Abstract
Objectives:
Levodopa-carbidopa intestinal gel (LCIG) was developed to reduce motor complications in Parkinson’s disease (PD) caused by pulsatile levodopa plasma concentrations following oral levodopa administration. Dyskinesia and ‘wearing off’ symptoms can vary between Asian and Caucasian patients with PD, thus highlighting the importance of assessing the effectiveness of LCIG in an Asian population. Efficacy and safety of LCIG were previously assessed in a 12-week open-label study; we report the efficacy and safety of at least 52 weeks of LCIG treatment in Japanese, Taiwanese, and Korean patients with advanced PD in the ongoing extension study.
Methods:
In this interim analysis of a phase III, open-label, multicenter extension study in Japan, South Korea, and Taiwan [ClinicalTrials.gov identifier: NCT02082249/JapiCTI-142482], the mean change from baseline to final visit in ‘off’ time, as reported in the PD symptom diary, was normalized to a 16-h waking day. Changes in Parkinson’s Disease Questionnaire-39 (PDQ-39) summary index and domains scores were also analyzed. Adverse events (AEs) were recorded.
Results:
Of the 28 patients enrolled (21 Japanese, 3 Taiwanese, 4 Korean), 27 completed at least 52 total weeks of treatment, and 25 patients were continuing in the study at data cutoff. The mean [standard deviation (SD)] ‘off’ time was significantly reduced by 4.6 (3.1) h/day (p < 0.001, n = 28). Patients experienced significant improvements in quality of life, as recorded by the mean change from baseline in PDQ-39 summary index (p < 0.001). All patients had at least one AE; three patients (11%) discontinued due to an AE. There were two deaths (sepsis and drowning), both of which the investigator considered unrelated to LCIG treatment.
Conclusions:
These data suggest that LCIG treatment is efficacious, safe, and well tolerated in Japanese, Taiwanese, and Korean patients with advanced PD, thus confirming the consistency of LCIG treatment in patients with advanced PD.
Keywords: levodopa-carbidopa intestinal gel, Parkinson’s disease
Introduction
Treatment with oral levodopa is associated with the development of motor complications (e.g. dyskinesia and ‘on’/‘off’ fluctuations) that are often disabling in some patients with advanced Parkinson’s disease (PD).1,2 These motor complications are due, in part, to non-physiologic pulsatile stimulation of striatal dopamine receptors produced by the short half-life of levodopa and a loss of dopaminergic neurons as the disease progresses.3 In addition, the narrowing therapeutic window that results as the disease progresses contributes to an increased prevalence of dyskinesia that cannot be adequately controlled by oral dopaminergic medications.1
Levodopa-carbidopa intestinal gel (LCIG, known as ABT-SLV187 in Japan, and in the United States as carbidopa-levodopa enteral suspension) was developed to minimize pulsatile levodopa plasma concentrations by providing continuous levodopa infusion directly into the jejunum via percutaneous gastrojejunostomy (PEG-J).4 Continuous administration of LCIG into the jejunum provides more consistent plasma levodopa concentrations than when levodopa is administered orally.3–5 Results from a global randomized, placebo-controlled trial and several open-label trials in patients with advanced PD indicate that LCIG is well tolerated and demonstrates significantly improved ‘off’ time and better quality of life.6–9
Symptoms of dyskinesia and ‘wearing off’ signs can vary between Asian and Caucasian patients with PD, highlighting the importance of assessing the effectiveness of LCIG in an Asian population.10,11 In the initial open-label trial in Japanese, Taiwanese, and Korean patients with advanced PD, 12 weeks of LCIG treatment reduced mean daily ‘off’ time by 4.6 h, and was generally well tolerated.12 These results were consistent with previous reports of LCIG efficacy and safety in predominantly Caucasian patient populations.6 Patients in the initial study were eligible to enroll in a two-part extension study. Herein we report on part one of the extension study, including the efficacy and safety of at least 52 weeks of LCIG treatment in Japanese, Taiwanese, and Korean patients with advanced PD.
Methods
Patients
Patients who completed 12 weeks of LCIG via PEG-J in a previous open-label study12 were eligible to enroll in this two-part extension study. Patients had to be at least 30 years old, diagnosed with idiopathic PD according to the UK Parkinson’s Disease Society Brain Bank Criteria, and levodopa-responsive.12 Eligible patients had to have severe motor fluctuations with at least 3 h of ‘off’ time/day at baseline of the initial study, despite optimized oral anti-PD therapy. Two patients from a separate open-label study [ClinicalTrials.gov identifier: NCT01479127], who had previously been exposed to LCIG were allowed access to the drug as part of this study due to the absence of commercial approval. These patients had previous naso-jejunal LCIG experience but returned to oral PD medication for a few years before enrolling in this extension study; because of the LCIG treatment gap, their data are not reported in this article. Another patient was included in this study because there is no compassionate-use program in Japan; this patient did not have efficacy data collected and was not included in this report. All patients provided written, informed consent.
Study design and treatment
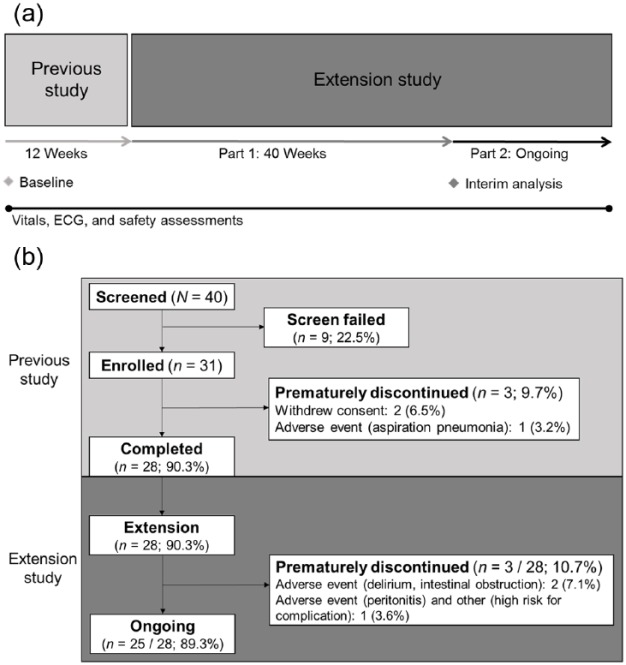
This was an interim analysis of the first part of a phase III, open-label, multicenter extension study in Japan, South Korea, and Taiwan [ClinicalTrials.gov identifier: NCT02082249/JapiCTI-142482]. In the initial study, patients were converted to oral levodopa/carbidopa, titrated to LCIG via naso-jejunum, and then received a stable dose of LCIG via PEG-J for 12 weeks.12 This current extension study includes two parts: in completed part one, patients continued LCIG for 40 weeks for a cumulative LCIG treatment of 52 weeks; in the ongoing part two portion of the study, patients have continued access to treatment (Figure 1(a)). LCIG was administered as monotherapy via PEG-J during 16 waking hours. Patients had the option to take oral levodopa-carbidopa at night.
Figure 1.
(a) Study design and (b) patient disposition. Patients who prematurely discontinued the study and did not continue into the separate extension study had a follow-up visit 7 days after discontinuation.
ECG, electrocardiogram.
This study was conducted in accord with the Good Clinical Practice Guideline as defined by the International Council on Harmonisation, the Declaration of Helsinki, and/or all applicable federal and local regulations and independent ethics committees (IECs)/institutional review board (IRB) guidelines. For all IEC/IRBs, their corresponding approval references include: National Hospital Organization Sagamihara National Hospital IRB (approval #2540), Seoul National University Hospital IRB (approval #H-14010-123-620), National Hospital Organization Asahikawa Medical Center IRB (approval #14-a-6), Chang Gung Memorial Hospital IRB (approval #102-3953C), Osaka University Hospital IRB (approval #135060), National Center of Neurology and Psychiatry IRB (approval #治-202), Ehime University Hospital IRB (approval #13-14), National Hospital Organization Utano Hospital (no IRB approval number), Juntendo University Hospital IRB (approval #2013-038), Kyoto University Hospital IRB (approval #1610), and China Medical University Hospital Research Ethics Committee (approval #CMUH102-REC1-057).
Assessments
Efficacy
Efficacy was evaluated using the PD symptom diary assessments of daily ‘off’ time, ‘on’ time with troublesome dyskinesia (TSD) and ‘on’ time without TSD (sum of ‘on’ time without dyskinesia and ‘on’ time with non-TSD). Other efficacy assessments included Unified Parkinson’s Disease Rating Scale (UPDRS) total score, UPDRS Parts II and III scores (during ‘on’ time), and UPDRS Part IV scores, Parkinson’s Disease Questionnaire-39 (PDQ-39) summary index score, Clinical Global Impression of Change (CGI-C) score, and Patient Global Impression of Change (PGI-C) score.
Safety
Treatment-emergent adverse events (AEs) included all AEs with onset on or after the date of PEG-J placement and within 30 days of the end of LCIG treatment. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA, version 17.1) and were tabulated by MedDRA preferred term. Local study investigators assessed each AE to determine if there was a reasonable possibility that the LCIG system (drug and/or device) had a causal relationship with an AE. AEs of special interest were tabulated by the MedDRA query for gastrointestinal (GI) and GI procedure-related events. Clinical laboratory values, electrocardiograms, and vital sign measurements were collected throughout the study.
Statistical analysis
Safety and efficacy data as of 8 December 2015 were included. Daily totals in the PD symptom diary were normalized to 16-h days and averaged for the 3 days prior to the visit. For efficacy measures, the change from baseline to each time point was evaluated by a mixed-model, repeated-measures analysis, which included fixed effects of country and visit, baseline value as a covariate, and baseline-by-visit interaction. Baseline was defined as the last non-missing observation that was on or before the date of the patient’s first dose of oral study drug in the initial study. Last visit was defined as the last non-missing observation that was no more than 1 day after the last infusion of LCIG at the time of data cutoff. The daily levodopa dose included the morning dose, continuous maintenance dose, and extra doses of LCIG, but not oral levodopa-carbidopa taken at night. The mean change from baseline in ‘off’ time was evaluated in subgroups defined by ethnicity (Japanese, Korean, and Taiwanese) using a one-sample Student’s t test.
Results
Patients
Of the 28 patients who enrolled in the extension study after completing 12 weeks of LCIG treatment via PEG-J, 27 (96%) completed at least 52 total weeks of treatment (Figure 1(b)). As of the data cutoff date, 25 patients were continuing in the study. Patient baseline characteristics are summarized in Table 1. Of 28 patients, most (75%) were Japanese. Based on the dosing diary of patients who completed at least 52 weeks of treatment (n = 27), the mean (SD) total daily levodopa dose from LCIG was 1125.3 (487.1) mg/day at week 52. The median (range) time of patient exposure to LCIG via PEG-J was 408 (184–696) days. Less than half of the patients were taking concomitant PD medication during the study, the most common of which was rotigotine (five patients, 17.9%) (Table 2).
Table 1.
Baseline demographics and characteristics.
| Characteristic | N = 28 |
|---|---|
| Age, years, mean (SD) | 60.4 (10.0) |
| Range | 45.0–77.0 |
| Sex, n (%) | |
| Female | 16 (57) |
| Male | 12 (43) |
| Ethnicity, n (%) | |
| Japanese | 21 (75) |
| Taiwanese | 3 (11) |
| Korean | 4 (14) |
| Mean (SD) duration of PD, years | 11.9 (5.0) |
| Mean (SD) levodopa dose, mg/daya | 1131.3 (597.7) |
| Mean (SD) ‘off’ time, h/day | 7.4 (2.3) |
| Range | 3.0–11.6 |
| Mean (SD) ‘on’ time without TSD, h/day | 7.4 (2.5) |
| Range | 1.0–11.8 |
| Mean (SD) ‘on’ time with TSD, h/day | 1.2 (2.3) |
| Range | 0.0–9.2 |
| Mean (SD) PDQ-39 summary index | 35.2 (13.5) |
| Range | 8–67 |
| Mean (SD) UPDRS total score | 26.3 (15.0) |
| Range | 2–60 |
| Mean (SD) UPDRS Part II score | 8.4 (5.5) |
| Range | 0–23 |
| Mean (SD) UPDRS Part III score | 16.1 (9.8) |
| Range | 1–42 |
| Mean (SD) UPDRS Part IV score | 8.6 (3.2) |
| Range | 4–17 |
Last full daily levodopa dose of oral levodopa-carbidopa prior to naso-jejunal procedure in the initial 12-week open-label study.
PD, Parkinson’s disease; PDQ-39, Parkinson’s Disease Questionnaire; SD, standard deviation; TSD, troublesome dyskinesia; UPDRS, Unified Parkinson’s Disease Rating Scale.
Table 2.
Concomitant Parkinson’s disease medication during the study.
| Medication | Patients, n (%) (N = 28) |
|---|---|
| Any | 12 (42.9) |
| Rotigotine | 5 (17.9) |
| Amantadine | 3 (10.7) |
| Pramipexole | 3 (10.7) |
| Ropinirole | 3 (10.7) |
| Trihexyphenidyl | 2 (7.1) |
| Cabergoline | 1 (3.6) |
| Istradefylline | 1 (3.6) |
| Levodopa | 1 (3.6) |
| Selegiline | 1 (3.6) |
Efficacy
The mean (SD) ‘off’ time was significantly reduced from 7.4 (2.3) h/day at baseline to 2.8 (2.6) h/day at the last visit [reduction of 4.6 (3.1) h/day; p < 0.001]. The mean (SD) ‘on’ time without TSD significantly increased from 7.4 (2.5) h/day at baseline to 12.4 (3.3) h/day at the last visit [improvement of 5.0 (3.1) h/day; p < 0.001]. Significant improvements in ‘off’ time and ‘on’ time with and without TSD were observed as early as week 2 and persisted for more than a year (Figure 2). At last visit, Japanese and Taiwanese patients experienced significant mean (SD) reductions in ‘off’ time from baseline of 4.3 (3.0) h/day (p < 0.001) and 7.5 (2.8) h/day (p < 0.05), respectively; although not significant, Korean patients experienced a mean (SD) reduction in ‘off’ time from baseline of 3.8 (3.5) h/day. LCIG treatment led to significant improvements in ‘off’ time, regardless of sex and age (Figure 3).
Figure 2.
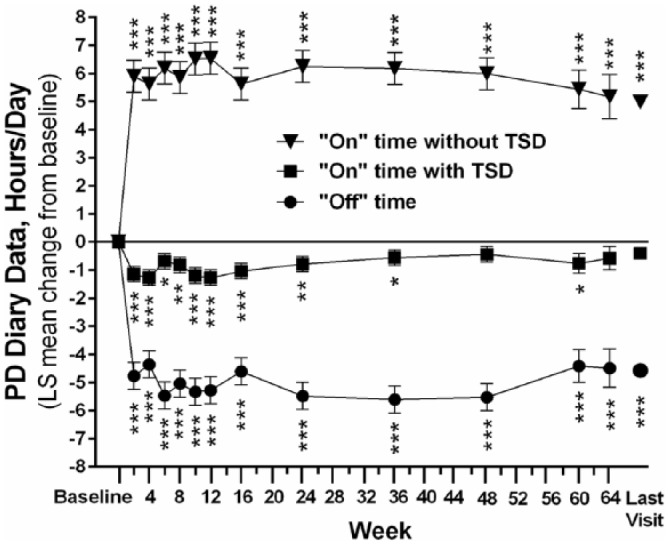
Change from baseline in PD diary measures.
Daily totals were normalized to a 16-h waking day and the 3 days prior to the visit were averaged. ‘On’ time without troublesome dyskinesia is the sum of ‘on’ time without dyskinesia and ‘on’ time with non-troublesome dyskinesia. Last visit refers to the last study visit at the time of the data cutoff. Error bars indicate standard deviation. Probability values indicate statistically significant mean change from baseline of p ⩽ 0.05 (*), p ⩽ 0.01 (**), and p ⩽ 0.0001 (***). The sample size was 28 at every time point except weeks 8, 36, and 48 (n = 27), and weeks 60 (n = 14) and 64 (n = 10).
LS, least squares; PD, Parkinson’s disease; TSD, troublesome dyskinesia.
Figure 3.
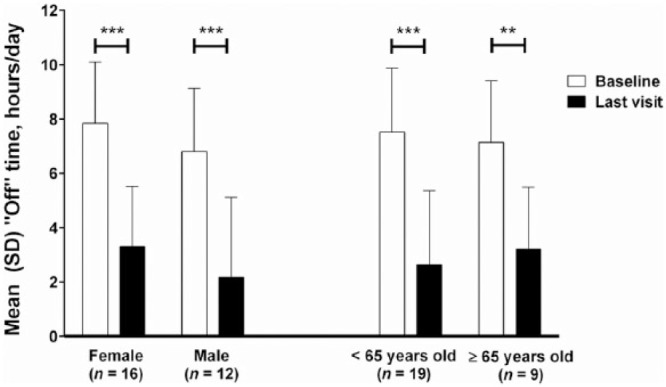
Mean (SD) hours of ‘off’ time/day in sex and age subgroups.
Daily totals were normalized to a 16-h waking day and the 3 days prior to the visit were averaged. Last visit refers to the last study visit at the time of the data cutoff. Error bars indicate standard deviation. Probability values indicate statistically significant mean change from baseline of p ⩽ 0.05 (*) and p ⩽ 0.0001 (***).
At last visit, patients also experienced a significant improvement in motor symptoms (motor fluctuations and dyskinesia), based on the mean change from baseline in UPDRS Part IV score (Table 3). There were no significant mean changes in the activities of daily living (UPDRS Part II) or motor function (UPDRS Part III) when assessed in the patients’ best ‘on’ state.
Table 3.
Changes in PDQ-39 summary index, UPDRS Parts II, III, and IV.
| Mean (SD) | p value compared with baseline | |||
|---|---|---|---|---|
| Baseline | Last visita | Change from baseline | ||
| PDQ-39 summary index | 35.2 (13.5) | 25.9 (13.2) | −9.3 (11.4) | <0.001 |
| UPDRS Part II score (ADL) during ‘on’ time | 8.4 (5.5) | 9.0 (5.8) | 0.7 (6.0) | 0.552 |
| UPDRS Part III score (motor) during ‘on’ time | 16.1 (9.8) | 17.6 (12.8) | 1.5 (11.1) | 0.482 |
| UPDRS Part IV score (motor fluctuations and dyskinesia) | 8.6 (3.2) | 5.7 (2.4) | −2.9 (3.6) | <0.001 |
Last visit refers to the last study visit at the time of data cutoff.
ADL, activities of daily living; PDQ-39, Parkinson’s Disease Questionnaire-39; SD, standard deviation; UPDRS, Unified Parkinson’s Disease Rating Scale.
In addition to improvements in diary measures, there was a significant improvement in quality of life, based on the mean change from baseline in PDQ-39 summary index (p < 0.001; Table 3). Notably, the quality of life significantly improved in five of nine subdomains in the PDQ-39, including the subdomains of mobility, activities of daily living, stigma, cognition, and bodily discomfort (Figure 4). Much like what was reported at week 12, more than half (n = 6/11, 54.5%) of patients rated their change in quality of life on the PGI-C from before initiation of treatment as ‘very much improved’ or ‘much improved’ at the last visit; five of 11 patients (45.5%) reported minimal improvement on the PGI-C, and no patients reported any worsening or ‘no change’ on the PGI-C. The mean (SD) PGI-C score of 2.2 (0.87) was significantly different (p < 0.001) from a hypothesized mean score of 4 (no change). Clinician-reported improvements (CGI-C) were consistent with patient-reported improvements (PGI-C).
Figure 4.
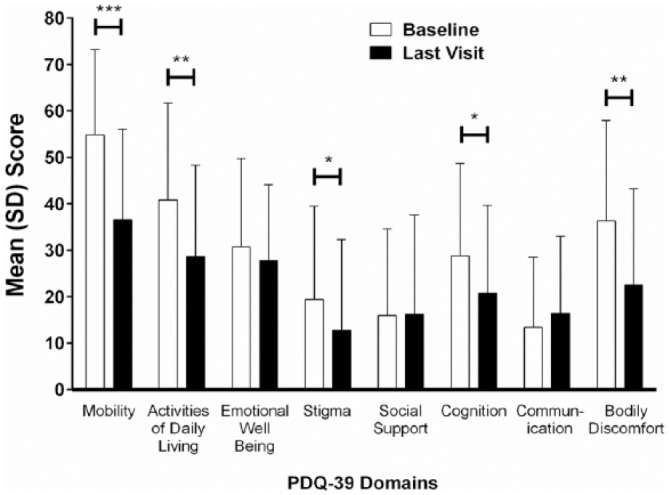
Mean PDQ-39 domain scores at baseline and last visit.
N = 28. Error bars indicate standard deviation. Probability values from a two-sided, one-sample t test indicate statistically significant mean change from baseline: p ⩽ 0.05 (*), p ⩽ 0.01 (**), and p ⩽ 0.001 (***).
Safety
All patients had at least one AE, and all patients had an AE that the study investigator considered to have a reasonable possibility of being related to the treatment system (Table 4). Eight patients (29%) had serious AEs, four of whom had events that the study investigator considered to have a reasonable possibility of being related to the treatment system: one patient had severe aspiration pneumonia, device (J tube) kink, device (J tube) dislocation, and GI perforation; one patient had moderate abdominal pain and constipation; one patient had delirium; one patient had mild aspiration pneumonia. There were two deaths, one due to sepsis and the other to non-treatment-emergent AE of drowning after discontinuation of the study treatment. Both deaths were considered by the investigators to have no reasonable possibility of being related to LCIG. Three patients (11%) discontinued due to an AE (peritonitis, delirium, and intestinal obstruction).
Table 4.
Summary of adverse events.
| Patients, n (%) (N = 28) |
|
|---|---|
| Any AE | 28 (100) |
| Any AE with reasonable possibility of being related to LCIG (drug/device) | 28 (100) |
| Any serious AE | 8 (28.6) |
| Any severe AE | 3 (10.7) |
| Any AE leading to a discontinuation | 3 (10.7) |
| Death | 2 (7.1) |
| AEs occurring in ⩾15% of patients by preferred terma | |
| Excessive granulation tissue | 21 (75.0) |
| Incision site pain | 14 (50.0) |
| Nasopharyngitis | 10 (35.7) |
| Constipation | 9 (32.1) |
| Diarrhea | 8 (28.6) |
| Incision site erythema | 7 (25.0) |
| Weight decreased | 7 (25.0) |
| Tinea pedis | 6 (21.4) |
| Dyskinesia | 5 (17.9) |
| Blood homocysteine increased | 5 (17.9) |
| Fall | 5 (17.9) |
| Procedural pain | 5 (17.9) |
| Nausea | 5 (17.9) |
| Vitamin B6 deficiency | 5 (17.9) |
| Vomiting | 5 (17.9) |
Preferred terms in the AEs of special interest gastrointestinal and gastrointestinal procedure-related standard MedDRA query are in bold type.
AE, adverse event; LCIG, levodopa-carbidopa intestinal gel; MedDRA, Medical Dictionary for Regulatory Activities.
There were no clinically meaningful changes in vital sign measurements, electrocardiogram results, and laboratory values compared with baseline. Eight patients (29%) experienced vitamin B6 deficiency or decrease, of which the most common action was B6 supplementation. One patient experienced two events in the category of polyneuropathy broad search: muscular weakness and radiculopathy; the investigator indicated that both events were not related to LCIG treatment.
At the data cutoff, seven patients (25%) had their PEG tube replaced and 24 (86%) had their J tube replaced at least once; 21 patients (75%) had the original PEG tube and 4 (14%) retained the original J tube (Table 5). J tube replacement was required every 6 months in Japanese patients. All four patients who did not have a J tube replacement were Korean. In the first 12 months post PEG-J placement, there was a mean of 0.3 PEG tube replacements and 1.8 J tube replacements. There were two patients (7.1%) who had three or more J tube replacements.
Table 5.
PEG and J tube replacements.
| Tube replacements, n | Replacements, n (%) | |
|---|---|---|
| PEG tube | J tube | |
| 0 | 21 (75) | 4 (14) |
| 1 | 7 (25) | 1 (3.6) |
| 2 | 0 | 21 (75) |
| 3 | 0 | 2 (7.1) |
N, 28; J, jejunum; PEG, percutaneous endoscopic gastrostomy.
Discussion
In a disease in which limited therapeutic options are available, LCIG has the potential to address a significant unmet medical need in the advanced PD patient population. Because most patients on oral therapy eventually develop uncontrolled motor fluctuations,13 it is essential to find treatment options that address such development. In Japan, deep brain stimulation is frequently used to treat patients with advanced PD;14 however, levodopa-responsive patients may desire other treatment options due to the invasiveness and potential side effects of deep brain stimulation.15
Long-term treatment with LCIG demonstrated sustained significant and clinically beneficial reductions in ‘off’ time. The mean (SD) improvement from baseline in ‘off’ time [mean (SD) reduction of 4.6 (3.1) h/day] was comparable to the improvement in ‘off’ time reported in a similar-length study performed in a predominantly Caucasian patient population [mean (SD) reduction of 4.4 (2.9) h/day].6 Improvements in ‘off’ time corresponded with an increase in ‘on’ time without an exacerbation of TSD. Previous studies have also demonstrated improvements in ‘off’ and ‘on’ time without an increase in ‘on’ time with TSD.6,7,16 The efficacy of LCIG was independent of sex, age, and ethnicity. Although the reduction in ‘off’ time in Korean patients was not significant due to the small sample size, the reductions were clinically significant and numerically similar in ‘off’ time reported for Japanese and Taiwanese patients. Japanese and Taiwanese patients did experience significant improvements in ‘off’ time.
Patients in this study also experienced significant reductions from baseline in motor fluctuations and dyskinesia (p < 0.001). The mean (SD) reduction from baseline of 2.9 (3.6) in UPDRS Part V score was comparable to several European-based open-label studies that reported a significant mean reduction from baseline of 2.0 after 1 year of treatment (p < 0.05),17 a reduction of 3.0 after 36 months of treatment (p < 0.001),18 and a reduction of 2.8 after 87 months of LCIG treatment (p < 0.0001).19
Improvements demonstrated by the PD diary data were further confirmed by improvements in quality-of-life measurements. PDQ-39 summary index scores indicated a significant improvement from baseline (p < 0.001), most notably in five of nine PDQ-39 subdomains (mobility, activities of daily living, stigma, cognition, and bodily discomfort). A 54-week study in a predominantly Caucasian population demonstrated similar improvements in PDQ-39 summary index score.6 Improvements in quality-of-life data were confirmed in the current study by the patients’ and investigators’ impressions of improvement from baseline, as indicated by a majority of ratings of ‘very much improved’ or ‘much improved’ on both the PGI-C and CGI-C.
The mean levodopa daily dose remained relatively stable over the course of the study. Similar to previous studies,6,16 most of the AEs reported were mild or moderate in severity and were generally those expected for the device-related procedure and for patients with advanced PD who are treated with levodopa. Less than one-third of patients experienced vitamin B6 deficiency during this study. Although it has been reported that vitamin B deficiency (primarily vitamin B12) may be linked to LCIG infusion and possibly to polyneuropathy,20,21 only one patient in this study experienced muscular weakness and radiculopathy that were deemed unrelated to LCIG treatment by the investigator. Three patients discontinued from the study due to AEs. Two patients died in the study; both deaths were considered by the investigator to have no reasonable possibility of being related to LCIG. Despite being an ambulatory population, a majority of patients did not require replacement of their PEG tube. It was not unexpected that most patients had at least one J tube replacement, given that Japanese patients were required to have the J tube replaced every 6 months.
This study was limited by the small sample size, particularly for Korean and Taiwanese patients. The open-label nature of the study, as well as entry criteria that may not reflect the characteristics of patients in the clinic (e.g. baseline MMSE score), may cause the outcomes to be influenced by selection bias.
This is the longest study to date of LCIG use in treating Asian patients with advanced PD. These data suggest that LCIG treatment is efficacious, safe and generally well tolerated in Japanese, Taiwanese, and Korean patients with advanced PD, thus confirming the consistency of LCIG treatment in patients with advanced PD.
Acknowledgments
This study was supported by AbbVie, Inc., North Chicago, Illinois, USA. The authors acknowledge the contributions of the study investigators Takashi Nakajima of National Hospital Organization Niigata National Hospital, Ryosuke Takahashi of Kyoto University Hospital, Yasushi Shimo of Juntendo University Hospital, Takashi Kimura of National Hospital Organization Asahikawa Medical Center, Chin-Song Lu of Chang Gung Memorial Hospital, and Ruey-Meei Wu of National Taiwan University Hospital. The authors thank Masayoshi Yanagawa, of AbbVie, for his contribution to the study. We also thank Kelly M. Cameron, of JB Ashtin, who developed the first draft and assisted in implementing author revisions.
Footnotes
Funding: This study was funded by AbbVie, Inc. AbbVie participated in the study design, research, analysis, data collection, interpretation of data, and review and approval of the manuscript prior to submission. Writing support related to this article was funded by AbbVie, Inc.
Conflict of interest statement: The authors declare the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: Miho Murata, Masahito Mihara, Kazuko Hasegawa, Chon-Haw Tsai, Noriko Nishikawa and Tomoko Oeda were study investigators and received research support from AbbVie, Inc. Beomseok Jeon was a study investigator and received research support from the Ministry of Health and Welfare, Seoul National University Hospital, Sinyang Cultural Foundation, Song Foundation, Korean Movement Disorder Society, Samil Pharmaceuticals, AbbVie Korea, UCB Korea, Ipsen Korea, Sandoz Korea, Lundbeck Korea, and Novartis Korea; travel support from Korea Research-Based Pharmaceutical Industry Association, Korean Pharmaceutical Manufacturers Association, Seoul National University, Seoul National University Hospital; and compensation from Lundbeck Korea for consulting and Ipsen and UCB for speaking. Masayuki Yokoyama, Weining Robieson, Maurizio Facheris, and Janet Benesh are employees of AbbVie, Inc., and may hold stock or stock options. Krai Chatamra was an employee of AbbVie at the time of the study; he is currently employed at Lundbeck.
Contributor Information
Miho Murata, National Center of Neurology and Psychiatry, 4-1-1 Ogawa-Higashi, Kodaira 187-8551, Tokyo, Japan.
Masahito Mihara, Osaka University Graduate School of Medicine, Suita, Japan.
Kazuko Hasegawa, National Hospital Organization Sagamihara National Hospital, Sagamihara, Japan.
Beomseok Jeon, Seoul National University Hospital, Seoul, Republic of Korea.
Chon-Haw Tsai, Department of Neurology, China Medical University Hospital Medical College, China Medical University, Taichung, Taiwan.
Noriko Nishikawa, Ehime University Hospital, Matsuyama, Japan.
Tomoko Oeda, National Hospital Organization Utano Hospital, Kyoto, Japan.
Masayuki Yokoyama, AbbVie Inc., Tokyo, Japan.
Weining Z. Robieson, AbbVie Inc., North Chicago, Illinois, USA
Krai Chatamra, AbbVie Inc., North Chicago, Illinois, USA.
Maurizio F. Facheris, AbbVie Inc., North Chicago, Illinois, USA
Janet Benesh, AbbVie Inc., North Chicago, Illinois, USA.
References
- 1. Mouradian MM, Heuser IJ, Baronti F, et al. Pathogenesis of dyskinesias in Parkinson’s disease. Ann Neurol 1989; 25: 523–526. [DOI] [PubMed] [Google Scholar]
- 2. Obeso JA, Olanow CW, Nutt JG. Levodopa motor complications in Parkinson’s disease. Trends Neurosci 2000; 23(Suppl. 10): S2–S7. [DOI] [PubMed] [Google Scholar]
- 3. Olanow CW, Obeso JA, Stocchi F. Continuous dopamine-receptor treatment of Parkinson’s disease: scientific rationale and clinical implications. Lancet Neurol 2006; 5: 677–687. [DOI] [PubMed] [Google Scholar]
- 4. Nyholm D, Askmark H, Gomes-Trolin C, et al. Optimizing levodopa pharmacokinetics: intestinal infusion versus oral sustained-release tablets. Clin Neuropharmacol 2003; 26: 156–163. [DOI] [PubMed] [Google Scholar]
- 5. Othman AA, Chatamra K, Mohamed ME, et al. Jejunal infusion of levodopa-carbidopa intestinal gel versus oral administration of levodopa-carbidopa tablets in Japanese subjects with advanced Parkinson’s disease: pharmacokinetics and pilot efficacy and safety. Clin Pharmacokinet 2015; 54: 975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fernandez HH, Standaert DG, Hauser RA, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s disease: final 12-month, open-label results. Mov Disord 2015; 30: 500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olanow CW, Kieburtz K, Odin P, et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol 2014; 13: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lang AE, Rodriguez RL, Boyd JT, et al. Integrated safety of levodopa-carbidopa intestinal gel from prospective clinical trials. Mov Disord 2016; 31: 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Epstein M, Johnson DA, Hawes R, et al. Long-term PEG-J tube safety in patients with advanced Parkinson’s disease. Clin Transl Gastroenterol 2016; 7: e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rivera-Calimlim L, Reilly DK. Difference in erythrocyte catechol-O-methyltransferase activity between Orientals and Caucasians: difference in levodopa tolerance. Clin Pharmacol Ther 1984; 35: 804–809. [DOI] [PubMed] [Google Scholar]
- 11. Bhidayasiri R, Hattori N, Jeon B, et al. Asian perspectives on the recognition and management of levodopa ‘wearing-off’ in Parkinson’s disease. Expert Rev Neurother 2015; 15: 1285–1297. [DOI] [PubMed] [Google Scholar]
- 12. Murata M, Mihara M, Hasegawa K, et al. Efficacy and safety of levodopa-carbidopa intestinal gel from a study in Japanese, Taiwanese, and Korean advanced Parkinson’s disease patients. NPJ Parkinson’s Dis 2016; 2: 16020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 2001; 16: 448–458. [DOI] [PubMed] [Google Scholar]
- 14. Taniguchi A, Narita Y, Naito Y, et al. Analysis of application form for Parkinson’s disease provided by the specific diseases treatment research program of Ministry of Health, Labour and Welfare of Japan. Rinsho Shinkeigaku 2008; 48: 106–113. [DOI] [PubMed] [Google Scholar]
- 15. Moldovan AS, Groiss SJ, Elben S, et al. The treatment of Parkinson’s disease with deep brain stimulation: current issues. Neural Regen Res 2015; 10: 1018–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Slevin JT, Fernandez HH, Zadikoff C, et al. Long-term safety and maintenance of efficacy of levodopa-carbidopa intestinal gel: an open-label extension of the double-blind pivotal study in advanced Parkinson’s disease patients. J Parkinsons Dis 2015; 5: 165–174. [DOI] [PubMed] [Google Scholar]
- 17. Antonini A, Mancini F, Canesi M, et al. Duodenal levodopa infusion improves quality of life in advanced Parkinson’s disease. Neurodegener Dis 2008; 5: 244–246. [DOI] [PubMed] [Google Scholar]
- 18. Palhagen SE, Sydow O, Johansson A, et al. Levodopa-carbidopa intestinal gel (LCIG) treatment in routine care of patients with advanced Parkinson’s disease: an open-label prospective observational study of effectiveness, tolerability and healthcare costs. Parkinsonism Relat Disord 2016; 29: 17–23. [DOI] [PubMed] [Google Scholar]
- 19. Zibetti M, Merola A, Artusi CA, et al. Levodopa/carbidopa intestinal gel infusion in advanced Parkinson’s disease: a 7-year experience. Eur J Neurol 2014; 21: 312–318. [DOI] [PubMed] [Google Scholar]
- 20. Merola A, Romagnolo A, Zibetti M, et al. Peripheral neuropathy associated with levodopa-carbidopa intestinal infusion: a long-term prospective assessment. Eur J Neurol 2016; 23: 501–509. [DOI] [PubMed] [Google Scholar]
- 21. Uncini A, Eleopra R, Onofrj M. Polyneuropathy associated with duodenal infusion of levodopa in Parkinson’s disease: features, pathogenesis and management. J Neurol Neurosurg Psychiatry 2015; 86: 490–495. [DOI] [PubMed] [Google Scholar]