Abstract
Background
Endoscopic screening for gastric cancer is debatable in countries with an intermediate risk.
Objective
The objective of this article is to determine the cost-utility of screening strategies for gastric cancer in a European country.
Methods
We conducted a cost-utility analysis using a Markov model comparing three screening strategies versus no screening: stand-alone upper endoscopy, endoscopy combined with a colorectal cancer screening colonoscopy after a positive faecal occult blood test or pepsinogens serologic screening. Clinical data were collected from systematic reviews, costs from published national data and utilities as quality-adjusted life years (QALY). The primary outcome was the incremental cost-effectiveness ratio (ICER). Deterministic and probabilistic sensitivity analyses were performed. The threshold was set at €37,000 (2016 prices).
Results
Upper endoscopy combined with screening colonoscopy (every 10 or 5 years) had an ICER of 15,407/QALY and €30,908/QALY respectively, stand-alone endoscopic screening (every five years) an ICER of €70,693/QALY and pepsinogens screening an ICER of €143,344/QALY. Sensitivity analyses revealed that only endoscopic costs <€75, a provision of only three endoscopies per patient or a gastric cancer risk >25/100,000 would make stand-alone endoscopic screening cost-effective.
Conclusion
Endoscopic gastric cancer screening in Europe can be cost-effective if combined with a screening colonoscopy in countries with a gastric cancer risk ≥10 per 100,000.
Keywords: Stomach neoplasm, gastrointestinal endoscopy, early detection of cancer, costs and cost analysis, Markov chains
Established knowledge on this subject:
Endoscopic screening for gastric cancer is advocated only in high-risk countries.
In countries with an intermediate risk for gastric cancer, endoscopic screening is debatable.
Gastric cancer endoscopic screening cost-effectiveness in European countries has never been evaluated.
What are the significant and/or new findings of this study?
Endoscopic gastric cancer screening combined with screening colonoscopy is cost-effective in some European countries.
Endoscopic resources allocated to colorectal screening can provide further benefit for gastric cancer prevention.
Introduction
Gastric cancer is the fifth most common malignancy and is the third leading cause of cancer-related death worldwide.1 Its prognosis is closely related to stage of diagnosis with an overall five-year survival below 25%. This can be improved by early detection by means of screening strategies as well as surveillance of patients at higher risk.2 At present, screening for gastric cancer is performed only in countries with a high risk of disease (defined as an age-standardised rate (ASR) ≥20 per 100,000) such as Japan and Korea (29.9 and 41.3, respectively).3
Screening is mainly performed by upper endoscopy, improves survival and is cost-effective in high-incidence regions.4 Screening enables the detection of gastric cancer at earlier stages, eventually as early gastric cancer, defined as carcinoma limited only to the mucosa or submucosa, regardless of lymph node involvement; this is usually accessible by endoscopic treatment, such as endoscopic submucosal dissection.5,6
In contrast, in countries with a low incidence of gastric cancer (defined as an ASR <10 per 100,000) such as the United States of America (USA) (3.9 per 100,000), there is no rationale for endoscopic screening in terms of allocation of resources and costs.7
In countries with an intermediate risk for gastric cancer the decision on endoscopic screening is less clear and economic analyses are needed to define the best strategy in terms of health benefit and use of economic resources. Guidelines recommend endoscopic surveillance every three years for patients at higher risk of gastric cancer progression due to the presence of extensive atrophy or intestinal metaplasia. This surveillance strategy proved to be cost-effective in 50- to 75-year-old individuals, but it might apply to only 7% of the population, while screening is intended for the whole population in the target age group.8–10
The main aim of our study was to evaluate the cost-utility by means of a Markov model of upper endoscopic screening versus no screening in a European country with an intermediate risk for gastric cancer.
Materials and methods
Target population
The target population was defined as all Portuguese men and women aged 50 to 75 years, but because this study is an economic model no human participants were used. The age range was based on the fact that most gastric adenocarcinomas are diagnosed after the age of 50 years, and is similar to the European colorectal cancer screening recommendations.11 Portugal has organised cancer screening programs in most of the country for breast, cervical and colorectal cancer, in this case by faecal occult blood testing with colonoscopy for positive cases. The same structural organisations could be used for the implementation of gastric cancer screening without a relevant increase of organisation costs, beyond the cost for the endoscopic exams and need for endoscopic resources.
Model structure
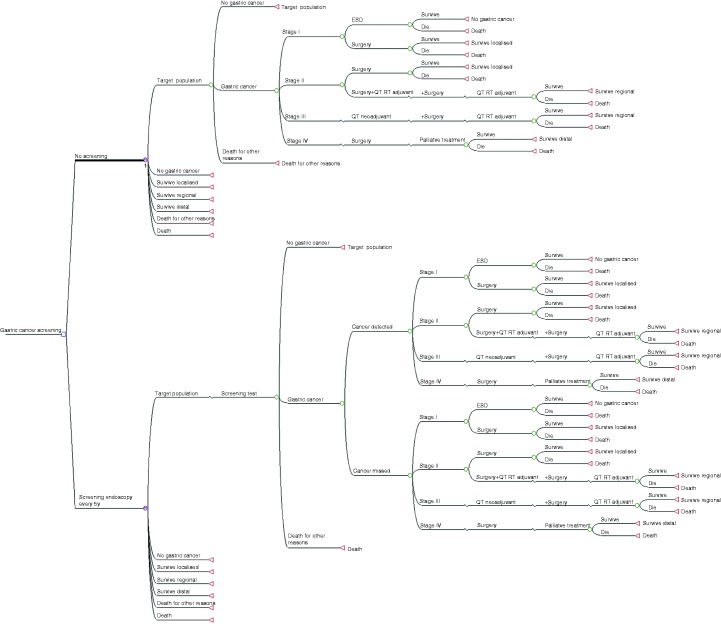
A Markov model was designed to compare every screening strategy versus the current no screening option. The model incorporated all stages of disease from a healthy stomach to early and advanced cancer, as well as post-treatment follow-up. It determined post-cancer survival, death by gastric cancer and death by other causes (Figure 1). In the model, every branch corresponds to an individual state of disease (circle), and Markov cycles start at the beginning of each branch for any of the screening strategies, in the figure represented as a single alternative for simplification of presentation (circles with an M). On the far right are the terminal stages (triangles) for the Markov cycles and patients at these stages return to the beginning of the next cycle after a one-year cycle, unless deceased.
Figure 1.
Markov model for comparison of gastric cancer screening versus no screening.
Tree diagram representing all possible options for patients at risk for gastric cancer in Portugal. The main research question in this study corresponds to the first dichotomous branch on the far left side comparing no screening versus screening. For simplicity of the figure only two options (screening strategy versus no screening) are represented although for the model three options were tested: stand-alone endoscopy, endoscopy along with a screening colonoscopy and serologic pepsinogens. Every branch corresponds to an option for every state (circle) and Markov cycles start at the beginning of each starting branch, no screening or screening respectively (circle with an M). On the far right are the terminal states (triangles) for the Markov cycles and patients at these stages return to the beginning of the next cycle after a one-year cycle, except for the Death stage.
ESD: endoscopic submucosal dissection; QT: chemotherapy; RT: radiotherapy.
Three hypotheses were modelled versus the no screening strategy. Strategy number one, the main intervention under study, was stand-alone endoscopic screening for gastric cancer by upper digestive endoscopy between 50 and 75 years old every five years. This choice of a five-year interval upper endoscopy is based on a conservative approach considering the minimum safe gap between endoscopies to prevent any interval gastric cancer (no guidelines or studies exist on this issue).
Strategy number two was endoscopic screening combined with an already performed colorectal cancer screening colonoscopy after a positive faecal occult blood test every 5–10 years (and only the extra endoscopy costs were accounted). This 5- to 10-year interval is a mix between the recommended colonoscopy intervals for the 45% of patients without polyps after a positive faecal occult blood (10 years according to recent guidelines) and the 55% of patients with polyps after a positive faecal occult blood (between three and five years based on the pathology results).12,13
Strategy number three was biennial serology screening by means of pepsinogen I and II followed by endoscopy only in positive cases, defined as a pepsinogen I ≤70 ng/ml and a pepsinogen I/II ratio ≤3.14,15
In accordance with the recommendations for reporting cost-effectiveness analyses, a societal perspective was adopted. This means the inclusion of costs charged to the health system, patients, families and employers, thereby representing the public interest rather than that of any specific group.16 Also according to guidelines recommendations, a cost-utility economic analysis was adopted to adjust life years saved to their quality by using community utilities in terms of quality-adjusted life years (QALY).
For the elaboration of the model and drafting of the manuscript we adopted the suggestions of the Consolidated Health Economic Evaluation Reporting Standards (CHEERS).17 The software used was TreeAge Pro 2009 (TreeAge Software, Williamstown, MA, USA).
Clinical data
An extensive review of the available literature was conducted in PubMed to look for the best available estimates for each variable in terms of transition probability for gastric cancer risk, distribution by cancer stage, efficacy and adverse events of treatments, and disease-specific survival. This was performed by means of the following search terms: gastric cancer, endoscopy, endoscopic submucosal dissection, gastrectomy, chemotherapy, radiotherapy, adverse events and costs. Where available, systematic reviews, meta-analyses and studies specifically conducted for the Portuguese population were preferred.18 All variables and respective references inserted in the model are presented in a supplementary file with point estimates for the base case scenario and their plausible ranges according to the published literature.
Cost data
Costs were calculated in euros (€) and given in 2016 prices. Prices from previous years were adjusted for inflation with an online conversion tool provided by Pordata.19 A discount rate of 3% was incorporated for both costs and effectiveness, ranging in the sensitivity analysis between 0 and 5% in accordance with published recommendations.16
Costs were estimated from national sources for endoscopic costs (endoscopy costs, related procedures and user fees but without administration-related costs), health state costs (related to disease stage and corresponding treatments), adverse event costs (endoscopic procedures and gastric cancer treatments such as surgery, chemotherapy and radiotherapy) and indirect costs (working days lost for patients and transportation). Resource use such as appointments to healthcare was also included, but no assumptions were made for issues such as nurse time or time lost for relatives or caregivers. Employers’ costs were based on the cost per hour reported by the Portuguese Institute of Statistics, but no changes in productivity were included in the model.
Utility data
Health states and utilities for individuals with present gastric cancer and those who survived after treatment were obtained with a single standardised health measurement instrument through a cross-sectional nationwide study of patients undergoing upper gastrointestinal endoscopy (n = 1434).20 The EQ-5D-5L quality of life questionnaire (EuroQol) was used. The results allowed adjustment for age and gender for inclusion in the present model.
Assumptions
To allow for comparability to other publications we used the same stages of disease without screening and the same improved stages after screening used in previous models.21–23 Also for comparability, the proportion of stage I early cancers manageable by endoscopic treatment after screening (30%) and the proportion of stage II cancers treated only by surgery (60%) were similar to a previous published model.24 We assumed that without screening only 1% of all gastric cancers would be detected at an early stage. The effectiveness of upper endoscopy in terms of early gastric cancer diagnosis was assumed to be the same for all screening strategies. The strategies differed only in terms of costs and adherence rates.
The diagnosis and surveillance of premalignant conditions, increased risk due to a positive family history of early-onset gastric cancer as well as endoscopic yield with the new high-resolution endoscopic technologies were out of the scope of the present model.8,9 Also in the present model, every gastric cancer patient received the same treatment according to stage of disease, irrespective of diagnosis with or without endoscopic screening.
Assumptions had to be made on expenses on transportation of patients and relatives; asymptomatic patients or patients without gastric cancer were assigned with a utility of 1 and for comparison of strategies a full compliance with endoscopic screening was used although it is well known from countries with screening programmes running that the adherence is well below 100%.25–28
Cost-utility analysis
The primary outcome measure was the incremental cost-effective ratio (ICER) between the screening strategies versus the no screening option. Costs were included in the numerator and effectiveness in the denominator in terms of QALY.
The willingness-to-pay was set at twice the Portuguese gross national income per capita in 2016 prices, according to the Atlas method of the World Bank, as suggested by the Commission for Macroeconomics and Health of the World Health Organisation, corresponding in US dollars ($) to 2 × $20,530 = $41,060 or in Euros (€) to €37,000 after conversion at 2016 rates.29 A strategy that would fall below this threshold was considered to be cost-effective or to have cost-utility.
One-way deterministic sensitivity analysis was performed for every single variable to identify parameters relevant to the model. Further probabilistic sensitivity analysis was conducted with all the parameters running at the same time. To assign the respective distributions for these variables, the mean and standard deviation were approximated using the calculations provided by the TreeAge software. A half-cycle correction was used on all transitions in state, in both costs and outcomes.
Results
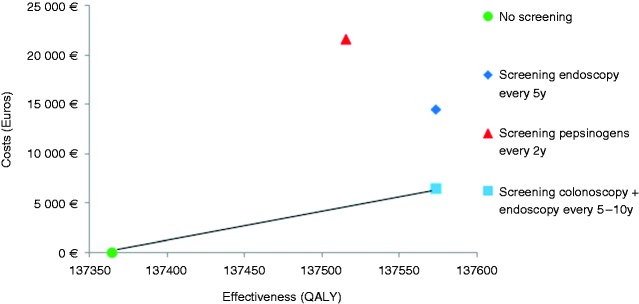
The results of the base case scenario are presented in Figure 2 and detailed in Table 1. In Portugal, performing a screening upper endoscopy in combination with an already performed colorectal cancer screening colonoscopy after a positive faecal occult blood test was cost-effective, with additional endoscopy costs of just €60. This strategy provided an ICER of €15,407–30,908/QALY, below the adopted threshold of €37,000/QALY, depending on the endoscopic interval every 10 or 5 years, respectively. The strategy with a stand-alone upper endoscopy every five years at a mean cost of €137 (including endoscopic, anaesthetic, work lost and transportation cost) was not cost-effective with an ICER result of €70,396/QALY. Serologic pepsinogen screening every two years at a cost of €100 plus the endoscopic cost for patients with a positive test resulted in an ICER of €143,344/QALY.
Figure 2.
Cost-effectiveness analysis comparing strategies for gastric cancer screening.
Cost-effectiveness analysis comparing three different gastric cancer screening strategies versus no screening, from the age of 50 to 75 years old. The x-axis represents the effectiveness in quality-adjusted life years (QALY) and the y-axis represents the cost in euros (€). The best cost-effective strategy was upper endoscopic screening combined with screening colonoscopy, providing more effectiveness at lower costs than the other options.
Light blue square: screening endoscopy associated with an already performed colonoscopy for colorectal cancer screening, every 5 to 10 years, where only the extra endoscopy cost is accounted for.
Dark blue diamond: stand-alone screening endoscopy every five years accounting for endoscopic, anaesthetic, work lost and transportation costs.
Red triangle: screening pepsinogen I and II every two years followed by endoscopy for positive cases only.
Green circle: no screening option.
Table 1.
Base case results of the endoscopic or serologic screening options versus no screening, according to different gastric cancer risk rates.
| Age standardised rate per 100,000 (gastric cancer risk) | Strategy | Cost € | Incremental cost € | Effectiveness QALY | Incremental effectiveness QALY | Cost- effectiveness €/QALY | Incremental cost-effectiveness ICER) €/QALY | Threshold of willingness- to-pay | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| 13.1 (intermediate risk) (rate in Portugal) | No screening | 44 | 137.364 | 0 | 37,000 | Cost-effective in Portugal | |||
| Endoscopy combined with colonoscopy every 10 years | 3,265 | 3,221 | 137.573 | 0.209 | 24 | 15,407 | |||
| Endoscopy combined with colonoscopy every five years | 6,506 | 6,462 | 137.573 | 0.209 | 47 | 30,908 | |||
| Stand-alone endoscopic screening every five years | 14,485 | 14,781 | 137.573 | 0.209 | 108 | 70,396 | Not cost-effective in Portugal | ||
| Pepsinogens screening every two years | 21,631 | 21,587 | 137.515 | 0.151 | 157 | 143,344 | |||
| 29.9 (high risk) (rate in Japan) | No screening | 101 | 136.441 | 0 | 66,000 | Cost-effective (if in Japan) | |||
| Stand-alone endoscopic screening every five years | 14,814 | 14,714 | 136.916 | 0.475 | 108 | 30,984 | |||
| 8.2 (intermediate risk) (rate in Singapore) | No screening | 28 | 137.635 | 0 | 93,400 | Not cost-effective (if in Singapore) | |||
| Stand-alone endoscopic screening every five years | 14,828 | 14,800 | 137.766 | 0.131 | 108 | 112,924 | |||
| 3.9 (low risk) (rate in USA) | No screening | 13 | 137.874 | 0 | 99,000 | Not cost -effective (if in USA) | |||
| Stand-alone endoscopic screening every 5 years | 14,813 | 14,818 | 137.936 | 0.062 | 108 | 237,406 |
Screening for gastric cancer in the model starts at the age of 50 until age 75. Endoscopy combined with colonoscopy means that, if associated with a colonoscopy already performed for colorectal cancer screening, every 5 to10 years (with or without polyps), only the extra endoscopy cost is accounted for; stand-alone endoscopic screening every five years means that the price includes endoscopic, anaesthetic, work lost and transportation costs; pepsinogen screening every two years means serologic screening measure of pepsinogens I and II, followed by endoscopy for positive cases only (positive cases if pepsinogen I ≤70 ng/ml and pepsinogen I/II ratio ≤3). The threshold of willingness-to-pay was set at 2× the gross national income per capita in euros for the year 2016.
ICER: incremental cost-effectiveness ratio; QALY: quality-adjusted life years; USA: United States of America; €: euros; $US: United States dollars.
Table 1 also shows the scenarios of the model for Japan, Singapore and the USA. This illustrates how the results depend on the gastric cancer incidence in the population. Stand-alone endoscopic screening every five years would be cost-effective in Japan (high ASR of 29.9/100,000) with an ICER of €30,984/QALY for a threshold of €66,000/QALY, but not cost-effective in Singapore (intermediate ASR of 8.2/100,000) with an ICER of €112,924/QALY for a high threshold of €93,400/QALY nor in the USA (low ASR of 3.9/100,000) with an ICER of €237,406/QALY for a high threshold of €99,000/QALY.
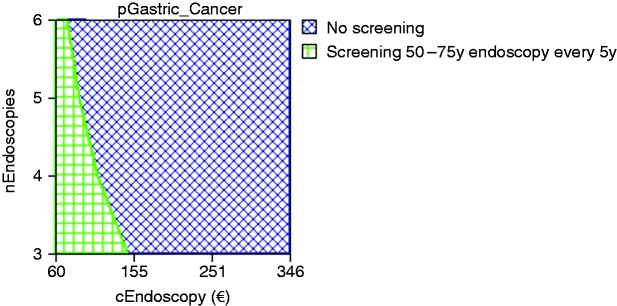
In deterministic one-way sensitivity analysis, three variables proved to be relevant to the model, and their possible range of values affected the ICER and cost-utility for endoscopic screening. These variables were the endoscopy costs, the number of endoscopies per patient over the screening age range and the ASR of gastric cancer. Table 2 presents the threshold values that would change the conclusion of the model and would make screening for gastric cancer cost-effective in Portugal. For the option of a screening endoscopy combined with a screening colonoscopy, the model remained cost-effective as long as the gastric cancer incidence in terms of ASR was ≥10/100,000. The option of a stand-alone screening endoscopy every five years was cost-effective only if the endoscopy costs were ≤€75, only three screening exams were performed per screened person (1 every 10 years) or if the ASR was ≥25/100,000. Figure 3 demonstrates these combined results for the stand-alone endoscopy under the current Portuguese ASR of 13.1/100,000, showing that the model would be cost-effective only for any screening strategy every five years (six exams per patient) if the endoscopic or serologic cost would be ≤ €75 or for an endoscopic cost of €160 if only three exams could be performed per screened person (one endoscopic exam every 10 years).
Table 2.
Deterministic one-way sensitivity analysis results of the endoscopic screening strategies versus no screening.
| Variable | Base case value | Range | Threshold to change the cost-effective strategy | Explanation for screening to be cost-effective |
|---|---|---|---|---|
| Scenario: endoscopic screening combined with screening colonoscopy | ||||
| Gastric cancer risk (age standardised rate) | 13.1 per 100,000 (rate in Portugal) | 3.9–29.9 per 100,000 | 10 per 100,000 | An age standardised rate ≥10 |
| Scenario: stand-alone endoscopic screening | ||||
| Endoscopic cost (from a societal point of view) | €137 (considering fees, hospital costs, anaesthesia, transportation and work lost) | €60–€398 | €75 | Endoscopic cost between €60 and €75 |
| Number of screening exams (between 50 and 75 years old) | 6 (one screening exam every five years) | 3–6 | 3 | Only three screening exams per patient (1 every 10 years) |
| Gastric cancer risk (age standardised rate) | 13.1 per 100,000 (rate in Portugal) | 3.9–29.9 per 100,000 | 25 per 100,000 | An age standardised rate ≥25 |
€: euros.
Figure 3.
Deterministic three-way sensitivity analysis on relevant variables for the model for the option of stand-alone endoscopic screening every five years versus no screening.
Deterministic sensitivity analysis combining the three variables that proved in the model to be relevant: cost of endoscopy (cEndoscopy), number of endoscopies to perform from 50 to 75 years of age (nEndoscopies) and risk of gastric cancer (pGastric Cancer). For the model to be cost-effective the combination of these three variables needs to fall within the green horizontal squared area of the graph. Any combination of these three factors that falls within the blue transversal square area means that the model is not cost-effective.
€: euros
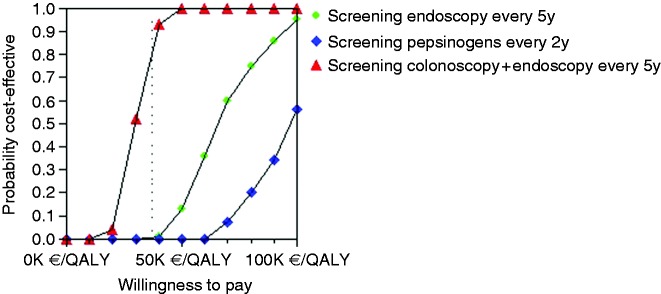
Probabilistic multi-way sensitivity analysis based on 1000 Monte Carlo simulations showed that the stand-alone endoscopic screening every five years strategy would be cost-effective in only 0.1% of cases (Figure 4). Figure 5 illustrates that endoscopic screening every five years has a probability of 86% to be cost-effective for the Portuguese population if combined with screening colonoscopy at a reduced price of only €60. Stand-alone screening endoscopy (mean cost of €137) and pepsinogen screening (cost of €100) followed by endoscopy in positive cases are not cost-effective.
Figure 4.
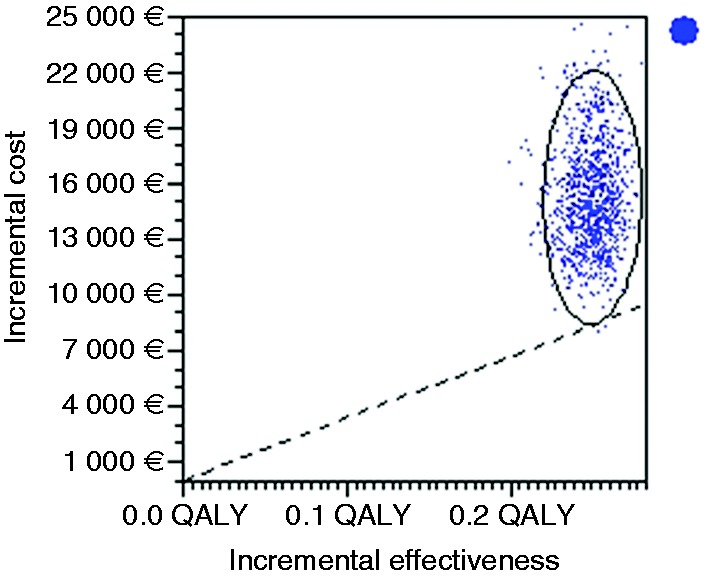
Incremental cost effectiveness. Scatter plot for probabilistic Monte Carlo sensitivity analysis of the model for the option of stand-alone endoscopic screening every five years versus no screening.
Scatter plot representing 1000 simulations in a Monte Carlo probabilistic sensitivity analysis where the x-axis represents incremental effectiveness in terms of quality-adjusted life years (QALY) and the y-axis represents incremental costs in euros (€). Each dot represents one simulation of the model and the ellipse surrounds the simulations that fall within the 95% confidence intervals. Cost effective simulations are mainly present on the right-hand side and above the dotted line representing the willingness-to-pay threshold, set at €37,000. A cost-effective option would have most of the dots below the dotted willingness-to-pay threshold line (only 0.1% of all simulations in this case).
Figure 5.
Acceptability curves for each gastric cancer screening strategy versus no screening.
Acceptability curves comparing all screening strategies versus no screening, where the x-axis represents the willingness-to-pay in euros per quality-adjusted life years (QALY) and the y-axis represents the probability of cost-effectiveness, ranging from 0 to 100%. The vertical dotted line represents the threshold of willingness-to-pay set at €37,000 above for the Portuguese population in 2016. The intersection of each screening strategy with the dotted line represents the probability for that strategy to be cost-effective according to the model, meaning that a screening endoscopy along with an already scheduled screening colonoscopy has a probability of 86% to be cost-effective, a stand-alone screening endoscopy has only a 0.1% probability and screening pepsinogens plus endoscopy for positive cases is never cost-effective due to their elevated cost.
K: thousand.
Discussion
The main conclusion of the present model is that in an intermediate-risk European country endoscopic screening for gastric cancer is cost-effective only if combined with an already scheduled screening colonoscopy after a positive faecal occult blood test. Although Portugal has an intermediate gastric cancer incidence with an ASR of 13.1/100.000, a stand-alone screening endoscopy every five years provides an ICER of €70,396/QALY that clearly exceeds the adopted threshold of €37,000/QALY. For a screening test to be cost-effective in Portugal according to 2016 prices, the test would have to cost less than €75. This might be achieved only when the associated societal costs for endoscopy such as sedation, work absence and transportation are already accounted for, for instance if the screened person is already undergoing a colonoscopy for colorectal cancer screening. Although prospective studies on the use of pepsinogen as a screening method are ongoing in some European countries, its high cost is the main limitation from a cost-effectiveness perspective.15
This means that even in Europe, if a screening colorectal cancer screening programme is already in place, by means of faecal occult blood or stand-alone colonoscopy, countries at an intermediate to higher risk for gastric cancer such as Albania, Belarus, Macedonia, Russia, Latvia, Ukraine, Estonia, Lithuania, Portugal, Moldova, Romania, Slovenia, Bulgaria and Croatia (presented according to their ASR, from 20.1 to 10.3) might benefit by providing their populations an upper endoscopy screening in conjunction with colonoscopy. As in any economic model, these conclusions should be modelled in each country after further adjustments to the local costs, gross national income per capita and clinical proportions; as such, this financial background may not be applicable to all intermediate gastric cancer’s risk countries.
Additionally, the possibility of adding an upper endoscopy to an already scheduled colonoscopy might also speed up the clinical investigation usually required in cases of positive faecal occult blood test and a negative colonoscopy.
Another relevant conclusion is that the results strongly depend on the national gastric cancer incidence. Implementation of screening in countries like Japan (where gastric cancer endoscopic screening is current practice) is cost-effective. This does not pertain to other intermediate-incidence countries like Singapore (where the gross national income per capita is much higher than in Portugal) nor to countries with a low risk of gastric cancer such as the USA despite the high income per capita.
To the best of our knowledge only nine economic studies have been published so far on the issue of endoscopic screening for gastric cancer, seven in Asian populations and two from the USA, but none from Europe. Studies in high-incidence countries concluded that endoscopic screening was cost-effective.4,30–32
In countries with an intermediate risk of gastric cancer like Singapore one study concluded that two-yearly endoscopic mass screening was cost-effective only in a high-risk population of men aged 50–70 years with an ASR of 25.9 but not cost-effective for the entire population;33 others concluded that a two-yearly surveillance strategy was the most cost-effective option for patients at increased risk aged 50–70 years,24 and another concluded that endoscopic surveillance was cost-effective for high-risk individuals with an odds ratio for cancer >3.93,34 in accordance with our previous model for high-risk patients.9
In countries with a low risk of gastric cancer, one concluded that gastric cancer would have to increase by 337% to become cost-effective while another concluded it was not cost-effective for the American population.7,35
The definition of the endoscopy costs is also relevant within this context. When using a societal point of view for the model, costs are relevant if they apply to the health system but also to the screenee and relatives. This means that the costs of endoscopy can easily rise up to €137 (the cost used for the base case scenario) if we account for fees, transportation, work time lost, and sedation. This reflects that the overall costs are much higher than the reimbursement costs for endoscopy alone.
There are, however, some limitations in the present study. First, as only positive faecal occult blood test cases would be invited to perform a colonoscopy, only those would be offered for the additional upper endoscopy screening benefit and recent data point to only 7% of positive faecal occult blood test cases.13 This strategy also implies the assumption that the risk of gastric cancer is similar among patients with positive, negative or no faecal occult blood test. Also, the best interval between screening endoscopies is not yet defined and it is not possible to say if 5 or 10 years are enough or too much. Finally, utilities valuation is open to bias and, as in any economic model, it was conceived for a specific population so adjustments would need to be made for other countries.
In conclusion, endoscopic gastric cancer screening in conjunction with a scheduled colonoscopy may be cost-effective in countries with an intermediate risk for gastric adenocarcinoma, namely in Eastern Europe and Portugal. This implies that endoscopic resources already allocated to colorectal screening programmes could be used to provide gastric cancer screening, both for detection of high-risk individuals with extensive premalignant conditions or early gastric cancer patients. This opens new opportunities to consider for prevention of a mostly incurable cancer when diagnosed at a symptomatic stage.
Supplementary Material
Acknowledgments
Guarantor of article: Miguel Areia.
M Areia and M Dinis-Ribeiro created the model and conducted the systematic search; MC Spaander and EJ Kuipers verified model consistency and checked for coherence of results; M Areia wrote the manuscript; and MC Spaander, EJ Kuipers and M Dinis-Ribeiro provided a critical review of the manuscript. All authors approved the final version of the manuscript.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Not applicable.
Informed consent
Not applicable.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2.Areia M, Pimentel-Nunes P, Marcos-Pinto R, et al. Gastric cancer: An opportunity for prevention. Acta Med Port 2013; 26: 627–629. [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Ervik M, et al. Cancer incidence and mortality worldwide: IARC CancerBase No. 11. GLOBOCAN 2012 v10. Lyon, France: International Agency for Research on Cancer, 2013.
- 4.Zhou L, Guan P, Sun LP, et al. Health economic assessment for screening of gastric cancer in a high risk population in northeastern China. Chin J Cancer Res 2011; 23: 21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pimentel-Nunes P, Mourao F, Veloso N, et al. Long-term follow-up after endoscopic resection of gastric superficial neoplastic lesions in Portugal. Endoscopy 2014; 46: 933–940. [DOI] [PubMed] [Google Scholar]
- 6.Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015; 47: 829–854. [DOI] [PubMed] [Google Scholar]
- 7.Yeh JM, Hur C, Ward Z, et al. Gastric adenocarcinoma screening and prevention in the era of new biomarker and endoscopic technologies: A cost-effectiveness analysis. Gut 2016; 65: 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinis-Ribeiro M, Areia M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (MAPS): Guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy 2012; 44: 74–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Areia M, Dinis-Ribeiro M, Rocha Gonçalves F. Cost-utility analysis of endoscopic surveillance of patients with gastric premalignant conditions. Helicobacter 2014; 19: 425–436. [DOI] [PubMed] [Google Scholar]
- 10.Marques-Silva L, Areia M, Elvas L, et al. Prevalence of gastric precancerous conditions: A systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2014; 26: 378–387. [DOI] [PubMed] [Google Scholar]
- 11.von Karsa L, Patnick J, Segnan N. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition – Executive summary. Endoscopy 2012; 44(Suppl 3): SE1–SE8. [DOI] [PubMed] [Google Scholar]
- 12.Hassan C, Quintero E, Dumonceau JM, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2013; 45: 842–851. [DOI] [PubMed] [Google Scholar]
- 13.Quintero E, Castells A, Bujanda L, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 2012; 366: 697–706. [DOI] [PubMed] [Google Scholar]
- 14.Dinis-Ribeiro M, Yamaki G, Miki K, et al. Meta-analysis on the validity of pepsinogen test for gastric carcinoma, dysplasia or chronic atrophic gastritis screening. J Med Screen 2004; 11: 141–147. [DOI] [PubMed] [Google Scholar]
- 15.Lomba-Viana R, Dinis-Ribeiro M, Fonseca F, et al. Serum pepsinogen test for early detection of gastric cancer in a European country. Eur J Gastroenterol Hepatol 2012; 24: 37–41. [DOI] [PubMed] [Google Scholar]
- 16.Russell LB, Gold MR, Siegel JE, et al. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA 1996; 276: 1172–1177. [PubMed] [Google Scholar]
- 17.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) – explanation and elaboration: A report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013; 16: 231–250. [DOI] [PubMed] [Google Scholar]
- 18.Areia M, Carvalho R, Cadime AT, et al. Screening for gastric cancer and surveillance of premalignant lesions: A systematic review of cost-effectiveness studies. Helicobacter 2013; 18: 325–337. [DOI] [PubMed] [Google Scholar]
- 19.Pordata. Lisbon: Francisco Manuel dos Santos Foundation, 2013.
- 20.Areia M, Alves S, Brito D, et al. Health-related quality of life and utilities in gastric premalignant conditions and malignant lesions: A multicentre study in a high prevalence country. J Gastrointestin Liver Dis 2014; 23: 371–378. [DOI] [PubMed] [Google Scholar]
- 21.Whiting JL, Sigurdsson A, Rowlands DC, et al. The long term results of endoscopic surveillance of premalignant gastric lesions. Gut 2002; 50: 378–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi KS, Suh M. Screening for gastric cancer: The usefulness of endoscopy. Clin Endosc 2014; 47: 490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassan C, Zullo A, Di Giulio E, et al. Cost-effectiveness of endoscopic surveillance for gastric intestinal metaplasia. Helicobacter 2010; 15: 221–226. [DOI] [PubMed] [Google Scholar]
- 24.Zhou HJ, Dan YY, Naidoo N, et al. A cost-effectiveness analysis evaluating endoscopic surveillance for gastric cancer for populations with low to intermediate risk. PLoS One 2013; 8: e83959–e83959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogoshi K, Narisawa R, Kato T, et al. Endoscopic screening for gastric cancer in Niigata city [article in Japanese]. Jpn J Endoscopic Forum Digestive Disease 2010; 26: 5–16. [Google Scholar]
- 26.Lee YY, Oh DK, Choi KS, et al. The current status of gastric cancer screening in Korea: Report on the National Cancer Screening Programme, 2009. Asian Pac J Cancer Prev 2011; 12: 3495–3500. [PubMed] [Google Scholar]
- 27.National Cancer Center: Cancer Facts & Figures 2014. Goyang: National Cancer Center, 2014, p.124.
- 28.Kwon YM, Lim HT, Lee K, et al. Factors associated with use of gastric cancer screening services in Korea. World J Gastroenterol 2009; 15: 3653–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sachs JD. Macroeconomics and health: Investing in health for economic development, Geneva: World Health Organization, 2001. [Google Scholar]
- 30.Tashiro A, Sano M, Kinameri K, et al. Comparing mass screening techniques for gastric cancer in Japan. World J Gastroenterol 2006; 12: 4873–4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee HY, Park EC, Jun JK, et al. Comparing upper gastrointestinal X-ray and endoscopy for gastric cancer diagnosis in Korea. World J Gastroenterol 2010; 16: 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang HS, Park EC, Chung W, et al. Comparing endoscopy and upper gastrointestinal X-ray for gastric cancer screening in South Korea: A cost-utility analysis. Asian Pac J Cancer Prev 2012; 13: 2721–2728. [DOI] [PubMed] [Google Scholar]
- 33.Dan YY, So JB, Yeoh KG. Endoscopic screening for gastric cancer. Clin Gastroenterol Hepatol 2006; 4: 709–716. [DOI] [PubMed] [Google Scholar]
- 34.Wu JT, Zhou J, Naidoo N, et al. Determining the cost-effectiveness of endoscopic surveillance for gastric cancer in patients with precancerous lesions. Asia Pac J Clin Oncol 2016; 12: 359–368. [DOI] [PubMed] [Google Scholar]
- 35.Gupta N, Bansal A, Wani SB, et al. Endoscopy for upper GI cancer screening in the general population: A cost-utility analysis. Gastrointest Endosc 2011; 74: 610–624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.