Abstract
Generating neocartilage with suitable mechanical integrity from a cell source that can circumvent chondrocyte scarcity is indispensable for articular cartilage regeneration strategies. Costal chondrocytes of the rib eliminate donor site morbidity in the articular joint, but it remains unclear how neocartilage formed from these cells responds to mechanical loading, especially if the intent is to use it in a load-bearing joint. In a series of three experiments, this study sought to determine efficacious parameters of passive axial compressive stimulation that would enable costal chondrocytes to synthesize mechanically robust cartilage. Experiment 1 determined a suitable time window for stimulation by its application during either the matrix synthesis phase, the maturation phase, or during both phases of the self-assembling process. The results showed that compressive stimulation at either time was effective in increasing instantaneous moduli by 92% and 87% in the synthesis and maturation phases, respectively. Compressive stimulation during both phases did not further improve properties beyond a one-time stimulation. The magnitude of passive axial compression was examined in Experiment 2 by applying 0, 3.3, 5.0, or 6.7 kPa stresses to the neocartilage. Unlike 6.7 kPa, both 3.3 and 5.0 kPa significantly increased neocartilage compressive properties by 42% and 48% over untreated controls, respectively. Experiment 3 examined how the passive axial compression regimen developed from the previous phases interacted with a bioactive regimen (transforming growth factor [TGF]-β1, chondroitinase ABC, and lysyl oxidase-like 2). Passive axial compression significantly improved the relaxation modulus compared with bioactive treatment alone. Furthermore, a combined treatment of compressive and bioactive stimulation improved the tensile properties of neocartilage 2.6-fold compared with untreated control. The ability to create robust articular cartilage from passaged costal chondrocytes through appropriate mechanical and bioactive stimuli will greatly extend the clinical applicability of tissue-engineered products to a wider patient population.
Keywords: : costal chondrocyte, articular cartilage, passive axial compression, stimulation time window, stimulation magnitude, bioactive
Introduction
Engineering cartilage implants from a suitable alternative cell source of autologous or allogeneic origin are a critical aspect in articular cartilage tissue engineering strategies. Current clinical methods involve harvesting tissue from the articular cartilage of damaged joints to obtain chondrocytes for engineering tissue implants.1 This procurement procedure creates donor site morbidity in joints, which can exacerbate joint degeneration. Donor site morbidity and the dearth of healthy articular cartilage suitable for harvest both limit the usefulness of existing clinical procedures. To overcome these limitations, alternative cell sources, such as mesenchymal stem cells (MSCs) of various origins, have been widely explored.2 Progress in using MSCs has been hindered by the low efficiency of differentiating these cells into a chondrogenic phenotype.3,4 Identification of a nonarticular cartilage cell source consisting of a differentiated, chondrogenic phenotype would be significant in advancing cartilage tissue engineering.
Recently, costal cartilage has emerged as a promising, differentiated cell source that allows autologous chondrocyte harvest from a nonarticular hyaline cartilage source. The procedures for harvesting and using costal cartilage as graft material for jaw and craniofacial reconstructions are well established,5,6 causing minimal donor site morbidity and few complications.6 Although costal cartilage is nonarticular cartilage in the native form, costochondral cells can be induced to produce superficial zone protein (SZP) in vitro,7 which serves to lubricate the articular surface to reduce friction and wear.8,9 This property is highly desirable for applications in articulating joint environments. Costal chondrocytes appear to be attractive because of their chondrogenic phenotype, high cellularity,10 ease of harvest, and ability to secrete SZP. Furthermore, these cells expand in vitro quickly and continue to maintain a chondrogenic phenotype in passage through aggregate redifferentiation.11 Despite these advantages, costal chondrocytes have not been widely employed for tissue engineering articular cartilage.
Achieving appropriate neocartilage mechanical properties is critical for survival and functionality of neocartilage implants. A plethora of mechanical and chemical stimuli to improve neocartilage properties have been applied on various cell sources to engineer cartilage. Mechanical stimuli such as compression and shear can improve neocartilage properties and potentially assist in neocartilage development.12–16 Compressive stimulation has been shown to be effective in enhancing neocartilage properties by stimulating extracellular matrix (ECM) synthesis and by improving tissue mechanical properties. For example, compression stimulation applied on chondrocyte-seeded hydrogels increased glycosaminoglycan (GAG) content by almost threefold and collagen content by almost ninefold.17 The increased ECM is associated with improved mechanical properties.13,18 These prior studies using compressive stimulation have focused on constructs produced from primary or passaged articular chondrocytes. Compressive stimulation has not been applied to costal chondrocytes for engineering neocartilage. It is not known whether costal chondrocytes would respond to mechanical stimuli, or, if they do, whether these cells would respond in a similar manner as articular chondrocytes. The field of mechanical stimulation for costal cartilage-derived neocartilage is unexplored but can potentially benefit neocartilage quality to achieve clinically relevant properties.
Previously, passive axial compression applied to fibrocartilage neotissue has been shown to enhance mechanical and biochemical properties.19 It is also effective and simple to apply, making it a good first choice for examining costal chondrocyte responses to mechanical stimulation. The timing of stimulation with respect to neocartilage development can have a tremendous influence on neocartilage response. In self-assembled neocartilage, the developmental stages are generally divided into four phases. The first two phases are associated with cell–cell adhesion and neocartilage formation; in the third phase, the neocartilage actively synthesizes ECM, and, in the fourth phase, the neocartilage undergoes maturation.20 For this work, the third and fourth phases are referred to as the ECM synthesis (S) phase and maturation (M) phase, respectively. The S phase has been shown to be receptive to mechanical stimulation; stimulation at 10–14 days after seeding showed increases in aggregate modulus and GAG content.21 Stimulation during the M phase has not been examined before, but this additional time window may allow flexibility in when the stimuli can be applied.
In addition to the timing of stimulation, the magnitude of stress is a critical factor in mechanical stimulation. Many studies attempt to recapitulate the in vivo loading environment.22 Although moderate stresses induce regeneration, excessive stresses on cartilage have been associated with cartilage degeneration.23,24 With different magnitudes, some studies have reported increased ECM synthesis whereas others observed no change.12–14 The lack of a consensus is likely because of differences in stimulation magnitude and also because of differences in in vitro culture systems.18 Therefore, although the existing literature may serve as a guide, it is essential to examine stimulation magnitudes specifically for each culture system of interest. Since low magnitude passive axial compression has been shown to be effective in self-assembled constructs, a range of low magnitudes was chosen for this study.
Equally important as mechanical stimulation, soluble bioactive stimuli are essential in modulating neocartilage biochemical and mechanical properties. The bioactive stimuli regimen consisting of transforming growth factor (TGF)-β1, chondroitinase ABC (cABC), and lysyl oxidase-like protein 2 (LOXL2) has been shown as highly effective in modulating neocartilage ECM.25 TGF-β1 stimulates synthesis of GAG and collagen. Treatment with cABC temporarily depletes GAG, bringing the collagen fibers closer together as LOXL2 enzymatically crosslinks the collagen fibers, strengthening the tensile integrity of neocartilage. This bioactive regimen has been applied to engineered fibrocartilage of bovine cell source as well as to porcine costochondral neocartilage to result in improved neocartilage tensile properties.25,26 Although promising, this bioactive regimen has yet to be used in conjunction with compressive stimulation; it is unclear whether compressive stimulation would work in synergy or antagonism with biochemical stimuli.
This study aimed to develop mechanically robust engineered neocartilage from ovine costal chondrocytes through mechanical and bioactive stimulation. We expected that a low magnitude, passive axial compression19 would be efficacious in promoting neocartilage tissue integrity. The study was conducted in three experiments: Experiment 1 focused on identifying a stimulation time window during neocartilage development to maximize neocartilage mechanical properties. The hypothesis was that both the matrix synthesis phase and maturation phase would be amenable to mechanical stimulation, resulting in improved neocartilage properties. Experiment 2 examined the magnitude of passive axial compression. It was hypothesized that neocartilage properties would increase with stimulation magnitude up to a specific threshold, after which continued increases in stimulation magnitude would no longer be effective. Experiment 3 focused on enhancing the compressive stimulation with bioactive stimuli (TGF-β1, cABC, and LOXL2). The hypothesis was that compressive stimulation would increase compressive properties, whereas bioactive stimuli would increase tensile properties. Through the examination of mechanical and bioactive stimuli, the objective was to achieve mechanically robust neocartilage from costal chondrocytes toward offering an attractive alternative cell source for the treatment of articular cartilage diseases.
Materials and Methods
Cell isolation, expansion, and redifferentiation
Costal cartilage was obtained from 1-year-old Suffolk-Dorset crossbred sheep (Animal Sciences Facility, University of California, Davis) within 2 days of slaughter. Muscle, fat, connective tissues, and perichondrium were removed from floating ribs, leaving only costal cartilage, which was then minced into ∼1 mm3 pieces. The cartilage pieces were digested for 18 h in 0.2% type II collagenase (Worthington, Lakewood, NJ) in Dulbecco's modified Eagle's medium (DMEM) with 1% penicillin/streptomycin/fungizone (PSF) (BD Biosciences, Bedford, MA) and 3% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) on an orbital shaker.
After cell isolation, chondrocytes were seeded in tissue culture-treated flasks at the density of 1.3 × 104 cells per cm2. They were expanded to passage 2 (P2) in DMEM supplemented with 1%/vol PSF, 1%/vol ITS+ premix, 1%/vol nonessential amino acids, 10 nM dexamethasone, 40 μg/mL l-proline, 50 μg/mL ascorbate-2-phosphate, 100 μg/mL sodium pyruvate, 1 ng/mL TGF-β1, 10 ng/mL platelet-derived growth factor, and 5 ng/mL basic fibroblast growth factor. This growth factor combination has been shown to enhance the chondrogenic and proliferative potentials of costal chondrocytes during expansion.27 After expansion, P2 cells were redifferentiated into chondrocytic phenotype in three-dimensional aggregate culture supplied with 10 ng/mL TGF-β1 and chondrogenic medium (CHG) that contains DMEM, 1%/vol PSF, 1%/vol ITS+ premix, 1%/vol nonessential amino acids, 100 nM dexamethasone, 40 μg/mL l-proline, 50 μg/mL ascorbate-2-phosphate, and 100 μg/mL sodium pyruvate for 11 days.11
Aggregates were then digested in collagenase solution as already described to dissociate into single cells, which were seeded into agarose cast wells at 7 M cells per construct to begin the self-assembling process. Two days after construct formation, they were unconfined from agarose wells and were cultured in six-well plates until mechanical stimulation was applied. CHG was supplied to constructs and medium exchange was performed every other day. The constructs were cultured for a total of 28 days, then were assessed for tissue properties. It is important to note that four holes were created into the neocartilage during the self-assembling process. These holes would allow tensile loading of neocartilage in future studies. In this study, only compressive stimulation was examined, and therefore, these holes did not serve any purpose; however, to be consistent with future studies, constructs of this shape were created.
Compressive stimulation setup
At the time of stimulation, constructs were placed in the compressive stimulation chambers (Fig. 1), which were custom fabricated in the TEAM facility (UC Davis). These chambers were designed such that they could house and protect constructs in six-well plates and stabilize compressive loads placed on individual constructs. To apply passive axial compression, tissue culture-treated six-well plates were coated with agarose at the bottom, constructs were placed in the center of the well, and a thin layer of agarose was placed on top of constructs (Fig. 1). Stainless steel weights corresponding to the compressive stresses already listed were applied on top of constructs. The agarose layers allowed medium diffusion to construct. The chambers restricted the lateral movement of the stainless steel weights to ensure uniform stress across the surface of the constructs. After stimulation period, constructs were returned to regular tissue culture well plates.
FIG. 1.
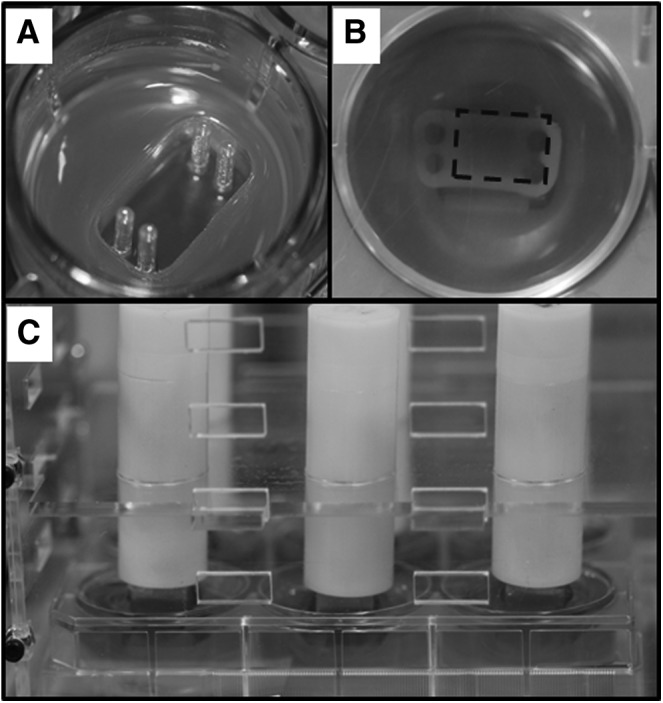
The setup of passive axial compression. (A) Agarose molds of specific shape were created for cell seeding. (B) Neocartilage seen from the bottom of the well plate. The dotted rectangle indicates the area of neocartilage that received passive axial compression. (C) Compressive stimulation chambers hold the weights directly on top of neocartilage, which were placed in six-well tissue culture plates.
Experiment 1: stimulation time
To determine an appropriate stimulation time during neocartilage development in self-assembling process, constructs were stimulated during ECM synthesis phase, maturation phase, or both phases of the self-assembling process. Previously, compressive magnitude of up to 5 g, which is calculated to be equivalent to ∼3.3 kPa, was examined in engineered fibrocartilage and shown to have positive effects on neocartilage properties.19 Therefore, 3.3 kPa and higher magnitudes were examined in this study. Six treatment groups were included examining two different stimulation times. One group received 3.3 kPa compressive stress stimulation only during the synthesis phase on days 10–14 continuously (denoted as “S(3.3)”). One group received 3.3 kPa compressive stress stimulation only during the maturation phase on days 18–22 continuously (denoted as “M(3.3)”). One group received 3.3 kPa compressive stress stimulation both times (denoted as “S(3.3)M(3.3)”). The last two groups were included to examine whether higher stress is necessary in the maturation phase. Therefore, these groups received 3.3 kPa during the synthesis phase and 5.0 or 6.7 kPa during the maturation phase (denoted as “S(3.3)M(5)” and “S(3.3)M(6.7),” respectively). The control group did not receive any stimulation (denoted “ctrl”). From these six treatments, the treatment that led to the highest improvements in neocartilage mechanical and biochemical properties was determined as the best treatment to be carried forward to Phase 2.
Experiment 2: stimulation magnitude
Low magnitude compressive stress up to 3.3 kPa was examined in engineered fibrocartilage,19 and this phase utilized 3.3 kPa as a starting point and examined whether 3.3 kPa or higher magnitudes positively affect neocartilage. Thus, compressive stresses of 3.3, 5.0, and 6.7 kPa were examined and compared with the untreated control. The magnitude that was the most beneficial to neocartilage in this phase was determined based on the highest increases in neocartilage properties, as in Phase 1. The best stimulation time and magnitude were carried forward to Phase 3.
Experiment 3: bioactive and compressive stimulations
In Experiment 3 of the study, the effects of compressive stimulation and bioactive treatment were examined. Three groups were studied—untreated control, bioactive stimulation alone (BA), and bioactive and compressive stimulation together (BA+S(5)). Bioactive treatment was a combination of TGF-β1, cABC, and LOXL2. TGF-β1 was supplied at 10 ng/mL in medium during days 1–28. cABC treatment was given on day 7 for 4 h at 1.5 units/mL. LOXL2 along with copper sulfate (1.6 μg/mL) and hydroxylysine (0.146 μg/mL) was given on days 14–28 at 0.15 μg/mL. To the neocartilage group that received both compressive and bioactive stimulation, compressive stress and stimulation time determined in previous phases were also applied.
Neocartilage assessment
After 4 weeks of neocartilage culture, constructs were assessed by their gross morphology, and mechanical, biochemical, and histological properties. Only the center portion of the neocartilage that received passive axial compression was tested. The regions with four holes were not included in testing. Compressive properties were measured by viscoelastic stress-relaxation test, as previously described.28 In brief, a cylindrical punch was taken from the center of neocartilage and was compressed at 10% and 20% strains after preconditioning at 5% strain for 15 cycles using an Instron tester (Instron, Inc.). The resulting data were analyzed and fit to the viscoelastic linear solid model, as described previously.28 Instantaneous moduli and relaxation moduli were reported from the model.
Tensile properties were measured by uniaxial tensile tests. In brief, dog-bone shaped specimens were cut out from the neocartilage samples and were stained until failure at 1% strain rate per second by gripping in a TestResources mechanical tester (TestResources, Inc.). Tensile modulus and ultimate tensile strength (UTS) were calculated and reported from the stress–strain curves.
Biochemical samples taken from a portion of neocartilage were weighed and lyophilized, and the water content of each sample was calculated by analyzing the wet weight and the dry weight of the samples. The samples were digested in 125 μg/mL papain solution (Sigma) for 18 h. The amount of GAG in the sample was measured by Blyscan Glycosaminoglycan assay (Biocolor). The amount of collagen was quantified by a modified colorimetric chloramine-T hydroxyproline assay using a Sircol collagen standard (Biocolor). The amount of DNA was measured by Picogreen assay (Quant-iT Picogreen dsDNA assay kit). The measured GAG and collagen amounts in milligram were normalized by the tissue wet weight (WW) in milligram, and the resulting ratios in percentages were reported.
Statistics
Data analyses were performed using JMP 12 statistical software. All data were reported as mean ± SD. One-way analysis of variance (ANOVA), followed by Tukey's post hoc tests, were performed to compare all groups, using p < 0.05 as statistical significance. Different statistical groups are indicated by different alphabetical letters.
Results
Experiment 1: stimulation time window
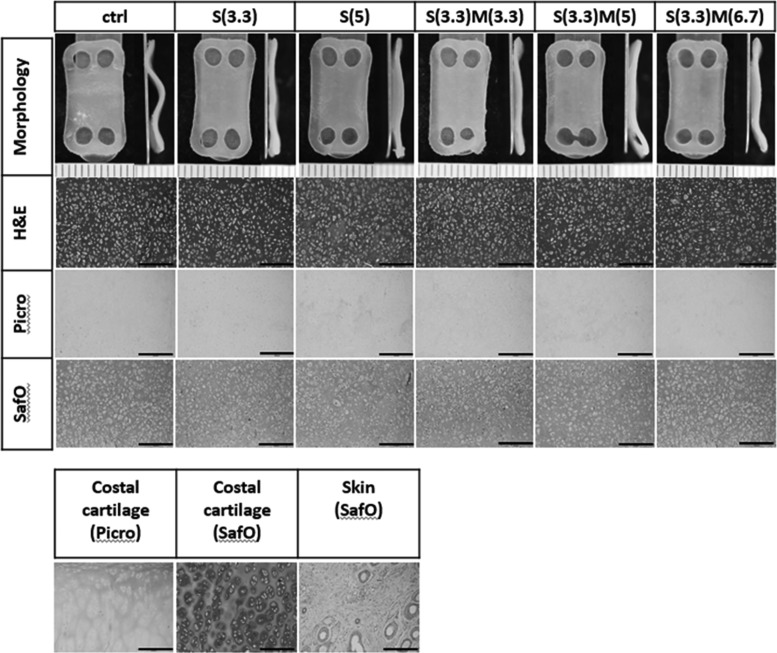
At 4-week assessment, constructs had slightly curved morphology, and the curvature was most pronounced in the control group. Constructs achieved flatter and more uniform morphology with passive uniaxial compression (Fig. 2). Neocartilage thicknesses were 0.67 ± 0.07, 0.65 ± 0.05, 0.62 ± 0.07, 0.62 ± 0.07, 0.63 ± 0.04, and 0.63 ± 0.06 mm for control, S(3.3), M(3.3), S(3.3)M(3.3), S(3.3)M(5), and S(3.3)M(6.7), respectively.
FIG. 2.
Representative gross morphology and histology of neocartilage engineered to examine the effects of the compressive stimulation time window. Morphology of neocartilage was generally similar among groups; however, the untreated control displayed wavy or curved morphology, whereas all neocartilage treated with passive axial compressive stimulation attained flat and uniform morphology. H&E staining showed highly cellular but homogeneous tissue in all groups. Safranin-O staining indicated that neocartilage was rich in GAG, and picrosirius staining showed that neocartilage was low in collagen. The scale bars represent 0.5 mm. GAG, glycosaminoglycan.
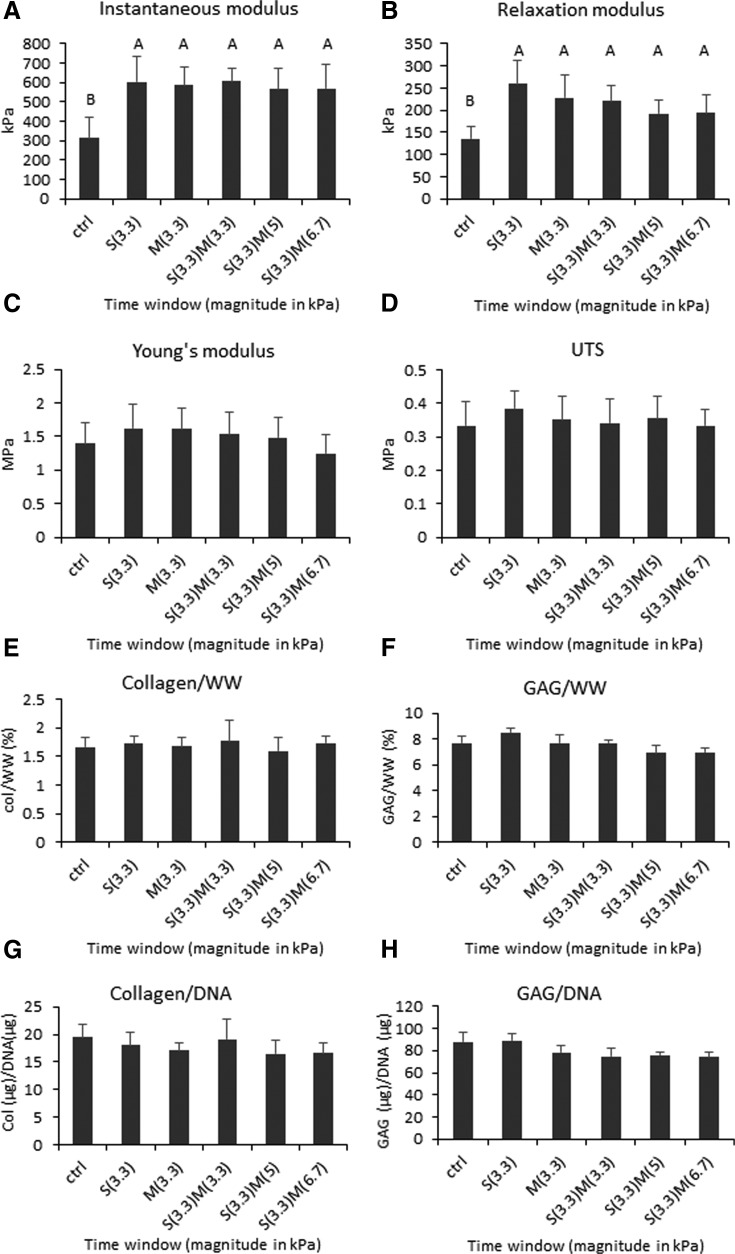
Examining timing of compressive stimulation showed that stimulation once either during the synthesis or maturation phases increased compressive properties compared with control. Stimulation during both phases did not provide additional benefits. All groups treated with passive axial compression increased both instantaneous and relaxation moduli significantly (Fig. 3). Tensile properties trended higher in stimulated groups; however, there was no statistical significance. No statistical difference was found in GAG, collagen, and DNA contents among groups (Fig. 3). It was noted, however, that GAG content in the stimulated group during synthesis trended the highest among groups. DNA contents were also similar among groups, and the number of cells per construct at 4 weeks was similar to the number of cells seeded originally, confirming that cells were retained in neocartilage and viability was maintained. Histological assessment showed that neocartilage of all groups was homogeneous with healthy cells distributed throughout the tissue. All groups stained strongly for GAG and faintly for collagen (Fig. 2).
FIG. 3.
Mechanical and biochemical properties of neocartilage engineered to examine the effects of the compressive stimulation time window. The groups examined are indicated by S and M for synthesis phase and maturation phase, respectively, and the magnitudes of stress in kilopascals are shown in parentheses. One-way ANOVAs were performed using p < 0.05 as statistical significance; letters above the bars show statistical significance among groups. All groups treated with passive axial compressive stimulation significantly increased in instantaneous modulus and relaxation modulus (A, B). S(3.3) trended higher than all other groups in the relaxation modulus (B). Tensile properties (C, D) and biochemical content were not affected significantly by stimulation (E–H). ANOVA, analysis of variance.
Experiment 2: stimulation magnitude
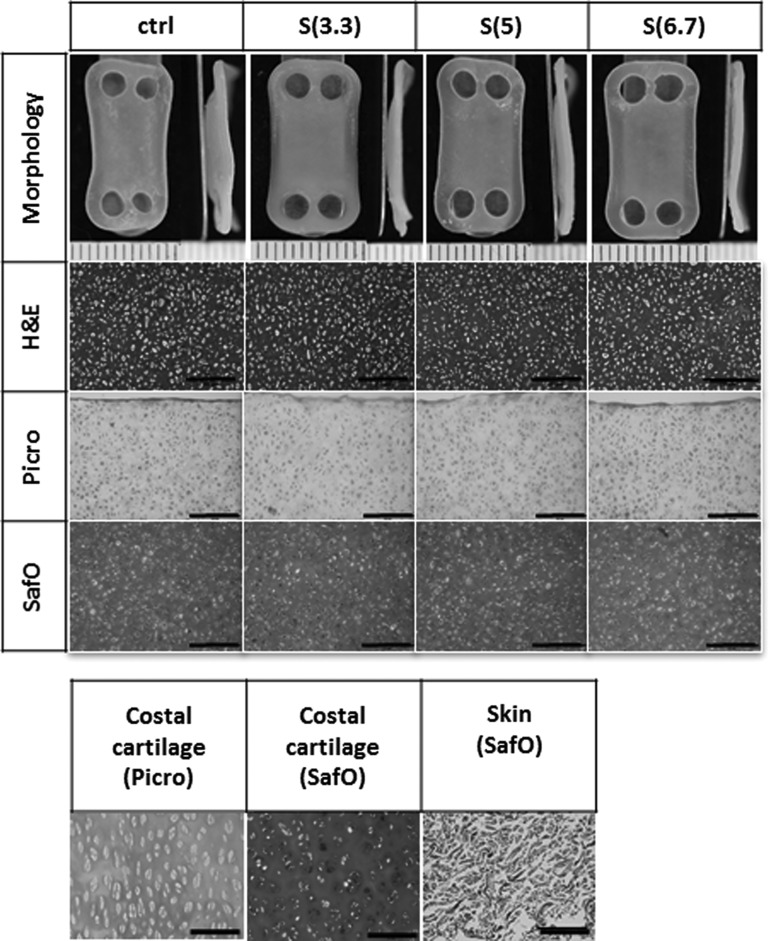
Constructs were slightly curved, similar to Phase 1 constructs, and compressive stimulation promoted flatter morphology. Neocartilage thicknesses were 0.83 ± 0.08, 0.80 ± 0.07, 0.78 ± 0.05, and 0.75 ± 0.06 mm for control, 3.3, 5.0, and 6.7 kPa stimulated groups, respectively (Fig. 4). Neocartilage was thinner with higher compressive loads; in particular, the 6.7 kPa group was significantly thinner than control.
FIG. 4.
Representative gross morphology and histology of neocartilage engineered to examine the effects of compressive magnitude. Untreated control neocartilage displayed wavy or curved morphology, similar to that seen in Experiment 1, whereas all neocartilage constructs treated with passive axial compressive stimulation were flat. H&E staining showed highly cellular, homogeneous tissue, and no differences were found among groups. Neocartilage stained strongly for GAG and weakly for collagen. The scale bars represent 0.5 mm.
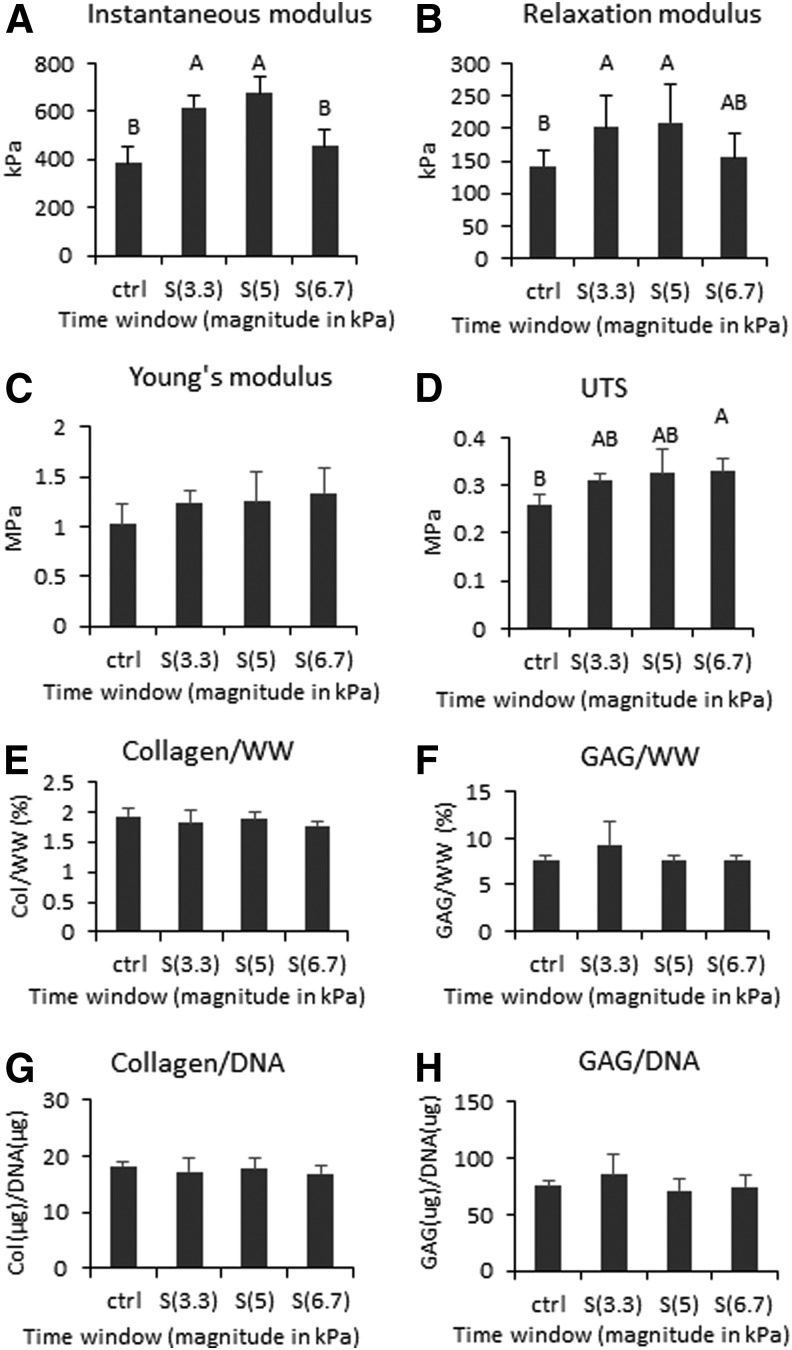
Compressive stimulation with 3.3 and 5.0 kPa significantly increased the compressive properties of neocartilage. Instantaneous and relaxation moduli of these two groups significantly increased over control. Stimulation with the 6.7 kPa magnitude did not significantly increase neocartilage compressive properties over control but increased UTS. Compressive stimulation slightly improved the GAG content of neocartilage treated with the 3.3 kPa stress, although it was not statistically significant compared with control. GAG contents of the 5.0 and 6.7 kPa groups were similar to that of control. Collagen and DNA contents were similar across all groups (Fig. 5). Histological assessment showed that neocartilage of all groups was homogeneous and had similar morphology. All groups stained strongly for GAG and weakly for collagen (Fig. 4).
FIG. 5.
Mechanical and biochemical properties of neocartilage engineered to examine the effects of compressive magnitude. Neocartilage was stimulated in the synthesis (S) phase. The applied magnitudes of stress in kilopascals are shown in parentheses. One-way ANOVAs were performed using p < 0.05 as statistical significance; letters above the bars show statistical significance among groups. Both groups treated with 3.3 and 5 kPa were significantly higher in the instantaneous and relaxation moduli than control (A, B). In contrast, the group treated with 6.7 kPa did not significantly improve in these properties compared with control (A, B). Tensile properties trended higher in the stimulated groups than control, and UTS of S(6.7) was significantly higher than control (C, D). Biochemical properties did not change with stimulation (E–H). UTS, ultimate tensile strength.
Experiment 3: stimulation in combination with bioactive stimuli
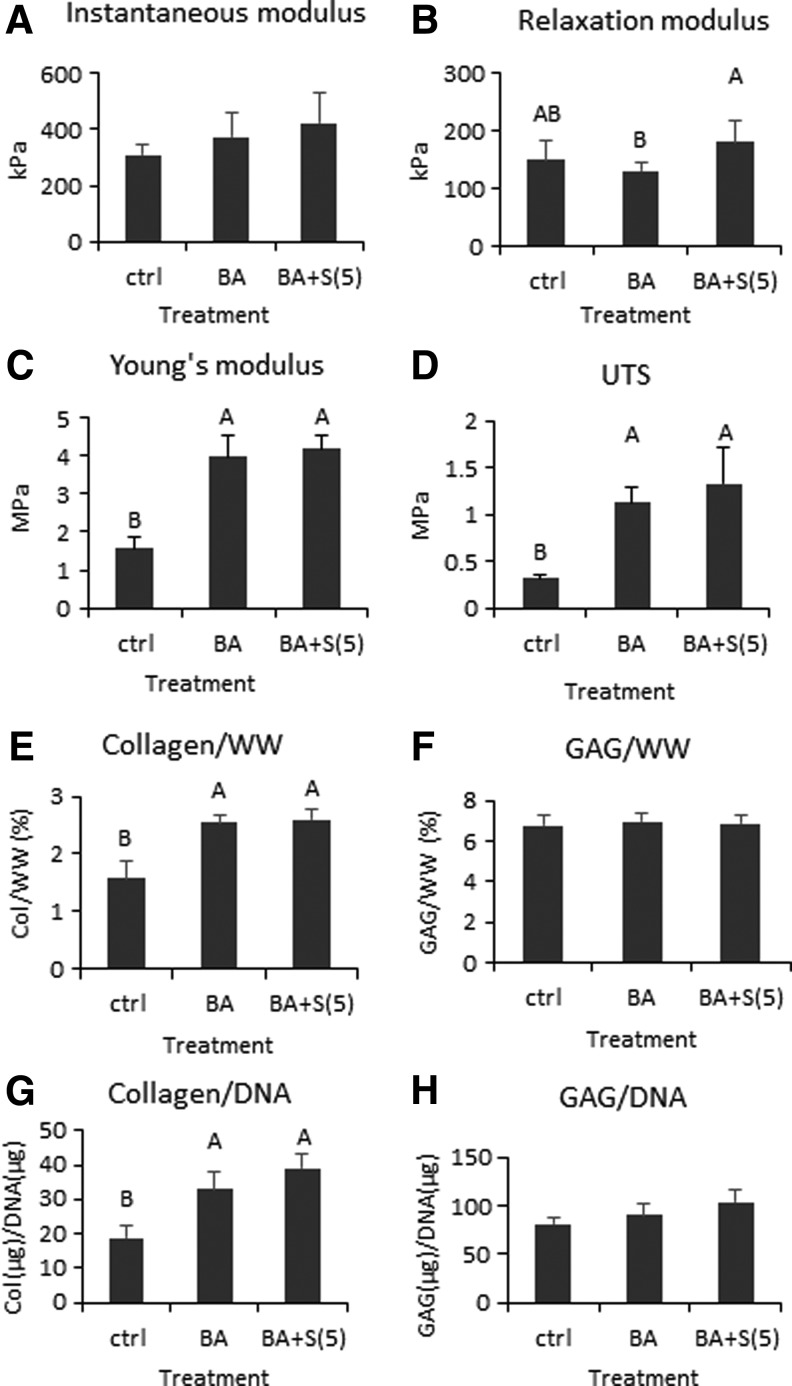
Neocartilage thicknesses were 0.59 ± 0.02, 0.55 ± 0.05, and 0.52 ± 0.05 mm for control, BA, and BA+S(5), respectively. Bioactive treatment significantly increased the tensile stiffness and UTS of neocartilage compared with control, whereas it did not significantly change the compressive properties (Fig. 7). Neocartilage that received compressive stimulation and BA treatment (BA+S(5)) also significantly increased in tensile properties compared with control; it also increased in compressive relaxation modulus compared with BA treatment alone. The instantaneous modulus also trended higher with this combination treatment but did not significantly increase over control.
FIG. 7.
Mechanical and biochemical properties of neocartilage engineered to examine the interaction of bioactive (BA) and compressive (S(5)) stimulation. One-way ANOVAs were performed using p < 0.05 as statistical significance; letters above the bars show statistical significance among groups. The instantaneous modulus trended higher in the BA and BA+S(5) groups than control (A). The relaxation modulus trended lower in the BA group, but when compressive stimulation was added, the modulus significantly increased (B). Both Young's modulus and UTS increased significantly in the BA and BA+S(5) groups compared with control (C, D). GAG content did not change with stimulation (F, H); however, collagen content increased significantly in the BA and BA+S(5) groups (E, G).
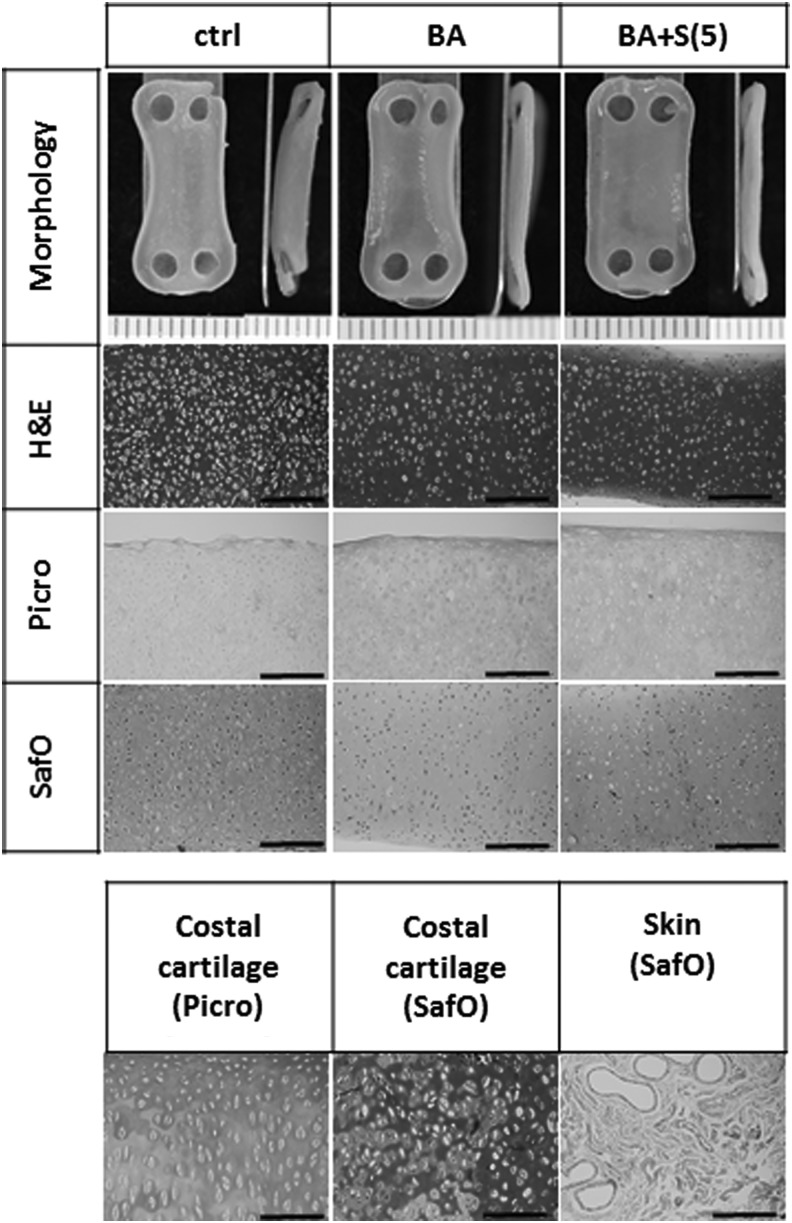
Collagen contents increased significantly in the BA and BA+S(5) groups compared with control (Fig. 7). Histological assessment also showed that these two groups stained more strongly for collagen than for control (Fig. 6). GAG and DNA contents did not change with treatment. Staining with Safranin-O showed that the BA group stained more weakly than control and BA+S(5) groups.
FIG. 6.
Representative gross morphology and histology of neocartilage engineered to examine the interaction of bioactive (BA) and compressive (S(5)) stimulation. Flat morphology was observed with compressive stimulation. H&E staining showed that cellularity decreased in groups treated with BA, reaching a density similar to native cartilage. Staining for collagen was stronger in groups treated with BA. Staining for GAG was weak in the BA group, whereas the staining was stronger for the BA+S(5) and control groups. The scale bars represent 0.5 mm.
Discussion
Developing engineered neocartilage with robust mechanical properties is imperative for the survival of the implant in vivo and for immediate load bearing upon implantation. With the goal of creating neocartilage from costal chondrocytes to offer flexibility in cell source, as well as to generate neocartilage with appropriate mechanical properties, this study examined the effects of passive axial compression and bioactive stimulation on neocartilage in three experiments. In Experiment 1, a suitable stimulation time window for passive axial compression was determined in self-assembled neocartilage during two different neocartilage development phases. The hypothesis that compressive stimulation during the ECM synthesis or maturation phase would yield enhanced mechanical properties was supported by increases in instantaneous modulus by 92% and 87% in the synthesis and maturation phases, respectively, compared with control. Experiment 2 was performed to determine a compressive stress magnitude that led to the greatest improvement in mechanical properties. The hypothesis that neocartilage properties would increase with increasing stimulation magnitude up to a specific threshold was supported by the identification of a peak at 5.0 kPa in the stimulation magnitude; properties diminished >5.0 kPa of compressive stress. Experiment 3 examined the use of compressive stimulation and bioactive stimuli together to enhance neocartilage compressive and tensile properties. The hypothesis that compressive stimulation would improve neocartilage compressive properties, while bioactive stimulation would improve tensile properties simultaneously, was supported by a 92% increase in compressive properties and a 262% increase in UTS. Through these experiments, robust neocartilage with enhanced compressive and tensile properties was created from costal chondrocytes. This study represents one of the first studies to examine mechanical stimulation on neocartilage engineered from a costal cartilage source. For the first time, costal chondrocytes have been shown to respond to passive axial compression by synthesizing mechanically robust tissue, paving the way for this abundant, less invasive cell source to be used in articular cartilage repair.
The ECM synthesis phase and maturation phase were both effective stimulation periods; however, stimulation in both phases did not yield added benefits. Instantaneous modulus values increased by 92% and 87%, and relaxation moduli increased by 92% and 68%, because of passive axial compression during only the S or M phases, respectively, compared with untreated control (Fig. 3). When neocartilage was stimulated in both phases, the instantaneous and relaxation modulus values increased by 92% and 63%, respectively, over untreated control, and, therefore, treatment during both phases was not better than a one-time treatment in either phase. A reason why stimulation during both phases did not lead to further improvements may be that the neocartilage became accustomed to the mechanical stimulus upon its first round of application, that is, the cells became saturated by the stimulus during the S phase. If this was the case, it was thought that increasing the magnitude of stimulus during the M phase would elicit a measurable effect. However, the S(3.3)M(5) and S(3.3)M(6.7) groups, both of which received higher magnitudes in the M phase, did not yield added benefits. The lack of further improvement, therefore, was unlikely because of insufficient applied stress magnitude. Although it is possible that a compressive magnitude beyond the 6.7 kPa examined here during the M phase might elicit a positive response, this, too, is unlikely. Groups that underwent treatment twice showed a decreasing trend in relaxation modulus and Young's modulus compared with one-time treatment. Collectively, these data indicate that passive axial compression is an effective method to improve compressive properties of scaffold-free neocartilage derived from costal chondrocytes, and that one-time stimulation is sufficient to yield significant improvements in properties.
Both synthesis and maturation phases were suitable stimulation time windows; however, improvements in neocartilage properties trended higher when the stimulus was applied during the S rather than the M phase. Specifically, the relaxation modulus, GAG/WW, Young's modulus, and UTS all trended higher when neocartilage was treated in the S phase than in the M phase. This finding is similar to the results found in the study performed previously21 because both studies showed that stimulation during days 10–14 was better than stimulation at a later time. Of the three time windows selected between day 6 and day 18, treatment during days 10–14 was better than treatment during days 6–10 or during days 14–18. Thus, both of these studies point to the same time window to be the most suitable, despite the differences in the study design such as cell source used (primary articular chondrocytes vs. passaged costal chondrocytes) and mode of mechanical stimulation (hydrostatic pressure vs. compression).
A study in another scaffold-free cartilage engineering system also showed that compressive stimulation was more effective when applied as early as day 1.18 Constructs became unresponsive to stimulation when a similar stimulus was applied later in the culture.18 When chondrocytes undergo the self-assembling process, ECM synthesis is most prevalent during days 10–14, whereas maturation is more prevalent during days 18–22.29 It is likely that the newly laid ECM in the synthesis phase was better able to adapt to the mechanical environment than the more mature ECM, thus stimulation was more effective in the S phase than in the M phase. Owing to the trend that treatment in the S phase was better than in the M phase, one-time compressive stimulation during the S phase was selected to be carried forward to subsequent experiments in this study.
The magnitude of stress applied in compressive stimulation was also a key parameter to achieve beneficial effects. In examining low magnitudes between 3.3 and 6.7 kPa, it was found that 3.3 and 5.0 kPa were the most effective compressive stresses for costal chondrocyte-derived neocartilage, exhibiting 60% and 75% improvement, respectively, in the instantaneous modulus, compared with control. Similar trends were observed in the relaxation modulus (Fig. 5). Interestingly, tensile properties of neocartilage were observed to have an upward trend with increasing compressive stimulation magnitude. Young's moduli increased by 21%, 21%, and 30%, whereas UTS increased by 19%, 25%, and 27%, respectively, with 3.3, 5.0, and 6.7 kPa stimulation magnitudes. The ECM biochemical content did not change significantly in concordance with the mechanical properties, although a 20% increase in GAG/WW was observed in the group stimulated with 3.3 kPa. It is speculated that the ECM organization may have been altered by the compression leading to changes in the mechanical properties. A peak in beneficial magnitude was observed at 5.0 kPa, as increasing to 6.7 kPa was no longer beneficial to neocartilage. High compressive stresses have been shown to cause detrimental effects on cartilage.30–32 Most studies commonly apply stress magnitudes from 0.5 to 10 MPa,33 which is substantially higher than the magnitude used in this study. In the self-assembling system, compressive magnitude much higher than 6.7 kPa may cause detrimental effects to neocartilage. It is crucial that the magnitude of applied stress is examined in tissue culture systems employing mechanical stimulation to improve neocartilage properties.
This study revealed that neocartilage derived from passaged costal chondrocytes responds differentially to the various magnitudes of mechanical stimulus. Previously, fibrocartilage engineered from primary articular chondrocytes and fibrochondrocytes was shown to respond differently based on magnitude of passive axial compression.34 These fibrocartilage constructs displayed up to a 96% increase in compressive properties, and, in this study, neocartilage from passaged costal chondrocytes displayed up to a 92% increase in compressive properties. The similarity indicates that that passaged costal chondrocytes respond to mechanical stimulus to the same extent as primary articular chondrocytes. Although in vitro expansion of chondrocytes typically leads to dedifferentiation, causing changes in gene expression and chondrocytes' response to mechanical environment,35 the aggregate culture process employed in this study sufficiently redifferentiated expanded cells to chondrocytes. Therefore, it is an exciting finding to note that costal chondrocytes, even after expansion, still displayed beneficial responses to mechanical stimulus. It opens new doors for manipulating costochondral neocartilage properties through other types of mechanical stimuli, such as tension and perfusion.
Treatment with bioactive stimuli, containing TGF-β1, cABC, and LOXL2, significantly enhanced neocartilage tensile properties, whereas the addition of compressive stimulation significantly increased neocartilage relaxation modulus. The BA treatment increased neocartilage Young's modulus by 152% and UTS increased by 262% compared with untreated control. When both compressive stimulation and bioactive stimuli were applied to neocartilage, Young's modulus increased by 165% and UTS by 326% compared with untreated control. Associated with these changes in tensile properties, collagen/WW increased by 61% compared with untreated control. These mechanical and biochemical improvements tremendously contribute toward the robustness of neocartilage, which is critical for implant survival and load bearing in vivo. The bioactive treatment has been shown to have similar improvements in engineered fibrocartilage25 and porcine costal neocartilage.26 This is the first time that the treatment was tested and reported in ovine costal-derived neocartilage, and the results further demonstrate the versatility and success of the treatment in neocartilage derived from different cell sources. The bioactive stimulation was not beneficial for compressive properties, however, as also seen previously.25 When compression stimulation was applied in combination with the BA treatment, compressive properties improved as the relaxation modulus significantly increased 39% more than that with bioactive stimulation alone (Fig. 7). Therefore, bioactive stimuli were required to improve neocartilage tensile properties and compressive stimulation was required to improve the compressive properties; thus, mechanical and bioactive stimuli complemented each other, ensuing in robust mechanical integrity of costal chondrocyte-derived neocartilage.
Using costal cartilage presents advantages over using articular cartilage by eliminating the need to harvest cells from joints, thus sparing the joints from further damage. For treating larger defects in more severely injured joints, it is even more crucial to preserve as much healthy tissue as possible. Thus, being able to tissue engineer mechanically robust neocartilage implants from alternative autologous cell sources would inarguably extend the clinical applicability of cartilage tissue engineering technologies. This study is one of the first studies to examine mechanical stimulation on scaffold-free neocartilage derived from passaged costal chondrocytes. It demonstrated that neocartilage derived from costal chondrocytes is responsive to mechanical stimulation and that neocartilage properties can be improved by the use of passive axial compression. Through this stimulation, neocartilage matrix was modified to result in enhanced mechanical properties. Identifying stimulation magnitude and critical time windows is key in benefiting from a simple system of passive axial compression. By determining when neocartilage is most amenable to these mechanical stimuli, such as when ECM synthesis is active, the effectiveness of the treatment can also be maximized. Other types of mechanical stimuli, such as tension, shear, and perfusion, should be examined on costal chondrocyte-derived neocartilage in the future. These findings and techniques should also be translated to human costal chondrocyte-derived neocartilage to realize these cells' true potential in clinical applications. This study contributes to the progression of tissue engineering technology to create mechanically robust neocartilage from an alternative autologous cell source, with the goal of widening treatment options for articular cartilage defects.
Acknowledgment
This work was supported by NIH R01AR067821.
Disclosure Statement
No competing financial interests exist.
References
- 1.Huang B.J., Hu J.C., and Athanasiou K.A. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 98, 1, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fellows C.R., et al. Adipose, bone marrow and synovial joint-derived mesenchymal stem cells for cartilage repair. Front Genet 7, 213, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Somoza R.A., et al. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng Part B Rev 20, 596, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz I.B., et al. Regenerative potential of the cartilaginous tissue in mesenchymal stem cells: update, limitations, and challenges. Rev Bras Ortop 52, 2, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karagoz H., et al. Mandibular reconstruction after hemimandibulectomy. J Craniofac Surg 23, 1373, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Wee J.H., et al. Complications associated with autologous rib cartilage use in rhinoplasty: a meta-analysis. JAMA Facial Plast Surg 17, 49, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Murphy M.K., et al. Inducing articular cartilage phenotype in costochondral cells. Arthritis Res Ther 15, R214, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanimoto K., et al. Superficial zone protein affects boundary lubrication on the surface of mandibular condylar cartilage. Cell Tissue Res 344, 333, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Neu C.P., et al. Increased friction coefficient and superficial zone protein expression in patients with advanced osteoarthritis. Arthritis Rheum 62, 2680, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J., et al. Comparison of articular cartilage with costal cartilage in initial cell yield, degree of dedifferentiation during expansion and redifferentiation capacity. Biotechnol Appl Biochem 48(Pt 3), 149, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Murphy M.K., et al. Engineering a fibrocartilage spectrum through modulation of aggregate redifferentiation. Cell Transplant 24, 235, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter C.J., Mouw J.K., and Levenston M.E. Dynamic compression of chondrocyte-seeded fibrin gels: effects on matrix accumulation and mechanical stiffness. Osteoarthritis Cartilage 12, 117, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Ng K.W., et al. Duty cycle of deformational loading influences the growth of engineered articular cartilage. Cell Mol Bioeng 2, 386, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicodemus G.D., and Bryant S.J. Mechanical loading regimes affect the anabolic and catabolic activities by chondrocytes encapsulated in PEG hydrogels. Osteoarthritis Cartilage 18, 126, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Connelly J.T., Vanderploeg E.J., and Levenston M.E. The influence of cyclic tension amplitude on chondrocyte matrix synthesis: experimental and finite element analyses. Biorheology 41, 377, 2004 [PubMed] [Google Scholar]
- 16.Vanderploeg E.J., et al. Oscillatory tension differentially modulates matrix metabolism and cytoskeletal organization in chondrocytes and fibrochondrocytes. J Biomech 37, 1941, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Appelman T.P., et al. The influence of biological motifs and dynamic mechanical stimulation in hydrogel scaffold systems on the phenotype of chondrocytes. Biomaterials 32, 1508, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Waldman S.D., et al. A single application of cyclic loading can accelerate matrix deposition and enhance the properties of tissue engineered cartilage. Osteoarthritis Cartilage 14, 323, 2006 [DOI] [PubMed] [Google Scholar]
- 19.MacBarb R.F., et al. Passive strain-induced matrix synthesis and organization in shape-specific, cartilaginous neotissues. Tissue Eng Part A 20, 3290, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Athanasiou K.A., et al. Self-organization and the self-assembling process in tissue engineering. Annu Rev Biomed Eng 15, 115, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elder B.D., and Athanasiou K.A. Effects of temporal hydrostatic pressure on tissue-engineered bovine articular cartilage constructs. Tissue Eng Part A 15, 1151, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sauerland K., Raiss R.X., and Steinmeyer J. Proteoglycan metabolism and viability of articular cartilage explants as modulated by the frequency of intermittent loading. Osteoarthritis Cartilage 11, 343, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Buschmann M.D., et al. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci 108 (Pt 4), 1497, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Grodzinsky A.J., et al. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng 2, 691, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Makris E.A., et al. Combined use of chondroitinase-ABC, TGF-beta1, and collagen crosslinking agent lysyl oxidase to engineer functional neotissues for fibrocartilage repair. Biomaterials 35, 6787, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy M.K., et al. Neocartilage integration in temporomandibular joint discs: physical and enzymatic methods. J R Soc Interface 12, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy M.K., et al. Enhancing post-expansion chondrogenic potential of costochondral cells in self-assembled neocartilage. PLoS One 8, e56983, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen K.D., and Athanasiou K.A. Viscoelastic characterization of the porcine temporomandibular joint disc under unconfined compression. J Biomech 39, 312, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Ofek G., et al. Matrix development in self-assembly of articular cartilage. PLoS One 3, e2795, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loening A.M., et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys 381, 205, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Kim Y.J., et al. Mechanical regulation of cartilage biosynthetic behavior: physical stimuli. Arch Biochem Biophys 311, 1, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Davisson T., et al. Static and dynamic compression modulate matrix metabolism in tissue engineered cartilage. J Orthop Res 20, 842, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Natenstedt J., et al. What quantitative mechanical loading stimulates in vitro cultivation best? J Exp Orthop 2, 15, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacBarb R.F., et al. Engineering functional anisotropy in fibrocartilage neotissues. Biomaterials 34, 9980, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das R.H., et al. In vitro expansion affects the response of chondrocytes to mechanical stimulation. Osteoarthritis Cartilage 16, 385, 2008 [DOI] [PubMed] [Google Scholar]