Abstract
Temporomandibular joint (TMJ) disorders are often associated with development of osteoarthritis-like changes in the mandibular condyle. Discoidin domain receptor 2 (DDR2), a collagen receptor preferentially activated by type I and III collagen found in the TMJ and other fibrocartilages, has been associated with TMJ degeneration, but its role in normal joint development has not been previously examined. Using Ddr2 LacZ-tagged mice and immunohistochemistry, we found that DDR2 is preferentially expressed and activated in the articular zone of TMJs but not knee joints. To assess the requirement for Ddr2 in TMJ development, studies were undertaken to compare wild-type and smallie (slie) mice, which contain a spontaneous deletion in Ddr2 to produce an effective null allele. Analysis of TMJs from newborn Ddr2slie/slie mice revealed a developmental delay in condyle mineralization, as measured by micro–computed tomography and histologic analysis. In marked contrast, knee joints of Ddr2slie/slie mice were normal. Analysis of older Ddr2slie/slie mice (3 and 10 mo) revealed that the early developmental delay led to a dramatic and progressive loss of TMJ articular integrity and osteoarthritis-like changes. Mutant condyles had a rough and flattened bone surface, accompanied by a dramatic loss of bone mineral density. Mankin scores showed significantly greater degenerative changes in the TMJs of 3- and 10-mo-old Ddr2slie/slie mice as compared with wild-type controls. No DDR2-dependent degenerative changes were seen in knees. Analysis of primary cultures of TMJ articular chondrocytes from wild-type and Ddr2slie/slie mice showed defects in chondrocyte maturation and mineralization in the absence of Ddr2. These studies demonstrate that DDR2 is necessary for normal TMJ condyle development and homeostasis and that these DDR2 functions are restricted to TMJ fibrocartilage and not seen in the hyaline cartilage of the knee.
Keywords: temporomandibular disorders (TMD), growth/development, joint disease, craniofacial biology/genetics, cartilage, cell-matrix interactions
Introduction
Temporomandibular joint (TMJ) disorders affect 10% to 40% of the US population. A large proportion of affected individuals develop osteoarthritis (OA)–like degenerative changes that are characterized by cartilage degradation, osteophyte formation, and joint mineralization (Carlsson 1999; Liu and Steinkeler 2013). Although TMJ-OA shares a similar pathology with OA in other joints, it also has unique characteristics including early onset and preferential occurrence in women. This is to be contrasted with OA in hyaline cartilage joints such as the knee, which increases with age and exhibits no sexual preference (Zhang and Jordan 2010). The basis for these differences in OA etiology remains unknown.
We believe that an understanding of interactions between TMJ articular fibrochondrocytes and their extracellular matrix may provide critical cues for understanding unique aspects of TMJ development and pathogenesis. Unlike hyaline cartilage extracellular matrix, which principally contains type II collagen, TMJ fibrocartilage also contains appreciable amounts of type I and III collagen (Wiberg and Wanman 1998; Arden and Nevitt 2006). Discoidin domain receptor 2 (DDR2) is an important mediator of cell-collagen interactions that may be important for understanding interactions between TMJ fibrochondrocytes and collagen. The discoidin domain receptor tyrosine kinases DDR1 and DDR2 each have a unique tissue distribution and sensitivity to activation by specific collagens. DDR1 is expressed in epithelium and weakly activated by collagens I through IV. In contrast, DDR2 is predominantly expressed by mesenchymal cells and is strongly activated by type I and III collagen but is less responsive to collagens II, IV, and V (Vogel et al. 1997; Benjamin and Ralphs 2004). Because DDR2 is preferentially activated by collagens found primarily in TMJ fibrocartilage, we hypothesized that it has a unique and specific role in TMJ development and contributes to the pathogenesis of TMJ degeneration.
Here we use a genetic approach to address this concept by studying the distinct expression pattern of DDR2 in the TMJ, evaluating the early effects of DDR2 deficiency on TMJ versus knee joint development as well as the long-term consequences of deficiency on TMJ cartilage and bone. As shown, DDR2 has a unique role in TMJ development and aging.
Materials and Methods
Animals
Ddr2 LacZ-tagged mice were used to determine the distribution of Ddr2 expression. Mice were developed from embryonic stem cell clone Ddr2tm1a(EUCOMM)Wtsi (EPD0607__B01) obtained from the European Mutant Mouse Repository. This clone contains a “knockout first” allele in the endogenous mouse Ddr2 locus containing a bacterial LacZ cassette and neomycin resistance gene 5′ to exon 2 of Ddr2 with appropriately placed loxP and FLP sites (see Fig. 1A for map). Crossing mice containing this allele with mice containing a Cre expressed in the germline, such as Sox2-Cre, leads to excision of the neo cassette and exon 2 to generate a mouse expressing LacZ under the control of the endogenous Ddr2 gene. Embryonic stem cell transplantation was performed by the University of Michigan Transgenic Model Core. Ddr2 LacZ-tagged mice were developed by crossing knockout-first allele mice with Sox2-Cre mice (Hayashi et al. 2002; Fig. 1A). Two-month-old male heterozygous Ddr2 LacZ-tagged mice (Ddr2+/LacZ) maintained in a C57BL/J6 background were used for LacZ analysis. All studies examining effects of Ddr2 deficiency used Smallie mice (Ddr2slie/slie mice), which contain a spontaneous 150-kb deletion in Ddr2 that removes exons 2 through 17 to produce an effective null allele (Kano et al. 2008). Mice were obtained from the Jackson Laboratory, BKS(HRS)-Ddr2slie/JngJ, and bred into a C57BL6 background for at least 10 generations (Ge et al. 2016). Mice were genotyped with a quantitative real-time polymerase chain reaction (qPCR) protocol provided by Jackson Labs. Whole skull and knee joints were dissected and analyzed from newborn, 3-, and 10-mo-old wild-type (WT) and Ddr2slie/slie mice (n = 6 to 8).
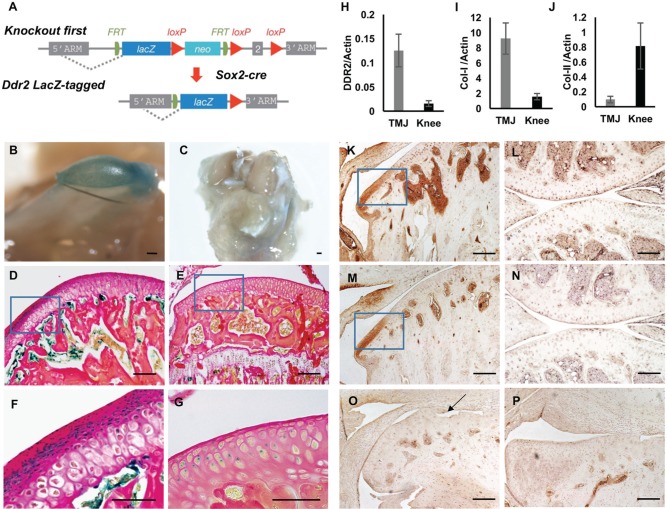
Figure 1.
Discoidin domain receptor 2 (DDR2) is preferentially expressed and activated in temporomandibular joint (TMJ) articular fibrocartilage. (A) Strategy for developing Ddr2 LacZ-tagged allele mice. Mice with recombinant allele knocked into the Ddr2 locus (“knockout first” allele) were developed as described in the methods and crossed with global Cre (Sox2-Cre) mice to generate a germline Ddr2 LacZ-tagged allele (Ddr2+/LacZ). (B–G) LacZ localization in mandibular condyle (B, D, F) and knee joint (C, E, G) from 2-mo-old Ddr2+/LacZ mice. Whole mount LacZ staining (B, C). Low- (D, E) and high-magnification (F, G) histologic sections; high-magnification images are of boxed areas in D, E. (H–J) Reverse transcription quantitative real-time polymerase chain reaction detection of Ddr2 (H), Col1a1 (I), and Col2a1 (J) mRNA in TMJ and knee articular cells from 2-mo-old wild-type mice (n = 6). Values were normalized to β-actin mRNA. (K–P) Immunohistochemistry: TMJ (K, M, O, P) and knee joint (L, N). Antibodies: anti-total DDR2 (K, L, O) and anti-phospho-DDR2 (Y740; M, N, P). (O, P) To determine background staining, TMJs from Ddr2slie/slie mice were stained with total (O) and phosphor-DDR2 (P) antibodies. Scale bars: 0.2 mm in panel B; 0.5 mm for panel C; 40 µm for panels D, E, K–P; 20 µm for panels F, G. Arrow (O) indicates defects in condylar morphology of Ddr2slie/slie mice.
TMJ and Knee Joint Chondrocyte Isolation for RNA Analysis
Articular chondrocytes were isolated from 12-wk-old WT mice with an established method (Gosset et al. 2008). Briefly, mandibular condyles and tibial plateaus were exposed by removing capsules and disc/meniscus. The articular cartilage was cut from mandible condyle and tibia along the mandible/tibial neck. Articular chondrocytes were then isolated by collagenase A digestion and total RNA extracted with TRIzol reagent for mRNA analysis by reverse transcription quantitative real-time PCR (RT-qPCR).
Micro–computed tomography Analysis of Bone
Whole skull and knee joints were scanned by micro–computed tomography (µCT) with a Scanco Model 100 (Scanco Medical). Scan settings were as follows: voxel size of 12 µm, 70 kVp, 114 µA, 0.5-mm aluminum filter, and integration time of 500 ms. All scans were analyzed with fixed thresholds (180 for bone volume). For quantification of subarticular bone volume of TMJ condyles and tibia heads, the mineralized tissue volume of each section was measured between the process from anterior to posterior and the line along the mandible/tibia neck. Total bone volume was determined by adding all the sections between the exterior and interior of each joint.
Tissue Preparation and Histopathologic Analysis
For whole mounts, tissue was fixed in 2% paraformaldehyde, 0.25% glutaraldehyde, and 0.01% NP40 in phosphate-buffered saline, while for tissue sections, samples were first decalcified with 10% ethylenediaminetetraacetic acid for 1 wk and then embedded in OTC and frozen. Fixed tissue and frozen sections were incubated with 1 mg/mL of X-gal overnight. Whole mount alizarin red and alcian blue staining and tissue clarification were conducted as previously described (Ge et al. 2007). For immunohistochemistry, tissue was fixed in 4% formalin and embedded in paraffin. Sections were incubated with total DDR2 antibody (ab5520; Abcam) or phospho-DDR2 (Y740) antibody (MAB25382; R&D). For safranin O staining, paraffin-embedded sections were stained with 0.001% fast green and 0.1% safranin O. A modified Mankin scoring system was used to evaluate pathologic changes in TMJ and knee articular cartilage (Xu et al. 2009; Xu et al. 2011).
Cell Cultures and In Vitro Differentiation
Primary articular chondrocytes were harvested from TMJ condyles dissected from 3-mo-old WT and Ddr2slie/slie mice as described previously (Park et al. 2015). Primary cells were stimulated to undergo chondrocyte differentiation by culturing for 15 d in α-MEM/10% fetal bovine serum containing 50 µg/mL of ascorbic acid, 10mM β-glycerophosphate, and 20 μg/mL of TGF-β1. Visualization of chondrocyte hypertrophy was performed by alcian blue staining and immunostaining with collagen X antibody (ab58632; Abcam) at day 5. Mineralization was assessed by alizarin red S staining (Ge et al. 2016). Cell images were taken with an inverted phase contrast microscope (Nikon D300). To measure mRNA expression, total RNA was isolated at the times indicated, and mRNA levels were measured by RT-qPCR.
Statistical Analysis
All statistical analyses were performed with SPSS 16.0 (IBM). Values are presented as mean ± SD. For in vivo studies, at least 6 animals were used per group. For cell culture studies, triplicate independent samples were used. Statistical significance was assessed with 1-way analysis of variance. P < 0.05 is considered a significant difference between groups.
Results
DDR2 Is Preferentially Expressed and Activated in the Articular Zone of the TMJ
DDR2 has been localized to mesenchymal cells including osteoblasts, chondrocytes, muscle, ligaments, and gonadal tissue (Leitinger 2014). However, its detailed expression pattern in fibrocartilage joints such as the TMJ and in hyaline cartilage joints including the knee has not been previously examined. To address this issue, a Ddr2-LacZ mouse line was generated from knockout-first Ddr2-targeted embryonic stem cell clones as described in the methods. As shown by whole mount LacZ staining of heterozygous Ddr2 LacZ-tagged mice, Ddr2 was strongly expressed in the TMJ condyle with little or no staining in the articular surface of the knee joint (Fig. 1B, C). LacZ staining of histologic sections showed Ddr2 expression in the TMJ articular surface, where multiple layers of cells exist (Fig. 1D, F). In contrast, the knee joint articular surface contains only a single cell layer that has little or no LacZ staining (Fig. 1E, G). Weak LacZ staining was detected in the subarticular zones in the TMJ and knee joint (Fig. 1F, G). The localized expression of DDR2 protein in the TMJ condyle articular surface was confirmed by immunohistochemistry with anti-total DDR2 antibody (boxed area, Fig. 1K). Staining was observed in disc and mandibular (glenoid) fossa regions, although this was not investigated in detail. Consistent with the LacZ distribution, little staining was seen in sections of the knee joint articular surface (Fig. 1I). Separate studies isolated chondrocytes from condyles and knee joints (tibial surface), and Ddr2 mRNA levels were measured by RT-qPCR. Consistent with LacZ staining and immunohistochemical analysis, Ddr2 mRNA was enriched 10-fold in chondrocytes from the TMJ relative to the knee (Fig. 1H). When compared with the knee joint, TMJ samples were enriched in Col1a1 and depleted in Col2a1 mRNA, as would be expected for this fibrocartilage (Fig. 1I, J).
DDR2 is preferentially activated and autophosphorylated at Y740 by type I and III collagen found in TMJ fibrocartilage but is less responsive to collagens II, IV, and V (Ikeda et al. 2002; Yang et al. 2005). To determine if TMJ-associated DDR2 is in a phosphorylated, activated state, immunohistochemistry was conducted with a phospho-Y740 DDR2 antibody. As shown in Figure 1M, strong phospho-DDR2 staining was seen in the TMJ articular zone and disc. In contrast, phosphorylated DDR2 was undetectable in the articular zone of the knee joint (Fig. 1N). The specificity of total and P-DDR2 antibody staining was confirmed by their inability to stain TMJ sections from Ddr2slie/slie mice (Fig. 1O, P).
DDR2 Deficiency in Ddr2slie/slie Mice Causes a Selective Delay in TMJ Development
To determine the consequences of global Ddr2 deficiency in TMJ development, we examined WT and Ddr2slie/slie mice. These animals contain a spontaneous 150-kb deletion in the Ddr2 gene to produce an effective null allele (Kano et al. 2008). As shown in our previous study, Ddr2slie/slie mice have major defects in craniofacial, trabecular, and, to a lesser extent, cortical bone formation (Ge et al. 2016). The preferential expression of Ddr2 in the TMJ versus knee joint suggests that it may also have selective functions in TMJ development. In the study shown in Figure 2, we compared TMJ and knee joints from newborn Ddr2slie/slie mice with WT littermates. Mineralization of the mandibular condyle as measured by µCT was delayed in Ddr2slie/slie mice (Fig. 2A, B). Whole mounts showed a larger volume of unmineralized cartilage (alcian blue stain) in mutant condyles (Fig. 2C, D, boxed area). Consistent with these findings, histologic analysis showed the TMJ condyle to contain safranin O–staining chondrocytes (red) in newborn Ddr2slie/slie mice, while cartilage in condyles from WT mice had largely been replaced by mineral (Fig. 2E, F). Mineralization of glenoid fossa articular surfaces within the temporal bone was delayed in Ddr2slie/slie mice (Fig. 2E, F). In contrast, knee joints from WT and Ddr2slie/slie mice were indistinguishable at this time point (Fig. 2G, H).
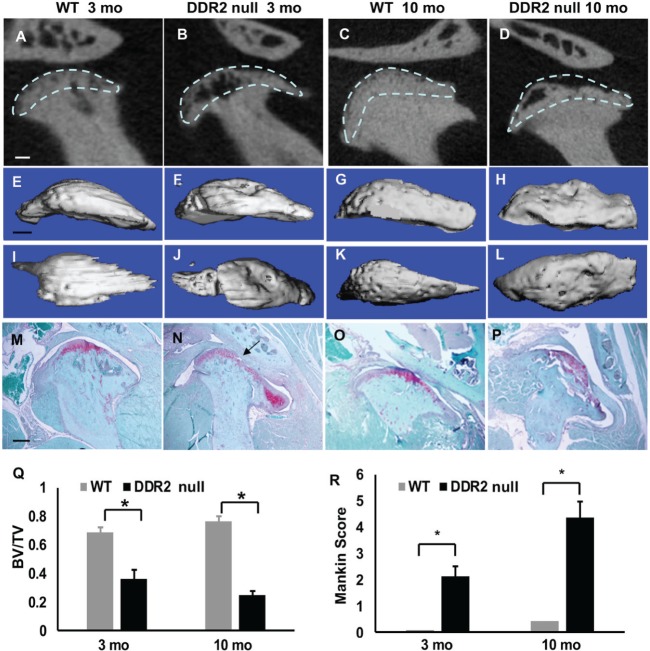
Figure 2.
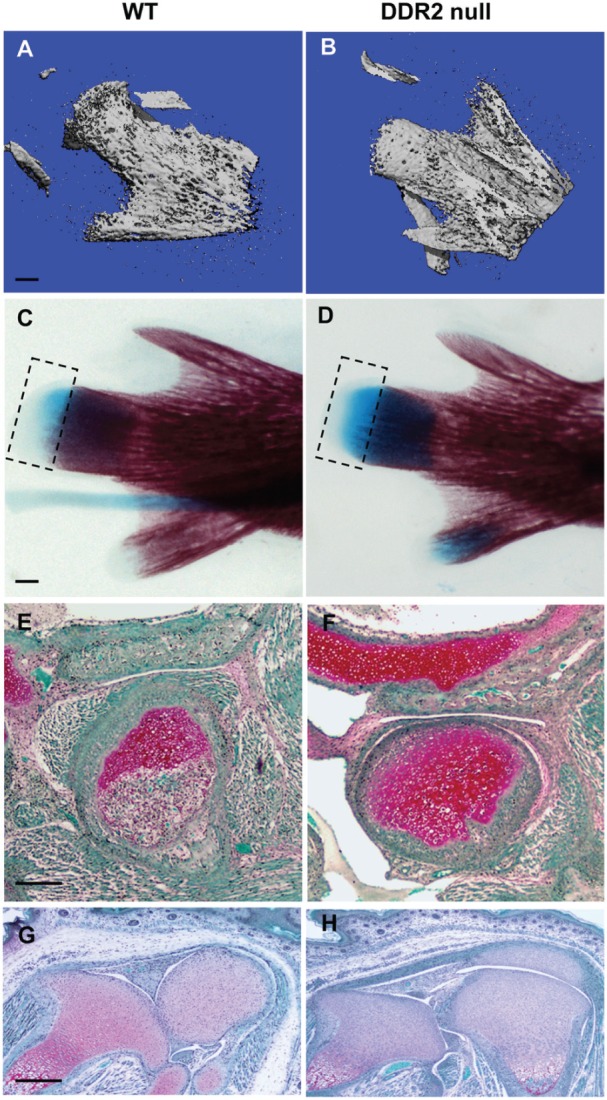
Ddr2-deficient mice exhibit tissue-selective defects in temporomandibular joint (TMJ) development. TMJ condyle structure was compared in newborn wild-type (WT) and Ddr2slie/slie mice with 3-dimensional reconstructed micro–computed tomography images (A, B), alizarin red and alcian blue whole mount staining (C, D), and safranin O staining of TMJ sections (E, F). Safranin O staining of knee joint histologic sections is also shown (G, H). Scale bars: 0.2 mm in panel A (A, B); 0.5 mm in panel C (C, D); 40 µm in panel E (E, F); 100 µm in panel G (G, H). Boxed area in panels C and D indicates alcian blue–stained region.
Adult Ddr2slie/slie Mice Develop Spontaneous and Selective TMJ Degeneration
To determine whether DDR2 deficiency leads to pathologic changes in adult mice, we next characterized TMJ and knee joints from 3- and 10-mo-old mice with µCT and histopathologic analysis. Clear changes in TMJ structure were evident in 3-mo-old Ddr2slie/slie mice when compared with WT littermates. Two-dimensional µCT analysis showed a dramatic loss of subchondral bone (Fig. 3A, B). These phenotypic bone changes were even more extensive in 10-mo-old Ddr2slie/slie mice, where loss of articular integrity and subchondral bone was observed (Fig. 3C, D). Three-dimensional µCT reconstruction of whole mandibular condyles showed severe bone loss on the condyle surface from 3-mo-old Ddr2slie/slie mice (Fig. 3E, F, I, J). By 10 mo of age, the condyle surface became flattened in Ddr2slie/slie mice due to loss of subchondral bone (Fig. 3G, H, K, L). Histopathologic analysis further confirmed loss of tissue integrity starting in 3-mo-old Ddr2slie/slie mice (arrows in Figs. 1O, 3N) that was progressively more severe in 10-mo-old animals. The pathologic changes included a decrease in safranin O–stained proteoglycan (Fig. 3N) that expanded to include >50% of the TMJ condyle surface in 10-mo-old Ddr2slie/slie mice (Fig. 3P). Subchondral bone loss is considered to be an early indicator of OA formation (Li et al. 2013). TMJ subchondral bone volume fraction, as measured by µCT, decreased approximately 50% in 3-mo-old Ddr2slie/slie mice and 70% in 10-mo-old animals (Fig. 3Q). The OA severity, quantified with a modified Mankin OA scoring system (Xu et al. 2009), showed small age-dependent degenerative changes in TMJs from WT mice and dramatically higher OA scores in 3- and 10-mo-old Ddr2slie/slie animals (Fig. 3R).
Figure 3.
Spontaneous temporomandibular joint (TMJ) degeneration in 3- and 10-mo-old Ddr2slie/slie mice. (A–D) Two-dimensional micro–computed tomography (µCT) reconstruction images of TMJs from 3- and 10-mo-old wild-type (WT) and Ddr2slie/slie mice. (E–H) Three-dimensional µCT lateral views of mandibular condyles. (I–L) Three-dimensional superior views of mandibular condyles. (M–P) Safranin O staining of TMJ sections. Arrow in panel N indicates early degeneration in 3-mo-old DDR2-null section. (Q) µCT quantification of subchondral bone volume fraction at mandibular condyle surfaces (area of analysis indicated by dashed lines; see Materials and Methods). For panel Q, values are presented as ratio of bone volume / tissue volume; for panel R, as histopathologic score. (R) Histopathologic scoring of TMJ osteoarthritis based on modified Mankin score. Scale bars: 0.25 mm in panel A (A–D); 0.5 mm in panel E (E–L); 40 µm in panel M (M–P). *P < 0.05, n = 8.
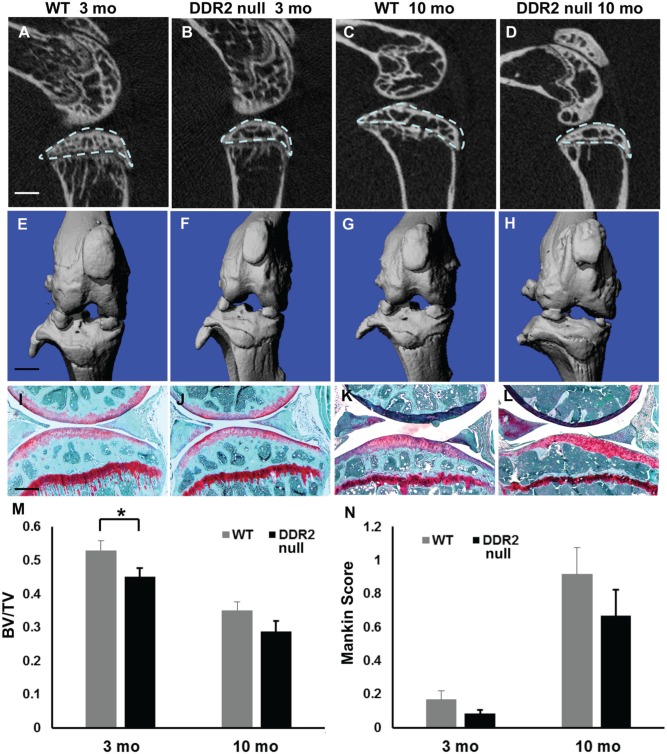
In contrast to the profound changes in TMJ cartilage and bone in Ddr2-null mice, DDR2 deficiency had no obvious effect on knee joints. µCT analysis detected slight subchondral bone loss in WT and Ddr2slie/slie mice when 3- and 10-mo-old mice were compared (Fig. 4A–H). Although bone volume fraction was reduced by approximately 10% in 3-mo-old Ddr2-null mice (Fig. 4M), this was not seen at 10 mo and is in marked contrast to the 50% loss seen in TMJs from Ddr2-deficient mice. Additionally, the integrity of the articular surface remained intact, and no defects in proteoglycan staining were apparent (Fig. 4I–L). Histopathologic analysis showed an age-related increase in OA score in knee joints of WT and Ddr2slie/slie mice, but the severity of joint degeneration on Ddr2slie/slie mice was similar to that of WT animals (Fig. 4N).
Figure 4.
Ddr2 deficiency does not affect knee joints. (A–D) Micro–computed tomography (µCT) 2-dimensional reconstruction images of knee joints from 3- and 10-mo-old wild-type (WT) and Ddr2slie/slie mice. (E–H) Three-dimensional µCT images of knee joints. (I–L) Safranin O staining of knee joint sections. (M) µCT analysis of tibia subchondral bone volume fraction (area of analysis indicated by dashed line in panel A). (N) Histopathologic scoring of knee joint osteoarthritis with modified Mankin score. Scale bars: 0.25 mm in panel A (A–D); 0.25 mm in panel E (E–H); 40 µm in panel I (I–L). *P < 0.05, n = 6.
DDR2 Deficiency Inhibits Differentiation and Hypertrophy of Primary TMJ Fibrochondrocytes
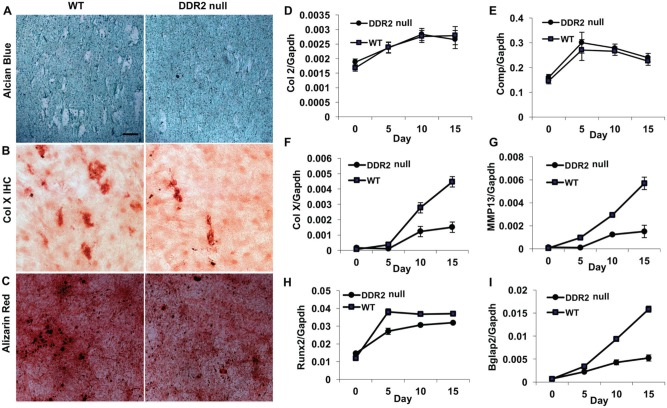
DDR2 has broad effects on cell proliferation, migration, survival, differentiation, and tissue remodeling (Leitinger 2014). In calvarial and marrow stromal cell cultures, DDR2 deficiency severely reduced differentiation to osteoblasts (Ge et al. 2016), but its possible role in fibrochondrocyte differentiation has not been previously examined. To address this issue, fibrochondrocytes were isolated from WT and DDR2-deficient condyles and grown in differentiation media for 2 weeks. Defects in chondrocyte differentiation/maturation were apparent on visual inspection of histochemically stained cultures (Fig. 5A–C). These findings show that enlarged alcian blue–negative cells having the appearance of hypertrophic chondrocytes were greatly reduced in DDR2-deficient cultures (Fig. 5A). In addition, immunostaining for type X collagen–positive cells and mineralization, additional properties of hypertrophic chondrocytes were reduced (Fig. 5B, C). Consistent with these findings, while chondrocyte markers such as Col2a1 and Comp, which are expressed during early chondrocyte differentiation stages, were not affected by loss of DDR2 (Fig. 5D, E), markers of chondrocyte hypertrophy and maturation, including Col10a1, Mmp13, Runx2, and Bglap2, were all significantly decreased in Ddr2-deficient cells (Fig. 5F–I).
Figure 5.
Decreased in vitro differentiation and maturation of primary temporomandibular joint chondrocyte cultures from Ddr2slie/slie mice. Primary temporomandibular joint condyle chondrocytes were isolated from 3-mo-old wild-type (WT) and homozygous Ddr2slie/slie mice (DDR2) and grown in chondrogenesis medium for up to 15 d. Five-day cultures were used for alcian blue staining (A) and immunohistochemistry for collagen X (B). Fifteen-day cultures were stained for mineral with alizarin red (C). Cultures from day 0 to day 15 were used for reverse transcription quantitative real-time polymerase chain reaction detection of temporal changes in chondrocyte gene expression: (D) Col2a1 (Col 2), (E) cartilage oligomeric matrix protein (Comp), (F) Col10a1 (Col X), (G) matrix metalloproteinase 13 (MMP13), (H) runt-related transcription factor 2 (Runx2), and (I) bone gamma carboxyglutamate protein 2 (Bglap 2). Values were normalized to Gapdh mRNA. Scale bar: 40 µm for panels A–C.
Discussion
Although DDR2 has been studied as a possible mediator of OA induction, its role in joint development and maturation has not been previously explored. Here we show that DDR2 is preferentially expressed in TMJ fibrocartilage versus hyaline cartilage of the knee. Furthermore, TMJ development is delayed in newborn Ddr2-deficient Ddr2slie/slie mice, and this developmental defect leads to spontaneous and progressive TMJ degeneration as animals age. In contrast, DDR2 deficiency does not affect knee joint development or degeneration. This selectivity may be explained by the preferential expression of DDR2 in TMJ versus hyaline joints and specific activation of DDR2 by the type I collagen–enriched extracellular matrix of TMJ fibrocartilage.
DDR2 levels have not been compared between the TMJ and hyaline joints such as the knee. In earlier work, DDR2 was not detectable by immunohistochemistry in healthy knees and was seen only after induction of OA by surgical destabilization (Xu et al. 2010). In our study, levels of DDR2 in knee articular cartilage were very low, whether detected by LacZ staining of Ddr2+/LacZ mice, immunohistochemistry, or quantitation of Ddr2 mRNA. In contrast, DDR2 was highly enriched in TMJ condyle fibrocartilage regardless of the detection method used (Fig. 1). The abundance of DDR2 in the TMJ may make it particularly dependent on this collagen receptor for normal development and homeostasis.
Immunohistochemical analysis localized DDR2 to certain cells of the TMJ disc and regions of the glenoid fossa (Fig. 1). This was also seen in LacZ-stained samples (not shown). Furthermore, in addition to delaying condyle mineralization, loss of DDR2 delayed mineralization of the glenoid fossa in newborn mice (Fig. 2). The relationship between these early developmental changes and the subsequent TMJ degeneration shown in Figure 3 is not currently understood. Abnormal articulation between the condyle and glenoid fossa is clearly apparent even in newborn animals and becomes more severe as animals age (Figs. 2, 3). Furthermore, DDR2 deficiency leads to abnormalities in skull shape with a resulting decrease in the distance between the external occipital protuberance and incisors (Dullin et al. 2007; Kano et al. 2008). These abnormalities, combined with delays in cartilage maturation and mineralization in the condyle and glenoid fossa, could clearly alter the mechanical environment of the TMJ, which is critical for normal maturation and may be related to subsequent joint degeneration (Kuroda et al. 2009; Ding et al. 2010). To determine the relative contribution of DDR2 deficiency–related developmental defects versus possible postnatal functions of DDR2 and to identify critical DDR2-positive cell populations, it will be necessary to utilize tissue-specific inducible Ddr2 knockout models that are currently under development in the project laboratory.
In a recent study, knockout of the other mammalian discoidin receptor, DDR1, was shown to induce TMJ defects in the absence of knee joint pathology (Schminke et al. 2014). Thus, deficiencies of mammalian discoidin receptors represent the only known mouse genetic models that selectively affect the TMJ. However, Ddr1- and Ddr2-deficient animals each exhibit distinct pathologies. In contrast to the early effects of Ddr2 deficiency, in the Ddr1 knockout, no TMJ developmental defects were reported. Instead, early-onset TMJ OA was seen beginning at 9 wk of age. The basis for the different phenotypes of DDR1- and DDR2-deficient mice is not known, although, interestingly, loss of DDR1 was accompanied by a compensatory upregulation of TMJ-associated DDR2. We and others previously showed that DDR2 functions to increase bone formation and cartilage hypertrophy (Zhang et al. 2011; Ge et al. 2016). This requirement for DDR2 for cartilage hypertrophy is seen in the results presented in Figure 5, where condyle fibrochondrocytes from Ddr2slie/slie mice were shown to have a diminished capacity to undergo hypertrophic maturation. It is therefore possible that DDR2 upregulation in Ddr1-deficient mice may explain subsequent abnormal TMJ mineralization and OA. Consistent with this concept, surgical destabilization of TMJ or knee joints leads to upregulation of DDR2 and OA induction (Xu et al. 2007), a response that is reduced in mice haploinsufficient for Ddr2 (Xu et al. 2010).
Taken together, these results are consistent with a model where DDR2 is required for chondrocytic maturation and subchondral bone formation and associated TMJ development. The selective role of DDR2 in TMJ versus knee joint development may be explained by the restricted expression of this collagen receptor in the TMJ articular zone and characteristic responsiveness of DDR2 to the type I and III fibrocartilage collagens present in the TMJ. In contrast, the hyaline cartilage of the knee normally expresses only low levels of DDR2 and is devoid of type I and III collagen necessary for its activation. For this reason, DDR2 may not be necessary for knee joint development. Only after the trauma of surgical destabilization does DDR2 become active in the knee joint, where it can then mediate pathologic mineralization and OA.
Author Contributions
C. Ge, contributed to conception, design, and data acquisition, drafted and critically revised the manuscript; F. Mohamed, A. Binrayes, contributed to conception, data acquisition, and analysis, critically revised the manuscript; S. Kapila, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript; R.T. Franceschi, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank Dr. Yuji Mishina (University of Michigan School of Dentistry) for the generous gift of Sox2-Cre mice.
Footnotes
This work was supported by National Institute of Dental and Craniofacial Research Career Development Award K12 DE023574 (C.G.; principal investigator, S.K.) and grant DE11723 (R.T.F.), as well as a scholarship from the Ministry of Higher Education and Scientific Research, Libyan Transitional Government (F.M.), and a scholarship from King Saud University (A.B.).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Arden N, Nevitt MC. 2006. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol. 20(1):3–25. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. 2004. Biology of fibrocartilage cells. Int Rev Cytol. 233:1–45. [DOI] [PubMed] [Google Scholar]
- Carlsson GE. 1999. Epidemiology and treatment need for temporomandibular disorders. J Orofac Pain. 13(4):232–237. [PubMed] [Google Scholar]
- Ding L, Heying E, Nicholson N, Stroud NJ, Homandberg GA, Buckwalter JA, Guo D, Martin JA. 2010. Mechanical impact induces cartilage degradation via mitogen activated protein kinases. Osteoarthritis Cartilage. 18(11):1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dullin C, Missbach-Guentner J, Vogel WF, Grabbe E, Alves F. 2007. Semi-automatic classification of skeletal morphology in genetically altered mice using flat-panel volume computed tomography. PLoS Genet. 3(7):e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge C, Wang Z, Zhao G, Li B, Liao J, Sun H, Franceschi RT. 2016. Discoidin receptor 2 controls bone formation and marrow adipogenesis. J Bone Miner Res. 31(12):2193–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge C, Xiao G, Jiang D, Franceschi RT. 2007. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J Cell Biol. 176(5):709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosset M, Berenbaum F, Thirion S, Jacques C. 2008. Primary culture and phenotyping of murine chondrocytes. Nat Protoc. 3(8):1253–1260. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Lewis P, Pevny L, McMahon AP. 2002. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech Dev. 119 Suppl 1:S97–S101. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Wang LH, Torres R, Zhao H, Olaso E, Eng FJ, Labrador P, Klein R, Lovett D, Yancopoulos GD, et al. 2002. Discoidin domain receptor 2 interacts with Src and Shc following its activation by type I collagen. J Biol Chem. 277(21):19206–19212. [DOI] [PubMed] [Google Scholar]
- Kano K, Marin de, Evsikova C, Young J, Wnek C, Maddatu TP, Nishina PM, Naggert JK. 2008. A novel dwarfism with gonadal dysfunction due to loss-of-function allele of the collagen receptor gene, Ddr2, in the mouse. Mol Endocrinol. 22(8):1866–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S, Tanimoto K, Izawa T, Fujihara S, Koolstra JH, Tanaka E. 2009. Biomechanical and biochemical characteristics of the mandibular condylar cartilage. Osteoarthritis Cartilage. 17(11):1408–1415. [DOI] [PubMed] [Google Scholar]
- Leitinger B. 2014. Discoidin domain receptor functions in physiological and pathological conditions. Int Rev Cell Mol Biol. 310:39–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Yin J, Gao J, Cheng TS, Pavlos NJ, Zhang C, Zheng MH. 2013. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 15(6):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Steinkeler A. 2013. Epidemiology, diagnosis, and treatment of temporomandibular disorders. Dent Clin North Am. 57(3):465–479. [DOI] [PubMed] [Google Scholar]
- Park Y, Hosomichi J, Ge C, Xu J, Franceschi R, Kapila S. 2015. Immortalization and characterization of mouse temporomandibular joint disc cell clones with capacity for multi-lineage differentiation. Osteoarthritis Cartilage. 23(9):1532–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schminke B, Muhammad H, Bode C, Sadowski B, Gerter R, Gersdorff N, Burgers R, Monsonego-Ornan E, Rosen V, Miosge N. 2014. A discoidin domain receptor 1 knock-out mouse as a novel model for osteoarthritis of the temporomandibular joint. Cell Mol Life Sci. 71(6):1081–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel W, Gish GD, Alves F, Pawson T. 1997. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1(1):13–23. [DOI] [PubMed] [Google Scholar]
- Wiberg B, Wanman A. 1998. Signs of osteoarthrosis of the temporomandibular joints in young patients: a clinical and radiographic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 86(2):158–164. [DOI] [PubMed] [Google Scholar]
- Xu L, Peng H, Glasson S, Lee PL, Hu K, Ijiri K, Olsen BR, Goldring MB, Li Y. 2007. Increased expression of the collagen receptor discoidin domain receptor 2 in articular cartilage as a key event in the pathogenesis of osteoarthritis. Arthritis Rheum. 56(8):2663–2673. [DOI] [PubMed] [Google Scholar]
- Xu L, Polur I, Lim C, Servais JM, Dobeck J, Li Y, Olsen BR. 2009. Early-onset osteoarthritis of mouse temporomandibular joint induced by partial discectomy. Osteoarthritis Cartilage. 17(7):917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Polur I, Servais JM, Hsieh S, Lee PL, Goldring MB, Li Y. 2011. Intact pericellular matrix of articular cartilage is required for unactivated discoidin domain receptor 2 in the mouse model. Am J Pathol. 179(3):1338–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Servais J, Polur I, Kim D, Lee PL, Chung K, Li Y. 2010. Attenuation of osteoarthritis progression by reduction of discoidin domain receptor 2 in mice. Arthritis Rheum. 62(9):2736–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Kim JH, Kim HJ, Park IS, Kim IY, Yang BS. 2005. Tyrosine 740 phosphorylation of discoidin domain receptor 2 by Src stimulates intramolecular autophosphorylation and Shc signaling complex formation. J Biol Chem. 280(47):39058–39066. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jordan JM. 2010. Epidemiology of osteoarthritis. Clin Geriatr Med. 26(3):355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Su J, Yu J, Bu X, Ren T, Liu X, Yao L. 2011. An essential role of discoidin domain receptor 2 (DDR2) in osteoblast differentiation and chondrocyte maturation via modulation of Runx2 activation. J Bone Miner Res. 26(3):604–617. [DOI] [PubMed] [Google Scholar]