Abstract
Conditioned stimuli contribute to the resilience of nicotine addiction in that nicotine-associated cues can influence smokers and promote relapse. These stimuli are thought to acquire incentive motivational properties through a Pavlovian mechanism, and this phenomenon can be measured in animals by observing conditioned approach to the conditioned stimulus (sign-tracking) or to the location of unconditioned stimulus delivery (goal-tracking). Goal-tracking is thought to be more flexible than sign-tracking in response to changes in expected outcome. Nicotine exposure can increase the expression of conditioned responses, and we hypothesized that animals exposed to nicotine would also exhibit less flexible conditioned responses after a change in the expected unconditioned stimulus. Adult male rats were exposed to nicotine (0.4mg/kg, s.c.) or saline before Pavlovian conditioned approach training sessions. After training, animals underwent test sessions that reduced (water substitution) or withheld (omission) the unconditioned stimulus (US, 20% sucrose). As expected, nicotine enhanced sign- and goal-tracking. Water substitution moderately and nonspecifically reduced both sign- and goal-tracking in all rats. In contrast, US omission only reduced goal-tracking, with robust effects in saline-exposed rats and smaller effects in nicotine-exposed rats. These data support the hypothesis that both sign-tracking and nicotine exposure confer behavioral inflexibility under US omission.
Keywords: Nicotine, Pavlovian conditioning, sign-tracking, goal-tracking, extinction
The influence of drug-associated cues is of particular importance in addiction, as exposure to these cues can precipitate craving and relapse (Childress et al., 1993). Cues can develop strong associations with a drug through Pavlovian learning, during which a conditioned stimulus (CS) is repeatedly paired with an unconditioned stimulus (US), such as a drug or natural reward. Over time, the CS can acquire conditioned motivational properties. Multiple drugs of abuse, including nicotine, have been shown to promote the attribution of incentive salience to a CS in animals (Palmatier et al., 2013; Saunders and Robinson, 2011; Tomie et al., 2008). Pavlovian processes are thought to underlie attentional bias to smoking cues in human smokers, measured as excessive allocation of attention to these cues in attentional tasks; such attentional bias often correlates with subjective nicotine craving (Chanon et al., 2010; Field et al., 2009; Mogg et al., 2003). Thus, developing strategies to reduce the salience of conditioned cues after nicotine exposure could promote smoking cessation.
Pavlovian conditioned approach can be used to measure the incentive properties of a CS in animals. As animals learn the association between CS presentation and US delivery, they begin to exhibit conditioned responses to CS presentation by approaching and interacting with the CS (sign-tracking) or the location of US delivery (goal-tracking). While the expression of any conditioned response indicates the learning of a predictive relationship between the CS and US, sign-tracking is specifically thought to indicate that the CS has become an incentive stimulus (Saunders and Robinson, 2013). Importantly, the enhanced attribution of salience to a CS can emerge in the absence of drug exposure (Flagel et al., 2007), when drugs are the US (Peters and De Vries, 2014; Uslaner et al., 2006), or after drug exposure outside of training (Guy and Fletcher, 2014; Olausson et al., 2003; Yager and Robinson, 2015). In particular, nicotine exposure enhances both goal-tracking (Olausson et al., 2003; Stringfield et al., 2017) and sign-tracking (Guy and Fletcher, 2014; Stringfield et al., 2017; Yager and Robinson, 2015). Addictive drugs may promote the attribution of incentive properties to a CS, and sign-tracking specifically is linked to other behaviors associated with addiction vulnerability (Saunders and Robinson, 2013; Tomie et al., 2008).
Behavioral studies in this paradigm typically focus on animals pre-classified as sign-trackers or goal-trackers. Sign-trackers may show less behavioral flexibility in response to changes in the previously learned CS-US relationship. While sign-trackers perform similarly to goal-trackers during extinction of instrumental drug self-administration (Saunders and Robinson, 2011; Versaggi et al., 2016), they are slower to update their behavior under extinction conditions in a Pavlovian task (Ahrens et al., 2016). Moreover, when animals are trained to exhibit sign-tracking and goal-tracking to separate stimuli, the sign-tracking behavior is more resistant to extinction of the US (Beckmann and Chow, 2015). Thus, animals classified as sign-trackers appear less flexible in updating their conditioned behavior after a change in the CS-US relationship, and sign-tracking may be less flexible than goal-tracking regardless of the animals’ classification. Exposure to drugs such as nicotine enhances the expression of sign-tracking conditioned responses, but the degree to which drugs further reduce the flexibility of conditioned behavior has yet to be established.
To address this knowledge gap, we investigated the extent to which nicotine exposure blunted the flexibility of sign- and goal-tracking after a change in US. Specifically, we evaluated conditioned behavior after delivery of an unexpected and less valuable US (water) and under US omission (single extinction session). We hypothesized that animals exposed to nicotine would be less likely to update conditioned responses after a change in the expected US.
Rats in the present experiments were used in a previous study (Stringfield et al., 2017). Adult male Sprague Dawley rats (225–250g on arrival) were purchased from Harlan/Envigo (Indianapolis, IN, USA) and pair-housed during initial training, then individually housed after surgery. Animals were provided with food and water ad libitum during the entire study. Rats were housed in a vivarium on a 12:12 hour light:dark cycle, and experiments occurred during the light cycle. All experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Training behavior for this cohort of animals was published previously (Fig. 4 in Stringfield et al., 2017), during which nicotine exposure enhanced sign- and goal-tracking. Animals were assigned to either a nicotine-exposure group (NIC, n=12) or a saline-exposed control group (SAL, n=12). Nicotine hydrogen tartrate salt (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile saline with pH adjusted to 7.0±0.2. Rats received 0.4mg/kg nicotine (s.c., dose calculated from the free base form) or an equivalent volume of saline once/day for two days to habituate them to the injection procedure, then 15 minutes before each behavioral session. Training was conducted in behavioral chambers (MedAssociates, St. Albans, VT), assembled with a recessed fluid receptacle with photobeam detector, cue light, and retractable lever on one wall of the chamber, and a house light located on the opposite wall. At least 25 daily Pavlovian conditioning sessions were conducted Monday-Friday, and each session included 15 CS-US pairings on a variable interval (VI) 120-second reinforcement schedule. The CS consisted of illumination of the stimulus light and extension of the lever located directly below the light. CS presentations lasted 30s, and were immediately followed by 0.1ml of 20% sucrose in the receptacle. Lever deflections and head entries into the receptacle were recorded but had no programmed consequences. After training, all animals were habituated to behavioral session in custom-built Plexiglas chambers inside sound-attenuated boxes for an additional 5 days before surgery to implant microelectrode arrays (used for a separate study, Stringfield et al., 2017). Prior to the experiments described below, animals underwent electrophysiological recordings during behavioral sessions, and the results of those studies were previously reported (Stringfield et al., 2017). Animals completed 3–5 standard sessions before initiation of these experiments, and testing occurred over 2 weeks.
Two challenge sessions were conducted to test the flexibility of sign- and goal-tracking in NIC and SAL animals: a ‘Water’ session in which water was dispensed after CS offset instead of the expected sucrose US, and an ‘Omission’ session in which the sucrose US was withheld. The order of the challenges was counterbalanced across rats and challenge sessions were separated by at least 3 standard Pavlovian sessions to confirm that conditioned behavior recovered after testing. Behavior during each challenge session was compared to behavior during the prior day (‘Baseline’).
Behavioral measures were compared across exposure group (NIC, SAL) and within each group. Sign- and goal-tracking were measured using latency to approach the receptacle or the lever, total lever presses per trial, total receptacle entries per trial, and probability of entering the receptacle or pressing the lever at least once per trial. In addition, as the receptacle was always present (while the lever was only available as part of the CS), a receptacle elevation score was computed by taking the number of receptacle entries during the 30-s cue presentation and subtracting from that the number of receptacle entries during the 30-s period immediately before CS presentation. Behavioral measures were compared between SAL and NIC groups for each challenge session and its corresponding ‘Baseline’ session by 2-way, repeated-measures ANOVA. Within each group, behavior during the ‘Baseline’ and challenge sessions was collapsed into five 3-trial blocks and compared by 2-way, repeated-measures ANOVA. Tukey’s HSD was used for post-hoc comparisons.
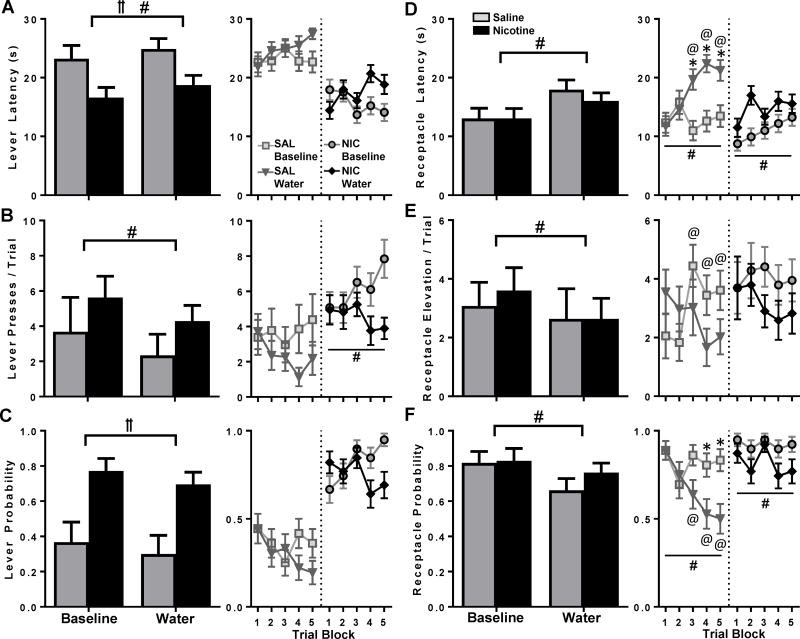
When water replaced the expected sucrose US, both NIC and SAL rats drank the water (data not shown) and slightly decreased both sign- and goal-tracking as compared to the corresponding ‘Baseline’ session (Figure 1). NIC rats pressed the lever faster than SAL rats [Figure 1A; main effect of group, F(1,23)=9.4, p<0.01], and all animals slightly but reliably increased the lever-press latency during the ‘Water’ session [Figure 1A; main effect of session, F(1,23)=6.8, p<0.05]. Rats in both exposure groups reduced lever pressing on ‘Water’ day, reflected in a main effect of session [Figure 1B; F(1,23)=9.4, p<0.01]. Additionally, while there was an expected main effect of group for lever probability [Figure 1C; F(1,23)=16.2, p<0.01] as NIC rats were more likely to press the lever, this was not significantly altered on ‘Water’ versus ‘Baseline’ day. Within-group behavior was also compared by trial blocks across both sessions. Only lever presses significantly varied in NIC rats, with a main effect of session [Figure 1B; F(1,11)=5.1, p<0.05]. Other main effects or group-by-session interactions for sign-tracking measures across the whole session or by trial blocks did not reach statistical significance.
Figure 1.
Water substitution nonspecifically reduced sign- and goal-tracking. Behavioral measures were compared between ‘Water’ and ‘Baseline’ days: between groups over the whole session (left, bar graphs), and within groups during each session (right, line graphs, collapsed into 5 blocks of 3 trials each). Measures of sign- and goal-tracking (mean ± SEM) are displayed as latency to press the lever (A), lever presses (B), probability of pressing the lever (C), latency to enter the receptacle (D), receptacle elevation score (E), and probability of entering the receptacle (F). □ main effect of group, # main effect of session, * trial block different between sessions, @ different from first block on same session, p<0.05 for all noted analyses.
Goal-tracking was reduced in both NIC and SAL rats during the ‘Water’ session: main effects of session were present for receptacle latency [Figure 1D; F(1,23)=31.1, p<0.01], receptacle elevation score [Figure 1E; F(1,23)=4.3, p<0.05] and receptacle probability [Figure 1F; F(1,23)=21.4, p<0.01]. No group differences or group-by-session interactions emerged for whole-session goal-tracking indices. Within-group analyses comparing within-session trial blocks in NIC rats resulted in a main effect of session for receptacle latency [Figure 1D; F(1,11)=9.1, p<0.05] and receptacle probability [Figure 1F; F(1,11)=5.6, p<0.05]. For SAL rats, main effects of session also emerged for receptacle latency [Figure 1D; F(1,11)=18.5, p<0.05] and receptacle probability [Figure 1F; F(1,11)=11.5 p<0.01]. Session-by-trial-block interactions occurred for elevation score [Figure 1E; F(4,44)=4.9, p<0.01], where trial block 3 differed from blocks 1-2 on ‘Baseline’ day. An interaction also occurred for receptacle latency [Figure 1D; F(4,44)=5.0, p<0.01], where blocks 3-5 differed between sessions and differed from block 1 within the ‘Water’ session. A similar interaction emerged for receptacle probability [Figure 1F; F(4,44)=4.0, p<0.01], where blocks 4-5 differed between sessions and blocks 3-5 differed from block 1 on ‘Water’ day. These results indicated that while both NIC and SAL rats reduced goal-tracking during the ‘Water’ session, SAL rats showed more pronounced behavioral change.
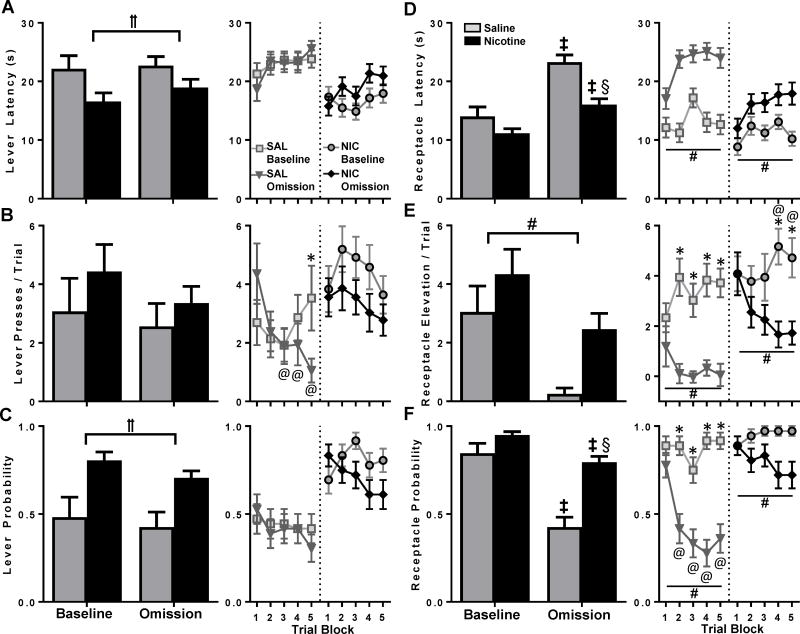
On ‘Omission’ day, the CS was presented but sucrose delivery did not occur. Rats in both the NIC and SAL groups reduced goal-tracking but not sign-tracking during this session as compared to the corresponding ‘Baseline’ session, with the greatest behavioral change occurring in the SAL group (Figure 2). Compared to SAL rats, NIC rats were faster to press the lever [Figure 2A; main effect of group, F(1,23)=5.3, p<0.05] and more likely to press the lever [Figure 2C; main effect of group, F(1,23)=9.6, p<0.01]. No other main effects of ‘Omission’ day or group-by-session interactions occurred for any sign-tracking measures. Within group analysis indicated that only SAL rats showed a trial block by session interaction for lever presses [Figure 2B; F(4,44)=3.2, p<0.05], where trial block 5 differed between sessions, and blocks 3-5 differed from block 1 within the ‘Omission’ session.
Figure 2.
Withholding the US preferentially reduced goal-tracking, especially in SAL rats. Behavioral measures were compared between the ‘Omission’ and ‘Baseline’ days. Behaviors and statistical notations are as described in Fig. 1. Additionally, ‡ interaction, change in responding from ‘Baseline’ to ‘Omission’ day within exposure groups, § interaction, difference between NIC and SAL only on ‘Omission’ day.
For goal-tracking behaviors, both groups of animals reduced receptacle elevation score on ‘Omission’ day [Figure 2E; F(1,23)=28.7, p<0.001], with no difference between groups. Group-by-session interactions were significant for receptacle latency [Figure 2D; F(1,23)=7.9, p<0.05] and receptacle probability [Figure 2F; F(1,23)=7.9, p<0.05]. Post-hoc comparisons indicated that both SAL and NIC animals slowed the latency and decreased the probability of receptacle entry on ‘Omission’ day (p<0.01), and that while the groups did not differ on ‘Baseline’ day, SAL animals displayed longer latency and lower probability than NIC animals on ‘Omission’ day (both p’s<0.001). Within group analyses indicated main effects of session for receptacle latency in NIC [F(1,11)=32.6, p<0.001] and SAL [F(1,11)=42.91, p<0.001] groups (Figure 2D). In NIC rats, a main effect of session emerged for elevation score [Figure 2E; F(1,11)=19.3, p<0.01] along with a trial-block-by-session interaction [F(4,44)=3.1, p<0.05], where blocks 4-5 differed between sessions and from block 1 on ‘Omission’ day. Elevation score in SAL rats also produced a main effect of session [Figure 2E; F(1,11)=13.9, p<0.01] and a trial-block-by-session interaction [F(4,44)=3.3, p<0.05], with blocks 2-5 differing between sessions. For receptacle probability, a main effect of session occurred in NIC [F(1,11)=18.6, p<0.01] and SAL [F(1,11)=40.6, p<0.001] groups (Figure 2F), as well as a trial-block-by-session interaction for SAL animals [F(4,44)=4.1, p<0.01], with blocks 2-5 differing between sessions and from block 1 on ‘Omission’ day.
In this study, we investigated the flexibility of Pavlovian approach by manipulating the expected outcome predicted by a conditioned cue, using water substitution and US omission. NIC and SAL animals moderately reduced both sign- and goal-tracking after water substitution to a similar degree, but no interactive effects emerged based on drug exposure or conditioned response. In contrast, rats reduced only goal-tracking in the ‘Omission’ session, with SAL rats showing greater reductions than NIC rats. Thus, in rats that express both sign- and goal-tracking upon CS presentation, sign-tracking is resistant to adaptation after US omission. In addition, goal-tracking is less flexible under this extinction condition in NIC-exposed rats than in SAL controls. These data support our hypothesis that nicotine reduces the flexibility of conditioned behavior after a change in the expected outcome.
Withholding the expected US produced large changes in goal-tracking, but not sign-tracking. Moreover, nicotine-exposed rats exhibited smaller changes in goal-tracking, suggesting less behavioral flexibility than exhibited by controls. While this study is limited by the fact that there was only one extinction session and, thus, sign-tracking was not extinguished, the present findings are consistent with prior reports of Pavlovian extinction that detected differences in sign- and goal-trackers on the first day of extinction and on subsequent test days. In one study (Ahrens et al., 2016), rats were trained to discriminate between rewarding and non-rewarding blocks of trials, and goal-trackers were more likely to reduce behavior specifically during non-rewarded blocks than sign-trackers. Similarly, when Beckmann and Chow (2015) trained rats to sign-track to a lever CS and goal-track to a tone CS, sign-tracking was resistant to extinction over multiple extinction sessions, including the first day. The present study further differentiates between sign- and goal-tracking by concurrently measuring both behaviors during Pavlovian conditioning sessions in which animals typically express both conditioned responses, but to different degrees. Here, we found not only that sign-tracking is resistant to updating, but that animals that express enhanced sign-tracking due to nicotine exposure are also less able to change their goal-tracking behavior than saline-exposed rats.
In contrast, during the water substitution test, we manipulated the expected outcome by delivering water instead of the expected sucrose solution. Substitution with water conserved the consummatory behavior of licking, as animals still drank the water. Under this condition, we found similar reductions in sign- and goal-tracking between nicotine- and saline-exposed rats. This nonspecific reduction in behavior is presumably due to the fact that animals still received a US, even though it was less valuable than the sucrose US.
The combination of these results and previous studies suggest a difference in the flexibility of sign-tracking and goal-tracking. Responding despite reward devaluation is typical of habitual behavior, and while sign-tracking would not necessarily be classified as “habitual,” it demonstrates the characteristic of inflexibility after a change in the value of an expected outcome. While others have reported that sign-tracking is resistant to US devaluation (Morrison et al., 2015), we observed small but reliable reductions when the sucrose US was replaced with water. Sign-tracking appears much more resistant to US omission, potentially because the CS has become an incentive stimulus and is able to act as a conditioned reinforcer (Guy and Fletcher, 2014; Yager and Robinson, 2015). Devaluing the CS requires a reduction in the attributed incentive value of the cue itself, separate from the US. As nicotine enhances the attribution of salience to a CS through its reinforcement-enhancing and incentive-amplifying effects (Palmatier et al., 2006), sign-tracking may be less flexible in nicotine-exposed animals, and our data suggest that goal-tracking is less flexible as well.
One limitation of this study is the use of only male rats for these experiments. Both female rats and humans exhibit diverse pharmacological or behavioral responses to nicotine compared to males (Chaudhri et al., 2005; Perkins et al., 2002). While female rats only differ slightly from males in the expression of sign- or goal-tracking (Pitchers et al., 2015) there may be differences in the way nicotine influences behavior such that females may be less flexible than males after nicotine exposure. Additionally, this study was conducted with passive-administration of nicotine instead of self-administration in order to explore the effects of a consistent concentration of drug on-board during the task. Future studies could investigate how the motivated component of nicotine-taking may influence the attribution of salience to a conditioned cue and the subsequent flexibility of sign- and goal-tracking behaviors.
In this study, we demonstrate that changing the expected outcome reduced both sign-tracking and goal-tracking, depending on the US change. Under US omission, sign-tracking was less flexible than goal-tracking, and goal-tracking was less flexible in nicotine-exposed rats than controls. The difference in resiliency of sign-tracking and goal-tracking after nicotine exposure suggests that multiple strategies may be needed to target separate aspects of nicotine-associated conditioning. Focusing on the elevated salience of a CS, perhaps via prolonged or targeted extinction learning (e.g., Unrod et al., 2014) may be a future method for achieving smoking cessation.
Highlights.
Conditioned stimuli and responses are key components of nicotine use and relapse
Conditioned responses can be modeled in animals with Pavlovian conditioned approach
Nicotine increased the expression of Pavlovian conditioned approach responses
Nicotine reduced the flexibility of approach responses after a change in outcome
Acknowledgments
The authors would like to thank Dr. Matthew Palmatier for critical feedback on these experiments, Dr. Alexander Gómez-A for comments on the manuscript, Drs. Aric Madayag, Margaret Broadwater, Rebecca Fanelli and Tatiana Shnitko as well as Kevin Caref, Brandi Lawrence, Yue Dong, and Jesse Sharp for outstanding technical assistance.
Funding: This research was funded by the National Institutes of Health [P60 AA011605, Project #3] and the UNC Bowles Center for Alcohol Studies. SJS was supported on 5P60AA011605-17S1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Notes: The authors declare no competing financial interest.
References
- Ahrens AM, Singer BF, Fitzpatrick CJ, Morrow JD, Robinson TE. Rats that sign-track are resistant to Pavlovian but not instrumental extinction. Behav Brain Res. 2016;296:418–30. doi: 10.1016/j.bbr.2015.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Chow JJ. Isolating the incentive salience of reward-associated stimuli: value, choice, and persistence. Learn Mem. 2015;22:116–127. doi: 10.1101/lm.037382.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanon VW, Sours CR, Boettiger CA. Attentional bias toward cigarette cues in active smokers. Psychopharmacology (Berl) 2010;212:309–320. doi: 10.1007/s00213-010-1953-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl) 2005;180:258–266. doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. doi: 10.1037/e495912006-006. [DOI] [PubMed] [Google Scholar]
- Field M, Munafò M, Franken I. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull. 2009;135:589–607. doi: 10.1037/a0015843.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- Guy EG, Fletcher PJ. The effects of nicotine exposure during Pavlovian conditioning in rats on several measures of incentive motivation for a conditioned stimulus paired with water. Psychopharmacology (Berl) 2014;231:2261–2271. doi: 10.1007/s00213-013-3375-3. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Field M, De Houwer J. Eye movements to smoking-related pictures in smokers: Relationship between attentional biases and implicit and explicit measures of stimulus valence. Addiction. 2003;98:825–836. doi: 10.1046/j.1360-0443.2003.00392.x. [DOI] [PubMed] [Google Scholar]
- Morrison SE, Bamkole MA, Nicola SM. Sign tracking, but not goal tracking, is resistant to outcome devaluation. Front Neurosci. 2015;9:1–12. doi: 10.3389/fnins.2015.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances reward-related learning in the rat. Neuropsychopharmacology. 2003;28:1264–71. doi: 10.1038/sj.npp.1300173. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Marks KR, Jones SA, Freeman KS, Wissman KM, Sheppard BA. The effect of nicotine on sign-tracking and goal-tracking in a Pavlovian conditioned approach paradigm in rats. Psychopharmacology (Berl) 2013b;226:247–59. doi: 10.1007/s00213-012-2892-9. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Jacobs L, Sanders M, Caggiula AR. Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology (Berl) 2002;163:194–201. doi: 10.1007/s00213-002-1168-1. [DOI] [PubMed] [Google Scholar]
- Peters J, De Vries TJ. Pavlovian conditioned approach, extinction, and spontaneous recovery to an audiovisual cue paired with an intravenous heroin infusion. Psychopharmacology (Berl) 2014;231:447–453. doi: 10.1007/s00213-013-3258-7. [DOI] [PubMed] [Google Scholar]
- Pitchers KK, Flagel SB, O’Donnell EG, Solberg Woods LC, Sarter M, Robinson TE. Individual variation in the propensity to attribute incentive salience to a food cue: Influence of sex. Behav Brain Res. 2015;278:462–469. doi: 10.1016/j.bbr.2014.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in resisting temptation: Implications for addiction. Neurosci Biobehav Rev. 2013;37:1955–1975. doi: 10.1016/j.neubiorev.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual Variation in the Motivational Properties of Cocaine. Neuropsychopharmacology. 2011;36:1668–1676. doi: 10.1038/npp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringfield SJ, Palmatier MI, Boettiger CA, Robinson DL. Orbitofrontal participation in sign- and goal-tracking conditioned responses: Effects of nicotine. Neuropharmacology. 2017;116:208–223. doi: 10.1016/j.neuropharm.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unrod M, Drobes DJ, Stasiewicz PR, Ditre JW, Heckman B, Miller RR, Sutton SK, Brandon TH. Decline in Cue-Provoked Craving During Cue Exposure Therapy for Smoking Cessation. Nicotine Tob Res. 2014;16:306–315. doi: 10.1093/ntr/ntt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, Acerbo MJ, Jones SA, Robinson TE. The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine. Behav Brain Res. 2006;169:320–4. doi: 10.1016/j.bbr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Versaggi CL, King CP, Meyer PJ. The tendency to sign-track predicts cue-induced reinstatement during nicotine self-administration, and is enhanced by nicotine but not ethanol. Psychopharmacology (Berl) 2016 doi: 10.1007/s00213-016-4341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. Individual variation in the motivational properties of a nicotine cue: sign-trackers vs. goal-trackers. Psychopharmacology (Berl) 2015;232:3149–3160. doi: 10.1007/s00213-015-3962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]