateral, its effect on oropharyngeal swallowing is bilateral. Primarily the premotor neurons in the NA and their connections seem to be affected and consequently a disruption and/or disconnection of their linkage to swallowing-related cranial motor neuron pools bilaterally and to the contralateral NA could produce the swallowing disorders.15
The head rotation maneuver is mainly used for patients afflicted with unilateral deficits, which are frequently observed in LMS. Rotation of the head toward the weakened side before the patient attempts to swallow results in the swallowing tract, or piriform sinus, on the damaged side being narrowing or even closing off, and this maneuver directs the bolus down the stronger side.16 The cricoid cartilage is pulled away from the posterior pharyngeal wall, reducing the pressure in the cricopharyngeal sphincter and thereby increasing the size of sphincter opening. These effects serve to reduce the amount of bolus residue after swallowing, and also diminishes the risk of aspiration.17
When the condition of severe dysphagia is prolonged, conversion to percutaneous endoscopic gastrostomy (PEG) should be considered for patients who require long-term enteral nutrition. In a recent study, feeding via PEG was found to be the preferable means of nutrition delivery if it is anticipated that the patient’s nutritional intake is likely to be inadequate and that supplementary artificial enteral nutrition will likely be necessary for a period exceeding 2–3 weeks.18 Based on the results of this study, invasive intervention such as PEG should be considered more carefully and ordered more judiciously, even in severe cases of dysphagia secondary to LMS. We can reduce the suffering of patients and excessive and unnecessary expense by avoiding early invasive intervention if at all possible.
There is yet to be enough research completed to explain the differences in the severity of dysphagia in LMS patients. Some neuro-anatomical differences, such as cephalic lesions with greater vertical spread, have been suggested to be the reason.19 Further studies that seek to explore the reasons for the differences, in terms of neuro-anatomical factors, should be carried out in the future. This study implements the first timeline approach to examining the progression of the clinical course and the outcome of the severe dysphagia seen in 11 LMS patients. We described the markers of recovery from dysphagia in terms of esophageal passage of bolus, necessity of postural modification such as head rotation and diet modification.
Conclusion
Each patient with severe dysphagia, post-LMS, could probably safely commence partial oral feeding at approximately 5 weeks following onset of the problem. Patients could be advanced to a normal diet (without any dietary modifications or limitations) at about 10 weeks and continuing thereafter. This documented clinical course and the outcome might prove useful in predicting the prognosis and planning a comprehensive, informed and appropriate rehabilitation program for patients afflicted with severe dysphagia as sequelae of LMS.
Supplementary Material
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics statement: Dongguk University Ilsan Hospital IRB approved this study. Approval number: 2012-1-41
Supplementary materials: Supplement 1. The average time (inside circle) of each clinical course in severe dysphagia patients after LMS are shown. Start of partial oral diet, full oral diet and cessation of postural modification were possible in 34.7 ± 18.3 days, 52.2 ± 21.5 days, and 68.1 ± 25.1 days on average, respectively.
ORCID iD: Jin-Woo Park  https://orcid.org/0000-0003-4989-2575
https://orcid.org/0000-0003-4989-2575
Contributor Information
Hyojun Kim, Department of Physical Medicine and Rehabilitation, Dongguk University Ilsan Hospital, Gyeonggi-do, Republic of Korea.
Ho Jun Lee, Department of Physical Medicine and Rehabilitation, Dongguk University Ilsan Hospital, Gyeonggi-do, Republic of Korea.
Jin-Woo Park, Department of Physical Medicine and Rehabilitation, Dongguk University Ilsan Hospital, 27 Dongguk-ro, Ilsandong-gu, Goyang-si, Gyeonggi-do, 10326, Republic of Korea.
References
- 1. Kim JS, Lee JH, Suh DC, et al. Spectrum of lateral medullary syndrome: correlation between clinical findings and magnetic resonance imaging in 33 subjects. Stroke 1994; 25: 1405–1410. [DOI] [PubMed] [Google Scholar]
- 2. Norrving B, Cronqvist S. Lateral medullary infarction: prognosis in an unselected series. Neurology 1991; 41: 244–248. [DOI] [PubMed] [Google Scholar]
- 3. Sacco RL, Freddo L, Bello JA, et al. Wallenberg’s lateral medullary syndrome: clinical-magnetic resonance imaging correlations. Arch Neurol 1993; 50: 609–614. [DOI] [PubMed] [Google Scholar]
- 4. Ertekin C, Aydogdu I, Tarlaci S, et al. Mechanisms of dysphagia in suprabulbar palsy with lacunar infarct. Stroke 2000; 31: 1370–1376. [DOI] [PubMed] [Google Scholar]
- 5. Kim H, Chung CS, Lee KH, et al. Aspiration subsequent to a pure medullary infarction: lesion sites, clinical variables, and outcome. Arch Neurol 2000; 57: 478–483. [DOI] [PubMed] [Google Scholar]
- 6. Logemann JA. Evaluation and treatment of swallowing disorders. 2nd ed. Austin, TX: PRO-ED, 1998, p.xiii. [Google Scholar]
- 7. Rosenbek JC, Robbins JA, Roecker EB, et al. A penetration-aspiration scale. Dysphagia 1996; 11: 93–98. [DOI] [PubMed] [Google Scholar]
- 8. Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil 2005; 86: 1516–1520. [DOI] [PubMed] [Google Scholar]
- 9. Barrett RT, Bao X, Miselis RR, et al. Brain stem localization of rodent esophageal premotor neurons revealed by transneuronal passage of pseudorabies virus. Gastroenterology 1994; 107: 728–737. [DOI] [PubMed] [Google Scholar]
- 10. Jean A. Control of the central swallowing program by inputs from the peripheral receptors: a review. J Auton Nerv Syst 1984; 10: 225–233. [DOI] [PubMed] [Google Scholar]
- 11. Kessler JP, Jean A. Identification of the medullary swallowing regions in the rat. Exp Brain Res 1985; 57: 256–263. [DOI] [PubMed] [Google Scholar]
- 12. Bieger D. Neuropharmacologic correlates of deglutition: lessons from fictive swallowing. Dysphagia 1991; 6: 147–164. [DOI] [PubMed] [Google Scholar]
- 13. Broussard DL, Lynn RB, Wiedner EB, et al. Solitarial premotor neuron projections to the rat esophagus and pharynx: implications for control of swallowing. Gastroenterology 1998; 114: 1268–1275. [DOI] [PubMed] [Google Scholar]
- 14. Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev 2001; 81: 929–969. [DOI] [PubMed] [Google Scholar]
- 15. Aydogdu I, Ertekin C, Tarlaci S, et al. Dysphagia in lateral medullary infarction (Wallenberg’s syndrome): an acute disconnection syndrome in premotor neurons related to swallowing activity? Stroke 2001; 32: 2081–2087. [DOI] [PubMed] [Google Scholar]
- 16. Logemann JA, Kahrilas PJ, Kobara M, et al. The benefit of head rotation on pharyngoesophageal dysphagia. Arch Phys Med Rehabil 1989; 70: 767–771. [PubMed] [Google Scholar]
- 17. Ekberg O. Dysphagia: diagnosis and treatment. New York: Springer, 2012, p.xix. [Google Scholar]
- 18. Loser C, Aschl G, Hebuterne X, et al. ESPEN guidelines on artificial enteral nutrition: percutaneous endoscopic gastrostomy (PEG). Clin Nutr 2005; 24: 848–861. [DOI] [PubMed] [Google Scholar]
- 19. Oshima F, Yokozeki M, Hamanaka M, et al. Prediction of dysphagia severity: an investigation of the dysphagia patterns in patients with lateral medullary infarction. Intern Med 2013; 52: 1325–1331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.