Abstract
Background:
One of the mandates of the Canadian Society of Nephrology’s (CSN) Vascular Access Working Group (VAWG) is to inform the nephrology community of the current status of vascular access (VA) practice within Canada. To better understand VA practice patterns across Canada, the CSN VAWG conducted a national survey.
Objectives:
(1) To inform on VA practice patterns, including fistula creation and maintenance, within Canada. (2) To determine the degree of consensus among Canadian clinicians regarding patient suitability for fistula creation and to assess barriers to and facilitators of fistula creation in Canada.
Design:
Development and implementation of a survey.
Setting:
Community and academic VA programs.
Participants:
Nephrologists, surgeons, and nurses who are involved in VA programs across Canada.
Measurements:
Practice patterns regarding access creation and maintenance, including indications and contraindications to fistula creation, as well as program-wide facilitators of and barriers to VA.
Methods:
A small group of CSN VAWG members determined the scope and created several VA questions which were then reviewed by 5 additional VAWG members (4 nephrologists and 1 VA nurse) to ensure that questions were clear and relevant. The survey was then tested by the remaining members of the VAWG and refinements were made. The final survey version was submitted electronically to relevant clinicians (nephrologists, surgeons, and nurses) involved or interested in VA across Canada. Questions centered around 4 major themes: (1) Practice patterns regarding access creation (preoperative assessment and maturation assessment), (2) Practice patterns regarding access maintenance (surveillance and salvage), (3) Indications and contraindications for arteriovenous (AV) access creation, and (4) Facilitators of and barriers to fistula creation and utilization.
Results:
Eighty-two percent (84 of 102) of invited participants completed the survey; the majority were nurses or VA coordinators (55%) with the remainder consisting of nephrologists (21%) and surgeons (20%). Variation in practice was noted in utility of preoperative Doppler ultrasound, interventions to assist nonmaturing fistulas, and procedures to salvage failing or thrombosed AV-access. Little consensus was seen regarding potential contraindications to AV-access creation (with the exception of limited life expectancy and poor vasculature on preoperative imaging, which had high agreement). Frequent barriers to fistula utilization were primary failure (77% of respondents) and long maturation times (73%). Respondents from centers with low fistula prevalence also cited long surgical wait times as an important barrier to fistula creation, whereas those from centers with high fistula prevalence cited access to multidisciplinary teams and interventional radiology as keys to successful fistula creation and utilization.
Conclusions:
There is significant variation in VA practice across Canada and little consensus among Canadian clinicians regarding contraindications to fistula creation. Further high-quality studies are needed with regard to appropriate fistula placement to help guide clinical practice.
Keywords: hemodialysis, vascular access, arteriovenous fistula
Abrégé
Contexte:
L’un des mandats du Groupe de travail en accès vasculaire (GTAV) de la Société canadienne de néphrologie consiste à informer la communauté en néphrologie des schémas de pratique actuels en accès vasculaire au Canada. Le GTAV a mené un sondage pancanadien pour mieux comprendre les schémas de pratique existants au pays en matière d’accès vasculaire.
Objectifs:
(1) Informer la communauté des schémas de pratique canadiens (notamment en ce qui a trait à la création et au maintien fistulaire). (2) Déterminer le niveau de consensus parmi les cliniciens canadiens concernant l’admissibilité d’un patient à la création d’une fistule et les facteurs facilitant ou entravant la procédure.
Type d’étude:
Il s’agit de la conception et de la réalisation d’un sondage.
Cadre de l’étude:
Programmes d’accès vasculaire en milieu universitaire ou communautaire.
Participants:
Ont été invités à participer néphrologues, chirurgiens et membres du personnel infirmier intervenant dans un programme d’accès vasculaire canadien.
Mesures:
Nous avons sondé les participants à propos de leur schéma de pratique concernant la création et l’entretien fistulaire, notamment les indications et contre-indications à la création d’une fistule, ainsi que des facteurs facilitant ou entravant la procédure dans leur milieu de pratique.
Méthodologie:
Quelques membres du GTAV ont défini le cadre du sondage et ont rédigé une série de questions. Cinq autres membres du GTAV (quatre néphrologues et un membre du personnel infirmier en accès vasculaire) ont ensuite validé la clarté et la pertinence des questions soumises. Finalement, le sondage a été testé auprès des autres membres du GTAV pour y apporter des ajustements. La version définitive du sondage a été envoyée électroniquement à des cliniciens (néphrologues, chirurgiens et membres du personnel infirmier) canadiens intervenant en accès vasculaire ou qui s’y intéressent. Les questions abordaient quatre thèmes : i) les schémas de pratique en création fistulaire (évaluation préopératoire, évaluation de la maturation fistulaire); ii) les schémas de pratique en entretien fistulaire (surveillance et rétablissement de l’accès vasculaire); iii) les indications et contre-indications à la création d’une fistule artérioveineuse; iv) les facteurs facilitant et entravant la création fistulaire et son utilisation.
Résultats:
Des 102 personnes invitées à participer au sondage, 84 (82 %) ont répondu au questionnaire. La majorité (55 %) était constituée de membres du personnel infirmier et de coordonnateurs en accès vasculaire. La différence se composait essentiellement de néphrologues (21 %) et de chirurgiens (20 %). On a noté une variabilité des habitudes de pratique quant au recours à une échographie Doppler en préopératoire, aux interventions en cas de fistules non formées et aux procédures de rétablissement d’un accès artériovasculaire défaillant ou thrombosé. Il n’existe pas de consensus sur les éventuelles contre-indications à la création d’un accès artériovasculaire, à l’exception de deux points : une espérance de vie limitée et une structure vasculaire faible (révélée par imagerie préopératoire). La défaillance primitive et un long délai de maturation ont été cernés comme obstacles au recours à la fistule par une majorité de répondants (77 % et 73 %, respectivement). Les répondants de centres où l’on pratique peu d’interventions fistulaires ont mentionné les longs délais d’attente préopératoire comme entrave; les répondants de centres où l’on pratique fréquemment l’intervention fistulaire ont quant à eux souligné deux facteurs facilitant la création fistulaire et son utilisation : l’accès à des équipes multidisciplinaires et à la radiologie interventionnelle.
Conclusion:
Au Canada, les schémas de pratique clinique en accès vasculaire varient fortement, et les indications et contre-indications à la création fistulaire ne font pas consensus au sein des cliniciens. D’autres études rigoureuses sur les conditions adéquates pour la pratique d’une intervention fistulaire sont nécessaires afin d’orienter la pratique clinique.
What was known before
Canada has one of the lowest fistula prevalence of any Dialysis Outcomes and Practice Patterns Study (DOPPS)-contributing nation. In addition, there is regional variation in fistula usage across Canada.
What this adds
Our survey highlights the significant variation that exists in practice across Canada and emphasizes the need for high-quality studies in the field of vascular access to help determine best practices.
Introduction
An important aspect of every hemodialysis program is ensuring that dialysis patients have a functional and reliable form of vascular access (VA). Careful consideration is necessary when selecting a suitable access for each patient, as complications arising from VA can result in considerable morbidity.1 Clinical practice guidelines, including those from the Canadian Society of Nephrology (CSN), advocate for arteriovenous fistulas (AVFs) as the VA of choice; this recommendation is based on observational data reporting that fistulas are associated with fewer infections, hospitalizations, interventions, and lower mortality.2-5 As such, there has been a global effort to increase the proportion of AVFs in hemodialysis patients over the last 2 decades. Despite these efforts, Canada has one of the lowest prevalence of fistulas of any Dialysis Outcomes and Practice Patterns Study (DOPPS)-contributing nation, and the proportion of patients using a central venous catheter (CVC) in Canada continues to increase.6,7 Furthermore, within Canada, there is a wide variation in AVF and CVC use across centers.7
The CSN Vascular Access Working Group (VAWG) is comprised of clinicians and decision makers in the field of VA with representation from each major center or region in Canada. The purpose of the VAWG is to inform the Canadian nephrology community on the national status of VA practice and to promote VA education. One of the goals of the VAWG is to increase the number of functioning fistulas in suitable hemodialysis patients. To achieve this goal, an understanding of current VA practice related to fistulas is required.
Here, we report on a nationwide survey of VA practice patterns across Canada. The main purpose of the survey was to identify variation in VA practice and in opinions regarding indications, contraindications, and barriers to AVF creation.
Methods
The survey (Online Appendix 1) was developed by the CSN VAWG. Working group members were asked to create questions which centered around 4 major themes: (1) Practice patterns regarding access creation (preoperative assessment and maturation assessment), (2) Practice patterns regarding access maintenance (surveillance and salvage), (3) Indications and contraindications for AVF/AVG (arteriovenous graft) creation, and (4) Facilitators of and barriers to AVF creation and utilization. A draft survey was created by a core group of VAWG members (J.M., M.K., Lisa Miller, M.J.O., C.E.L., Louise Moist) and then reviewed by 5 additional VAWG members (4 nephrologists and 1 VA nurse) to ensure that the questions were clear and that they captured the relevant content. The survey was then sent to the remaining members of the VAWG to test the survey and provide further suggestions. Refinements were made by the core group to create the final survey version, and it was submitted electronically to participants. Participants (VA coordinators, access surgeons, and nephrologists) were identified by members of the VAWG on the basis of their involvement in VA programs across Canada. The survey was distributed with an initial email link in February 2013 and a follow-up email link in April of 2013. A research ethics board review was not required for implementation of the survey as participation in the survey was considered implied consent for reporting of data. Responses were recorded in Microsoft Excel Version 15.11.2. Demographic information and practice pattern data were analyzed and reported for all respondents. Surveys that were less than 95% complete were excluded from data analysis.
Results
A total of 102 surveys were sent out and 88 were responded to (response rate of 86%). Four surveys were excluded from analysis because the majority of the survey was incomplete, leaving a total of 84 (82%) included in the analyses (all of which were over 95% complete).
Demographics
Demographic information for survey respondents is shown in Table 1. Over half of the survey respondents were VA nurses and/or coordinators. All Canadian provinces were represented, with the exception of Prince Edward Island (for which Nova Scotia provides the VA services). Of the 84 respondents, 11 (13%) came from centers with populations less than 100 000, with the remaining respondents being from larger centers. The majority (52 of 84, 62%) were from academic centers. Three respondents did not identify their gender, age, or job title; 4 did not identify the population of their community or the province of their practice; and 5 did not identify their hospital affiliation.
Table 1.
Demographics of Survey Respondents.
| Gender | |
| Male | 36.9% (31/84) |
| Female | 59.5% (50/84) |
| Unknown | 3.6% (3/84) |
| Age (years) | |
| <35 | 4.8% (4/84) |
| 35-55 | 72.6% (61/84) |
| 56-65 | 16.7% (14/84) |
| >65 | 2.4% (2/84) |
| Unknown | 3.6% (3/84) |
| Job title | |
| Nephrologist | 21.4% (18/84) |
| Surgeon | 20.2% (17/84) |
| VA coordinator | 54.8% (46/84) |
| Unknown | 3.6% (3/84) |
| Affiliation | |
| University | 61.9% (52/84) |
| Urban community-based | 31.0% (26/84) |
| Rural | 1.2% (1/84) |
| Unknown | 6.0% (5/84) |
| Province | |
| BC | 14.3% (12/84) |
| AB | 19.0% (16/84) |
| SK | 4.8% (4/84) |
| MB | 3.6% (3/84) |
| ON | 36.9% (31/84) |
| QC | 7.1% (6/84) |
| NB | 3.6% (3/84) |
| NS | 4.8% (4/84) |
| NL | 1.2% (1/84) |
| Unknown | 4.8% (4/84) |
| Community population | |
| <10 000 | 1.2% (1/84) |
| 10-50 000 | 1.2% (1/84) |
| 50-100 000 | 10.7% (9/84) |
| 100-50 000 | 10.7% (9/84) |
| 250-550 000 | 16.7% (14/84) |
| >550 000 | 54.8% (46/84) |
| Unknown | 4.8% (4/84) |
Practice Patterns Regarding Access Creation
Respondents were asked whether or not they felt they had adequate knowledge to answer questions regarding VA practice patterns at their center; this question had a 100% response rate (84 of 84). The majority (76 of 84, 90%) felt they did have adequate knowledge and completed the sections “Practice Patterns Regarding Access Creation.” All questions in this section had a 100% response rate from these 76 respondents.
Of the 76 respondents who completed these sections, 66 (87%) consider preoperative ultrasound mapping before AVF creation. Indications for ultrasound mapping varied between respondents. Fifty-eight percent (38 of 66) of those who perform ultrasound mapping do so for all patients. Others (28 of 66, 42%) reserve ultrasound mapping for specific indications such as the first attempt at arteriovenous (AV) access creation (7 of 66, 11%), those with no visible veins on physical exam (22 of 66, 33%), those with previous failed AV-access attempts (13 of 66, 20%), and/or those thought to benefit from mapping for other reasons (unspecified) (23 of 66, 35%).
Most respondents (60 of 66, 91%) who perform ultrasound mapping stated a minimum vein diameter is used to judge suitability for AVF creation. Of these 60, 53 (88%) were able to identify the minimum vein diameter used at their center. The minimum acceptable vein diameter was stated to be 2 mm by 42% (22 of 53), 2.5 mm by 26% (14 of 53), and 3 mm or more by 32% (17 of 53).
Maturation assessments are routinely done on all newly created fistulas in the centers of 82% (62 of 76) of the respondents. Most maturation assessments are done in a dedicated VA clinic (36 of 62, 58%) or in a hemodialysis unit (22 of 62, 35%) with the remainder (4 of 62, 6%) being done in the office of an individual nephrologist or surgeon. Of those who perform maturation assessments, 90% (56 of 62) do so within 6 to 8 weeks after access creation while the remaining 10% routinely perform an assessment at more than 8 weeks after creation.
If an AVF is deemed immature at the time of assessment, 55% (42 of 76) of respondents attempt to promote maturation with fistulogram ± angioplasty regardless of whether the patient is on dialysis or not. Twenty-one percent (16 of 76) send the patient for fistulogram/angioplasty only if they are already on hemodialysis, and 24% (18 of 76) send the patient for a surgical consultation for consideration of AVF revision rather than send for fistulogram. No respondents stated the AVF would routinely be abandoned without further assessment.
Practice Patterns Regarding Access Maintenance
The majority of respondents (76 of 84, 90%) felt they had adequate knowledge to answer questions regarding VA practice patterns at their center and completed the sections “Practice Patterns Regarding Access Maintenance.” All questions in this section have a 100% response rate (76 of 76).
AV-access monitoring or surveillance on hemodialysis is done monthly by 50% (38 of 76), every 2 months by 24% (18 of 76), and every 3 months by 8% (6 of 76) of respondents; 18% (14 of 76) of respondents either stated that frequency of surveillance depended on the type of access and/or results of recent surveillance or they did not specify a timeline. Clinical monitoring of the access is performed by 83% (63 of 76). The most common forms of surveillance were ultrasound dilution technique for intra-access flow (64 of 76, 84%) and dynamic venous pressure monitoring at a blood pump speed of 200 mL/min (31 of 76, 41%). Other methods cited include Duplex ultrasound exam (27 of 76, 36%) and surveillance of access flow by thermal dilution (17 of 76, 22%) or ionic dialysance (15 of 76, 20%).
Seventy-five percent (57 of 76) of respondents stated that thrombosed AV-access (AVF or AVG) are always sent for attempted salvage. Twenty percent (15 of 76) attempt AV-access salvage only if resources are available. The remaining 5% (4 of 76) stated attempts to salvage thrombosed AV-access are not routinely made and that a CVC is placed when access thrombosis occurs.
Indications and Contraindications to AVF/AVG Creation
All 84 respondents answered questions regarding indications and contraindications for AVF/AVG creation. Seventy-one percent (60 of 84) agreed with the statement “AVFs are the first choice of access for all patients”; 23% (19 of 84) disagreed with this statement, 4% (3 of 84) stated they were neutral, and 2% (2 of 84) did not answer.
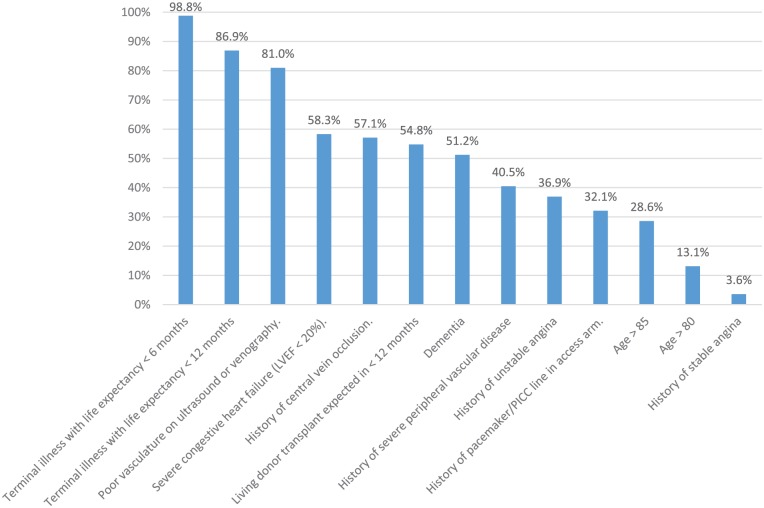
A list of possible contraindications to AVF/AVG creation was provided, and respondents were asked to state whether or not they agreed that each statement was indeed a contraindication. All statements in this section had a 100% (84 of 84) response rate, with the exception of 4 specific contraindications (“poor cardiac function with left ventricular ejection fraction less than 20%,” “history of stable angina,” “history of unstable angina,” and “history of pacemaker/peripherally inserted central catheter (PICC) in planned access extremity”) which had a response rate of 99% (83 of 84). The proportion of respondents who agreed with each proposed contraindication is shown in Figure 1. “Terminal illness with life expectancy less than 6 months,” “terminal illness with life expectancy less than 12 months,” and “poor vasculature on ultrasound or venography” were cited as contraindications by the majority of respondents (83 of 84, 98.8%; 73 of 84, 86.9%; and 68 of 84, 81.0%, respectively). Thirteen percent (11 of 84) agreed age over 80 is a contraindication to AVF/AVG creation. Fifteen percent (13 of 84) responded that there are no contraindications to AVF/AVG creation.
Figure 1.
Contraindications to AVF/AVG creation.
Note. Survey respondents were provided with a list of possible contraindications to AVF/AVG creation and asked whether they agreed or disagreed that each statement was truly a contraindication. Bars represent the proportion of respondents who agreed with each statement. “Severe congestive heart failure (LVEF < 20%),” “history of unstable angina,” “history of pacemaker/PICC line in access arm,” and “history of stable angina” had a response rate of 99% (83 of 84). All other statements had a response rate of 100% (84 of 84). AVF = arteriovenous fistula; AVG = arteriovenous graft; LVEF = left ventricular ejection fraction; PICC = peripherally inserted central catheter.
Facilitators of and Barriers to AVF Creation and Utilization
All 84 respondents answered questions in this section; statements without a 100% response rate are identified in Figures 2 to 5.
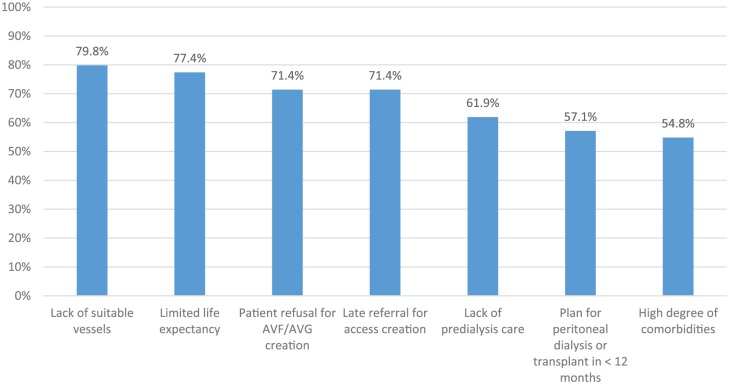
Figure 2.
Reasons patients initiate dialysis with a catheter.
Note. Survey respondents were provided with a list of reasons patients may initiate dialysis with a catheter and asked whether they agreed or disagreed that each statement was a valid reason. Bars represent the proportion of respondents who agreed with each statement. “Lack of suitable vessels,” “plan for peritoneal dialysis or transplant in <12 months,” “patient refusal for AVF/AVG creation,” “limited life expectancy,” and “lack of predialysis care” had a response rate of 98% (82 of 84). “High degree of comorbidities” and “late referral for access creation” had a response rate of 99% (83 of 84). AVF = arteriovenous fistula; AVG = arteriovenous graft.
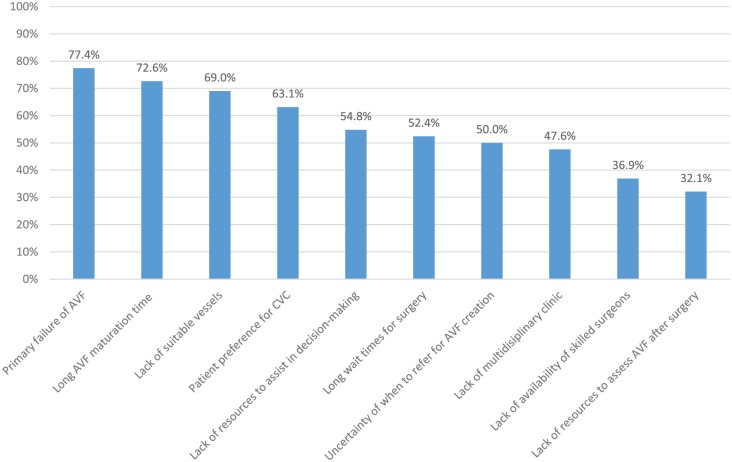
Figure 3.
Barriers to CKD patients initiating dialysis with an AVF.
Note. Survey respondents were provided with a list of possible barriers to AVF creation/utilization and asked whether they agreed or disagreed that each statement was a barrier at their center. Bars represent the proportion of respondents who agreed with each statement. All statements had a response rate of 100% (84 of 84). CKD = chronic kidney disease; AVF = arteriovenous fistula; CVC = central venous catheter.
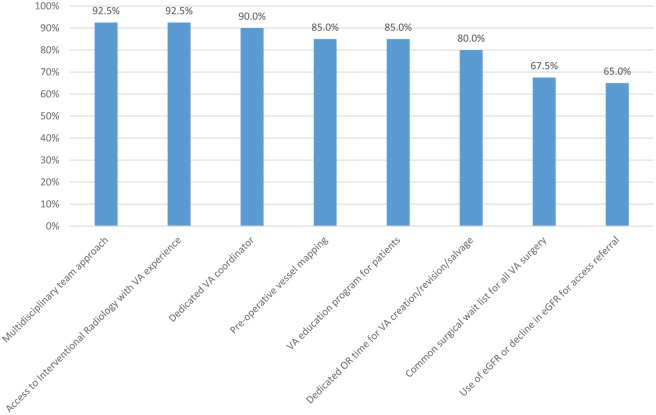
Figure 4.
Contributors to high AVF prevalence.
Note. Survey respondents from centers with AVF prevalence >50% were provided with a list of factors that may contribute to successfully obtaining high AVF prevalence and asked whether they agreed or disagreed each statement was a contributor at their center. Bars represent the proportion of respondents who agreed with each statement. “Use of eGFR or decline in eGFR for access referral” had a response rate of 98% (39 of 40). All other statements had a response rate of 100% (40 of 40). AVF = arteriovenous fistula; VA = vascular access; eGFR = estimated glomerular filtration rate.
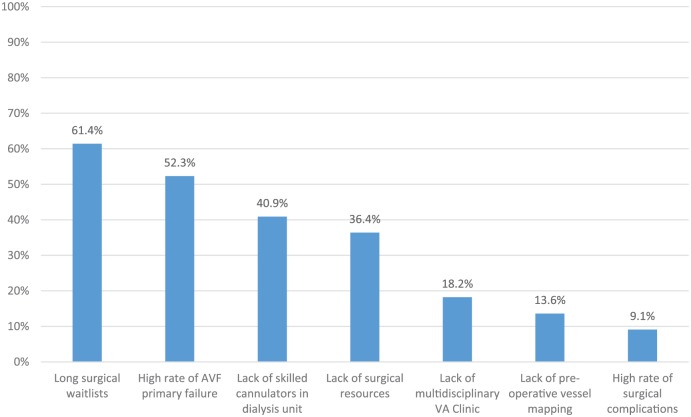
Figure 5.
Contributors to low AVF prevalence.
Note. Survey respondents from centers with AVF prevalence <50% were provided with a list of factors that may constitute barriers to increasing AVF prevalence and asked whether they agreed or disagreed each statement was a barrier at their center. Bars represent the proportion of respondents who agreed with each statement. “Lack of preoperative vessel mapping” had a response rate of 95% (42 of 44). All other statements had a response rate of 100% (44 of 44). AVF = arteriovenous fistula; VA = vascular access.
A list of reasons explaining why chronic kidney disease (CKD) patients might initiate dialysis with a CVC was provided; Figure 2 shows the proportion of respondents who agreed that each statement was a valid reason. The 2 statements with the most consensus were “lack of suitable vessels” (67 of 84, 79.8%, agreed) and “limited life expectancy” (65 of 84, 77.4%, agreed).
Respondents were provided with a list of potential barriers that may keep patients with CKD from initiating dialysis with an AVF; Figure 3 shows the proportion of respondents who agreed that each statement constituted a barrier at their center. The 2 statements with the most consensus were “primary failure of AVF to mature” (77%) and “long AVF maturation time” (73%).
Forty-eight percent (40 of 84) of respondents reported an AVF prevalence rate ≥50% in their unit. Respondents from “high AVF centers” were asked about factors that contributed to successful AVF creation and utilization. Responses are shown in Figure 4. “Multidisciplinary team approach” (92.5%) and “access to interventional radiology with VA experience” (92.5%) were the 2 most highly cited factors.
Fifty-two percent (44 of 84) of respondents reported an AVF prevalence <50% in their unit. Respondents from “low AVF centers” were asked about factors leading to difficulties creating and utilizing AVFs. Responses are shown in Figure 5. “Long surgical wait times” (61.4%) and “high rates of AVF primary failure” (52.3%) were the 2 most highly cited factors resulting in low AVF prevalence.
Discussion
This is one of the first surveys to obtain information on VA practice patterns and opinions among multidisciplinary Canadian clinicians involved and interested in VA.
Practice Patterns Regarding Access Creation
Our results showed significant variation across Canada regarding preoperative assessment for AVF creation. Of the clinicians utilizing preoperative Doppler ultrasound (DUS), 58% are using ultrasound for all patients while others (42%) perform mapping in selected instances only. Clinicians from centers with a high AVF prevalence were more likely to use preoperative ultrasound than those from centers with low AVF prevalence (88% vs 70%, respectively). The European Best Clinical Practice Guidelines (2007) and the National Kidney Foundation Kidney Disease Outcomes Quality Initiative Guidelines (NKF-KDOQI) (2006) advocate for the routine use of preoperative DUS.2,4 These recommendations were based on observational studies that showed routine DUS increased rates of AVF creation, improved AVF patency, and decreased rates of AVF failure.8-11However, more recently published randomized controlled trials and a Cochrane Library systematic review have failed to show a consistent benefit to routine preoperative DUS, prompting some authors to advocate for “selective” DUS (use of DUS only in patients with questionable physical exam findings) rather than routine DUS.12-16 At this time, the indication for preoperative ultrasound is unclear. Our results suggest that many Canadian clinicians individualize the use of DUS based on patient characteristics, a practice that is likely to continue until more data supporting the routine use of DUS becomes available.
Many clinicians utilize a minimum vein diameter on preoperative DUS to determine whether a vein is eligible for AVF creation. Our results showed variation in the minimum vein diameter being used across Canada. While the majority of Canadian clinicians are using minimum vein sizes in the 2.0 to 2.5-mm range, approximately one-third of clinicians stated the minimum acceptable vein size in their center is 3.0 mm. (It should be noted that the survey did not specify whether these measurements were taken with or without tourniquet application or the anatomical location of the fistula, which may have influenced the response of some respondents). To date, there have been no randomized trials validating a minimum vein size that is predictive of AVF maturation. All studies have been observational and have shown that vein diameters <1.6 mm are predictive of AVF failure, whereas veins with a minimum size of 2.0 to 2.5 mm (some studies with tourniquet and others without) have acceptable rates of success.17-19 Given the lack of high-quality evidence, no recommendation can be made as to a minimum vein diameter that Canadian practitioners should be using. However, given the lack of evidence to support using vein diameters of 3.0 mm or higher, and good success rates with smaller vein diameters, this practice may be effectively reducing the number of potential AVF candidates at some centers.
The majority of clinicians responded that maturation assessments are routinely done within 6 to 8 weeks of AVF creation. NKF-KDOQI guidelines suggest that if a fistula fails to mature by 6 weeks, a fistulogram or other imaging study should be obtained to determine the cause.2 Just over half of Canadian clinicians would refer all such patients for fistulogram, while approximately 20% stated they would send the patient for fistulogram only if that patient was already on hemodialysis and another 25% would instead send the patient for surgical consultation for potential revision. When fistulas fail to mature in the first 6 weeks after creation, early intervention with percutaneous angioplasty of stenotic lesions or embolization/ligation of accessory veins can often result in fistula maturation.20-25 Our survey results do not explain why 25% of Canadian clinicians would opt for surgical referral rather than fistulogram; it may be that a lack of interventional radiology in some centers is a contributing factor. This was not explicitly addressed in this survey; however, access to interventional radiology was cited by many centers with high AVF prevalence as a contributing factor to successfully increasing AVF prevalence. Advocating for timely investigation and intervention in patients with nonmaturing fistulas may help to increase AVF prevalence in Canada and avoid unnecessary use of CVCs.
Clinicians who only refer patients on dialysis for fistulogram are likely deterred by the potential risk of contrast-induced nephropathy (CIN). This risk has been examined in 2 small studies in which CKD stage IV/V patients underwent venography for nonmaturing AVF or preoperative assessment; neither study demonstrated a significantly increased risk of acute kidney injury or requirement for dialysis.26,27 While these studies are small, they suggest that patients with advanced CKD may safely undergo interventions to assist nonmaturing fistulas, provided that low volumes of contrast (8-20 mL) are used.
Practice Patterns: Access Maintenance
Both the CSN Hemodialysis Guidelines and the NKF-KDOQI Clinical Practice Guidelines for Vascular Access recommend regular access surveillance using either access flow measurements or measurements of venous pressures.2,3 These recommendations are based on early studies which suggested early detection and correction of stenosis reduces the risk of thrombosis and improves access survival.28,29 Seventy-four percent of Canadian clinicians responded that their centers perform regular access surveillance at least every 2 months. In those centers performing routine surveillance, access flow measurement using ultrasound dilution technique is the most common adjunct to physical exam and clinical markers of access dysfunction.
In recent years, some have questioned the utility of routine access surveillance.30 In 2008, Tonelli et al published a systematic review and meta-analysis of 12 randomized-control trials comparing surveillance with either access blood flow or ultrasound with standard care (physical exam and clinical monitoring).31 They found that routine surveillance significantly reduced the risk of fistula thrombosis, but not the risk of fistula loss. For patients with grafts, there was no decrease in risk of thrombosis or risk of access loss with routine surveillance. A 2016 systematic review by Ravani et al showed that access surveillance and preemptive intervention on stenoses do not prolong access longevity compared with waiting for clinical signs of access dysfunction.32 Given the discrepancy between VA guidelines and the results of recent studies, the wide variation in practice is not surprising. Similar to DUS use, many Canadian clinicians seem to individualize the use of access surveillance based on the clinical situation.
Clinical practice guidelines recommend rapid intervention upon detection of thrombosis to salvage the AV-access and minimize the need for temporary access.3 However, 20% of clinicians indicate that their ability to do so may be impacted by limited resources while another 5% indicate that the standard practice is to abandon a thrombosed AV-access and arrange for CVC insertion. Both endovascular thrombectomy and surgical thrombectomy can restore flow and provide acceptable patency rates at 6 to 12 months; of the 2, endovascular thrombectomy is less expensive and requires fewer postintervention procedures.33-38 Limited access to interventional radiology or surgical resources may be a barrier to AVF salvage in some centers, and encouraging timely referral to a center where intervention is available may help reduce a significant proportion of fistulas which are lost to thrombosis.
Indications and Contraindications for AVF/AVG Creation
Two-thirds of clinicians agreed that fistulas are the first choice of access for all patients. A 2007 publication by the CSN VAWG cited limited life expectancy, high likelihood of AVF nonmaturation, and expected transition to peritoneal dialysis or transplantation in the near future as reasons for which AVF creation may be deferred.39 However, current guidelines do not detail contraindications to AVF or AVG creation, and decisions regarding contraindications are often clinician-dependent.2-5 While the majority of clinicians (85%) felt that contraindications to AV-access creation exist, there was little consensus on suggested contraindications. Only “terminal illness with life expectancy less than 6 months,” “terminal illness with life expectancy less than 12 months,” and “inadequate vasculature on preoperative vessel mapping” were agreed upon as contraindications by the majority. Surprisingly, consensus was not reached on the following contraindications: “very elderly age (older than 85 years),” “dementia,” “expected transplant within 1 year,” “severe congestive heart failure (LVEF < 20%),” “severe peripheral vascular disease,” and “central vein occlusion.” A previous survey of Canadian and American nephrologists also showed a similar lack of consensus regarding contraindications to AV-access.40 The lack of consensus regarding contraindications to AVF creation may explain, in part, the regional variation in AVF prevalence seen in Canada. However, differences in patient characteristics across dialysis centers may also explain some of the regional variation. A 2007 single-center study from Ottawa found that the majority of patients utilizing CVCs do so because of patient-specific factors (for example, unsuitable vessels or medical comorbidities) rather than differences in physician opinions regarding AVF eligibility.41
Facilitators of and Barriers to AVF Creation and Utilization
A few common themes emerged when examining facilitators of and barriers to AVF creation and utilization. “Primary failure of AVF” was the most highly cited barrier to patients with CKD utilizing a fistula at the time of dialysis initiation; primary failure was also highly cited as a contributor to low AVF prevalence by respondents at low AVF centers. Primary failure is a problem that requires further research, and an ideal approach to reducing primary failure is beyond the scope of this article. However, this survey has identified that many centers in Canada do not currently refer all patients with primary failure for procedures to assist maturation by 6 weeks post creation. As described above, evidence supports early intervention on fistulas that fail to mature in the first 4 to 6 weeks. Increasing the number of patients referred for assessment and intervention on nonmaturing fistulas may reduce rates of primary failure at some centers.
“Long surgical waitlists” was cited as the most common contributor to low AVF prevalence and was cited by approximately 50% of respondents as a barrier to CKD patients initiating dialysis with a functional fistula. Conversely, 80% of respondents from centers with high AVF prevalence stated “dedicated OR time for vascular access creation/revision/salvage” was a key contributor to high AVF prevalence at their center. This would suggest that higher AVF rates might be achieved in Canada if surgical wait times for AVF creation could be shortened and timely access to salvage interventions was available at many centers that currently have low AVF prevalence.
“Multidisciplinary team approach” and “access to interventional radiologists with VA experience” were the 2 most highly cited reasons for high AVF prevalence at roughly half the centers involved in this survey. This data may be useful to support the resources required for clinicians looking to establish or improve VA programs at their own centers.
Summary: The Canadian Picture
Our survey suggests that Canadian VA clinicians are, for the most part, practicing in concordance with existing clinical practice guidelines with regard to VA creation, surveillance, and salvage. Our results do not fully explain why Canada has a low AVF prevalence compared with other DOPPS-contributing nations. In fact, very few contraindications to AVF creation were agreed upon, suggesting that the majority of Canadian dialysis patients would be considered “AVF eligible.” However, other nations may take an even more aggressive approach to AVF creation; American nephrologists were less likely to identify advanced age, severe congestive heart failure, short life expectancy, patient preference for CVC, dementia, and history of multiple failed AV-access attempts as contraindications to AV-access creation than Canadians.40 In addition, 43% of American nephrologists felt there was no absolute contraindication to AV-access creation as compared with 27% of Canadian nephrologists. While Canadian nephrologists are more likely to place CVCs in older patients, patients with increased comorbidities, or patients with previously failed fistulas, American nephrologists were more likely to choose a graft, an approach rarely used in Canada.42
The explanation for the difference in CVC prevalence between Canada and the United States is also unclear. There are more patients over the age of 75 on dialysis in Canada (30% vs 23% in the United States), and as age is a risk factor for primary failure, this may contribute to the low fistula prevalence in Canada.43,44 In addition, there appears to be a more aggressive approach to permanent AV-access creation in the United States. Fistulas are more heavily promoted in the United States due to reimbursement based on AVF prevalence.45 In contrast, Canadian clinicians have no financial incentive to promote one form of VA over another and may follow a more individualized approach based on patient choice.
Figure 2 shows reasons that respondents felt were acceptable justification to utilize a CVC rather than AVF at dialysis initiation. The 2 most highly cited reasons, “lack of suitable vessels” and “limited life expectancy,” are likely consistent across DOPPS nations. However, the third most highly cited reason was “patient refusal for AVF creation”; “patient preference for CVC” was also a highly cited barrier to incident hemodialysis patients utilizing an AVF (Figure 3). Over the past decades, medical practice within Canada has become increasingly patient-centered. Patients may desire a CVC rather than AV-access for a number of reasons (fear of needling, dislike of the appearance of AVFs, arm discomfort during dialysis, etc). Our survey shows that 71% of respondents agree patient refusal for AVF creation is a valid reason to utilize CVC; it is difficult to say whether this is different from other DOPPS nations, but this may explain, in part, the relatively high CVC usage in Canada.
Study Limitations
There are potential limitations to interpretation of our survey results. The primary limitation is that those surveyed were VA “experts” in Canada. As such, the majority of respondents were from large, urban, academic centers. Their answers may not be reflective of VA practice patterns or opinions in smaller, rural, or community centers, where access to resources such as preoperative ultrasound mapping or interventional radiology may be limited. The answers of VA experts may also not be reflective of the practice or opinions of general nephrology practitioners. A follow-up survey to a wider population may yield different results.
In addition, the respondents in our survey included a disproportionate number of VA nurses and coordinators. Opinions and attitudes may vary depending on whether the respondent is a nurse, nephrologist, or surgeon, and our survey results may be biased by an overrepresentation of nurses. However, VA nurses and coordinators typically have thorough knowledge of practice patterns in their center and usually play a vital role in decisions regarding access referral and intervention. Their opinions, therefore, likely align quite closely with the opinions of other VA clinicians at their center.
Finally, our survey included a number of response formats, including questions in which participants were asked to answer “yes” or “no” to express their agreement or disagreement with given statements. Such questions may lead to acquiescence bias, in which participants have a tendency to agree with provided statements or questions, especially when in doubt of the answer.46 Such questions in our survey, therefore, may have different results than if they had a different response format.
Conclusion
Our survey of VA clinicians revealed that there is significant variation in VA practice across Canada. This variation may reflect either the lack of high-quality evidence to support current clinical practice guidelines, an individualized approach to patient care, or discrepancies in resource availability across Canada.
Increased education regarding patient suitability for AVF creation may help increase AVF prevalence in suitable patients. Unfortunately, at this point in time, no standardized criteria exist for appropriate AVF placement. Furthermore, there is a lack of consensus regarding contraindications to AVF creation. Further studies are clearly needed to help guide clinicians in this matter.
Footnotes
Ethics Approval and Consent to Participate: A research ethics board review was not required for implementation of the survey as participation in the survey was considered implied consent for reporting of data.
Consent for Publication: We have all authors consent for publication.
Availability of Data and Materials: Data related to the survey responses is available upon request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplementary material for this article is available online.
References
- 1. MacRae J, Dipchand C, Oliver M, et al. Arteriovenous access: infection, neuropathy, and other complications. Can J Kidney Health Dis. 2016;3:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vascular Access Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48:S248-S273. [DOI] [PubMed] [Google Scholar]
- 3. Canadian Society of Nephrology Vascular Access Working Group. Clinical practice guidelines for vascular access. J Am Soc Nephrol. 2006;17:S1-S27. [DOI] [PubMed] [Google Scholar]
- 4. Tordoir J, Canaud B, Haage P, et al. European best practice guidelines on vascular access. Nephrol Dial Transplant. 2007;22(suppl 2):ii88-ii117. [DOI] [PubMed] [Google Scholar]
- 5. Polkinghorne KR, Chin GK, MacGinley RJ, et al. KHA-CARI Guideline: vascular access—central venous catheters, arteriovenous fistulae, and arteriovenous grafts. Nephrology. 2013;18(11):701-705. [DOI] [PubMed] [Google Scholar]
- 6. Pisoni R, Zepel L, Port F, Robinson BM. Trends in US vascular access use, patient preferences, and related practices: an update from the US DOPPS practice monitor with international comparisons. Am J Kidney Dis. 2015;65(6):905-915. [DOI] [PubMed] [Google Scholar]
- 7. Canadian Society of Nephrology Vascular Access Working Group. Report of the Canadian Society of Nephrology Vascular Access Working Group. Semin Dial. 2012;25(1):22-25. [DOI] [PubMed] [Google Scholar]
- 8. Silva M, Jr, Hobson R, II, Pappas P, et al. A strategy for increasing use of autogenous hemodialysis access procedures: impact of pre-operative noninvasive evaluation. J Vasc Surg. 1998;27:302-307. [DOI] [PubMed] [Google Scholar]
- 9. Allon M, Lockhart M, Lilly R, et al. Effect of pre-operative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int. 2001;60:2013-2020. [DOI] [PubMed] [Google Scholar]
- 10. McGill R, Marcus R, Healy D, Brouwer DJ, Smith BC, Sandroni SE. AV fistula rates: changing the culture of vascular access. J Vasc Access. 2005;6:13-17. [DOI] [PubMed] [Google Scholar]
- 11. Ilhan G, Esi E, Bozok S, et al. The clinical utility of vascular mapping with Doppler ultrasound prior to arteriovenous fistula construction for hemodialysis access. J Vasc Access. 2013;14:83-88. [DOI] [PubMed] [Google Scholar]
- 12. Ferring M, Claridge M, Smith S, Wilmink T. Routine preoperative vascular ultrasound improves patency and use of arteriovenous fistulas for hemodialysis: a randomized trial. Clin J Am Soc Nephrol. 2010;5(12):2236-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mihmanli I, Besirli K, Kurugoglu S, et al. Cephalic vein and hemodialysis fistula: surgeon’s observation versus color Doppler ultrasonographic findings. J Ultrasound Med. 2001;20:217-222. [DOI] [PubMed] [Google Scholar]
- 14. Nursal T, Oguzkurt L, Tercan F, et al. Is routine preoperative ultrasonographic mapping for arteriovenous fistula creation necessary in patients with favorable physical examination findings? Results of a randomized controlled trial. World J Surg. 2006;30:1100-1107. [DOI] [PubMed] [Google Scholar]
- 15. Kosa S, Al-Jaishi A, Moist L, Lok CE. Preoperative vascular access evaluation for haemodialysis patients (review). Cochrane Database Syst Rev. 2015;9:CD007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith G, Barnes R, Chetter I. Randomized clinical trial of selective versus routine preoperative duplex ultrasound imaging before arteriovenous fistula surgery. Br J Surg. 2014;101:469-474. [DOI] [PubMed] [Google Scholar]
- 17. Wong V, Ward R, Taylor J, Selvakumar S, How TV, Bakran A. Factors associated with early failure of arteriovenous fistulae for haemodialysis access. Eur J Vasc Endovasc Surg. 1996;12:207-213. [DOI] [PubMed] [Google Scholar]
- 18. Mendes R, Farber M, Marston W, Dinwiddie LC, Keagy BA, Burnham SJ. Prediction of wrist arteriovenous fistula maturation with preoperative vein mapping with ultrasonography. J Vasc Surg. 2002;36:460-463. [DOI] [PubMed] [Google Scholar]
- 19. Lauvao L, Ihnat D, Goshima K, Chavez L, Gruessner AC, Mills JL., Sr Vein diameter is the major predictor of fistula maturation. J Vasc Surg. 2009;49(6):499-504. [DOI] [PubMed] [Google Scholar]
- 20. Beathard G, Settle S, Shields M. Salvage of the nonfunctioning arteriovenous fistula. Am J Kidney Dis. 1999;33(5):910-916. [DOI] [PubMed] [Google Scholar]
- 21. Faiyaz R, Abereo K, Zaman F, Pervez A, Zibari G, Work J. Salvage of poorly developed arteriovenous fistulae with percutaneous ligation of accessory veins. Am J Kidney Dis. 2002;39(4):824-827. [DOI] [PubMed] [Google Scholar]
- 22. Berman S, Gentile A. Impact of secondary procedures in autogenous arteriovenous fistula maturation and maintenance. J Vasc Surg. 2001;34:866-871. [DOI] [PubMed] [Google Scholar]
- 23. Voormolen E, Jahrome A, Bartels L, Moll F, Mali W, Blankestijn P. Nonmaturation of arm arteriovenous fistulas for hemodialysis access: a systematic review of risk factors and results of early treatment. J Vasc Surg. 2009;49:1325-1336. [DOI] [PubMed] [Google Scholar]
- 24. Bhimani B, Asif A. Diagnosis and salvage of an immature fistula. Kidney Int. 2007;72:126-130. [DOI] [PubMed] [Google Scholar]
- 25. Turmel-Rodrigues L, Mouton A, Birmele B, et al. Salvage of immature forearm fistulas for haemodialysis by interventional radiology. Nephrol Dial Transplant. 2001;16:2365-2371. [DOI] [PubMed] [Google Scholar]
- 26. Kian K, Wyatt C, Schon D, Packer J, Vassalotti J, Mishler R. Safety of low-dose radiocontrast for interventional AV fistula salvage in stage 4 chronic kidney disease patients. Kidney Int. 2006;69:1444-1449. [DOI] [PubMed] [Google Scholar]
- 27. Asif A, Cherla G, Merrill D, et al. Venous mapping using venography and the risk of radiocontrast-induced nephropathy. Semin Dial. 2005;18:239-242. [DOI] [PubMed] [Google Scholar]
- 28. Besarab A, Sullivan K, Ross R, Moritz M. Utility of intra-access pressure monitoring in detecting and correcting venous outlet stenoses prior to thrombosis. Kidney Int. 1995;47:1364-1373. [DOI] [PubMed] [Google Scholar]
- 29. Schwab S, Raymond J, Saeed M, et al. Prevention of hemodialysis fistula thrombosis: early detection of venous stenoses. Kidney Int. 1989;36:707-711. [DOI] [PubMed] [Google Scholar]
- 30. Paulson W, Moist L, Lok C. Vascular access surveillance: an ongoing controversy. Kidney Int. 2012;81:132-142. [DOI] [PubMed] [Google Scholar]
- 31. Tonelli M, James M, Wiebe N, Jindal K, Hemmelgarn B. Ultrasound monitoring to detect access stenosis in hemodialysis patients: a systematic review. Am J Kidney Dis. 2008;51:630-640. [DOI] [PubMed] [Google Scholar]
- 32. Ravani P, Quinn R, Oliver M, et al. Preemptive correction of arteriovenous access stenosis: a systematic review and meta-analysis of randomized controlled trials. Am J Kidney Dis. 2016;67(3):446-460. [DOI] [PubMed] [Google Scholar]
- 33. Turmel-Rodrigues L, Pengloan J, Rodrigue H, et al. Treatment of failed native arteriovenous fistulae for hemodialysis by interventional radiology. Kidney Int. 2000;57:1124-1140. [DOI] [PubMed] [Google Scholar]
- 34. Huang H, Chen C, Chang S, et al. Combination of duplex ultrasound-guided manual declotting and percutaneous transluminal angioplasty in thrombosed native dialysis fistulas. Ren Fail. 2005;27:713-719. [DOI] [PubMed] [Google Scholar]
- 35. Palmer R, Cull D, Kalbaugh C, et al. Is surgical thrombectomy to salvage failed autogenous arteriovenous fistulae worthwhile? Am Surg. 2006;72:1231-1233. [DOI] [PubMed] [Google Scholar]
- 36. Ponikvar R. Surgical salvage of thrombosed arteriovenous fistulas and grafts. Ther Apher Dial. 2005;9:245-249. [DOI] [PubMed] [Google Scholar]
- 37. Lipari G, Tessitore N, Poli A, et al. Outcomes of surgical revision of stenosed and thrombosed forearm arteriovenous fistulae for haemodialysis. Nephrol Dial Transplant. 2007;22:2605-2612. [DOI] [PubMed] [Google Scholar]
- 38. Yang S, Lok C, Arnold R, Rajan D, Glickman M. Comparison of post-creation procedures and costs between surgical and an endovascular approach to arteriovenous fistula creation. J Vasc Access. 2017;18(suppl 2):8-14. [DOI] [PubMed] [Google Scholar]
- 39. Canadian Society of Nephrology Vascular Access Working Group. Report of the CSN VAWG. https://www.csnscn.ca/images/Docs_Misc/VAWG/CSN_VAWG_INITIATIVE_AUGUST_18_2011.pdf. Published 2011. Accessed May 30, 2017. [DOI] [PubMed]
- 40. Xi W, MacNab J, Lok C, et al. Who should be referred for a fistula? A survey of nephrologists. Nephrol Dial Transplant. 2010;25(8):2644-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Graham J, Hiremath S, Magner PO, Knoll GA, Burns KD. Factors influencing the prevalence of central venous catheter use in a Canadian haemodialysis centre. Nephrol Dial Transplant. 2008;23(11):3585-3591. [DOI] [PubMed] [Google Scholar]
- 42. Arbor Research Collaborative for Health. DOPPS Practice Monitor Canada: vascular access in use. http://www.dopps.org/DPM/Canada/. Published 2017. Accessed May 30, 2017.
- 43. Arbor Research Collaborative for Health. DOPPS Practice Monitor Canada: demographics. http://www.dopps.org/DPM/Canada/. Published 2017. Accessed May 30, 2017.
- 44. Arbor Research Collaborative for Health. DOPPS Practice Monitor United States: demographics. http://www.dopps.org/DPM/DPMSlideBrowser.aspx?type=Topic&id=33. Published 2017. Accessed May 30, 2017.
- 45. Centers for Medicare Medicaid Services. ESRD quality incentive program. https://www.cms.gov/Medicare/Quality-Initiatives-patient-Assessment-Instruments/ESRDQIP/. Published 2017. Accessed May 30, 2017.
- 46. Hinz A, Michalski D, Schwarz R, Herzberg P. The acquiescence effect in responding to a questionnaire. Psychosoc Med. 2007;4:Doc07. [PMC free article] [PubMed] [Google Scholar]