Abstract
Background
Citrulline has been described as a marker of intestinal function or absorption but evidence varies according to clinical settings.
Objective
The objective of this article is to examine the evidence of plasma citrulline as a marker of intestinal function and absorption in various clinical settings.
Methods
Studies were examined for p values, means and standard deviations, correlation coefficients or other metrics depicting the association of citrulline with intestinal function. A random effects model was used to produce a pooled estimate. A hierarchical summary receiver operating curve model was fitted for diagnostic accuracy measures.
Results
Citrulline levels are correlated strongly with small bowel length in short bowel syndrome patients (r = 0.67). Citrulline is strongly negatively correlated (r = –0.56) with intestinal disease severity with regards to enteropathies (coeliac disease, tropical enteropathy, Crohn’s disease, mucositis, acute rejection in intestinal transplantation). Citrulline cut-off levels have an overall sensitivity and specificity of 80% and 84% respectively. Citrulline levels in untreated coeliac patients compared to controls were reduced by 10 µmol/l. Citrulline levels increase with gluten-free diet and with improvement of enteropathy. Citrulline is decreased in critical illness and sepsis.
Conclusion
These findings allow us to advocate quite reasonably that citrulline is a marker of acute and chronic intestinal insufficiency.
Keywords: Citrulline, meta-analysis, short bowel syndrome, enteropathy, systematic review
Key summary
1. Summarise the established knowledge on this subject
Citrulline is a non-protein amino acid, and in humans its plasma content is derived largely from the amount produced in enterocytes of the small bowel.
Certain clinical conditions have been identified in which citrulline has been used as a marker of intestinal function.
It is not clear whether citrulline levels reflect intestinal function (notably absorption), enterocyte mass, both or other.
2. What are the significant and/or new findings of this study?
Citrulline is positively correlated with small bowel length in short bowel syndrome with lower citrulline levels being indicative of intestinal insufficiency.
Citrulline is moderately correlated with enteral absorption in various conditions.
Citrulline is negatively correlated with disease severity in intestinal enteropathies.
Citrulline cut-off levels have a sensitivity and specificity of 80% and 84%; 20 µmol/l seems to be the most prevalent cut-off level.
Introduction
Citrulline is a non-protein amino acid, and in humans its plasma content is derived largely from the amount produced in enterocytes of the small bowel.1 Citrulline’s first isolation from the juice of the watermelon has been attributed to Koga and Ohtake2,3 and Wada.4 Certain clinical conditions have been identified in which citrulline has been used as a marker of intestinal function.5–7 However, it is not clear whether citrulline levels reflect intestinal function (notably absorption), enterocyte mass, or both, with its current use being interchangeable. Hence, due to citrulline’s unique metabolism, this systematic review aims to answer whether citrulline is a successful indicator of intestinal enterocyte mass and absorption and what clinical conditions it has been utilised in as a marker.
Methods
The inclusion criterion for this systematic review was any empirical study describing investigation of citrulline in relation to intestinal function. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and Meta-analysis of Observational Studies in Epidemiology (MOOSE) checklist for systematic reviews and meta-analyses were used.8,9 Electronic database searches were conducted with no year limits. The quality of studies was assessed with elements from Cochrane Collaboration’s tool10 and the RTI Item Bank for Observational Studies.11,12 For the meta-analysis, studies were examined for p values, means and standard deviations, correlation coefficients (CCs) or other metrics depicting the association of citrulline with intestinal function. Metrics were converted to the standardised mean difference (SMD),13,14 mean difference (MD),15 and/or CC. A random effects model was used to produce a pooled estimate of the SMDs/MDs/CCs. Heterogeneity was quantified using the I2 statistic and further investigated with subgroup analysis and meta-regression. Publication bias was assessed using funnel plots, Egger’s test, Begg’s test and Rosenthal’s number.16–19 The CC is converted to the Fisher’s z for all analyses.15 Regarding diagnostic accuracy data, a hierarchical summary receiver operating curve (HSROC) model was fitted to provide a summary receiver operating curve (SROC) and to allow derivation of pooled sensitivity and specificity estimates.20 The methods are described in more detail in the Supplementary Materials.
Results
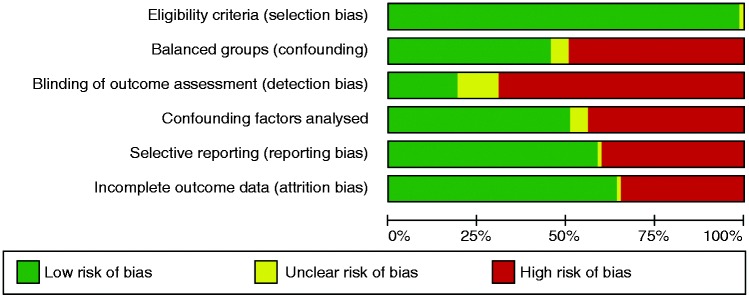
From 463 initial studies, 131 were included in the systematic review and 63 in the meta-analyses performed (Figure 1). Overall number of patients was 4292 (mean 68, range 6–847) with mean age 31.6 years, male percentage 50.9% and body mass index (BMI) 21.9 kg/m2. Twenty-three studies involved children and 40 involved adults, and the majority of studies were conducted in Europe (45 studies). Mean citrulline value from all studies was 23.2 µmol/l and citrulline was mostly measured with high-performance liquid chromatography (HPLC). Main findings from all studies are shown in Supplementary Tables 1–6, grouped by condition. There was a strong presence of detection bias and almost 50% confounding bias (Figure 2, Supplementary Figure 1). Reporting bias was also an issue that arose from the papers.
Figure 1.
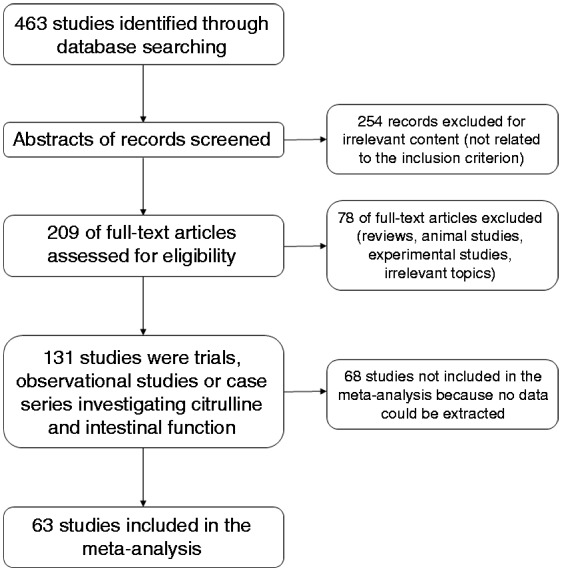
Flowchart for systematic review of studies.
Figure 2.
Risk of bias graph: Review authors’ judgements about each risk of bias item presented as percentages across all included studies (all studies).
Necrotising enterocolitis (NEC)
Four studies have assessed citrulline levels in patients with NEC (Supplementary Table 1). Risk of bias was low in most studies. The MD indicated a significant decrease in citrulline levels by –7.8 µmol/l (95% confidence interval (CI) (–14.7, –0.9); I2 = 98%) compared to controls, which indicated a strong decrease when the SMD was analysed –1.44 (95% CI (–2.80, –0.07); I2 = 96%). Celik et al.21 described that the area under the receiver operating characteristic (ROC) curve for citrulline to differentiate NEC from controls was 0.88 (95% CI (0.77, 0.99)) and the cut-off level of citrulline was 13.15 µmol/l with a sensitivity of (80% and a specificity of 82% but no association with duration of parenteral nutrition was noted. Similarly, Ioannou and colleagues22 noted that the area under the ROC curve for plasma citrulline to discriminate neonates with NEC from control neonates was 0.86 (95% CI (0.77, 0.96)). The citrulline level that maximised the test’s sensitivity and specificity was 17.75 µmol/l, with a sensitivity of 76% and a specificity of 87%.
Intestinal transplantation
Measurement of citrulline levels has been investigated as a possible indicator of intestinal transplant rejection. Thirteen studies were identified in the literature search and two groups have published quite extensively in the field: the University of Miami, School of Medicine23 and the Mount Sinai School of Medicine24 (Supplementary Table 2).
These studies’ focus is the ability of citrulline levels to predict the grade of acute cellular rejection and the cut-off value of citrulline levels that yield a high possibility of acute cellular rejection. There was presence of detection bias and confounding because not all studies assessed possible confounders of associations. Also, there is a strong possibility of reporting bias and attrition bias, because many papers are published in Transplantation Proceedings, which publishes short reports from transplant centres (Supplementary Figure 1). The initial studies by the Miami group described a moderate negative CC of citrulline levels with rejection (Pappas et al.25 reported CC = –0.590) but in the recent studies by Ruiz and colleagues26 and Hibi et al.,27 which include up to around 10,000 plasma citrulline samples, correlation reaches up to a strong –0.977 with acute cellular rejection. The CCs are shown in Supplementary Figure 2 without meta-analysis due to severe heterogeneity.
Two other trends were noted in the transplantation articles: First, citrulline appears to normalise after a certain amount of time post-transplantation and this is a significant factor against rejection;28–31 secondly, the cut-off value of citrulline predicting rejection varies. The Miami group have described that citrulline levels have a very high negative predictive value for moderate or severe acute rejection (negative prognostic value = 99% with cut-off level 13 µmol/l; sensitivity = 96.4% with particularly high specificity in adult patients);27,32,33 but the Mount Sinai Group did not find that citrulline had satisfactory diagnostic accuracy to discern rejection.34 Multiple parameters need to be taken into account when measuring citrulline in this group, including time after surgery, renal function, graft pathology, infection, sepsis, and donor and patient anthropometrics.24,29,34
Short bowel syndrome (SBS)
Thirty-five papers and abstracts were identified which included eventually 26 studies (Supplementary Table 3). Quality assessment showed possibilities of reporting, attrition and detection bias (Figure 2).
Citrulline and residual small bowel length
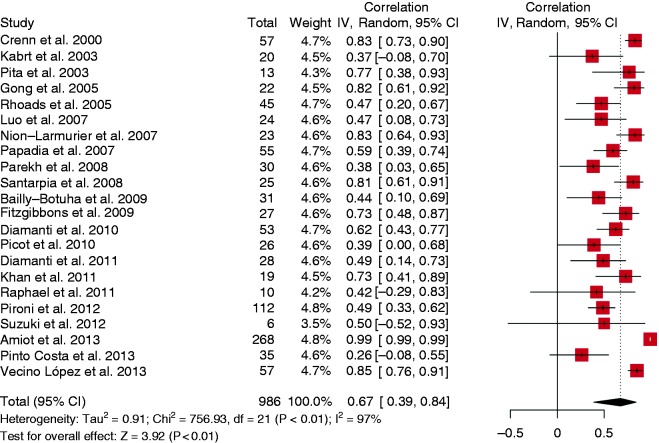
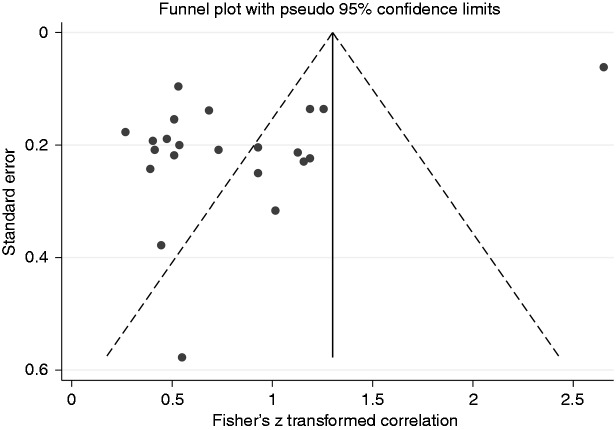
Twenty-one studies were analysed and the random-effects analysis of CCs produced a pooled effect of 0.67 (95% CI (0.39, 0.84), range (0.26, 0.99)), which indicates a strong correlation (Figure 3). In addition there was evidence of publication bias (funnel plot asymmetry, Egger’s test p = 0.001 but Begg’s test p = 0.156, Fail-safe N = 5286) and high heterogeneity (I2 = 97%, p < 0.001) (Figure 4).
Figure 3.
Forest plot. Correlation coefficient with small bowel length in short bowel syndrome.
CI: confidence interval. IV: Inverse Variance.
Figure 4.
Funnel plot. Correlation coefficient with small bowel length in short bowel syndrome – there is asymmetry in the plot indicating publication bias.
When analysed by subgroups, heterogeneity remained high with respect to patient type, and measurement method, but was reduced in studies from the United States, Spain and Italy (Supplementary Figure 3). Meta-regression also did not identify any heterogeneity with respect to male percentage, mean age, mean BMI, mean citrulline concentration and mean small bowel length (Supplementary Table 7).
Citrulline between SBS patients and healthy controls
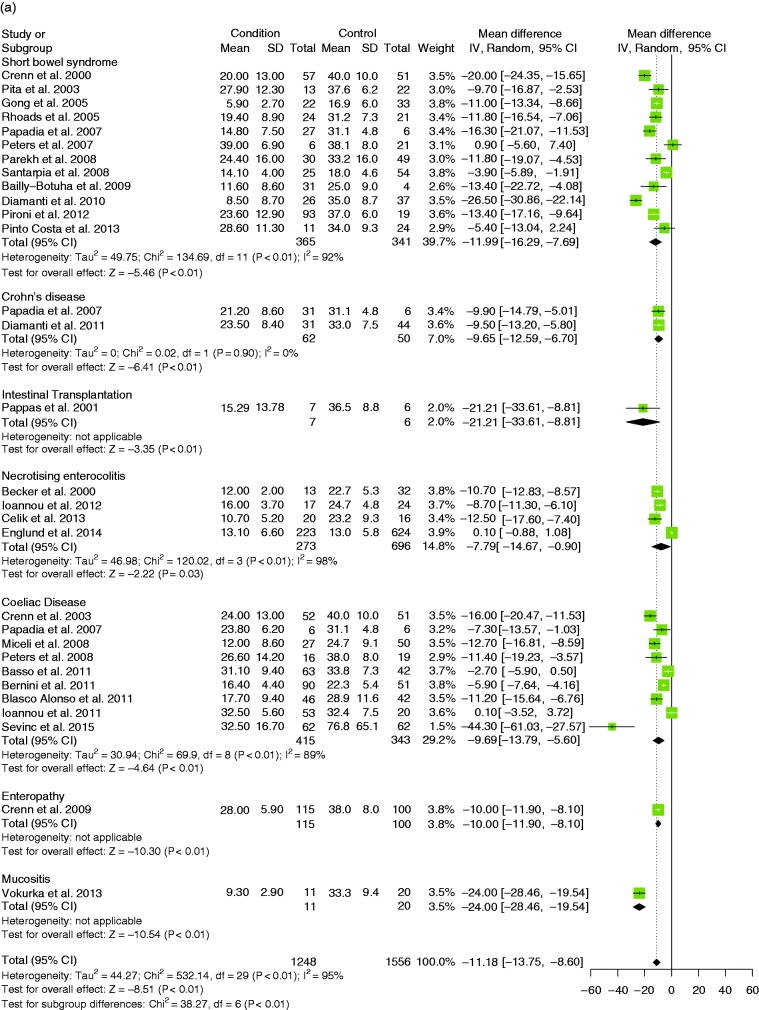
Twelve studies were analysed and the random-effects model showed that citrulline levels were decreased by –12 µmol/l (95% CI (–16.3, –7.7)) (SMD –1.34, 95% CI (–1.77, –0.91)); there was heterogeneity (MD: I2 = 92%, p < 0.001; SMD: I2 = 80%, p < 0.001) (Figure 5(a)) but no publication bias (symmetric funnel plot, Egger’s test p = 0.606, Begg’s test p = 0.537, Fail-safe N = 853) (Supplementary Figure 5(b)).
Figure 6.
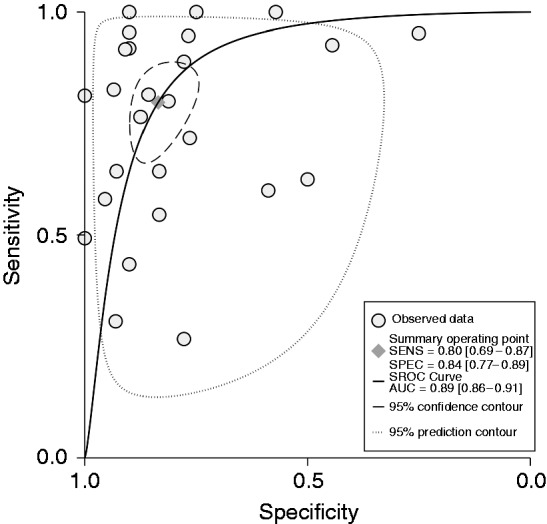
Summary receiver operating characteristic (SROC) curve for all studies of diagnostic accuracy (26 studies).
SENS: sensitivity; SPEC: specificity; AUC: area under the curve.
Citrulline levels in parenteral nutrition (PN)-dependent vs PN-independent patients
Twelve studies were analysed comparing levels of citrulline in patients who needed PN against patients who were weaned off PN. The random-effects model showed that citrulline levels were decreased by –13.3 µmol/l (95% CI (–17.6, –9.0)) (SMD –1.58, 95% CI (–2.09, –1.08)); there was heterogeneity (MD: I2 = 89%, p < 0.001; SMD: I2 = 79%, p < 0.001) but no publication bias (symmetric funnel plot, Egger’s test p = 0.174, Begg’s test p = 0.451, Fail-safe N = 753) (Supplementary Figures 4(a) and 5(d)).
Sixteen studies described diagnostic accuracy results (Supplementary Table 8). Overall, citrulline levels have a sensitivity of 82.5% and specificity 82% (Supplementary Table 9, Supplementary Figure 6). Since 13 studies compared citrulline levels at a cut-off level of 20 µmol/l to discern among SBS patients who needed PN or not, the meta-analysed sensitivity and specificity reflect mostly that. Any heterogeneity present is due to different cut-off levels and comparison groups.
Teduglutide and citrulline levels
There have been four studies studying the effect of teduglutide – a GLP-2 analogue which acts as a growth factor in patients with SBS – on citrulline levels (Supplementary Table 3). Three studies compared citrulline levels in patients who received teduglutide against patients who received placebo in Crohn’s disease and SBS. The random-effects model showed that citrulline levels in teduglutide vs placebo were increased by 12.4 µmol/l (95% CI (5.5, 19.3)) (SMD 1.02, 95% CI (0.47, 1.58)) and there was heterogeneity (MD: I2 = 85%, p = 0.001; SMD: I2 = 73%, p = 0.02). Four studies provided citrulline levels of patients who received teduglutide at the end of treatment compared to their baseline. The random-effects model showed that citrulline levels in teduglutide at end of treatment vs baseline were increased by 15.3 µmol/l (95% CI (12.5, 18.2)) (SMD 1.21, 95% CI (1.00, 1.43)); there was no heterogeneity (MD: I2 = 16, p = 0.31; SMD: I2 = 0%, p = 0.56) (Supplementary Figures 4(b) and (c)).
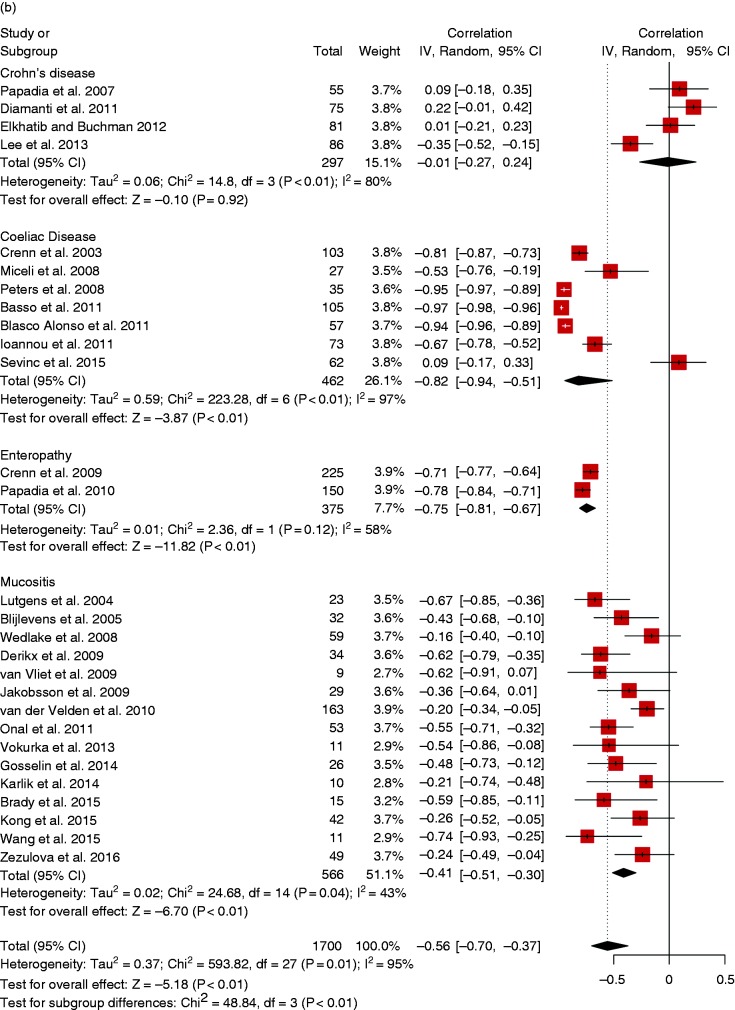
Enteropathies
Villous atrophy syndrome
Eleven studies were used in meta-analyses in this category which included cases that had coeliac disease or other enteropathy (Supplementary Table 4). Meta-analyses firstly compare citrulline levels in diseased patients against controls, then those who had received a gluten-free diet (GFD) compared to those who had not, and finally association of citrulline levels with disease severity. Severity of disease was categorised broadly and included either histological diagnoses, worsening symptoms or any other metric reported by the authors which indicated severity of the enteropathy. Severity is to be considered as a scale by which higher values indicate more severe disease and lower values indicate less severe disease. The random-effects model showed that citrulline levels in coeliac disease patients compared to controls were decreased by –9.7 µmol/l (95% CI (–13.8, –5.6)) (SMD –0.99, 95% CI (–1.30, –0.67)); there was heterogeneity (MD: I2 = 89%, p < 0.001; SMD: I2 = 78%, p < 0.001) but no publication bias (symmetric funnel plot, Egger’s test p = 0.247, Begg’s test p = 0.283) (Figure 6(a), Supplementary Figure 5(c)). The random-effects model showed that citrulline levels were decreased by –8.2 µmol/l (95% CI (–10.4, –5.9)) (SMD –1.08, 95% CI (–1.42, –0.75)) in those patients who had not received a GFD compared to those who had (Supplementary Figure 4(d)).35–39
Crohn’s disease
Citrulline levels were compared between Crohn’s disease patients and controls in two studies.40,41 The random-effects model showed that citrulline levels in patients vs controls was decreased by –9.7 µmol/l (95% CI (–12.6, –6.7)) (SMD –1.19, 95% CI (–1.63, –0.75)); there was no heterogeneity (MD: I2 = 0%, p = 0.90; SMD: I2 = 0%, p = 0.99) (Figure 5(a)).
Acute mucosal enteropathy and cancer treatments
Acute mucosal enteropathy can cause a significant loss of enterocytes. Fourteen studies were used in a meta-analysis in this category which included cases of patients who had received chemoradiation for bone marrow transplant, cancer or other malignant disorder (Supplementary Table 5). Generally:
Citrulline decreases in the initial phase of treatment and then increases while the initial gastrointestinal toxicity related to treatment seems to reside.
Citrulline decrease is related to higher doses of treatment and is usually inversely correlated with severity of gastrointestinal toxicity. Meta-analysis was performed on this outcome since 14 studies were identified.
The random-effects model showed that citrulline levels were negatively correlated with severity of gastrointestinal toxicity with a moderate correlation of –0.41 (95% CI (–0.51, –0.30)); there was no heterogeneity (I2 = 43%, p = 0.04) and no publication bias (symmetric funnel plot, Egger’s test p = 0.009, Begg’s test p = 0.102) (Figure 5(b), Supplementary Figure 5(f)).
Critical illness patients
Twenty-five studies were diagnosed investigating citrulline levels in patients with critical illness (Supplementary Table 6). The majority of studies involve patients in intensive care settings which attempt to correlate decrease in citrulline levels with severity of condition or other sepsis markers. No meta-analyses were performed on these studies due to different measurement methods and inability to extract common outcomes. The following comments can be made:
Citrulline appears decreased in most studies and is related to critical illness and markers of sepsis or inflammation.
This decrease in citrulline does not necessarily mean that there is intestinal dysfunction since in inflammatory responses and severe critical illness, nitric oxide and arginine are depleted through inflammatory pathways hence leading to the reduction of citrulline.42 This is also corroborated by the fact that citrulline levels increase once the critical condition is overcome.
Citrulline seems to act as a negative inflammatory marker.
Citrulline levels: An overall assessment
Diagnostic accuracy
Overall sensitivity of citrulline levels appear to be satisfactory 80% (95% CI (69%–87%)), specificity was 84% (95% CI (77%–89%)) and the diagnostic odds ratio was 20.03 (Supplementary Table 9, Supplementary Figure 7). The SROC curve indicates overall satisfactory diagnostic accuracy of citrulline levels (Figure 6).
Citrulline levels in diseased patients vs controls
The random-effects model showed that citrulline levels in patients vs controls (30 studies) was decreased by –11.2 µmol/l (95% CI (–13.8, –8.6)) (SMD –0.53, 95% CI (–0.69, –0.36)); there was heterogeneity (MD: I2 = 95%, p = 0.002; SMD: I2 = 68.7%, p < 0.001) (Figure 5(a)). No publication bias was observed (symmetric funnel plot, Egger’s test p = 0.969, Begg’s test p = 0.986) (Supplementary Figure 5(a)).
Figure 5.
(a) Forest plot with overall weighted mean difference of patients with a condition against controls (30 studies). (b) Forest plot with correlation coefficients with severity in all available studies (28 studies).
CI: confidence interval. IV: Inverse Variance, SD: Standard Deviation.
Citrulline levels as a marker of intestinal disease severity
Citrulline levels were described in association with disease severity in 28 studies. The random-effects model showed that citrulline levels were negatively correlated with severity of disease with a moderate correlation of –0.56 (95% CI (–0.70, –0.37)) (Figure 5(b)); there was heterogeneity (I2 = 95%, p < 0.001); but no publication bias (symmetric funnel plot, Egger’s test p = 0.356, Begg’s test p = 0.722) (Supplementary Figure 5(e)). Interestingly, we can see that only in Crohn’s disease is citrulline not associated with disease severity (Figure 5(b)).
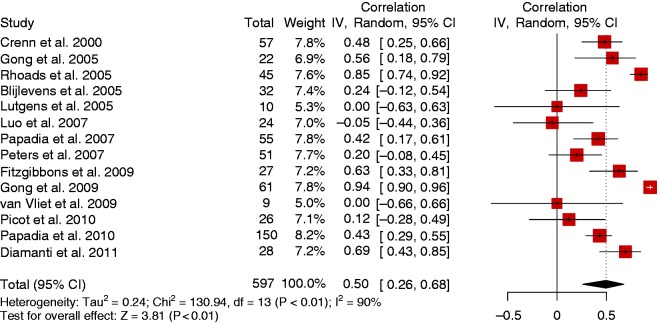
Citrulline and absorptive function
Fourteen studies reported an association of citrulline levels with the level of intestinal absorption. Absorption was assessed with the D-xylose absorption test,40,43–48 oral or enteral nutrition tolerance,49–51 and nutrient absorption tests with bomb calorimetry and measuring oral/enteral intake in comparison to faecal and other loses.52–54 The random-effects model showed that citrulline levels were positively correlated with enteral absorption with a moderate correlation of 0.50 (95% CI (0.26, 0.68)) (Figure 7) but there was heterogeneity (I2 = 90%, p < 0.001).
Figure 7.
Forest plot with correlation coefficients of citrulline levels with enteral absorption (14 studies).
CI: confidence interval. IV: Inverse variance.
Conclusion
The present study is the first meta-analysis on the association of citrulline with gut function. Although citrulline appears to be a strong marker of enterocyte mass, its correlation with intestinal absorption is weaker. This correlation appears clinically significant in SBS. In other conditions in which short bowel is not an issue, there is a decrease in mean citrulline compared to healthy controls, and citrulline decrease can be correlated to the degree of disease severity. Its interpretation however needs to take into account other factors because its diagnostic accuracy is satisfactory but not completely exclusive of negative cases and it might as well produce false-positive cases. There were various thresholds for discerning a high from low citrulline level but the level of 20 µmol/l seems to be most prevalent.
In critical illness the interpretation of a low citrulline as a marker of intestinal dysfunction should be treated with caution – in a similar manner that a low albumin in a critical ill patients needs to be cautiously interpreted as malnutrition. The availability of nitric oxide and arginine during septic and inflammatory states is decreased hence decreasing citrulline and in this context citrulline could be a negative inflammatory marker – without excluding enteropathy of acute illness.
Limitations of the present meta-analysis stem from various sources of heterogeneity and possibility of publication bias, detection bias and confounding bias. It was a pattern in the present review that many studies did not analyse confounding factors such as other amino acids, renal function (citrulline’s pathways involve a renal component) and inflammatory state. Also different methods exist for plasma citrulline measurement, sample preparation, population parameters, disease severity, absorption, and small bowel length. Although heterogeneity is partially explained by geographical factors, ultimately this reflects different clinical and analytical practices throughout the world. The random-effects models performed take heterogeneity into account and thus were the preferred method of analysis. Standardisation of measurement methods and practices will possibly allow for comparable and more homogeneous results, resulting in meaningful clinical interpretation.
Although interest in citrulline originated from intestinal failure and intestinal transplant medicine, we believe that its application extends to the general gastroenterologist since it has to do with intestinal function per se. Is the bowel working? This exact question has led investigation into citrulline’s response in enteropathies such as Crohn’s disease, coeliac disease, critical care enteropathy, and mucositis related to bone marrow transplant and chemo-radiotherapy so far. Serial citrulline measurements seem to reflect patterns of mucosal barrier injury and hence are associated with septic episodes in transplant and critical illness patients, setting the ground for use as a marker of early intestinal dysfunction. Its use in SBS is multifactorial, assisting towards the decision of the absorptive capacity of the bowel and hence the need for PN. Nevertheless, citrulline needs be considered within the individual patient context due to measurement variations between centres, countries and populations, with dissimilar normal ranges and average values. The presence of heterogeneous results in the present systematic review reflects this, possibly also explaining its current niche status (as yet).
In conclusion, citrulline concentration is decreased in patients with compromised intestinal states compared to controls; it has a sensitivity and specificity of ∼80%; it is negatively correlated with disease severity in intestinal enteropathies; it is positively correlated with small bowel length in SBS; and it is moderately correlated with enteral absorption in various conditions. Overall, lower citrulline levels are indicative of acute or chronic intestinal insufficiency.
Supplementary Material
Acknowledgement
Any research materials related to the present manuscript (for example, datasets or models) can be obtained by contacting the corresponding author.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Windmueller HG, Spaeth AE. Source and fate of circulating citrulline. Am J Physiol Endocrinol Metab 1981; 241: E473–E480. [DOI] [PubMed] [Google Scholar]
- 2.Fragkos KC, Forbes A. Was citrulline first a laxative substance? The truth about modern citrulline and its isolation. Nihon ishigaku zasshi [Journal of Japanese History of Medicine] 2011; 57: 275–292. [PubMed] [Google Scholar]
- 3.Koga Y, Ohtake R. Study report on the constituents of squeezed watermelon [article in Japanese]. Tokyo Kagaku Kaishi [Journal of the Tokyo Chemical Society] 1914; 35: 519–528. [Google Scholar]
- 4.Wada M. Über Citrullin, eine neue Aminosäure im Preßsaft der Wassermelone, Citrullus vulgaris schrad. Biochem Z 1930; 224: 420–429. [Google Scholar]
- 5.Crenn P. Citrulline et métabolisme protéique. Nutrition clinique et métabolisme 2008; 22: 75–79. [Google Scholar]
- 6.Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr 2008; 27: 328–339. [DOI] [PubMed] [Google Scholar]
- 7.Curis E, Crenn P, Cynober L. Citrulline and the gut. Curr Opin Clin Nutr Metab Care 2007; 10: 620–626. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol 2009; 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 9.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JPT and Green S (eds) Cochrane handbook for systematic reviews of interventions. Chichester, England; Hoboken, NJ: Wiley-Blackwell, 2008, p.xxi, p.649.
- 11.Viswanathan M, Berkman ND. Development of the RTI item bank on risk of bias and precision of observational studies. J Clin Epidemiol 2012; 65: 163–178. [DOI] [PubMed] [Google Scholar]
- 12.Viswanathan M, Berkman ND, Dryden DM, et al. Assessing risk of bias and confounding in observational studies of interventions or exposures: Further development of the RTI Item Bank, Rockville, MD: Agency for Healthcare Research and Quality, 2013. [PubMed] [Google Scholar]
- 13.Lipsey MW, Wilson DB. Practical meta-analysis, Thousand Oaks, CA, US: Sage Publications Inc, 2001. p.ix, p.247. [Google Scholar]
- 14.Cooper HM, Hedges LV, Valentine JC. The handbook of research synthesis and meta-analysis, 2nd ed New York: Russell Sage Foundation, 2009. p.xvi, p.615. [Google Scholar]
- 15.Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to meta-analysis, Chichester, UK: John Wiley & Sons, 2008. [Google Scholar]
- 16.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 17.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull 1979; 86: 638–641. [Google Scholar]
- 19.Fragkos KC, Tsagris M, Frangos CC. Publication bias in meta-analysis: Confidence intervals for Rosenthal’s Fail-Safe number. Int Sch Res Notices 2014; 2014: 825383–825383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harbord RM, Deeks JJ, Egger M, et al. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007; 8: 239–251. [DOI] [PubMed] [Google Scholar]
- 21.Celik IH, Demirel G, Canpolat FE, et al. Reduced plasma citrulline levels in low birth weight infants with necrotizing enterocolitis. J Clin Lab Anal 2013; 27: 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ioannou HP, Diamanti E, Piretzi K, et al. Plasma citrulline levels in preterm neonates with necrotizing enterocolitis. Early Hum Dev 2012; 88: 563–566. [DOI] [PubMed] [Google Scholar]
- 23.David AI, Gaynor JJ, Zis PP, et al. An association of lower serum citrulline levels within 30 days of acute rejection in patients following small intestine transplantation. Transplant Proc 2006; 38: 1731–1732. [DOI] [PubMed] [Google Scholar]
- 24.Gondolesi G, Fishbein T, Chehade M, et al. Serum citrulline is a potential marker for rejection of intestinal allografts. Transplant Proc 2002; 34: 918–920. [DOI] [PubMed] [Google Scholar]
- 25.Pappas PA, Saudubray JM, Tzakis AG, et al. Serum citrulline and rejection in small bowel transplantation: A preliminary report. Transplantation 2001; 72: 1212–1216. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz P, Tryphonopoulos P, Island E, et al. Citrulline evaluation in bowel transplantation. Transplant Proc 2010; 42: 54–56. [DOI] [PubMed] [Google Scholar]
- 27.Hibi T, Nishida S, Garcia J, et al. Citrulline level is a potent indicator of acute rejection in the long term following pediatric intestinal/multivisceral transplantation. Am J Transplant 2012; 12: S27–S32. [DOI] [PubMed] [Google Scholar]
- 28.Pappas PA, Saudubray JM, Tzakis AG, et al. Serum citrulline as a marker of acute cellular rejection for intestinal transplantation. Transplant Proc 2002; 34: 915–917. [DOI] [PubMed] [Google Scholar]
- 29.Gondolesi GE, Kaufman SS, Sansaricq C, et al. Defining normal plasma citrulline in intestinal transplant recipients. Am J Transplant 2004; 4: 414–418. [DOI] [PubMed] [Google Scholar]
- 30.Pappas PA, Tzakis G, Gaynor A JJ, et al. An analysis of the association between serum citrulline and acute rejection among 26 recipients of intestinal transplant. Am J Transplant 2004; 4: 1124–1132. [DOI] [PubMed] [Google Scholar]
- 31.Pappas PA, Tzakis AG, Saudubray JM, et al. Trends in serum citrulline and acute rejection among recipients of small bowel transplants. Transplant Proc 2004; 36: 345–347. [DOI] [PubMed] [Google Scholar]
- 32.David AI, Selvaggi G, Ruiz P, et al. Blood citrulline level is an exclusionary marker for significant acute rejection after intestinal transplantation. Transplantation 2007; 84: 1077–1081. [DOI] [PubMed] [Google Scholar]
- 33.David AI, Szutan LA, Gaynor J, et al. Critical value of citrulline for complications of intestinal transplant graft [article in Portuguese]. Rev Assoc Med Bras 2008; 54: 426–429. [DOI] [PubMed] [Google Scholar]
- 34.Gondolesi G, Ghirardo S, Raymond K, et al. The value of plasma citrulline to predict mucosal injury in intestinal allografts. Am J Transplant 2006; 6: 2786–2790. [DOI] [PubMed] [Google Scholar]
- 35.Crenn P, Vahedi K, Lavergne-Slove A, et al. Plasma citrulline: A marker of enterocyte mass in villous atrophy-associated small bowel disease. Gastroenterology 2003; 124: 1210–1219. [DOI] [PubMed] [Google Scholar]
- 36.Hozyasz KK, Szaflarska-Popławska A, Ołtarzewski M, et al. Whole blood citrulline levels in patients with coeliac disease [article in Polish]. Pol Merkur Lekarski 2006; 20: 173–175. [PubMed] [Google Scholar]
- 37.Miceli E, Poggi N, Missanelli A, et al. Is serum citrulline measurement clinically useful in coeliac disease? Intern Emerg Med 2008; 3: 233–236. [DOI] [PubMed] [Google Scholar]
- 38.Blasco Alonso J, Serrano Nieto J, Navas Lopez VM, et al. Plasma citrulline as a marker of loss of enterocitary mass in coeliac disease in childhood [article in Spanish]. Nutr Hosp 2011; 26: 807–813. [DOI] [PubMed] [Google Scholar]
- 39.Ioannou HP, Fotoulaki M, Pavlitou A, et al. Plasma citrulline levels in paediatric patients with celiac disease and the effect of a gluten-free diet. Eur J Gastroenterol Hepatol 2011; 23: 245–249. [DOI] [PubMed] [Google Scholar]
- 40.Papadia C, Sherwood RA, Kalantzis C, et al. Plasma citrulline concentration: A reliable marker of small bowel absorptive capacity independent of intestinal inflammation. Am J Gastroenterol 2007; 102: 1474–1482. [DOI] [PubMed] [Google Scholar]
- 41.Diamanti A, Knafelz D, Panetta F, et al. Plasma citrulline as surrogate marker of intestinal inflammation in pediatric and adolescent with Crohn’s disease: Preliminary report. Int J Colorectal Dis 2011; 26: 1445–1451. [DOI] [PubMed] [Google Scholar]
- 42.Luiking YC, Poeze M, Ramsay G, et al. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am J Clin Nutr 2009; 89: 142–152. [DOI] [PubMed] [Google Scholar]
- 43.van Vliet MJ, Tissing WJ, Rings EH, et al. Citrulline as a marker for chemotherapy induced mucosal barrier injury in pediatric patients. Pediatr Blood Cancer 2009; 53: 1188–1194. [DOI] [PubMed] [Google Scholar]
- 44.Blijlevens NM, Donnelly JP, DePauw BE. Inflammatory response to mucosal barrier injury after myeloablative therapy in allogeneic stem cell transplant recipients. Bone Marrow Transplant 2005; 36: 703–707. [DOI] [PubMed] [Google Scholar]
- 45.Lutgens LC, Blijlevens NM, Deutz NE, et al. Monitoring myeloablative therapy-induced small bowel toxicity by serum citrulline concentration: A comparison with sugar permeability tests. Cancer 2005; 103: 191–199. [DOI] [PubMed] [Google Scholar]
- 46.Papadia C, Kelly P, Caini S, et al. Plasma citrulline as a quantitative biomarker of HIV-associated villous atrophy in a tropical enteropathy population. Clin Nutri 2010; 29: 795–800. [DOI] [PubMed] [Google Scholar]
- 47.Jianfeng G, Weiming Z, Ning L, et al. Serum citrulline is a simple quantitative marker for small intestinal enterocytes mass and absorption function in short bowel patients. J Surg Res 2005; 127: 177–182. [DOI] [PubMed] [Google Scholar]
- 48.Gong JF, Zhu WM, Yu WK, et al. Role of enteral nutrition in adult short bowel syndrome undergoing intestinal rehabilitation: The long-term outcome. Asia Pac J Clin Nutr 2009; 18: 155–163. [PubMed] [Google Scholar]
- 49.Rhoads JM, Plunkett E, Galanko J, et al. Serum citrulline levels correlate with enteral tolerance and bowel length in infants with short bowel syndrome. J Pediatr 2005; 146: 542–547. [DOI] [PubMed] [Google Scholar]
- 50.Fitzgibbons S, Ching YA, Valim C, et al. Relationship between serum citrulline levels and progression to parenteral nutrition independence in children with short bowel syndrome. J Pediatr Surg 2009; 44: 928–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diamanti A, Panetta F, Gandullia P, et al. Plasma citrulline as marker of bowel adaptation in children with short bowel syndrome. Langenbecks Arch Surg 2011; 396: 1041–1046. [DOI] [PubMed] [Google Scholar]
- 52.Luo M, Fernández-Estívariz C, Manatunga AK, et al. Are plasma citrulline and glutamine biomarkers of intestinal absorptive function in patients with short bowel syndrome? JPEN J Parenter Enteral Nutr 2007; 31: 1–7. [DOI] [PubMed] [Google Scholar]
- 53.Peters JH, Wierdsma NJ, Teerlink T, et al. Poor diagnostic accuracy of a single fasting plasma citrulline concentration to assess intestinal energy absorption capacity. Am J Gastroenterol 2007; 102: 2814–2819. [DOI] [PubMed] [Google Scholar]
- 54.Picot D, Garin L, Trivin F, et al. Plasma citrulline is a marker of absorptive small bowel length in patients with transient enterostomy and acute intestinal failure. Clin Nutr 2010; 29: 235–242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.