Abstract
The load-bearing dentoalveolar fibrous joint is composed of biomechanically active periodontal ligament (PDL), bone, cementum, and the synergistic entheses of PDL-bone and PDL-cementum. Physiologic and pathologic loads on the dentoalveolar fibrous joint prompt natural shifts in strain gradients within mineralized and fibrous tissues and trigger a cascade of biochemical events within the widened and narrowed sites of the periodontal complex. This review highlights data from in situ biomechanical simulations that provide tooth movements relative to the alveolar socket. The methods and subsequent results provide a reasonable approximation of strain-regulated biochemical events resulting in mesial mineral formation and distal resorption events within microanatomical regions at the ligament-tethered/enthesial ends. These biochemical events, including expressions of biglycan, decorin, chondroitin sulfated neuroglial 2, osteopontin, and bone sialoprotein and localization of various hypertrophic progenitors, are observed at the alkaline phosphatase–positive widened site, resulting in mineral formation and osteoid/cementoid layers. On the narrowed side, tartrate-resistant acid phosphatase regions can lead to a sequence of clastic activities resulting in resorption pits in bone and cementum. These strain-regulated biochemical and subsequently biomineralization events in the load-bearing periodontal complex are critical for maintenance of the periodontal space and overall macroscale joint biomechanics.
Keywords: periodontal ligament, mechanobiology, X-ray computed tomography, entheses, dentoalveolar, interface
Introduction
In most vertebrates, biological events arising from passive and active mechanical forces are fundamental for development, growth, and maturation of tissues and organs (Ingber 1997, 2003; Carter et al. 1998). Developmentally, biomechanical forces and resulting strain gradients in tissues stimulate cell differentiation, polarization, and migration. Strain-mediated cellular activities induce syntheses of organic and inorganic constituents to maintain, develop, remodel, and/or repair the extracellular matrices (Ingber 1997) of tissues within an organ.
The dental, oral, and craniofacial complex is a paramasticatory organ system that includes the periodontal complex of the dentoalveolar fibrous joint, cranial and palatal sutures, and the diarthrodial temporomandibular joint (TMJ), all of which are affected by chewing forces (Hiiemae 1967; Vinyard et al. 2008). In addition to chewing forces, this organ system is subjected to other types of multiple loads with varying magnitudes and frequencies that include speech and intracranial pressure (Vinyard et al. 2008). Using fossil data and animal models, morphologists and experimentalists interpreted temporal and morphologic adaptations of the cranium with plausible insights into pathologic adaptations of the mandible (Yamamoto 1996; Margvelashvili et al. 2013; Jang et al. 2015). The effects of chewing magnitude and frequency continue to be investigated to explain the cranial morphology of early homo sapiens (Vinyard et al. 2008). The relationship between the mechanical strains within tissues from mastication and the subsequent form of the alveolar bony socket, including cranial morphology, has been well established (Hylander 1979). Several gaps exist in current knowledge about the plausible cause-and-effect relationship. It is thus imperative to establish a mechanistic link to map strain-mediated adaptive processes in the hopes of developing effective clinical interventions with mechanical loads as a therapeutic tool that would reverse common pathologic conditions of the affected tissues and related organs.
Specific to the dentoalveolar fibrous joint, the magnitude and duration of chewing forces that act directly on the crown of a tooth are subsequently felt by the periodontal ligament (PDL) of the fibrous joint as food is first incised by the anterior teeth and then crushed by the molars (Cate and Nanci 2013). As other organs of the paramasticatory complex are also affected by the magnitude and duration of chewing forces, the current challenge is identifying the direct causality of functional loads and decoupling their effects as related to joint-specific tissue adaptation. From a clinical standpoint, the intertwined nature of joint-level biomechanics with tissue-level mechanobiology can be significantly altered in treatments that include distraction osteogenesis. A similar clinical intervention that lies at the intersection of biomechanics and mechanobiology of the fibrous joint is orthodontic tooth movement and related effects on the TMJ.
Over the years, the masticatory behaviors and biomechanics of the fibrous joint have been studied with several animal models, from their developing stages through adulthood (Herring 1976; Hylander 1979; Kiliaridis 1986b; Daegling et al. 1992). These behaviors are attributable to occlusal loads on the crown caused by mastication of harder foods (higher magnitude but lower frequency; Kiliaridis 1986a) and softer foods (lower magnitude but higher frequency; Kiliaridis 1986a). To investigate the subsequent “functional adaptation” of the fibrous joint systematically, a multiscale approach (Fig. 1) has been utilized based on guiding principles from mechanics of materials, materials science, imaging with in situ microscopy, and mechanical loading (Jang et al. 2009; Lin et al. 2013; Jang et al. 2014; Jang et al. 2015). Of particular interest is the link between tooth movement within the alveolar socket and the resulting deformations in the mechanoresponsive PDL. This movement stimulates the mineral-forming and mineral-resorbing cells at the strain-amplified tethered/attachment/enthesial sites of PDL-bone and PDL-cementum (Ho et al. 2010; Kurylo et al. 2016). As a result, spatiotemporal reorganization of physical and biochemical events of bone and cementum within the fibrous joint is also discussed.
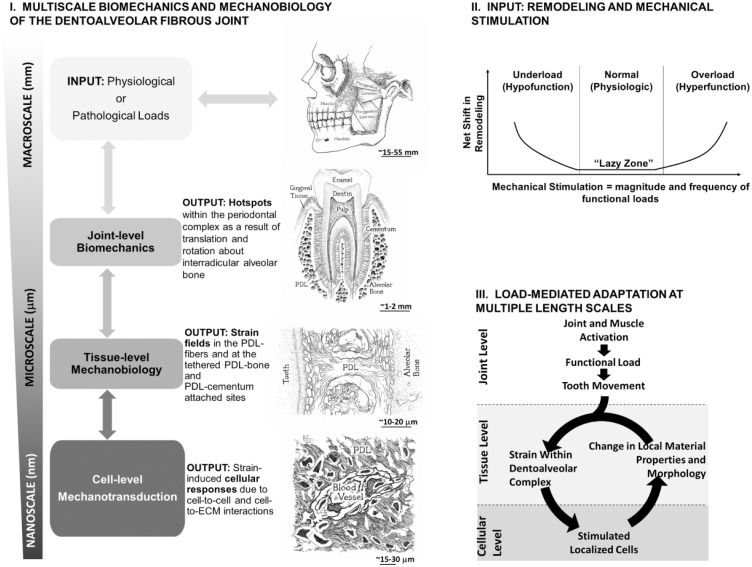
Figure 1.
General layout of multiscale biomechanics (I), physiologic and pathologic inputs (II), and load-mediated adaptations in the oral masticatory complex (III), in particular, the dentoalveolar fibrous joint. (I) Multiscale biomechanics and mechanobiology of the dentoalveolar fibrous joint: Chart illustrates joint-level biomechanics to tissue-level biological events commonly addressed as mechanobiology of tissues within the load-bearing fibrous joint. Biological events within tissues and interfaces resulting in mineral formation and resorption can be mapped within the context of joint biomechanics. (II) Input: net shifts in remodeling rates and mechanical stimulation. Figure illustrates relationships between mechanical stimulation (magnitude and frequency of load) of the joint and thereby changes in remodeling rates of tissues within the periodontal complex. This graph was adapted from Turner and Pavalko (1998). (III) Load-mediated adaptation at multiple-length scales. At a joint level, functional loads (physiologic or pathologic) on teeth cause tooth movement within the alveolar socket. Tooth movement causes local deformations within periodontal tissues. Cells within tissues sense localized strains and respond through adaptation identified as shifts in material properties and morphologies. This cycle will continue to optimize material properties and structure to accommodate functional demands, albeit shifting to pathologic adaptations. ECM, extracellular matrix; PDL, periodontal ligament.
Physiologic and Pathologic Loads and Dentoalveolar Biomechanics
Physiologic and Pathologic Loads within the Oral Cavity and the Craniofacial Complex
Functional loads within the oral cavity can be divided into several types: physiologic and nonphysiologic (pathologic and therapeutic; Fig. 1II). Prolonged physiologic and pathologic loads on the tooth crown can cause significant tooth displacement relative to the surface of the alveolar socket resulting in altered root and bone morphologies (Koivumaa et al. 1971). While there exists a range in direction, magnitude, and frequency for physiologic loads, loads that go below or beyond the normal range of 100 to 200 N for humans are pathologic by nature. A common pathologic load is a dramatic decrease or increase in magnitude and/or frequency (load rate) on a noncompromised fibrous dentoalveolar joint. Examples of pathologic loads include disuse, bruxism, or primary occlusal trauma leading to joint injury and localized sterile inflammation (as opposed to nonsterile inflammation arising from periodontitis) of the fibrous joint (Hallmon 1999). In addition, physiologic loads applied to a compromised fibrous joint can result in a traumatic inflammatory response, referred to as “secondary occlusal trauma” (Hallmon 1999). From a translational and practical standpoint, loads continue to be used as a therapeutic tool (e.g., orthodontics) to reposition teeth for optimal occlusal contact. This practice can cause root shortening through mineral resorption in bone and cementum (Krishnan 2005) and are mechanobiological processes that occur below the tooth crown and within the periodontal complex. By extrapolating philosophies specific to load-mediated adaptation of long bone, investigating the prolonged effect of magnitude and the rate of loading on the tooth crown and tissues of the periodontal complex (Figs. 1I, III) is equally important to prevent a traumatic injury to the tooth and the alveolar bone to which it is attached (Turner and Pavalko 1998).
Over the years, several groups have investigated the effects of physiologic and pathologic loads, including therapeutic loading regimes on adaptation of the oral and craniofacial masticatory system (Appendix Table 1). Within each loading regime, the effects of magnitude and load rate on the dentoalveolar complex were investigated. Tooth morphology plausibly influenced by diet hardness and/or environment continued to be discussed over centuries and was included by Tomes in 1882. Most relevant to this topic is Hiiemae, who in 1967 provided an elegant extensive review on the form of the masticatory complex and its function in mammals—specifically, rats that were given foods of varying consistencies. The magnitude and frequency of chewing forces were altered by providing animals either softer foods (lower loads at higher frequencies) or harder foods (higher loads at lower frequencies). This straightforward systematic approach to investigate load-mediated adaptation of the masticatory complex of small-scale animal models revealed significantly altered chewing patterns, including muscle activity, and was illustrated by Thomas and Peyton in 1983. Currently, pathologic loads continue to be programmed in mammals by either placing impedance on the occlusal surface (e.g., bite blocks) or causing hypofunction with an unopposed tooth molar model.
Pathologic loads leading to hyper- or hypofunction continue to result in altered bone volume density (Jang et al., 2015). Orthodontics is a common load-mediated clinical intervention. Significant tooth movement is programmed in rats and mice with springs of known stiffness values to prompt forces on incisors and crown of molars and, in turn, result in biochemical expressions within site-specific regions of the periodontal complex (Krishnan and Davidovitch 2009). Despite these many studies, there exists limited information on the causality of the functional load (regardless of its type—physiologic or pathologic) toward adaptation of the periodontal complex—that is, cause and effect of loads on adaptation of the periodontal complex. One primary reason is the challenge to link the change in force on the tooth crown to a corresponding change in site-specific biological signals within its periodontal complex.
Experimental Mechanics to Map Strains within Periodontal Tissues and Their Interfaces
Interdisciplinary approaches with several experimental mechanics techniques to map deformations and strains within periodontal tissues and their interfaces continue to be implemented. Deformations and strains caused by forces on pieces of tissues reduced from whole dentoalveolar complexes were mapped with experimental mechanics techniques that included strain gauges (Jantarat et al. 2001; Popowics et al. 2004), photoelasticity (Asundi and Kishen 2001), Moiré interferometry (Wang and Weiner 1998; Wood et al. 2003), electronic speckle pattern interferometry (ESPI; Zaslansky et al. 2005), and digital image correlation (Qian et al. 2009; Zhang et al. 2009; Appendix Table 2). Observed PDL mechanics was limited to cut sections from the dentoalveolar complex (Komatsu 1988; Chiba et al. 1990). Strain maps with photoelastic, finite element, and numerical methods are limited by assumptions of constitutive properties of tissues and their interfaces (Provatidis 1999; Cattaneo et al. 2005; Ziegler et al. 2005; Bourauel et al. 2007). Among other technique-related problems Moiré and ESPI (Wang and Weiner 1998; Wood et al. 2003; Zaslansky et al. 2005) are surface sensitive and cannot provide root-bone association unless the joint is sectioned to expose internal structures.
Multiscale Biomechanics and Mechanobiology of the Dentoalveolar Complex
Functional Loads and Biomechanics
Masticatory function in vertebrates is affected by the structure of the mandible and the efficiency of masseter, temporalis, and medial pterygoid muscles (Fig. 1). Masticatory function is also affected by the interdigitating contacts from the opposing dentition and the motion of the TMJ producing translational (vertical and horizontal) and rotational movements of the tooth about the interradicular bone and within the alveolar bony socket. Forces within the periodontal tissues arising from tooth movements within the alveolar socket are thought to have a regulatory action on the growth and remodeling of tissues within the periodontal complex (Weinmann 1955).
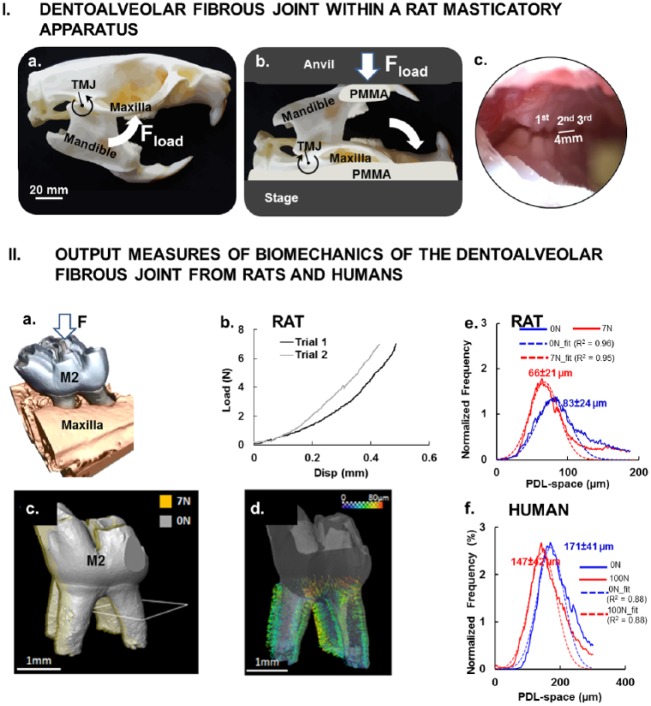
Functional loads on the fibrous joint traverse as forces on the tissues of a tooth (enamel, dentin, and cementum) and the tissues of the periodontal complex (the PDL, alveolar bone, cementum; Fig. 1). As such, mechanical testing of intact joints, as shown in Figure 2, is needed to investigate the intimate interconnected nature of these periodontal tissues that defines this continuum. Subsequent adaptive processes are not within a single tissue but multiple tissues that crosstalk and compose the fibrous joint. For this reason, a multiscale biomechanics approach with intact specimens (Fig. 2) is proposed, as adaptations within joints exist at different hierarchical length scales (Fig. 1). Adaptations within load-bearing joints are indeed a manifestation of biological events that occur at cellular and subsequently tissue levels (Fig. 1I). The link between forces on a tooth crown and physical changes in the PDL-space to biological events within the periodontal complex must be mapped in 3-dimensional (3D) space to understand the relevance of mechanically induced biological events in the context of joint biomechanics (Fig. 1). The approach highlighted in this review challenges the aforementioned limitations by using X-rays to expose the internal structures at loads equivalent to in vivo conditions on intact specimens (Figs. 2I, IIa) as described by Naveh et al. in 2012 and Lin et al. in 2013. Through the use of an in situ loading device in an X-ray computed tomography unit, load-displacement curves (Fig. 2IIb) of joints of interest under wet conditions can be obtained (Jang et al. 2014). X-ray tomography will provide 3D spatial association of an intact mineralized tooth within the alveolar bony socket (Jang et al. 2014; Fig. 2IcI, IId). Specific to the study presented in this review, mapping of the 3D tooth-bone association will allow identification of changes in the PDL-space (Fig. 2IId), which can then be labeled as widened or narrowed spaces between alveolar bone of the socket and cementum of the tooth. Additionally, in situ imaging based on X-ray computed tomography will provide multiscale imaging without disturbing the loading scheme. Imaging based on X-ray computed tomography to measure shifts in the PDL-space is noninvasive, as it can be performed on intact specimens, and generates digital 3D images of the periodontium for quantitative analyses. This eliminates the need for specimen preparation, such as reducing an intact fibrous joint into smaller pieces, as done by others. Insights into strain-induced tissue mechanobiology can be gathered by correlating X-ray computed tomography data of mineralized tissues at unloaded and loaded conditions within the realm of joint form and its function (Lin et al. 2013; Jang et al. 2015; Pal et al. 2017).
Figure 2.
Dentoalveolar fibrous joint within a rat masticatory apparatus. (I) Natural articulation was encouraged by maintaining the (a) rotational guidance about the temporomandibular joint of the masticatory complex and (b) loading (Fload) thereby interdigitation of maxillary and mandibular molars (zoomed view) (c) by loading a freshly dissected rat head with a mechanical testing device. (II) Output measures of biomechanics of the dentoalveolar fibrous joints from rats and humans. (a) Rat maxillary second molar was digitally isolated to determine the change in position of the tooth relative to the alveolar socket when loaded. (b) Load-displacement curves illustrate a nonlinear behavior of the dentoalveolar fibrous joint as the tooth is loaded and traverses into the alveolar socket of a rat. (c) Image registration of no-load (gray) and loaded (7 N, yellow) second molar of a rat allows evaluation of (d) surface distance representative of rat PDL deformation, which is illustrated by vectors (see scale bar). Histogram: distribution of PDL-space to normalized frequency at which a specific PDL-space occurs illustrates peak shifts as a result of tooth loading in rats (e) and humans (f), respectively. PDL, periodontal ligament.
In vertebrates (rats; nonhuman and human primates) under loaded conditions, tooth rotation was first described by Christiansen and Burstone (1969) and subsequently by other investigators (Lin et al. 2013). More recently, biomechanical testing in situ of a developed tooth and visualization of its association with the alveolar socket with X-ray micro–computed tomography were performed by Naveh et al. (2012) and Lin et al. (2013). Their study described the rotation of the tooth about the interradicular bone as a “screw-like” motion within the alveolar socket. This observation led to insights into the shear-dominated deformations within the PDL. Chewing loads are about 10 to 15 N in rats (Nies and Ro 2004). By comparison, occlusal loads can be 10 to 20 times more, on average, in humans (Kikuchi et al. 1997) and are recorded to be different with measurement technique, age, and sex. Regardless of the magnitude of load, mapping in situ via an experimental mechanics technique indicated a comparable net decrease in the PDL-space in respective mammalian periodontal complexes loaded to equivalent occlusal loads (20% net decrease in the PDL-space in rats loaded to 8 N vs. 14% in humans loaded to 100 N; Fig. 2IIe, IIf). These data pose the important question, in that, do net changes in respective PDL spaces of rats and humans reflect similar mechanobiological events at their respective PDL entheses?
While mapping in situ has shown tooth displacement relative to alveolar bone (Lin et al. 2013), the effect of mechanical load on PDL deformation and, consequently, the expressions of matrix-related proteins and proteases near mineral-forming and mineral-resorbing ligament entheses is unclear. Colocalization of these molecules associated with mineral formation and resorption-related events at the PDL entheses has suggested the “sculpting” of bone, cementum (McCulloch et al. 2000; Lee et al. 2015), and, subsequently, the periodontal complex, thus redefining joint biomechanics. Within the dentoalveolar fibrous joint, the normal adaptive role of the PDL entheses is amplified with the addition of external perturbations, including therapeutic loads caused by orthodontic forces or disease, as is the case with periodontitis. Shifts in mechanical strain can prompt higher incidence of inflammation at the PDL entheses. These mechanically strained regions over time become local “landscapes” as they continue to regulate cells and matrix protein expressions forming self-governing zones, and they are ultimately observed as chronically inflamed sites that undergo erratic mineral formation and resorption in alveolar bone of the socket and in cementum of the tooth root (Ho et al. 2013; Grandfield et al. 2015; see Mineral-Forming and Mineral-Resorbing Zones section).
Strained Microanatomic Locations within the Loaded Dentoalveolar Fibrous Joint
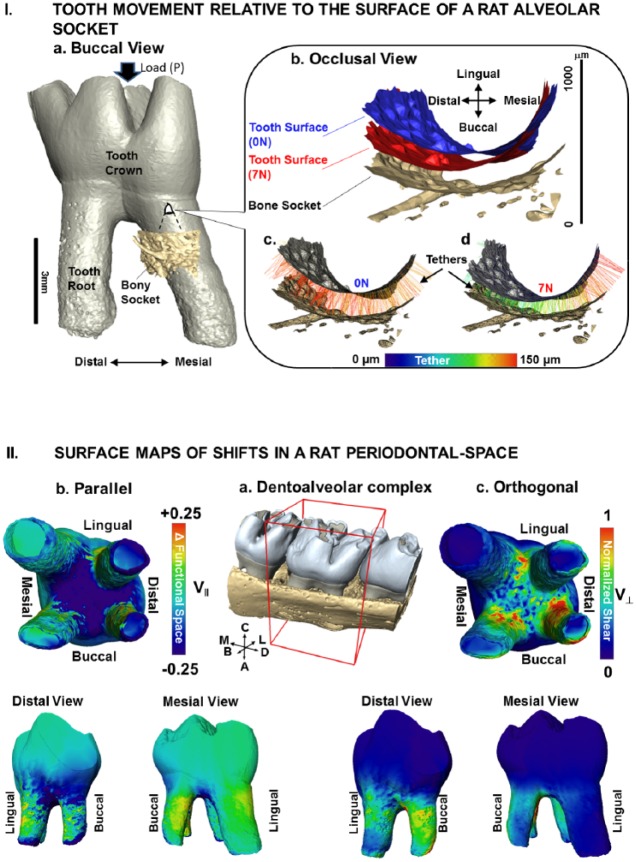
Dentoalveolar joint biomechanics is governed by anatomically specific coordinated mineral appositional growth and resorption events that are a consequence of occlusal loads on teeth. These loads continue to play a role in functional adaptation of the joint, albeit in a reduced capacity under normal physiologic conditions (Weinmann 1955). Insights into functional adaptation can be gathered by mapping PDL deformations (Fig. 3) in narrowed and widened regions of the periodontal complex and, consequently, mechanotransduction of cells (Ingber 1997, 2003). Deformations in the periodontal complex can result from tooth translation within the socket space and rotation about the interradicular bone (Christiansen and Burstone 1969; Naveh et al. 2012; Lin et al. 2013). Tooth translation and rotational movements are manifested into PDL deformations in directions parallel and orthogonal to the tooth-root surface in a 3D space (Fig. 3II; Appendix Fig.). From a conceptual standpoint, parallel effects in the defined tethers (programmed pseudo-ligament; Pal et al. 2017) are similar to “compression (narrowing, -ve)” and “tension (widening, +ve)” of the functional space (Fig. 3II-b; Appendix Fig.), while orthogonal effects are seen as “shear-induced strains” (Fig. 3II-c; Appendix Fig.) and could be related to the time-dependent nature of the PDL that is leveraged for orthodontic tooth movement (the effect of load over time to promote adequate tooth translation). The patterns found in parallel and orthogonal PDL deformations add to the current understanding of the relation between the natural direction of a tooth and the power stroke of a mastication cycle.
Figure 3.
Tooth movement relative to the surface of a rat alveolar socket. (I) (a) Volume-rendered 3-dimensional structure of a tooth relative to a portion of the alveolar bony socket. (b) A magnified region of the bony socket with positions of the tooth-root relative to the alveolar socket surface at no-load (0 N; c) and loaded (7 N; d) conditions illustrates colored tethers indicative of different lengths (see scale bar). (II) Surface maps of shifts in a rat periodontal space. (a) Surface-rendered 3-dimensional structure of molars in alveolar bone with the second molar for which V|| and V⊥ vectors were evaluated is highlighted in a box with global directions. (b) Components parallel to the tether were defined as narrowed or widened regions (compression and tension; V||) while (c) orthogonal components were defined as shear (V⊥) of the PDL and are illustrated as surface maps on the second molar. Widened PDL-space and less shear are seen in the mesial direction, while narrowing and more shear are seen in the distal direction. Global directions: A, apical; B, buccal; C, coronal; D, distal; L, lingual; M, mesial. PDL, periodontal ligament.
Across vertebrates, primary contributors to tooth motion within an alveolar socket during function are most likely the morphology of the crown cusps and the orientation of cusps and root apices. The eventual increase in shear and narrowing of the functional space separating the tooth and alveolar bone occurs primarily as parallel and/or orthogonal modes of deformations within the PDL and relative to the socket surface (Fig. 3). These PDL-related deformations eventually initiate “blastic” or “clastic” responses at the ligament entheses (Fig. 4). This synergistic behavior of blastic and clastic cellular activities at the ligament entheses (Fig. 4) is thought to maintain a uniform PDL-space within the fibrous joint (Applebaum 1947). Synergistic cellular activities in general have been linked to mesial tooth drift in humans with appositional growth (supported by the observation of stratified layers of alveolar bone in developing and functionally active complexes; Weinmann 1955) on the distal side, as compared with resorption on the mesial aspect of the complex. The biological events arising from similar types of strain across vertebrates are the same, but tooth drift between rats/mice and humans occurs in opposite directions (Schneider and Meyer 1965). This result indicates that mapping deformation attributable to compression, tension, and shear modes within tissues and along their interfaces is important in understanding mechanically induced biological events resulting in tooth drift. However, tipping of the cellular balance predominantly toward clastic or blastic function can lead to an adapted PDL-space with a net change toward compression or tension, resulting in altered directions in tooth drift (Lin et al. 2013). It is this mechanobiologically related fundamental that is also leveraged in orthodontics.
Figure 4.
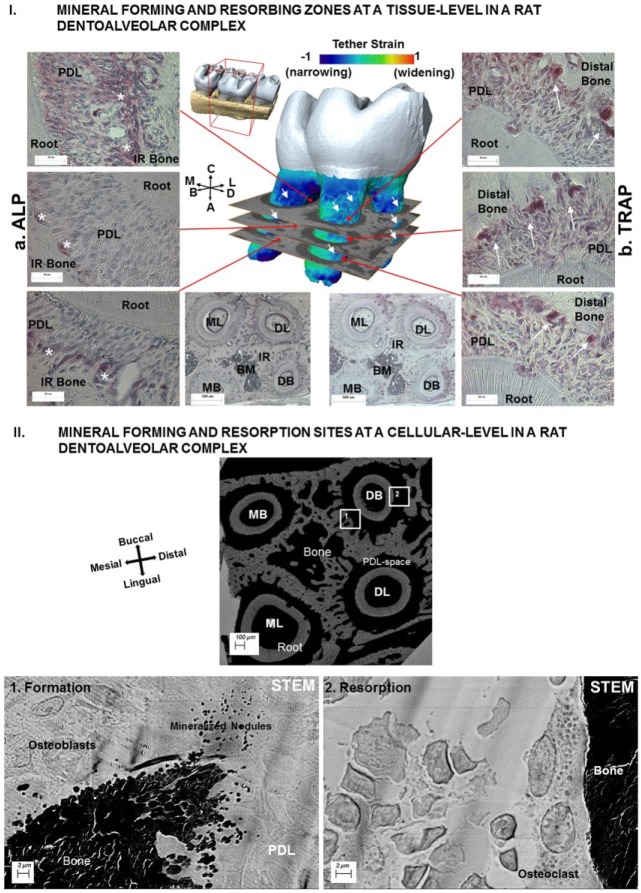
Mineral-forming and mineral-resorbing zones at a tissue level in a rat dentoalveolar complex. (I) Spatial distribution of ALP and TRAP staining is shown in left (a) and right (b) panels: (a) ALP was mainly observed at the PDL-bone enthesis in the interradicular regions and within the interradicular endosteal spaces. (b) TRAP was observed at the PDL-bone enthesis on the distal regions of the complex. (II) Mineral-forming and mineral-resorption sites at a cellular level in a rat dentoalveolar complex. Morphologic features at an ultrastructural level between mineral-forming (1) regions of the second molar containing 4 roots are shown in the STEM micrograph. Higher-resolution micrographs of regions 1 and 2 illustrate mineralized nodules and osteoblasts at the PDL-bone enthesis. In region 2 opposite to the formation site (right image), the PDL-bone enthesis was smooth, and mineralized nodules were not observed. Instead, a multinucleated cell adhered to bone surface, representing an osteoclast responsible for active resorption, was observed. ALP, alkaline phosphatase; B, buccal; D, distal; L, lingual; M, mesial; PDL, periodontal ligament; STEM, scanning transmission electron microscope; TRAP, tartrate-resistant acid phosphatase.
Mineral-Forming and Mineral-Resorbing Zones and Resulting Changes in Root-Alveolar Socket Association within the Periodontal Complex
Tissue Composition
As noted here and described by others (Hiiemae 1967), the fibrous joint is a biomechanical continuum. The dentoalveolar fibrous joint functions as a single biomechanical unit, albeit consisting of anatomically distinct tissues that include enamel, dentin, cementum, bone, and the PDL (Fig. 1). Under physiologic or pathologic loading conditions (Fig. 2), pullout forces are generated within the soft-hard tissue interfaces. Pullout forces impart a detectable strain within tissues and near the entheses (Fig. 3). Detectable strains can be correlated to biochemical expressions of cells at interfaces within the periodontal complex (Fig. 4). The shift in strain (either an abrupt or gradual shift) stimulates cells, and the ensuing extracellular output can grossly be quantified through measured and/or immunolocalized molecules (mechanobiology). Strains within softer tissues are governed by macromolecules that are elastic (e.g., collagen, elastin, fibronectin, tenascin, and laminin; Berkovitz 1990; Lukinmaa et al. 1991) and viscous (e.g., proteoglycans, noncollagenous proteins, and other constituents of ground substance bound to water; Berkovitz 1990) by nature. The viscous components contribute to time-related tissue response (e.g., creep). Viscous components such as proteoglycans also exist in mineralized tissues, including cementum and alveolar bone (McCulloch et al. 2000; Chiu et al. 2012; Kurylo et al. 2016). Calcium and phosphate minerals reinforce the proteoglycan-rich fronts of the PDL entheses of the fibrous joint (Fig. 4I), thereby strengthening the osteoid and cementoid fronts to bear loads. These observations imply a possible crosstalk among varied cell types at the PDL entheses that could shift toward expressing a net increase or decrease in organic to inorganic matrix constituents. A consequence of such a change specifically at the entheses could lead to formation and/or resorption of mineral in the 3D space of the periodontal complex, and these biological activities are constantly leveraged to promote natural drift under physiologic loading and gross tooth movement under pathologic conditions, including orthodontic loads.
Proteins, Proteases, Proteoglycans, and PDL Entheses
Mapping mechanobiological factors within microanatomic locations of the PDL entheses, as well as at a tissue level within the PDL, cementum, and bone, continues to provide insights into regeneration of soft-hard tissue interfaces. The major challenge in regenerating soft and hard tissue interfaces lies in their ability to sustain function of load-bearing joints. From a mechanics standpoint, these interfaces serve as key targets for strain concentrations (McCulloch et al. 2000; Ho et al. 2010). From a biological standpoint, these interfaces contain a higher concentration of proteoglycans and an increased vascular density needed to supply nutrients and molecules for progenitor cell migration and differentiation (Lukinmaa et al. 1991; McCulloch et al. 2000; Lee et al. 2015). Within the microenvironment of the periodontal complex, cell migration may occur through shifts in matrix stiffness (durotaxis) and chemical concentrations (hepotaxis; Discher et al. 2009). The local matrix/interstitial fluid–related pH may shift toward an alkaline or an acidic range. These shifts are indicated by the presence of alkaline phosphatase (Fig. 4Ia) or the acidic range per the presence of tartrate-resistant acid phosphatase (TRAP; Fig. 4Ib). Such changes may initiate formation or resorption of biomineral (Arnett 2008), such as apatite, at specific locations within the complex (Fig. 4II). Narrowed functional space in the distal complex may be correlated with TRAP+ cells (Fig. 4Ib). On the widened side of the joint space, the net change in PDL strain is converted into a cascade of biochemical events through transmembrane proteins (e.g., integrins) and triggers biochemical factors from cells, including molecules that can alter extracellular pH (Arnett 2008). Putative mechanosensitive proteins (e.g., bone sialoprotein and dentin matrix protein 1) and mechanosensitive genes (e.g., osterix) are also expressed (George and Veis 2008). A multitude of matrix molecules and aggregating and nonaggregating proteoglycans contributing to time-related properties (viscous component) includes biglycan and decorin (which are small leucine-rich proteoglycans), chondroitin sulfate neuroglial 2 (Chiu et al. 2012; Lee et al. 2015; Kurylo et al. 2016), osteopontin, bone sialoprotein (small integrin binding ligand N-glycoprotein, globular proteins; Grandfield et al. 2015), and various progenitors (hypertrophic cells), all of which seem to be activated specifically on the widened (mesial) side of the complex (Lee et al. 2015) when under load. On the narrowed side, the presence of TRAP is indicative of osteoclastic activity resulting in resorption pits. Close association with progenitor cells includes those within osteoid layers of the alveolar bone and within cementoid layers of the cementum. Rat and mouse models have consistently shown mineral formation in mesial-buccal widened volumes of the periodontal complex (correlate Figs. 3, 4). This observation contrasts mineral resorption in distal-lingual/buccal narrowed volumes (correlate Figs. 3, 4). It is thought that these opposing biological events in addition to the “form” of the roots regulate distal tooth drift in the commonly studied rat and mouse in vivo models (Applebaum 1947). These balanced mineral formation– and mineral resorption–related biological events have also been proposed to explain mesial tooth drift in higher mammals, such as humans (Weinmann 1955). These adaptations are necessary to meet physiologic functional demands that continue to encourage the needed distal drift in rats and mice and mesial drift of teeth in humans.
Pathologic loads, including those used for orthodontic tooth movement, can however result in differential expressions of pro- and anti-inflammatory cytokines in compressed PDL (e.g., tumor necrosis factor α, receptor activator of nuclear factor κB ligand, and matrix metalloproteinase 1—promoting osteoclastic activity resulting in mineral resorption) and stretched PDL (e.g., tissue inhibitor of metalloproteinase 1, type I collagen, osteoprotegerin, and osteocalcin—indicative of osteoblastic activity resulting in mineral formation) during orthodontic tooth movement (Garlet et al. 2007). Extrapolation from these events suggests that molecular activities at the ligament entheses play a key role in maintaining the functional space.
Conclusion
Within the topic of biomechanics and mechanobiology, this review highlights the need to investigate biology of the periodontal complex (tissue-level adaptations) within the context of force on the tooth crown (joint function). During mastication, mechanical events on the dentoalveolar fibrous joint can cause tooth translation and rotation about the interradicular bone within the alveolar socket (Fig. 5a–c). These micromotions of the tooth within the alveolar socket cause PDL deformations and pullout forces at the PDL-bone and PDL-cementum tethered/enthesial sites. These localized deformations at the PDL entheses and within the alveolar bone (Fig. 5c, d) prompt site-specific strain-amplified regions for bone and cementum mineralization- and resorption-related biological events (Fig. 5d). Tooth morphology and direction of the prolonged force (Fig. 5a; physiologic or pathologic) and the change in the PDL-space (Fig. 5e) by virtue of mineralized and resorbed volumes in the periodontal complex direct and can alter the direction and rate of tooth drift.
Figure 5.
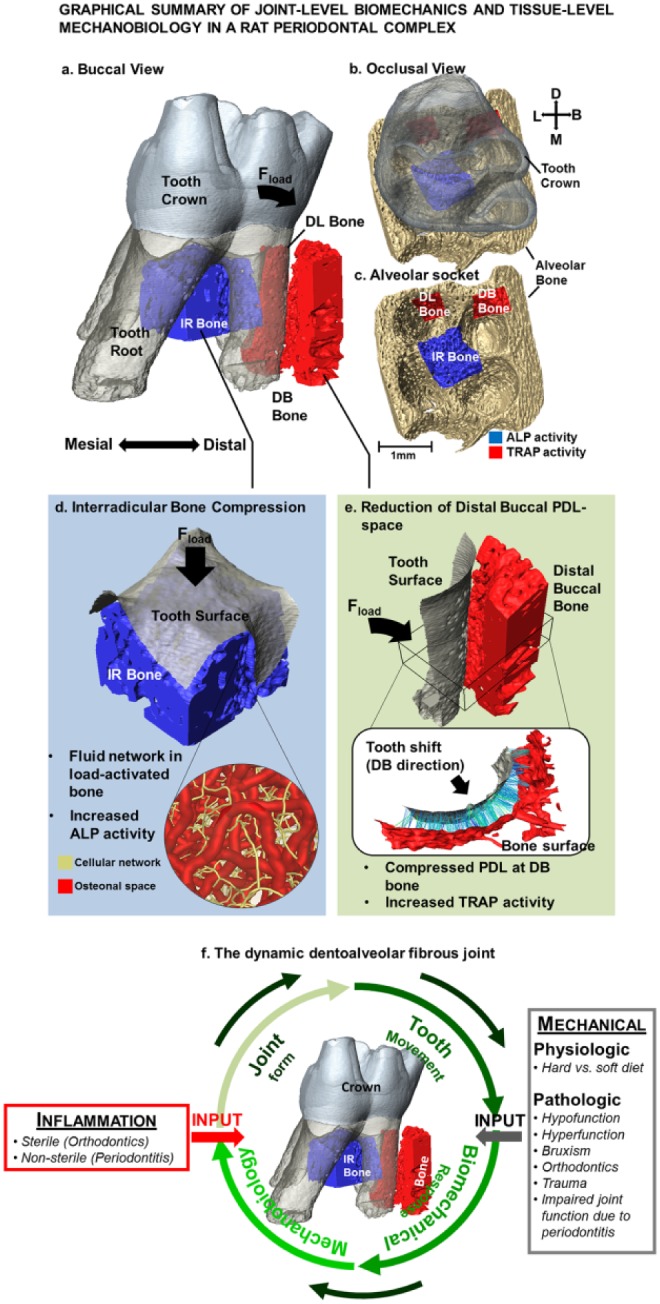
Graphic summary of joint-level biomechanics and tissue-level mechanobiology in a rat periodontal complex. (a–c) Following mechanical loading, the tooth undergoes a combination of vertical movement and a rotation in the distal-buccal direction. Consequently, the anatomy-specific affected primary regions are IR bone as well as the distal-buccal and distal-lingual roots. (d) IR bone bears majority of compression during mastication, as it first provides a reactionary force by serving as a fulcrum prompting tooth rotation. The subsequent deformation causes shifts in mechanical strain within the solid matrix of the alveolar bone and fluid flow through the osteonal and canalicular networks signaling local osteocytes and bone-lining cells to initiate mineral formation. (e) Within the distal-buccal side of the tooth, the space between the tooth and bone is narrowed causing signaling of osteoclastic activities at the PDL entheses. It is these events that aid in carving the periodontal complex to maintain natural tooth drift in case of physiologic loads or prompt altered rates of mineral resorption and formation per the demands from pathologic loads on the tooth crown. (f) Various inputs to the dynamic dentoalveolar fibrous joint. Functional loads on a tooth can be either lower or higher than the physiologic force or within the physiologic force range. These forces can prompt a need to achieve a balance (“tug of war”) between clastic and blastic activities within the periodontal complex. Regardless of force type, the periodontal complex meets the functional demands through mineral formation– and mineral resorption–related biological activities at the PDL entheses in an attempt to maintain a uniform PDL-space. Uniform PDL-space is maintained under physiologic conditions resulting from balanced clastic and blastic cellular activities. However, pathologic forces can cause aberrant PDL-space. As demonstrated in this review, the link between these physical changes in PDL-space and the biological activities at the microanatomic locations of the PDL entheses can be established by using the principles of biomechanics and mechanobiology of a load-bearing dentoalveolar complex. Encircling green arrows represent the processes involved in joint maintenance and adaptation. The red arrow represents inflammatory perturbations as induced by sterile (mechanical stimulation) and/or nonsterile (bacteria) methods and other systemic diseases related to metabolic syndromes (diabetes/hypertension) that invariably induce acerbated mechanobiological adaptation by altering the joint structure. The gray arrow represents physiologic and pathologic perturbations that can also induce strain-mediated adaptation through altered tooth movement in the actively remodeling alveolar socket. ALP, alkaline phosphatase; B, buccal; D, distal; IR, interradicular; L, lingual; M, mesial; PDL, periodontal ligament; TRAP, tartrate-resistant acid phosphatase.
Data from the highlighted in situ biomechanical simulation approach are the first to measure tooth movements relative to the alveolar socket. Mimicking the occlusion in an intact craniofacial complex has provided insights into tissue adaptations at microanatomic locations of the periodontal complex. It is recognized that the chewing behavior of rats (and humans) involves many complex patterns, which can affect docking of the tooth relative to alveolar bone. These complex patterns that relate functional tooth movement relative to alveolar bone (Christiansen and Burstone 1969; Lin et al. 2013) are difficult to model consistently in silico. The methods and subsequent results presented within this study, however, provide a reasonable approximation and highlight the need for hierarchical biomechanical experimental modeling to couple the effects of joint-level displacements to tissue-level adaptations within the periodontal complex.
Author Contributions
A.T. Jang, contributed to conception, design, and data analysis, drafted the manuscript; L. Chen, contributed to data analysis, drafted the manuscript; A.R. Shimotake, contributed to conception and design, drafted the manuscript; W. Landis, M. Ryder, contributed to conception and design, critically revised the manuscript; V. Altoe, S. Aloni, contributed to data analysis, critically revised the manuscript; S.P. Ho, contributed to conception, design, and data analysis, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
The authors thank the Biomaterials and Bioengineering Correlative Microscopy Core (http://bbcmc.ucsf.edu), University of California San Francisco, for the use of the MicroXCT-200 X-ray system and the Sigma 500 electron microscope.
Footnotes
A supplemental appendix to this article is available online.
Support was provided by the National Institutes of Health / National Institute of Dental and Craniofacial Research (R01DE02 2032; S.P.H.); the National Institutes of Health / National Center for Research Resources (S10RR026645; S.P.H.); and the Department of Preventive and Restorative Dental Sciences, School of Dentistry, University of California San Francisco. Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy (contract DE-AC02-05CH11231).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Applebaum E. 1947. Development of rat molar crowns and jaws. J Dent Res. 26(1):73–78. [DOI] [PubMed] [Google Scholar]
- Arnett TR. 2008. Extracellular pH regulates bone cell function. J Nutr. 138(2):415S–418S. [DOI] [PubMed] [Google Scholar]
- Asundi A, Kishen A. 2001. Advanced digital photoelastic investigations on the tooth-bone interface. J Biomed Opt. 6(2):224–230. [DOI] [PubMed] [Google Scholar]
- Berkovitz BK. 1990. The structure of the periodontal ligament: an update. Eur J Orthod. 12(1):51–76. [DOI] [PubMed] [Google Scholar]
- Bourauel C, Keilig L, Rahimi A, Reimann S, Ziegler A, Jager A. 2007. Computer-aided analysis of the biomechanics of tooth movements. Int J Comput Dent. 10(1):25–40. [PubMed] [Google Scholar]
- Carter DR, Beaupre GS, Giori NJ, Helms JA. 1998. Mechanobiology of skeletal regeneration. Clin Orthop Relat Res. 355:S41–S55. [DOI] [PubMed] [Google Scholar]
- Cate T, Nanci A. 2013. Ten Cate’s—oral histology: development, structure, and function. St. Louis, MO: Elsevier. [Google Scholar]
- Cattaneo PM, Dalstra M, Melsen B. 2005. The finite element method: a tool to study orthodontic tooth movement. J Dent Res. 84(5):428–433. [DOI] [PubMed] [Google Scholar]
- Chiba M, Yamane A, Ohshima S, Komatsu K. 1990. In vitro measurement of regional differences in the mechanical properties of the periodontal ligament in the rat mandibular incisor. Arch Oral Biol. 35(2):153–161. [DOI] [PubMed] [Google Scholar]
- Chiu R, Li W, Herber RP, Marshall SJ, Young M, Ho SP. 2012. Effects of biglycan on physico-chemical properties of ligament-mineralized tissue attachment sites. Arch Oral Biol. 57(2):177–187. [DOI] [PubMed] [Google Scholar]
- Christiansen RL, Burstone CJ. 1969. Centers of rotation within the periodontal space. Am J Orthod. 55(4):353–369. [DOI] [PubMed] [Google Scholar]
- Daegling DJ, Ravosa MJ, Johnson KR, Hylander WL. 1992. Influence of teeth, alveoli, and periodontal ligaments on torsional rigidity in human mandibles. Am J Phys Anthropol. 89(1):59–72. [DOI] [PubMed] [Google Scholar]
- Discher D, Dong C, Fredberg JJ, Guilak F, Ingber D, Janmey P, Kamm RD, Schmid-Schonbein GW, Weinbaum S. 2009. Biomechanics: cell research and applications for the next decade. Ann Biomed Eng. 37(5):847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlet TP, Coelho U, Silva JS, Garlet GP. 2007. Cytokine expression pattern in compression and tension sides of the periodontal ligament during orthodontic tooth movement in humans. Eur J Oral Sci. 115(5):355–362. [DOI] [PubMed] [Google Scholar]
- George A, Veis A. 2008. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem Rev. 108(11):4670–4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandfield K, Herber RP, Chen L, Djomehri S, Tam C, Lee JH, Brown E, Woolwine WR, 3rd, Curtis D, Ryder M, et al. 2015. Strain-guided mineralization in the bone-PDL-cementum complex of a rat periodontium. Bone Rep. 3:20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmon WW. 1999. Occlusal trauma: effect and impact on the periodontium. Ann Periodontol. 4(1):102–108. [DOI] [PubMed] [Google Scholar]
- Herring SW. 1976. The dynamics of mastication in pigs. Arch Oral Biol. 21(8):473–480. [DOI] [PubMed] [Google Scholar]
- Hiiemae KM. 1967. Masticatory function in the mammals. J Dent Res. 46(5):883–893. [DOI] [PubMed] [Google Scholar]
- Ho SP, Kurylo MP, Fong TK, Lee SS, Wagner HD, Ryder MI, Marshall GW. 2010. The biomechanical characteristics of the bone–periodontal ligament–cementum complex. Biomaterials. 31(25):6635–6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SP, Kurylo MP, Grandfield K, Hurng J, Herber RP, Ryder MI, Altoe V, Aloni S, Feng JQ, Webb S, et al. 2013. The plastic nature of the human bone–periodontal ligament–tooth fibrous joint. Bone. 57(2):455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylander WL. 1979. The functional significance of primate mandibular form. J Morphol. 160(2):223–240. [DOI] [PubMed] [Google Scholar]
- Ingber DE. 1997. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 59:575–599. [DOI] [PubMed] [Google Scholar]
- Ingber DE. 2003. Tensegrity II: how structural networks influence cellular information processing networks. J Cell Sci. 116(Pt 8):1397–1408. [DOI] [PubMed] [Google Scholar]
- Jang AT, Lin JD, Seo Y, Etchin S, Merkle A, Fahey K, Ho SP. 2014. In situ compressive loading and correlative noninvasive imaging of the bone–periodontal ligament–tooth fibrous joint. J Vis Exp. 85. doi: 10.3791/51147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang AT, Merkle A, Fahey K, Gansky SA, Ho SP. 2015. Multiscale biomechanical responses of adapted bone–periodontal ligament–tooth fibrous joints. Bone. 81:196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang IG, Kim IY, Kwak BB. 2009. Analogy of strain energy density based bone-remodeling algorithm and structural topology optimization. J Biomech Eng. 131(1):011012. [DOI] [PubMed] [Google Scholar]
- Jantarat J, Palamara JE, Messer HH. 2001. An investigation of cuspal deformation and delayed recovery after occlusal loading. J Dent. 29(5):363–370. [DOI] [PubMed] [Google Scholar]
- Kikuchi M, Korioth TW, Hannam AG. 1997. The association among occlusal contacts, clenching effort, and bite force distribution in man. J Dent Res. 76(6):1316–1325. [DOI] [PubMed] [Google Scholar]
- Kiliaridis S. 1986. a. Masticatory muscle function and craniofacial morphology: an experimental study in the growing rat fed a soft diet. Swed Dent J Suppl. 36:1–55. [PubMed] [Google Scholar]
- Kiliaridis S. 1986. b. The relationship between masticatory function and craniofacial morphology: III. The eruption pattern of the incisors in the growing rat fed a soft diet. Eur J Orthod. 8(2):71–79. [DOI] [PubMed] [Google Scholar]
- Koivumaa KK, Mäkilä E, Honka O. 1971. Histological changes in human periodontium of teeth in masticatory hyper- and hypofunction. Suom Hammaslaak Toim. 67(2):122–136. [PubMed] [Google Scholar]
- Komatsu K. 1988. In vitro mechanics of the periodontal ligament in impeded and unimpeded rat mandibular incisors. Arch Oral Biol. 33(11):783–791. [DOI] [PubMed] [Google Scholar]
- Krishnan V. 2005. Critical issues concerning root resorption: a contemporary review. World J Orthod. 6(1):30–40. [PubMed] [Google Scholar]
- Krishnan V, Davidovitch Z. 2009. On a path to unfolding the biological mechanisms of orthodontic tooth movement. J Dent Res. 88(7):597–608. [DOI] [PubMed] [Google Scholar]
- Kurylo MP, Grandfield K, Marshall GW, Altoe V, Aloni S, Ho SP. 2016. Effect of proteoglycans at interfaces as related to location, architecture, and mechanical cues. Arch Oral Biol. 63:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Pryce BA, Schweitzer R, Ryder MI, Ho SP. 2015. Differentiating zones at periodontal ligament–bone and periodontal ligament–cementum entheses. J Periodontal Res. 50(6):870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JD, Ozcoban H, Greene JP, Jang AT, Djomehri SI, Fahey KP, Hunter LL, Schneider GA, Ho SP. 2013. Biomechanics of a bone–periodontal ligament–tooth fibrous joint. J Biomech. 46(3):443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukinmaa PL, Mackie EJ, Thesleff I. 1991. Immunohistochemical localization of the matrix glycoproteins—tenascin and the ED-sequence-containing form of cellular fibronectin—in human permanent teeth and periodontal ligament. J Dent Res. 70(1):19–26. [DOI] [PubMed] [Google Scholar]
- Margvelashvili A, Zollikofer CP, Lordkipanidze D, Peltomäki T, Ponce de, León MS. 2013. Tooth wear and dentoalveolar remodeling are key factors of morphological variation in the Dmanisi mandibles. Proc Natl Acad Sci U S A. 110(43):17278–17283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch CA, Lekic P, McKee MD. 2000. Role of physical forces in regulating the form and function of the periodontal ligament. Periodontol 2000. 24:56–72. [DOI] [PubMed] [Google Scholar]
- Naveh GR, Shahar R, Brumfeld V, Weiner S. 2012. Tooth movements are guided by specific contact areas between the tooth root and the jaw bone: a dynamic 3D microCT study of the rat molar. J Struct Biol. 177(2):477–483. [DOI] [PubMed] [Google Scholar]
- Nies M, Ro JY. 2004. Bite force measurement in awake rats. Brain Res Brain Res Protoc. 12(3):180–185. [DOI] [PubMed] [Google Scholar]
- Pal A, Chen L, Yang L, Yang F, Meng B, Jheon AH, Ho SP. 2017. Micro-anatomical responses in the periodontal complex to calibrated orthodontics forces on the crown. Orthod Craniofac Res. 20 Suppl 1:100–105. [DOI] [PubMed] [Google Scholar]
- Popowics TE, Rensberger JM, Herring SW. 2004. Enamel microstructure and microstrain in the fracture of human and pig molar cusps. Arch Oral Biol. 49(8):595–605. [DOI] [PubMed] [Google Scholar]
- Provatidis CG. 1999. Numerical estimation of the centres of rotation and resistance in orthodontic tooth movement. Comput Methods Biomech Biomed Engin. 2(2):149–156. [DOI] [PubMed] [Google Scholar]
- Qian L, Todo M, Morita Y, Matsushita Y, Koyano K. 2009. Deformation analysis of the periodontium considering the viscoelasticity of the periodontal ligament. Dent Mater. 25(10):1285–1292. [DOI] [PubMed] [Google Scholar]
- Schneider BJ, Meyer J. 1965. Experimental studies on the interrelations of condylar growth and alveolar bone formation. Angle Orthod. 35:187–199. [DOI] [PubMed] [Google Scholar]
- Thomas NR, Peyton SC. 1983. An electromyographic study of mastication in the freely-moving rat. Arch Oral Biol. 28:939–945. [DOI] [PubMed] [Google Scholar]
- Tomes CS. 1882. A manual of dental anatomy human and comparative. London (UK): J & A Churchill. [Google Scholar]
- Turner CH, Pavalko FM. 1998. Mechanotransduction and functional response of the skeleton to physical stress: the mechanisms and mechanics of bone adaptation. J Orthop Sci. 3(6):346–355. [DOI] [PubMed] [Google Scholar]
- Vinyard C, Ravosa MJ, Christine W. 2008. Primate craniofacial function and biology. In: Tuttle RH. editor. Developments in primatology: progress and prospects. New York (NY): Springer Science + Business Media, LLC. [Google Scholar]
- Wang RZ, Weiner S. 1998. Strain-structure relations in human teeth using moire fringes. J Biomech. 31(2):135–141. [DOI] [PubMed] [Google Scholar]
- Weinmann JP. 1955. Bone formation and bone resorption. Oral Surg Oral Med Oral Pathol. 8(10):1074–1078. [DOI] [PubMed] [Google Scholar]
- Wood JD, Wang R, Weiner S, Pashley DH. 2003. Mapping of tooth deformation caused by moisture change using moire interferometry. Dent Mater. 19(3):159–166. [DOI] [PubMed] [Google Scholar]
- Yamamoto S. 1996. The effects of food consistency on maxillary growth in rats. Eur J Orthod. 18(6):601–615. [DOI] [PubMed] [Google Scholar]
- Zaslansky P, Currey JD, Friesem AA, Weiner S. 2005. Phase shifting speckle interferometry for determination of strain and Young’s modulus of mineralized biological materials: a study of tooth dentin compression in water. J Biomed Opt. 10(2):024020. [DOI] [PubMed] [Google Scholar]
- Zhang D, Mao S, Lu C, Romberg E, Arola D. 2009. Dehydration and the dynamic dimensional changes within dentin and enamel. Dent Mater. 25(7):937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler A, Keilig L, Kawarizadeh A, Jager A, Bourauel C. 2005. Numerical simulation of the biomechanical behaviour of multi-rooted teeth. Eur J Orthod. 27(4):333–339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.