Abstract
Low collagen accumulation in the extracellular matrix is a pressing problem in cartilage tissue engineering, leading to a low collagen-to-glycosaminoglycan (GAG) ratio and poor mechanical properties in neocartilage. Soluble factors have been shown to increase collagen content, but may result in a more pronounced increase in GAG content. Thyroid hormones have been reported to stimulate collagen and GAG production, but reported outcomes, including which specific collagen types are affected, are variable throughout the literature. Here we investigated the ability of thyroxine (T4) to preferentially stimulate collagen production, as compared with GAG, in articular chondrocyte-derived scaffold-free engineered cartilage. Dose response curves for T4 in pellet cultures showed that 25 ng/mL T4 increased the total collagen content without increasing the GAG content, resulting in a statistically significant increase in the collagen-to-GAG ratio, a fold change of 2.3 ± 1.2, p < 0.05. In contrast, another growth factor, TGFβ1, increased the GAG content in excess of threefold more than the increase in collagen. In large scaffold-free neocartilage, T4 also increased the total collagen/DNA at 1 month and at 2 months (fold increases of 2.1 ± 0.8, p < 0.01 and 2.1 ± 0.4, p < 0.001, respectively). Increases in GAG content were not statistically significant. The effect on collagen was largely specific to collagen type II, which showed a 2.8 ± 1.6-fold increase of COL2A1 mRNA expression (p < 0.01). Western blots confirmed a statistically significant increase in type II collagen protein at 1 month (fold increase of 2.2 ± 1.8); at 2 months, the fold increase of 3.7 ± 3.3 approached significance (p = 0.059). Collagen type X protein was less than the 0.1 μg limit of detection. T4 did not affect COL10A1 and COL1A2 gene expression in a statistically significant manner. Biglycan mRNA expression increased 2.6 ± 1.6-fold, p < 0.05. Results of this study show that an optimized dosage of T4 is able to increase collagen type II content, and do so preferential to GAG. Moreover, the upregulation of COL2A1 gene expression and type II collagen protein accumulation, without a concomitant increase in collagens type I or type X, signifies a direct enhancement of chondrogenesis of hyaline articular cartilage without the induction of terminal differentiation.
Keywords: : thyroxine, engineered cartilage, collagen type II, collagen-to-GAG ratio, chondrogenesis, extracellular matrix composition
Introduction
Low collagen content in the neocartilage extracellular matrix (ECM) is a chronic problem in cartilage tissue engineering. The ECM of hyaline cartilage is comprised primarily of type II collagen, followed by glycosaminoglycan (GAG). Collagen is reported to account for 67% of the dry weight of human medial femoral condylar cartilage,1 whereas GAG is reported to account for 21%,2 resulting in a collagen-to-GAG ratio of 3.2-to-1. Native rabbit articular cartilage demonstrates a nearly identical collagen-to-GAG ratio of 3.3-to-1.3 Tissue-engineered cartilage (TEC) commonly contains >75% of the GAG content found in native cartilage (NC),3–5 whereas collagen content is usually <40%4,6 of that found in NC (a collagen-to-GAG ratio of ∼1.7). Studies in TEC and NC have shown a correlation of mechanical properties to both collagen7,8 and GAG8–10 content, whereas studies on osteoarthritic cartilage (OAC) have found that the mechanical properties of OAC are highly correlated with the extent of collagen degradation but not GAG content.11
Based on these studies, and owing that collagen is usually greatly deficient in TEC, both in an absolute sense and when compared with GAG content, low collagen content has been identified as a major cause of the characteristically poor mechanical, and functional, properties of TEC.12 Recently, we demonstrated that scaffold-free engineered cartilage deficient in collagen content is susceptible to cracking and surface peeling when subjected to even subphysiological frictional shear forces.13 To enhance the ability of TEC to withstand the demanding mechanical environment of weight-bearing diarthroidal joints, increased collagen content is a requisite.
Many different soluble factors have been tested for their effects on the ECM of TEC. This work has been extensively reviewed by Demoor et al.14 Although some factors may increase collagen accumulation, the increase in GAG content is usually greater than the increase in collagen.15 As the collagen-to-GAG ratio in TEC is usually already lower than that in NC, the ability to selectively increase collagen content more so than GAG content is needed to achieve ECM content comparable with that of NC.
Thyroxine (T4) is a hormone produced by the thyroid gland and is instrumental in various developmental processes.16 The literature contains multiple studies describing a range of effects on TEC, including accelerated maturation of growth plate chondrocytes, including stimulation of alkaline phosphatase,17 and the upregulation of type X collagen mRNA.18 Induction of chondrocyte hypertrophy has also been reported, even in nongrowth plate-derived chondrocytes.17 Although both type X collagen expression and chondrocyte hypertrophy can be seen as undesirable for articular cartilage chondrogenesis, because of their well-known colocalization at sites of endochondral ossification, that is, at the growth plate,19 the effect of each of these on the functional performance of TEC is not actually known.
Furthermore, the effect of T4 on the accumulation of cartilage-specific collagen types II, XI, and X by articular chondrocytes is not well known, because the majority of published collagen type studies were done at the gene expression level, rather than at the protein level, and the studies often examined growth plate chondrocytes rather than articular chondrocytes. One recent study does show that thyroid hormones T3 and T4 can preferentially stimulate total collagen versus GAG and improve uniaxial mechanical properties, but individual collagen types were not measured quantitatively.20
This study is the first of a two-part investigation to determine the biochemical and biomechanical effects of T4 on scaffold-free TEC generated from articular chondrocytes. This study focuses on the compositional and biochemical effects of T4 supplementation, with emphasis on protein level effects on specific collagen types. Attention is also given to the collagen-to-GAG ratio, which may be an important consideration for mechanical properties.
Materials and Methods
Dose response of T4 and TGFβ1 on articular chondrocytes
Dose responses were assessed in high-density pellet cultures. Neocartilage pellets were generated with 2.5 × 105 culture expanded rabbit articular chondrocytes as previously described,21 and stimulated with T4 (12.5, 25, 50, and 100 ng/mL) or TGFβ1 (5, 10, 15, and 20 ng/mL) from day 2 in culture. Control pellet cultures that did not receive either growth factor were also included. The T4 dose response was characterized on pellets from five different donors and the TGFβ1 response on pellets from two different donors. For each donor–concentration combination, four pellets were generated, although at some conditions a single pellet was lost, resulting in a minimum of three and a maximum of four pellets for all donor–concentration combinations. Cartilage pellets were harvested at 1 month, and GAG, hydroxyproline (HYP), and DNA were measured as previously described,3 and expressed as a relative increase compared with the untreated controls.
Effect of T4 on scaffold-free neocartilage
Neocartilage generation
Articular chondrocytes were isolated from cartilage harvested from the knees and shoulders of skeletally mature New Zealand White male rabbits as previously described.3 Chondrocytes were then culture expanded in low-glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), and 1% Antibiotic-Antimycotic (expansion medium). Owing to the large number of chondrocytes needed for neocartilage generation, chondrocytes were cultured on a devitalized ECM generated by porcine synoviocytes22 as previously described,23 or on tissue culture-treated plastic in expansion medium supplemented with 10 ng/mL fibroblast growth factor 2 to increase chondrocyte proliferation.21 Chondrocytes were culture expanded through two passages, then seeded onto a porous membrane suspended in chondrogenic defined medium by a custom metal frame, as previously described,3 with the exception that the portion of the assembly into which the cells were seeded was 1.65 cm deep, rather than 0.55 cm. The chondrogenic defined medium consisted of DMEM with 4.5 g/L glucose supplemented with 1% ITS + premix (BD Biosciences, San Jose, CA), 10−7 M dexamethasone (Sigma-Aldrich), 0.13 mM l-ascorbate-2-phosphate (Wako Chemicals, Richmond, VA), and 1:100 dilutions of GlutaMAX, nonessential amino acids, 100 mM sodium pyruvate, and Antibiotic-Antimycotic (Invitrogen).
Frame–membrane assemblies were placed into Nalgene™ (Nunc, Penfield, NY) containers with 255 mL of chondrogenic medium on the outside portion of the frame–membrane assembly,3 and cells were seeded into the inner portion of the assembly (onto the porous polyester membrane) at 6.5 × 106 cells/cm2 in 27 mL of chondrogenic medium, then incubated at 37°C in 5% CO2. Two neocartilages per donor were generated: a control and a T4-treated neocartilage (+T4 neocartilage). For the +T4 neocartilage, all additional culture medium supplementation and medium exchange from this stage forward was with chondrogenic medium supplemented with T4 at a concentration of 25 ng/mL. The control neocartilage was given chondrogenic medium throughout the duration of tissue culture.
After the initial 24 h of culture, an additional 60 mL of medium was added, resulting in fluid communication between the inner and outer portions of the frame–membrane assembly, and the chamber was placed on an orbital shaker set to 55 RPM to induce fluid mixing. Culture medium was exchanged with 65% replacement twice per week.
After 1 month of culture, part of each neocartilage was sampled (1-month samples) with 5-mm diameter biopsy punches for assaying, as described hereunder. The remainder of the neocartilage was transferred to a petri dish and cultured in 30 mL chondrogenic medium on the orbital shaker for an additional month at 37°C in 5% CO2. Culture medium was completely exchanged twice per week. After an additional 4 weeks, the remainder of the neocartilage was sampled using 5 mm biopsy punches (2-month samples). After each harvest, samples were weighed (wet weight), then frozen at −80°C.
Water content, cellularity, GAG, and total collagen
Frozen samples were lyophilized then weighed (dry weight) and water content was calculated as (wet weight − dry weight)/(wet weight) × 100. Lyophilized samples were then papain digested and assayed for DNA and GAG.3 HYP assays were adapted to a 96-well plate format and 10%, rather than 5%, Ehrlich's reagent was used.3 Cellularity was estimated by assuming 7.7 × 10−12 g of DNA/cell24 and normalizing to wet weight. Total collagen estimates were calculated as HYP × 7.5.25
Collagen cross-link analysis
Frozen samples were lyophilized, weighed, and then hydrolyzed in 6 N HCl at 110°C for 24 h. An aliquot of the hydrolysate was colorimetrically assayed for HYP. Pyridinoline cross-links were quantified in these samples by C-18 reverse-phase high-perfomance liquid chromatography and fluorometry and expressed as moles per mole of collagen.26–28
Sample thickness
Sample thickness was measured with a micrometer by averaging a total of six measurements from two different biopsy punches per neocartilage.
Gene expression
One-month samples were used for gene expression studies. After harvest, tissue samples were stabilized in RNAlater® (Qiagen, Inc., Toronto, Canada) and stored at −80°C. Gene expression analysis was performed as previously described29 with the exception that samples were minced before homogenization. After mincing samples with a razor blade, total RNA was extracted using a combination of Trizol (Molecular Research Centre, Inc., Burlington, Canada) and a High Pure RNA Isolation Kit (Roche Applied Science, Laval, Canada) in accordance with the manufacturer's instructions. Total isolated RNA was resuspended in RNase-free (diethylpyrocarbonate-treated) water and quantified using a Nano-Drop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE). Reverse transcription of total RNA was conducted using a High Capacity cDNA Reverse Transcription Kit (Life Technologies, Carlsbad, CA). The reverse transcription was primed with oligo-dT18 primers for 1 h and then with random nonamers during the second hour. Real-time quantitative polymerase chain reaction (PCR) was then performed using a Roche LightCycler® 480 II real-time PCR system (Roche Scientific, Laval, Canada) with the Kapa SYBR Fast PCR kit (Kapa Biosystems, D-Mark Biosciences, Toronto, Canada) with primer sequences listed in Table 1, and additional information given by Brenner et al.29 Gene expression levels were calculated from standard curves of each gene. Expression levels of mRNA for collagens type I (α2) [COL1A2], type II (α1) [COL2A1], and type X (α1) [COL10A1], along with biglycan (BGN), and superficial zone protein (PRG4) were measured and normalized to 18s ribosomal RNA.
Table 1.
Primer Sequences for Gene Expression Analysis
| Gene | Primer sequence | Size (bps) | |
|---|---|---|---|
| Collagen X (COL10α1) | Forward | 5′-CCATCCTGGATCTCACAGAA-3′ | 115 |
| Reverse | 5′-GCCACTAGGAATCCTGAGAA-3′ | ||
| Collagen II (COL2α1) | Forward | 5′-GCTGGAGAAGAAGGCAAG-3′ | 391 |
| Reverse | 5′-CAGGTTCACCATTGGCAC-3′ | ||
| Collagen I (COL1α2) | Forward | 5′-CTCACCACCTTCTCTCAGAC-3′ | 298 |
| Reverse | 5′-GGCCTTGGAGCTCTTATAC-3′ | ||
| Superficial zone protein (PRG4) | Forward | 5′-CCACATCACCACCATCTTC-3′ | 344 |
| Reverse | 5′-GCCACTTCCAGCTTCATC-3′ | ||
| Biglycan (BGN) | Forward | 5′-AAGAACCACCTGGTGGAGA-3′ | 209 |
| Reverse | 5′-GAGATGCGCAGGTAGTTGA-3′ | ||
| 18S | Forward | 5′-CACATCCAAGGAAGGCAG-3′ | 145 |
| Reverse | 5′-CCTCCAATGGATCCTCGT-3′ | ||
Histological and immunohistochemical evaluation
Specimen preparation
The tissues for histology were fixed in 10% neutral buffered formalin, dehydrated through a graded series of ethanol (EtOH), and embedded in paraffin. Eight micrometer-thick paraffin sections were used for all staining. Seven donor-matched pairs of control and +T4 neocartilage were used for 1-month samples, and five pairs were used for 2-month samples. Where available, two sections were stained for each antibody used, and up to four sections were stained with safranin O. For immunolocalization, paraffin sections were first deparaffinized and rehydrated in phosphate-buffered saline (PBS), then incubated with pronase for epitope retrieval (1 mg/mL in PBS containing 5 mM CaCl2; Sigma) for 10 min. Slides were then incubated with PBS/bovine serum albumin (BSA, 1%) for 10 min. to block nonspecific binding and incubated overnight at 4°C with primary antibodies diluted in PBS/BSA (mouse antitype I collagen, 1/1000, 631703—MP Biomedical; mouse antitype II collagen, 1/500, II-II6B3—DSHB; mouse antitype X collagen, 1/1000—kindly provided by Gary Gibson, Henry Ford Hospital, Detroit, MI).
After overnight incubation, slides were rinsed twice with PBS and once with PBS/BSA. They were then incubated with secondary antibody (biotin-conjugated horse antimouse, 1/2000 dilution in PBS/BSA, BA2000; Gibco) for 1 h at room temperature (RT) followed by streptavidin-horseradish peroxidase (1/5000 in PBS/BSA, 43-4323; Invitrogen) at RT for 30 min. Sections were rinsed and developed with peroxidase (SK4600; Vector Labs) for 10 min. Slides were then counterstained with 0.0125% fast green, dehydrated, and mounted with Permount™ (Fisher Scientific). Positive staining was confirmed with control slides from human and rabbit cartilage, and negative controls were made with secondary antibody only incubations.
Sections for safranin O staining were deparaffinized and rehydrated with deionized water, then stained with hematoxylin solution for 2 min. After rinsing exhaustively with water, slides were stained with 0.1% fast green solution for 5 min, and then washed again. Next, they were stained with 1% safranin O for 12 min, washed, and then dehydrated in graded EtOH baths. Finally, slides were cleared with xylene and cover slipped with mounting medium.
Imaging
Low-magnification (10 × objective lens) secondary antibody only and type I collagen sections were imaged as a group at the same microscope settings; type II and type X collagen sections were imaged as a separate group. Excluding the native-knee sections, low-magnification safranin O-stained sections were imaged as a single group, as were high-magnification (40 × objective lens) safranin O-stained sections. All high-magnification immunostained sections were imaged as one group.
Postprocessing
Micrograph black and white levels, color balance, and exposure were adjusted in Photoshop (Adobe, San Jose, CA). Low-magnification immunostained images and secondary and type I collagen images were adjusted as one group, and type II and type X collagen sections were adjusted as a separate group. For high-magnification immunostained images, all images were adjusted identically as one group. Safranin O-stained sections were adjusted as groups of low- or high-magnification images, except in the case of the native-knee sections that were each adjusted individually.
Histomorphometry
Location-dependent histomorphometric analysis was performed as described previously.13 In brief, safranin O-stained histological micrographs were manually segmented in GIMP (The Gimp Team) or Photoshop (Adobe) to indicate the tissue boundary and cells. When possible, two histological sections were analyzed for each neocartilage to reduce effects from heterogeneity within individual neocartilages. A custom Matlab (Mathworks, Natick, MA) program was used to divide each segmented image into five equal-thickness regions throughout the depth of the histological slice and calculate the number of cells and area occupied by the cells within each region. Cell density was defined as cells/mm2 and a statistic termed “cell area fraction” was defined as the fraction of the area of each region occupied by cells.
Electrophoresis and densitometry
After harvest, samples were frozen at −80°C until use. Samples were first lyophilized then dry weights obtained. Next, proteoglycans were extracted using 4 M guanidine hydrochloride (GuHCl). The residue was exhaustively rinsed using MilliQ water to remove residual GuHCl and the cross-linked collagen network depolymerized using equal volumes of pepsin (0.5 mg/mL in 0.5 M acetic acid).30 Equivalent aliquots of pepsin-extracted collagen were loaded on 6% polyacrylamide gels. After electrophoresis, collagen chains were stained using Coomassie blue. For Western blots, after 7.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis, protein was electrophoretically transferred onto poly vinyl difluoride membrane and type X collagen was probed with an antitype X collagen antibody (kindly provided by Gary Gibson). Rabbit auricular cartilage was used as a positive control because type X collagen has been immunolocalized in this cartilage.19
After imaging of gels, densitometry was performed in ImageJ (NIH, Bethesda, MD) for semiquantitative analysis of collagen types II and XI. The area under the pixel intensity plot curve (AUC) was measured for the peaks of the bands for the α1, β1, and δ1 chains of type II collagen, and for the α1 and α2 chains of type XI collagen. AUCs of individual chains were summed for each collagen type, and then the summed AUC was normalized by the dry weight of the sample multiplied by the fraction of the digest loaded on the gel; n = 6 for 1-month and n = 5 for 2-month samples.
Statistics
All statistical analyses other than for histomorphometry were performed in GraphPad Prism 6 (Graphpad, La Jolla, CA). Histomorphometry analysis was performed in R31 using the lme4 package.32 Summary statistics are presented as mean ± standard deviation when given.
For growth factor dose response, a two-way analysis of variance was used to assess statistical significance of all measures. This analysis was selected to account for the large donor-to-donor variability that was observed. Sidak's multiple comparison test with significance set at p < 0.05 was used for post hoc testing wherein treatment conditions were compared with controls but not to each other. For T4, results from pellets originating from a single donor were averaged at each concentration, and then normalized by the average value of the control pellets from the same donor. Data are presented as the average and standard deviation across all donors (n = three or four pellets/donor for five donors). For TGFβ1, since pellet cultures from only two donors were available for analysis, each pellet was normalized by the average value of the control pellets from the matching donor. Data are presented as mean ± standard deviation of all pellets at each growth factor concentration (n = minimum of seven and a maximum of eight pellets/donor for two donors because of some loss as already described).
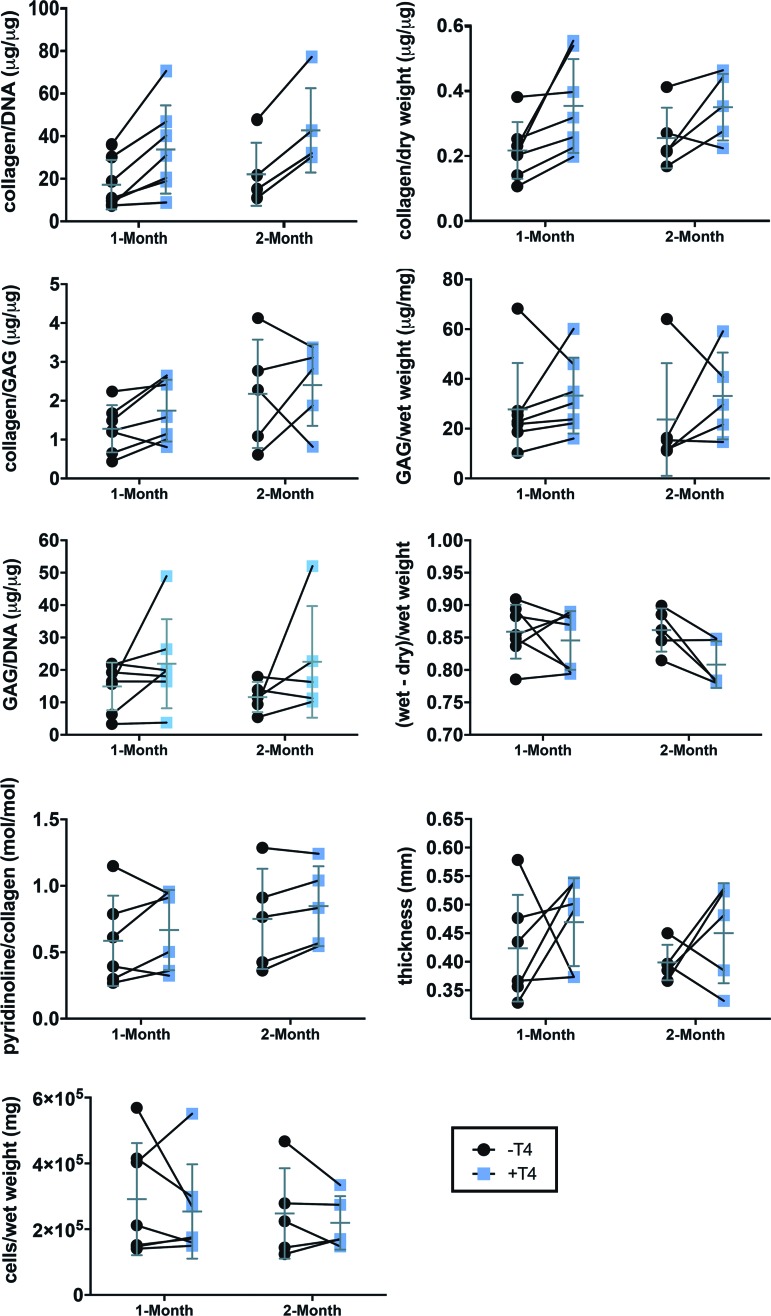
For cellularity, water content, and ECM composition, two-tailed paired t-tests were used to assess differences between the matched control and +T4 neocartilage for each donor. The number of observations in each analysis is listed alongside the results from statistical testing in Table 2.
Table 2.
Statistical Significance Testing for Tissue Composition in Figure 2
| 1 month | 2 months | |||
|---|---|---|---|---|
| Property | n | p | n | p |
| Water content | 7 | 0.5081 | 5 | 0.0415 |
| Thickness | 6 | 0.4652 | 5 | 0.3461 |
| Cells/wet weight | 7 | 0.5069 | 5 | 0.4378 |
| GAG/DNA | 7 | 0.1995 | 5 | 0.2609 |
| GAG/wet weight | 7 | 0.4114 | 5 | 0.4350 |
| Collagen/dry weight | 7 | 0.0325 | 5 | 0.1043 |
| Collagen/DNA | 7 | 0.0072 | 5 | 0.0009 |
| GAG/collagen | 7 | 0.1586 | 5 | 0.6153 |
| rp: 0.0704 | rp: 0.6514 | |||
| Pyridinole/collagen | 6 | 0.3601 | 5 | 0.0728 |
GAG, glycosaminoglycan; rp, ratio paired.
For histomorphometry, the effect of T4 treatment on cell size, cell density, and the fraction of tissue occupied by cells (cell area fraction) was determined (1-month: n = 4, 2-month: n = 3). To maintain consistency with our previous study13 on depth-dependent histomorphometry, linear mixed effect models were used to assess these differences. For each of the three response variables, the model included fixed effects for T4 treatment condition, depth, and the interaction of treatment and depth region. Random effects were included for donor to perform a paired test between neocartilages originating from the same donor. Uncorrected significance was set at p < 0.05, which was then modified to p < 0.01 to account for five multiple comparisons at each time point, that is, five regions were compared.
For gene expression, two-tailed paired t-tests were used to assess differences between the matched control and +T4 neocartilage for each gene assayed (n = 7).
For densitometry, two-tailed paired t-tests were used to assess differences between the matched control and +T4 neocartilage for each donor; n = 6 for 1-month neocartilages and n = 5 for 2-month neocartilages.
Results
Dose response of T4 and TGFβ1 on articular chondrocytes
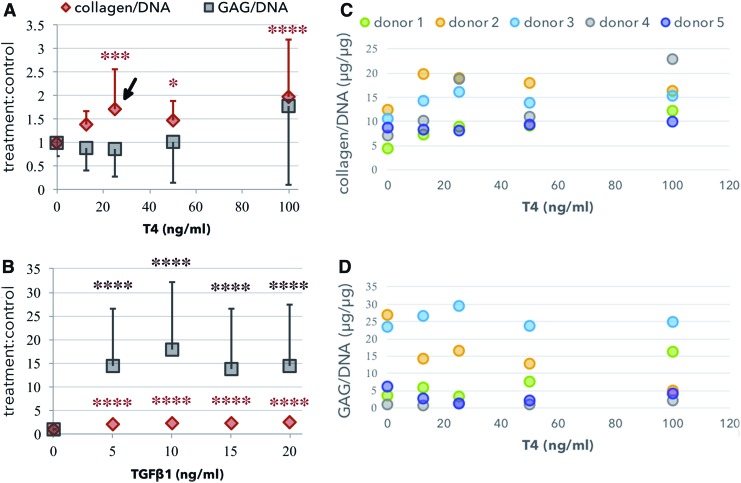
T4 increased collagen/DNA but not GAG/DNA at 25 and 50 ng/mL, and increased both collagen/DNA and GAG/DNA at 100 ng/mL (Fig. 1A). This resulted in a statistically significant increased collagen-to-GAG ratio of 2.28 ± 1.18, p < 0.05, at 25 ng/mL. The change in the collagen-to-GAG ratio was not statistically significant at any other concentration. When treated with TGFβ1, the relative change in GAG/DNA was greater than three times the relative change in collagen/DNA for all concentrations tested (Fig. 1B).
FIG. 1.
Growth factor dose response in micromass culture. T4 (A, C, D) and TGFβ1 (B) dose response for HYP and GAG. In both (A) and (B), change relative to control is fold change, treatment:control. T4 increased the HYP/DNA but not the GAG/DNA at 25 ng/mL (arrow). Asterisks indicated statistical significance compared with the control (0 ng/mL), *p < 0.05, ***p < 0.001, ****p < 0.0001. The mean response of each donor is shown in (C) and (D). GAG, glycosaminoglycan; HYP, hydroxyproline. Color images available online at www.liebertpub.com/tea
Effect of T4 on scaffold-free neocartilage
Cellularity, water content, and ECM composition
Large donor-to-donor variability caused high variance across observations within each treatment group. When effects were analyzed based on the effect on each donor by use of the paired t-test, collagen was found to have increased in a statistically significant manner at both time points on a per DNA basis (Fig. 2, Table 2; fold increase: 1 month = 2.1 ± 0.8, 2 months = 2.1 ± 0.4) and at 1 month on a per dry weight basis (Fig. 2, Table 2; fold increase: 1.7 ± 0.6). A statistically significant decrease in water content (Fig. 2, Table 2; decrease of 5.3% ± 3.6%) was also observed, but only at 2 months. No other measure achieved a p < 0.05 (Fig. 2, Table 2). However, the increase in pyridinoline/collagen neared significance at 2 months (Fig. 2, Table 2), and by the ratio-paired t-test (assessment of the ratio between the treated and control samples) the decrease in GAG/collagen neared significance at 1 month (Fig. 2, Table 2).
FIG. 2.
Tissue characterization and ECM composition. Lines between −T4 and +T4 samples indicate matched neocartilages (same donor) paired for statistical analysis. The mean and standard deviation of each group are overlaid in gray. Table 2 contains p-values from paired t-tests for all groups. ECM, extracellular matrix. Color images available online at www.liebertpub.com/tea
Gene expression
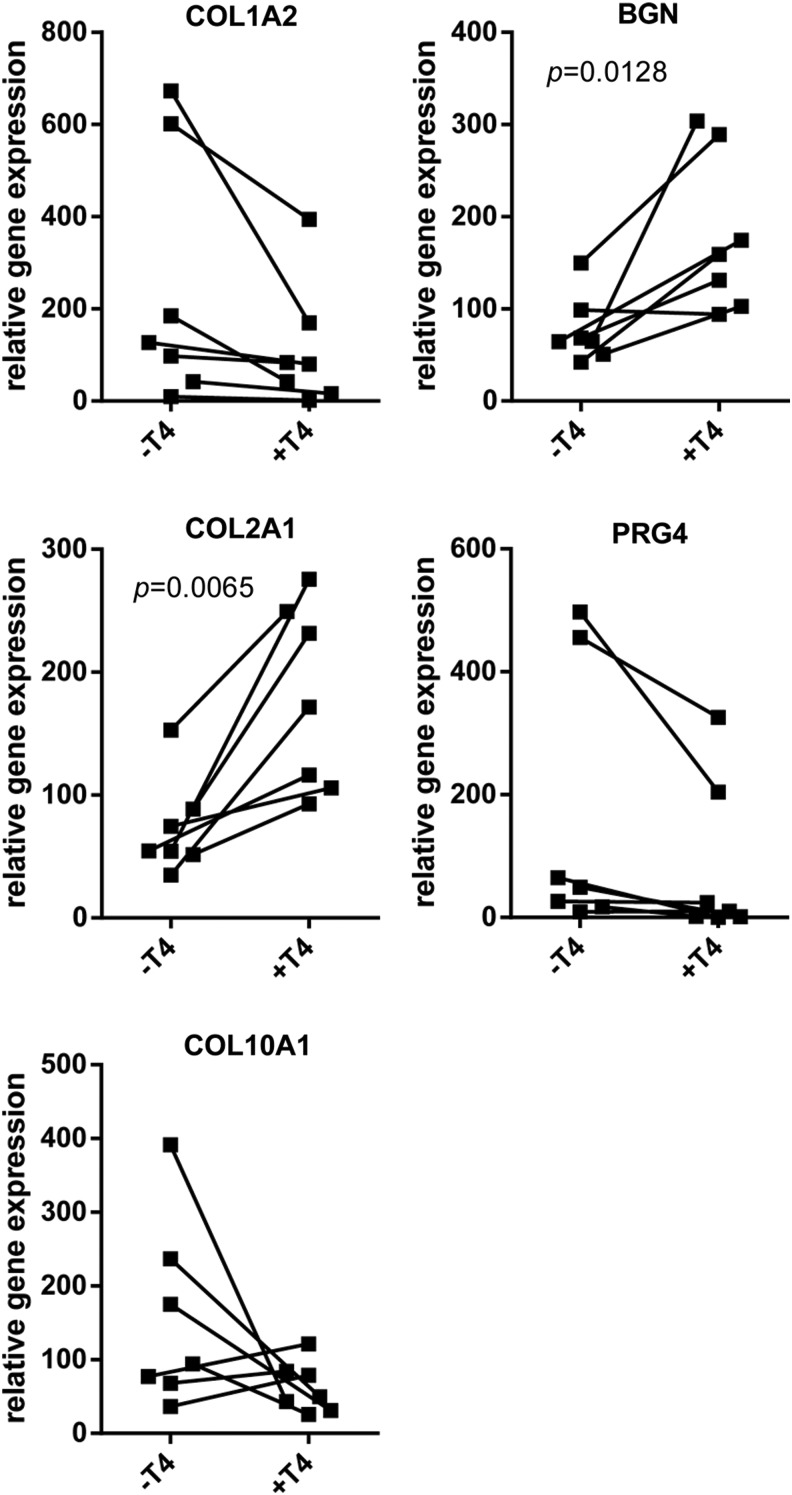
As seen in Figure 3, gene expression at 1 month showed a 2.8 ± 1.6-fold increase of COL2A1 (p = 0.007), and 2.6 ± 1.6-fold increase of BGN, mRNA expression for +T4 neocartilages versus paired controls. Although donor-to-donor variability precluded significance, COL1A2 expression was decreased in every T4-treated sample as compared with that in the paired controls. PRG4 expression decreased as a result of T4 treatment in six out of seven paired neocartilages. The effect on COL10A1 was inconsistent, and not significant, although there was a greater than twofold reduction in the mean for +T4 neocartilages.
FIG. 3.
ECM gene expression at 1 month. ECM gene expression is relative to 18S gene expression. Lines between −T4 and +T4 samples indicate matched constructs (same donor) paired for statistical analysis. Differences between control and +T4 samples in genes without an indicated p-value were not significant.
Histological and immunohistochemical evaluation
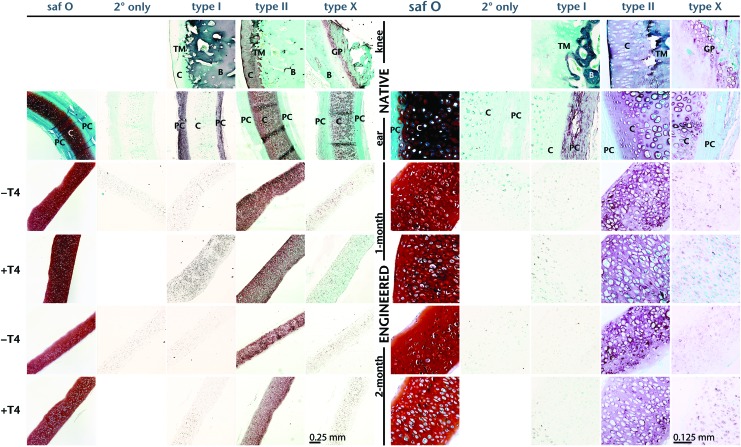
Both control and T4-treated neocartilage exhibited strong safranin O staining for GAG in the ECM. Both showed large rounded cells in lacunae near the center, with some flattened cells at the surface (Figs. 4 and 5). The lacunae in the +T4-treated tissue appeared larger than those in the control. Immunohistochemistry localized type II collagen in the ECM and not in the cells. Little to no type I collagen was detected in the ECM in either control or +T4 neocartilage, and type X collagen was immunolocalized to a limited extent within cells in both control and +T4 neocartilage (Fig. 4).
FIG. 4.
Histological evaluation. Micrographs of safranin O (saf O) and collagen type I, type II, and type X, immunostained histological cross-sections of native rabbit and TEC. In immunostained images, blue and green coloring is a result of a fast green counterstain, and darker coloring (red, brown, purple) is the result of the peroxidase reaction, indicating detection of the specific collagen type. B, bone; C, cartilage; GP, growth plate; PC, perichondrium; TEC, tissue-engineered cartilage; TM, tide mark. Color images available online at www.liebertpub.com/tea
FIG. 5.
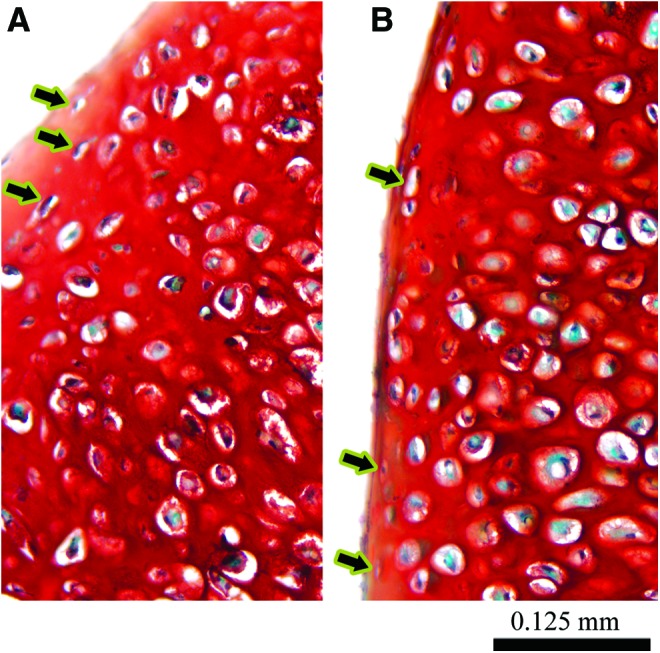
Magnified histological cross-sections showing flattened cells near the construct periphery. Both −T4 (A) and +T4 (B) constructs exhibited some flattened cells (arrows) near the periphery of the constructs. (A, B) are magnified views of the 1-month safranin O-stained images from Figure 4. Color images available online at www.liebertpub.com/tea
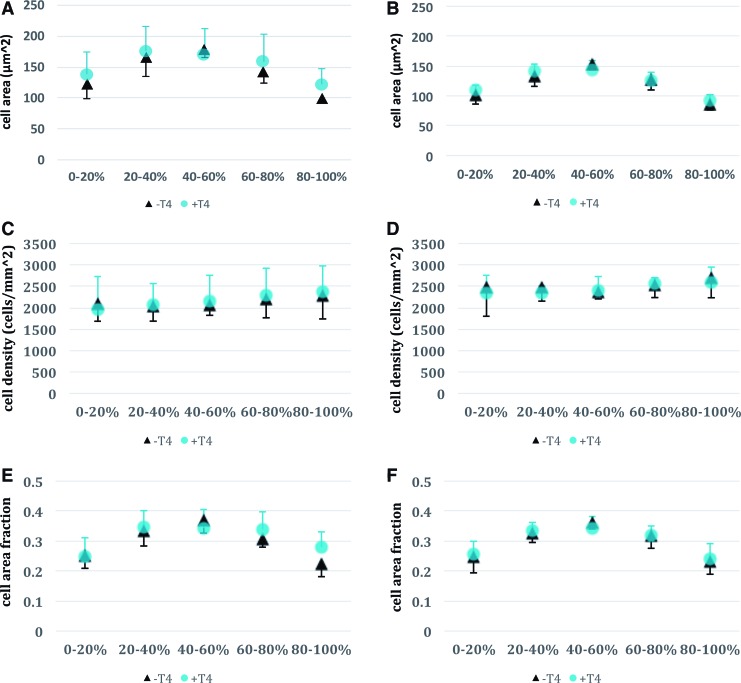
Histomorphometry
Cell size, cell density, and cell area fraction exhibited nearly identical depth-dependent patterns for both control and T4-treated neocartilage (Fig. 6). There were no statistically significant differences between control and treatment groups for matched regions, for example, comparing the 0–20% depth region in the control group with the 0–20% depth region in the treatment group. It is worth noting that even without correcting for multiple comparisons, no differences would have achieved statistical significance.
FIG. 6.
Histomorphometry of control and T4-treated constructs at 1 and 2 months. (A, C, E) 1-month metrics, (B, D, F) 2-month metrics. A 0–20% depth corresponds to the upper surface of the construct during the initial membrane attached culture, whereas 80–100% depth corresponds to the lower membrane contacting region. Cell area (A, B) increased toward the central regions of the constructs for both control (−T4) and T4-treated (+T4) constructs, whereas cell density was nearly uniform throughout (C, D). Cell area fraction (E, F) also increased in the central region for both −T4 and +T4 constructs, apparently as a result of increased cell area rather than increased cell density. Differences between −T4 and +T4 constructs were minimal, with differences only reaching statistical significance in the cell area fraction metric, and only at the membrane-contacting surface of the construct (80–100% region). There were no statistically significant cell size differences between control and treatment groups when comparing matched depth regions. Color images available online at www.liebertpub.com/tea
It appeared that there were differences between depth regions, specifically that cell size and cell area fraction were larger in the 40–60% depth region as compared with the upper and lower regions for both control and treatment. However, this was not tested statistically as it was not the focus of this study. Region-specific average cell area was measured to be between 125 and 175 μm2 for both control and T4-treated neocartilage at month 1, and between 100 and 150 μm2 at month 2.
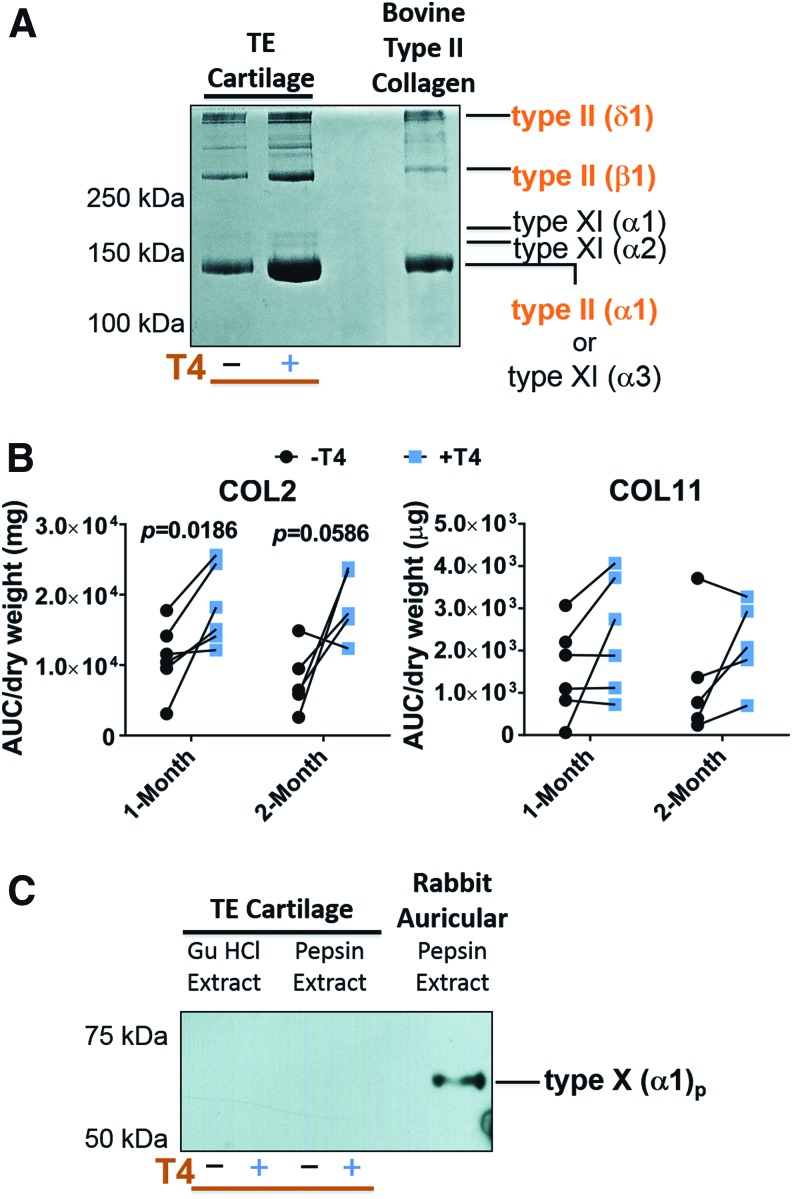
Electrophoresis and densitometry for collagen characterization
Figure 7A shows the Coomassie blue-stained collagen bands after electrophoresis. The major bands in TEC extracts were similar to that for type II collagen controls. The two additional bands in the TEC at ∼170 kDa (Fig. 7A),were α1(XI) and α2(XI) collagen chains as we have identified by mass spectrometry.27,28,33 Type II collagen generally appeared to be more abundant in T4-treated samples verses paired controls (Fig. 7A). Densitometry confirmed a statistically significant increase in type II collagen (fold change of 2.2 ± 1.8) on a mass basis, at 1 month; at 2 months the difference approached significance (p = 0.059) even with a reduced sample size compared with 1-month samples (Fig. 7B). No significant differences in collagen type XI (α1 and α2) were found between controls and +T4 neocartilage at either time point.
FIG. 7.
The effect of T4 on types of collagen in neocartilage. (A) Coomassie blue-stained sodium dodecyl sulfate polyacrylamide gel electrophoresis showing the presence of type II and type XI collagen in pepsin extracts of TEC. Bovine type II collagen was electrophoresed as a control. (B) Densitometric semiquantitative analysis of type II and type XI collagen in TEC. AUC is the area under the pixel intensity plot curve. (C) Western blot probed with type X collagen antibody in 4 M GuHCl and pepsin extracts of TEC and native auricular cartilage as a positive control. In (A, C) the molecular weights of globular protein standards are shown. GuHCl, guanidine hydrochloride. Color images available online at www.liebertpub.com/tea
Western blotting revealed the pepsinized chain of type X collagen [αI(X)p] in native auricular cartilage extracts as expected.19 In control or T4-treated neocartilages, however, type X collagen chains were not detected in 4 M GuHCl extracts (noncross-linked collagen) or in pepsin extracts (cross-linked collagen) (Fig. 7C).
Discussion
In this study, the thyroid hormone T4 has been shown to enhance chondrogenesis at both the molecular level (increased collagen content at both 1 and 2 months of culture, and increased expression of COL2A1 and BGN mRNA) and the bulk tissue level (water content closer to that of NC at 2 months of culture), in neocartilage originating from articular chondrocytes (Figs. 2 and 3).
The effect on collagen type II was seen both at the gene expression level (Fig. 3) and in protein accumulation in the ECM (Fig. 7). Furthermore, this increase in collagen type II content was achieved without a significant increase in GAG content. Despite the GAG content not being significantly altered, the increased collagen accumulation in treated samples did not produce a significant increase in the collagen-to-GAG ratio of neocartilages. One cause of this may be that the T4 concentration was optimized in pellet cultures—in that culture system, a statistically significant increase in the collagen-to-GAG ratio was observed (Fig. 2, Table 2). Testing whether the collagen-to-GAG ratio could be significantly improved in the large neocartilage cultures through optimization of the T4 concentration in that culture platform could be an important question for a future study.
It is important to note that although the stimulation of collagen type II gene expression and protein accumulation appear relatively small in magnitude, the change is consistent between gene expression and protein accumulation, with both being approximately twofold higher in treatment versus control neocartilages. It should also be noted that collagen content in engineered neocartilage is typically reported to be on the order of 40%,4,6 that of NC, and that the tensile mechanical properties are proportional to the collagen content.7,8
The immunohistochemistry assay, designed only to observe spatial distribution, not quantity of collagen, was very similar between treatment and control neocartilages. Taken together with the results of the gene expression and protein accumulation assays, these results indicate that type II collagen quantity, not localization, is affected by T4. This finding will be important as the effect of T4-related collagen effects on mechanical properties continues to be elucidated; collagen quantity and distribution are both important factors regulating mechanical properties.
Quantitative measures of chondrocyte terminal differentiation indicators, hypertrophy and expression of collagen type X, indicated that T4 did not drive articular chondrocytes toward terminal differentiation in this system (Figs. 3 and 6). Limited intracellular type X collagen staining was observed by immunohistochemistry, but not to the extent seen in native rabbit growth plate cartilage or native auricular cartilage. (Fig. 4). Type X collagen was below the level of detection in the ±T4-treated neocartilages in the Western blot assay (Fig. 7C). These results indicate that T4 enhances chondrogenesis at a precise dosage, and that this can be tuned to favor collagen accumulation over GAG accumulation.
Although the primary objectives in this study were to determine whether T4 could increase the collagen-to-GAG ratio, and specifically whether collagen type II expression and accumulation were increased, the findings on the effect on type X collagen localization and accumulation are also noteworthy. Multiple studies on the effects of thyroid hormones on type X collagen in chondrocytes are present in the literature, yet the range of culture conditions, chondrocyte sources, and specific thyroid hormone used have resulted in a wide variety of findings.15,34–36 The results in this study are similar to those of Liu et al., who found that the thyroid hormone, tri-iodothyronine (T3), increased collagen type II and reduced the stimulation of collagen type X that was induced by a combination of bone morphogenetic protein 2 (BMP2) and insulin.15 The findings of Liu et al. are especially relevant to the current study given that T4 is canonically viewed as the prohormone of T3, because T4 is converted to the potent genomic regulator T3 by deiodinases in the cytoplasm of various cells.34 Recent evidence also shows that T4 itself regulates certain signaling pathways, such as MAPK, by directly binding to cell surface receptors.16,34,36 However, the results in this study, indicating that a precise dosage of T4, like that shown for T3,15 demonstrate that thyroid hormones are able to induce collagen type II mRNA and protein synthesis, without a concomitant increase of type X collagen.
Our results also corroborate those of Lee et al., who found that T3 and T4 can selectively upregulate total collagen accumulation without a concomitant increase in GAG accumulation.20 However, in their system, collagen type X was also upregulated by T3 and T4. Dosage differences between the two studies may account for the apparent contradiction, because we used one-fourth the concentration of T4 used by Lee et al.
Our results may also appear to run counter to other published reports demonstrating thyroid hormone-induced upregulation of type X collagen mRNA and protein synthesis by chondrocytes18,37; evidence suggests that cell source may be an important factor in the apparent discrepancy. We used rabbit articular chondrocytes; Ballock and Reddi, using rat growth plate chondrocytes, showed that T4 increased the type X collagen expression in pellet cultures,37 whereas Boehme et al. (1992) found that T4 stimulated type X collagen expression 17-day-old chick embryo sternal chondrocytes cultured in agarose.18
In addition to cell source, the level of chondrocyte differentiation/dedifferentiation while in cell culture has also been implicated. Lassová et al. have demonstrated collagen type X expression as a result of T3 stimulation, but that T3 exerted this effect through BMP4, and that the BMP antagonist, noggin, was able to counteract T3-induced collagen type X expression.38 In the same study, they also found that in culture, chondrocytes expressed noggin initially, but that during cell culture, noggin expression quickly dropped to approximately one-third of the initial level. Thus, thyroid hormones themselves are not sufficient to drive maturation, and varying effects of thyroid hormones on chondrocyte maturation may result from confounding effects of other soluble molecules present in culture medium, or those produced by chondrocytes themselves at various stages of development. Findings from a study by Alini et al.39 support this, as they found that hormone concentration may also determine whether hypertrophy is induced. When taken together with this study, evidence suggests that, when added to articular chondrocytes at select dosages, T4 can induce type II collagen, rather than type X collagen. This may depend on the current differentiation state of the chondrocytes, and it is possible that thyroid hormone stimulation further enhances chondrocyte subtype maturation along a predetermined fate-committed lineage, rather than an obligatory terminal differentiation.
The upregulation of BGN in T4-treated neocartilage is also an interesting finding. Moreno et al. (2005) have described a role for BGN in inactivating BMP4 by enhancing binding of BMP4 to chordin,40 another BMP antagonist. Thus, considering the interplay between T4 and BMP4 in chondrocyte maturation already described, T4 could potentially downregulate collagen type X by stimulating BGN. In addition, Heinegård and Oldberg have reported that BGN is more abundant in tissues that carry load,41 and in implantation studies of scaffold-free rabbit TEC generated in vitro. Brenner et al. also found that the expression of BGN correlated positively with in vivo outcomes.29
Although there are several positive findings in this study, it is not without limitations. First, since the level of protein expression of type X collagen was below detectable limits, an exact determination of the effect on type X collagen protein cannot be made. However, there was no consistent effect on gene expression of this terminal differentiation marker. In a similar regard, some isolated hypertrophy was qualitatively observed in +T4 neocartilages (Fig. 4), although the increase in cell size was not statistically significant when measured quantitatively at the gross level (Fig. 6).
Even isolated hypertrophy in +T4 neocartilages raises the question as to whether these neocartilages would be converted to bone through endochondral ossification if implanted. Hypertrophy itself is not sufficient for induction of the endochondral pathway though. Auricular cartilage comprises mainly hypertrophic chondrocytes19 (Fig. 4), yet does not become ossified under normal physiological conditions. Furthermore, type X collagen is abundant in auricular cartilage19 (Fig. 4), so the limited type X collagen immunolocalized in this study is not necessarily an indicator of susceptibility to endochondral ossification. If these neocartilages did prove susceptible to ossification, their use would be limited in joint repair, but could prove beneficial in bone regeneration applications.
Second, although total collagen content increased in these neocartilages in a statistically significant manner and GAG content did not, the change in the collagen-to-GAG ratio did not achieve statistical significance (Fig. 2, Table 2). This is in contrast to the T4 dose response in pellet cultures. One possible reason for this difference is that culture conditions and neocartilage geometry differ between the two, and are likely to influence mass transfer and cellular signaling. Optimization of the concentration in the larger neocartilage format may prove able to increase the collagen-to-GAG ratio. Donor-to-donor variability was also a factor in this study, and for future studies with human chondrocytes, it may be necessary to screen each donor for the optimal T4 concentration. Interestingly, the collagen-to-GAG ratio did approach significance at 1 month (but not at 2 months) when the fold change (or ratio) was compared (ratio-paired t-test); the 1-month time point corresponds to the same time point at which pellet cultures were harvested. Thus, although statistically significant effects on collagen content were seen at 2 months of culture, additional studies are needed to determine the optimal timing and the duration of the T4 effect.
Another limitation may be that the expression of PRG4 decreased in five out of seven neocartilages treated with T4. In addition to positive correlation between BGN and in vivo outcomes, Brenner et al. also found a positive correlation between PRG4 and in vivo outcomes.29
Lastly, harvest of 1-month samples (biopsy punches) caused localized damage to the neocartilages, and it is known that disrupting the ECM causes metabolic changes in cartilage, in an apparent attempt at repair. Results at 2 months are, therefore, potentially affected by the prior sampling at 1 month.
In conclusion, results of this study show that optimized dosage of T4 is able to preferentially increase collagen content without causing large increases in GAG that are often caused by other soluble factors. Moreover, the upregulation of collagen type II gene and protein expression, without a concomitant increase in collagen type I or collagen type X, signifies a direct enhancement of chondrogenesis of hyaline articular cartilage.
Acknowledgments
The authors would like to thank Geoff Traeger for performing electrophoresis, and cross-linking experiments, and also Dr. Gary J. Gibson, Breech Research Laboratory, Bone and Joint Center, Henry Ford Hospital and Medical Centers, for providing the anticollagen type X antibody. The II-II6B3 monoclonal antibody developed by T.F. Linsenmayer was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA. This work was supported by the NIH NIDCR grant number R01 DE015322 (J.E.D.), NIH NIAMS grant numbers P01 AR053622 (J.M.M.) and RO1 AR057025 (R.J.F.), by the Canadian Institutes of Health Research CHRP grant number 413680 (S.W.), and under the Ruth L. Kirschstein National Research Service Award T32 AR007505 from the NIH NIAMS (G.A.W.).
Disclosure Statement
The authors have no financial interests to disclose. This study, less the histomorphometry results, was first published in dissertation form in Characterization of the Frictional Shear Damage Properties of Scaffold-Free Engineered Cartilage and Reduction of Damage Susceptibility by Upregulation of Collagen Content, by G. Adam Whitney, PhD, Case Western Reserve University, November 2014.
References
- 1.Lipshitz H., Etheredge R., and Glimcher M.J. In vitro wear of articular cartilage. J Bone Joint Surg Am 57, 527, 1975 [PubMed] [Google Scholar]
- 2.Anderson C.E., Ludowieg J., Harper H.A., and Engleman E. The composition of the organic component of human articular cartilage: relationship to age and degenerative joint disease. J Bone Joint Surg Am 46, 1176, 1964 [PubMed] [Google Scholar]
- 3.Whitney G.A., Mera H., Weidenbecher M., Awadallah A., Mansour J.M., and Dennis J.E. Methods for producing scaffold-free engineered cartilage sheets from auricular and articular chondrocyte cell sources and attachment to porous tantalum. Biores Open Access 1, 157, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vunjak-Novakovic G., Martin I., Obradovic B., Treppo S., Grodzinsky A.J., Langer R., et al. . Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res 17, 130, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Scotti C., Osmokrovic A., Wolf F., Miot S., Peretti G.M., Barbero A., et al. . Response of human engineered cartilage based on articular or nasal chondrocytes to interleukin-1β and low oxygen. Tissue Eng Part A 18, 362, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bian L., Zhai D.Y., Mauck R.L., and Burdick J.A. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A 17, 1137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt M.B., Mow V.C., Chun L.E., and Eyre D.R. Effects of proteoglycan extraction on the tensile behavior of articular cartilage. J Orthop Res 8, 353, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Basalo I.M., Mauck R.L., Kelly T.-A.N., Nicoll S.B., Chen F.H., Hung C.T., et al. . Cartilage interstitial fluid load support in unconfined compression following enzymatic digestion. J Biomech Eng 126, 779, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kempson G.E., Muir H., Swanson S.A., and Freeman M.A. Correlations between stiffness and the chemical constituents of cartilage on the human femoral head. Biochim Biophys Acta 215, 70, 1970 [DOI] [PubMed] [Google Scholar]
- 10.Treppo S., Koepp H., Quan E.C., Cole A.A., Kuettner K.E., and Grodzinsky A.J. Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. J Orthop Res 18, 739, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Bank R.A., Soudry M., Maroudas A., Mizrahi J., and Tekoppele J.M. The increased swelling and instantaneous deformation of osteoarthritic cartilage is highly correlated with collagen degradation. Arthritis Rheum 43, 2202, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Bastiaansen-Jenniskens Y.M., Koevoet W., de Bart A.C.W., van der Linden J.C., Zuurmond A.M., Weinans H., et al. . Contribution of collagen network features to functional properties of engineered cartilage. Osteoarthritis Cartilage 16, 359, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Whitney G.A., Jayaraman K., Dennis J.E., and Mansour J.M. Scaffold-free cartilage subjected to frictional shear stress demonstrates damage by cracking and surface peeling. J Tissue Eng Regen Med 11, 412, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demoor M., Ollitrault D., Gomez-Leduc T., Bouyoucef M., Hervieu M., Fabre H., et al. . Cartilage tissue engineering: molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim Biophys Acta 1840, 2414, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Liu G., Kawaguchi H., Ogasawara T., Asawa Y., Kishimoto J., Takahashi T., et al. . Optimal combination of soluble factors for tissue engineering of permanent cartilage from cultured human chondrocytes. J Biol Chem 282, 20407, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Bhargava M., Lei J., and Ingbar D.H. Nongenomic actions of L-thyroxine and 3,5,3′-triiodo-L-thyronine. Focus on “L-Thyroxine vs. 3,5,3′-triiodo-L-thyronine and cell proliferation: activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase”. Am J Physiol Cell Physiol 296, C977, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burch W.M., and Lebovitz H.E. Triiodothyronine stimulates maturation of porcine growth-plate cartilage in vitro. J Clin Invest 70, 496, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Böhme K., Conscience-Egli M., Tschan T., Winterhalter K.H., and Bruckner P. Induction of proliferation or hypertrophy of chondrocytes in serum-free culture: the role of insulin-like growth factor-I, insulin, or thyroxine. J Cell Biol 116, 1035, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naumann A., Dennis J.E., Awadallah A., Carrino D.A., Mansour J.M., Kastenbauer E., et al. . Immunochemical and mechanical characterization of cartilage subtypes in rabbit. J Histochem Cytochem 50, 1049, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Lee J.K., Gegg C.A., Hu J.C., Reddi A.H., and Athanasiou K.A. Thyroid hormones enhance the biomechanical functionality of scaffold-free neocartilage. Arthritis Res Ther 17, 28, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mounts T., Ginley N., Schluchter M., and Dennis J. Optimization of the expansion and differentiation of rabbit chondrocytes in vitro. Cartilage 3, 181, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kean T.J., and Dennis J.E. Synoviocyte derived-extracellular matrix enhances human articular chondrocyte proliferation and maintains re-differentiation capacity at both low and atmospheric oxygen tensions. PLoS One 10, e0129961, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kean T.J., and Dennis J.E. Tissue engineering, scaffold free, human cartilage sheets. Int Conf Tissue Eng October 1–3, 2012. Available at: https://www.omicsonline.org/proceedings/tissue-engineered-scaffold-free-human-cartilage-sheets-5342.html (accessed June23, 2017)
- 24.Kim Y., Sah R., and Doong J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem 174, 168, 1988 [DOI] [PubMed] [Google Scholar]
- 25.Baker L.C., Lampitt L.H., and Brown K.P. Connective tissue of meat. III.—Determination of collagen in tendon tissue by the hydroxyproline method. J Sci Food Agric 5, 226, 1954 [Google Scholar]
- 26.Fernandes R.J., Wilkin D.J., Weis M.A., Wilcox W.R., Cohn D.H., Rimoin D.L., et al. . Incorporation of structurally defective type II collagen into cartilage matrix in Kniest chondrodysplasia. Arch Biochem Biophys 355, 282, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Fernandes R.J., Weis M., Scott M.A., Seegmiller R.E., and Eyre D.R. Collagen XI chain misassembly in cartilage of the chondrodysplasia (cho) mouse. Matrix Biol 26, 597, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McAlinden A., Traeger G., Hansen U., Weis M.A., Ravindran S., Wirthlin L., et al. . Molecular properties and fibril ultrastructure of types II and XI collagens in cartilage of mice expressing exclusively the α1(IIA) collagen isoform. Matrix Biol 34, 105, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenner J.M., Ventura N.M., Tse M.Y., Winterborn A., Bardana D.D., Pang S.C., et al. . Implantation of scaffold-free engineered cartilage constructs in a rabbit model for chondral resurfacing. Artif Organs 38, E21, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Fernandes R.J., Schmid T.M., and Eyre D.R. Assembly of collagen types II, IX and XI into nascent hetero-fibrils by a rat chondrocyte cell line. Eur J Biochem 270, 3243, 2003 [DOI] [PubMed] [Google Scholar]
- 31.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. Available at: https://www.R-project.org [Google Scholar]
- 32.Bates D., Mächler M., Bolker B., and Walker S. Fitting linear mixed-effects models using lme4. arXiv:1406.5823, 2014
- 33.Murdoch A.D., Hardingham T.E., Eyre D.R., and Fernandes R.J. The development of a mature collagen network in cartilage from human bone marrow stem cells in Transwell culture. Matrix Biol 50, 16, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis P., Davis F., and Lin H. L-thyroxine acts as a hormone as well as a prohormone at the cell membrane. IEMAMC 6, 235, 2006 [Google Scholar]
- 35.Casey R.C., and Oegeman T.R. Caspase regulation of hypertrophy in articulate chondrocytes. Transactions of the 51st Annual Meeting of the Orthopaedic Research Society 2005. Available at: http://www.ors.org/Transactions/51/1043.pdf (accessed June23, 2017) [Google Scholar]
- 36.Davis P.J., Shih A., Lin H.Y., Martino L.J., and Davis F.B. Thyroxine promotes association of mitogen-activated protein kinase and nuclear thyroid hormone receptor (TR) and causes serine phosphorylation of TR. J Biol Chem 275, 38032, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Ballock R.T., and Reddi A.H. Thyroxine is the serum factor that regulates morphogenesis of columnar cartilage from isolated chondrocytes in chemically defined medium. J Cell Biol 126, 1311, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lassová L., Niu Z., Golden E.B., Cohen A.J., and Adams S.L. Thyroid hormone treatment of cultured chondrocytes mimics in vivo stimulation of collagen X mRNA by increasing BMP 4 expression. J Cell Physiol 219, 595, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Alini M., Kofsky Y., Wu W., Pidoux I., and Poole A.R. In serum-free culture thyroid hormones can induce full expression of chondrocyte hypertrophy leading to matrix calcification. J Bone Miner Res 11, 105, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Moreno M., Muñoz R., Aroca F., Labarca M., Brandan E., and Larraín J. Biglycan is a new extracellular component of the Chordin-BMP4 signaling pathway. EMBO J 24, 1397, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heinegard D., and Oldberg A. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J 3, 2042, 1989 [DOI] [PubMed] [Google Scholar]