Abstract
Radial artery thrombosis is a rare complication of cannulation. There are no reported cases of acute thrombosis and severe acute neuropathy in the setting of cannula discontinuation. We report a case of acute radial nerve mono-neuropathy following thrombosis after radial arterial line removal. The thrombus was immediately evident on exam and diagnostic imaging after cannula discontinuation. The patient was consented and promptly taken to OR for immediate repair. Mild radial neuropathy persisted despite immediate repair. Immediate recognition of signs and symptoms is essential for diagnosis and management, especially in the high-risk population.
Keywords: Radial artery, thrombosis, radial neuropathy, radial artery line
Introduction
Radial artery cannulation is a reliable and safe method for continuous hemodynamic monitoring in the intensive care setting.1 However, severe cases of radial artery thrombosis can cause ischemic loss of limb.2–4 In rare occasions when flow is restored, ischemic neuropathy can persist. We present a case of acute radial artery thrombosis and radial neuropathy after discontinuation of cannula. Informed patient consent was obtained for publication of this case report including images.
Case presentation
A 65-year-old male was referred to our center for treatment of severe coronary artery disease. The patient had a medical history significant for extensive tobacco use, diabetes, hypertension, and peripheral arterial disease requiring an aorto-bi-femoral bypass, lower extremity bypass, and left carotid endarterectomy. He reported having intermittent right upper extremity numbness for over 5 years without weakness. After completion of the preoperative cardiac surgery evaluation, the patient underwent coronary artery bypass using his left internal mammary artery in addition to a saphenous vein graft to re-perfuse his left anterior descending and obtuse marginal coronary arteries, respectively. Prior to his operation, a left radial artery cannula was placed by an experienced anesthesiology staff for close hemodynamic monitoring. A 10 cm 20-gauge arterial cannula was placed under ultrasound guidance after the first attempt. There were no intra-operative issues with the arterial cannula, and it remained in place when the patient was transferred to the intensive care unit for recovery. On postoperative day 1, arterial cannula was discontinued, by first disconnecting the IV from pressure bag followed by immediate removal of the cannula and non-occlusive digital pressure for 5 min. The patient immediately complained of left-hand numbness and weakness. On examination, he had loss of left radial artery pulse and poikilothermia. Noninvasive vascular studies showed occlusion of the left radial artery, proximal peak systolic velocity (PSV) of 10 cm/s, and no distal flow at the wrist with absence of digital pressures (Figure 1). There was a patent ulnar artery feeding the palmar arch and no other clinically significant lesions in the subclavian, axillary, or brachial arteries. After obtaining surgical consent, an emergent cut-down and open thrombo-embolectomy under local anesthesia and sedation was performed within 2 hours of initial consultation. A 5 cm fresh thrombus was removed via an arteriotomy and several passes of fogarty balloons. We elected to repair the arteriotomy with a bovine patch angioplasty. Although vein patch angioplasty might have been a better option, both lower extremity saphenous veins were used in previous vascular and cardiac operations and the patient did not have adequate upper extremity veins. Postoperatively, the patient had return of his left radial pulse; however, he had persistent weak left wrist extension, finger extension at the metacarpophalangeal joint, and thumb extension which were graded as 2/5 on the Medical Research Council (MRC) scale.5–7 Thumb range of motion, finger and wrist flexion, hand grasp were all 4/5, as well as an intact forearm supination and pronation (Figure 2). Sensation to pinprick and light touch in the left hand was intact. The examination was consistent with involvement of the deep branch of the radial nerve in the forearm and the posterior interosseous nerve.
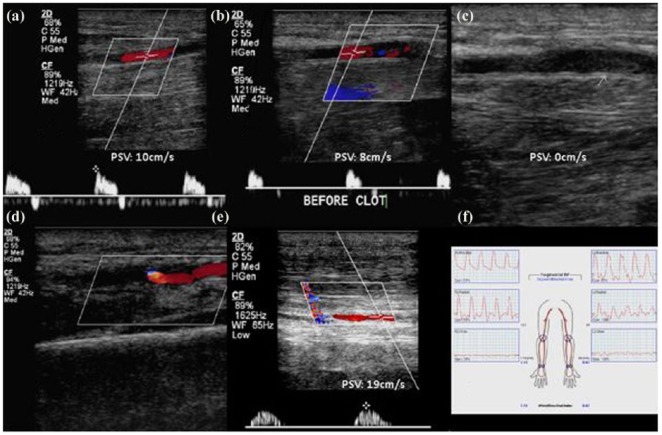
Figure 1.
Duplex ultrasound and Doppler evaluation of radial artery. Preoperative Doppler evaluation demonstrated normal flow in proximal and mid radial artery (a). Mid distal flow is decreased (b) and stops at thrombus (c). Radial artery beyond the wrist is fed through collaterals (d). Ipsilateral ulnar artery had normal flow (e); however, segmental pressure (f) demonstrated decreased wrist brachial index.
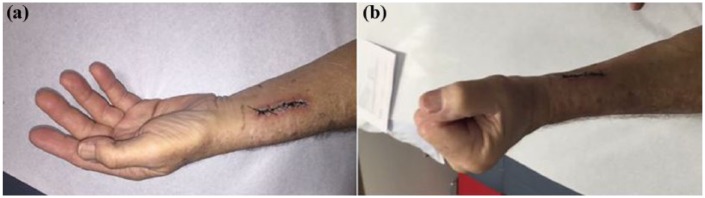
Figure 2.
Neurological exam of left-hand post repair. At 2-week postoperative follow-up, the wound is well healed (a). The patient still has weak wrist extension, finger extension at the metacarpophalangeal joint, and thumb extension (2/5 MRC) (b). Thumb adduction, abduction and flexion, finger, and wrist flexion were 4+/5, grasp +4/5, and forearm supination and pronation were intact. Sensation to pinprick and light touch in left hand was intact.
Discussion
Continuous hemodynamic monitoring in critically ill patients can be safely and effectively performed through arterial cannulation. Rates of complications, specifically arterial thrombosis, have been variable and reported to range from 10% to 40% in different case series.1,3,4 Some reports have suggested that smaller gauge cannula can decrease the risk for radial artery thrombosis; however, they may provide lower quality signals.8,9 Management of this complication is dependent on the patient’s symptoms and severity of the underlying comorbidities. As such, traumatic radial artery thrombosis has been treated with several modalities including thrombolysis, anticoagulation, open repair, or endovascular therapy.10
In the case of our patient, he underwent preoperative screening utilizing the Allen11,12 bedside exam and the procedure was uncomplicated with a single needle pass under ultrasound guidance. We believe that during the cannulation process, there was a traumatic dissection flap that remained stented while the arterial cannula was in place. Upon removal of the cannula with digital compression to prevent a hematoma or pseudoaneurysm, the distal radial artery thrombosed. Due to his recent cardiac surgery, endovascular lysis therapy would have been high risk of re-bleeding, as such we opted for open exploration and repair under local anesthesia and sedation. The patient’s hand was warm with resolution of numbness and pain postoperatively; however, he continued to complain of weakness.
Although radial artery thrombosis after cannulation is not an uncommon complication, persistent radial neuropathy after reperfusion is not commonly reported. To our knowledge, this is the first case reported in the literature of radial artery thrombosis after removal of an arterial cannula. It is also the first case that the arterial thrombosis is associated with a severe and acute onset radial neuropathy. Data on radial artery occlusion and associated neuropathies mainly come from larger cardiac surgery series. These series report long-term sensory neuropathies after harvesting radial arteries for coronary artery bypasses without any significant motor symptoms.8,12 These events are also well documented in patients with Arterio-Venous (AV) fistulas causing ischemic steal syndrome. Steal syndrome develops in 2%–20% of patients with AV fistulas and presents with paresthesia, pain, hand stiffness and pallor, and diminished sensation on physical examination.13 Radial nerve sensory and motor neuropathy following arterial line removal is an extremely rare complication and the suggested mechanism is embolic occlusion of the distal radial artery and its branches leading to ischemia. Ischemic neuropathies can occur in patients who have risk factors for vascular disease such as diabetes mellitus, hypertension, obesity, and hyperlipidemia, as is the case of our patient.
In retrospect, the patient did have a normal Allen test performed by anesthesia professional prior to cannulation. This, however, did not predict ulnar artery adequate blood supply to the affect hand. In addition to the Allen test, the Barbeau test14 can better predict palmar flow and anticipate symptomatic complications in cases of radial artery injury. It is essential to emphasize that both of these exams do not prevent possible complications. In high-risk populations, such as our patient, vigilant monitoring is key for early identification and management of arterial injury.
Conclusion
Radial artery cannulation is a routine procedure performed in critical care settings and complications can occur as a consequence. Bedside evaluation of upper extremity ulnopalmar flow allows providers to predict risk of symptomatic complications if radial artery injury were to occur. However, it does not predict occurrence of the complication itself. In addition to limb loss, a very rare complications of radial artery occlusion includes focal sensory and motor neuropathies. Patients with extensive comorbidities for peripheral vascular disease may be at increased risk for such complications. As is the case in this scenario, vigilant monitoring led to early detection of complication and immediate intervention for revascularization.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: Ali Khalifeh  https://orcid.org/0000-0003-1420-2669
https://orcid.org/0000-0003-1420-2669
References
- 1. Martin C, Saux P, Papazian L, et al. Long-term arterial cannulation in ICU patients using the radial artery or dorsalis pedis artery. Chest 2001; 119(3): 901–906. [DOI] [PubMed] [Google Scholar]
- 2. Türker T, Capdarest-Arest N. Acute hand ischemia after radial artery cannulation resulting in amputation. Chir Main 2014; 33(4): 299–302. [DOI] [PubMed] [Google Scholar]
- 3. Wilkins RG. Radial artery cannulation and ischaemic damage: a review. Anaesthesia 1985; 40(9): 896–899. [DOI] [PubMed] [Google Scholar]
- 4. Valentine RJ, Modrall JG, Clagett GP. Hand ischemia after radial artery cannulation. J Am Coll Surg 2005; 201(1): 18–22. [DOI] [PubMed] [Google Scholar]
- 5. Bove GM. Epi-perineurial anatomy, innervation, and axonal nociceptive mechanisms. J Bodyw Mov Ther 2008; 12(3): 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Medical Research Council. Aids to examination of the peripheral nervous system. Memorandum no. 45. London: Her Majesty’s Stationary Office, 1976, https://www.mrc.ac.uk/documents/pdf/aids-to-the-examination-of-the-peripheral-nervous-system-mrc-memorandum-no-45-superseding-war-memorandum-no-7/ [Google Scholar]
- 7. Paternostro-Sluga T, Grim-Stieger M, Posch M, et al. Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J Rehabil Med 2008; 40(8): 665–671. [DOI] [PubMed] [Google Scholar]
- 8. Ikizler M, Ozkan S, Dernek S, et al. Does radial artery harvesting for coronary revascularization cause neurological injury in the forearm and hand? Eur J Cardiothoracic Surg 2005; 28(3): 420–424. [DOI] [PubMed] [Google Scholar]
- 9. Saito S, Ikei H, Hosokawa G, et al. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Catheter Cardiovasc Interv 1999; 46(2): 173–178. [DOI] [PubMed] [Google Scholar]
- 10. Goswami R, Oliphant CS, Youssef H, et al. Radial artery occlusion after cardiac catheterization: significance, risk factors, and management. Curr Probl Cardiol 2016; 41(6): 214–227. [DOI] [PubMed] [Google Scholar]
- 11. WHO. Best practices in phlebotomy, 2010, http://www.euro.who.int/__data/assets/pdf_file/0005/268790/WHO-guidelines-on-drawing-blood-best-practices-in-phlebotomy-Eng.pdf; http://www.ncbi.nlm.nih.gov/pubmed/23741774
- 12. Allen RH, Szabo RM, Chen JL. Outcome assessment of hand function after radial artery harvesting for coronary artery bypass. J Hand Surg Am 2004; 29(4): 628–637. [DOI] [PubMed] [Google Scholar]
- 13. Suding PN, Wilson SE. Strategies for management of ischemic steal syndrome. Semin Vasc Surg 2007; 20(3): 184–188. [DOI] [PubMed] [Google Scholar]
- 14. Barbeau GR, Arsenault F, Dugas L, et al. Evaluation of the ulnopalmar arterial arches with pulse oximetry and plethysmography: comparison with the Allen’s test in 1010 patients. Am Heart J 2004; 147(3): 489–493. [DOI] [PubMed] [Google Scholar]