Abstract
Background
In pivotal studies with direct-acting antivirals (DAAs), rates of sustained virological response in hepatitis C genotype 1 infection are >90%.
Objective
The objective of this article is to assess real-world safety and effectiveness of DAA treatment in a prospective multicenter registry study.
Methods
The German Hepatitis C-Registry includes 6606 patients with genotype 1 from 246 centers, treated between February 2014 and June 2016 at the discretion of the physician.
Results
A total of 4846 patients completed treatment and follow-up; 51% of these patients were treatment experienced and 28% had liver cirrhosis. Comorbidities were reported in 76% of patients, including HIV co-infection in 8%. SVR12 was 92% with 91% in GT1a and 93% in GT1b. HIV co-infected patients (n = 247) had an SVR12 of 92%. Treatment was discontinued prematurely in 2.5%. In multivariate analysis, SVR12 was dependent on the choice of antiviral regimen (OR 1.33 (1.24–1.43); p < 0.001), negatively associated with presence of liver cirrhosis (OR 0.71 (0.56–0.89); p < 0.003) and positively associated with female gender (OR 1.52 (1.21–1.91); p < 0.001).
Conclusion
Data from this real-world registry show SVR12 rates close to those obtained in clinical studies. Discontinuation rates are low, confirming good tolerance of the regimens and good adherence of patients (Trial registration number DRKS00009717, German Clinical Trials Register, DRKS).
Keywords: Hepatitis C virus, chronic hepatitis C, genotype 1, direct-acting antivirals, treatment, therapy, effectiveness
Key summary
Summarize the established knowledge on this subject
With modern direct-acting antivirals, rates of sustained virological response in hepatitis C genotype 1 infection are >90% in phase III studies.
Successful treatment of HCV infection depends not only on the efficacy of the antiviral drugs, but also on patient and health care-related factors.
Data on real-world effectiveness are spare.
What are the significant and/or new findings of this study?
Data from this real-world registry show sustained virological response at 12 weeks (SVR12) rates close to those obtained in clinical studies.
Efficacies of interferon-free treatment regimens were superior to interferon-containing therapy for GT1.
Physician-tailored therapy according to cirrhosis status achieved high response rates notwithstanding a remaining lower SVR in cirrhotic patients.
Discontinuation rates are low, confirming good tolerance of the regimens and good adherence of patients.
Introduction
Worldwide up to 103 million people are chronically infected with hepatitis C (HCV) and it is estimated that about 1 million to 4 million people die from HCV-associated diseases per year.1,2 Sequelae of HCV infection include development of liver cirrhosis and hepatocellular carcinoma, which are reduced after successful treatment of HCV infection.3 Starting in 2011 with the approval of the first direct-acting antivirals (DAAs) telaprevir and boceprevir, used in combination with pegylated-interferon (IFN) and ribavirin (RBV), rates of sustained virological response (SVR) have continuously improved.4 The approval of sofosbuvir (SOF), simeprevir (SIM) and daclatasvir (DCV) in 2014 led to improved efficacy combined with a much better tolerability.5,6 The combination of SIM/SOF showed SVR rates of 79%–100%7–9 and the combination of SOF/DCV 95%–100%.6 In early 2015 sofosbuvir and ledipasvir (SOF/LDV) and ombitasvir (OBV), paritaprevir/ritonavir (PTV/r) and dasabuvir (DSV) were approved following phase III studies with SVR rates >90% for HCV genotype 1 (GT1).10,11 High SVR results were also achieved in difficult-to-treat patients, albeit treatment had to be prolonged to 24 weeks or RBV had to be added.11–15
Presently, several of the aforementioned combinations of DAAs are recommended for the treatment of HCV infection by current guidelines, varying in treatment duration from 8 to 24 weeks and the possible addition of RBV tailored to the condition of the patient.16
However, treatment with the new DAAs is associated with significant immediate costs for health care systems.17 In addition, population-based modeling suggests that a significant effect on HCV mortality on a population level can be observed only if a substantial increase in the number of successfully treated patients is achieved.18 Therefore, successful treatment of HCV infection depends not only on the efficacy of the antiviral drugs, but also on patient and health care-related factors. Accordingly, effectiveness of antiviral therapy may vary in a real-world setting.
The health care system in Germany allows initiation of antiviral therapy with any European Medicine Agency (EMA)-approved drug by physician discretion. Furthermore, treatment is not centralized and can be initiated by any physician. However, costs per SVR are estimated to be between 41,766€ and 80,824€ in Germany, with higher prices paid for longer treatment durations in difficult-to-treat patients.19 Physicians may be penalized by claim backs of medication costs if they did not use the most cost-efficient therapy. We here assess the effectiveness of the new DAA in the German health care system based on data from a large nationwide cohort.
Material and methods
German Hepatitis C-Registry
The German Hepatitis C-Registry (Deutsches Hepatitis C-Register, DHC-R) is a prospective, open-label, multicenter, non-interventional study as described previously.20,21 Inclusion criteria were chronic hepatitis C virus (HCV) infection with detectable HCV-RNA and ≥18 years of age. Exclusion criteria were pregnancy and non-reliable contraception. The choice of antiviral therapy was at the discretion of the physician. Main parameters of interest included demographic data, medical history, viral genotype and viral load, laboratory parameters, fibrosis assessment (transient elastography and/or histology), concomitant medication, virological outcomes and adverse events. Data were recorded via a web browser-based Electronic Data Capture system and reviewed by study monitors (see supplements for details).
Patient population
Patient enrollment started November 24, 2014. For documentation of antiviral therapies that started on or after February 1, 2014, a retrospective documentation was allowed until June 30, 2015.
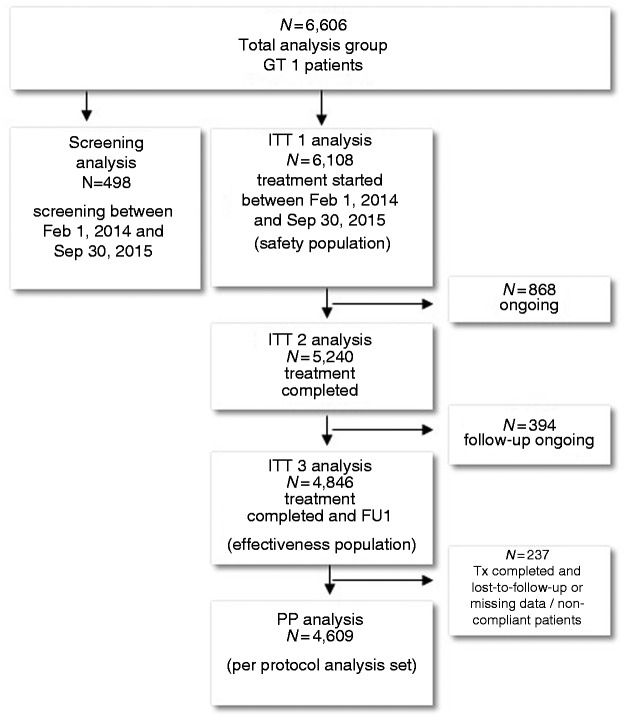
The present analysis is based on 6606 patients with HCV genotype 1 infection (GT1) who were documented between February 1, 2014 and June 30, 2016. The screening population consists of patients who were enrolled until September 30, 2015 but not treated until June 30, 2016. The treatment population comprises patients who started antiviral therapy on or before September 30, 2015 (Figure 1).
Figure 1.
Study design.
GT: genotype; ITT: intention-to-treat; PP: per protocol analysis; FU: follow-up 12 to 24 weeks after end of therapy; Tx: therapy.
Assessment of endpoints
The safety population (intention-to-treat, ITT1) consists of patients who started antiviral therapy and for whom at least the baseline visit was documented. The modified ITT effectiveness analysis (ITT3) included all patients from the safety population (ITT1) with SVR12 data. For the per protocol analysis (PP), non-adherent patients, patients with missing data or patients lost-to-follow-up were excluded (Figure 1). Primary endpoint was the proportion of patients who achieved SVR12. SVR12 was defined as undetectable HCV-RNA 70–153 days after end of treatment (EoT).
Definitions
Duration of therapy
Therapy duration between 50 and 61 days after treatment initiation was defined as “8 weeks,” between 78 and 90 days as “12 weeks,” between 162 and 174 as “24 weeks” and between 308 and 364 as “48 weeks.” Duration outside these definitions was considered as “other duration.”
Liver cirrhosis
Clinical evidence of liver cirrhosis was given if one of the following criteria was fulfilled: liver biopsy showing cirrhosis (Metavir score F4), transient elastography (Fibroscan) >12.5 kPa, ultrasound confirming cirrhosis, clinical judgment of cirrhosis (e.g. presence of ascites, esophageal varices).
Treatment periods
To analyze changing treatment patterns over time five treatment periods were defined as follows: period 1 from February 1, 2014 to May 15, 2014 (recent approval of SOF), period 2 from May 16, 2014 to November 20, 2014 (approval of SMV and DCV), period 3 from November 21, 2014 to April 15, 2015 (approval of SOF/LDV and OBV/PTV/r + DSV), period 4 from April 16, 2015 to September 1, 2015 and period 5 from September 2, 2015 to June 30, 2016. Listed in brackets are the DAA approved during the treatment period or in case of SOF one month before.
Statistics
Because of the ongoing nature of the study, the status of data for this analysis was frozen on June 30, 2016. The statistical analysis was descriptive to reflect the clinical routine as intended by the physicians. Further details are described in the supplements.
Results
Patient population and treatment regimens
Overall, 6606 patients with HCV GT1 infection were included in the study. A total of 498 patients were only assessed for therapy, but not treated. A total of 6108 patients received treatment (safety population, ITT1) and 4846 patients received treatment and a follow-up visit 12 weeks after end of treatment (effectiveness population, ITT3). There were 237 patients who were lost to follow-up or had missing data, thus, the per-protocol-analysis consists of 4609 patients (Figure 1).
Baseline characteristics of the screened and treated patients are shown in Table 1. In summary, patients who were treated were older (median 55 ± 12.5 vs 51.5 ± 13.5), more likely to be treatment experienced (49.7% vs 27.5%) and had a higher prevalence of cirrhosis (27.8% vs 7.4%). However, human immunodeficiency virus (HIV) prevalence was slightly lower (8.2% vs 12.0%).
Table 1.
Baseline characteristics.
| Patients characteristics | Patients only screened | % of patients only screened | Safety population (ITT 1) | % of patients (safety population) | Effectiveness population (ITT 3) | % of patients (effectiveness c population) | Per-protocol analysis | % of patients (per-protocol analysis) |
|---|---|---|---|---|---|---|---|---|
| Total | 498 | 6108 | 4846 | 4609 | ||||
| Male | 304 | 61.0 | 3458 | 56.6 | 2700 | 55.7 | 2550 | 55.3 |
| Age, years (mean) | 52.1 ± 13.5 | 54.2 ± 12.5 | 54.3 ± 12.6 | 54.5 ± 12.4 | ||||
| Treatment-naïve | 361 | 72.5 | 3070 | 50.3 | 2397 | 49.5 | 2238 | 48.6 |
| IFN pretreatment | 133 | 26.7 | 2929 | 48.0 | 2365 | 48.8 | 2292 | 49.7 |
| DAA pretreatment | 13 | 2.6 | 704 | 11.5 | 590 | 12.2 | 564 | 12.2 |
| MELD score (mean, min–max; cirrhotic patients)a | n.a. | 8.7 ± 3.0/(6–32) | 8.7 ± 2.9/(6–32) | 8.7 ± 2.9/(6–32) | ||||
| Cirrhosis | 37 | 7.4 | 1697 | 27.8 | 1378 | 28.4 | 1316 | 28.6 |
| CHILD Ab | 28 | 82.4 | 1175 | 86.7 | 955 | 86.9 | 916 | 87.2 |
| CHILD B | 4 | 11.8 | 156 | 11.5 | 126 | 11.5 | 119 | 11.3 |
| CHILD C | 2 | 5.9 | 24 | 1.8 | 18 | 1.6 | 15 | 1.4 |
| Any comorbidities | 394 | 79.1 | 4615 | 75.6 | 3690 | 76.1 | 3513 | 76.2 |
| GFR <30 ml/minc | n.a. | 43 | 0.8% | 36 | 0.8 | 36 | 0.9 | |
| Diabetes mellitus | 36 | 7.2 | 617 | 10.1 | 500 | 10.3 | 485 | 10.5 |
| Opioid substitution therapy (OST) | 111 | 22.3 | 499 | 8.2 | 366 | 7.6 | 327 | 7.1 |
| Solid organ transplantation | 11 | 2.2 | 131 | 2.1 | 105 | 2.2 | 102 | 2.2 |
| HIV | 60 | 12.0 | 499 | 8.2 | 378 | 7.8 | 366 | 7.9 |
| Plateletsd/nl (mean) | 202.9 ± 85.0 | 192.9 ± 78.7 | 192.3 ± 78.5 | 193.7 ± 78.9 | ||||
| Platelets <90/nld | 18 | 6.9 | 550 | 9.7 | 457 | 9.9 | 438 | 10.0 |
| Albumin g/l (mean) | n.a. | 40.4 ± 7.9 | 40.7 ± 7.5 | 40.7 ± 7.5 | ||||
| Albumin <35 g/le | 313 | 12.1 | 244 | 11.2 | 233 | 11.2 | ||
| HCV-RNA <800,000 IU/ml | 197 | 39.6 | 2368 | 38.8 | 1899 | 39.2 | 1803 | 39.1 |
| HCV-RNA >800,000–2 × 106 IU/ml | 106 | 21.3 | 1435 | 23.5 | 1151 | 23.8 | 1099 | 23.8 |
| HCV-RNA > 2 × 106–6 × 106 IU/ml | 96 | 19.3 | 1464 | 24.0 | 1210 | 25.0 | 1154 | 25.0 |
| HCV-RNA > 6 × 106 IU/ml | 47 | 9.4 | 647 | 10.6 | 516 | 10.6 | 487 | 10.6 |
Data for MELD were available from 477 treated patients (ITT1), patients with follow-up (ITT3) and 373 pts in the per-protocol analysis
Data for Child-Pugh score were available for 34 screened patients, 1355 treated patients (ITT1), 1099 patients in the follow-up (ITT3) group and 1050 patients in the per-protocol analysis
Data for glomerular filtration rate (GFR) were available for 5355 treated patients (ITT1), 4405 patients in the follow-up (ITT3) group and 4200 patients in the per-protocol analysis
Data on platelet count were available for 263 screened and 5653 treated patients (ITT 1), 4613 patients with follow-up (ITT 3) and 4393 in the per-protocol analysis
Data on albumin were available for 2592 treated patients (ITT 1), 2182 patients with follow-up (ITT3) and 2085 in the per-protocol analysis
ITT: intention-to-treat; IFN: interferon; DAA: direct-acting antivirals; MELD: Model for End-Stage Liver Disease; HIV: human immunodeficiency virus; HCV: hepatitis C virus; n.a.: not available.
Among all patients who started treatment (ITT1), cirrhosis was diagnosed in 1355 patients, of whom 1175 patients were classified as Child-Pugh A (86.7%), 156 patients as Child-Pugh B (11.5%) and 24 patients as Child-Pugh C (1.8%). Comorbidities were present in 4615 patients (75.6%). The most common comorbidities were cardiovascular diseases (27.2%), psychiatric diseases (15.1%), diabetes (10.1%) and thyroid dysfunction (9.8%). Chronic respiratory diseases were present in 5.3% of patients, malignancies, renal insufficiency and neurological comorbidities in 3.9%, 2.6% and 2.8%, respectively. Liver-related behavioral risk factors were drug abuse, which affected 14.4% of all treated patients, and alcohol abuse in 3.9% of patients. No major differences could be detected between the safety population and the effectiveness population (ITT3) as well as the population included in the per-protocol analysis (Table 1).
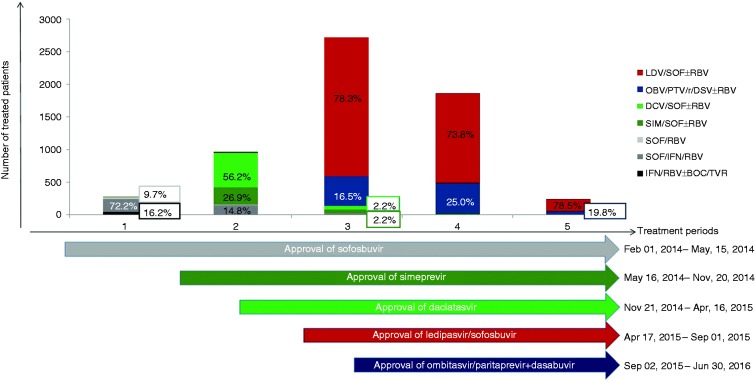
The choice of treatment regimens varied distinctly by time period (Figure 2). After approval of SOF in January 2014 the majority of patients (88.4%) were treated with PEG-IFN/RBV + DAA (SOF/TVR/BOC). IFN-free treatment was rapidly adopted after approval of SIM in May 2014 and DCV in August 2014 with 84.2% of patients receiving an SOF-based IFN-free treatment. LDV/SOF was approved in November 2014 and OBV/PTV/r/DSV in January 2015, accounting for 94.8% of all treatments after their approval. IFN-based treatment declined to less than 1% of all treatments prescribed during this period. In addition to the uptake of IFN-free treatment a 10-fold increase in absolute numbers of treated patients within the registry was observed (Figure 2).
Figure 2.
Treatment periods and treatment regimens (ITT1 population).
ITT: intention-to-treat; LDV: ledipasvir; SOF: sofosbuvir; RBV: ribavirin; OBV: ombitasvir; PTV/r: paritaprevir/ritonavir; DSV: dasabuvir; DCV: daclatasvir; SIM: simeprevir; IFN: pegylated-interferon; BOC: boceprevir; TVR: telaprevir.
In total, 373 patients completed an IFN-based therapy (7.7%) and 4473 patients completed IFN-free treatment (92.3%). Among these patients, 890 patients (19.9%) were treated for eight weeks, 2794 patients for 12 weeks (62.5%) and 422 patients for 24 weeks (9.5%). A total of 363 patients received a therapy with individualized treatment length other than eight, 12 or 24 weeks (8.1%). Complete data on used treatment regimens can be found in Supplemental Table S1. Treatment regimens varied also by the presence of cirrhosis. Usage of IFN-free treatment regimens for 24 weeks was highly associated with presence of cirrhosis (346/422 patients, 82.0%, p < 0.0001). However, the majority of patients with cirrhosis received IFN-free treatment for 12 weeks or less (n = 849 patients, 61.6% of all cirrhotics; data available for 4106 patients with distinct treatment durations).
Effectiveness of treatment
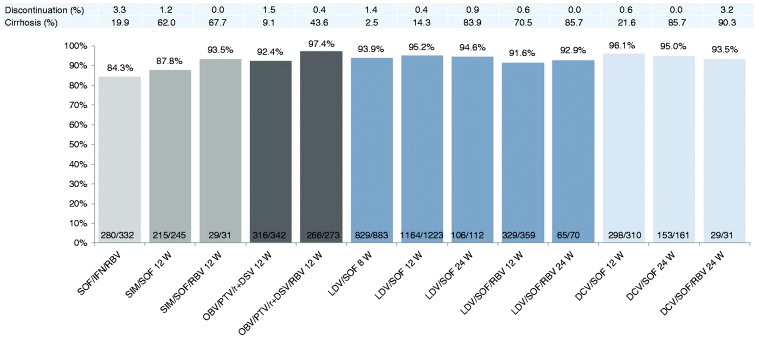
Overall, SVR12 was achieved in 4445/4846 patients (91.7%; ITT3). SVR12 rates were >90% for approved IFN-free treatment regimens except the combination of SIM/SOF without RBV for 12 weeks in mostly cirrhotic patients, which showed an SVR of 87.8%. The IFN-based combination SOF/RBV/PEG-IFN resulted in an SVR12 rate of 84.3% (Figure 3(a)). Complete data for all treatment regimens are shown in Supplemental Table S1. Because of the real-world nature of this study, the baseline characteristics of the patients treated with the various treatment regimens are different, thus, SVR12 rates of the different regimens can’t be directly compared to each other.
Figure 3(a).
SVR12 rates of the most frequently used approved antiviral regimens (effectiveness population, ITT3).
ITT: intention-to-treat; SOF: sofosbuvir; IFN: interferon; RBV: ribavirin; SIM: simeprevir; DCV: daclatasvir; LDV: ledipasvir; OBV: ombitasvir; PTV/r: paritaprevir/ritonavir; DSV: dasabuvir. Because of different baseline characteristics, SVR12 rates cannot be directly compared to each other.
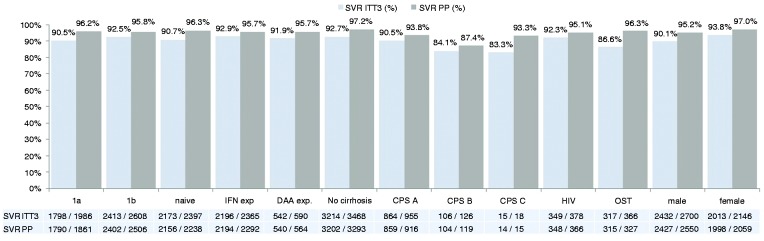
SVR12 rates for HCV genotype 1b were higher with 92.5% (2413/2608) vs 90.5% (1798/1986) for genotype 1a (p < 0.05) (Figure 3(b)). Previous treatment with (PEG)-IFN or DAAs did not result in lower SVR12 rates in comparison to treatment-naïve patients with SVR12 rates of 92.9%, 91.9% and 90.7%, respectively. Of the 590 patients with previous DAA-based treatment, none of the patients had a non-response to treatment. The majority of these patients received retreatment with a combination of LDV/SOF ± RBV or DCV/SOF for 12–24 weeks. Details about specific treatment regimens in DAA treatment-experienced patients and the respective SVR rates are shown in Table 2.
Figure 3(b).
SVR12 in subgroups (effectiveness population, ITT3).
ITT: intention-to-treat; SVR: sustained virological response, ITT: intention-to-treat, PP: per-protocol, 1a: hepatitis C virus genotype 1a, 1b: hepatitis C virus genotype 1b, naïve: treatment-naïve, IFN exp: pre-treatment with (pegylated)-interferon, DAA exp: pre-treatment with direct-acting antivirals, CPS: Child-Pugh-Score, OST: opioid-substitution therapy.
Table 2.
Treatment regimens and outcomes of DAA pre-treated patients (ITT3).
| Treatment regimen | Duration of treatment | Total number and frequency of patients (ITT3) | Treatment discontinuation | Non-response | Relapse | SVR | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (weeks) | N | % | N | % | N | % | N | % | N | % | |
| LDV/SOF | 12 | 144 | 24.4% | 0 | 0.0% | 0 | 0.0% | 2 | 1.4% | 139 | 96.5% |
| DCV/SOF | 24 | 70 | 11.9% | 0 | 0.0% | 0 | 0.0% | 1 | 1.4% | 68 | 97.1% |
| LDV/SOF/RBV | 12 | 57 | 9.7% | 1 | 1.8% | 0 | 0.0% | 1 | 1.8% | 50 | 87.7% |
| DCV/SOF | 12 | 40 | 6.8% | 1 | 2.5% | 0 | 0.0% | 0 | 0.0% | 38 | 95.0% |
| LDV/SOF/RBV | 24 | 36 | 6.1% | 0 | 0.0% | 0 | 0.0% | 2 | 5.6% | 33 | 91.7% |
| OBV/PTV/r + DSV/RBV | 12 | 35 | 5.9% | 0 | 0.0% | 0 | 0.0% | 1 | 2.9% | 34 | 97.1% |
| SIM/SOF | 12 | 34 | 5.8% | 0 | 0.0% | 0 | 0.0% | 3 | 8.8% | 31 | 91.2% |
| LDV/SOF | 24 | 32 | 5.4% | 0 | 0.0% | 0 | 0.0% | 1 | 3.1% | 31 | 96.9% |
| OBV/PTV/r + DSV | 12 | 29 | 4.9% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 28 | 96.6% |
| SOF/IFN/RBV | n.a. | 24 | 4.1% | 0 | 0.0% | 0 | 0.0% | 4 | 16.7% | 17 | 70.8% |
| DCV/SOF/RBV | 24 | 13 | 2.2% | 1 | 7.7% | 0 | 0.0% | 0 | 0.0% | 12 | 92.3% |
| OBV/PTV/r + DSV/RBV | other | 13 | 2.2% | 4 | 30.8% | 0 | 0.0% | 0 | 0.0% | 9 | 69.2% |
| LDV/SOF | other | 11 | 1.9% | 1 | 9.1% | 0 | 0.0% | 0 | 0.0% | 9 | 81.8% |
| DCV/SOF | other | 10 | 1.7% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 10 | 100.0% |
| SIM/SOF/RBV | 12 | 6 | 1.0% | 0 | 0.0% | 0 | 0.0% | 1 | 16.7% | 5 | 83.3% |
| LDV/SOF/RBV | other | 6 | 1.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 5 | 83.3% |
| OBV/PTV/r + DSV/RBV | 24 | 6 | 1.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 6 | 100.0% |
| SOF/RBV | 24 | 5 | 0.8% | 0 | 0.0% | 0 | 0.0% | 2 | 40.0% | 3 | 60.0% |
| SIM/SOF/RBV | other | 4 | 0.7% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 25.0% |
| LDV/SOF | 8 | 4 | 0.7% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 4 | 100.0% |
| DCV/SOF/RBV | other | 3 | 0.5% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 2 | 66.7% |
| SIM/SOF | other | 2 | 0.3% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 50.0% |
| DCV/SOF/RBV | 12 | 2 | 0.3% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 2 | 100.0% |
| OBV/PTV/r + DSV | other | 2 | 0.3% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 2 | 100.0% |
| SIM/SOF/RBV | 24 | 1 | 0.2% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 100.0% |
| OBV/PTV/r | n.a. | 1 | 0.2% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 100.0% |
| Total | Total | 590 | 100.0% | 8 | 1.4% | 0 | 0.0% | 18 | 3.1% | 542 | 91.9% |
ITT: intention-to-treat; DAA: direct-acting antivirals; SVR: sustained virological response; pts: patients; SOF: sofosbuvir; IFN: interferon; RBV: ribavirin; SIM: simeprevir; DCV: daclatasvir; LDV: ledipasvir; OBV: ombitasvir; PTV/r: paritaprevir/ritonavir; DSV: dasabuvir; n.a.: not available.
Patients with HIV co-infection had similar SVR12 rates with 92.3%. Lower SVR12 rates were observed in patients with advanced cirrhosis (Child-Pugh A 90.5%, Child-Pugh B 84.1% and Child-Pugh C 83.3%) and on opioid-substitution therapy (SVR12 86.6%, OST). The per-protocol analysis showed comparable SVR12 rates in comparison to the ITT3 analysis except for patients with Child-Pugh C cirrhosis (83.3% vs 93.3%, 15/18 vs 14/15) and OST (86.6% vs 96.3%, 317/366 vs 315/327). In these groups the difference was approximately 10%, indicating a substantial proportion of patients lost to follow-up. Virological response in the OST group was non-inferior to that in patients without OST. Non-response and relapse rates were 0.1% and 2.7% for patients without OST and 0.0% and 1.1% for patients with OST, respectively. Treatment discontinuation was more common in patients with OST (3.8% vs 1.3%).
Univariate analysis for genotype subtype, cirrhosis, age ≥60 years, choice of HCV therapy, gender, platelets <100/nl and initial viral load was performed to assess risk factors for virological non-response. The analysis revealed significant differences for the presence of cirrhosis, platelets, age, HCV therapy and gender (Table 3(a)). On multivariate analysis chance of SVR was significantly dependent on the choice of antiviral therapy, negatively correlated with presence of cirrhosis and positively correlated with female gender (Table 3(b)). However, HCV therapy was chosen by the physician based on the preconditions of the patient; therefore, baseline criteria of the populations treated with the various treatment regimens are different and SVR rates of the various treatment regimens are not directly comparable.
Table 3.
Analysis of parameters associated with SVR (ITT3 population, GT1a and GT1b patients only).
| Sig. | OR | 95% CI for OR |
||
|---|---|---|---|---|
| Lower | Upper | |||
| (a) Univariate analysis | ||||
| GT1 subtype | 0.016 | 13.149 | 1.619 | 106.772 |
| Cirrhosis | 0.001 | .683 | 0.549 | 0.850 |
| Age <60 vs ≥60 years | 0.018 | 1.334 | 1.050 | 1.695 |
| HCV therapy | 0.000 | 1.325 | 1.238 | 1.418 |
| Gender | 0.000 | 1.671 | 1.338 | 2.085 |
| Platelets <100 vs ≥100/nl | 0.001 | 1.648 | 1.232 | 2.205 |
| Initial viral load | 0.770 | .951 | 0.680 | 1.331 |
| (b) Multivariate analysis | ||||
| Cirrhosis | 0.003 | 0.705 | 0.560 | 0.886 |
| Gender | 0.000 | 1.519 | 1.207 | 1.911 |
| HCV therapy | 0.000 | 1.333 | 1.242 | 1.432 |
Sig.: significance level; OR: odds ratio; CI: confidence interval; GT: genotype; ITT: intention-to-treat; HCV: hepatitis C virus; SVR: sustained virological response.
Safety of treatment
Adverse events were reported in 3293 patients, affecting 53.9% of the study population (safety population). The most common adverse events affecting >10% of the study population were fatigue and headache (Table 4). Serious adverse events occurred in 240 patients (3.9%). Common serious adverse events were typical complications of advanced liver disease (i.e. liver malignancy, variceal bleeding; Table 4). Death during the study period was noted for 30 patients (0.5%) as the most common serious adverse event. Eight of these patients died while being on treatment. The reasons for death during treatment were upper gastrointestinal bleeding (n = 1), acute or chronic liver failure (n = 2), renal failure, septic peritonitis as complication of diverticulitis and unknown causes in three patients each. Among all patients, 13 patients died from liver-related complications (43.3%), whereas 12 died from reasons not related to liver disease (40.0%). In five patients reason for death was not reported (16.6%). Anemia was a frequent cause of adverse or serious adverse events with 156 (2.6%) and 13 (0.21%) of the safety population affected and is a very common side effect of RBV. Dose adjustment of the antiviral therapy occurred in 181 patients (3.0%).
Table 4.
Twenty most common adverse (AE) and serious adverse events (SAEs).
| AE | N | % of safety population (ITT1) | SAE | N | % of safety population (ITT1) |
|---|---|---|---|---|---|
| Fatigue | 1444 | 23.6% | Death | 30 | 0.49% |
| Headache | 955 | 15.6% | Anemia | 13 | 0.21% |
| Nausea | 405 | 6.6% | Liver malignancy | 11 | 0.18% |
| Insomnia | 342 | 5.6% | Variceal bleeding | 9 | 0.15% |
| Pruritus | 328 | 5.4% | Abdominal pain | 9 | 0.15% |
| Arthralgia | 288 | 4.7% | Dyspnea | 8 | 0.13% |
| Abdominal pain | 261 | 4.3% | Pneumonia | 6 | 0.10% |
| Skin disease | 256 | 4.2% | Atrial fibrillation | 5 | 0.08% |
| Diarrhea | 188 | 3.1% | Liver failure | 5 | 0.08% |
| Depressive mood | 181 | 3.0% | Gastrointestinal bleeding | 4 | 0.07% |
| Myalgia | 176 | 2.9% | Fever | 4 | 0.07% |
| Attention disorder | 159 | 2.6% | Fatigue | 4 | 0.07% |
| Anemia | 156 | 2.6% | Diarrhea | 4 | 0.07% |
| Dizziness | 153 | 2.5% | Rash | 4 | 0.07% |
| Irritability | 150 | 2.5% | Back pain | 3 | 0.05% |
| Dyspnea | 141 | 2.3% | Acute renal failure | 3 | 0.05% |
| Restlessness | 129 | 2.1% | Chronic renal failure | 3 | 0.05% |
| Alopecia | 121 | 2.0% | Myocardial infarction | 3 | 0.05% |
| Rash | 115 | 1.9% | Liver transplantation | 3 | 0.05% |
| Flu-like symptoms | 91 | 1.5% | Icterus | 3 | 0.05% |
n = number of patients; ITT: intention-to-treat.
Treatment discontinuations were assessed in the effectiveness population (ITT3). Overall, 122 patients (2.5%) discontinued treatment prematurely. Discontinuations were higher in patients treated with SOF/RBV + PEG-IFN with 3.3% (Figure 3(a)). Reasons for premature discontinuation were loss to follow-up in 43 patients (35.2%), poor adherence in 35 patients (28.7%), adverse events in 24 patients (19.7%), inadequate virological response in 16 patients (13.1%), death in eight patients (6.6%) and other reasons in six patients (4.9%). Although IFN-based treatment was used in only a minority of patients (373 patients, 7.7%), IFN-based treatments accounted for 25% (6/24) of all treatment discontinuations due to adverse events.
Discussion
The results from this large real-world registry with 6606 patients with HCV genotype 1 infection confirm the high SVR rates >90% from pivotal studies in a real-world scenario. Effectiveness was high in HCV genotype 1a and 1b, as well as compensated cirrhosis and HIV co-infected patients. This confirms the finding of several studies that HIV co-infection is not associated with reduced SVR rates anymore. In this cohort HIV co-infected patients had a slightly higher SVR rate of 92.3% in comparison to non-HIV infected patients. Similar results have also been shown in the German GECCO study for HIV co-infected patients.22 Patients with compensated cirrhosis had comparable SVR rates to non-cirrhotic patients at 90.5%. However, treatment regimens were modified according to cirrhosis status (Figure 3(a)). Lower rates are observed in patients with decompensated cirrhosis with 84.1% and 83.3% for Child-Pugh B and Child-Pugh C cirrhosis, respectively. Comparable results have been demonstrated in pivotal studies with combinations of SOF/LDV and SOF/DCV.23,24
Several dynamic changes in the use of DAA combinations in this real-world setting were observed. Whereas INF-based therapy was the mainstay of therapy in early 2014, a dramatic shift to INF-free treatment to late 2014 could be detected. This may reflect guideline recommendations and the results of pivotal studies showing higher SVR rates and lower frequency of adverse events with IFN-free treatment. SVR rates of IFN-based therapy were lower with 84.3% for SOF + PEG-IFN + RBV representing a mix of premature treatment discontinuation together with lower efficacy.
Simultaneously with the decrease in IFN-based treatment, a 10-fold increase in treated patients can be observed within the registry. Although this finding cannot generalize an overall uptake in HCV treatment in Germany, this finding may resemble an increased acceptance of HCV treatment by physicians and patients. Certainly, the awareness of the results from phase III and IV studies is a main driver for treatment uptake and the choice of treatment. In a recent paper the increase in treated patients with hepatitis C in Germany was reported to triple from 2014 to 2015.25
All health care systems operate under cost constraints and costs can influence treatment decisions, which may lead to suboptimal effectiveness or undertreatment. Regarding the choice of treatment, a key finding is that treatment for 24 weeks was strongly associated with presence of cirrhosis, essentially reserving the longest and most costly treatment strategy for a minority of difficult-to-treat patients. Importantly, most patients with early-stage cirrhosis were still treated for 12 weeks with comparable SVR rates, mainly with a regimen including ribavirin. This is associated with substantial cost savings for the health care system and shows that physicians achieved very good results by tailoring treatment according to the patient’s preconditions taking treatment cost into account.
However, patients with decompensated cirrhosis Child-Pugh stage B and C demonstrated lower SVR rates of 84.1% and 83.1%. These results are in line with outcomes from phase II and III studies.23,24 It is important to note that only a few patients with Child-Pugh C cirrhosis were treated in this large cohort (24/6108, 0.4%). This may be due to center selection, as these patients are candidates for liver transplantation. A Model for End-Stage Liver Disease (MELD) score ≥27 has been suggested as a threshold above which treatment may have detrimental effects on the patient and treatment after liver transplantation may be the better option.26
Treatment retention was high, indicated by a low treatment discontinuation rate of 2.3%. However, discrepancies between the per-protocol analysis and the ITT analysis were observed for patients on OST. Although the virological response was not inferior to patients without OST, rates of treatment discontinuation and loss of follow-up were higher in these patients, explaining the differences in observed SVR rates and partly contradicting findings from phase III studies, showing no influence of OST on treatment.27 Supportive measures have been shown to increase retention and SVR rates in these difficult-to-treat patients.28
In conclusion, the data of the real-world DHC-R validate effectiveness and safety for treatment regimens that have been approved with limited data for certain subgroups. It shows dynamic changes in treatment uptake and treatment choice based on several events, demonstrating timely treatment adjustments by the responsible physicians after release of new treatment options and guideline recommendations. A high success rate was achieved even in more difficult-to-treat patients, thereby demonstrating a balance between patients’ needs and demands of the health care system.
Supplementary Material
Acknowledgments
Special thanks to Bianka Wiebner and Yvonne Serfert for the management of the German Hepatitis C-Registry.
Declaration of conflicting interests
Christoph Höner zu Siederdissen reports personal fees from Gilead, MSD and Roche outside the submitted work. Peter Buggisch reports speaker bureau and consultancy fees from AbbVie, BMS, Gilead, Janssen and MSD outside the submitted work. Klaus H.W. Böker reports personal fees from Gilead Sciences, MSD Sharp & Dohme/Merck and AbbVie, and others from Janssen, outside the submitted work. Eckart Schott reports personal fees from Abbvie, BMS, Gilead and MSD outside the submitted work. Hartwig Klinker reports personal fees from AbbVie, BMS, Gilead, Hexal, Janssen, MSD, Arrowhead and Novartis, and grants/research support from AbbVie, BMS, Gilead, Janssen, MSD, Arrowhead and Novartis outside the submitted work. Anita Pathil reports personal fees from AbbVie, Gilead and Bristol-Myers Squibb outside the submitted work. Heike Pfeiffer-Vornkahl is an employee of e.factum (CRO) working for AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Leberstiftungs-GmbH Deutschland, MSD and Roche. Thomas Berg reports grants, personal fees and non-financial support from Abbvie, grants, personal fees and non-financial support from Gilead, grants and personal fees from BMS, personal fees from Bayer, grants and personal fees from Janssen, grants and personal fees from Roche, personal fees from Vertex, personal fees from Tibotec, personal fees from Intercept, and grants and personal fees from Sequana Medical, outside the submitted work. Christoph Sarrazin reports grants and personal fees from Abbott, Gilead, Janssen, Qiagen, Roche and Siemens, and personal fees from AbbVie, BMS and Merck/MSD outside the submitted work. Dietrich Hüppe reports personal fees from AbbVie, Bristol-Myers Squibb, Boehringer-Ingelheim, Echosens, Falk, Gilead Sciences, Janssen-Cilag, MSD Sharp&Dohme/Merck and Roche Pharma outside the submitted work. Michael P. Manns reports grants, personal fees and non-financial support from Roche, BMS, Gilead, Boehringer Ingelheim, Biotest, AbbVie, Merck/MSD, Janssen and GlaxoSmithKline, and grants from Novartis outside the submitted work. Stefan Mauss reports personal fees from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, MSD Sharp&Dohme/Merck and ViiV, outside the submitted work.
Funding
The German Hepatitis C-Registry (DHC-R) is a project of the German Liver Foundation (Deutsche Leberstiftung) managed by Leberstiftungs-GmbH Deutschland in cooperation with the Association of German gastroenterologists in private practice (bng, Bund Niedergelassener Gastroenterologen) with financial support from the German Center for Infection Research (DZIF) as well as from the companies AbbVie Deutschland GmbH & Co. KG, Bristol-Myers Squibb GmbH & Co. KGaA, Gilead Sciences GmbH, Janssen-Cilag GmbH, MSD Sharp & Dohme GmbH, and Roche Pharma AG. The authors are independent from the funding companies in data analysis, data interpretation, report writing, and publication.
Ethics approval
This study protocol was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol was approved by the central Institutional Review Board (Ethics Committee of Aerztekammer Westfalen-Lippe) on September 2, 2014. The registry was registered at the Federal Institute for Drugs and Medical Devices (BfArM; study number 2493) and the German Clinical Trials Register (DRKS; ID DRKS00009717).
Informed consent
All participants had to provide written informed consent before being enrolled in the registry.
References
- 1.Gower E, Estes C, Blach S, et al. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014; 61(1 Suppl): S45–S57. [DOI] [PubMed] [Google Scholar]
- 2.Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386: 743–800. [DOI] [PMC free article] [PubMed]
- 3.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012; 308: 2584–2593. [DOI] [PubMed] [Google Scholar]
- 4.Hézode C, Fontaine H, Dorival C, et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC)-NCT01514890. J Hepatol 2013; 59: 434–441. [DOI] [PubMed] [Google Scholar]
- 5.Lawitz E, Ghalib R, Rodriguez-Torres M, et al. Simeprevir plus sofosbuvir with/without ribavirin in HCV genotype-1 prior null-responder/treatment-naive patients (COSMOS study): Primary endpoint (SVR12) results in patients with METAVIR F3-4 (Cohort 2). J Hepatol 2014; 60: S524–S524. [Google Scholar]
- 6.Sulkowski M, Gardiner D, Rodriguez-Torres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 2014; 370: 211–221. [DOI] [PubMed] [Google Scholar]
- 7.Lawitz E, Matusow G, DeJesus E, et al. Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrhosis: A phase 3 study (OPTIMIST-2). Hepatology 2016; 64: 360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sulkowski MS, Vargas HE, Di Bisceglie AM, et al. Effectiveness of simeprevir plus sofosbuvir, with or without ribavirin, in real-world patients with HCV genotype 1 infection. Gastroenterology 2015; 150: 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwo P, Gitlin N, Nahass R, et al. Simeprevir plus sofosbuvir (12 and 8 weeks) in HCV genotype 1-infected patients without cirrhosis: OPTIMIST-1, a phase 3, randomized study. Hepatology 2016; 64: 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014; 370: 1879–1888. [DOI] [PubMed] [Google Scholar]
- 11.Ferenci P, Bernstein D, Lalezari J, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med 2014; 370: 1983–1992. [DOI] [PubMed] [Google Scholar]
- 12.Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014; 370: 1483–1493. [DOI] [PubMed] [Google Scholar]
- 13.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370: 1889–1898. [DOI] [PubMed] [Google Scholar]
- 14.Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 2014; 370: 1973–1982. [DOI] [PubMed] [Google Scholar]
- 15.Feld JJ, Moreno C, Trinh R, et al. Sustained virologic response of 100% in HCV genotype 1b patients with cirrhosis receiving ombitasvir/paritaprevir/r and dasabuvir for 12 weeks. J Hepatol 2015; 64: 301–307. [DOI] [PubMed] [Google Scholar]
- 16.Pawlotsky JM, Negro F, Aghemo A, et al. EASL recommendations on treatment of hepatitis C 2016. J Hepatol 2017; 66: 153–194. [DOI] [PubMed] [Google Scholar]
- 17.Younossi ZM, Park H, Dieterich D, et al. Assessment of cost of innovation versus the value of health gains associated with treatment of chronic hepatitis C in the United States. Medicine (Baltimore) 2016; 95: e5048–e5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wedemeyer H, Duberg AS, Buti M, et al. Strategies to manage hepatitis C virus (HCV) disease burden. J Viral Hepat 2014; 21(Suppl 1): 60–89. [DOI] [PubMed] [Google Scholar]
- 19.Stahmeyer J, Rossol S, Bert F, et al. Kosten einer leitliniengerechten Versorgung von Hepatitis-C-Patienten im Zeitalter Interferon-freier Therapien. Z Gastroenterol 2016; 54: 760–769. [DOI] [PubMed] [Google Scholar]
- 20.Tacke F, Günther R, Buggisch P, et al. Treatment of HCV genotype 2 with sofosbuvir and ribavirin results in lower sustained virological response rates in real life than expected from clinical trials. Liver Int 2017; 37: 205–211. [DOI] [PubMed] [Google Scholar]
- 21.Cornberg M, Petersen J, Schober A, et al. Real-world use, effectiveness and safety of anti-viral treatment in chronic hepatitis C genotype 3 infection. Aliment Pharmacol Ther 2017; 45: 688–700. [DOI] [PubMed] [Google Scholar]
- 22.Ingiliz P, Christensen S, Kimhofer T, et al. Sofosbuvir and ledipasvir for 8 weeks for the treatment of chronic hepatitis C virus (HCV) infection in HCV-monoinfected and HIV-HCV–coinfected individuals: Results from the German Hepatitis C Cohort (GECCO-01). Clin Infect Dis 2016; 63: 1320–1324. [DOI] [PubMed] [Google Scholar]
- 23.Manns M, Samuel D, Gane EJ, et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: A multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis 2016; 16: 685–697. [DOI] [PubMed] [Google Scholar]
- 24.Poordad F, Schiff ER, Vierling JM, et al. Daclatasvir with sofosbuvir and ribavirin for HCV infection with advanced cirrhosis or post-liver transplant recurrence. Hepatology 2016; 63: 1493–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmermann R, Kollan C, Ingiliz P, et al. Real-world treatment for chronic hepatitis C infection in Germany: Analyses from drug prescription data, 2010–2015. J Hepatol. Epub ahead of print 9 February 2017. DOI: 10.1016/j.jhep.2017.01.024. [DOI] [PubMed]
- 26.Chhatwal J, Samur S, Kues B, et al. Optimal timing of hepatitis C treatment for patients on the liver transplant waiting list. Hepatology 2017; 65: 777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grebely J, Mauss S, Brown A, et al. Efficacy and safety of ledipasvir/sofosbuvir with and without ribavirin in patients with chronic HCV genotype 1 infection receiving opioid substitution therapy: Analysis of phase 3 ION trials. Clin Infect Dis 2016; 63: 1405–1411. [DOI] [PubMed] [Google Scholar]
- 28.Reimer J, Schmidt CS, Schulte B, et al. Psychoeducation improves hepatitis C virus treatment during opioid substitution therapy: A controlled, prospective multicenter trial. Clin Infect Dis 2013; 57(Suppl 2): S97–S104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.