Abstract
Osteonecrosis of the jaws (ONJ) is a rare but severe complication of antiresorptive medications, such as bisphosphonates, used in the treatment of bone malignancy or osteoporosis. Tooth extraction and dental disease have been strongly associated with ONJ development. Here, we investigated molecular and cellular markers of socket healing after extraction of healthy or teeth with experimental periodontitis (EP) in Wistar-Han rats treated with zoledronic acid (ZA). We included 4 experimental groups: vehicle-treated animals with extraction of healthy teeth or teeth with ligature-induced EP and ZA-treated animals with extraction of healthy teeth or teeth with EP. Animals were pretreated with vehicle or ZA for a week, and EP was induced. Four weeks later, the second maxillary molars were extracted; sockets were allowed to heal for 4 wk; animals were euthanized; and maxillae were isolated. Radiographically, extraction sockets in groups 1, 2, and 3 demonstrated normal healing. Contrary incomplete socket healing was noted after extraction of teeth with EP in ZA-treated rats of group 4. Histologically, persistent inflammation and extensive osteonecrosis were seen in group 4. Disorganization of the collagen network, collagen type III predominance, and lack of collagen fiber insertion in the necrotic bone were associated with impaired socket healing. Cells positive for MMP-9, MMP-13, and α-SMA expression were present at the areas of epithelial invagination and adjacent to osteonecrotic bone. Importantly, human biopsies from patients with ONJ showed similar findings. Our data emphasize the importance of dental disease and tooth extraction in ONJ pathogenesis and help delineate an altered profile in wound-healing markers during ONJ development.
Keywords: osteoclast(s), osteonecrosis, periodontal disease(s)/periodontitis, matrix metalloproteinases (MMPS), collagen(s), wound healing
Introduction
Osteonecrosis of the jaws (ONJ) is a rare but severe complication of antiresorptive medications, specifically bisphosphonates and receptor activator of nuclear factor kappa-B ligand (RANKL) inhibitors, or antiangiogenic medications (Ruggiero et al. 2014; Allen 2015). Duration, dose, and potency of antiresorptives, as well as concomitant diseases and additional medications, are known to increase the incidence and severity of ONJ. However, the detailed pathophysiologic mechanisms of ONJ remain poorly understood, largely due to its low incidence and the proposed conservative treatment protocols that do not allow extensive sampling. Animal models provide significant insight but need to be critically considered, since bone homeostasis of experimental animals and interventional approaches do not always parallel clinical scenarios (Khan et al. 2015). Altered inflammatory response, infection, defective angiogenesis, bisphosphonate toxicity to soft tissues, increased turnover of alveolar bone, oversuppression of bone remodeling, and genetic predisposition might contribute to ONJ pathophysiology (Yamashita and McCauley 2012).
ONJ may occur spontaneously, underneath a denture or at areas with periodontal or periapical infection. However, the most common local instigating factor for ONJ development is trauma, particularly tooth extraction (Ruggiero et al. 2004; Manfredi et al. 2017). Teeth in adults are mainly extracted due to periodontal or pulpal disease (Chrysanthakopoulos 2011). Clinical studies support an association between dental disease with ONJ occurrence (Fung et al. 2017), while dental preventive measures reduce ONJ incidence (Dimopoulos et al. 2009).
Extraction socket healing involves several cellular processes and the interplay of soft tissue and bone healing. The wound/socket-healing process is divided into inflammatory, proliferative, and modeling/remodeling phases (Gosain and DiPietro 2004; Olczyk et al. 2014). Early events include hemostasis, formation of a provisional wound matrix, and neutrophil and monocyte infiltration. In the proliferation phase, granulation tissue forms, and the vascular network is restored. Layers of woven bone are deposited adjacent to the socket borders. Finally, in the modeling/remodeling phase, changes occur in the connective tissue and bone architecture, including replacement of woven with lamellar bone and bone marrow (Araújo et al. 2015).
Animal models reproduce clinical, radiographic, and histologic features of ONJ (Aguirre et al. 2012; Kang et al. 2013; Williams et al. 2014). Zoledronic acid (ZA), a potent bisphosphonate, or RANKL inhibitors and periapical or periodontal disease cause osteonecrosis of alveolar bone in rats and mice (Aghaloo et al. 2011; Aghaloo et al. 2014; de Molon et al. 2014). These findings led us to hypothesize that in antiresorptive-treated animals, extraction of teeth with experimental periodontitis (EP) and thus with preexisting osteonecrosis would result in compromised socket healing and progression of ONJ-like findings. Indeed, extraction of teeth with spontaneous periradicular disease but not healthy (H) teeth in mice treated with ZA or a RANKL inhibitor induced clinical bone exposure and osteonecrosis (Soundia et al. 2016).
Although socket healing is delayed in tooth extraction sites of most human patients receiving strong antiresorptives, ONJ occurs in only a fraction of such tooth extractions (Ruggiero et al. 2014). An improved understanding of this topic—especially concerning preexisting circumstances that increase the risk that a specific tooth extraction will lead to ONJ—is of great clinical importance. The focus of our studies herein is to investigate changes in socket- and wound-healing markers (areas with positive expression of type III collagen and cells with positive expression of matrix metalloproteinases MMP-9 and MMP-13, and α-SMA) after extraction of H teeth or teeth with preexisting EP in vehicle (veh)– or ZA-treated rats.
Materials and Methods
Animal Care
Forty-five rats were randomly assigned to receive saline (veh) or 200 μg/kg of ZA (LKT Laboratories) intraperitoneally, 2 times per week in the morning. At time of injection, body weight was recorded for all animals.
Rats received veh or ZA for a week, and 4.0 silk ligatures were placed around the left maxillary second molar to induce EP. Of 22 veh-treated rats, 10 received molar ligation, and 12 (12 of 22) did not. Of 23 ZA-treated animals, 11 received molar ligation, and 12 did not. Four weeks later, in vivo micro–computed tomography was performed, and the following day, the first and second maxillary molars were extracted. Thus, our study included 1) veh-H and veh-EP groups—veh-treated animals with extraction of H teeth or teeth with EP; 2) ZA-H and ZA-EP groups—ZA-treated animals with extraction of H teeth or teeth with EP. Four weeks after tooth extraction, without discontinuation of veh and ZA treatment, rats were euthanized utilizing CO2.
Specimen Scanning
Deidentified maxillae were imaged, as described (de Molon et al. 2014).
Radiographic Assessment of Socket Healing
Qualitative and quantitative radiographic assessment details are provided in the Appendix. Examples of normal, partial, and limited socket healing are seen in Appendix Figure 1.
Histology and TRAP Staining
Histology and tartrate-resistant acid phosphatase (TRAP) details are provided in the Appendix. All specimens were stained with a picrosirius red stain kit (Polysciences Inc.) and visualized under bright field and polarized light.
Immunohistochemistry
Anti-collagen I (ab34710), anti-collagen III (ab7778), anti-MMP-9 (ab38898), anti-MMP-13 (ab39012), and anti-α-SMA (ab5694) antibodies (ABCAM) were used.
Human Specimens
Anonymous unstained slides and associated nonidentifiable clinical information from 2 cases with histologic diagnosis of ONJ were received from records of the University of California, Los Angeles (UCLA) School of Dentistry. The first patient was a 70-y-old woman with Fosamax treatment, recent extractions, and drainage of the upper left maxilla. The second patient was a 68-y-old woman with intravenous ZA history and chronic bone exposure in the right posterior mandible. Hematoxylin and eosin and immunohistochemistry staining was performed for both patients.
Statistics
The experimental unit was a single animal. Data were analyzed with GraphPad Prism (GraphPad Software, Inc.). Descriptive statistics (mean and SEM), a 2-way analysis of variance for multiple comparisons, and Fisher exact test for socket healing were used.
Results
Radiographic Findings
In vivo micro–computed tomography scanning revealed similar bone appearance around the second molar of veh or ZA animals without ligature. In veh rats, ligature caused periodontal bone loss (Appendix Fig. 2A, white arrows). In ZA rats, bone loss around molars with ligature was significantly greater than nonligated molars but attenuated versus veh animals (Appendix Fig. 2A, white arrows, B).
To assess the ZA effect on alveolar morphology, bone volume/total volume (BV/TV) of the mandibular trabecular bone was measured, which showed a significant increase in ZA animals (Appendix Fig. 3A). In the maxillae of these animals, woven bone filled the sockets of veh-H and veh-EP animals (Fig. 1A, white arrows). In ZA-H animals, woven bone occupied the socket. In ZA-EP animals, impaired socket healing was noted (Fig. 1A, white arrowheads). Qualitative (Fig. 1B) and quantitative (Fig. 1C) assessment demonstrated significantly compromised socket healing of ZA-EP animals. These animals also demonstrated decreased BV/TV of the second molar socket (Appendix Fig. 3B).
Figure 1.
Radiographic and histologic assessment of socket healing. (A) In vitro assessment of the edentulous maxillary alveolar ridge. Sagittal sections of sockets after extraction of healthy teeth in vehicle (Veh) or zoledronic acid (ZA) treatment groups. White arrows point to complete socket healing, and white arrowheads point to impaired socket healing. (B, C) Qualitative and quantitative assessment of socket healing after extraction of healthy or diseased teeth in various treatment groups. Representative hematoxylin and eosin–stained images from maxillae of all groups: alveolar ridges of vehicle (D) or ZA (E) treatment animals after extraction of healthy or diseased teeth, viewed at 4× and 20× magnification. Yellow arrows point to absence of inflammation in the connective tissue, green arrows to the periosteum, blue arrows to the healing of the original extraction socket, white arrows to the original socket outline, aqua arrows to inflammatory infiltrate, and black arrows to osteonecrotic areas. (F–H) Quantification of osteonecrotic area and empty osteocyte lacunae and number of TRAP+ cells per bone area respectively. For all statistical comparisons: **P < 0.01. ***P < 0.001. ****P < 0.0001. Differences among groups were calculated by 2-way analysis of variance for multiple comparisons. Data represent mean ± SEM. For qualitative socket healing, differences among groups were calculated by Fisher exact test.
Histologic Findings
Histologically, veh-H and veh-EP animals showed socket filling with woven bone (Fig. 1D, blue arrows), presence of prominent reversal lines, and a pronounced periosteum at the alveolar crest (green arrows). The mucosa consisted of keratinized epithelium, dense connective tissue, and a lack of marked inflammatory infiltrate (yellow arrows). ZA-H animals demonstrated filling with woven bone (Fig. 1E, blue arrows) and prominent reversal lines. The original socket borders were easily discernible (white arrows). The mucosa consisted of keratinized epithelium and a lamina propria with a dense connective tissue lacking a marked inflammatory infiltrate. In contrast, ZA-EP animals showed impaired bone healing, epithelial invagination, and osteonecrotic areas with empty osteocyte lacunae (black arrows). A marked inflammatory infiltrate was noted in the nonhealing socket adjacent to osteonecrotic areas, extending into the submucosa and the basal epithelial layer (aqua arrows). The percentage empty osteocyte lacunae (Fig. 1F), the absolute number of empty osteocyte lacunae in the alveolar bone (Appendix Fig. 3C), and the area of osteonecrosis (Fig. 1G) were significantly increased.
Osteoclast number was significantly increased in the EP versus H groups in both veh and ZA rats. As previously reported (de Molon et al. 2015), the number of osteoclasts normalized to bone area (Fig. 1H) and the absolute osteoclast number in the alveolar ridge (Appendix Fig. 3D) were significantly elevated in the ZA versus veh groups. However, the osteoclasts in the ZA groups demonstrated altered round morphology with pyknotic nuclei and appeared detached from the bone surface.
Picrosirius red staining revealed differences in collagen organization among the groups. In veh-H and veh-EP animals, collagen fibers extended from the lamina propria, just below the epithelium into the alveolar bone, forming a dense, well-organized network (Fig. 2A, A1, A2, B, B1, B2, aqua arrows). Collagen fiber birefringence had a yellow signal, suggestive of collagen type I prevalence (Junqueira et al. 1986). In ZA-H animals, collagen fibers were markedly dense, maintained insertion in the alveolar bone, and demonstrated a yellow signal (Fig. 2C, C1, C2, aqua arrows). In contrast, ZA-EP animals revealed collagen fibers with an overall green birefringence, suggesting collagen type III predominance (Junqueira et al. 1986; Fig. 2D, D1, D2, green arrows). These collagen bundles did not extend into the alveolar bone (Fig. 2D2, magenta arrows); signal absence (yellow arrows) was noted bordering the areas of osteonecrosis.
Figure 2.
Picrosirius red staining of maxillary sockets of vehicle (veh; A–A2, B–B2) or zoledronic acid (ZA; C–C2, D–D2) treatment animals after extraction of healthy (A–A2, C–C2) or diseased (B–B2, D–D2) teeth, visualized under bright field (A–D) or polarized light filter (A1, A2, B, B2, C1, C2, D1, D2), viewed at 4× (A, B, C, D, A1, B1, C1, D1) and 10× (A2, B2, C2, D2) magnification. Aqua arrows point to yellow birefringence signal, green arrows to green birefringence signal, yellow arrows to absence of signal, and magenta arrows to lack of signal extension into the alveolar bone. Collagen type III immunohistochemistry of maxillary sockets in vehicle (E, E1, F, F1) or ZA (G, G1, H, H1) treatment animals after extraction of healthy (E, E1, G, G1) or diseased (F, F1, H, H1) teeth, viewed in 10× (E–H) or 20× (E1, F1, G1, H1). White arrows point to positive signal underneath the epithelium, blue arrows to signal adjacent to osteonecrotic bone, green arrows to positive signal near inflammation, and red arrows to positive signal adjacent to epithelial invagination.
Immunohistochemical Findings in Rats
Collagen type I immunostaining was evident in all groups. As expected, no staining was present in regions of marked inflammatory infiltrate adjacent to areas of osteonecrosis (Appendix Fig. 4).
For veh-H, veh-EP, and ZA-H animals, collagen type III immunoreactivity was noticeable underneath the epithelium (Fig. 2E–G, white arrows) and faint and diffuse in the remaining submucosa to the bone level (Fig. 2E1, F1, G1). However, in the ZA-EP animals, a pronounced collagen type III signal was noted (Fig. 2H, H1): the signal was especially prominent below the epithelium (white arrow) around osteonecrotic bone (blue arrow), and adjacent to inflammation (green arrows) and epithelial invagination (red arrows).
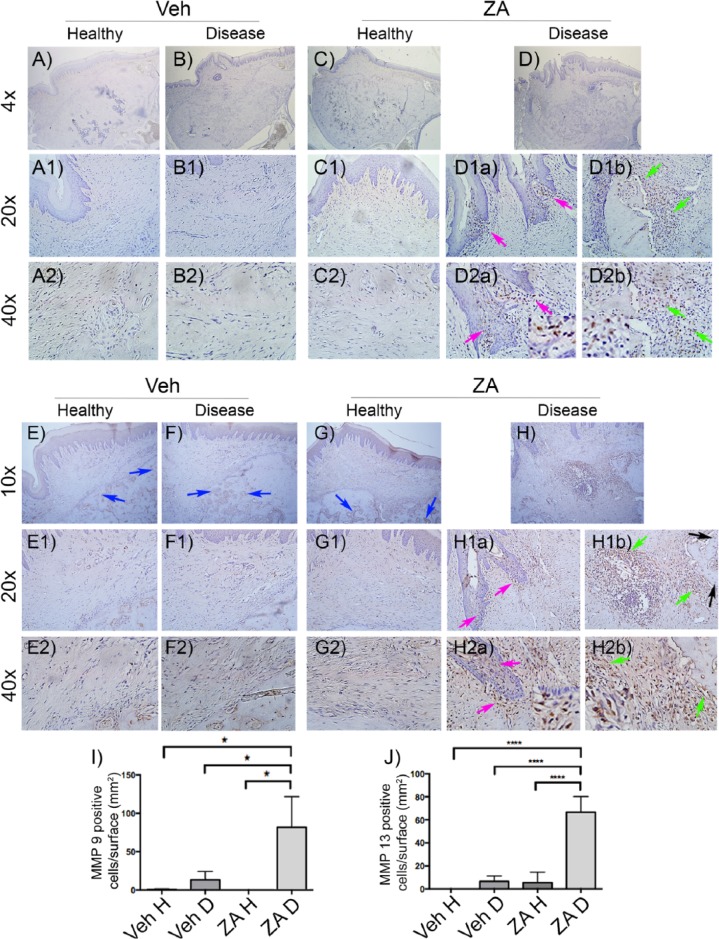
MMP-8, MMP-9, and MMP-13 are molecules that can modify matrix structure. No expression of MMP-8 was detected (not shown). MMP-9 positive cells were mostly observed in ZA-EP rats (Fig. 3D, D1a, D1b, D2a, D2b arrows, I). Significantly more positively immunoreactive cells were noted within the epithelium at areas of epithelial invagination (Fig. 3D1a, D2a, magenta arrows) and around areas of osteonecrosis (Fig. 3D1b, D2b, green arrows).
Figure 3.
MMP-9 immunohistochemistry of edentulous alveoli in vehicle (veh; A–A2, B–B2) or zoledronic acid (ZA; C–C2, D, D1a, D1b, D2a, D2b) treatment animals after extraction of healthy (A, A1, A2, C, C1, C2) or diseased (B, B1, B2, D, D1a, D1b, D2a, D2b) teeth, viewed at 4× (A–D), 20× (A1, B1, C1, D1a, D1b), and 40× (A2, B2, C2, D2a, D2b) magnification. Magenta arrows point to positive cells around epithelial invagination and green arrows to positive cells at inflammatory areas and adjacent to necrotic bone. MMP-13 immunohistochemistry of maxillary alveolar sockets in vehicle (E–E2, F–F2) or ZA (G–G2, H, H1a, H1b, H2a, H2b) treatment animals after extraction of healthy (E, E1, E2, G, G1, G2) or diseased (F, F1, F2, H, H1a, H1b, H2a, H2b) teeth, viewed at 10× (E–H), 20× (E1, F1, G1, H1a, H1b) or 40× (E2, F2, G2, H2a, H2b) magnification. Blue arrows point to positive signal in areas of reversal lines, magenta arrows to positive cells around epithelial invagination, and green arrows to positive cells at inflammatory areas and adjacent to necrotic bone. Quantification of (I) MMP-9 and (J) MMP-13 positively immunostained cells. D, diseased; H, healthy. *P < 0.05. ****P < 0.0001. Differences among groups were calculated by 2-way analysis of variance for multiple comparisons. Data represent mean ± SEM.
Few weakly immunostained MMP-13 cells were seen in the periosteum at the extraction site but not in the submucosal area of veh-H, veh-EP, and ZA-H animals (Fig. 3E, E1, E2, F, F1, F2, G, G1, G2). In socket-healing areas, MMP-13 immunoreactivity was noted along the reversal lines and the bone–bone marrow interface (Fig. 3E–G, blue arrows). However, in ZA-EP rats MMP-13 immunostain was significantly increased (Fig. 3J). Positive cells were localized within epithelium in areas of epithelial invagination (Fig. 3H, H1a, H2a, magenta arrows). Exuberant MMP-13 staining was noted at areas of inflammation and peripheral to the necrotic alveolar ridge (Fig. 3H, H1b, H2b, green arrows). Select MMP-13-positive osteocytes were seen adjacent to osteonecrotic areas (Fig. 3H1b, black arrows).
We finally tested α-SMA, a cytoskeletal scaffold protein expressed in myofibroblasts (Hinz et al. 2001). Veh animals demonstrated low α-SMA signal, confined to blood vessel walls (Fig. 4A, A1, A2, B, B1, B2, white arrows). In ZA-H animals, α-SMA signal was also present on vessel walls (Fig. 4C, C1, C2, white arrows). Few scattered positive cells were dispersed in the submucosa (Fig. 4C1, C2, yellow arrows) and marrow spaces. However, no statistical difference from the veh groups was detected (Fig. 4E). Interestingly, significantly increased numbers of α-SMA positive cells were noted in the submucosa of ZA-EP animals localized around blood vessels but also adjacent to necrotic bone (Fig. 4D, D1, D2, green arrows, E).
Figure 4.

α-SMA immunohistochemistry of maxillary edentulous alveoli in vehicle (veh; A–A2, B–B2) or zoledronic acid (ZA; C–C2, D–D2) treatment animals after extraction of healthy (A, A1, A2, C, C1, C2) or diseased (B, B1, B2, D, D1, D2) teeth, viewed in 10× (A–D), 20× (A1, B1, C1, D1), or 40× (A2, B2, C2, D2) magnification. White arrows point to positive signal at the walls of blood vessels, yellow arrows to positive cells in the connective tissue, and green arrows to positive cells around necrotic bone. (E) Quantification of α-SMA positively immunostained cells. D, diseased; H, healthy. ****P < 0.0001. Differences among groups were calculated by 2-way analysis of variance for multiple comparisons. Data represent mean ± SEM.
Immunohistochemical Findings in Patient Specimens
To investigate whether the same markers were relevant in a patient setting, we explored collagen type III, MMP-13, and α-SMA presence in ONJ patient specimens (Fig. 5). Adjacent to osteonecrotic areas (blue arrows), high levels of collagen type III in the extracellular matrix (red arrows), as well as cells positive for expression of MMP-13 (yellow arrows) and α-SMA (magenta arrows) were noted. Blood vessel walls also demonstrated α-SMA immunoreactivity (white arrows).
Figure 5.
Representative collagen type III (COL III), MMP-13, and α-SMA immunohistochemistry of human biopsies in 2 women (68 and 70 y old) with a history of osteonecrosis of the jaws after Fosamax or intravenous zoledronic acid treatment. Blue arrows point to necrotic bone, red arrows to positive COL III signal in the matrix around the areas of osteonecrosis, yellow arrows to cells with positive signal for MMP-13 immunostain adjacent to osteonecrotic areas, magenta arrows to cells with positive signal for α-SMA immunostain in the proximity of necrotic bone, and white arrows to positive signal at the walls of blood vessels.
Discussion
In rodents treated with antiresorptives, dental disease can induce radiographic and histologic ONJ-like lesions (Aghaloo et al. 2011; Kang et al. 2013; de Molon et al. 2014), while subsequent extraction of diseased but not H teeth results in clinically exposed bone in mice (Soundia et al. 2016). Here, we investigated alveolar ridge healing in veh- or ZA-treated animals after extraction of H or EP teeth in a larger animal, the rat. First, we established that ligature placement induced experimental periodontal bone loss by in vivo micro–computed tomography. This important step confirmed the effectiveness of ligature placement to induce periodontal bone loss. Radiographic assessment revealed that extraction of teeth with EP in ZA animals but not in other groups resulted in impaired osseous healing of extraction sockets after 4 wk. Histology confirmed these findings and established extensive alveolar osteonecrosis and inflammatory infiltrate adjacent to necrotic areas, pointing to a temporal association between the inflammatory environment and osteocyte death.
Consequently, the main focus of our studies was to investigate potential changes in socket- and wound-healing markers among the 4 treatment groups. We explored extracellular matrix changes that might be associated with the observed structural differences around necrotic areas. Collagen, the major extracellular matrix component (Cuttle et al. 2005), acts as a structural scaffold in tissues, while during wound healing, it modulates cell proliferation and migration (Tracy et al. 2016). Type III collagen is an important ECM component and the predominant collagen during early phases of wound healing, synthesized by fibroblasts in the granulation tissue (Olczyk et al. 2014). With maturation and wound closure, type III collagen undergoes degradation, while type I collagen synthesis increases (Olczyk et al. 2014). Chronic inflammatory processes, such as dermatitis or myocardial inflammation, are associated with increased type III expression (Pauschinger et al. 1998; Hirota et al. 2003). Persistence of type III collagen paralleled presence of inflammation and osteonecrosis in the ZA-EP animals. Interestingly, areas where type I collagen immunostaining decreased and type III collagen increased coincided with weak picrosirius red birefringence.
During wound remodeling and maturation, extracellular matrix undergoes continuous modifications. MMPs can degrade ECM components, growth factors, and cytokines (Li et al. 2016). MMP-9 and MMP-13, in particular, play key roles in the periodontium and alveolar bone homeostasis during health and disease (Hernández Ríos et al. 2009; Honibald et al. 2012). MMP-9- and MMP-13-positive cells were abundantly present in ZA-EP rats at inflamed sites around necrotic bone and within the epithelium. Importantly, select MMP-13-positive osteocytes were noted adjacent to osteonecrotic areas, probably as a result of osteocyte activation.
Progressive wound healing depends on the coordinated function of several cell populations. Among them, myofibroblasts, characterized by the presence of α-SMA-positive stress fibers, play a central role in wound tissue closure through their capacity to produce a strong contractile force in later healing stages (Hinz et al. 2001). As healing progresses, the myofibroblastic population decreases (Wynn and Ramalingam 2012). Persistent presence of myofibroblasts is associated with pathologic conditions, such as hypertrophic scarring and cardiac or lung fibrosis (Slemp and Kirschner 2006; Chistiakov et al. 2016; Pardo and Selman 2016). In our studies, the marked increase of α-SMA-positive cells in close proximity to the inflammatory infiltrate and to the necrotic bone in ZA-EP animals suggests prolonged presence of the myofibroblastic population.
In these experiments, we did not observe consistent exposure of necrotic bone. Although there were many animals with clinically apparent mucosal defects in the ZA-EP group, an epithelial layer was present. Presence of epithelial invagination toward the necrotic area was noted, suggesting that a longer period might have allowed for epithelial migration to the bone surface. Alternatively, other parameters might be necessary, such as infection by specific microbes, genetic predisposition, systemic disease, or concomitant treatment interventions that might compromise soft tissue responses. Our findings resemble stage 0 ONJ in patients, where necrotic bone is not clinically exposed (Ruggiero et al. 2014). Interestingly, in our previous study of extraction of teeth with periradicular disease in mice, we observed bone exposure in ZA animals (Soundia et al. 2016). Differences of animal species (mice vs. rats), duration of ZA treatment (12 vs. 8 wk), and spontaneous periradicular versus EP lesions might account for this discrepancy.
The tissue formation phase of wound healing follows the inflammatory phase and is characterized by the development of granulation tissue. Subsequently, at the maturation phase, granulation tissue develops into fibrotic tissue, marked by replacement of the provisional matrix with collagen (Lucas et al. 2010). Our findings suggest excessive retention of granulation tissue in the ZA-EP animals, while resolution of granulation tissue and healing occurred in all other groups. Failure of granulation tissue resolution reflects a defective wound-healing process. In the ZA-EP animals, the presence of inflammatory infiltrate around large areas of osteonecrosis, the lack of an organized collagen network without fiber insertion in the necrotic bone, and epithelial invagination suggest a compromise of socket healing beyond granulation tissue retention. Whether the composition of the granulation tissue around the osteonecrotic areas is unique to the ZA animals or similar to the granulation tissue during normal socket healing warrants further investigation.
Some limitations need to be considered in translating our findings toward human disease. We used only male rats to parallel our previous observations (Aghaloo et al. 2011). However, sex differences might affect socket healing. Although rodents provide useful animal models to study bone diseases, including ONJ, they demonstrate differences in skeletal homeostasis when compared with humans (Frost and Jee 1992). In our studies, we used a ZA dose of 200 µg/kg, which is 3 times greater than the dose for a patient with bone malignancy (Ruggiero et al. 2014). We elected to utilize this higher dose to increase the prevalence of the disease and generate more prominent tissue responses. It should be noted that ONJ incidence in cancer patients appears to plateau between 2 and 3 y of treatment (Ruggiero et al. 2014) or 24 to 36 ZA infusions. The animals in our study received 18 ZA injections, which is within the clinically relevant treatment regimen. Finally, our studies included a single observational time point. It would be important to expand these studies for multiple points and longer periods such that socket healing in ZA animals could be characterized in more detail.
Importantly, our observations in rats were replicated in biopsy specimens from patients with clinically, radiographically, and histologically diagnosed ONJ, thus supporting the potential significance of our findings. Clinical studies with larger numbers of human samples focusing on markers of socket healing could begin delineating the process of ONJ pathogenesis in patients.
Collectively, our data point to the disruption of the physiologic process of wound healing during the extraction of teeth with periodontitis in the presence of bisphosphonates. The prolonged presence of inflammatory cells in the healing alveolar ridge, the predominance of a type III collagen–rich matrix, increased MMP-9 and MMP-13 expression in resident and inflammatory cells, and persistence of a prominent myofibroblastic population were key observations that could underlie ONJ pathophysiology.
Author Contributions
A. Soundia, S.M. Dry, T. Aghaloo, S. Tetradis, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; D. Hadaya, contributed to conception, design, and data acquisition, critically revised the manuscript; N. Esfandi, contributed to design, data acquisition, and analysis, critically revised the manuscript; I. Gkouveris, contributed to data analysis, critically revised the manuscript; R. Christensen, N. Nikitakis, contributed to data analysis and interpretation, critically revised the manuscript; O. Bezouglaia, contributed to data acquisition and analysis, critically revised the manuscript; F. Pirih, contributed to conception, design, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Footnotes
A supplemental appendix to this article is available online.
This work was supported by grant support from Amgen Inc. (research agreement 2014586784; S.T.) and the National Institutes of Health/National Institute of Dental and Craniofacial Research (DE019465; S.T.). D.H. was supported by NIH/NIDCR T90/R90 DE007296. All histology and digital imaging were performed at the Translational Pathology Core Laboratory at the David Geffen School of Medicine at UCLA.
S.T. has served as a paid consultant for and has received grant support from Amgen Inc. The other authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aghaloo TL, Cheong S, Bezouglaia O, Kostenuik P, Atti E, Dry SM, Pirih FQ, Tetradis S. 2014. RANKL inhibitors induce osteonecrosis of the jaw in mice with periapical disease. J Bone Miner Res. 29(4):843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghaloo TL, Kang B, Sung EC, Shoff M, Ronconi M, Gotcher JE, Bezouglaia O, Dry SM, Tetradis S. 2011. Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J Bone Miner Res. 26(8):1871–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre JI, Akhter MP, Kimmel DB, Pingel JE, Williams A, Jorgensen M, Kesavalu L, Wronski TJ. 2012. Oncologic doses of zoledronic acid induce osteonecrosis of the jaw-like lesions in rice rats (Oryzomys palustris) with periodontitis. J Bone Miner Res. 27(10):2130–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MR. 2015. Medication-related osteonecrosis of the jaw: basic and translational science updates. Oral Maxillofac Surg Clin North Am. 27(4):497–508. [DOI] [PubMed] [Google Scholar]
- Araújo MG, Silva CO, Misawa M, Sukekava F. 2015. Alveolar socket healing: what can we learn? Periodontol 2000. 68(1):122–134. [DOI] [PubMed] [Google Scholar]
- Chistiakov DA, Orekhov AN, Bobryshev YV. 2016. The role of cardiac fibroblasts in post-myocardial heart tissue repair. Exp Mol Pathol. 101(2):231–240. [DOI] [PubMed] [Google Scholar]
- Chrysanthakopoulos NA. 2011. Reasons for extraction of permanent teeth in Greece: a five-year follow-up study. Int Dent J. 61(1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttle L, Nataatmadja M, Fraser JF, Kempf M, Kimble RM, Hayes MT. 2005. Collagen in the scarless fetal skin wound: detection with picrosirius-polarization. Wound Repair Regen. 13(2):198–204. [DOI] [PubMed] [Google Scholar]
- de Molon RS, Cheong S, Bezouglaia O, Dry SM, Pirih F, Cirelli JA, Aghaloo TL, Tetradis S. 2014. Spontaneous osteonecrosis of the jaws in the maxilla of mice on antiresorptive treatment: a novel ONJ mouse model. Bone. 68:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Molon RS, Shimamoto H, Bezouglaia O, Pirih FQ, Dry SM, Kostenuik P, Boyce RW, Dwyer D, Aghaloo TL, Tetradis S. 2015. OPG-Fc but not zoledronic acid discontinuation reverses osteonecrosis of the jaws (ONJ) in mice. J Bone Miner Res. 30(9):1627–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos MA, Kastritis E, Bamia C, Melakopoulos I, Gika D, Roussou M, Migkou M, Eleftherakis-Papaiakovou E, Christoulas D, Terpos E, et al. 2009. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol. 20(1):117–120. [DOI] [PubMed] [Google Scholar]
- Frost HM, Jee WS. 1992. On the rat model of human osteopenias and osteoporoses. Bone Miner. 18(3):227–236. [DOI] [PubMed] [Google Scholar]
- Fung P, Bedogni G, Bedogni A, Petrie A, Porter S, Campisi G, Bagan J, Fusco V, Saia G, Acham S, et al. ; GENVABO Consortium. 2017. Time to onset of bisphosphonate-related osteonecrosis of the jaws: a multicentre retrospective cohort study. Oral Dis. 23(4):477–483. [DOI] [PubMed] [Google Scholar]
- Gosain A, DiPietro LA. 2004. Aging and wound healing. World J Surg. 28(3):321–326. [DOI] [PubMed] [Google Scholar]
- Hernández Ríos M, Sorsa T, Obregón F, Tervahartiala T, Valenzuela MA, Pozo P, Dutzan N, Lesaffre E, Molas M, Gamonal J. 2009. Proteolytic roles of matrix metalloproteinase (MMP)-13 during progression of chronic periodontitis: initial evidence for MMP-13/MMP-9 activation cascade. J Clin Periodontol. 36(12):1011–1017. [DOI] [PubMed] [Google Scholar]
- Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. 2001. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 12(9):2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota A, Ebihara T, Kusubata M, Kobayashi M, Kobayashi K, Kuwaba K, Tanaka K, Kiriyama T, Irie S, Koyama Y. 2003. Collagen of chronically inflamed skin is over-modified and upregulates secretion of matrix metalloproteinase 2 and matrix-degrading enzymes by endothelial cells and fibroblasts. J Invest Dermatol. 121(6):1317–1325. [DOI] [PubMed] [Google Scholar]
- Honibald EN, Mathew S, Padmanaban J, Sundaram E, Ramamoorthy RD. 2012. Perioceutics: matrix metalloproteinase inhibitors as an adjunctive therapy for inflammatory periodontal disease. J Pharm Bioallied Sci. 4(Suppl 2):S417–S421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junqueira LC, Assis Figueiredo MT, Torloni H, Montes GS. 1986. Differential histologic diagnosis of osteoid: a study on human osteosarcoma collagen by the histochemical picrosirius-polarization method. J Pathol. 148(2):189–196. [DOI] [PubMed] [Google Scholar]
- Kang B, Cheong S, Chaichanasakul T, Bezouglaia O, Atti E, Dry SM, Pirih FQ, Aghaloo TL, Tetradis S. 2013. Periapical disease and bisphosphonates induce osteonecrosis of the jaws in mice. J Bone Miner Res. 28(7):1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Morrison A, Hanley DA, Felsenberg D, McCauley LK, O’Ryan F, Reid IR, Ruggiero SL, Taguchi A, Tetradis S, et al. ; International Task Force on Osteonecrosis of the Jaw. 2015. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res. 30(1):3–23. [DOI] [PubMed] [Google Scholar]
- Li W, Zhu Y, Singh P, Ajmera DH, Song J, Ji P. 2016. Association of common variants in MMPs with periodontitis risk. Dis Markers. 2016:1545974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, Eming SA. 2010. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 184(7):3964–3977. [DOI] [PubMed] [Google Scholar]
- Manfredi M, Mergoni G, Goldoni M, Salvagni S, Merigo E, Meleti M, Vescovi P. 2017. A 5-year retrospective longitudinal study on the incidence and the risk factors of osteonecrosis of the jaws in patients treated with zoledronic acid for bone metastases from solid tumors. Med Oral Patol Oral Cir Bucal. 22(3):e342–e348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olczyk P, Mencner L, Komosinska-Vassev K. 2014. The role of the extracellular matrix components in cutaneous wound healing. BioMed Res Int. 2014:747584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo A, Selman M. 2016. Lung fibroblasts, aging, and idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 13(Suppl 5):S417–S421. [DOI] [PubMed] [Google Scholar]
- Pauschinger M, Doerner A, Remppis A, Tannhauser R, Kühl U, Schultheiss HP. 1998. Differential myocardial abundance of collagen type I and type III mRNA in dilated cardiomyopathy: effects of myocardial inflammation. Cardiovasc Res. 37(1):123–129. [DOI] [PubMed] [Google Scholar]
- Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, O’Ryan F; American Association of Oral and Maxillofacial Surgeons. 2014. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg. 72(10):1938–1956. [DOI] [PubMed] [Google Scholar]
- Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. 2004. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 62(5):527–534. [DOI] [PubMed] [Google Scholar]
- Slemp AE, Kirschner RE. 2006. Keloids and scars: a review of keloids and scars, their pathogenesis, risk factors, and management. Curr Opin Pediatr. 18(4):396–402. [DOI] [PubMed] [Google Scholar]
- Soundia A, Hadaya D, Esfandi N, de Molon RS, Bezouglaia O, Dry SM, Pirih FQ, Aghaloo T, Tetradis S. 2016. Osteonecrosis of the jaws (ONJ) in mice after extraction of teeth with periradicular disease. Bone. 90:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy LE, Minasian RA, Caterson EJ. 2016. Extracellular matrix and dermal fibroblast function in the healing wound. Adv Wound Care (New Rochelle). 5(3):119–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DW, Lee C, Kim T, Yagita H, Wu H, Park S, Yang P, Liu H, Shi S, Shin KH, et al. 2014. Impaired bone resorption and woven bone formation are associated with development of osteonecrosis of the jaw-like lesions by bisphosphonate and anti-receptor activator of NF-κB ligand antibody in mice. Am J Pathol. 184(11):3084–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Ramalingam TR. 2012. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 18(7):1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita J, McCauley LK. 2012. Antiresorptives and osteonecrosis of the jaw. J Evid Based Dent Pract. 12(Suppl 3):233–247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.