Abstract
Background
This study aimed to (1) provide a comprehensive characterization of depressive symptoms profiles, and (2) examine the cross-sectional association between depressive symptom profiles and cardio-metabolic outcomes, including metabolic syndrome and obesity, while controlling for sociodemographic variables, health behaviors and inflammation.
Methods
Our sample was comprised of 1085 participants (55.80% female) enrolled in the MIDUS-II biomarker study. Latent profile analysis (LPA) was used to derive depressive symptom profiles using subscales of the Mood and Anxiety Symptom Questionnaire (MASQ) and the Center for Epidemiologic Studies Depression Scale (CES-D) subscales as well as Pittsburgh Sleep Quality Index (PSQI) global score. Metabolic syndrome was defined according to the Interim Joint Statement definition. CRP was used as a marker of inflammation.
Results
Four depressive symptom profiles were identified. The “No Symptoms” subgroup (60.65% of the sample) had the lowest overall scores across subscales. The “Mild Symptoms” subgroup (26.73%) was characterized by lower scores across indicators, with subscales measuring somatic symptoms being the highest within group. The “Moderate Symptoms” subgroup (10.32%) had higher scores across subscales (1 SD above the mean), with subscales measuring negative affect/loss of interest being the highest within group. Finally, the “Acute symptoms” subgroup (2.30%) was characterized by the highest overall scores (1.5–3 SD above the mean) on all indicators. After controlling for sociodemographic characteristics and health behaviors, the “Moderate Symptoms” subgroup was significantly associated with metabolic syndrome (OR=1.595, p=0.035) and obesity (OR=1.555, p=0.046). Further, there was a trend between the “Mild Symptoms” subgroup and the presence of obesity (OR=1.345, p=0.050). Inflammation attenuated these associations.
Conclusions
Four depressive symptom profiles were identified among healthy mid-life individuals in the US. These profiles are differentially associated with cardio-metabolic outcomes. Future work should examine whether distinct symptom profiles may reflect differential pathways to increased risk, and whether tailored management of symptoms is needed.
Keywords: Depression, metabolic syndrome, obesity, inflammation, lipoproteins, blood pressure, hyperglycemia, latent class analysis
1. Introduction
Depressive symptoms are important predictors of cardiovascular morbidity and mortality (Pan et al., 2011). Recently, researchers have shifted their attention to cardio-metabolic conditions, as they are thought to partially account for the association between depression and increased cardiovascular risk (Goldbacher et al., 2009). Obesity and metabolic syndrome, a cluster of anthropometric and metabolic disturbances, which includes central adiposity, hypertension, dyslipidemia and abnormal glucose regulation (Alberti et al., 2009), are two highly prevalent cardio-metabolic conditions (Gee and Bailey, 2013), and widely recognized precursors of cardiovascular disease (Galassi et al., 2006).
Studies examining the link between depressive symptoms and cardio-metabolic conditions have yielded inconsistent results (Luppino et al., 2011). Methodological differences in the assessment of depressive symptoms may account for the contradictory findings in this area. Some studies have opted for a categorical diagnostic approach to depression, using the Diagnostic and Statistical Manual of mental disorders (DSM) or a clinical interview to classify individuals as depressed or non-depressed (Goldbacher et al., 2009). Others have used continuous self-report measures of depressive symptoms (Luppino et al., 2011). While dimensional measures of depression have some disadvantages, including their inability to diagnose clinical depression, the fact that they measure symptoms within 1–2 weeks of administration only, as well as their assessment of symptoms that may overlap with somatic disease, they also have numerous advantages over categorical outcomes. In a recent meta-analysis, there was a stronger association between metabolic syndrome and depression when measured by self-reported scales rather than a structured clinical interview or clinical diagnosis (Pan et al., 2012). In another recent study, depressive symptoms (per standard deviation higher) were associated with 1.17–1.25 increased odds of metabolic syndrome incidence after 15 years, suggesting they may predict the development of cardio-metabolic conditions, even at subclinical levels (Womack et al., 2016). Most importantly, depression scales allow researchers to examine not only the presentation but also the severity of symptoms, whereas categorical dichotomization may lump together different symptoms clusters and severities into a single diagnosis.
Individuals can be highly heterogeneous in their presentation of depressive symptoms, even when having the same diagnosis. For example, some individuals primarily endorse somatic symptoms, while others primarily report cognitive symptoms. Somatic depressive symptoms often include loss of energy, sleep disturbances, changes in appetite, and irritability, whereas cognitive symptoms are characterized by sadness, loss of interest, pessimism, guilt, indecisiveness and worthlessness (Dozois et al., 1998). Researchers have reported differential associations between depressive symptom dimensions (cognitive vs. somatic) and cardiovascular outcomes (Luppino et al., 2011). The association between somatic, and not cognitive depressive symptoms, appear to be stronger among individuals with established heart disease (Doyle et al., 2010), but somatic complaints are also predictive of subclinical disease (Stewart et al., 2007) and cardio-metabolic conditions (Luppino et al., 2011). Fewer studies have found that cognitive symptoms only (Pedersen et al., 2007), or both symptom domains are associated with cardiac events (Hoen et al., 2010).
There is some controversy surrounding the differential associations between depressive symptom dimensions and cardio-metabolic risk. Somatic complaints, in the absence of sadness or loss of interest (cardinal depressive cognitive symptoms), cannot be classified as representing clinical depression (Association, 2013a). However, individuals who do not meet criteria for major depression but who endorse somatic symptoms also tend to have family and personal histories of mood disorders, shortened rapid eye movement (REM) latency, and increased proportion of sleep time spent in REM, all of which are factors associated with clinical depression (Akiskal et al., 1997). Similarly, somatic symptoms of depression, particularly sleep disturbances, often precede major depressive episodes and are better predictors of depressive episodes than are cognitive symptoms (Cho et al., 2008).
Inconsistent findings may also be a result of statistical limitations. Authors have pointed out the inadequacy of including two highly overlapping factors (cognitive and somatic subscales) in the same statistical model, as multicollinearity (high correlation between two predictor variables) may be present (Carney and Freedland, 2012). Clearly, a better characterization of depressive symptom profiles in the context of cardio-metabolic risk is needed. Advanced statistical modeling techniques offer an important opportunity to advance our knowledge in this regard. Latent profile analysis (LPA), in particular, is specifically designed to use actual empirical data to create quantitatively and qualitatively distinct profiles based on individual’s presentation of symptoms (Collins and Lanza, 2013).
In this study, our primary aim was to provide a comprehensive characterization of depressive symptom profiles in a national sample of healthy adults. We applied LPA to evaluate whether distinct subtypes of symptom profiles could be identified based on continuous measures of depressive symptom domains, which included negative affect, loss of interest, somatic complaints, sleep disturbances, interpersonal difficulties and positive affect. Positive affect was included as it has been shown to be predictive of cardio-metabolic conditions independent of symptoms of negative affect (Steptoe et al., 2005), and may therefore be conceptualized as a correlated but separate dimension. Further, we examined the association between age, gender, race, ethnicity, and anti-depressant use on symptom profiles. Based on previous research, we hypothesized that subgroups with increased severity of depressive symptoms as well as separate subgroups with different dimensions of depressive symptoms will emerge (somatic vs cognitive).
In addition to a proper characterization of symptoms, adequate covariate adjustment is needed when examining the association between depressive symptom profiles and cardio-metabolic conditions. Specifically, sociodemographic characteristics, such as age or gender, are important predictors of cardio-metabolic risk and should be accounted for (Ford, 2004). Furthermore, as suggested by some authors, depressive symptoms may not be the principal contributors to elevated cardio-metabolic risk, but rather may increase the risk by adding to the burden of pro-inflammatory cytokines (Vogelzangs et al., 2011). In fact, a leading mechanistic hypothesis proposes that inflammation serves as a biological pathway linking depression to cardio-metabolic risk (Shelton and Miller, 2010). In light of this, a secondary aim of this study was to examine the association between depressive symptom profiles and cardio-metabolic conditions, including metabolic syndrome and obesity, after accounting for socio-demographic characteristics, health behaviors, anti-depressant use, and inflammation. We hypothesized that depressive symptom profiles of increased severity would be associated with greater odds of having cardio-metabolic conditions, even after accounting for relevant covariates.
2. Material and Methods
2.1. Study Sample
Our sample was comprised of participants enrolled in the Midlife in the Unites States (MIDUS) study. This large scale research study aimed to examine predictors of mental and physical health in middle-aged adults (Radler and Ryff, 2010). The MIDUS study was originally established in 1995 and recruited 7,108 non-institutionalized English-speaking individuals ages 25–74 from random digit dialing from across the US, including siblings for some respondents and some pairs of twins. The second wave of the study (MIDUS-II) occurred in 2004–2008 and followed up 4963 (70%) of the original sample. Participants who participated in MIDUS II were more likely to be Caucasian, female, married, more highly educated and in better health than those lost to follow-up of diseased. MIDUS II involved expanded assessments and newly recruited a total of 592 African American participants from Milwaukee, WI. These participants were recruited using area probability sampling methods along with population counts from the 2000 United States Census to identify potential respondents. Field interviewers screened households to determine if they contained any African American adults. Milwaukee respondents were interviewed at home. All measures paralleled those used in the larger MIDUS sample.
The current study is based on the subset of MIDUS-II participants who completed the Biomarker Project which included 1,255 individuals from both the longitudinal survey sample (n=1,054) and the Milwaukee sample (n=201). The biomarker project included an in-person visit that was carried out at three General Clinical Research Centers (at UCLA, University of Wisconsin, and Georgetown University). Details on the biomarker sample and protocol have been previously described (Dienberg Love et al., 2010). All participants provided informed consent and the study was approved by the Institutional Review Boards at each participating center. Our analytical sample included a total of 1,085 participants. A total of 74 participants were excluded from analysis due to CRP levels ≥10 mg/L (Pearson et al., 2003), as these values are likely a sign of infection. In addition, 96 participants were excluded due to missing data psychosocial scales (N=80), metabolic syndrome components (N=8) or demographic variables (N=8). Compared to participants excluded due to missing data, participants retained in the analytical sample were comparable in terms of age, gender and education. However, excluded participants were significantly more likely to be Caucasian (prace=<0.001) and to identify as Hispanic Latino (pethnicity=0.020). Further, we compared our analytical sample with a larger sample that included MIDUSII national survey sample participants along with participants in the Milwaukee African American study. Our sample was comparable to this greater sample in terms of age, gender, race and ethnicity. Participants in our analytical sample, however, we more likely to be educated than those in the greater survey sample.
2.2. Measures
Depressive symptoms
Indicators used to derive latent profiles included subscales of three well-validated measures of depressive symptoms and/or sleep disturbances: the Mood and Anxiety Symptom Questionnaire (MASQ), the Center for Epidemiologic Studies Depression Scale (CES-D) and the Pittsburgh Sleep Quality Index (PSQI). The MASQ is a measure of symptoms of mood and anxiety disorders and has been well validated in healthy and clinical samples (Watson et al., 1995). Three subscales of the MASQ were used as indicators of depressive symptoms: the General Distress-Depressive symptoms, the Loss of Interest, and the Positive affect subscales. Higher scores on each subscale were reflective of higher depressive symptoms, loss of interest and positive affect, respectively. The CES-D is a widely utilized measure of depression (Radloff, 1977). A recent meta-analysis of the factor structure of the CES-D found a clear four-factor solution that distinguished somatic, negative affect, positive affect, and interpersonal symptoms (Shafer, 2006). Results indicate that items load robustly into one of the four factors. Accordingly, and consistent with previous studies that used a subscale approach to the CES-D (Leventhal et al., 2008), we computed subscale scores for each dimension by summing their respective items. Three subscales of the CES-D: Negative Affect, Somatic Features, and Interpersonal Disturbances were used as indicators in depressive symptom profiles. Higher scores on each subscale were indicative of greater depressive symptomatology. Finally, the PTSQI is a widely used instrument for the evaluation of sleep disturbances which consists of seven component scores that are aggregated in a global score with a range of 0–21 (Buysse et al., 1989a). Higher scores are indicative of greater psychopathology. The global score of the PSQI was used as an indicator of sleep-related complaints.
Metabolic syndrome
The Joint Interim Statement criteria were used to define metabolic syndrome (Alberti et al., 2009). Accordingly, participants were classified as having the metabolic syndrome if they met three or more of the following criteria: 1) waist circumference ≥102 cm in men and ≥88 cm in women; 2) triglycerides ≥150 mg/dl; 3) high-density lipoprotein (HDL) cholesterol <40 mg/dl in men and <50 mg/dl in women; 4) blood pressure ≥130 mm Hg systolic and/or ≥85 mm Hg diastolic and/or on medication; 5) fasting glucose ≥100 mg/dl and/or on medication. Waist circumference was measured at the narrowest point between the ribs and iliac crest. Blood pressure was measured after participants rested for 5 minutes. Three consecutive assessments in a seated position with a 30-second interval between each assessment were recorded, and the two most similar readings were averaged. Lipids and glucose were assessed from a fasting morning blood samples with automated instruments from Roche Diagnostics, Indianapolis, IN.
Obesity
Participant height and weight collected during the visit were used to calculate body mass index (BMI). Obesity was defined as a BMI of 30 km/m2 or higher.
Inflammation
Levels of CRP were used as markers of inflammation and were determined via immunoassays. CRP was measured by BNII nephelometer (Dade Behring Inc., Deerfield, IL).
Covariates
Participant age, gender, race, ethnicity, education, employment, marital status, antidepressant use, presence of chronic conditions and health behavior information were collected via clinical questionnaires and were included in regression models as covariates. These variables have been associated with the presence of cardio-metabolic conditions such as metabolic syndrome and obesity (Park et al., 2003), depressive symptoms (Djernes, 2006) and inflammation. The variable assessing presence of chronic conditions was computed using information from the medical history performed during the clinical visit. Participants were categorized as having a chronic condition if they reported having been diagnosed with heart disease, transient ischemic attack (TIA) or stroke, diabetes, asthma, chronic obstructive pulmonary disease, cancer or liver disease by a physician. Health behaviors included current smoking, drinking (at least one alcoholic beverage during the past month) as well self-reported regular physical exercise which was defined as exercising at least 20 minutes 3 times per week. Categorical variables including race (1=Non-Caucasian), ethnicity (1=Hispanic/Latino), education (1= college graduate or higher), employment (1=currently working [includes part-time workers]) and marital status (1=married) were dichotomized for regression analysis.
2.3. Statistical Analysis
Preliminary statistical analyses included descriptive statistics and assessment of normality distributions. Triglyceride, fasting glucose and C-reactive protein levels were log-transformed as they were found to have a non-normal distribution. Data for continuous variables are presented as means and standard deviations and were compared between groups using independent t-tests. Categorical variables are presented as percentages and were compared with the chi-squared test. The Statistical Package for the Social Sciences (SPSS) version 23.0 (SPSS, Chicago, IL, USA) was used for all preliminary analyses.
Latent Profile Analysis (LPA) was used to identify depressive symptom profiles. LPA is an empirically driven approach, which uses continuous variables (indicators) to derive subgroups of individuals. Patterns of interrelationships among individuals are examined with the goal of maximizing homogeneity within class (or subgroup) and heterogeneity between classes. LPA is an individual based approach given its emphasis on identifying similarities between individuals, rather than associations among variables (variable-based approach). Continuous indicators used in these analyses to characterize symptom profiles included: (1) the MASQ Depressive Symptom subscale, (2) the MASQ Loss of Interest subscale, (3) the MASQ Positive Affect subscale, (4) the CES-D Negative Affect subscale, (5) the CES-D Somatic Features subscale, (6) the CES-D Interpersonal Disturbance subscale, and the (7) PSQI Global Score. The optimal number of subgroups was determined after examination of the following fit indexes: the Akaike information criteria (AIC), the Bayesian information criteria (BIC), the sample-size adjusted BIC (ABIC), log-likelihood (LL), entropy, the Lo-Mendell-Rubin adjusted likelihood ratio test (LRT), and the parametric bootstrapped likelihood ratio test (BLRT). Better fitting models are determined by smaller AIC, BIC, ABID and LL values. Similarly, entropy values closer to 1.0 indicate better fit, with values over 0.80 being considered noteworthy (Roesch et al., 2010). The LRT and the BLRP provide a p-value for each solution indicating that a model with one less class is rejected in favor of the estimated model. Once subgroups were identified, age, gender, race and antidepressant use were included as covariates of emerging profiles. Mplus version 6.0 was used for all LPA analyses.
Profile membership was used as independent variables to examine the association between depressive symptom subgroup and cardio-metabolic outcomes. Logistic regression models were used to examine the association between depressive symptom profiles and dichotomous outcomes (metabolic syndrome and obesity). Control variables included age, gender, education, employment status, marital status, race, and ethnicity. Further adjustment for health behaviors including current smoking, drinking, self-reported exercises, and inflammation was conducted. All tests were two-sided and α<0.05 was considered to be statistically significant. SPSS version 23.0 was used for all logistic regression analyses.
3. Results
3.1. Descriptive Statistics
Our sample was comprised of 1085 individuals, 605 women (55.80%) and 280 men (44.20%). Approximately 81.6% of individuals were of Caucasian decent, whereas 14.70%, 1.10%, 0.20% and 2.20% identified as African American, Native American, Asian, and other race, respectively. Further, 3.00% of the sample was of Hispanic/Latino ethnicity. Mean age was 54.71 (SD=11.81) and 43.40% of the sample were college graduates. Metabolic syndrome was present in 40.30% of the sample, whereas obesity was present in 39.70%. Detailed descriptive characteristics of the study sample are presented in Table 1.
Table 1.
Descriptive characteristics of the study sample
| All (n=1085) M (SD)/ Median (IQR) |
Women (605) M (SD)/ Median (IQR) |
Men (480) M (SD)/ Median (IQR) |
p- value |
|
|---|---|---|---|---|
| Demographic | ||||
| Age, years | 54.71 (11.81) | 54.31 (11.63) | 55.22 (12.02) | 0.207 |
| Education, % College Graduates | 43.40 | 39.80 | 47.90 | 0.008 |
| Work Status, % Employed | 54.70 | 51.60 | 58.50 | |
| Marital Status, % Married | 65.90 | 58.20 | 75.60 | <0.001 |
| Race, % | 0.149 | |||
| Caucasian | 81.80 | 79.50 | 84.60 | |
| African American | 14.70 | 17.20 | 11.70 | |
| Native American | 1.10 | 1.20 | 1.00 | |
| Asians | 0.20 | 0.20 | 0.20 | |
| Other | 2.20 | 2.00 | 2.50 | |
| Ethnicity, % Hispanic/Latino | 3.00 | 3.30 | 2.70 | 0.569 |
| Psychosocial | ||||
| MASQ, Depression | 18.50 (6.54) | 18.92 (6.76) | 17.97 (6.22) | 0.018 |
| MASQ, Loss of Interest | 11.96 (4.07) | 12.11 (4.25) | 11.79 (3.82) | 0.202 |
| MASQ, Positive Affect | 44.67 (10.12) | 45.19 (10.06) | 44.01 (10.15) | 0.057 |
| CES-D, Total Score | 8.44 (8.01) | 8.76 (8.18) | 8.04 (7.80) | 0.145 |
| CES-D, Negative Affect | 1.90 (3.10) | 2.13 (3.26) | 1.61 (2.87) | 0.005 |
| CES-D, Interpersonal Disturbance | 0.41 (0.84) | 0.40 (0.80) | 0.43 (0.88) | 0.556 |
| CES-D, Somatic Features | 3.50 (3.17) | 3.64 (3.32) | 3.32 (2.97) | 0.105 |
| PSQI, Global score | 6.08 (3.53) | 6.49 (3.68) | 5.57 (3.27) | <0.001 |
| Antidepressant Use, % | 13.50 | 16.50 | 9.80 | 0.001 |
| Health Behaviors | ||||
| Regular Exercise, % | 78.20 | 77.50 | 79.20 | 0.514 |
| Current Smoking, % | 14.30 | 13.10 | 15.80 | 0.194 |
| Drinker, % | 65.80 | 71.00 | 61.70 | 0.001 |
| Medical | ||||
| Metabolic Syndrome, % | 40.30 | 34.80 | 46.40 | <0.001 |
| Obesity, % | 39.70 | 38.80 | 39.90 | 0.506 |
| Presence of Chronic Conditions, % | 41.00 | 41.30 | 40.60 | 0.824 |
| Waist Circumference, cm | 97.07 (16.82) | 91.49 (15.34) | 104.10 (15.96) | <0.001 |
| Systolic Blood Pressure, mmHg | 131.28 (18.00) | 129.82 (19.65) | 133.11 (15.490 | 0.003 |
| Diastolic Blood Pressure, mmHg | 75.55 (10.66) | 73.29 (10.65) | 78.41 (9.97) | <0.001 |
| Triglycerides, ng/dL€ | 106.00 (77.00–156.00) | 98.00 (72.00–139.50) | 123.00 (84–181.00) | <0.001 |
| HDL-Cholesterol, mg/dL€ | 53.00 (43.00–66.00) | 59.00 (48.00–72.00) | 45.00 (37.00–55.00) | <0.001 |
| Fasting Glucose, mg/dL€ | 96.00 (90.00–104.00) | 95.00 (88.00–102.00) | 98.00 (92.00–107.00) | <0.001 |
| Inflammatory Markers | ||||
| C-reactive Protein, ug/mL€ | 1.33 (0.68–3.10) | 1.59 (0.75–3.70) | 1.13 (0.59–2.43) | <0.001 |
MASQ=Mood and Anxiety Symptom Questionnaire, CES-D=Center for Epidemiological Studies Depression Scale, PSQI=Pittsburgh Sleep Quality Inventory; HDL=High density lipoprotein cholesterol;
values presented are medians and 25%–75% Interquartile Ranges).
3.2. Characterization of Depressive Symptom Profiles
LPA was used to derive depressive symptom profiles. Multiple LPA models were fitted with the number of subgroups (or clusters) ranging from 1 to 8. Fit indexes for all models are presented in Table 2. Entropy values ranged between 0.888 and 0.978 indicating excellent fit to the data across all cluster solutions. The BLRT was significant across comparisons of greater number of profiles, which suggests that a greater number of subgroups fit the data progressively better. The LRT indicated that a 2-class solution was significantly better than a 1-class solution (p<0.001) and a 4-class solution was significantly better than a 3-class solution (p=0.003). The AIC, BIC, aBIC and LL values decreased as the number of classes increased; however, only small decreases were noted in these indexes (<300) for the 5, 6, 7 and 8-cluster solutions. Further, the proportion of individuals belonging to each cluster pronouncedly declined as the number of classes increased. After collectively accounting for model fit indexes, the size of each cluster, and theoretical considerations, the 4-cluster solution was selected as best representing the data.
Table 2.
Fit indexes for latent profile analysis
| No. of clusters | No. of parameters | AIC | BIC | aBIC | LL | Entropy | ALRT (p) | BLRT (p) |
|---|---|---|---|---|---|---|---|---|
| 1 | 14 | 40998.513 | 41068.364 | 41023.897 | −20485.257 | - | - | - |
| 2 | 22 | 37832.411 | 37942.177 | 37872.300 | −18894.206 | 0.978 | <0.001* | <0.001* |
| 3 | 30 | 36942.717 | 37092.397 | 36997.111 | −18441.358 | 0.922 | 0.482 | <0.001* |
|
| ||||||||
| 4 | 38 | 36282.364 | 36471.959 | 36351.262 | −18103.182 | 0.897 | 0.007* | <0.001* |
|
| ||||||||
| 5 | 46 | 36005.325 | 36234.834 | 36088.728 | −17956.662 | 0.888 | 0.288 | <0.001* |
| 6 | 54 | 35809.824 | 36079.248 | 35907.732 | −17850.912 | 0.915 | 0.508 | <0.001* |
| 7 | 62 | 35627.192 | 35936.531 | 35739.606 | −17751.596 | 0.923 | 0.434 | <0.001* |
| 8 | 70 | 35414.576 | 35763.829 | 35541.494 | −17637.288 | 0.915 | 0.415 | <0.001* |
p<0.05;
AIC=Akaike information criterion; BIC=Bayesian information criterion; aBIC=Adjusted BIC; LL=log-likelihood; ALRT=Lo-Medell-Rubin Adjusted likelihood ratio test; BLRT= Bootstrapped likelihood ratio test.
The four latent profiles identified were labeled “No Symptoms”, “Mild Symptoms”, “Moderate Symptoms” and “Acute Symptoms” subgroups based on the presentation and severity of symptoms. As expected of a healthy sample of individuals, the “No Symptoms” subgroup was the largest comprising of 658 (60.65%) individuals, followed by the “Mild Symptoms”, “Moderate Symptoms”, and “Acute Symptoms” subgroups with 290 (26.73%), 112 (10.32%) and 25 (2.30%) individuals, respectively.
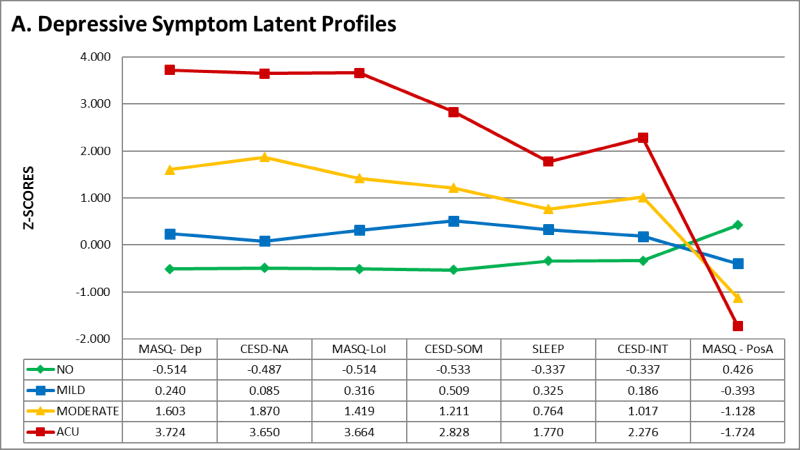
Mean and standard deviations for all indicators across subgroups are presented in Table 3. The graphical representation of the four symptom profiles is shown in Figure 1, which depicts z-scores for each indicator across subgroups. As seen on the figure, the “No Symptoms” subgroup has the lowest scores in subscales measuring negative affect (MASQ Depressive symptoms and CES-D Negative affect subscales), loss of interest (MASQ Loss of Interest subscale), somatic symptoms (CES-D Somatic Features subscale and PSQI Global Score) and interpersonal difficulties (CES-D Interpersonal Disturbance subscale), as well as the highest scores on measures of positive affect (MASQ Positive Affect subscale). The “Mild Symptoms” subgroup was characterized by overall lower scores across indicators, with subscales measuring somatic symptoms being the highest within group. Further, the “Moderate Symptoms” subgroup had higher scores across subscales (1 SD above the mean), with negative affect/loss of interest being the highest within group. Finally, the “Acute symptoms” subgroup was characterized by the highest overall scores (1.5–3.7 SD above the mean) on all indicators, which may be representative of a very severe/acute depressive state.
Table 3.
Indicators and unadjusted predictors/outcomes by symptom profile
| No Symptoms Subgroup n=658 (60.65%) M (SD)/ Median (IQ) |
Mild Symptoms Subgroup 290 (26.73%) M (SD)/ Median (IQ) |
Moderate Symptoms Subgroup 112 (10.32%) M (SD)/ Median (IQ) |
Acute Symptoms Subgroup 25 (2.30%) M (SD)/ Median (IQ) |
|
|---|---|---|---|---|
| Indicators | ||||
| MASQ- Depression | 15.186 (3.591) | 20.050 (7.374) | 28.855 (7.567) | 42.546 (8.690) |
| CESD-Negative Affect | 0.389 (0.975) | 2.164 (3.661) | 7.695 (4.022) | 13.211 (3.515) |
| MASQ-Loss of Interest | 9.878 (2.539) | 13.251 (4.513) | 17.730 (4.530) | 26.854 (5.400) |
| CESD-Somatic Features | 1.807 (2.873) | 5.113 (3.372) | 7.338 (3.662) | 12.466 (4.705) |
| PSQI- Global Score | 4.890 (3.463) | 7.226 (4.070) | 8.777 (3.969) | 12.329 (4.550) |
| CESD- Int. Disturbance | 0.130 (0.462) | 0.568 (1.107) | 1.263 (1.344) | 2.316 (1.725) |
| MASQ - Positive Affect | 48.976 (9.696) | 40.694 (12.278) | 33.263 (9.123) | 27.229 (6.500) |
| Depression Caseness | ||||
| CES-D Score > 16, % | 0.02 | 6.20 | 87.50 | 100.00 |
| Predictors | ||||
| Age, years | 55.96 (11.58) | 54.11 (12.50) | 50.42 (10.51) | 48.12 (7.37) |
| Gender, female % | 53.60 | 57.90 | 60.70 | 64.00 |
| Race, % Caucasian | 84.70 | 81.00 | 70.50 | 64.00 |
| Antidepressant use, % | 9.60 | 16.20 | 24.10 | 40.00 |
| Outcomes | ||||
| Metabolic Syndrome, % | 37.50 | 42.80 | 47.30 | 54.2 |
| Obesity, % | 35.70 | 44.50 | 49.10 | 48.00 |
| Waist Circumference, cm | 96.11 (15.91) | 98.67 (19.44) | 98.11 (14.52) | 98.83 (16.17) |
| Systolic Blood Pressure, mmHg | 132.29 (18.16) | 130.82 (18.23) | 128.49 (15.85) | 122.32 (16.87) |
| Diastolic Blood Pressure, mmHg | 75.73 (10.42) | 75.34 (10.94) | 75.54 (11.52) | 73.52 (10.06) |
| Triglycerides, ng/dL€ | 102.00 (74.00–152.25) | 114.50 (83.00–156.25) | 110.50 (80.00–183.50) | 120.00 (84.00–210.50) |
| HDL-Cholesterol, mg/dL€ | 54.00 (44.00–67.00) | 52.00 (41.00–62.00) | 48.50 (41.00–60.75) | 51.00 (40.50–63.50) |
| Fasting Glucose, mg/dL€ | 95.00 (90.00–104.00) | 96.00 (90.00–104.25) | 98.50 (90.25–106.00) | 94.00 (87.00–100.50) |
MASQ=Mood and Anxiety Symptom Questionnaire, CES-D=Center for Epidemiological Studies Depression Scale, PSQI=Pittsburgh Sleep Quality Inventory; HDL=High density lipoprotein cholesterol;
values presented are medians and 25%-75% Interquartile Ranges).
Figure 1.
Graphical repressive of depressive symptoms latent profiles. Z-scores of indicators are plotted by subgroup.
The inclusion of covariates to the model (age, gender, race and antidepressant use) did not alter the means across profiles, which further confirms the stability of the 4-cluster solution. Gender was not a significant predictor of group membership, whereas age significantly predicted membership to the “Mild Symptoms” (OR=0.984, p=0.042) and “Moderate Symptoms” (OR=0.956, p<0.001) and “Acute Symptoms” (OR=0.938, p=0.001) subgroups when compared to the “No/Low Symptoms” subgroup. This indicates that for every one-year increase in age, there is a 1.6%, 4.4% and 6.2% reduction in the odds of belonging to the “Mild Symptoms”, “Moderate Symptoms” or “Acute Symptoms” subgroups, respectively, when compared to the “No Symptoms” subgroup. Similarly, race significantly predicted group membership with Non-Caucasian individuals (African American, Native American or Asian) participants being most likely to belong to the “Moderate Symptoms” (OR=2.462, p<0.001) and “Acute Symptoms” subgroups (OR=3.684, p=0.011). Finally, antidepressant use was also a significant predictor of group membership. After controlling for age and gender, individuals taking antidepressants were significantly more likely to belong to the “Mild Symptoms” (OR=2.046 p=0.003), “Moderate Symptoms” (OR=3.691, p<0.001) or “Acute Symptoms” (OR=8.872, p<0.001) subgroup, when compared to the “No Symptoms” subgroup.
3.3. Association with Cardio-metabolic Outcomes
Logistic regression models were fitted to examine the cross-sectional associations between subgroups (or clusters) and cardio-metabolic outcomes, including metabolic syndrome and obesity. Results from all multivariate models are presented in Table 4. The “No Symptoms” subgroup was used as the reference group in all analyses. After controlling for age, gender, education, employment status, marital status, race, ethnicity, presence of chronic conditions, and antidepressant use, there was a significant relationship between membership to the “Moderate Symptoms” subgroup (OR=1.571, p=0.039) and the presence of metabolic syndrome. This indicates that individuals that belong to the “Moderate Symptoms” subgroup have a 57.1% increase in their odds of having metabolic syndrome, when compared to individuals in the “No Symptoms” subgroup. Associations between depressive symptom subgroups and metabolic syndrome components are presented in Supplementary Table 1. Briefly, these additional analyses indicated that the association between the “Moderate Symptoms” subgroup is primarily driven by triglycerides and HDL-cholesterol levels. In regards to obesity, a significant association was found between the “Mild Symptoms” subgroup and obesity (OR=1.359, p=0.040). Similarly, a trend was found for the “Moderate Symptoms” subgroup (OR=1.491, p=0.066). No association was found between the membership to the “Acute Symptoms” subgroup and cardio-metabolic outcomes.
Table 4.
Associations between subgroup membership and cardio-metabolic outcomes
| Metabolic Syndrome | Obesity | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Model 1 | ||||||
| Age | 1.005 | 0.993–1.018 | 0.384 | 0.995 | 0.983–1.007 | 0.413 |
| Gender | 0.588 | 0.454–0.762 | <0.001* | 0.825 | 0.637–1.067 | 0.143 |
| Education | 0.646 | 0.498–0.839 | 0.001* | 0.722 | 0.557–0.936 | 0.014 |
| Employment | 1.061 | 0.803–1.402 | 0.679 | 1.163 | 0.881–1.535 | 0.287 |
| Marital Status | 1.210 | 0.909–1.611 | 0.191 | 0.992 | 0.748–1.315 | 0.954 |
| Race | 1.349 | 0.948–1.92 | 0.096 | 2.111 | 1.492–2.986 | <0.001* |
| Ethnicity | 0.742 | 0.342–1.608 | 0.449 | 0.911 | 0.432–1.92 | 0.806 |
| Presence of Chronic Conditions | 1.517 | 1.166–1.973 | 0.002* | 1.151 | 0.884–1.498 | 0.296 |
| Antidepressant Use | 1.573 | 1.087–2.276 | 0.016* | 1.445 | 1.002–2.086 | 0.049* |
| Model 2¥ | ||||||
| Mild Symptoms Subgroup | 1.240 | 0.923–1.665 | 0.154 | 1.359 | 1.015–1.821 | 0.040* |
| Moderate Symptoms Subgroup | 1.571 | 1.023–2.413 | 0.039* | 1.491 | 0.973–2.283 | 0.066 |
| Acute Symptoms Subgroup | 1.752 | 0.750–4.093 | 0.195 | 1.297 | 0.56–3.004 | 0.544 |
| Model 3¥ | ||||||
| Regular Exercise | 0.606 | 0.445–0.824 | <0.001* | 0.541 | 0.399–0.735 | <0.001* |
| Current Smoking | 0.863 | 0.587–1.268 | 0.453 | 0.551 | 0.371–0.82 | 0.003* |
| Drinker (past month) | 0.723 | 0.552–0.946 | 0.018* | 0.890 | 0.68–1.166 | 0.398 |
| Mild Symptoms Subgroup | 1.225 | 0.909–1.65 | 0.182 | 1.345 | 1–1.808 | 0.050 |
| Moderate Symptoms Subgroup | 1.595 | 1.032–2.463 | 0.035* | 1.555 | 1.008–2.4 | 0.046* |
| Acute Symptoms Subgroup | 1.653 | 0.697–3.923 | 0.254 | 1.280 | 0.539–3.038 | 0.576 |
| Model 3¥,€ | ||||||
| C-reactive Protein | 1.780 | 1.551–2.043 | <0.001* | 2.080 | 1.801–2.402 | <0.001* |
| Mild Symptoms Subgroup | 1.218 | 0.895–1.657 | 0.210 | 1.347 | 0.986–1.842 | 0.061 |
| Moderate Symptoms Subgroup | 1.400 | 0.891–2.201 | 0.144 | 1.325 | 0.835–2.103 | 0.232 |
| Acute Symptoms Subgroup | 1.963 | 0.791–4.873 | 0.146 | 1.551 | 0.6–4.008 | 0.365 |
p<0.05;
OR=Odds Ratio; Low/No Symptoms Subgroup is reference group;
controlled for age, gender, education, employment, marital status, race, ethnicity, antidepressant use and presence of chronic conditions;
controlled for health behaviors.
3.4. The Role of Health Behaviors and Inflammation
Further analyses were conducted in order to control for health behavior variables including regular physical exercise, current smoking, and drinking (in the past month) as well as inflammation, measured by C-reactive protein (Tables 4, Models 3 and 4). After controlling for health behaviors, the association between the “Moderate Symptoms” subgroup and metabolic syndrome remained significant (OR=1.595, p=0.035). Further, the association between the “Mild Symptoms” subgroup and obesity became slightly attenuated (OR=1.345, p=0.050) while the association for the “Moderate Symptoms” subgroup was strengthened (OR=1.555, p=0.046). Finally, adjustment for CRP attenuated all association between depressive symptom profiles and cardio-metabolic conditions.
4. Discussion
In this paper, we used a sample of non-institutionalized adults to identify empirically derived depressive symptom profiles based on continuous measures of negative affect, loss of interest, somatic complaints, sleep disturbances, interpersonal difficulties and positive affect: a “No Symptoms” a “Mild Symptoms”, a “Moderate Symptoms” and an “Acute Symptoms” subgroup. In addition, we provided novel data on the differential associations between depressive symptom profiles and cardio-metabolic outcomes, and examine the role of health behaviors and inflammation in these associations. Our results indicated that the “Moderate Symptoms” subgroup was significantly associated with metabolic syndrome and both the “Mild Symptoms” (trend) and “Moderate Symptoms” subgroups were associated with obesity after controlling for demographic factors, anti-depressant use, presence of chronic conditions and health behaviors. Inflammation attenuated the association between these subgroups and cardio-metabolic outcomes suggesting inflammation may be a biological mediator linking depressive symptoms profiles and cardio-metabolic outcomes.
Given depressive symptoms are highly heterogeneous; investigators have for long focused on gaining a better understanding of their presentation. In fact, a seminal paper published in 1966 aimed at classifying patients in distinct depression subtypes and examining their differential response to drugs (Overall et al., 1966). Similarly, the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) included several diagnostic categories for distinct depressive disorders (Association, 2013b). This nosology, however, is used to classify individuals with significant symptoms or clinical depression. Far fewer efforts have been devoted to identifying depressive symptoms profiles at subclinical levels of depression or in the general population. This study fills this gap in the literature.
Self-report scales may help characterize profiles as they provide valuable information on the nature of symptoms (e.g. cognitive vs. somatic) as well as their severity (e.g. higher scores are typically characteristic of more severe depression). In this study, we used several depressive symptom subscales to create comprehensive profiles of symptoms. In addition to subscales measuring negative affect, loss of interest and somatic complaints, this is the first study to incorporate subscales specifically measuring positive affect, interpersonal difficulties and sleep complaints into one comprehensive profile. Low positive affect and sleep disturbances, in particular, have strong associations with coronary heart disease (Boehm et al., 2011); therefore, studying positive affect and sleep in the context of cardio-metabolic health is important.
Integrating scores on multiple self-report scales measuring distinct dimensions of symptoms, however, is a challenge. Simply adding or averaging scores may result in the loss of individual differences and hampers investigators’ ability to recognize response patterns. Investigators themselves may choose to classify individuals based on scores across scales, but this may introduce bias and requires high interrater reliability (concordance across raters). In this study, we overcome these challenges by using a state-of-the-art statistical modeling approach, LPA. The primary strength of LPA is that it uses continuous observed variables, in our case depression subscales, to classify individuals into subgroups that consist of comparable individuals (homogeneity within groups), while still ensuring these groups are distinct from each other (heterogeneity across groups) (Collins and Lanza, 2013).
In this study we identified four depressive symptom profiles. Individuals in three of these subgroups endorsed at least mild symptoms of depression. The “Mild Symptoms” subgroup had overall low symptoms across scales but higher within group scores on subscales measuring somatic symptoms (CES-D Somatic Complaints and PSQI). It is worth noting that participants in this group did not endorse somatic symptoms only, but rather had slightly higher scores among somatic items than they do on items measuring negative affect, positive affect or interpersonal disturbances. Historically, investigators argued that the differential associations between somatic depressive symptoms and health outcomes are a result of the overlap between somatic depressive symptoms and symptoms of medical illness in spite of rigorous methodological designs that controlled for illness severity (Silverstein and Patel, 2011). Similarly, our study controlled for the presence of chronic conditions such as heart disease, TIA or stroke, diabetes, asthma, chronic obstructive pulmonary disease, cancer and liver disease, however, the presence of this conditions were assessed using participant’s self-report of a physical diagnosis. The characteristics of this profile in addition to the high proportion of individuals belonging to this subgroup (26.73%) highlight the need for tailored interventions. Previous reports indicate that individuals with predominantly somatic symptomatology have poor responses to antidepressant medication (Silverstein and Patel, 2011). Further, the mean scores on the PSQI within this profile was in the clinical range (<5) (Buysse et al., 1989b) indicating that incorporating intervention components to address sleep disturbances (e.g. cognitive behavioral therapy for insomnia) may be beneficial. Future intervention studies are needed to examine whether tailored interventions result in decreases in symptoms among members of this subgroup.
We identified two other subgroups in which individuals endorsed primarily cognitive symptoms at different degrees of severity. The “Moderate Symptoms” subgroup was characterized by primarily high scores across all subscales with those measuring negative affect and loss of interest being the highest within group. Scores within this subgroup were within a clinical range across subscales, and individuals within this subgroup were the more likely to be under anti-depressant treatment. The “Acute Symptoms” profile had a similar presentation but at a greater degree of severity. In fact, individuals within this subgroup had the highest overall mean scores across subscales, most of which were at least 2 or 3 standard deviations above the overall mean. Due to the severity of the symptoms, it is possible that individuals in this subgroup were undergoing a major depressive episode at time of assessment, or that they had recently experienced a highly stressful event. This subgroup was also most likely to be on anti-depressant treatment and the youngest across all subgroups with an unadjusted mean age on 48 years. Interestingly, Non-Caucasian individuals (those of African American, Native American or Asian descent) were also most likely to belong to the “Moderate” or “Acute Symptoms” subgroup. Given the primarily cognitive nature of symptoms within these two subgroups, it is possible that individuals among these subgroups would respond well to interventions targeting negative cognitions. Interventions addressing negative thoughts and their impact on emotions and behaviors, such as cognitive behavioral therapy for depression may prove beneficial for individuals in this subgroup. Future research should examine whether tailored interventions with primarily cognitive components result in a reduction of symptoms among members of these subgroups.
Our results indicate that only two of the four depressive symptom profiles identified result in increased cardio-metabolic risk. It is worth noting that while the “Acute Symptoms” subgroup was not significantly associated with greater risk for cardio-metabolic conditions, the odds ratios for these groups were comparable to those of the “Mild” and “Moderate Symptoms” subgroups, particularly in the case of metabolic syndrome. However, due to the size of this group, confidence intervals were rather large, and therefore no significant associations were detected. No study, to date, has examined the association between depressive symptom profiles, yet other studies have reported on differential associations between somatic and cognitive symptoms of depression and cardio-metabolic outcomes (Carney and Freedland, 2012; Luppino et al., 2011). While comparability with other studies is a challenge, the presence of somatic depressive symptoms, even at subclinical levels have been associated with greater risk of both metabolic syndrome (Luppino et al., 2011) and obesity (Marijnissen et al., 2011). Similarly, a diagnosis of depression, which is likely comparable to our “Moderate” and “Acute Symptoms” subgroups was also associated with greater cardio-metabolic risk in other studies (Luppino et al., 2011). Interestingly, studies looking at metabolic syndrome components have also reported independent associations between depression and individual components, particularly lipid levels (van Reedt Dortland et al., 2009) and glucose (Golden et al., 2008). It is worth noting that in our study, the association between the “Moderate Symptom” subgroup and metabolic syndrome was primarily driven by lipid levels (triglycerides and HDL-cholesterol).
Various efforts have been devoted to identifying subtypes of major depressive disorder (MDD) which are more closely linked to cardio-metabolic conditions (Lamers et al., 2010; Lasserre et al., 2014). For example, using a population based cohort in Switzerland investigators found that only participants with the atypical subtype of MDD had greater prevalence and incidence of obesity (Lasserre et al., 2014). Another study used a similar methodology to the one used in this study to derive classes of symptoms among patients with MDD and found that a severe atypical symptoms class was associated with higher body mass index and metabolic syndrome (Lamers et al., 2010). Future research should aim at validating the presence of these and other depressive symptom profiles, and further elucidate the differential associations with cardio-metabolic risk.
Inflammation attenuated the association between depressive symptoms profiles and cardio-metabolic outcomes. These results suggest that inflammation is likely an important biological pathway linking depression to cardio-metabolic risk. In line with these findings are other reports on a mediating role of inflammatory markers and cardiovascular risk (Stewart et al., 2009). Inflammation, however, is likely not the only biological outcome linking these two conditions. Other candidate biological pathways in this relationship include sympathetic activation (Carney et al., 2000), pro-coagulant factors (Strike and Steptoe, 2004), endothelial dysfunction (Yasunari et al., 2006), and adipokines secreted by adipose tissue such as leptin (Chirinos et al., 2013). Behavioral factors may also play an important role. Previous work has linked depressive symptoms to unhealthy behaviors that promote weight gain, such as increased fat intake and decreased physical activity (Raikkonen et al., 2007). Furthermore, depressive symptoms have been associated with smoking (Brummett et al., 2003), a known predictor of cardiovascular disease endpoints (Lorenz et al., 2007). Of note is the fact that our study controlled for self-reported physical activity, smoking as well as drinking (in the past month). Inclusion of this covariates strengthened the association between symptom profiles and cardio-metabolic conditions.
Important strengths of this study include its large sample size which increases the power to detect associations among variables. Further, this study used rigorous methodology and state-of-the-art statistical analysis. The present study is limited by the cross sectional research design. As a result, causality between depressive symptom profiles and cardio-metabolic outcomes cannot be determined; however, available theoretical and empirical evidence suggests that depressive symptoms are prospective predictors of cardio-metabolic outcomes (Pan et al., 2011). Findings from the present study extend this literature by demonstrating the importance of considering depressive symptom profiles. The predominantly Caucasian sample limits the generalizability of the findings to more diverse populations. Although evaluation of inflammation as a mechanism linking depressive symptom profiles and cardio-metabolic outcomes is a clear strength of the present study, other potential mechanisms (e.g., pro-coagulant factors, endothelial dysfunction, adipokines) were not evaluated.
5. Conclusions
Given depressive symptoms are heterogeneous; investigators have historically focused on understanding their presentation. This is the first study to identify empirically-derived depressive symptom profiles and link them to cardio-metabolic outcomes. Future work should examine differential pathways to increased risk among across depressive symptom profiles and examine whether tailored interventions have an impact on cardio-metabolic risk.
Supplementary Material
Highlights.
Depressive symptoms can be highly heterogeneous.
We identified empirically derived depressive symptom profiles.
Four subgroups were identified.
The Mild and Moderate Symptoms subgroups have higher cardiometabolic risk.
Inflammation attenuated these associations.
Acknowledgments
This research was supported by a grant from the National Institute on Aging (P01-AG020166) to conduct a longitudinal follow-up of the MIDUS (Midlife in the U.S.) investigation. The original study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development.
We thank the staff of the Clinical Research Centers at the University of Wisconsin-Madison, UCLA, and Georgetown University for their support in conducting this study. Supported by the following grants M01-RR023942 (Georgetown), M01-RR00865 (UCLA) from the General Clinical Research Centers Program and 1UL1RR025011 (UW) from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health.
Authors of this manuscript were supported by the National Heart, Lung, and Blood Institute (1R01HL127260-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors of this manuscript have no conflict of interest to disclose.
References
- Akiskal HS, Judd LL, Gillin JC, Lemmi H. Subthreshold depressions: clinical and polysomnographic validation of dysthymic, residual and masked forms. Journal of affective disorders. 1997;45:53–63. doi: 10.1016/s0165-0327(97)00059-1. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr International Diabetes Federation Task Force on, E., Prevention, Hational Heart, L., Blood, I., American Heart, A., World Heart, F., International Atherosclerosis, S., International Association for the Study of, O. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and statistical manual of mental disorders (DSM-5®) American Psychiatric Pub. 2013a doi: 10.1590/s2317-17822013000200017. [DOI] [PubMed] [Google Scholar]
- Association AP. DSM 5. American Psychiatric Association 2013b [Google Scholar]
- Boehm JK, Peterson C, Kivimaki M, Kubzansky LD. Heart health when life is satisfying: evidence from the Whitehall II cohort study. European heart journal. 2011;32:2672–2677. doi: 10.1093/eurheartj/ehr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummett BH, Babyak MA, Siegler IC, Mark DB, Williams RB, Barefoot JC. Effect of smoking and sedentary behavior on the association between depressive symptoms and mortality from coronary heart disease. Am. J. Cardiol. 2003;92:529–532. doi: 10.1016/s0002-9149(03)00719-7. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989a;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Iii, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry research. 1989b;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE. Are somatic symptoms of depression better predictors of cardiac events than cognitive symptoms in coronary heart disease? Psychosomatic medicine. 2012;74:33–38. doi: 10.1097/PSY.0b013e3182405ac4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Stein PK. Anxiety, depression, and heart rate variability. Psychosom Med. 2000;62:84–87. doi: 10.1097/00006842-200001000-00013. [DOI] [PubMed] [Google Scholar]
- Chirinos DA, Goldberg R, Gellman M, Mendez AJ, Gutt M, McCalla JR, Llabre MM, Schneiderman N. Leptin and its association with somatic depressive symptoms in patients with the metabolic syndrome. Ann Behav Med. 2013;46:31–39. doi: 10.1007/s12160-013-9479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Lavretsky H, Olmstead R, Levin MJ, Oxman MN, Irwin MR. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. The American journal of psychiatry. 2008;165:1543–1550. doi: 10.1176/appi.ajp.2008.07121882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Lanza ST. Latent class and latent transition analysis: With applications in the social, behavioral, and health sciences. John Wiley & Sons; 2013. [Google Scholar]
- Dienberg Love G, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS national study: protocol, measures, sample, and comparative context. Journal of aging and health. 2010;22:1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djernes JK. Prevalence and predictors of depression in populations of elderly: a review. Acta psychiatrica Scandinavica. 2006;113:372–387. doi: 10.1111/j.1600-0447.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- Doyle F, Conroy R, McGee H, Delaney M. Depressive symptoms in persons with acute coronary syndrome: specific symptom scales and prognosis. Journal of psychosomatic research. 2010;68:121–130. doi: 10.1016/j.jpsychores.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Dozois DJ, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory-II. Psychological assessment. 1998;10:83. [Google Scholar]
- Ford ES. The metabolic syndrome and mortality from cardiovascular disease and all-causes: findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis. 2004;173:309–314. doi: 10.1016/j.atherosclerosis.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. The American journal of medicine. 2006;119:812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Gee DL, Bailey RL. Prevalence of metabolic syndrome (MetS) and hyperglycemia in US adults: NHANES 2003–06 and 2007–10. Faseb J. 2013:27. [Google Scholar]
- Goldbacher EM, Bromberger J, Matthews KA. Lifetime history of major depression predicts the development of the metabolic syndrome in middle-aged women. Psychosomatic medicine. 2009;71:266–272. doi: 10.1097/PSY.0b013e318197a4d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SH, Lazo M, Carnethon M, Bertoni AG, Schreiner PJ, Roux AVD, Lee HB, Lyketsos C. Examining a bidirectional association between depressive symptoms and diabetes. Jama. 2008;299:2751–2759. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoen PW, Conradi HJ, Denollet J, Martens EJ, de Jonge P. Interview-based ratings of somatic and cognitive symptoms of depression and their impact on cardiovascular prognosis. Psychotherapy and psychosomatics. 2010;79:319–320. doi: 10.1159/000319528. [DOI] [PubMed] [Google Scholar]
- Lamers F, de Jonge P, Nolen WA, Smit JH, Zitman FG, Beekman AT, Penninx BW. Identifying depressive subtypes in a large cohort study: results from the Netherlands Study of Depression and Anxiety (NESDA) The Journal of clinical psychiatry. 2010;71:1582–1589. doi: 10.4088/JCP.09m05398blu. [DOI] [PubMed] [Google Scholar]
- Lasserre AM, Glaus J, Vandeleur CL, Marques-Vidal P, Vaucher J, Bastardot F, Waeber G, Vollenweider P, Preisig M. Depression with atypical features and increase in obesity, body mass index, waist circumference, and fat mass: a prospective, population-based study. JAMA Psychiatry. 2014;71:880–888. doi: 10.1001/jamapsychiatry.2014.411. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Ramsey SE, Brown RA, LaChance HR, Kahler CW. Dimensions of depressive symptoms and smoking cessation. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2008;10:507–517. doi: 10.1080/14622200801901971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- Luppino FS, van Reedt Dortland AK, Wardenaar KJ, Bouvy PF, Giltay EJ, Zitman FG, Penninx BW. Symptom dimensions of depression and anxiety and the metabolic syndrome. Psychosomatic medicine. 2011;73:257–264. doi: 10.1097/PSY.0b013e31820a59c0. [DOI] [PubMed] [Google Scholar]
- Marijnissen RM, Bus BA, Holewijn S, Franke B, Purandare N, de Graaf J, den Heijer M, Buitelaar JK, Oude Voshaar RC. Depressive symptom clusters are differentially associated with general and visceral obesity. Journal of the American Geriatrics Society. 2011;59:67–72. doi: 10.1111/j.1532-5415.2010.03228.x. [DOI] [PubMed] [Google Scholar]
- Overall JE, Hollister LE, Johnson M, Pennington V. NOsology of depression and differential response to drugs. Jama. 1966;195:946–948. [PubMed] [Google Scholar]
- Pan A, Keum N, Okereke OI, Sun Q, Kivimaki M, Rubin RR, Hu FB. Bidirectional association between depression and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Diabetes care. 2012;35:1171–1180. doi: 10.2337/dc11-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. Jama. 2011;306:1241–1249. doi: 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y-W, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Archives of internal medicine. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Pedersen SS, Denollet J, Daemen J, van de Sande M, de Jaegere PT, Serruys PW, Erdman RA, van Domburg RT. Fatigue, depressive symptoms, and hopelessness as predictors of adverse clinical events following percutaneous coronary intervention with paclitaxel-eluting stents. Journal of psychosomatic research. 2007;62:455–461. doi: 10.1016/j.jpsychores.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Radler BT, Ryff CD. Who participates? Accounting for longitudinal retention in the MIDUS national study of health and well-being. Journal of aging and health. 2010;22:307–331. doi: 10.1177/0898264309358617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Applied psychological measurement. 1977;1:385–401. [Google Scholar]
- Raikkonen K, Matthews KA, Kuller LH. Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women: a comparison of World Health Organization, Adult Treatment Panel III, and International Diabetes Foundation definitions. Diabetes Care. 2007;30:872–877. doi: 10.2337/dc06-1857. [DOI] [PubMed] [Google Scholar]
- Roesch SC, Villodas M, Villodas F. Latent class/profile analysis in maltreatment research: A commentary on Nooner et al., Pears et al., and looking beyond. Child abuse & neglect. 2010;34:155–160. doi: 10.1016/j.chiabu.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. Journal of clinical psychology. 2006;62:123–146. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Miller AH. Eating ourselves to death (and despair): the contribution of adiposity and inflammation to depression. Progress in neurobiology. 2010;91:275–299. doi: 10.1016/j.pneurobio.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein B, Patel P. Poor response to antidepressant medication of patients with depression accompanied by somatic symptomatology in the STAR*D Study. Psychiatry research. 2011;187:121–124. doi: 10.1016/j.psychres.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proceedings of the National academy of Sciences of the United States of America. 2005;102:6508–6512. doi: 10.1073/pnas.0409174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JC, Janicki DL, Muldoon MF, Sutton-Tyrrell K, Kamarck TW. Negative emotions and 3-year progression of subclinical atherosclerosis. Archives of general psychiatry. 2007;64:225–233. doi: 10.1001/archpsyc.64.2.225. [DOI] [PubMed] [Google Scholar]
- Stewart JC, Rand KL, Muldoon MF, Kamarck TW. A prospective evaluation of the directionality of the depression-inflammation relationship. Brain Behav Immun. 2009;23:936–944. doi: 10.1016/j.bbi.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strike PC, Steptoe A. Psychosocial factors in the development of coronary artery disease. Prog Cardiovasc Dis. 2004;46:337–347. doi: 10.1016/j.pcad.2003.09.001. [DOI] [PubMed] [Google Scholar]
- van Reedt Dortland AK, Giltay EJ, van Veen T, van Pelt J, Zitman FG, Penninx BW. Associations between serum lipids and major depressive disorder: results from the Netherlands Study of Depression and Anxiety (NESDA) The Journal of clinical psychiatry. 2009;71:729–736. doi: 10.4088/JCP.08m04865blu. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Beekman AT, Boelhouwer IG, Bandinelli S, Milaneschi Y, Ferrucci L, Penninx BW. Metabolic depression: a chronic depressive subtype? Findings from the InCHIANTI study of older persons. The Journal of clinical psychiatry. 2011;72:598–604. doi: 10.4088/JCP.10m06559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of abnormal psychology. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Womack VY, De Chavez PJ, Albrecht SS, Durant N, Loucks EB, Puterman E, Redmond N, Siddique J, Williams DR, Carnethon MR. A Longitudinal Relationship Between Depressive Symptoms and Development of Metabolic Syndrome: The Coronary Artery Risk Development in Young Adults Study. Psychosomatic medicine. 2016;78:867–873. doi: 10.1097/PSY.0000000000000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunari K, Matsui T, Maeda K, Nakamura M, Watanabe T, Kiriike N. Anxiety-induced plasma norepinephrine augmentation increases reactive oxygen species formation by monocytes in essential hypertension. Am J Hypertens. 2006;19:573–578. doi: 10.1016/j.amjhyper.2005.10.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.