Abstract
Background
Cannabis abuse has been associated with psychopathology, including negative emotionality and a higher risk of psychosis, particularly with early age of initiation. However, the mechanisms underlying this association are poorly understood. Because aberrant dopamine (DA) signaling is implicated in cannabis-associated psychopathology, we hypothesized that regular cannabis abuse (CA) would be associated with altered resting functional connectivity in dopamine midbrain-striatal circuits.
Methods
We examined resting brain activity of subcortical regions in 441 young adults from the Human Connectome Project, including 30 CA meeting DSM criteria for dependence, and 30 controls matched on age, sex, education, BMI, anxiety, depression, and alcohol/tobacco usage.
Results
Across all subjects, local functional connectivity density (lFCD) hubs in subcortical regions were most prominent in ventral striatum, hippocampus, amygdala, dorsal midbrain, and the posterior-ventral brainstem. As hypothesized, CA showed markedly increased lFCD relative to controls in ventral striatum (where nucleus accumbens is located) and midbrain (where substantia nigra/ventral tegmental nuclei are located) but also in brainstem and lateral thalamus. These effects were observed in the absence of significant differences in subcortical volumes, and were most pronounced in the individuals who began cannabis use earliest in life and who reported high levels of negative emotionality.
Conclusions
Together, these findings suggest that chronic cannabis abuse is associated with changes in resting brain function, particularly in dopaminergic nuclei implicated in psychosis but that are also critical for habit formation and reward processing. These results shed light on neurobiological differences that may be relevant to psychopathology associated with cannabis use.
Keywords: resting state functional connectivity, fMRI, graph theory, addiction, basal ganglia, emotionality, marijuana
Introduction
Cannabis is one of the most widely used addictive substances in the United States, with 44% of individuals over the age of 12 reporting cannabis use at least once in their lifetime (1). Despite current efforts to legalize cannabis, little is known about the long-term effects of cannabis abuse (CA) on brain function and neuropsychiatric outcomes. Of particular concern has been the association between regular CA and psychiatric symptoms such as amotivation, negative emotionality (2, 3) and a heightened risk for psychosis (4). Indeed, CA was associated with up to a 6 fold increase in the risk of schizophrenia in early-onset users (5, 6) and with the use of cannabis with high THC (7). The increased risk remains after controlling for other substances of abuse and for familial risk of psychosis (8). Aberrant dopaminergic function in the midbrain-striatal circuitry, a hallmark feature of schizophrenia, may underlie this association (9). Accordingly, CA with genetic variants that confer high midbrain-striatal dopamine (DA), including the DRD2 rs1076560 T allele, the DAT1 3′ 9-repeat allele, and the AKT1 rs2494732 C allele, have an increased risk of psychosis compared with CA that do not have these genetic variants (10–12). However, the effects of chronic CA on the functional organization of subcortical regions modulated by DA, and their relevance for psychiatric symptoms, is poorly understood.
Resting-state functional magnetic resonance imaging (rsfMRI) offers a non-invasive method for probing the functional connectedness of neural circuits. By measuring correlations among spontaneous low-frequency blood-oxygen-level dependent (BOLD) signals, studies have revealed the involvement of functional changes in subcortical circuits in psychiatric diseases including schizophrenia. For instance, functional connectivity between reward processing regions, such as nucleus accumbens (NAc) and orbitofrontal cortex (OFC), appears to be related to disrupted DA function, and as such, has clinical relevance: higher intrinsic connectivity correlated with amotivation syndrome (13) and with the duration that schizophrenia had been left untreated (14). Intriguingly, a similar pattern of NAc-OFC hyperconnectivity was reported in CA (15). However, the relevance of these effects for psychopathology in CA is unknown. Further, prior investigations in CA have relied mainly on seed-based connectivity analyses.
In contrast, local functional connectivity density (lFCD; the size of a local cluster of correlated voxels) is a data-driven method for identifying functional hubs in the brain (16). lFCD accounts for up to 70% of resting brain metabolism (17), and therefore is an index of local brain activity that has superior spatiotemporal resolution to PET imaging. We recently used this method to identify functional connectivity changes that were associated with cognitive and mood-related behaviors in heavy drinkers (18). To our knowledge, no studies have examined the effects of CA on subcortical functional hub organization, and its relevance to negative emotionality, which is elevated in CA (3) and schizophrenia (19). Intriguingly, recent studies using a very similar approach found subcortical hyperconnectivity in a cohort of 95 individuals with Schizophrenia (20). We hypothesized similar effects may be observed in CA. To test this hypothesis, we took advantage of the large dataset produced by the Human Connectome Project (21). While the HCP does not have targeted measures that specifically assess psychosis, they do offer measures of negative emotionality, a symptom shared between CA and Schizophrenia (2, 22) that we have previously found to be associated with subcortical dopaminergic function in CA (3). Thus, while the present study does not directly study individuals with schizophrenia, negative emotionality is relevant in light of the emerging view that psychiatric disorders represent clusters of symptoms and traits that are elevated over a spectrum of normal functioning (23–26), and that elevated negative emotionality predicts development of psychosis (27). We were particularly interested in one aspect of negative emotionality: symptoms of alienation (beliefs that others wish them harm, and that they are deceived by friends), after our recent investigation demonstrated that this may be particularly affected in CA and associated with aberrant brain function (2).
Materials and Methods
Participants
We analyzed data from the S500 release (https://www.humanconnectome.org/documentation/S500/index.html) of the WU-Minn HCP Consortium (21). We only included participants who had a) complete structural and rsfMRI imaging data that passed a quality assurance check, and b) complete measures of cognitive function and emotionality (total n=441 participants). The HCP initiative studied young adults aged 22–35 from a wide range of backgrounds and behavioral profiles representative of the population at large. Thus, while all participants are considered generally healthy, participants with sub-clinical psychiatric symptomatology and recreational drug use are included.
Of the 441 participants, 36 met the DSM-IV criteria for cannabis dependence (see Supplementary Material for a description). Three participants were excluded for comorbid alcohol dependence and one was excluded for anxiety and depression ratings > 3 SD from the group mean. Recent studies have indicated that it is critical in studies of cannabis abuse to select a well-matched control group, particularly on measures of alcohol and tobacco usage, e.g., (28). Therefore, we matched groups on age, sex, education, BMI, anxiety, depression, and alcohol and tobacco usage (we calculated composite tobacco/alcohol usage the same way as a recent study of HCP data; see Supplementary Material and (29)). Two subjects from each group were excluded to ensure groups were matched on tobacco usage (Supplementary Material), therefore the final sample included 30 CA and 30 controls; demographics and statistical tests are presented in Table 1.
Table 1.
Demographics of the CA and control samples. Values are reported as mean ± standard deviation. Depression, anxiety, tobacco, and alcohol use values were converted to Z-scores based on the larger population of 441 participants. See Tobacco and Alcohol Use section in Supplementary Material for a description of how the combined past- and present use measures were derived.
| CA | CTRL | t-test p-value | |
|---|---|---|---|
| Age | 29.17 ± 3.07 | 30.23 ± 2.74 | 0.161 |
| Sex | 22 Male | 20 Male | χ2 = 0.573 |
| Edu | 14.6 ± 1.89 | 14.6 ± 1.92 | 1.000 |
| BMI | 27.17 ± 3.6 | 26.83 ± 4.89 | 0.757 |
| DSM Depression | 0.03 ± 0.86 | −0.13 ± 0.83 | 0.452 |
| DSM Anxiety | 0.07 ± 1.04 | −0.16 ± 0.95 | 0.383 |
| Alcohol Use (Composite Z) | 0.27 ± 0.4 | 0.15 ± 0.38 | 0.250 |
| Tobacco Use (Composite Z) | 0.57 ± 0.83 | 0.32 ± 0.85 | 0.260 |
Behavioral Measures of Interest
We examined data related to cognitive function and negative emotionality, given the interest in potential chronic effects of cannabis in these domains (30). Participants completed various NIH toolbox measures as part of the HCP. We were particularly interested in relating the current work to our previous findings that CA are vulnerable to feelings of alienation, i.e. the belief that others wish them harm, and that they are betrayed or deceived by friends (2). However, our previous work used the Multidimensional Personality Questionnaire (MPQ) and this was not administered as part of the HCP protocol. Therefore, we attempted to find analogous measures for the three main domains of the MPQ: stress reactivity, aggression, and alienation. For stress reactivity, we used the “perceived stress” measure; for aggression, we averaged together the Z-scores of “Anger Hostility” and “Anger Aggression” (i.e., one’s own behavior in the anger and aggressive domains); and for alienation we averaged together the Z-scores of “Perceived Hostility” and “Perceived Rejection” (i.e., how one perceives others behaving towards them). We then averaged together these stress, aggression, and alienation measures together for a composite negative emotionality score. All three domains were included to examine if the effects were specific to alienation. More comprehensive descriptions of cognitive and emotional measures are available in Supplementary Material and https://wiki.humanconnectome.org/display/PublicData/HCP+Data+Dictionary+Public+500+Subject+Release.
MRI image acquisition and preprocessing
All brain images were collected on a Siemens 3T “connectome Skyra” scanner with a 32-channel coil at Washington University in St. Louis. T1- and T2-weighted anatomical scans were acquired (FOV=224 mm, matrix=320, 256 slices, 0.7 mm isotropic voxels). rsfMRI scans were acquired with an EPI sequence (Multiband factor = 8, TR = 720 ms, TE = 33.1 ms, flip angle = 52°, FOV = 208 mm, 104 × 90 matrix, 72 slices of 2 mm isotropic voxels, no gap). Two sessions were completed with two rsfMRI scans (one LR and one RL phase encoding) in each session. Each scan was 14:33 min, for a total of 54:15 min. For rsfMRI, participants were instructed to lie with eyes open, to relax and look at a white cross on a dark background, think of nothing and to not fall asleep. For further details on image acquisition, see https://www.humanconnectome.org/documentation/S500/HCP_S500+MEG2_Release_Appendix_I.pdf.
For analysis of rsfMRI data, we used the “minimal preprocessing” datasets (hp2000_clean.nii files), where preprocessing included: a) gradient distortion correction, b) rigid body realignment, c) field map processing, d) nonlinear normalization to MNI space, e) high-pass filtering with independent component analysis (ICA)-based denoising, and f) brain masking. In our own subsequent preprocessing, we removed timepoints that were severely affected by motion using a ‘scrubbing’ approach (Supplementary Material). Remaining motion effects on fMRI time series were regressed out using the six translation and rotation regressors. Finally, band-pass temporal filtering (0.01–0.10 Hz) was applied. lFCD was computed separately on each of the four runs of processed, unsmoothed data, masked by each participant’s FreeSurfer subcortical parcellation (wmparc.2.nii.gz), which included bilateral thalamus, caudate, putamen, pallidum, amygdala, nucleus accumbens, hippocampus, midbrain and brainstem (see Local FCD section below). Finally, the four resulting lFCD maps (LR/RL; REST1/REST2) were averaged together and averaged images were smoothed at 2mm FWHM.
Local FCD (lFCD) analysis
The Pearson correlation was used to assess the strength of functional connectivity, Cij, between voxels i and j. A positive correlation threshold of r = 0.2 (sufficient to Bonferroni correct for the number of correlations performed in the subcortical mask, p < 1 × 10−4) was used to compute the binary connectivity coefficients, aij = 1 (if Cij > 0.2) or aij = 0 (if Cij ≤ 0.2). This threshold was lower than previous investigations (16) to have sensitivity to detect effects in subcortical regions that have noisier signals than the neocortex, and hence have weaker observed resting-state correlations (31). The local FCD (or ‘local degree’) for voxel i was computed as the size of a continuous cluster of voxels with aij = 1, that are connected by surface. A ‘growing’ algorithm was used for time-efficient estimation of lFCD (16).
Seed-based functional connectivity analysis
To examine functional connectivity differences with other regions of the brain, we computed seed-based connectivity using the same methods as our previous work (32, 33; Supplementary Material).
Statistical analysis
Second-level statistical analyses were conducted in SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/) for imaging data and in Graphpad Prism 7.02 (San Diego, CA) for behavioral data. First, to examine lFCD across the larger population, we conducted a one-sample T-test of lFCD across all 441 participants. Next, to compare CA with the matched control group, we conducted a two-sample T-test of lFCD between groups. These analyses were thresholded at p < .001, uncorrected, with a cluster-level correction of p < .05 family-wise error (FWE) corrected, and a minimum cluster size of k = 100 voxels. To control cluster-level type I error rates (34), we calculated cluster corrections with the Statistical nonparametric mapping toolbox (SnPM13: http://warwick.ac.uk/snpm; 5000 permutations). Because lFCD has a power law distribution (16), we also conducted analyses with log-transformed lFCD values; this did not alter the findings, and so we report these data in Supplementary material. We also conducted two-sample T-tests on the volume of subcortical nuclei (from FreeSurfer output), as well as measures of cognition and negative emotionality. To examine if subcortical lFCD had relevance for aberrant cognition and/or negative emotionality in CA, we conducted correlation analysis between lFCD in regions showing significant group differences and in behavioral measures showing significant group differences.
Results
Demographics and behavioral measures
Demographics and lifestyle factors with descriptive statistics are presented in Table 1. The groups did not significantly differ on any of the DSM-oriented scales, including depression, ADHD, panic disorder, agoraphobia, anxiety, and somatic problems (all p’s > .15), except the CA group reported higher levels of antisocial behavior (p = .05) and more childhood conduct problems (p = .008). Cognitive scores and measures of negative emotionality are presented in Table 2. Notably, while there were no obvious differences in cognitive performance, the CA group showed significantly higher levels of negative emotionality (t(58) = 2.14, p = .036), particularly alienation (t(58) = 2.34, p = .023), in line with our previous work (2, 3).
Table 2.
Scores on measures of cognition and negative emotionality in CA and controls. Values are reported as mean ± standard deviation. Raw values for each measure were converted to Z-scores based on the larger population of 441 participants.
| CA | CTRL | t-test p-value | |
|---|---|---|---|
| Cognition (Composite Z) | 0.02 ± 0.49 | −0.05 ± 0.49 | 0.572 |
| Episodic Memory | −0.33 ± 1.16 | −0.11 ± 0.99 | 0.440 |
| Working Memory | −0.07 ± 0.9 | 0.17 ± 1.08 | 0.344 |
| Flexibility | 0.17 ± 0.96 | −0.05 ± 0.82 | 0.343 |
| Inhibitory Control | 0.03 ± 0.97 | 0.09 ± 0.93 | 0.799 |
| Processing Speed | 0.1 ± 0.85 | −0.09 ± 1.25 | 0.494 |
| Delay Discounting | 0.02 ± 0.85 | −0.28 ± 1.06 | 0.229 |
| Fluid Intelligence | 0.28 ± 0.79 | −0.03 ± 0.96 | 0.183 |
| Spatial Orientation | 0.22 ± 0.98 | 0.01 ± 0.96 | 0.393 |
| Verbal Episodic Memory | −0.24 ± 1.04 | −0.18 ± 0.93 | 0.799 |
| Negative Emotionality (Composite Z) | 0.35 ± 0.74 | −0.05 ± 0.71 | 0.036 |
| Aggression | 0.42 ± 0.96 | 0.14 ± 0.82 | 0.240 |
| Alienation | 0.43 ± 0.85 | −0.1 ± 0.92 | 0.022 |
| Stress | 0.2 ± 1.05 | −0.2 ± 1.02 | 0.138 |
Subcortical Volume
Volumetric data and descriptive statistics are reported in Supplementary Table S1. In line with recent work (28, 29), no subcortical regions showed significantly different volume between CA and controls. However, CA did show a trend towards smaller volume of the left hippocampus (p = .068), consistent with findings of structural hippocampal abnormalities by prior studies in CA (35).
lFCD Analyses
We first conducted a voxelwise one-sample t-test of lFCD across all 441 participants. Results showed widespread lFCD; to summarize, peaks were observed in ventral striatum, hippocampus, amygdala, midbrain, and the posterior-ventral brainstem (Figure 1; see Supplementary Figure S2 for maps restricted to CA and control groups). We then examined group differences in lFCD between CA and matched controls. In voxelwise two-sample T-tests, CA demonstrated significantly higher lFCD in the ventral striatum, dorsal midbrain (including substantia nigra and ventral tegmental area), brainstem, and lateral thalamus (all p’s < 1×10−5, Figure 2a,b). Motion estimates were highly similar between the CA and control groups (mean framewise displacement across all images for CA: 0.171 ± 0.05, and for controls: 0.163 ± 0.05; t(58) = −.557, p = .580); results were nearly identical when including motion or FreeSurfer-estimated subcortical volume as covariates in the model. Because the lFCD values across these four ROIs was highly correlated across subjects (mean bivariate correlation: r = 0.78), we averaged together the lFCD values across the four ROIs to increase statistical power; subsequent analyses refer to this averaged value. This averaged lFCD value did not significantly correlate with FreeSurfer subcortical volume estimates across subjects (r = −.10, p = .432).
Figure 1.
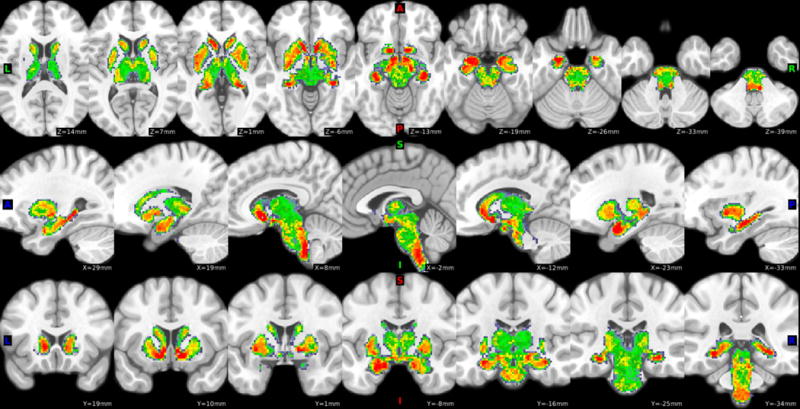
Subcortical lFCD results across the larger population of 441 HCP participants. Maps are thresholded at T > 10, for visualization. Hot colors indicate regions with high local connectivity density.
Figure 2.
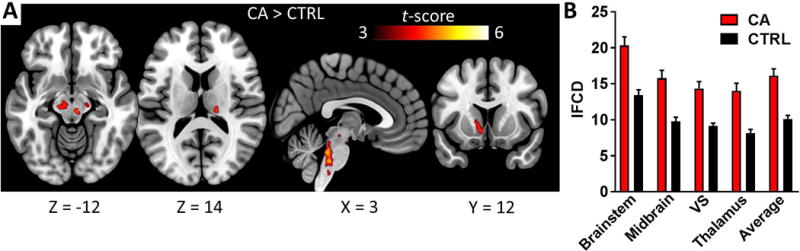
Subcortical regions where lFCD was significantly higher in CA than controls (two-sample t-test, CA > CTRL). Results are thresholded voxelwise at p < .001, with a nonparametric cluster-level threshold of p < .05 family-wise error (FWE) corrected, using SnPM13. See Table 3 for coordinates of each cluster in MNI space. Error bars represent standard error of the mean (SEM). NOTE: SN/VTA = Substantia Nigra/Ventral Tegmental Area; VS = Ventral Striatum
In whole-brain functional connectivity analysis using the four clusters from Figure 2A as seed regions, no significant between-group differences emerged at an exploratory threshold of p < .005 uncorrected.
Early onset of cannabis abuse in life is associated with a higher risk for poor neuropsychiatric outcomes (36). Therefore, we ran a one-way analysis of variance (ANOVA) between subcortical lFCD and self-reported age of first use. Indeed, subcortical lFCD was significantly different across age of first use, F(4,55) = 4.13, p = .005, such that higher lFCD was associated with earlier age of cannabis use onset (Figure 3a). In a two-way ANOVA including group and sex as factors, there was no significant main effect of sex on lFCD (F(1,56) = .49, p = .488), nor was there a significant group by sex interaction (F(1,56) = .09, p = .761). Finally, because CA reported significantly higher feelings of alienation than controls, in line with our previous work (2), we ran an across-subject correlation between the alienation scores and subcortical lFCD. CA showed a significant correlation between lFCD and alienation scores (r = .43, p = .019), whereas the control subjects did not (r = −.09, p = .615) (Figure 3b). The correlation among CA may be most strongly driven by lFCD near the midbrain; see Supplementary Figure S3 for a voxelwise regression analysis. These results remained significant when conducting a partial correlation to control for the FreeSurfer-estimated subcortical volume of each subject (CA: r = .43, p = .04; controls: r = .02, p = .895). The difference in slopes between CA and controls was significant, F(1,56) = 5.95, p = .018.
Figure 3.
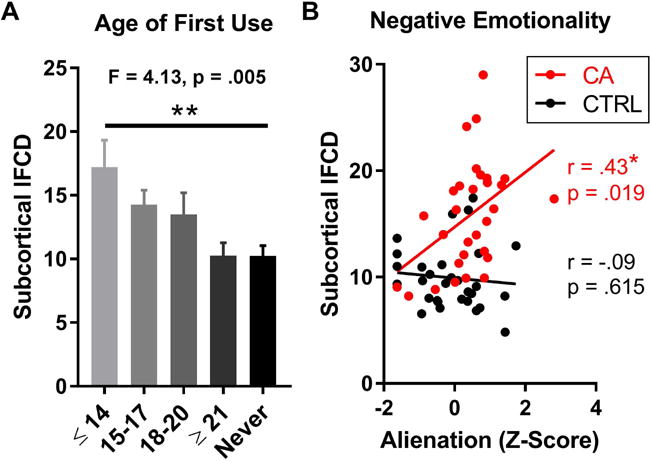
Associations between subcortical lFCD and A) age at first use of cannabis and B) self-reported feelings of alienation. The difference in slopes between CA and CTRL groups was significant, p = .018.
Discussion
Despite the high prevalence of cannabis use, little is known about potential chronic effects of CA on brain function and behavior. Here, we demonstrate that heavy CA is associated with a marked increase in subcortical lFCD, including the midbrain (where the main DA nuclei are located) and the ventral striatum, relative to a well-matched control group. These effects are not explained by volumetric differences, and they associate with critical features of CA: hyperconnectivity was most pronounced in early-onset CA, a demographic that is particularly vulnerable to the harmful effects of CA (36), and in those reporting the highest levels of negative emotionality, particularly alienation. These findings indicate that the resting functional organization of the subcortical regions is altered in CA, and this may have relevance for some of the adverse effects of early-onset CA, including emotional disturbance and increased risk for psychosis.
Increased lFCD in the ventral striatum (VS) and midbrain, including regions where the substantia nigra and ventral tegmental area are located, may be related to hyperdopaminergia in CA. Indeed, using PET and [11C]raclopride to measure DA-induced changes to methylphenidate we found that CA when compared to controls showed increased DA release in the midbrain, though they showed an attenuated response in striatal regions (3). Functional connectivity between VS and VTA is higher in patients with Schizophrenia with symptoms of hyperdopaminergia, such as auditory and visual hallucinations, than in patients who do not experience these symptoms (37). Further, in healthy adults and in rats, drugs that increase (levodopa) and decrease (haloperidol) DA signaling have been demonstrated to increase and decrease functional connectivity of these regions, respectively (38, 39). However, it is important to note that the findings from these seed-based connectivity studies are likely network-specific, because abnormal DA levels attenuate the connectivity between different resting state networks (33, 39). This may explain why CA show hypoconnectivity between nodes of the mesolimbic reward network and nodes of the salience network; e.g. between the dopaminergic midbrain and insula (40) and between NAc and dorsal anterior cingulate cortex (41). Interestingly, our seed-based connectivity analysis from these regions did not yield significant group differences. Thus, while previous studies have observed long-range subcortical-cortical connectivity alterations in CA, the current results appear to be confined to local hub differences in subcortical circuits. There are at least two possible explanations for this. First, this study carefully controlled for factors such as alcohol and tobacco usage, which may have influenced findings from previous studies. Second, the HCP uses a high-resolution sequence with an aggressive multiband factor, and this contributes to lower subcortical signal-to-noise ratio than is observed with low-resolution sequences. lFCD is more resilient to noise than seed-voxel correlations because lFCD capitalizes on locally shared synchrony and high sampling rate, which makes it possible to reach significant correlations in the absence of significant long-range synchrony. Nevertheless, our lFCD results seem to be broadly in line with previous studies using the FCD technique, although evidence is limited. For instance, subcortical global FCD (a measure that is highly correlated with lFCD, (42)) is increased in schizophrenia relative to healthy controls ((20), but see (35), where the dopaminergic medication status of the patients was unknown).
On the other hand, increased lFCD in the VS and midbrain may be a general consequence of pathology to these circuits, as this pattern is observed in various conditions with aberrant DA signaling. Subcortical lFCD is increased in aging (44), ADHD (45), and cocaine use disorder (46), which are all implicated in altered dopaminergic function (47–49). These results are also generally in line with the notion that altered connectivity in high-cost hubs is linked to neuropsychiatric disease burden (50). An important next step is to examine how tonic, resting subcortical hyperconnectivity may have consequences for phasic DA-dependent processes that are altered in CA, such as punishment-based learning. CA show altered subcortical activations and impaired learning from non-drug rewards and punishment (51, 52). If higher resting subcortical lFCD is indeed due to higher tonic DA transmission, then this increased baseline activity would confer weaker ability to generate the “dips” in activity necessary to learn from negative outcomes, in line with extant models of dopaminergic function (53). Future studies with combined PET-fMRI could examine this possibility.
We also observed heightened lFCD in CA relative to controls in the pulvinar nucleus of the thalamus and in the brainstem, regions critical for sensory processing and maintenance of autonomic functions, respectively. CA is hypothesized to increase thalamic neuronal excitability, disrupt burst firing patterns and impair thalamocortical connectivity, leading to impaired sensory processing (54). In correspondence, we find increased local thalamic connectivity while others found decreased thalamocortical connectivity in CA (55), and both are exacerbated in early-onset CA. In addition, CA show hyperactive thalamic responses to cannabis cues, which correlate with subjective craving of cannabis and are thought to contribute to sensorimotor deficits (56, 57). There has been comparatively less attention on changes to brainstem function in CA, perhaps because this region has lower concentrations of cannabinoid receptors than the basal ganglia (58). Yet CA impacts functions regulated by the brainstem region identified here, which includes the ventral raphe nuclei extending into the nucleus of the solitary tract. For instance, regular CA disrupts rapid eye movement (REM) sleep and increases insomnia (59, 60) and negatively influences mood (61). Interestingly, in individuals with post-traumatic stress disorder, sleep disturbance is associated with heightened brainstem glucose metabolism (62), a measure that strongly correlates with lFCD (17). More work is needed to describe how changes to brain functional organization in CA have relevance for sensory and autonomic functions.
Finally, subcortical hyperconnectivity was most pronounced in early-onset CA and correlated with feelings of alienation (especially in the midbrain). CA is thought to be particularly detrimental in adolescence because the brain is in a critical period of increased myelination and extensive synaptic pruning (63). Subcortical cannabinoid receptor development is ongoing at this time, and exogenous cannabis perturbs the normal development of the mesolimbic system, which is thought to contribute to psychopathology (64). It is well-established that early-onset CA have poor cognitive and emotional outcomes (36), but the neural basis of this phenomenon is not well understood. Prior rsfMRI studies suggested that increased functional connectivity within cortical networks involved in self-awareness, including the salience and default-mode networks, could lead to aberrant emotional/motivational processing (40). We also recently showed that glucose metabolism in inferior frontal gyrus negatively correlated with feelings of alienation in CA (2). These findings, together with the current data provide convergent evidence supporting the notion that impaired prefrontal regulation of subcortical activity contributes to the negative emotionality seen in addictions (3, 47).
The data presented here build on the small body of work in CA using HCP data. An initial investigation using structural MRI data found that effects of cannabis exposure on subcortical volumetry were minimal, but critically, they concluded that cannabis effects may be stronger in DA-rich regions including the VS and in the most frequent CA (65). Another diffusion-tensor imaging study examined 466 individuals reporting at least one lifetime experience with cannabis, and found that frequency of cannabis use did not associate with cortical volumes, but did associate with changes to the shape of the amygdala and hippocampus (29). Most notably, they observed that early-onset CA was associated with altered shape of the nucleus accumbens and loss of white matter integrity throughout the cortex. To our knowledge, the present study is the first to extend HCP investigations of CA to rsfMRI data. Because the HCP project is open access, there is a rich opportunity for further examination of the chronic effects of CA using a common dataset.
Limitations
The HCP does not have in vivo measures of subcortical DA release or receptor function, and so we could not directly assess the hypothesis of hyperdopaminergia and psychosis risk in CA. Resting-state lFCD is an indirect measure of neuronal activity and the true neurobiological basis of this measure needs further exploration. Further, how exactly hyperdopaminergia manifests at the neural level is disputed. While CA with psychosis do not show elevated striatal DA release, stimulant-induced changes in DA correlate with psychosis, suggesting that hyperdopaminergia may be more related to postsynaptic hypersensitivity than to total levels of synaptic DA (66, 67). Nevertheless, the final release of the HCP will include single-nucleotide polymorphism (SNP) data for all participants; future studies should examine how genetic differences predicting D2/3 function, e.g. Taq1A and C957T SNPs, predict risk for CA and subcortical lFCD, which would help shed light on this issue. Additionally, it remains unknown whether emotional disturbance is directly caused by CA or if individuals use cannabis to self-medicate feelings of negative emotionality (30). Finally, we cannot rule out the possibility that subcortical hyperconnectivity may be associated with cannabis withdrawal and the extent to which it abates with prolonged abstinence, as has been observed with other functional connectivity abnormalities in CA (41).
Conclusion
Despite increased usage of cannabis worldwide, little is known about the neuropsychiatric effects of CA, especially in early-onset users. Here we show that resting connectivity of subcortical functional hubs, particularly within dopaminergic nuclei implicated in psychopathology, is greatly increased in CA. This pattern was exaggerated in individuals who began using in early adolescence and were associated with high levels of negative emotionality. Thus, subcortical functional connectivity may be a useful marker for tracking the development of psychopathology with prolonged CA.
Supplementary Material
Table 3.
Subcortical regions where lFCD was significantly higher in CA than controls (two-sample t-test, CA > CTRL). Results are thresholded voxelwise at p < .001, with a nonparametric cluster-level threshold of p < .05 family-wise error (FWE) corrected, using SnPM13. Note that there were no significant results for the reverse contrast (i.e., CTRL > CA). NOTE: SN/VTA = Substantia Nigra/Ventral Tegmental Area; VS = Ventral Striatum
| MNI Coordinates (mm) |
||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Cluster Size (mm3) | FWE-corrected p-value | Peak t-value | X | Y | Z | Identified Region |
| 2544 | .008 | 5.20 | 20 | −20 | 6 | Right Thalamus |
| 4.15 | 18 | −22 | −6 | |||
| 4.11 | 6 | −26 | −12 | |||
| 3880 | .002 | 4.77 | 2 | −36 | −30 | Brainstem |
| 4.70 | 2 | −34 | −40 | |||
| 4.69 | −8 | −34 | −30 | |||
| 1968 | .002 | 4.58 | −16 | −20 | −10 | Midbrain (including SN/VTA) |
| 4.18 | −22 | −12 | −6 | |||
| 4.06 | −28 | −12 | 0 | |||
| 976 | .017 | 4.36 | −10 | 20 | −6 | VS |
| 3.94 | −8 | 12 | −8 | |||
| 3.84 | −12 | 12 | 2 | |||
Acknowledgments
The authors would like to thank Şükrü Barış Demiral, Corinde Wiers, and Ehsan Shokri Kojori for their helpful comments and discussions.
Funding
This work was accomplished with support from the National Institute on Alcohol Abuse and Alcoholism (Y1AA-3009).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.National survey on drug use and health. Cent Behav Heal Stat Qual. Rockville, MD: 2015. [Google Scholar]
- 2.Wiers CE, Shokri-Kojori E, Wong CT, Abi-Dargham A, Demiral ŞB, Tomasi D, et al. Cannabis Abusers Show Hypofrontality and Blunted Brain Responses to a Stimulant Challenge in Females but not in Males. Neuropsychopharmacology. 2016:1–10. doi: 10.1038/npp.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volkow ND, Wang G-J, Telang F, Fowler JS, Alexoff D, Logan J, et al. Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. Proc Natl Acad Sci. 2014;111:E3149–E3156. doi: 10.1073/pnas.1411228111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andréasson S, Engström A, Allebeck P, Rydberg U. Cannabis and Schizophrenia: A Longitudinal Study of Swedish Conscripts. Lancet. 1987;330:1483–1486. doi: 10.1016/s0140-6736(87)92620-1. [DOI] [PubMed] [Google Scholar]
- 6.Galvez-Buccollini JA, Proal AC, Tomaselli V, Trachtenberg M, Coconcea C, Chun J, et al. Association between age at onset of psychosis and age at onset of cannabis use in non-affective psychosis. Schizophr Res. 2012;139:157–160. doi: 10.1016/j.schres.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Forti M, Sallis H, Allegri F, Trotta A, Ferraro L, Stilo SA, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40:1509–1517. doi: 10.1093/schbul/sbt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Os J, Bak M, Hanssen M, Bijl RV, De Graaf R, Verdoux H. Cannabis use and psychosis: A longitudinal population-based study. Am J Epidemiol. 2002;156:319–327. doi: 10.1093/aje/kwf043. [DOI] [PubMed] [Google Scholar]
- 9.Sherif M, Radhakrishnan R, D’Souza DC, Ranganathan M. Human laboratory studies on cannabinoids and psychosis. Biol Psychiatry. 2016;79:526–538. doi: 10.1016/j.biopsych.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Colizzi M, Iyegbe C, Powell J, Ursini G, Porcelli A, Bonvino A, et al. Interaction between functional genetic variation of DRD2 and cannabis use on risk of psychosis. Schizophr Bull. 2015;41:1171–1182. doi: 10.1093/schbul/sbv032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharyya S, Atakan Z, Martin-Santos R, Crippa JA, Kambeitz J, Prata D et al. Preliminary report of biological basis of sensitivity to the effects of cannabis on psychosis: AKT1 and DAT1 genotype modulates the effects of δ-9-tetrahydrocannabinol on midbrain and striatal function. Mol Psychiatry. 2012;17:1152–1155. doi: 10.1038/mp.2011.187. [DOI] [PubMed] [Google Scholar]
- 12.Di Forti M, Iyegbe C, Sallis H, Kolliakou A, Falcone MA, Paparelli A, et al. Confirmation that the AKT1 (rs2494732) genotype influences the risk of psychosis in cannabis users. Biol Psychiatry. 2012;72:811–816. doi: 10.1016/j.biopsych.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Park H, Il, Lee BC, Kim J-J, Il Kim J, Koo M-S. Effort-based reinforcement processing and functional connectivity underlying amotivation in medicated patients with depression and schizophrenia. J Neurosci. 2017;37:2524–16. doi: 10.1523/JNEUROSCI.2524-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarpal DK, Robinson DG, Fales C, Lencz T, Argyelan M, Karlsgodt KH, et al. Relationship between Duration of Untreated Psychosis and Intrinsic Corticostriatal Connectivity in Patients with Early Phase Schizophrenia. Neuropsychopharmacology. 2017:1–8. doi: 10.1038/npp.2017.55. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, et al. Long-term effects of marijuana use on the brain. Proc Natl Acad Sci. 2014;111:16913–16918. doi: 10.1073/pnas.1415297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomasi D, Volkow ND. Functional connectivity density mapping. Proc Natl Acad Sci. 2010;107:9885–9890. doi: 10.1073/pnas.1001414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomasi D, Wang G, Volkow N. Energetic cost of brain functional connectivity. Proc Natl Acad Sci. 2013;110:13642–13647. doi: 10.1073/pnas.1303346110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shokri-Kojori E, Tomasi D, Wiers CE, Wang G-J, Volkow ND. Alcohol affects brain functional connectivity and its coupling with behavior: greater effects in male heavy drinkers. Mol Psychiatry. 2016:1–11. doi: 10.1038/mp.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson S, Sponheim SR. Dimensions underlying psychotic and manic symptomatology: Extending normal-range personality traits to schizophrenia and bipolar spectra. Compr Psychiatry. 2014;55:1809–1819. doi: 10.1016/j.comppsych.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Zhuo C, Zhu J, Wang C, Qu H, Ma X, Tian H, et al. Brain structural and functional dissociated patterns in schizophrenia. BMC Psychiatry. 2017;17:1–8. doi: 10.1186/s12888-017-1194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TEJ, Bucholz R, et al. The Human Connectome Project: A data acquisition perspective. Neuroimage. 2012;62:2222–2231. doi: 10.1016/j.neuroimage.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berenbaum H, Fujita F. Schizophrenia and personality: exploring the boundaries and connections between vulnerability and outcome. J Abnorm Psychol. 1994;103:148–158. doi: 10.1037//0021-843x.103.1.148. [DOI] [PubMed] [Google Scholar]
- 23.Watson D, Watson D, Clark LA, Clark LA, Harkness AR, Harkness AR. Structures of personality and their relevance to psychopathology. J Abnorm Psychol. 1994;103:18–18. [PubMed] [Google Scholar]
- 24.Kotov R, Gamez W, Schmidt F, Watson D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychol Bull. 2010;136:768–821. doi: 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- 25.Krueger RF, Tackett JL. Personality and psychopathology: working toward the bigger picture. J Pers Disord. 2003;17:109–128. doi: 10.1521/pedi.17.2.109.23986. [DOI] [PubMed] [Google Scholar]
- 26.Insel T, Cuthbert B, Garvie M, Heinssen R, Pine DS, Quinn K, et al. Research Domain Criteria (RDoC): Toward a New Classification Framework for Research on Mental Disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 27.Horan WP, Blanchard JJ, Clark LA, Green MF. Affective traits in schizophrenia and schizotypy. Schizophr Bull. 2008;34:856–874. doi: 10.1093/schbul/sbn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiland BJ, Thayer RE, Depue BE, Sabbineni A, Bryan AD, Hutchison KE. Daily Marijuana Use Is Not Associated with Brain Morphometric Measures in Adolescents or Adults. J Neurosci. 2015;35:1505–1512. doi: 10.1523/JNEUROSCI.2946-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orr JM, Paschall CJ, Banich MT. Recreational marijuana use impacts white matter integrity and subcortical (but not cortical) morphometry. NeuroImage Clin. 2016;12:47–56. doi: 10.1016/j.nicl.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, et al. Effects of Cannabis Use on Human Behavior, Including Cognition, Motivation, and Psychosis: A Review. JAMA psychiatry. 2016;73:292–297. doi: 10.1001/jamapsychiatry.2015.3278. [DOI] [PubMed] [Google Scholar]
- 31.DeDora DJ, Nedic S, Katti P, Arnab S, Wald LL, Takahashi A, et al. Signal fluctuation sensitivity: An improved metric for optimizing detection of resting-state fMRI networks. Front Neurosci. 2016;10:1–15. doi: 10.3389/fnins.2016.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manza P, Zhang S, Hu S, Chao HH, Leung H-C, Li C-SR. The effects of age on resting state functional connectivity of the basal ganglia from young to middle adulthood. Neuroimage. 2015;107:311–22. doi: 10.1016/j.neuroimage.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manza P, Zhang S, Li C-SR, Leung H-C. Resting-state functional connectivity of the striatum in early-stage Parkinson’s disease: Cognitive decline and motor symptomatology. Hum Brain Mapp. 2016;37:648–662. doi: 10.1002/hbm.23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci. 2016;201602413 doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nader DA, Sanchez ZM. Effects of regular cannabis use on neurocognition, brain structure, and function: a systematic review of findings in adults. Am J Drug Alcohol Abuse. 2017:1–15. doi: 10.1080/00952990.2017.1306746. [DOI] [PubMed] [Google Scholar]
- 36.Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RSE, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci. 2012;109:E2657–E2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rolland B, Amad A, Poulet E, Bordet R, Vignaud A, Bation R, et al. Resting-state functional connectivity of the nucleus accumbens in auditory and visual hallucinations in schizophrenia. Schizophr Bull. 2015;41:291–299. doi: 10.1093/schbul/sbu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cole DM, Oei NYL, Soeter RP, Both S, van Gerven JMA, Rombouts SARB, Beckmann CF. Dopamine-dependent architecture of cortico-subcortical network connectivity. Cereb Cortex. 2013;23:1509–16. doi: 10.1093/cercor/bhs136. [DOI] [PubMed] [Google Scholar]
- 39.Cole DM, Beckmann CF, Oei NYL, Both S, van Gerven JMA, Rombouts SARB. Differential and distributed effects of dopamine neuromodulations on resting-state network connectivity. Neuroimage. 2013;78:59–67. doi: 10.1016/j.neuroimage.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 40.Pujol J, Blanco-Hinojo L, Batalla A, López-Solà M, Harrison BJ, Soriano-Mas C, et al. Functional connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. J Psychiatr Res. 2014;51:68–78. doi: 10.1016/j.jpsychires.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Blanco-Hinojo L, Pujol J, Harrison BJ, Macià D, Batalla A, Nogué S, et al. Attenuated frontal and sensory inputs to the basal ganglia in cannabis users. Addict Biol. 2016 doi: 10.1111/adb.12370. [DOI] [PubMed] [Google Scholar]
- 42.Tomasi D, Volkow ND. Functional connectivity hubs in the human brain. Neuroimage. 2011;57:908–917. doi: 10.1016/j.neuroimage.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomasi D, Volkow ND. Mapping small-world properties through development in the human brain: Disruption in schizophrenia. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomasi D, Volkow N. Aging and Functional Brain Networks. Mol Psychiatry. 2012;17:549–558. doi: 10.1038/mp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomasi D, Volkow ND. Abnormal functional connectivity in children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;71:443–450. doi: 10.1016/j.biopsych.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konova AB, Moeller SJ, Tomasi D, Goldstein RZ. Effects of chronic and acute stimulants on brain functional connectivity hubs. Brain Res. 2015;1628:147–156. doi: 10.1016/j.brainres.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volkow ND, Wang G-J, Kollins SH, Wigal TL, Newcorn JH, Telang F, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. Jama. 2009;302:1084–91. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, Mcguire P, Bullmore ET. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain. 2014;137:2382–2395. doi: 10.1093/brain/awu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martz ME, Trucco EM, Cope LM, Hardee JE, Jester JM, Zucker RA, Heitzeg MM. Association of Marijuana Use With Blunted Nucleus Accumbens Response to Reward Anticipation. JAMA Psychiatry. 2016;370:2219–2227. doi: 10.1001/jamapsychiatry.2016.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Hell HH, Vink M, Ossewaarde L, Jager G, Kahn RS, Ramsey NF. Chronic effects of cannabis use on the human reward system: An fMRI study. Eur Neuropsychopharmacol. 2010;20:153–163. doi: 10.1016/j.euroneuro.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 53.Frank MJ. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. J Cogn Neurosci. 2005;17:51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- 54.Vukadinovic Z, Herman MS, Rosenzweig I. Cannabis, psychosis and the thalamus: A theoretical review. Neurosci Biobehav Rev. 2013;37:658–667. doi: 10.1016/j.neubiorev.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 55.Buchy L, Cannon TD, Anticevic A, Lyngberg K, Cadenhead KS, Cornblatt BA, et al. Evaluating the impact of cannabis use on thalamic connectivity in youth at clinical high risk of psychosis. BMC Psychiatry. 2015;15:276. doi: 10.1186/s12888-015-0656-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. Proc Natl Acad Sci U S A. 2009;106:13016–21. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Machielsen MW, Veltman DJ, van den Brink W, de Haan L. Comparing the effect of clozapine and risperidone on cue reactivity in male patients with schizophrenia and a cannabis use disorder: A randomized fMRI study. J Psychopharmacol. 2017 doi: 10.1177/0269881114527357. in press. [DOI] [PubMed] [Google Scholar]
- 58.Herkenham M, Lynn AB, Litrle MD, Johnsont MR, Melvin LS, De Costa BR, Riceo KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schierenbeck T, Riemann D, Berger M, Hornyak M. Effect of illicit recreational drugs upon sleep: Cocaine, ecstasy and marijuana. Sleep Med Rev. 2008;12:381–389. doi: 10.1016/j.smrv.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Conroy DA, Kurth ME, Strong DR, Brower KJ, Stein MD. Marijuana Use Patterns and Sleep among Community-Based Young Adults. J Addict Dis. 2016;35:0. doi: 10.1080/10550887.2015.1132986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chabrol H, Duconge E, Roura C, Casas C. Relations between anxious, depressive and borderline symptomatology and frequency of cannabis use and dependence. Encephale. 2004;30:141–146. doi: 10.1016/s0013-7006(04)95424-3. [DOI] [PubMed] [Google Scholar]
- 62.Hasler BP, Insana SP, James JA, Germain A. Evening-type military veterans report worse lifetime posttraumatic stress symptoms and greater brainstem activity across wakefulness and REM sleep. Biol Psychol. 2013;94:255–262. doi: 10.1016/j.biopsycho.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 64.Bossong MG, Niesink RJM. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol. 2010;92:370–385. doi: 10.1016/j.pneurobio.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 65.Pagliaccio D, Barch DM, Bogdan R, Wood PK, Lynskey MT, Heath AC, Agrawal A. Shared Predisposition in the Association Between Cannabis Use and Subcortical Brain Structure. JAMA psychiatry. 2015;72:994–1001. doi: 10.1001/jamapsychiatry.2015.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bloomfield MAP, Morgan CJA, Egerton A, Kapur S, Curran HV, Howes OD. Dopaminergic function in cannabis users and its relationship to cannabis-induced psychotic symptoms. Biol Psychiatry. 2014;75:470–478. doi: 10.1016/j.biopsych.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 67.Thompson JL, Urban N, Slifstein M, Xu X, Kegeles LS, Girgis RR, et al. Striatal dopamine release in schizophrenia comorbid with substance dependence. Mol Psychiatry. 2013;18:909–915. doi: 10.1038/mp.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


