Abstract
The effects of pH cycling immersion on the corrosion of glass-based ceramic materials were investigated by examining the silicon release level in the immersion solution and the surface morphology of the ceramic after immersion. The hypothesis that pH cycling causes more surface degradation than constant immersion was tested. An inductively coupled plasma atomic emission spectrometer was used for Si ion concentration determination and scanning electron microscopy for surface morphology analyses. Two pH cycling sequences (pH 2, 7, 10 and pH 10, 2, 7) were employed in this study. Glass-ceramic disks were immersed in each pH solution for 3 d, then cycled for 27 d. The silicon release levels during the pH cycling were significantly higher than those in the constant pH immersion. The silicon levels for both cycling sequences were around 47 and 2 times higher than that in constant pH conditions for 2 and 10, respectively. The morphology of the ceramic treated with cycling was also significantly degraded as compared with the ceramic immersed in the constant pH solution. Thus, the severity of glass-ceramic degradation depends not only on the pH of the immersed solution but also on the pH of the previous solution. Since the pH of the oral environment can vary depending on the diet and buffering capacity of saliva, materials testing in constant pH immersion might underestimate the in vivo corrosion. New mechanisms were proposed to account for the effect of pH cycling on glass-ceramic corrosion.
Keywords: pH, silicon, ion exchange, dental porcelain, scanning electron microscopy, inductively coupled plasma atomic emission spectrophotometry
Introduction
Glass-ceramic materials used for dental prostheses are susceptible to corrosion. This was clearly demonstrated when all-ceramic crowns in a clinical study (Esquivel-Upshaw, Rose, et al. 2013) exhibited significant roughening of crown surfaces. The current testing standard for chemical durability does not reflect changes in pH that occur in the oral cavity. ISO standard 6872 for dental ceramics requires evidence of minimal chemical solubility for all dental ceramic materials when exposed to 4% acetic acid solution. However, in vitro studies revealed that ceramics exhibit corrosion and surface degradation after exposure to liquids over a broad pH range (Milleding et al. 2002; Butler et al. 2004; Ccahuana et al. 2010; Junpoom et al. 2010; Kukiattrakoon et al. 2010c). In addition, in vitro analysis has shown that ceramics degrade more severely when exposed to a basic pH buffer solution (Esquivel-Upshaw, Dieng, et al. 2013). These conditions are not reflected adequately in the standards for testing.
The oral environment presents a major challenge for dental restorations because of alternating pH environments. The oral environment is routinely subjected to fluctuations in acidity and alkalinity, which can range from pH 2 to 14 (Bridges and Mattice 1939; “pH Values of Food Products” 1962). Ingested basic substances, such as spinach (pH 8.3), lima beans or soy beans (pH 12), and antacids (pH 10-14), can increase the pH of the local oral environment, while sodas (pH 2.6), apple juice (pH 3.8), and various candies (pH ≤3) reduce the pH (Bridges and Mattice 1939; Landry et al. 2001). Dietary habits and the buffering capacity of saliva also have a significant effect on the pH of the oral fluid (Bartlett et al. 2011), and they challenge the chemical durability of ceramic-based restorations. Moreover, ceramic corrosion has been shown to adversely affect the fracture resistance of these materials (Drummond et al. 1991; Pinto et al. 2008) and their surface integrity (Milleding et al. 1999). These effects can reduce the longevity of the ceramic-based restoration and damage the adjacent oral structures as a result of enamel wear, plaque accumulation, and periodontal disease. In addition, rougher surfaces are more likely to cause adhesive and abrasive wear of the opposing tooth structures. Ceramics undergo surface degradation and corrosion through a complex mechanism, which involves the breakdown of the glass phase and release of component ions from the microstructure. This breakdown is influenced by several factors, which include mechanical abrasion and a concurrent loss of ions as a result of reactions with the environment. The results of in vitro studies on chemical corrosion suggest that acidic environments adversely affect the microhardness (Kukiattrakoon et al. 2009; Kukiattrakoon et al. 2010a, 2010b), flexural strength (Pinto et al. 2008), and surface roughness (Esquivel-Upshaw et al. 2001; Butler et al. 2004; Ccahuana et al. 2010; Junpoom et al. 2010; Kukiattrakoon et al. 2010c, 2011) of ceramics during prolonged exposure. In addition, surface roughness has been shown to decrease flexural strength (Fischer et al. 2003), increase wear of the opposing enamel surfaces (Esquivel-Upshaw et al. 2006; Esquivel-Upshaw, Rose, et al. 2013; Preis et al. 2012), and promote plaque accumulation (Aksoy et al. 2006; Al-Marzok and Al-Azzawi 2009) that can eventually lead to secondary caries and periodontal disease.
From the aforementioned studies, there is evidence that acidic or basic solutions increase surface degradation and reduce structural strength. As the pH of the oral environment can vary from acidic to basic environments based on diet and saliva, the effect of alternating pH levels on the surface of ceramic needs to be determined. The objectives of this study are 1) to test the hypothesis that pH cycling will cause significantly more surface degradation as a result of greater ion release as compared with constant pH immersion and 2) to test the hypothesis that the severity of degradation of ceramic is affected by the pH of the initial solution in the cycling sequence.
Materials and Methods
Constant Immersion pH Group
The methodology and results for the constant pH group was reported in a previous publication (Esquivel-Upshaw, Dieng, et al. 2013). Thirty-six disks of a glass-ceramic material (IPS Eris for Empress 2 Ceramic Core; Ivoclar Vivadent) were fabricated (12 × 2.0 mm) from porcelain powder and liquid. Disks were sintered with a programmable furnace (Radiance Multi-Stage MSL Furnace; Jelrus International) according to manufacturer recommendations. The densities of selected disks were measured to ensure structural consistency prior to immersion.
The specimens were predried for 7 min, dried for 2 min, heated to 403 °C at a rate of 60 °C/min under full vacuum, heated further at 724 °C for 1.5 min, and subsequently cooled for 2 min. Specimens were fired twice. Specimens were then ground through 600-grit abrasive, rinsed, and dried on both sides.
Each disk was washed 3 times in ethyl alcohol, dried, weighed on a microbalance (precision, 0.001 g; BB240, Mettler Toledo), and placed in 15-mL polyethylene corrosion jars (Nalgene). The disks were immersed in pH 2, 7, or 10 buffer solution for 1, 3, 5, 10, 15, and 30 d inside the corrosion jars. The disks were placed vertically in the containers, allowing exposure of both faces and the surface edge to the solution. The solution pH was measured before and after immersion (SevenMulti pH Conductivity Meter; Mettler Toledo). The buffer solutions (pH 7.00, 2.00, 10.00; Fisher Scientific) were certified with a purity of 99.99%. The ratio of specimen surface area to solution volume was maintained at 0.16 cm2/cm3 to standardize dynamic corrosion rates. Each specimen was sealed with Teflon tape in each jar, which was then placed in a shaker bath (TSBS40; Techne) containing deionized distilled water at a temperature of 80 °C and a vibrating speed of 50 oscillations per minute. This temperature is specified as a test for chemical durability in ISO standard 6872.
pH Cycling Group
Further analysis was performed on the same material to determine the effect of pH cycling on the surface degradation of ceramics to simulate fluctuating pH of the oral environment. Six disks were assembled into 2 groups, with each group being exposed to the following sequence for the buffer solutions: 1) pH 2, 7, 10 and 2) pH 10, 2, 7. The disks were immersed in solution for 3 d, after which they were rinsed ultrasonically in deionized distilled water for 30 min before being immersed in the next buffer solution according to the test sequence. The total immersion time for all disks was 27 d, which resulted in nine 3-d cycles for each group.
Silicon Ion Concentration, Surface Morphology, and Statistical Analyses
The disk surfaces were rinsed with ethyl alcohol and dried for 40 min at room temperature (25 °C). The concentration of Si4+ in the corrosion solutions was analyzed by means of an inductively coupled plasma atomic emission spectrometer (3200RL; PerkinElmer). Si4+ released was deemed important because this is the main network former for these glass-ceramic materials. For the pH cycling group, Si4+ release for 3, 15, and 30 d was determined by adding Si4+ release at each 3-d cycle to total 27 d. These data were compared with the constant immersion levels for the same periods. Prior to analysis, Si4+ levels were tested against known concentrations to determine the accuracy of elemental detection. The instrument was calibrated to an accuracy of 1.5 mg/L with a detection limit of 50 ppb. Each element concentration was determined 5 times to ensure the reproducibility of results. Concentrations of each solution were derived 3 times and the mean of the 3 readings as the concentration for Si4+. The means of the 3 samples were averaged as the concentration for Si4+ released per period. Disk surfaces that were exposed to the test solutions for 27 d at each pH level were examined by scanning electron microscopy (JSM-6400; JEOL Ltd.).
The R statistical software package (version 3.0.2) was used to create mixed effects linear models. For all ions, the natural log of ion concentration was used as the outcome variable to meet linear modeling assumptions. Fixed factors were group (cycling pH or constant pH), time (3, 15, or 27 d), and the interaction between group and time. Disks were considered a random factor.
Simulated Calculation
Simulated weight loss and Si4+ release levels for cycling immersions were calculated. The assumption that different pH environments have no interaction was applied. The simulated cycling values were obtained by adding 3-d Si4+ release data or weight loss data obtained for each constant pH condition (pH 2, 7, and 10) equaling 27 d of immersion (i.e., adding the 3-d data for each pH, equaling 9 cycles) with the pH cycling sequence. For example, simulated release calculated for pH 2, 7, 10 meant adding 3 pH 2, 3 pH 7, and 3 pH 10 3-d constant values.
Results
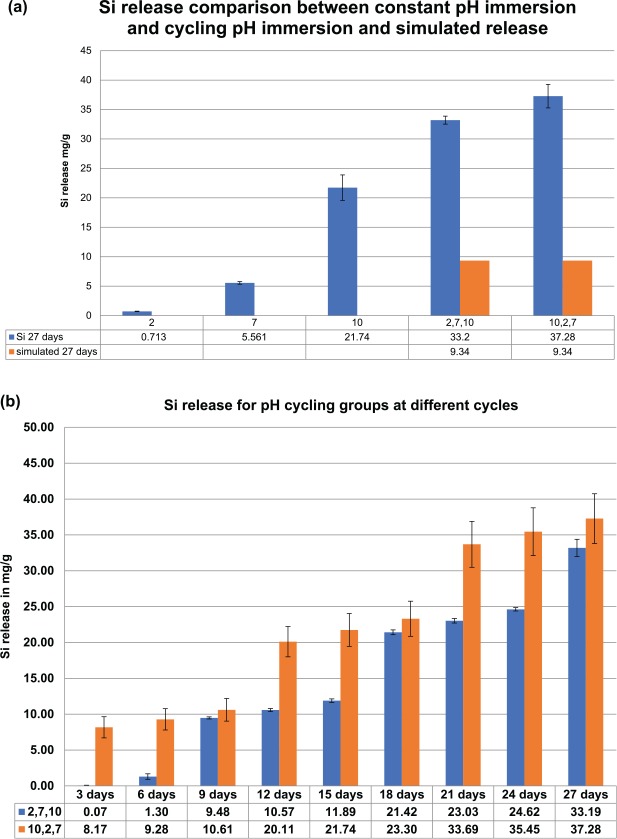
The pH for each solution stayed constant before and after immersion. Ion release for Si4+ measured for the constant pH group (Esquivel-Upshaw, Dieng, et al. 2013) was compared with the pH cycling groups. Figure 1a shows the silicon release levels after 27-d immersions for the constant and cycling pH groups and simulated cycling pH groups based on constant pH dissolution rates. Figure 1b shows the silicon release levels for each pH cycling sequence at 3-d intervals up to 27 d. Figure 1a shows a simulated Si4+ release level of 9.34 mg/g for sequence 1 (2, 7, 10) by adding 3-d constant Si4+ release data to total three 3-d release at pH 2, three 3-d release at pH 7, and three 3-d release at pH 10. The simulated release rate is far below the actual observed pH cycling value of 33.2 mg/g. The same scenario was evident for sequence 2 (10, 2, 7) where three 3-d release at pH 10, three 3-d release at pH 2, and three 3-d Si4+ release levels at pH 7 were added. The simulated amount for sequence 2 was 9.34 mg/g, which was almost 4 times less than the actual observed value of 37.28 mg/g.
Figure 1.
Si release comparison between: (a) constant immersion, cycling pH after 27 d, and simulated cycling; (b) both cycling pH sequences at each 3-d cycle up to 27 d.
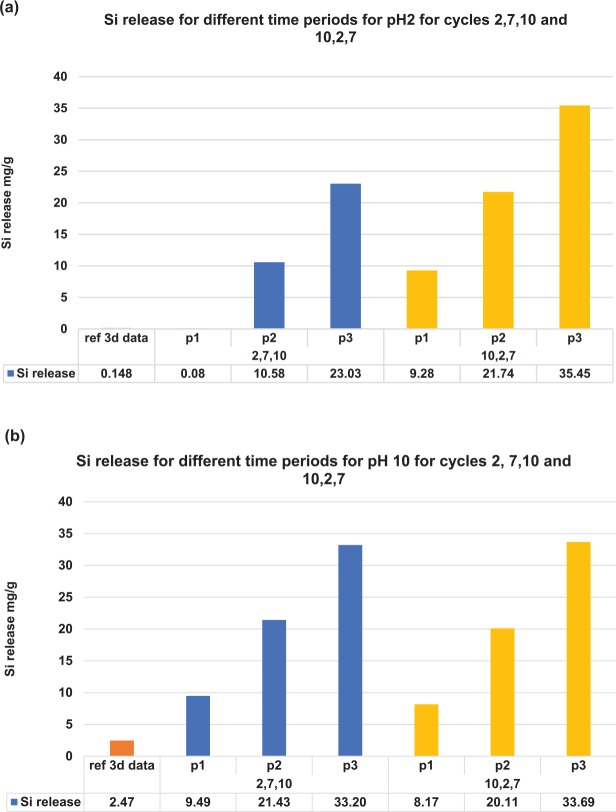
Figures 2 shows the 3-d Si release levels in pH 2 and 10 solutions for the constant pH groups and cycling pH groups. Figure 2a shows the 3-d Si release levels in pH 2 solution for the constant pH groups (“ref 3-d data”) and for different periods in the pH cycling groups. During the first period of pH cycling immersion, the Si4+ release level for sequence 1 (2, 7, 10) is 0.08 mg/g, which was in the same order for the constant pH groups. After 2 periods of cycling, the Si4+ release level reached a steady state level around 10.58 mg/g, which was 71.5 times higher than constant pH immersion release levels. Similar results were obtained for sequence 2 (10, 2, 7). The first period of pH 2 immersion for sequence 2 exhibited a higher Si4+ release level of 9.28 mg/g because the disk had already undergone a 3-d immersion in pH 10 based on the pH sequence. Figure 2b shows the 3-d Si4+ release levels in the pH 10 solution for the constant pH and cycling pH groups. For the first period, Si4+ release levels for sequence 1 (2, 7, 10) and sequence 2 (10, 2, 7) are similar, although the level for sequence 1 is slightly higher because it underwent cycling in pH 2 and 7. After 1 period of pH cycling, the Si4+ release levels increased to around 20 mg/g for sequence 2, which is double the previous release and 8.7 times larger than the levels for constant pH immersion.
Figure 2.
Si release at different periods along the sequence 1 and 2 cycles. p1, p2, and p3: the periods in each cycle where Si release was recorded during which the sequence cycled to either pH 2 or pH 10. ref 3d data: 3-d data in the constant cycling experiment for pH 2 or 10. Si release levels for the cycling group in both sequences when it cycles to (a) pH 2 solution and (b) pH 10 solution.
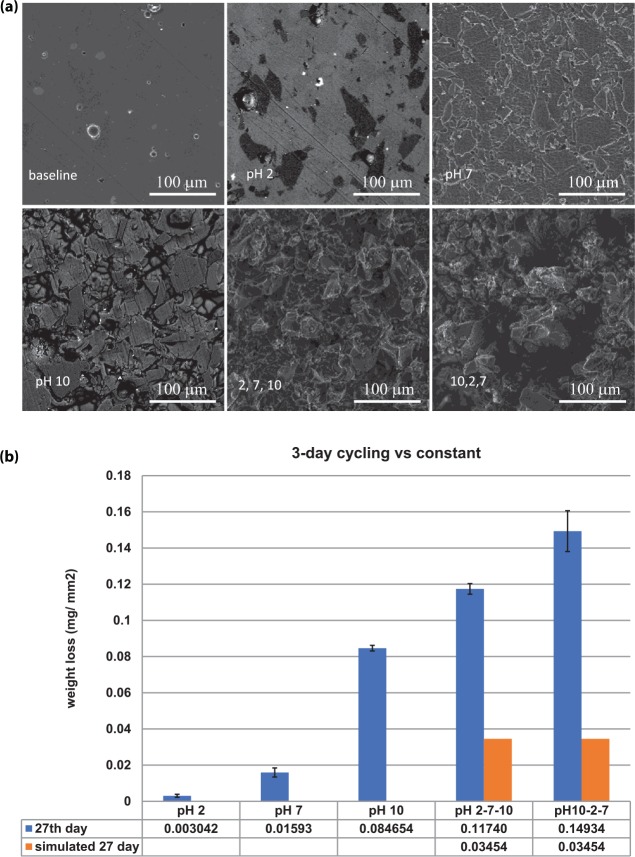
Representative areas of surface degradation were selected at random for scanning electron microscopy images since the surface degradation was homogeneous across the surfaces. Figure 3a illustrates the scanning electron microscopy pictures of surface morphology for ceramic disks, which were untreated; immersed in constant pH 2, 7, and 10 solutions; and cycled in pH 2, 7, 10 and pH 10, 2, 7 solutions for 27 d. As illustrated in Figure 3a, there is a stark contrast in surface morphology between the cycled samples and the ones immersed in constant solution. The results from these images are corroborated by the weight loss per area measured in the disks shown in Figure 3b. The weight loss from pH 10, 2, 7 is 49 times that of the pH 2 constant weight loss and 2 times that of the pH 10 constant weight loss based on a calculated value of 27 d for constant pH dissolution rates. Similar to the ion release, the trend for the weight loss shows that more surface degradation occurs in the cycled immersion than the constant immersion regimens.
Figure 3.
Scanning electron microscopy and weight loss per area of cycling. (a) Baseline: untreated ceramic material. pH 2, 7, 10: ceramic material immersed in pH 2 solution for 27 d, pH 7 solution for 27 d, and pH 10 solution for 27 d, respectively. 2, 7, 10: ceramic material immersed in cycling pH sequence 1 solutions for 27 d. 10, 2, 7: ceramic material immersed in cycling pH sequence 2 solutions for 27 d. (b) Weight loss per area of cycling vs. constant immersion with simulated weight loss data. Values are presented as mean ± SD.
Linear models for ion release show a significant interaction between pH type and day (P < 0.0001), indicating that ion release occurs significantly faster in the cycling group than in the constant pH group (the slope across time is significantly steeper in the cycling group). Model results also show a significant overall effect of group (P = 0.016). With removal of the interaction term from the model estimates, the 10, 2, 7 group shows Si release values that are on average 7.1 higher than the 2, 7, 10 group (95% CI, 3.31 to 10.9; P = 0.004). Additionally, the results clearly show that sequence 2, which starts at pH 10, has a higher initial ion release that continues throughout the whole sequence. Despite the boost in ion release that occurs in sequence 1 every time there is immersion in pH 10, the level of ion release never catches up to the ion release seen in sequence 2 (Fig. 1b).
Linear models for weight loss show a significant interaction between pH type and day (P < 0.0001), indicating that weight loss rises significantly faster in the cycling group than in the constant pH group. The 10, 2, 7 group has values that are on average 0.12 higher than the 2, 7, 10 group (95% CI, 0.085 to 0.155; P = 0.0002).
Discussion
The results of this study demonstrate that the new testing methodology of pH cycling with immersion in alternating basic and acidic environments was more detrimental to the integrity of silicate glasses than constant immersion in either environment (Esquivel-Upshaw, Dieng, et al. 2013). Since the human diet introduces different pH solutions in the mouth, this new testing method could be more representative of oral conditions than the current method of challenging ceramics only in an acidic environment. However, the in vitro methodology described in this experiment represents very extreme conditions in pH levels that will probably never be realized intraorally.
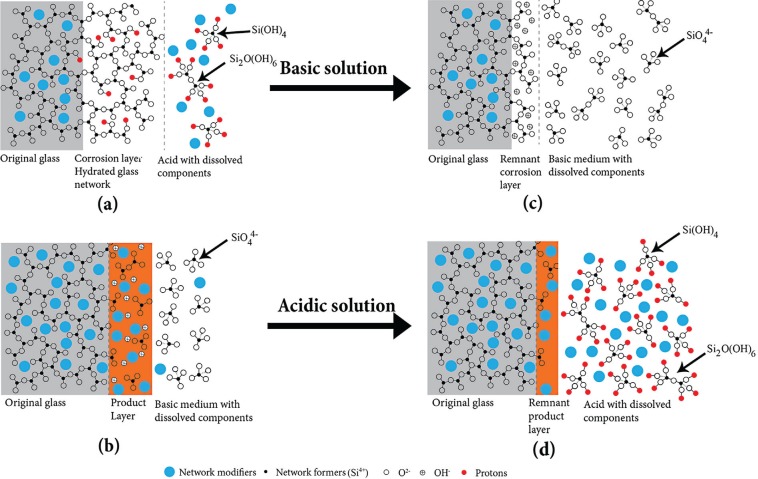
The Si4+ release levels, weight loss, and surface morphology of ceramic materials treated with cycling pH were completely different from those immersed in constant pH solution. The theory that the mechanism of Si4+ release from the ceramic surface must be completely different for the 2 methodologies of just basic and acidic exposure was postulated. Herrmann et al. (2013) proposed Si4+ release mechanisms of glass corrosion in constant acidic or basic solutions. A corroded layer with a hydrated glass network is formed in acidic environments by leaching the glass network modifiers and a minimal amount of SiO2 (H2O)n molecules in the aqueous solution, as illustrated in Figure 4a. The leaching process of network modifiers and the formation of a hydrated glass network have the following reaction:
Figure 4.
Schematic diagram of degradation mechanisms in (a) acidic and (b) basic solutions, proposed by Herrmann et al. (2013). New model of the degradation mechanisms (c) in a basic solution for those ceramics previously immersed in an acidic solution and (d) in an acidic solution for those ceramics previously immersed in a basic solution.
| (1) |
In contrast, the dissolution of the hydrated network reaction is
| (2) |
The dissolution of the hydrated network is the rate-limiting process. This explains why there was only a very small amount of Si4+ ions released for the glass ceramic material immersed in a constant pH 2 solution with 0.148 mg/g for 3-d and 0.713 mg/g for 27-d constant immersion. However, in a basic environment, the glass network dissolves as shown in Figure 4b, and the dissolution process follows the reaction as
| (3) |
The network modifiers are more stable in the base solution, and a layer of these network modifiers with fragments of SiO44− forms on the glass surface. Constant immersion in pH 10 demonstrated Si4+ concentrations of 2.47 mg/g for 3 d and 21.74 mg/g for 27 d. However, these proposed corrosion mechanisms in acidic and basic solutions cannot explain the increased Si4+ release levels during the pH cycling conditions, as shown in Figures 1 to 3.
Figure 4c and d shows the schematics of proposed new mechanisms for Si4+ release for the glass-ceramic materials previously exposed to a different pH solution. As mentioned earlier, a less dense hydrated glass network forms after the ceramic is immersed in the acidic solution. The interaction between the ceramic and acidic solution is governed by reaction 1, where the network fillers (alkali ions) exchange with protons in solution, creating a lower-density 3-dimensional glass structure with fewer network modifiers present. By further exposing this disk in the acidic solution for a longer time, there is less Si4+ released due to a diffusion control process of protons diffusing through the hollow 3-dimensional glass structure. In contrast, Si4+ ion release levels significantly increased by immersing this disk with a less dense defective surface layer in the basic solution. The hydroxyl ions in solution can easily penetrate through the hollow 3-dimensional glass structure and follow reaction 2, as shown in Figure 4c. This accounts for a much higher Si release level detected every time the cycling sequence cycled to pH 10, regardless of the sequence pattern (2, 7, 10 or 10, 2, 7). As shown in Figure 2b, instead of 2.47 mg/g of Si4+ released in constant pH 10 solution immersion for 3 d, around 10 mg/g of Si4+ was released for 3-d pH 10 solution immersion after 1 period of pH cycle for sequence 1 (2, 7, 10) and sequence 2 (10, 2, 7) immersions.
When the glass-ceramic disk previously immersed in basic solution was exposed to acidic solution, a significant amount of Si4+ ion was also detected. As illustrated in Figure 2a, instead of 0.08 mg/g of Si4+ released in constant pH 2 solution immersion for 3 d, around 10 mg/g of Si4+ was released for 3-d pH 2 solution immersion after 2 periods of pH cycling in sequence 1 (2, 7, 10) and 1 period in sequence 2 (10, 2, 7) immersions. This demonstrates that the Si4+ release process during the cycling experiment does not follow reaction 1. As shown in Figure 4b, fragments of SiO44− are trapped in the surface layer containing network modifiers without glass network formed during the immersion in the basic solution. After subsequent immersion in the pH 2 solution, SiO44− trapped in the network modifier layer was easily released to the acidic solution, as shown in Figure 4d. This accounts for the much higher Si concentration detected in the pH 2 solution during cycling.
There is also Si4+ release at pH 7, which is a neutral solution. pH 7 has a balance of protons and hydroxyl ions, which results in competing reactions—namely, ion exchange and a total dissolution of the surface, as mentioned previously. The hydroxyl ions dissolve the silicon-rich surface, leading to a release of Si4+ in solution, more than pH 2.
In addition to the much higher Si4+ release levels demonstrated by the cycling pH immersion group, the weight loss coupled with the surface morphology of these disks confirm significant degradation.
Conclusion
The results suggest a tendency for greater ion release and, thus, greater surface degradation from pH cycling than from exposure to a constant pH environment. At least 2 mechanisms control the surface degradation process as a function of the pH cycling method. Cycling of pH solutions showed greater surface degradation when the initial pH was 10. However, severe generalized degradation occurred within all ceramic surfaces, suggesting that the oral environment, with a wide range of pH exposure, may cause extensive degradation of glass-phase ceramics. Thus, the conventional testing methodology of constant pH immersion to test for chemical durability of ceramics could underestimate the in vivo corrosion. This is significant clinically, because saliva and interacting liquids cycle among several pH levels. Since these solutions can be constantly renewed, the approach with pH cycling immersion is more adequate in simulating ceramic corrosion in the oral environment. New mechanisms were also proposed to explain the rationale for the increased Si4+ release during the cycling pH immersion.
Author Contributions
J.F. Esquivel-Upshaw, contributed to conception, design, and data analysis, drafted and critically revised the manuscript; F. Ren, S.M. Hsu, contributed to data analysis and interpretation, critically revised the manuscript; F.Y. Dieng, contributed to data acquisition, critically revised the manuscript; D. Neal, contributed to data analysis and interpretation, drafted and critically revised the manuscript; A.E. Clark, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript.
Footnotes
National Institutes of Health National Institute of Dental and Craniofacial Research grant R01 DE025001 and the University of Florida Office of Research Seed Grant supported this study. Ceramic materials were supplied by Ivoclar Vivadent. Scanning electron microscopy–energy dispersive spectroscopy was performed at the Major Analytical Instrumentation Center of the University of Florida.
The University of Florida has filed a patent application covering aspects of the work detailed in this publication. The authors declare no other potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aksoy G, Polat H, Polat M, Coskun G. 2006. Effect of various treatment and glazing (coating) techniques on the roughness and wettability of ceramic dental restorative surfaces. Colloids Surf B Biointerfaces. 53(2):254–259. [DOI] [PubMed] [Google Scholar]
- Al-Marzok MI, Al-Azzawi HJ. 2009. The effect of the surface roughness of porcelain on the adhesion of oral Streptococcus mutans. J Contemp Dent Pract. 10(6):E017–E024. [PubMed] [Google Scholar]
- Bartlett DW, Fares J, Shirodaria S, Chiu K, Ahmad N, Sherriff M. 2011. The association of tooth wear, diet and dietary habits in adults aged 18–30 years old. J Dent. 39(12):811–816. [DOI] [PubMed] [Google Scholar]
- Bridges MA, Mattice MR. 1939. Over two thousand estimations of the ph of representative foods. Am J Dig Dis. 6(7):440–449. [Google Scholar]
- Butler CJ, Masri R, Driscoll CF, Thompson GA, Runyan DA, Anthony von Fraunhofer J. 2004. Effect of fluoride and 10% carbamide peroxide on the surface roughness of low-fusing and ultra low-fusing porcelain. J Prosthet Dent. 92(2):179–183. [DOI] [PubMed] [Google Scholar]
- Ccahuana VZ, Ozcan M, Mesquita AM, Nishioka RS, Kimpara ET, Bottino MA. 2010. Surface degradation of glass ceramics after exposure to acidulated phosphate fluoride. J Appl Oral Sci. 18(2):155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond JL, Novickas D, Lenke JW. 1991. Physiological aging of an all-ceramic restorative material. Dent Mater. 7(2):133–137. [DOI] [PubMed] [Google Scholar]
- Esquivel-Upshaw JF, Chai J, Sansano S, Shonberg D. 2001. Resistance to staining, flexural strength, and chemical solubility of core porcelains for all-ceramic crowns. Int J Prosthodont. 14(3):284–288. [PubMed] [Google Scholar]
- Esquivel-Upshaw JF, Dieng FY, Clark AE, Neal D, Anusavice KJ. 2013. Surface degradation of dental ceramics as a function of environmental pH. J Dent Res. 92(5):467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquivel-Upshaw JF, Rose W, Oliveira E, Yang M, Clark AE, Anusavice K. 2013. Randomized, controlled clinical trial of bilayer-ceramic and metal-ceramic crown performance. J Prosthodont. 22(3):166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquivel-Upshaw JF, Young H, Jones J, Yang M, Anusavice KJ. 2006. In vivo wear of enamel by a lithia disilicate-based core ceramic used for posterior fixed partial dentures: first-year results. Int J Prosthodont. 19(4):391–396. [PubMed] [Google Scholar]
- Fischer H, Schäfer M, Marx R. 2003. Effect of surface roughness on flexural strength of veneer ceramics. J Dent Res. 82(12):972–975. [DOI] [PubMed] [Google Scholar]
- Herrmann M. 2013. Corrosion of silicon nitride materials in aqueous solutions. J Am Ceram Soc. 96(10):3009–3022. [Google Scholar]
- Junpoom P, Kukiattrakoon B, Hengtrakool C. 2010. Surface characteristic changes of dental ceramics after cyclic immersion in acidic agents and titratable acidity. Eur J Prosthodont Restor Dent. 18(4):177–184. [PubMed] [Google Scholar]
- Kukiattrakoon B, Hengtrakool C, Kedjarune-Leggat U. 2010. a. Chemical durability and microhardness of dental ceramics immersed in acidic agents. Acta Odontol Scand. 68(1):1–10. [DOI] [PubMed] [Google Scholar]
- Kukiattrakoon B, Hengtrakool C, Kedjarune-Leggat U. 2010. b. Degradability of fluorapatite-leucite ceramics in naturally acidic agents. Dent Mater J. 29(5):502–511. [DOI] [PubMed] [Google Scholar]
- Kukiattrakoon B, Hengtrakool C, Kedjarune-Leggat U. 2010. c. The effect of acidic agents on surface ion leaching and surface characteristics of dental porcelains. J Prosthet Dent. 103(3):148–162. [DOI] [PubMed] [Google Scholar]
- Kukiattrakoon B, Hengtrakool C, Kedjarune-Leggat U. 2011. Effect of acidic agents on surface roughness of dental ceramics. Dent Res J (Isfahan). 8(1):6–15. [PMC free article] [PubMed] [Google Scholar]
- Kukiattrakoon B, Junpoom P, Hengtrakool C. 2009. Vicker’s microhardness and energy dispersive X-ray analysis of fluorapatite-leucite and fluorapatite ceramics cyclically immersed in acidic agents. J Oral Sci. 51(3):443–450. [DOI] [PubMed] [Google Scholar]
- Landry WL, Schwab AH, Lancette GA. 2001. Examination of canned foods. In: FDA bacteriological analytical manual. Gaithersburg (MD): AOAC International. [Google Scholar]
- Milleding P, Gerdes S, Holmberg K, Karlsson S. 1999. Surface energy of non-corroded and corroded dental ceramic materials before and after contact with salivary proteins. Eur J Oral Sci. 107(5):384–392. [DOI] [PubMed] [Google Scholar]
- Milleding P, Haraldsson C, Karlsson S. 2002. Ion leaching from dental ceramics during static in vitro corrosion testing. J Biomed Mater Res. 61(4):541–550. [DOI] [PubMed] [Google Scholar]
- pH values of food products. 1962. Food Eng. 34(3):98–99. [Google Scholar]
- Pinto MM, Cesar PF, Rosa V, Yoshimura HN. 2008. Influence of pH on slow crack growth of dental porcelains. Dent Mater. 24(6):814–823. [DOI] [PubMed] [Google Scholar]
- Preis V, Behr M, Handel G, Schneider-Feyrer S, Hahnel S, Rosentritt M. 2012. Wear performance of dental ceramics after grinding and polishing treatments. J Mech Behav Biomed Mater. 10:13–22. [DOI] [PubMed] [Google Scholar]