Abstract
Background
Distant metastatic breast cancer (MBC), including metastases found at diagnosis (de novo) and those occurring later (recurrence), represents the most severe form of the disease, when resource utilization is most intensive. Yet, the number of women living with MBC in the US is unknown. The objective of this paper is to use population-based data to estimate the prevalence of MBC.
Methods
We used a back-calculation method to estimate MBC prevalence from US BC mortality and survival from the Surveillance, Epidemiology and End Results (SEER) registries. Based on the illness-death process, this method assumes that each observed BC death is the result of MBC, either de novo or a recurrence with metastatic disease.
Results
We estimate that by January 1, 2017 there will be 154,794 women living with MBC in the United States, 3 in 4 initially diagnosed with stage I–III BC who later progressed to MBC.
Median survival and 5-year relative survival for de novo MBC increased over the years, especially in younger women. We estimate a 2-fold increase in 5-year relative survival from 18% to 36%, for women diagnosed with de novo MBC at age 15–49 between 1992–1994 and 2005–2012, respectively.
Conclusion
This study demonstrates an increasing number of women in the US living with MBC, likely the result of improvements in treatment and aging of the US population.
Impact
The increasing burden of MBC highlights the importance of documenting recurrence in order to foster more research into the specific needs of this understudied population.
Keywords: Metastasis, Recurrence, Breast Cancer, Prevalence, Backcalculation
In 2016, there are approximately 3.5 million women living with a history of breast cancer (BC) in the United States(1). This number includes newly diagnosed women with BC undergoing surgery and adjuvant treatment, long term survivors who may be cured of the disease, and women who have experienced a recurrence after a disease free interval. Distant metastatic cancers, including metastases found at diagnosis (de novo) and those occurring later in the disease course (distant recurrence), who represent the majority of cases, constitute the most advanced form of the disease. Many groups, including the Orphan Drug Program of the Food and Drug Administration (FDA), health services researchers and especially the cancer survivorship and advocacy community are increasingly interested in assessing the prevalence of women with metastatic breast cancer (MBC) as these women have significant health care needs when resource utilization tends to be continuous and intensive(2–6).
The prevalence of women initially diagnosed with MBC can be directly estimated (7) using population-based cancer registry data on de novo MBC and vital-status at the study cut-off date. However, estimating prevalence of those diagnosed with early stage BC who later have had a distant recurrence is challenging, since there are no nationally representative data that capture recurrence. Currently, registries in the US do not routinely collect or report recurrence data.
In the absence of empirical data on the incidence of recurrent MBC, a back-calculation method, Mortality Incidence Approach MODel (MIAMOD) (8, 9), has been used to reconstruct prevalence of recurrent cancer in Australia (10). This method calculates the incidence of MBC (de novo and distant recurrence) based on BC mortality and MBC survival. The method has also been used to estimate the prevalence of BC survivors in states within the US (11) when cancer incidence data is not available over the long term.
The objective of this paper is to use national data on BC mortality and MBC survival from Surveillance, Epidemiology and End Results (SEER) registries to estimate the prevalence of women living with MBC in the U.S., including both women initially diagnosed with MBC and those who have progressed to distant MBC. We also calculate separately the prevalence of women diagnosed with de novo MBC in SEER and the US (7). The SEER de novo MBC prevalence is compared with an estimate based on the backcalculation method to validate the method and calibrate survival (11).
Materials and Methods
Data sources and Definitions
The Surveillance Epidemiology and End Results (SEER) Program collects clinical, demographic, and vital status information on all cancer cases diagnosed in defined geographic areas. Data included in this report are from the SEER-9 and SEER-11 registries (November 2015 Submission) obtained using SEER*Stat software version 8.3.2 (www.seer.cancer.gov/seerstat). SEER-9 covers approximately 11% of the US population and includes: Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah. For survival analyses we used data from 1992–2012 from the SEER-11 registries which includes, SEER-9, Los Angeles and San-Jose Monterey. We only included malignant BCs.
Stage at diagnosis was defined using adjusted American Joint Committee on Cancer (AJCC) 6th edition staging classification (12). This stage definition uses extent of disease information for cases diagnosed in 1988–2003 and collaborative staging for cases diagnosed in 2004–2012. De novo MBC was defined as AJCC 6 stage IV which includes only tumors with distant metastasis. Stage IV from previous AJCC editions and distant stage from SEER historical summary staging classification include some locally advanced tumors without distant metastasis, e.g., tumors with positive supraclavicular lymph node involvement without distant metastasis. Recurrent MBC was used to designate women initially diagnosed with AJCC stages I–III BC, whose disease later progressed (metastasized) after treatment to distant organs or tissues.
Main inputs to the back-calculation methods are cancer deaths, all cause-deaths, population sizes and MBC survival. We obtained US female deaths due to BC and all causes, from 1990 to 2012 from the National Center for Health Statistics (NCHS) and US female populations from 1990 to 2020 from the US Census Bureau. The population projections are based on the July 1, 2013 population estimates, which are based on the 2010 Census, and provide projections of the population for 2014 through 2060, (https://www.census.gov/population/projections/data/national/2014.html). Deaths and populations were obtained by single calendar year and single age (0–99) using the SEER*Stat software.
Survival associated with cancer diagnosis was assessed via relative survival calculated using SEER*Stat. Relative survival is based on the ratio of overall survival (all causes of death) among cancer cases to the expected survival in individuals without cancer. The expected survival is estimated from US life tables matched to the group of cancer patients by age, sex, race, and calendar year. Relative survival captures all excess mortality among cancer cases including deaths attributable to treatment and as such serves as a proxy for disease-specific survival that accounts for treatment-related mortality. In calculating relative survival, we excluded women diagnosed through death certificate or autopsy, because of uncertainties in the diagnosis date. We also excluded cases with no follow-up information.
Prevalence of de novo MBC using the counting method
The prevalence of de novo MBC in the SEER-9 areas (counts and proportions) is calculated directly using the SEER*Stat counting method(7), which counts all women alive on 31st, December 2013 with a previous diagnosis of stage IV BC (1988–2012) in the SEER-9 areas. The method also adjusts for cases lost to follow-up. To estimate the de novo MBC prevalence counts in the US we applied the SEER-9 prevalence proportions by 5-year age group and race to the respective female US populations.
Modeling survival time from MBC including de novo and recurrence
To model survival for de novo MBC cases we estimated relative survival by age and year at diagnosis for women diagnosed with stage IV BC from 1992 to 2012 in the SEER-11 areas. To extrapolate survival beyond the observed data, as required by the back-calculation method, we fit a Weibull mixture cure survival model to de novo MBC relative survival data. The mixture cure survival model assumes that a proportion of cancer patients is cured of cancer while the remaining patients die following a Weibull survival distribution. While most stage IV BC patients die of their cancer, this model is used because it allows for modeling of long term survivors and extrapolation of survival beyond the observed data. We fit a separate model to each of the 5 age groups (15–49, 45–64, 65–74, 75–84, 85–99) and used calendar year as a covariate in the model using the CANSURV software(13, 14) (https://surveillance.cancer.gov/cansurv/). Details of the model are provided in the Supplemental Materials.
Because population-level data on survival from MBC recurrence are unavailable, we use an adjustment to the de novo MBC survival based on a University of Texas M. D. Anderson Cancer Center (MDACC) study that included 2,881 and 643 women, retrospectively identified and diagnosed between 1992 and 2007 with recurrent and de novo MBC, respectively (15). The comparison of the overall survival curves for recurrent and de novo MBC, Figure 1 in Dawood et al. (15), showed an average risk of death for recurrent relative to de novo disease of 1.35 (i.e., 1.35=log(recurrent survival)/log(de novo survival)). Recurrent MBC survival was estimated by applying the 1.35 relative risk adjustment to each of the modeled de novo MBC survival curves (recurrent MBC survival= de novo MBC survival exp(1.35) ). We also performed sensitivity analyses and provided prevalence estimates using a lower and a higher relative risk adjustment of 1.2 and 1.5, respectively.
To model survival from de novo or recurrent MBC we compute a weighted average of the de novo MBC survival and the recurrent MBC survival, i.e.,
where w is the fraction of BC deaths that are a consequence of de novo MBC and (1−w) is the fraction of BC deaths that are a consequence of recurrent MBC. We use incidence-based mortality by stage in SEER to estimate w. Details of the calculation are provided in the Supplemental Materials and Supplemental Figure 1 which shows that the resulting estimated w is 0.2, implying that 20% of BC deaths in a given year originate from women diagnosed with de novo MBC, while 80% are deaths from women diagnosed with earlier stage BC who progressed to recurrent MBC.
Back-calculation method
We used the Mortality Incidence Approach MODel (MIAMOD) (8, 9) to estimate incidence and prevalence from BC mortality and MBC survival. The method is based on the illness-death process and two equations relating incidence, survival, prevalence and mortality. The method assumes that each observed BC death is the result of MBC, either de novo or recurrent. The first equation specifies mortality as the sum of prior incidence and survival and back-calculates incidence of MBC (de novo or recurrent), by single-year ages and single calendar years, from BC deaths and MBC survival. The second equation is used to estimate prevalence from the estimated incidence and survival. The MIAMOD software can be downloaded from (http://www.eurocare.it/MiamodPiamod/tabid/60/Default.aspx) and details of this application are included in the Supplemental Materials. Prevalence projections from 2014 to 2020 assume constant BC mortality rates at 2014 levels and constant survival, but use dynamic population size projections for these years.
To adjust for data inconsistencies, such as underreporting of deaths and misclassification of deaths to site of metastasis as found elsewhere (11), we calibrate the back-calculation method by comparing the SEER-9 counting-method prevalence of de novo MBC with the one obtained from the MIAMOD method. The calibration suggests adjusting MBC survival by a factor of 0.92=exp(−0.08) to correct for 11% underestimation of the observed prevalence, i.e., . Results from the calibration are shown in the Supplemental Figures 2 and 3.
Results
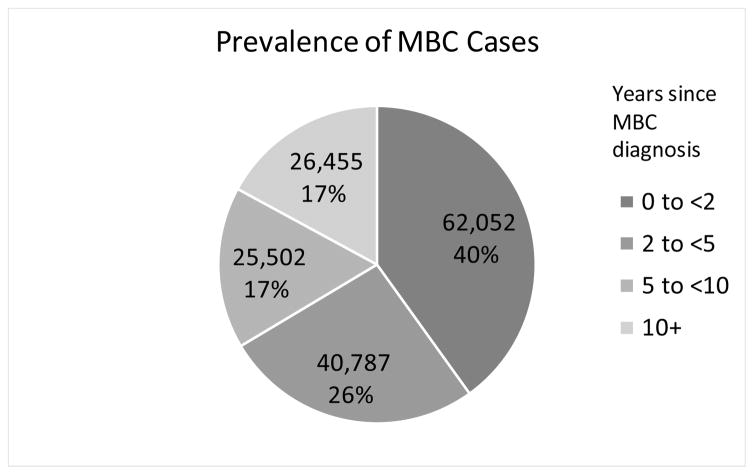
In 2013, the last year with observed data, we estimate a prevalence of MBC of 138,622 of which 38,897 (28%) are survivors who were initially diagnosed with de novo stage IV disease and 99,725 (72%) survivors initially diagnosed with stage I–III BC who later progressed to MBC (Table 1). The backcalculation method also estimates 50,344 new diagnoses of MBC in 2013, of which 12,966 (26%) are de novo and 37,378 (74%) recurrences, thus 3 in 4 are undocumented diagnoses of MBC. We project that by 1/1/2017 there will be 154,794 women living with MBC in the United States. Using relative risk adjustment of 1.5 and 1.2, instead of 1.35, we estimate 136,419 and 178,412, 2017 US MBC prevalence, respectively. Figure 1 shows that, based on our calculations, MBC prevalence in terms of the number of women living with MBC increased 4% from 1990 to 2000, 17% from 2000 to 2010 and is projected to increase by 31% from 2010 to 2020. Although the largest majority of prevalent cases are women who have been living with metastatic disease for 2 years or less (40%), one third (34%) have lived for 5 years or more with MBC (Figure 2).
Table 1.
Estimates (1/1/2013) and projections (1/1/2017) of breast cancer mortality, and incidence and prevalence of metastatic breast cancer (MBC) including de novo and recurrence disease in the US. Projections are based on dynamic projections of population growth and aging from the US Census Bureau and constant projections of breast cancer mortality and of MBC survival.
| Number of Women at 1/1/2013 | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| BC Deaths | MBC Incidence | MBC prevalence | |||||
|
|
|
|
|||||
| Age | US Female Population | Observed | Estimated | De novo (Observed) | De novo and recurrence (Estimated) | De novo (Observed) | De novo and recurrence (Estimated) |
| 15–39 | 52,450,844 | 996 | 976 | 705 | 1,870 | 1,604 | 4,205 |
| 40–49 | 21,199,116 | 3,530 | 3,416 | 1,422 | 5,129 | 4,736 | 15,684 |
| 50–59 | 22,400,308 | 7,979 | 8,095 | 2,994 | 9,962 | 8,950 | 30,642 |
| 60–69 | 17,143,155 | 10,071 | 9,888 | 3,200 | 11,481 | 11,002 | 36,194 |
| 70–70 | 10,011,131 | 8,650 | 8,617 | 2,678 | 9,342 | 7,740 | 27,232 |
| 80–99 | 7,338,871 | 11,216 | 11,176 | 1,967 | 12,560 | 4,865 | 24,665 |
| All ages | 130,543,425 | 42,442 | 42,169 | 12,966 | 50,344 | 38,897 | 138,622 |
|
| |||||||
| Number of Women at 1/1/2017 (Projections) | |||||||
|
|
|||||||
| BC Deaths | MBC Incidence | MBC prevalence | |||||
|
|
|
|
|||||
| Age | US Female Population | Observed | Estimated | De novo (Observed) | De novo and recurrence (Estimated) | De novo (Observed) | De novo and recurrence (Estimated) |
| 15–39 | 54,104,476 | - | 1,056 | - | 2,050 | - | 4,711 |
| 40–49 | 20,471,655 | - | 3,317 | - | 5,052 | - | 16,019 |
| 50–59 | 22,240,898 | - | 8,103 | - | 10,042 | - | 32,573 |
| 60–69 | 19,420,211 | - | 11,151 | - | 13,037 | - | 42,450 |
| 70–70 | 11,771,880 | - | 10,035 | - | 10,910 | - | 32,731 |
| 80–99 | 7,602,526 | - | 11,767 | - | 13,302 | - | 26,310 |
| All ages | 135,611,646 | - | 45,429 | - | 54,394 | - | 154,794 |
Figure 1.
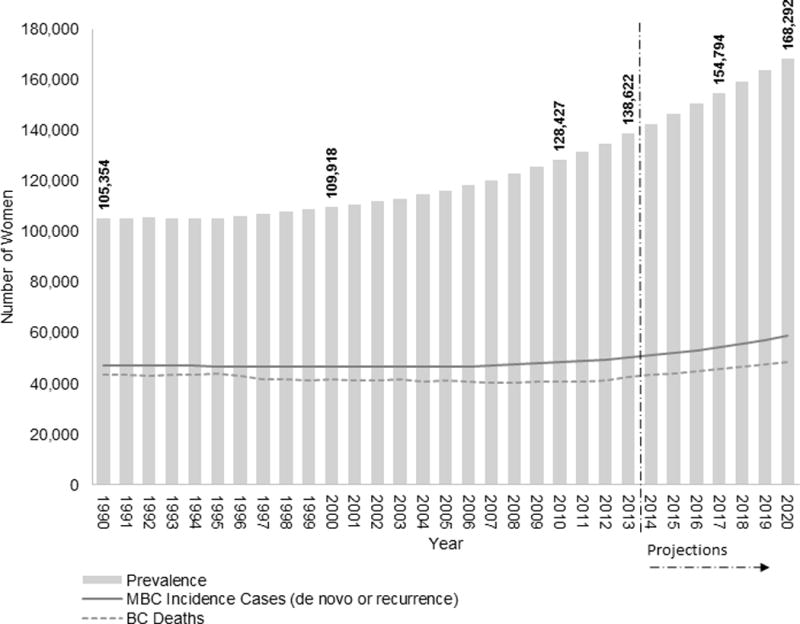
Estimates and projections of metastatic breast cancer (MBC) prevalence in the United States from 1990 to 2020 (gray bars). Observed number breast cancer (BC) deaths (dashed line) as used as input in the backcalculation model and estimated number of new cases with de novo and recurrent MBC (solid line).
Figure 2.
Number of women in the US alive at 1/1/2017 previously diagnosed with de novo or recurrent metastatic breast cancer (MBC) by time since diagnosis.
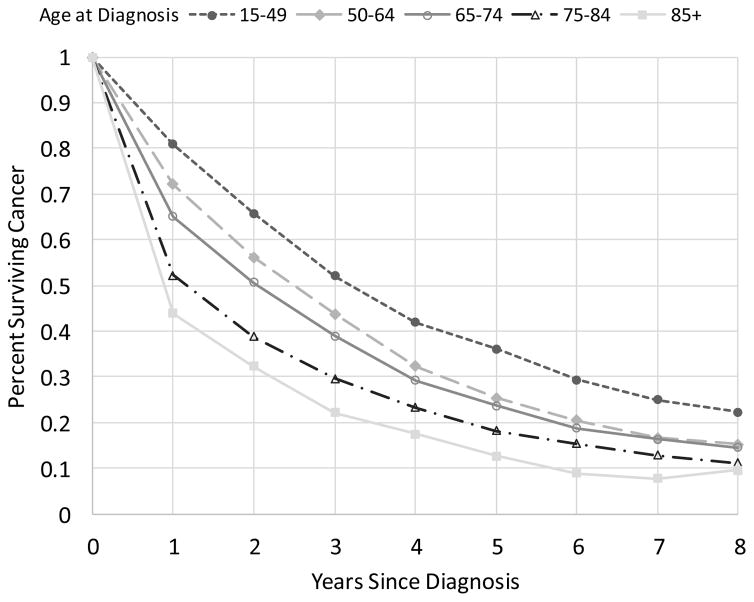
Relative survival estimates used in the modeling included 25,935 women diagnosed with de novo stage IV BC from 1992–2012 (Table 2). Median survival and 5-year relative survival increased over the years especially for younger women diagnosed after 1995 (Table 2). Median relative survival time increased from 22.3 to 38.7 months and from 19.1 to 29.7 months for women diagnosed between ages 15–49 and 50–64, respectively, during 1992–1994 versus 2005–2012. The 5-year relative survival had a 2-fold increase from 18% to 36%, for women diagnosed with de novo MBC at age 15–49 between 1992–1994 and 2005–2012, respectively. Despite a poor prognosis there is a small but meaningful percent of these cases who survive 10-years or more; more than 11% of women diagnosed between 2000–2004 under the age of 64 years survived 10 years or more. Younger women diagnosed with de novo MBC have higher survival compared to women diagnosed at older ages (Figure 3).
Table 2.
Number of women, median overall and relative survival (RS) in months and 5-year relative survival in percent (95% C.I.) for women diagnosed with de novo stage IV breast cancer in the SEER-11 areas by grouped age and year at diagnosis.
| Median (in months) | 5-year Relative Survival | 10-year Relative Survival | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Year | Age | N | Overall | Relative Survival | 95% C.I. | 95% C.I. | ||
| 1992–1994 | 15–49 | 430 | 22.2 | 22.3 | 18% | (14%,21%) | 10% | (8%,14%) |
| 1992–1994 | 50–64 | 777 | 18.4 | 19.1 | 15% | (13%,18%) | 8% | (6%,11%) |
| 1992–1994 | 65–74 | 598 | 16 | 17.6 | 15% | (12%,18%) | 7% | (5%,10%) |
| 1992–1994 | 75–84 | 442 | 10.1 | 10.9 | 16% | (12%,20%) | 7% | (4%,11%) |
| 1992–1994 | 85+ | 168 | 3.8 | 4.1 | 6% | (2%,13%) | 4% | (0%,16%) |
| 1992–1994 | All ages | 2,415 | 15.7 | 16.7 | 15% | (14%,17%) | 8% | (7%,9%) |
| 1995–1999 | 15–49 | 894 | 24.5 | 24.7 | 24% | (21%,27%) | 11% | (9%,13%) |
| 1995–1999 | 50–64 | 1,321 | 20.3 | 20.6 | 21% | (18%,23%) | 10% | (8%,12%) |
| 1995–1999 | 65–74 | 978 | 14.4 | 15.2 | 17% | (15%,20%) | 6% | (5%,8%) |
| 1995–1999 | 75–84 | 799 | 10.4 | 11.8 | 13% | (10%,16%) | 7% | (5%,10%) |
| 1995–1999 | 85+ | 292 | 4.7 | 5.5 | 16% | (10%,23%) | 8% | (2%,21%) |
| 1995–1999 | All ages | 4,284 | 16.5 | 17.7 | 19% | (17%,20%) | 8% | (8%,9%) |
| 2000–2004 | 15–49 | 1,307 | 29 | 29.3 | 29% | (26%,31%) | 14% | (12%,16%) |
| 2000–2004 | 50–64 | 2,270 | 24.6 | 25.1 | 24% | (23%,26%) | 11% | (10%,13%) |
| 2000–2004 | 65–74 | 1,319 | 18.9 | 20.3 | 20% | (18%,23%) | 8% | (6%,10%) |
| 2000–2004 | 75–84 | 1,142 | 10.3 | 11.4 | 15% | (13%,18%) | 8% | (6%,10%) |
| 2000–2004 | 85+ | 436 | 5.7 | 7.2 | 14% | (9%,20%) | 9% | (3%,19%) |
| 2000–2004 | All ages | 6,474 | 19.8 | 21.1 | 22% | (21%,23%) | 10% | (9%,11%) |
| 2005–2012 | 15–49 | 2,748 | 38.4 | 38.7 | 36% | (34%,38%) | - | - |
| 2005–2012 | 50–64 | 4,861 | 29 | 29.7 | 25% | (24%,27%) | - | - |
| 2005–2012 | 65–74 | 2,468 | 23.3 | 24.5 | 24% | (22%,26%) | - | - |
| 2005–2012 | 75–84 | 1,820 | 12 | 14 | 18% | (16%,21%) | - | - |
| 2005–2012 | 85+ | 865 | 6 | 8.2 | 13% | (9%,17%) | - | - |
| 2005–2012 | All ages | 12,762 | 25.2 | 26.9 | 26% | (25%,27%) | - | - |
Figure 3.
Relative survival by time since diagnosis for women diagnosed with de novo stage IV in the SEER-11 areas between 2005–2012 at different age groups.
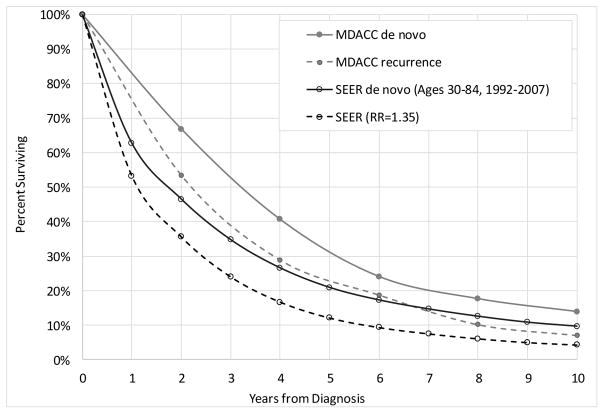
Figure 4 compares MBC survival in SEER and in the MDACC study cohort. The MDACC cohort included 2,881 and 643 women with recurrent and de novo MBC respectively, retrospectively identified and diagnosed between years 1992 and 2007 and ages 17 and 91. In order to be comparable, we selected women diagnosed with de novo MBC in SEER in the same calendar years (1992–2007) and ages 15 through 84. In the MDACC cohort the median age at diagnosis was 52 and 50 years for de novo and recurrent MBC, respectively, while in SEER the median age at diagnosis was 61 years of age. Relative survival for women diagnosed with de novo MBC in the SEER areas was lower than overall survival among women in the MDACC cohort. The 4-year relative survival of de novo MBC in SEER was 27% compared with 41% and 29% overall survival for de novo and recurrent MBC in the MDACC cohort, respectively. Relative survival is generally higher than overall survival for de novo MBC (Table 1, thus these results suggest that the MDACC cohort represents a lower-risk cohort than the general population. The absolute difference decreased with longer follow-up and 10-year relative survival was 10% in SEER versus 14% in the MDACC for women diagnosed with de novo MBC (Figure 4).
Figure 4.
Relative survival by time since diagnosis for women diagnosed with de novo stage IV in the SEER-11 areas in 1992–2007 at ages 15–84 (black) and the adjusted survival for recurrence stage IV for ages 15–84 based on a 1.35 relative risk adjustment (dashed black). The gray curves represent survival from a University of Texas M. D. Anderson Cancer Center (MDACC) study that included women diagnosed with de novo (gray) and recurrence MBC (dashed gray).
Discussion
Despite the progressive and incurable nature of almost all MBC, median survival after diagnosis with metastatic disease has been increasing, resulting in a growing number of women living with MBC in the United States. The increased survival is especially noted for women diagnosed at younger ages. We estimate a 2-fold increase in 5-year relative survival from 18% to 36%, for women diagnosed with de novo stage IV at age 15–49 between 1992–1994 and 2005–2012, respectively, translating into an increase of approximately one third in the number of women living with MBC, from 105,354 in 1990 to 138,622 in 2013. We further project that by 2017 there will be 154,794 women living with MBC in the United States.
To our knowledge, this is the first time that the number of women living with MBC in the United States has been estimated. These estimates provide a new perspective on the population burden of breast cancer and have great potential significance to the research and advocacy community working on behalf of MBC patients and their families.
Other studies have also shown improvement in survival for women with de novo distant disease or metastatic recurrence(16–18), attributed to improved treatment. The improvement in MBC survival may also be explained by changes in staging. A study using SEER data (19) has shown that incidence of distant BC has been increasing, especially among young women (Supplemental Figure 4). Also, incidence of stage III and unknown stage has been decreasing (Supplemental Figure 5). Thus, although survival may have increased because of improvements in treatment, part of the increase may be also due to stage migration from stage III or unstaged to stage IV or early detection of stage IV, likely due to increasing availability of better imaging techniques.
Strengths of our study include the large population size, the population-based setting, the long follow-up, and the fact that we used consistent definitions of staging and other variables across time. The calibrated back-calculation method showed a very good agreement with reported incidence and directly estimated prevalence of de novo MBC in the SEER areas. The calibration corrects for possible underreporting and misclassification of cause of death.
The main limitation of this study is the absence of population-based survival estimates following MBC recurrence. In order to represent survival/mortality associated with MBC recurrence, we used a 1.35 higher risk of cancer death (inflation factor) for recurrent MBC relative to de novo disease based on a single-institution study conducted at MDACC (15). This factor accounts for greater susceptibility to the cancer as well as greater vulnerability to treatment morbidities due to accumulation of cancer treatments received before the point of recurrence. Other causes of death, not associated with breast cancer or its treatment are assumed to be similar between de novo and recurrence MBC patients. Sensitivity analyses to this assumption showed that US prevalence of MBC estimates would vary from 136,000 to 178,000 in 2017 using a higher relative risk of death (RR=1.5) or a lower relative risk of death (RR=1.2) for recurrent MBC survival compared to de novo MBC survival. However, we noted that SEER survival was lower than survival in the MDACC. Possible explanations may be the fact that MDACC patients were younger compared to SEER patients and that, by definition, were in treatment at a major cancer center, and therefore more likely to receive optimal care. Given these differences, collection of additional data to estimate recurrent MBC survival would be of value.
We used the adjusted 6th edition stage IV to define MBC to only include tumors that have metastasized to distant sites. If instead we used SEER historical distant stage definition, prevalence would have been higher, as some tumors without a distant metastasis are included in this definition.
At one time, a diagnosis of distant recurrence or de novo Stage IV meant that death from BC was likely to be imminent. Today, with the development of new therapies that target the drivers of BC, and with improved palliative care, MBC is not the immediate death sentence it once was. With optimal care, women with MBC can and often do live for years with reasonable quality of life, albeit undergoing constant treatment to keep their disease under control.
This study demonstrates that there are a large number of women in the US living with MBC and that this number has increased in more recent years, likely the result of treatment and aging of the US population. This study demonstrates a growing burden of MBC in the US. It also makes clear that the majority of MBC patients, 3 out of 4 who are diagnosed with non-metastatic cancer but progress to distant disease, have never been properly documented. Given the growing burden of MBC, it is critical to collect data on recurrence in order to foster more research into the specific needs of this understudied population(5).
In an ideal world, a cancer registry would record the experiences of all patients throughout the entire cycle of disease, enabling researchers, health policy experts and planners, providers, patients and advocates to understand the full impact of cancer. Finding ways to incorporate information on metastatic disease progression would be an important advance and a key first step towards a comprehensive assessment of the population burden of disease.
Supplementary Material
Acknowledgments
Financial support: This work was supported by the National Cancer Institute at the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
The authors would like to thank Joan Warren and Julia Rowland whose comments/suggestions helped improve and clarify this manuscript. The authors acknowledge the members of the Metastatic Breast Cancer Alliance and patients living with MBC who inspired this work and encourage readers to learn more at www.mbcalliance.org.
References
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA: a cancer journal for clinicians. 2016 Jul;66(4):271–89. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011 Jan 19;103(2):117–28. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cleeland CS, Mayer M, Dreyer NA, Yim YM, Yu E, Su Z, et al. Impact of symptom burden on work-related abilities in patients with locally recurrent or metastatic breast cancer: Results from a substudy of the VIRGO observational cohort study. Breast. 2014 Dec;23(6):763–9. doi: 10.1016/j.breast.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Yang HC, Thornton LM, Shapiro CL, Andersen BL. Surviving recurrence: psychological and quality-of-life recovery. Cancer. 2008 Mar 1;112(5):1178–87. doi: 10.1002/cncr.23272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer M. Lessons learned from the metastatic breast cancer community. Semin Oncol Nurs. 2010 Aug;26(3):195–202. doi: 10.1016/j.soncn.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Aranda S, Schofield P, Weih L, Yates P, Milne D, Faulkner R, et al. Mapping the quality of life and unmet needs of urban women with metastatic breast cancer. Eur J Cancer Care. 2005 Jul;14(3):211–22. doi: 10.1111/j.1365-2354.2005.00541.x. [DOI] [PubMed] [Google Scholar]
- 7.Byrne J, Kessler LG, Devesa SS. The prevalence of cancer among adults in the United States: 1987. Cancer. 1992 Apr 15;69(8):2154–9. doi: 10.1002/1097-0142(19920415)69:8<2154::aid-cncr2820690823>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 8.De Angelis G, De Angelis R, Frova L, Verdecchia A. MIAMOD: a computer package to estimate chronic disease morbidity using mortality and survival data. Computer Methods and Programs in Biomedicine. 1994;44(2):99–107. doi: 10.1016/0169-2607(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 9.Verdecchia A, Capocaccia R, Egidi V, Golini A. A method for the estimation of chronic disease morbidity and trends from mortality data. Stat Med. 1989;8(2):201–16. doi: 10.1002/sim.4780080207. [DOI] [PubMed] [Google Scholar]
- 10.Clements MS, Roder DM, Yu XQ, Egger S, O’Connell DL. Estimating prevalence of distant metastatic breast cancer: a means of filling a data gap. Cancer Cause Control. 2012 Oct;23(10):1625–34. doi: 10.1007/s10552-012-0040-9. [DOI] [PubMed] [Google Scholar]
- 11.De Angelis R, Tavilla A, Verdecchia A, Scoppa S, Hachey M, Feuer EJ, et al. Breast cancer survivors in the United States: geographic Variability and Time Trends, 2005–2015. Cancer. 2009;115(9):1954–66. doi: 10.1002/cncr.24217. [DOI] [PubMed] [Google Scholar]
- 12.Surveillance Epidemiology and End Results Program (SEER) Adjusted AJCC 6th Edition Stage Classification [website] Available from https://seer.cancer.gov/seerstat/variables/seer/ajcc-stage/6th/#stage.
- 13.Yu B, Tiwari RC, Cronin KA, Feuer EJ. Cure fraction estimation from the mixture cure models for grouped survival data. StatMed. 2004;23(11):1733–47. doi: 10.1002/sim.1774. [DOI] [PubMed] [Google Scholar]
- 14.Yu B, Tiwari RC, Cronin KA, McDonald C, Feuer EJ. CANSURV: A Windows program for population-based cancer survival analysis. ComputMethods Programs Biomed. 2005;80(3):195–203. doi: 10.1016/j.cmpb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol. 2010 Nov;21(11):2169–74. doi: 10.1093/annonc/mdq220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giordano SH, Buzdar AU, Smith TL, Kau SW, Yang Y, Hortobagyi GN. Is breast cancer survival improving? Trends in survival for patients with recurrent breast cancer diagnosed from 1974 through 2000. Cancer. 2004 Jan 1;100(1):44–52. doi: 10.1002/cncr.11859. [DOI] [PubMed] [Google Scholar]
- 17.Chia SK, Speers CH, D’yachkova Y, Kang A, Malfair-Taylor S, Barnett J, et al. The impact of new chemotherapeutic and agents on survival in a population-based of women with metastatic breast cancer hormone cohort. Cancer. 2007 Sep 1;110(5):973–9. doi: 10.1002/cncr.22867. [DOI] [PubMed] [Google Scholar]
- 18.Pal SK, Dehaven M, Nelson RA, Onami S, Hsu J, Waliany S, et al. Impact of modern chemotherapy on the survival of women presenting with de novo metastatic breast cancer. Bmc Cancer. 2012 Sep;28:12. doi: 10.1186/1471-2407-12-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson RH, Chien FL, Bleyer A. Incidence of Breast Cancer With Distant Involvement Among Women in the United States, 1976 to 2009. Jama-J Am Med Assoc. 2013 Feb 27;309(8):800–5. doi: 10.1001/jama.2013.776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.