Abstract
Axial spondyloarthritis (axSpA) is a chronic inflammatory condition that encompasses ankylosing spondylitis (AS) as well as non-radiographic axial disease (nr-axSpA) and can lead to chronic pain, structural damage and disability. The introduction of tumour necrosis factor inhibitor (TNFi) drugs for AS heralded a new era of drug therapeutics for what was previously a largely untreatable disease. This has now been expanded with the licensing of secukinumab, an interleukin 17A (IL-17A) inhibitor for the treatment of AS. Although biologic disease modifying antirheumatic drugs (bDMARDs) are not a first line treatment option in AS or axSpA, they are highly effective following incomplete or no response to physiotherapy and non-steroidal anti-inflammatory drugs (NSAIDs). Current research strategies aim to test whether the desired treatment goal of disease remission may now be achievable with early and stratified use of bDMARDs in both AS and nr-axSpA. This review summarizes the current literature on axSpA including pathophysiology, treatment indications, radiographic progression and the evidence for new developments in the treatment of both AS and nr-axSpA.
Keywords: ankylosing spondylitis, SpA, axSpA, sacroiliitis, axial psoriatic arthritis, biologic therapies, anti-TNF, IL-17A, secukinumab, JAK inhibitors
Introduction
Axial spondyloarthritis (axSpA) has a broad phenotype which includes both ankylosing spondylitis (AS), the prototype spondyloarthropathy also known as radiographic axSpA, and the non-radiographic spectrum of the disease (nr-axSpA) that may represent either early or mild forms of disease with the potential to progress into AS. The term spondyloarthritis (SpA) refers to a group of heterogeneous conditions which share common aetiopathogenic and clinical manifestations underpinned by a complex genotype. Chronic inflammation affects musculoskeletal sites of synovium, entheses, and extra-articular targets such as the uvea and the aortic valve root. The all-inclusive clinical entity is axial spondyloarthritis (axSpA), of which AS is the extreme phenotypic manifestation characterized by sacroiliac joint and spinal damage which may range from mild erosive disease to new bone formation and joint fusion. Other subgroups are largely characterized by peripheral joint involvement and include psoriatic arthritis (PsA), arthritis related to inflammatory bowel disease and reactive arthritis, where the use of therapy in those with axial disease is extrapolated from AS. It is this diversity in clinical phenotype that has hindered research in SpA and led to the somehow artificial divide of axial versus peripheral SpA. In reality, individuals with a predominant axial disease may develop peripheral joint involvement, much the same as individuals with typical peripheral PsA can develop axial involvement indistinguishable from those with AS in an estimated 40% of cases.1
AxSpA can therefore be defined as a chronic inflammatory disease with a varied clinical phenotype which in its severe, advanced stage is identified by a combination of clinical symptoms and established radiographic changes which can be graded according to the definitions given in the 1984 modified New York criteria.2 This stage is called AS.
It is now understood, however that a large number of affected individuals may not be readily identifiable by the modified New York criteria, yet they can suffer the same burden of symptoms and disability as those who do. Although it was initially thought that the non-radiographic stage may represent an early phase of disease (early AS) for a subset of individuals; this will not be the case for all, as radiographic progression is not universal. The recent classification criteria for axSpA developed by the Assessment of Spondyloarthritis International Society (ASAS) incorporates both radiographic and non-radiographic disease stages, provided they include a combination of features such as sacroiliitis on either radiography or magnetic resonance imaging (MRI), HLA-B27, C-reactive protein (CRP), and other associated clinical characteristics.2,3 These criteria capture the broad spectrum of features accountable towards confirming a diagnosis of axSpA (Figure 1).
Figure 1.
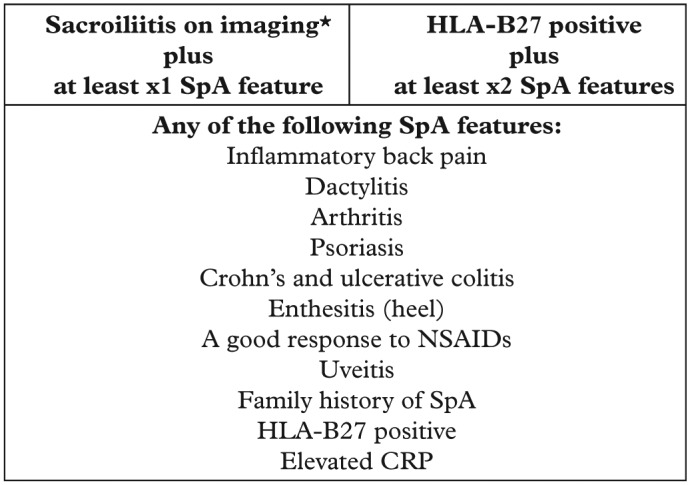
ASAS classification criteria for axial SpA in patients with back pain for at least 3 months and under 45 years of age.
*Sacroiliitis is defined by definite radiographic evidence by modified New York criteria or on MRI by ASAS consensus definition. Adapted from Rudwaleit and colleagues.4
ASAS, Assessment of Spondyloarthritis International Society; MRI, magnetic resonance imaging; SpA, spondyloarthritis.
Epidemiology
Axial SpA commonly starts in the second to third decade of life with a male to female ratio of approximately 2–3:1. Disease onset is about 5 years sooner in HLA-B27 positive individuals compared with those who are HLA-B27 negative.5 The majority of epidemiological studies performed to date have been in AS, which has an estimated prevalence of 0.5–1%.6 The overall prevalence of axSpA is variable with estimates between 0.32% and 1.4% depending upon geographical region and ethnicity.5 The average age of symptom onset in axSpA is slightly later in women than men with a lower prevalence of HLA-B27 in women which may account for slightly longer diagnostic delay.7
Pathophysiology of axSpA
AxSpA is characterized by a polyenthesitis which at the molecular level leads to an osteitis and secondary synovitis.8 The common denominator between enthesitis and subchondral osteitis that characterizes early sacroiliac joint disease, is disease localization to sub-fibrocartilaginous bone that is a site of high physical stress.9 Our current understanding is that bone repair leads to excessive bone formation by syndesmophyte formation and subsequent ankylosis typical of AS, but which can mark the evolution of the disease process from axSpA into AS. There are several mechanisms implicated in the pathogenesis of disease including HLA-B27, a class I surface antigen encoded by the B locus of the major histocompatibility complex (MHC). The basis for the association between HLA-B27 and AS remains unexplained but there are two major theories. Firstly, the fact that AS has, at the population level been associated with other MHC class-1 antigens and is genetically linked to single nucleotide polymorphisms involved in peptide loading to T-cells, invokes a CD8 T-cell driven disease, although a putative antigen has not been defined.10 The second theory holds that there is abnormal function of antigen presenting cells and a tendency of HLA-B27 to misfold, triggering the production of interleukins, IL-17 and IL-23.11 T-cell mediated mechanisms have been described for CD4+ and CD8+ T-cells resulting in further release of cytokines including tumour necrosis factor α (TNF-α), IL-22, IL 17 associated with bony destruction, osteoproliferation, and synovitis. Structural bony damage causes stimulation of repair mechanisms that involve osteoproliferation which is associated with the development of syndesmophyte formation and leads to bony ankylosis, the hallmark of AS and main cause for loss of functional ability.
The two major non-HLA-B27 loci specifically associated with AS are the endoplasmic reticulum aminopeptidase (ERAP) and the IL-23 receptor. The ERAP gene is specific to HLA-B27 positive patients and is involved in the processing of proteins, including those presented by HLA-B27, for the MHC class I presentation to immune effector cells.10 The IL-23 receptor activates proinflammatory cells including the T-helper cells leading to the secretion of IL-17.
The intestinal microbiome is also thought to play a pertinent role in the pathogenesis of disease that is not yet fully understood. Dysbiosis in the gut flora may drive proinflammatory cytokines resulting in intestinal inflammation.11 Barrier dysfunction in axSpA is associated with exposure of the immune system to microorganisms. Ruminococcus gnavus has recently been shown to be specific to the gut in SpA and is associated with disease activity.12 This contributes towards damage to dermal and mucosal surfaces by chronic inflammation leading to the subsequent development of skin psoriasis and clinical or subclinical intestinal inflammation.13 Although the microbiome has been posited to be central to most common diseases, the fact that AS patients often have abnormal mucosal permeability and that subclinical gut lesions correlate with MRI-determined sacroiliitis, suggests a very strong connection between the gut environment and clinical disease in AS and SpA.14 More recently, the presence of group 3 innate lymphoid cells, which form an essential part of the gut and skin barrier in SpA, were also identified in entheseal soft tissue and adjacent perientheseal bone suggesting a role in the pathogenesis of axSpA.15 Despite the improved understanding of AS and axSpA, the pathogenic insights are yet to show significant therapy advances. Indeed the greatest advances have come from empirical studies utilizing cytokine pathway blockade, resulting in some spectacular success, representative of the pivotal role of TNF and IL-17A thus far. These are further discussed below.
What are the indications for treatment in AS and axSpA?
Up to recently, the lack of understanding about the aetiopathogenic mechanisms in SpA translated into an absence of efficacious therapies. Exercise, however has always been understood to alleviate symptoms and perhaps ameliorate disease progression. Robust evidence behind the role of self-exercise or structured physiotherapy in axSpA is however lacking.16,17 The same applies to the use of non-steroidal anti-inflammatory drugs (NSAIDs) which remain the mainstay of initial therapy in AS and axial SpA.18 NSAIDs can alleviate symptoms very effectively but also have associated risks with long-term administration and may lead to gastrointestinal, cardiovascular and renal complications. However, there is the recognition that up to 40% of affected individuals may never require treatment above NSAIDs and physiotherapy. An estimated two-thirds, may have active disease suitable for biologic disease modifying anti-rheumatic drugs (bDMARDs) as defined by a Bath Ankylosing Spondylitis Disease activity Index (BASDAI) questionnaire score of greater than 4 and spinal visual analogue scale greater than 4, or an AS disease activity score (ASDAS) of 2.1 or above.19,20 The current ASAS-European League Against Rheumatism (ASAS-EULAR) guidelines recommend treating patients with bDMARDs when elevated CRP or radiological or MRI evidence of sacroiliitis is present, and there is a failure of at least two different NSAIDs each for 4 weeks in conjunction with the treating rheumatologist’s opinion.21 The American College of Rheumatology and the Spondyloarthritis Research and Treatment Network (SPARTAN) have published complementary treatment recommendations.22
The evidence for treatment with conventional disease modifying antirheumatic drugs (cDMARDs) is lacking but physicians may use these, especially for associated peripheral synovitis. The bDMARDs are the only proven efficacious therapies for the treatment of axSpA. Studies looking into the efficacy of TNF inhibitors identified that the best predictors of achieving a good response are: raised CRP, short symptom duration or young age, and active inflammation on MRI.5 On the other hand, the main indicator of poor response is smoking as shown by data from the German Spondyloarthritis Inception Cohort (GESPIC) demonstrating that smokers suffer a dose-dependent deterioration on their structural damage over 2 years shown by changes to the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS), a composite index score characterizing AS radiologic features in the spine used to determine radiographic structural progression in AS over time.
What precautions should be considered prior to bDMARD initiation?
It is recommended that prior to immunosuppression with bDMARDs, patients should undergo relevant screening to identify potential contraindications, comorbidities and risks. This process is the same for axSpA as it is for other rheumatic diseases such as rheumatoid arthritis (RA) and PsA and specifically includes checking for prior history of contraindications to anti-TNF, such as history of demyelinating disease, malignancy, moderate-to-severe congestive heart failure using the New York Heart Association grade III or IV, and case history risk assessment for tuberculosis (TB).23,24 It is routine practice for all patients to undergo a pretreatment chest radiograph, TB testing for latent and active disease with either interferon gamma release assay or tuberculin skin test or both, serology for hepatitis B and C, human immunodeficiency virus, varicella zoster virus (VZV), and antinuclear antibody. There is paucity of data in axSpA for infection risks with bDMARDs but the risk appears to be lower in AS compared with RA and PsA. In a meta-analysis of 71 clinical trials with adalimumab, 4 in AS, the serious infection risk was 1.4/100 patient-years (pys) compared with 4.6/100 pys for RA and 2.8/100 pys for PsA.25 An increased serious infection risk was also observed with etanercept at 3.01/100 pys in patients with AS compared with 3.75/100 pys for RA.26 There was an overall increased risk of any infection observed in 440 axSpA patients treated with TNFis at 15/100 pys, and for serious infections 1.3/100 pys.27 It is therefore strongly recommended that patients are administered vaccinations against inactivated influenza and pneumococcus to protect from infection whilst on bDMARDs.28 On the contrary, live attenuated vaccines such as measles mumps and rubella, live attenuated influenza, VZV, yellow fever, Ty21a oral typhoid, Bacillus Calmette–Guerin (BCG), and rotavirus should be avoided due to the increased risks of uninhibited bacterial or viral replication in patients on bDMARDs and in light of reports of severe complications.29 The risks and benefits of initiating a bDMARD should always be fully considered and patient education for shared decision making between the clinician and patient is key in the treatment discussion.30
Anti-TNF therapy and IL-17A inhibition in AS and axSpA
There are currently five TNFi bDMARDs available for the treatment of AS: adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab. Most of the evidence for these drugs comes from phase III randomized double-blind placebo-controlled clinical trials which included patients with confirmed AS that were treated with anti-TNF versus placebo. Evaluation of treatment responses in these studies was demonstrated by the ASAS response criteria (Figure 2). All the bDMARD therapies display efficacy against placebo, but no direct comparison on efficacy can be made between biologics given the absence of head-to-head studies. Improved understanding of the IL-23/IL-17 axis in the pathogenesis of SpA has led to the recent advent of drugs targeting IL-17A such as secukinumab, which has opened up a new treatment avenue for people affected with axSpA, PsA and skin psoriasis. Evidence from the MEASURE study programme confirmed secukinumab efficacy in AS.31,32 The blockade of IL-17A via subcutaneous secukinumab in AS, revealed an efficacious ASAS40 response, albeit slightly less at 36% compared with the combined mean ASAS40 of 44.5% overall from the TNFi trials for AS (Figure 2).31 A successful treatment response was sustained at 52 weeks in both studies for AS, but interestingly the 300 mg monthly dose of secukinumab, which has not yet been examined in clinical trials for AS, has been shown to be more effective than the 150 mg monthly dose in treating skin psoriasis alone.33
Figure 2.
ASAS40 and 20 responses to biologic DMARDs (bDMARDs) in ankylosing spondylitis (AS) and nonradiographic axial spondyloarthritis (nr-axSpA) in phase III clinical trials.
Direct comparison is limited given that there are no head-to-head studies comparing biologics. Partly adapted from Sieper et al.5
DMARD, disease modifying antirheumatic drug.
Although this review cannot go into detail on safety, we recommend continuing safe prescribing by the usual pre-bDMARD screen prior to TNFi or anti-IL17 therapies given the potential adverse effects. The safety profile of secukinumab in AS appears to be comparable with that seen in the trials for skin psoriasis.34,35 Despite the concerns over suicide rates that halted the brodalumab trials (an IL-17A receptor antagonist which also inhibits IL-17F, IL-17A/F heterodimer and IL-17E), none occurred in the treatment group for secukinumab, although one suicide was recorded in the placebo group (MEASURE 1).36 Exacerbations of Crohn’s disease and uveitis were adverse events reported in the clinical trials (MEASURE 1 and 2) which suggests TNFi, excluding etanercept, may have been more suitable treatment for those patients.34 The adverse gut effect in rare cases has been attributed to the protective role of IL-17 on enterocyte tight junction formation.37 Another IL-17A antagonist, ixekizumab, has demonstrated efficacy in phase III clinical trials for the treatment of skin psoriasis and PsA but there are none to date in axSpA or AS.38–40
Other bDMARDs
IL-12 and IL-23 blockade
Ustekinumab is a monoclonal antibody that inhibits the p40 subunit of IL-12, and IL-23. It is licensed for the treatment of skin psoriasis, PsA, and more recently for Crohn’s disease.41 Over expression of IL-23 has been linked to the development of enthesitis in animal models which resembles human SpA which suggests ustekinumab may be effective in AS. A proof-of-concept, prospective, open-label trial in AS reported a clinically efficacious response against placebo at 24 weeks with 65% of participants achieving an ASAS40 response.42 Secondary outcome measures at week 24 were achieved with an ASAS20 of 75%, ASAS5/6 of 60%, 30% achieved ASAS partial remission, 55% achieved a 50% response in BASDAI (BASDAI50), and 50% demonstrated a change in ASDAS ⩾ 1.1 as compared with baseline (clinically important improvement), 20% achieved a response in ASDAS ⩾ 2.0 (major improvement), and 35% achieved inactive disease defined by ASDAS < 1.3.42 Exploratory studies with IL-23 anti-p19 monoclonal antibodies have been performed but not reported as yet.43
IL-1 blockade
Interleukin-1 (IL-1) is a highly active proinflammatory cytokine that may play a role in certain patients particularly those with a high inflammatory burden of disease defined biochemically by a high CRP level. We already know from the treatment of patients with autoinflammatory syndromes, where there are typically high levels of inflammation, that the blockade of IL-1 alone results in rapid and sustained disease remission.44 There are limited data from open-label studies for the use of anakinra in AS, an antagonist to the IL-1 receptor. An early study from Leeds demonstrated efficacy to anakinra by clinical and MRI features in 20 patients with AS, however another study reported no significant effect as compared with placebo.45,46 The latter study had low CRP levels compared with the former and since CRP is a predictor of response, it would be premature to suggest that blockade of the IL-1 pathway has no role.
IL-6 blockade
The clinical trials using tocilizumab for the treatment of AS (BUILDER-1 and 2), failed to achieve their desired endpoint for demonstrating efficacy.47 Sarilumab is similar in its action except that it is the first fully human monoclonal IL-6α antagonist. It was tested in a phase II randomized controlled trial (ALIGN study) which demonstrated no statistical difference between ASAS20 response over placebo despite the use of multiple dosing regimens.48 A recent report has described refractory spondyloarthritis with peripheral synovitis treated successfully in some patients with tocilizumab, an IL-6 receptor blocker, which was used in patients with high CRP and demonstrated an impressive treatment response by clinical symptoms and CRP normalization.49 These patients were all TNFi nonresponders with the additional burden of aggressive peripheral arthritis and consisted of an AS-resistant disease phenotype. Interestingly, a TNF-independent and IL-6 dependent model where disease starts at the synovio-entheseal complex has been recently described suggesting that a subgroup of SpA cases may be IL-6 dependent.50 However, an anti-IL-6 strategy is an unlicensed use of these agents and further research is needed to define rare phenotypes.
Anti-T-cell co-stimulation and anti-B-cell targeted therapy
Abatacept is an inhibitor of T-cell co-stimulation and has also been used to treat AS in a prospective open-label study but without any significant difference as compared with placebo.51 B-cell inhibition has also been tested using rituximab, an anti-CD-20 monoclonal antibody. The trial was small with only 10 patients in each of the anti-TNF naïve and anti-TNF experienced patients. Interestingly, a higher response was achieved in the rituximab-treated group in the anti-TNF naïve patients as compared with placebo with the achievement of an ASAS40 response of 40% at week 24, despite no clinical efficacy seen in the anti-TNF experienced patients.52 We are not aware of this anti-CD20 strategy being developed in AS.
Targeted synthetic DMARDs
Targeted synthetic DMARDs are non-biologic smaller molecules and an emerging group of drugs within rheumatology. Apremilast inhibits phosphodiesterase 4, suppresses the activation of proinflammatory cytokines and activates anti-inflammatory mediators. It is effective for the treatment of psoriasis and PsA.53 It has been tried in AS patients in a small proof-of-concept trial with 36 patients who were treated for 12 weeks with either apremilast or placebo. A moderate reduction in the BASDAI was reported but did not achieve statistical significance.54 However results are still awaited from a larger phase III placebo-controlled trial.55
Tofacitinib is an oral Janus kinase (JAK) inhibitor and also a small molecule. It inhibits cell signaling through JAK3 and JAK1 receptors and to a lesser extent JAK2. In the United States, it is licensed for the treatment of RA, but is yet to be approved in Europe. A small placebo-controlled trial in AS patients was conducted without any significant benefit at 12 weeks.56 Given that this drug appears to work in PsA, including enthesitis, the basis for these findings is slightly unexpected.
Radiographic progression
True disease modification in terms of inhibition of radiographic disease remains an important consideration with regards to long-term treatment with immunomodulatory therapies. Studies that involve established AS patients followed up over 2–4 years showed no evidence to support the inhibitory effect of TNFi drugs on radiographic progression.57 However, there is some evidence that early treatment for more than 4 years may retard the radiographic progression if treatment with TNFi is given early.58 By comparison, NSAIDs have an inhibitory effect on osteoclast activity, and this translates to clinical trial evidence of inhibition of radiographic progression in AS over 2 years of continuous use, as compared with on demand use which was shown to have a lesser effect.59 Continuous NSAID use also slowed radiogtraphic progression in patients with raised acute phase reactants.60 In contrast with NSAIDs, TNFi has been shown to normalize the erthyrocyte sedimentation rate confirming its efficacy and potency in clearing the symptoms in AS.18 We are yet to fully understand axSpA, including which individuals will or will not progress to radiographic disease. We do know that individuals who are HLA-B27 positive, have elevated CRP levels, inflammation of the sacroiliac joints on MRI, and are smokers have been identified as the most likely to develop radiographic progression.61,62 It is estimated that 5–12% of nr-axSpA will progress to develop radiographic disease over a 2 year period and this increases to 20% in nr-axSpA patients with active spinal inflammation on MRI.61,62 An estimated 15–20% will never develop radiographic sacroiliitis.62 Further data has emerged indicating that MRI positive patients with axSpA predicts radiographic sacroiliac changes by 5–8 years through data from both the DESIR and Leeds cohorts respectively.63,64
However, our knowledge from recent animal models has provided a greater insight into the pathogenic link between IL-23, IL-22, IL-17 and early entheseal disease including periosteal bone formation. At the enthesis, specific types of IL-23 receptor-positive T-cells have been identified (retinoic acid receptor-related orphan nuclear receptor γt positive CD3+CD4-CD8-) that produce IL-22 and IL-17 in response to IL-23.65 Following the in vivo stimulation of entheseal cells by IL-23, expression of IL-22 and IL-17 downstream caused enthesitis in addition to entheseal new bone formation and occurred without any synovitis.66 Although our understanding of animal SpA is improving, we are yet to determine whether this knowledge can be extrapolated to human SpA although these current developments remain very promising.65
Discussion
AxSpA is the accepted term for all the individuals with AS, including those with non-radiographic disease that may represent milder or earlier disease. The advent of bDMARDs in the last two decades has changed the lives of many affected individuals and given clinicians effective options above NSAIDs and physiotherapy. There are five TNFi bDMARDs licensed for the treatment of AS, of which four can also be used in nr-axSpA. Although for many patients TNFi treatment has been highly successful, there remains an unmet need for those who do not respond or cannot tolerate them. Further understanding of axSpA disease phenotypes may unlock potential opportunities to identify and treat early with tailored therapy without having a ‘one size fits all’ sequence to follow before a response is obtained. The more recent availability of IL-17 blockade has brought an era of choice in mode of action of targeted immunotherapy beyond TNFi and although the clinical trial data are almost comparable to that for TNFi, we await the experience from real world clinical practice. Interestingly, secukinumab has also shown efficacy for treating skin psoriasis and PsA, including at the higher 300 mg monthly maintenance dose which has not yet been reported in AS studies.33 But conversely, the available evidence suggests that it is not suitable for individuals with Crohn’s disease due to increased adverse events including the triggering of exacerbations.67,68 This highlights that clinicians should be mindful of the paradoxical responses to treatment in diseases of the SpA family. The reverse has been reported following treatment for inflammatory bowel disease with a humanized immunoglobulin G1 (IgG1) monoclonal antibody to α4β7 integrin that resulted in the induction of sacroiliitis or arthritic flare.69 Despite limited responses with other biologics and targeted DMARDs, we tentatively await the potential for new therapies including those that have been licensed for the use in other conditions; namely the JAK inhibitors, which are already licensed for the treatment of RA in the United States (tofacitinib) and Europe (baricitinib), and ustekinumab which is licensed for use in psoriasis and Crohn’s disease in Europe.
Conclusion
The possibility of diagnosing axSpA early, together with the advent of new therapeutic options for the treatment of nr-axSpA and AS, have significantly improved the care of affected individuals. The introduction of IL-17A blockade increasing the choice of cytokine target in AS beyond TNF, marks a step forward in the management of AS, and may be suitable for the whole spectrum of axSpA. However, up to now only limited data exist on their effect on radiographic progression as possible disease modifiers. Defining what should be the most effective sequence of therapy with bDMARDs following non-response is an area of unmet need and together with the search for biomarkers of treatment response and the careful study of longitudinal axSpA cohorts, remain key domains to research for the coming decade.
Acknowledgments
The views expressed are those of the authors and not necessarily those of the National Health Service, UK, the National Institute for Health Research, UK or the Department of Health, UK.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research is supported by the National Institute for Health Research, Leeds Biomedical Research Centre, UK.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors Professor Dennis McGonagle and Dr Helena Marzo-Ortega have received grants and/or honoraria from Abbvie, Celgene, Janssen, Elli-Lilly, MSD, Novartis, Pfizer and UCB.
References
- 1. Lambert JR, Wright V. Psoriatic spondylitis: a clinical and radiological description of the spine in psoriatic arthritis. Q J Med 1977; 46: 411–425. [PubMed] [Google Scholar]
- 2. Linden S, Van Der Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. Arthritis Rheum 1984; 27: 361–368. [DOI] [PubMed] [Google Scholar]
- 3. Sieper J, Rudwaleit M, Baraliakos X, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 2009; 68(Suppl. 2): ii1–44. [DOI] [PubMed] [Google Scholar]
- 4. Rudwaleit M, van der Heijde D, Landewé R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009; 68: 777–783. [DOI] [PubMed] [Google Scholar]
- 5. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017; 42: 251–256. [DOI] [PubMed] [Google Scholar]
- 6. Braun J, Bollow M, Remlinger G, et al. Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum 1998; 41: 58–67. [DOI] [PubMed] [Google Scholar]
- 7. Ciurea A, Scherer A, Weber U, et al. Age at symptom onset in ankylosing spondylitis: is there a gender difference? Ann Rheum Dis 2014; 73: 1908–1910. [DOI] [PubMed] [Google Scholar]
- 8. McGonagle D, Gibbon W, Emery P. Classification of inflammatory arthritis by enthesitis. Lancet 1998; 352: 1137–1140. [DOI] [PubMed] [Google Scholar]
- 9. McGonagle D, Khan MA, Marzo-Ortega H, et al. Enthesitis in spondyloarthropathy. Curr Opin Rheumatol 1999; 11: 244–250. [DOI] [PubMed] [Google Scholar]
- 10. McGonagle D, Aydin SZ, Gül A, et al. ‘MHC-I-opathy’—unified concept for spondyloarthritis and Behçet disease. Nat Rev Rheumatol 2015; 11: 731–740. [DOI] [PubMed] [Google Scholar]
- 11. Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med 2016; 374: 2563–2574. [DOI] [PubMed] [Google Scholar]
- 12. Breban M, Tap J, Leboime A, et al. Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann Rheum Dis 2017; 76: 1614–1622. [DOI] [PubMed] [Google Scholar]
- 13. Sieper J, Braun J, Dougados M, et al. Axial spondyloarthritis. Nat Rev Dis Prim 2015; 1: 1–16. [DOI] [PubMed] [Google Scholar]
- 14. Brakenhoff LKPM, van der Heijde DM, Hommes DW, et al. The joint–gut axis in inflammatory bowel diseases. J Crohn’s Colitis 2010; 4: 257–268. [DOI] [PubMed] [Google Scholar]
- 15. Cuthbert RJ, Fragkakis EM, Dunsmuir R, et al. Group 3 innate lymphoid cells in human enthesis. Arthritis Rheumatol 2017; 69: 1816–1822. [DOI] [PubMed] [Google Scholar]
- 16. Millner JR, Barron JS, Beinke KM, et al. Exercise for ankylosing spondylitis: an evidence-based consensus statement. Semin Arthritis Rheum 2016; 45: 411–427. [DOI] [PubMed] [Google Scholar]
- 17. Sharan D, Rajkumar J. Physiotherapy for ankylosing spondylitis: systematic review and a proposed rehabilitation protocol. Curr Rheumatol Rev 2017; 13: 121–125. [DOI] [PubMed] [Google Scholar]
- 18. Haroon N, Kim T-H, Inman RD. NSAIDs and radiographic progression in ankylosing spondylitis Bagging big game with small arms? Ann Rheum Dis 2012; 71: 1593–1595. [DOI] [PubMed] [Google Scholar]
- 19. Barkham N, Kong KO, Tennant A, et al. The unmet need for anti-tumour necrosis factor (anti-TNF) therapy in ankylosing spondylitis. Rheumatology 2005; 44: 1277–1281. [DOI] [PubMed] [Google Scholar]
- 20. Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994; 21: 2286–2291. [PubMed] [Google Scholar]
- 21. van der Heijde D, Ramiro S, Landewé R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017; 76: 978–991. [DOI] [PubMed] [Google Scholar]
- 22. Ward MM, Deodhar A, Akl EA, et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2016; 68: 282–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ding T, Ledingham J, Luqmani R, et al. BSR and BHPR rheumatoid arthritis guidelines on safety of anti-TNF therapies. Rheumatology 2010; 49: 2217–2219. [DOI] [PubMed] [Google Scholar]
- 24. Singh JA, Saag KG, Bridges SL, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2016; 68: 1–25. [DOI] [PubMed] [Google Scholar]
- 25. Burmester GR, Pannaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis 2013; 72: 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamilton L, Barkham N, Bhalla A, et al. BSR and BHPR guideline for the treatment of axial spondyloarthritis (including ankylosing spondylitis) with biologics. Rheumatology 2017; 56: 313–316. [DOI] [PubMed] [Google Scholar]
- 27. Wallis D, Thavaneswaran A, Haroon N, et al. Tumour necrosis factor inhibitor therapy and infection risk in axial spondyloarthritis: results from a longitudinal observational cohort. Rheumatology 2015; 54: 152–156. [DOI] [PubMed] [Google Scholar]
- 28. van Assen S, Agmon-Levin N, Elkayam O, et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2011; 70: 414–422. [DOI] [PubMed] [Google Scholar]
- 29. Ferreira I, Isenberg D. Vaccines and biologics. Ann Rheum Dis 2014; 73: 1446–1454. [DOI] [PubMed] [Google Scholar]
- 30. Smolen JS, Schöls M, Braun J, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Annals of the Rheumatic Diseases Published Online First: 06 July 2017. DOI: 10.1136/annrheumdis-2017-211734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med 2015; 373: 2534–2548. [DOI] [PubMed] [Google Scholar]
- 32. Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis 2016; 76: 1070–1077. [DOI] [PubMed] [Google Scholar]
- 33. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis — results of two phase 3 trials. N Engl J Med 2014; 371: 326–338. [DOI] [PubMed] [Google Scholar]
- 34. Baeten D, Baraliakos X, Braun J, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet 2013; 382: 1705–1713. [DOI] [PubMed] [Google Scholar]
- 35. Blauvelt A. Safety of secukinumab in the treatment of psoriasis. Expert Opin Drug Safety 2016; 15: 1413–1420. [DOI] [PubMed] [Google Scholar]
- 36. Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti–interleukin-17–receptor antibody for psoriasis. N Engl J Med 2012; 366: 1181–1189. [DOI] [PubMed] [Google Scholar]
- 37. Lee JS, Tato CM, Joyce-Shaikh B, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity 2015; 43: 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nash P, Kirkham B, Okada M, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet 2017; 389: 2317–2327. [DOI] [PubMed] [Google Scholar]
- 39. Mease PJ, van der Heijde D, Ritchlin CT, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naïve patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis 2017; 76: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med 2016; 375: 345–356. [DOI] [PubMed] [Google Scholar]
- 41. Feagan B, Sandborn W, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016; 375: 1946–1960. [DOI] [PubMed] [Google Scholar]
- 42. Poddubnyy D, Geert K-, Hermann A, et al. Ustekinumab for the treatment of patients with active ankylosing spondylitis: results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann Rheum Dis 2014; 73: 817–823. [DOI] [PubMed] [Google Scholar]
- 43. U.S National Library of Medicine. An efficacy and safety study of ustekinumab in participants with active nonradiographic axial spondyloarthritis, https://clinicaltrials.gov/ct2/show/NCT02407223 (accessed 18 August 2017).
- 44. Dinarello CA, Simon A, van der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 2012; 11: 633–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tan AL, Marzo-Ortega H, O’Connor P, et al. Efficacy of anakinra in active ankylosing spondylitis: a clinical and magnetic resonance imaging study. Ann Rheum Dis 2004; 63: 1041–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Haibel H, Rudwaleit M, Listing J, et al. Open label trial of anakinra in active ankylosing spondylitis over 24 weeks. Ann Rheum Dis 2005; 64: 296–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sieper J, Porter-Brown B, Thompson L, et al. Assessment of short-term symptomatic efficacy of tocilizumab in ankylosing spondylitis: results of randomised, placebo-controlled trials. Ann Rheum Dis 2014; 73: 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sieper J, Braun J, Kay J, et al. Sarilumab for the treatment of ankylosing spondylitis: results of a Phase II, randomised, double-blind, placebo-controlled study (ALIGN). Ann Rheum Dis 2015; 74: 1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Merashli M, De Marco G, Podgorski M, et al. Evidence of response to IL-6 inhibition in some cases of refractory spondyloarthritis-associated peripheral synovitis. Ann Rheum Dis 2016; 75: 1418–1420. [DOI] [PubMed] [Google Scholar]
- 50. De Wilde K, Martens A, Lambrecht S, et al. A20 inhibition of STAT1 expression in myeloid cells: a novel endogenous regulatory mechanism preventing development of enthesitis. Ann Rheum Dis 2016; 76: 585–592. [DOI] [PubMed] [Google Scholar]
- 51. Song IH, Heldmann F, Rudwaleit M, et al. Treatment of active ankylosing spondylitis with abatacept: an open-label, 24-week pilot study. Ann Rheum Dis 2011; 70: 1108–1110. [DOI] [PubMed] [Google Scholar]
- 52. Song IH, Heldmann F, Rudwaleit M, et al. Different response to rituximab in tumor necrosis factor blocker-naive patients with active ankylosing spondylitis and in patients in whom tumor necrosis factor blockers have failed: a twenty-four-week clinical trial. Arthritis Rheum 2010; 62: 1290–1297. [DOI] [PubMed] [Google Scholar]
- 53. Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis 2016; 75: 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pathan E, Abraham S, Van Rossen E, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in ankylosing spondylitis. Ann Rheum Dis 2013; 72: 1475–1480. [DOI] [PubMed] [Google Scholar]
- 55. U.S National library of Medicine. Study of apremilast to treat subjects with active ankylosing spondylitis, https://clinicaltrials.gov/ct2/show/study/NCT01583374?term=NCT01583374&rank=1 (accessed 18 August 2017).
- 56. van der Heijde D, Deodhar A, Wei JC, et al. Tofacitinib in patients with ankylosing spondylitis: a phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis 2017; 76: 1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Van Der Heijde D, Landewé R, Baraliakos X, et al. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum 2008; 58: 3063–3070. [DOI] [PubMed] [Google Scholar]
- 58. Haroon N, Inman RD, Learch TJ, et al. The impact of tumor necrosis factor α inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum 2013; 65: 2645–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wanders A, Heijde D van der, Landewé R, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum 2005; 52: 1756–1765. [DOI] [PubMed] [Google Scholar]
- 60. Kroon F, Landewé R, Dougados M, et al. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann Rheum Dis 2012; 71: 1623–1629. [DOI] [PubMed] [Google Scholar]
- 61. Navarro-Compán V, Machado PM. Spondyloarthropathies: sacroiliac joint radiographic progression — speed and determinants. Nat Rev Rheumatol 2016; 12: 380–382. [DOI] [PubMed] [Google Scholar]
- 62. Sieper J Van Der, Heijde D. Review: nonradiographic axial spondyloarthritis: new definition of an old disease? Arthritis Rheum 2013; 65: 543–551. [DOI] [PubMed] [Google Scholar]
- 63. Bennett AN, Mcgonagle D, O’Connor P, et al. Severity of baseline magnetic resonance imaging–evident sacroiliitis and HLA–B27 status in early inflammatory back pain predict radiographically evident ankylosing spondylitis at eight years. Arthritis Rheum 2008; 58: 3413–3418. [DOI] [PubMed] [Google Scholar]
- 64. Dougados M, Sepriano A, Molto A, et al. Sacroiliac radiographic progression in recent onset axial spondyloarthritis: the 5-year data of the DESIR cohort. Ann Rheum Dis 2017; 76: 1823–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Breban M, Araujo LM, Chiocchia G. Editorial: animal models of spondyloarthritis: do they faithfully mirror human disease? Arthritis Rheumatol 2014; 66: 1689–1692. [DOI] [PubMed] [Google Scholar]
- 66. Sherlock JP, Joyce-Shaikh B, Turner SP, et al. IL-23 induces spondyloarthropathy by acting on ROR-[gamma]t+ CD3+CD4-CD8-entheseal resident T cells. Nat Med 2012; 18: 1069–1077. [DOI] [PubMed] [Google Scholar]
- 67. Colombel JF, Sendid B, Jouault T, et al. Secukinumab failure in Crohn’s disease: the yeast connection? Gut 2013; 62: 800–801. [DOI] [PubMed] [Google Scholar]
- 68. Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012; 61: 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Varkas G, Thevissen K, De Brabanter G, et al. An induction or flare of arthritis and/or sacroiliitis by vedolizumab in inflammatory bowel disease: a case series. Ann Rheum Dis 2017; 76: 878–881. [DOI] [PubMed] [Google Scholar]
- 70. Van Der Heijde D, Kivitz A, Schiff MH, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2006; 54: 2136–2146. [DOI] [PubMed] [Google Scholar]
- 71. Davis JC, van der Heijde DM, Braun J, et al. Sustained durability and tolerability of etanercept in ankylosing spondylitis for 96 weeks. Ann Rheum Dis 2005; 64: 1557–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Inman RD, Davis JC, Van Der Heijde D, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum 2008; 58: 3402–3412. [DOI] [PubMed] [Google Scholar]
- 73. Landewé R, Braun J, Deodhar A, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann Rheum Dis 2014; 73: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sieper J, van der Heijde D, Dougados M, et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann Rheum Dis 2012; 72: 815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dougados M, Van Der Heijde D, Sieper J, et al. Symptomatic efficacy of etanercept and its effects on objective signs of inflammation in early nonradiographic axial spondyloarthritis: a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol 2014; 66: 2091–2102. [DOI] [PubMed] [Google Scholar]
- 76. Sieper J, van der Heijde D, Dougados M, et al. A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2015; 67: 2702–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]



