Abstract
BACKGROUND
Cortisol has potent effects on learning and neuroplasticity, but little is known about its effects on negative memory biases in depression. Animal models show that aversive caregiving alters effects of glucocorticoids (primarily corticosterone in rodents; cortisol in primates) on learning and neuroplasticity into adulthood.
METHODS
We investigated whether history of childhood emotional abuse (EA) moderated effects of cortisol administration (CORT) vs. placebo on emotional memory formation in depression. Participants included 75 unmedicated women with varying levels of depression severity and/or EA history. In a double-blind crossover investigation, we used fMRI to measure effects of CORT (vs. Placebo) on neural function during emotional memory formation.
RESULTS
CORT eliminated the well-known relation between depression severity and negative memory bias, a finding explained by EA severity. For women with history of severe EA, CORT reduced depression-related negative memory bias and normalized recall for pleasant stimuli. EA severity also moderated CORT’s effects on neural function: in women with history of severe EA, CORT increased activation in supplementary motor area (SMA) during unpleasant relative to pleasant pictures. Additionally, SMA activation predicted reduced negative bias for pictures encoded during CORT.
CONCLUSIONS
These results suggest that increasing cortisol signaling may be neurocognitively beneficial in depressed women with history of maltreatment. The findings corroborate prior research suggesting that presence or absence of adverse caregiving is etiologically important in depression. The findings suggest potential neurocognitive mechanisms of therapeutics targeting cortisol signaling, which show promise in treating affective disorders.
Keywords: cortisol, depression, emotional memory, emotional abuse, fMRI, supplementary motor area
INTRODUCTION
Childhood maltreatment is a predisposing factor for psychiatric disorders and triggers various biobehavioral alterations (1–3). In animal models, aversive caregiving causes lifelong changes in offspring, including alterations in neuroplasticity and stress-related neuromodulators, such as glucocorticoid (GC) hormones (i.e., cortisol and corticosterone) (1, 4–6). GCs modulate neuroplastic mechanisms through binding at both Type I (mineralocorticoid receptors; MRs) and Type II (glucocorticoid receptors; GRs) corticosteroid receptors (7–9). It is not possible to directly measure neural signaling of GCs at corticosteroid receptors in humans, and little is known about how aversive caregiving alters cortisol’s effects on neural function in humans.
Early life stress in rodents causes lifelong alterations in GC cellular signaling (4, 6, 10), which is partially due to influences of maternal care on epigenetic programming of GR expression (11). Furthermore, aversive caregiving in rodents causes alterations in GCs’ effects on learning and neuroplasticity (4, 6, 10). Corticosterone eliminates reductions in hippocampal long-term potentiation associated with early experience of poor maternal care (4). Sullivan and colleagues showed that infant rats exposed to paired maternal odor-shock conditioning exhibited deficits in fear learning at later developmental stages, which were rescued with corticosterone administration (6). These findings suggest that GC administration may eliminate deficient neuroplastic processes in adult rats who experienced aversive parenting.
Recent research highlights the role of altered neuroplastic mechanisms in animal models of psychiatric disorders (12). It has been hypothesized that altered effects of stress and GCs on neuroplastic mechanisms are key etiological factors in depression (8, 13). Consistent with their effects on neuroplasticity, GCs have potent effects on emotional memory in humans (14–16). Despite decades of research implicating cortisol alterations in depression, relatively little is known about the role of GCs in biased emotional memory formation, which is a core feature of depression (17–20).
We used pharmacological manipulation of cortisol (CORT) vs. placebo during fMRI scanning and memory formation for emotional pictures. Recall of pictures encoded during fMRI was tested two days after scanning. Because GCs’ effects on emotional memory vary based on sex (21), only women were included. Women were recruited across a range of severity of childhood emotional abuse (EA) and depressive symptomatology. We hypothesized that CORT would reduce depression-related memory bias, and that EA would moderate this effect. We further hypothesized that brain regions associated with adrenal function and emotional memory would be related to CORT’s effects on memory bias. Our lab previously found that cortisol’s effects on hippocampal function were related to memory bias in depression (18). Regions involved in emotional enhancement of memory (amygdala and medial prefrontal cortex) are influential in corticosteroids’ effects on learning (22, 23). Recent research in nonhuman primates suggests a key role for premotor cortex (PMC) and supplementary motor area (SMA) in regulating adrenal function (24). These areas project to the adrenal gland and likely regulate the adrenal medulla sympathetic system, which moderates corticosteroids’ effects on learning (24). Because sympathetic nervous system activation affects emotional memory (25, 26), we tested whether variation in salivary alpha-amylase (sAA), as an index of sympathetic functioning (27), was related to CORT’s neurocognitive effects.
METHODS AND MATERIALS
Participants
We recruited women between the ages of 18 and 45 with varying levels of EA and/or depression (see Supplemental Information for inclusion/exclusion criteria). We did not specifically recruit women with anxiety disorder or PTSD, but these were not exclusionary. Of 85 eligible participants, 80 completed the study. Full data were available for 75 participants (mean age 27.6; 75% White, 17% Asian, 5% Black, 8% Hispanic). Data was lost due to experimenter error (1 participant), scanner malfunction (1 participant), fMRI signal drop out (2 participants), and a medical condition (1 participant). The University of Wisconsin Health Sciences IRB approved study procedures. Participants provided written informed consent and were paid for participation.
Measurement of Childhood Emotional Abuse and Depressive Symptoms
We retrospectively assessed childhood emotional abuse (EA), which predicts negative cognitive bias and incidence of depression over and above severity of physical and sexual abuse (28–31). To index severity of EA, we used the Emotional Abuse subscale of the Childhood Trauma Questionnaire (32). The Emotional Abuse subscale captures mild to severe aversive caregiving. The CTQ is a well-validated instrument that can be used continuously or to categorize participants into groups, which aids in interpreting results (32). Standard CTQ cut scores were used to categorize participants based on severity of EA. Of the final sample, 15 women experienced moderate-to-extreme (“severe”), 14 experienced low-to-moderate (“moderate”), and 46 experienced none-to-minimal (“minimal”) childhood EA. We examined timing of EA prior to age 18 using a life history calendar (33), which confirmed that all women endorsing EA experienced abuse prior to menarche, many of whom experienced ongoing emotional abuse from early childhood through adolescence.
Consistent with NIMH Research Domain Criteria (RDoC) framework (34), we recruited women with a range of severity of depressive symptomatology. Psychopathology was assessed using the SCID-I/P for DSM-IV-TR (35) with additional questions to assess DSM-5 criteria. Table 1 indicates DSM-5 diagnoses with respect to EA groups (full listing of DSM-5 diagnoses in Supplemental Information). We indexed depression severity by taking the average of Beck Depression Inventory-II (BDI-II) (36) scores from the two scan sessions. As in previous research (37, 38), we applied a square-root transformation of BDI-II data to reduce negative skew and undue influence of extreme BDI-II scores. BDI-II scores presented in scatter plots were back-transformed to preserve BDI-II score range. Because of the tight association between childhood EA and adult depression (28–31), it is not possible to disentangle variation in EA and depressive symptomatology (correlation in this sample is r(73) = 0.45, p < 0.01). Nonetheless, our goal was to recruit a sample in which EA and depressive symptoms were not entirely overlapping (see Table 1).
Table 1.
Demographic and Clinical Characteristics
| Characteristics | CTQ Emotional Abuse Groups | Group Comparisons | ||
|---|---|---|---|---|
| Minimal (n = 46) |
Moderate (n = 14) |
Severe (n = 15) |
||
| Age, Years | 26.1 ±6.4 | 31.4 ±7.1 | 28.6 ±7.9 | F(2, 74) = 3.37, p = .04 |
| Lifetime Depressive Disorder | 23 (50.0) | 9 (64.3) | 14 (93.3) | χ2(2, N =75) = 9.02, p = .01 |
| Current Depressive Disorder | 12 (26.1) | 7 (50.0) | 13 (86.7) | χ2(2, N =75) = 17.35, p < .001 |
| Current Anxiety Disorder | 12 (26.1) | 6 (42.9) | 9 (60.0) | χ2(2, N =75) = 7.02, p = .03 |
| Current PTSD | 0 | 3 (21.4) | 6 (40.0) | χ2(2, N =75) = 17.53, p < .001 |
| Racea, b | χ2(4, N =75) = 1.72, p = .79 | |||
| White | 34 (73.9) | 9 (64.3) | 13 (86.7) | |
| Asian | 8 (17.4) | 3 (21.4) | 2 (13.3) | |
| African American | 3 (6.5) | 1 (7.1) | 0 | |
| Unknown | 1 (2.2) | 1 (7.1) | 0 | |
| Ethnicitya | χ2(2, N =75) = 2.27, p = .32 | |||
| Hispanic/Latina | 4 (8.7) | 2 (14.3) | 0 | |
| Not Hispanic/Latina | 42 (91.3) | 11 (78.6) | 15 (100) | |
| Unknown | 0 | 1 (7.1) | 0 | |
| Education Levelc | 4.4 ±1.4 | 5.2 ±1.1 | 4.8 ±1.3 | F(2, 74) = 2.04, p = .14 |
| Childhood Caregivers’ Education Levelc | 4.5 ±1.7 | 4.9 ±1.2 | 4.9 ±1.8 | F(2, 74) = 0.56, p = .57 |
Values are mean ±SD or n (%).
CTQ, Childhood Trauma Questionnaire; PTSD, Post-Traumatic Stress Disorder.
Chi-squared tests confirmed the CTQ Emotional Abuse groups did not significantly differ by racial or ethnic composition.
Because of rounding, percentages may not total 100.
Education categories: 1 = Less than high school; 2 = High school diploma or equivalent (i.e., GED); 3 = Some college, no degree; 4 = Associate’s degree; 5 = Bachelor’s degree; 6 = Master’s degree; 7 = Doctoral degree.
Procedure
After screening, participation included a mock scan for acclimation to fMRI, two fMRI scans, and two recall test sessions (Figure 1). Cortisol was pharmacologically manipulated with oral administration of 20 mg encapsulated cortisol (CORT; i.e., hydrocortisone) vs. an identically appearing placebo capsule. Drug was administered 50 minutes after participants arrived and 90 minutes prior to the memory encoding task in the scanner. CORT and Placebo administration order was randomized and double-blinded. Capsules were prepared by the University of Wisconsin Pharmaceutical Research Center. The two scanning sessions began at ~4:15 PM (earliest start was 4:03 PM and latest start was 4:43 PM) and were typically separated by 1 week.
Figure 1. Study procedures and timeline.
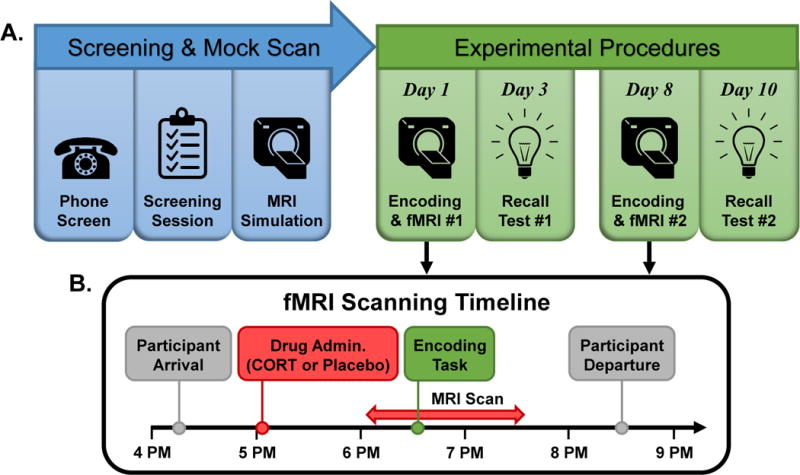
(A) Study timeline. Phone-based and in-person screening determined participant eligibility, after which participants completed a mock scan. In a double-blind crossover design, participants completed two memory encoding/fMRI sessions, which were typically separated by one week. During each memory encoding/fMRI session, participants were administered a pill containing either 20 mg cortisol (CORT) or placebo. Two days after each encoding/fMRI session participants returned to the lab to be tested for memory recall of encoded pictures. (B) Encoding/fMRI sessions timeline. Encoding/fMRI sessions were conducted in the evening when endogenous cortisol levels are low. Participants arrived at ~4:15 PM, received study drug at 5:05 PM, and underwent MRI scanning from ~6:05 PM to ~7:35 PM, with the encoding task beginning at ~6:35 PM. Participants departed the lab at ~8:30 PM. Saliva samples were collected throughout, including a sample immediately after the encoding task, to index salivary cortisol and alpha-amylase levels.
Memory Encoding Task and Free Recall for Emotional Pictures
For memory encoding tasks, we used emotionally-normed pictures from the International Affective Picture System (39) to create two sets of 84 pictures, which were matched on valence and arousal. Each set contained 28 each of pleasant, unpleasant, and neutral pictures. During each fMRI scan the encoding task entailed presenting one of the two picture sets. Participants engaged in a simple emotional response task during encoding, rating each picture as “positive,” “neutral,” or “negative” using a button box (Current Designs, Philadelphia, PA). Pictures were presented for 5s each, followed by a 3s response period and a jittered inter-stimulus-interval ranging from 4–9s. Stimuli were back-projected onto a screen inside the scanner bore.
Recall test sessions were conducted in the afternoon to early evening, within 48 hours of scanning sessions (except for one subject in the “minimal” EA group whose post-CORT recall session was 9 days after scanning). Free recall for pictures encoded during scans was assessed using methods based on our laboratory’s prior studies (14, 40). Participants were given 10 min to provide brief written descriptions of as many pictures as they could recall. If participants had not exhausted recall by 10 min, they were given additional time. Scoring was conducted blind to drug condition, depression severity, and EA. Recall descriptions were coded by two scorers. Any discrepancies between scorers were rectified by a third individual (RMH).
Salivary Analytes
Saliva samples were collected for measurement of cortisol and sAA (Table 2). We used Salivettes (Sarstedt, Nümbrecht, Germany) according to recommendations for cortisol and sAA collection (27). Cortisol concentrations were measured with high sensitivity chemiluminescence immunoassay (IBL International, Hamburg, Germany). sAA concentrations were measured with an enzyme kinetic method. Intra- and inter-assay CVs were below 8% for cortisol and below 11% for sAA. Log-transformed values for sAA and cortisol were used in analyses.
Table 2.
Salivary Analytes and Recall Performance
| Measure | CTQ Emotional Abuse Groups, Mean ± SD | Group Comparisons | ||
|---|---|---|---|---|
| Minimal (n = 46) |
Moderate (n = 14) |
Severe (n = 15) |
||
| Post-Encoding Salivary Cortisol Levels, nmol/L | ||||
| Placebo | 1.3 ±1.6 | 1.7 ±2.1 | 1.2 ±0.8 | F(2, 74) = 0.33, p = .72 |
| CORT | 55.4 ±33.6 | 54.2 ±32.7 | 49.7 ±40.6 | F(2, 74) = 0.15, p = .86 |
| Post-Encoding sAA Levels, U/ml | ||||
| Placebo | 191.8 ±155.9 | 172.9 ±155.6 | 227.9 ±189.8 | F(2, 74) = 0.61, p = .55 |
| CORT | 166.0 ±115.1 | 221.7 ±225.9 | 180.5 ±131.9 | F(2, 74) = 0.10, p = .90 |
| Recall for Pleasant Pictures | ||||
| Placebo | 10.8 ±4.1a | 11.9 ±6.1a | 7.5 ±3.4a, b | F(2, 74) = 4.09, p = .02 |
| CORT | 11.1 ±4.9 | 12.9 ±4.8 | 10.4 ±4.0b | F(2, 74) = 0.11, p = .35 |
| Recall for Unpleasant Pictures | ||||
| Placebo | 12.9 ±4.8 | 13.4 ±4.9 | 11.7 ±3.8 | F(2, 74) = 0.54, p = .59 |
| CORT | 12.8 ±4.6 | 13.5 ±3.7 | 12.3 ±2.6 | F(2, 74) = 0.32, p = .72 |
CORT, Cortisol administration; CTQ, Childhood Trauma Questionnaire; sAA, salivary alpha-amylase.
Note: Means for salivary cortisol and sAA samples taken immediately after the memory encoding task show no differences related to severity of childhood emotional abuse (EA). Means for recall performance show that a) participants with severe EA recalled fewer pleasant pictures encoded during Placebo administration than did participants with minimal or moderate EA,
F(2,74) = 4.09, p = .02, and b) participants with severe EA recalled more pleasant pictures encoded during CORT than Placebo administration,
t(14) = 2.34, p = .03.
Image Collection and Preprocessing
Brain images were collected using a 3T General Electric MRI scanner (Discovery MR750; GE Medical Systems, Waukesha, WI) equipped with an 8 channel RF coil (GE Healthcare, Waukesha, WI). Structural anatomical brain data were acquired using a T1-weighted BRAVO pulse sequence (TI: 450ms, TR/TE/flip:8.16ms/3.2ms/12°, matrix:256×256×160, FOV:215.6mm, slice thickness:1mm). Functional data were acquired using a series of sagittal T2*-weighted echo-planar images (TR/TE/flip:2150ms/22ms/79°, matrix:64×64:40, FOV:224mm, slice thickness:3mm with 0.5mm gap).
Data were processed in AFNI, unless otherwise indicated (41). First, a rigid-body volume registration was implemented to compensate for participants’ motion (3dvolreg, 4th volume as the base image volume for registration). Sagittal field maps were collected via a 3D SPGR sequence (TR/TE/flip:5ms/1.8ms/7°, matrix:192×128×44, FOV:230mm, slice thickness:3.5mm) to geometrically unwarp EPIs to reduce distortion caused by magnetic field inhomogeneities using FMRIB Software Library (42) and IDEAL sequence (43).
Functional EPI data were corrected for slice-timing differences (3dTshift), aligned to their respective T1-weighted anatomical image (align_epi_anat.py), and transformed to Talairach atlas space (44) in a single interpolation to 2×2×2mm3 voxels. The 3D+time series were despiked (3dDespike) and spatially smoothed with a 3D Gaussian kernel (FWHM=6mm; 3dmerge). Nuisance regressors, including the 6 estimated motion realignment parameters and constant and linear trend, were removed (3dDeconvolve). Activation was estimated using multiple linear regression (3dDeconvolve) modeling the picture viewing for each valence as a 5-second block convolved with the hemodynamic response function (the “BLOCK” function in AFNI’s 3dDeconvolve).
Data Analysis
Memory bias is expressed as , where UR and PR represent unpleasant and pleasant pictures recalled, respectively, to index the difference in recall for unpleasant and pleasant pictures while adjusting for variation in overall recall. Each participant has two memory bias scores, one for pictures encoded during CORT and one for pictures encoded during Placebo. Full models were analyzed first using continuous measures of EA severity. For purposes of interpretation, we also present results from analyses using categories for severity of EA based on standard CTQ cut scores (i.e., “EA groups”). We used ANCOVA (Proc GLM, SAS version 9.4, Cary, NC) to test whether EA, Depression Severity, or their interaction moderated effects of Drug (CORT vs. placebo) on memory bias. To identify neural activation related to effects of CORT on memory bias, we analyzed whole-brain fMRI data (during unpleasant vs. pleasant trials) using linear mixed-effects analysis in AFNI (3dLME) in 2 separate analyses, first including EA as continuous and second as a categorical independent variable, along with Drug (CORT vs. Placebo) as a categorical independent variable and depression severity and memory bias as continuous covariates. “Activation” refers to greater signal change during unpleasant with respect to pleasant stimuli. Data were corrected for multiple comparisons by first choosing an individual voxel p-value threshold (p = 0.005 for main effects and p = 0.01 for EA × Drug interaction in view of increased noise across multiple scan sessions), and then performing a Monte Carlo simulation (3dClustSim) to determine the minimum cluster size to achieve a false positive rate of 0.05. This simulation uses an estimate of the autocorrelation function, determined from pre-processed data (3dClustSim), in order to address recent concerns over inflated false positive rates (45, 46). To examine whether neural findings could be explained by variation in sympathetic nervous system arousal, we conducted hierarchical regression with fMRI signal change as the dependent variable and the following predictors: sAA level at mid-scan; severity of childhood EA; and memory bias. We also confirmed that variation in endogenous cortisol (using baseline and mid-scan samples from Placebo day) did not moderate findings.
RESULTS
Memory Bias
EA and depression severity jointly moderated effects of CORT on memory bias, as illustrated by an EA × depression severity × Drug (CORT vs. Placebo) interaction, F(1,71)=5.13, p<.03 (Figure 2). For pictures encoded during Placebo, depression severity was associated with negatively biased memory, F(1,69)=12.57, p<.001 (Figure 2a), which remains significant when accounting for variation in endogenous cortisol, p’s<.001. CORT abolished the relation between depression severity and memory bias, r(73)=.08, n.s. (Figure 2b). For pictures encoded during CORT, neither depression severity nor EA predicted negative memory bias, p’s>.43. However, the interaction between depression severity and EA predicted memory bias for pictures encoded during CORT, F(2,69)=5.63, p<.01 (Figure 2b),1 which remains significant when accounting for variation in endogenous cortisol, p’s<.01. CORT reversed the relation between depression severity and emotional memory bias only in women with severe EA (Figure 2b). Group means for recall performance (Table 2) show that women with severe EA had a deficit in recall for pleasant pictures encoded during Placebo, which was normalized for pictures encoded during CORT, t(14)=2.34, p=.03.
Figure 2. In participants with severe childhood emotional abuse (EA), CORT reversed the association between depression severity and negative memory bias.
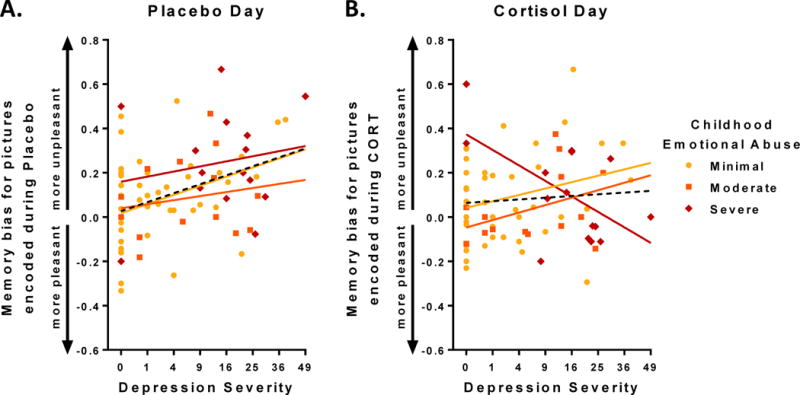
Dashed black lines represent regression fit for full sample. Colored lines represent regression fits for subjects with differing levels of childhood EA (see legend). Depression severity indexed with BDI-II (see text for details). (A) For pictures encoded following Placebo, women with greater levels of depression recalled more unpleasant relative to pleasant pictures, i.e. showed greater negative memory bias, r(74) = .38, p < .001. (B) Following CORT, there was no such correlation at the group level, r(74) = .08, n.s., illustrating a Drug (CORT vs. Placebo) × Depression Severity interaction effect, F(1,73)=5.31, p<.03, in which CORT abolishes the relation between Depression Severity and negative memory bias for the entire sample. Childhood EA moderated the effect of CORT: in women with history of severe EA, CORT reversed the relation between depression severity and negative memory bias, F(2,74) = 5.63, p < .01.
Effects of CORT on Neural Activation
Neural activation during Placebo was not related to EA or depression severity. During CORT, EA but not depression severity moderated neural activation in left supplementary motor area (SMA), inferior parietal, and cerebellar clusters (p<.05 corrected for multiple comparisons; Table 3). In addition, significant interactions (p<.05 corrected for multiple comparisons) showed that EA moderated effects of Drug in a cortical region spanning left lateral (PMC) and medial (SMA) extent of Brodmann area 6 (BA6), thalamus, and in right PMC (spanning from BA6 to BA40; Table 3). Interactive effects of Drug × EA are displayed in Figure 3 for the model using the continuous measure of EA. Figure 4 displays effects categorically for EA groups, illustrating the Drug × EA interaction in detail. Posthoc testing showed that CORT enhanced SMA activation in participants with severe childhood EA, F(2,74)=4.33, p<.02 (Figure 4),2 which remains significant when accounting for variation in endogenous cortisol, p’s<.02. The thalamus cluster significant for the EA × Drug interaction was centered in the pulvinar nucleus; we observed activation in the pulvinar in women with minimal EA during both CORT and Placebo, whereas women with moderate and severe EA showed pulvinar activation only during CORT (Figure 4).
Table 3.
Moderating Effects of Childhood Emotional Abuse (EA) Severity on BOLD Signal Contrasts for Unpleasant > Pleasant
| Region | Volume (voxels) |
Coordinate (peak) |
Coordinate (CM) |
Stat (peak) |
P Value (corrected) |
|---|---|---|---|---|---|
| Placebo | |||||
| -(none)- | – | – | – | – | – |
| CORT | |||||
| Left SMA | 3409 | (−4, −17, 62) | (−8, −25, 51) | Z = 4.8 | .01 |
| Cerebellum | 1023 | (24, −33, −47) | (18, −35, −45) | Z = 4.1 | .01 |
| Left Inf. Parietal Lobule | 781 | (−55, −28, 31) | (−46, −39, 33) | Z = 4.4 | .02 |
| CORT vs. Placebo | |||||
| Left BA6--whole clustera | 1425 | (−38, −13, 52) | (−18, 23, 51) | F = 12.4 | .01 |
| --medial aspect (SMA) | – | (−4, −17, 60) | (−6, −17, 58) | F = 10.2 | – |
| Bilateral Thalamus | 1188 | (14, −33, 7) | (4, −25, 5) | F = 11.2 | .02 |
| Right BA6/BA40 | 1148 | (28, −46, 59) | (28, −38, 52) | F = 9.1 | .02 |
SMA, supplementary motor area; BA, Brodmann area; CM, center of mass.
The full extent of left BA6 activation for EA × Drug (CORT vs. Placebo) includes both SMA and premotor cortex (PMC), and peak significance falls in PMC. Due to significant main effect of EA on CORT day in SMA and for theoretical reasons (24), we also report coordinates for the local maxima in SMA alone.
Figure 3. Brain regions showing significant Drug (CORT vs. Placebo) × EA interaction for the model using the continuous measure of EA (i.e., CTQ Emotional Abuse subscale scores).
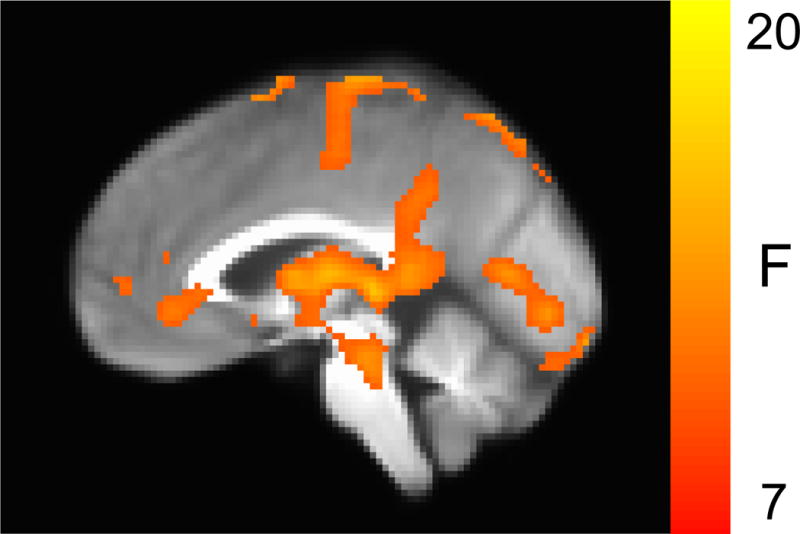
Also see Table 3 & Figure 4, which display the Drug × EA interaction in more detail for EA groups (based on CTQ Emotional Abuse cut scores).
Figure 4. CORT-related neural response to unpleasant > pleasant pictures varied by severity of childhood emotional abuse (EA).
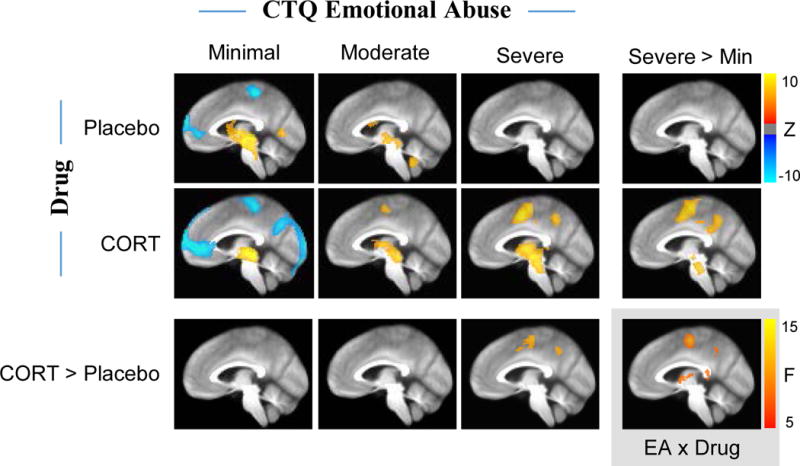
Shown are sagittal group difference maps for unpleasant > pleasant activations (centered on left SMA) for EA groups (Minimal, Moderate, and Severe) during Placebo and CORT conditions. Colors reflect Z-scores for CORT > Placebo and Severe > Minimal activation. At bottom-right (gray box), colors reflect F-values for significant clusters (SMA & thalamus) in the EA × Drug interaction. For cluster statistics see Table 3. Individual voxel p-value threshold for Placebo & CORT conditions was p < .005; a less stringent threshold of p < .01 was used for CORT > Placebo.
Neural Function, Sympathetic Activation, and Memory
Because recent research in non-human primates suggests that SMA has top-down control over the sympathetic adrenal medullary system (24), we further interrogated the left SMA cluster (which showed a main effect of EA during CORT and a EA × Drug interaction) by testing whether sympathetic activation (sAA levels during scanning) accounted for SMA activation. Hierarchical regression showed that during Placebo, SMA activation was not related to predictors (i.e., sAA level during Placebo scan, severity of EA, memory bias for pictures encoded during Placebo, or interactions among these variables; all p’s>.26). However, during CORT, sAA levels accounted for significant variance in SMA activation (Table 4). Even after accounting for sAA levels during the CORT scan, SMA activation significantly related to severity of EA and memory bias for pictures encoded during CORT but not interactions among these variables (Table 4). Zero-order correlations show that greater CORT-day activation of SMA was associated with higher sAA, r(73)=.29, p=.01, more severe EA, r(73)=.28, p=.01, and more positive memory bias for pictures encoded during CORT, r(73)=−.26, p=.02. When tested separately for each of the three EA groups, the relation between CORT-day SMA activation and memory bias was in the same direction for each group although not significantly in those with moderate or severe EA, Minimal: r(44)= −.33, p=.02, Moderate: r(12)=−.13, n.s., Severe: r(13)= −.21, n.s.
Table 4.
Hierarchical Regression Predicting Signal Change in left SMA during CORTa
| R2 | Increment in R2 | F | p valueb | |
|---|---|---|---|---|
| Mid - scan sAA | 0.08 | 0.08 | 6.71 | .01 |
| EA | 0.15 | 0.07 | 5.52 | .02 |
| Memory Bias | 0.21 | 0.07 | 5.80 | .02 |
| sAA × EA | 0.25 | 0.04 | 3.13 | .08 |
| sAA × Memory Bias | 0.25 | – | 0.18 | .67 |
| sAA × EA × Memory Bias | 0.25 | – | 0.05 | .83 |
CORT, Cortisol administration; EA, emotional abuse; CTQ, Childhood Trauma Questionnaire; sAA, salivary alpha-amylase; SMA, supplementary motor area.
This table shows results from a hierarchical regression predicting variation in left SMA (supplementary motor area) activation during CORT. The findings listed in the table show that during CORT, SMA activation is significantly related to mid-scan sAA (which indexes sympathetic activation). Even after accounting for variation in sAA, SMA activation during CORT is significantly related to severity of childhood EA (CTQ Emotional Abuse) and emotional memory bias for pictures encoded during CORT. The full model is significant, F(6,74) = 3.78, p < .003, and accounts for 25% of the variance in SMA signal change during CORT, which represents a medium-to-large effect size.
p values represent significance of increment in R2 attributable to each variable.
DISCUSSION
We replicated the well-known relationship between greater depression severity and negative bias in emotional memory formation for pictures encoded during Placebo administration (19). CORT eliminated the relation between depression severity and memory bias, a finding that was explained by severity of childhood EA. In women with severe EA, CORT reversed the relation between depression severity and memory bias and normalized deficient memory formation for pleasant pictures. Thus, in women with severe childhood EA, CORT normalized the emotional memory alterations associated with depression.
Our findings are consistent with rodent data showing that GCs can ameliorate alterations in neuroplasticity in rats with history of aversive caregiving (4, 6, 10). Studies in rodents suggest that prior experiences can shift the dose-response relationship between GCs and plasticity (47, 48). For instance, rats previously exposed to aversive caregiving show impairments in learning and neuroplasticity with low GCs but enhancements with high GCs (4, 6, 10). We can speculate that experience of aversive parenting may induce a cascade that results in persistent cellular resistance to GCs at non-stress levels, which may normalize or shift to cellular sensitization to GCs when elevated. Future research is needed to substantiate this speculation.
Our findings may suggest that individuals with vs. without childhood EA show different neurocognitive responses to acute GC elevations. Heim and colleagues have suggested that depressed adults with vs. without a history of childhood adversity represent different subtypes, in part because depressed patients with a history of adversity are more likely to show peripheral HPA dysregulation, negative feedback deficits, and GC resistance than depressed patients without adversity (49). A number of studies suggest that different measures of GC resistance are interrelated, and that peripheral measures of GC resistance may predict variation in GC effects on cognition (50–52). Future research should address to what extent peripheral measures of GC resistance reflect altered neural signaling of cortisol.
CORT’s Effects on Neural Function
We did not replicate altered CORT effects on hippocampal function in depression (18), and we did not observe effects of CORT on frontolimbic circuitry directly involved in emotional memory (53–55). However, we found that severity of childhood EA moderated effects of CORT on activity in a cluster spanning left PMC and SMA. In women with severe EA, CORT increased SMA activation. We further showed that sympathetic activation was related to SMA activation during CORT but not during Placebo administration. Despite this relation, levels of sympathetic activation did not account for relations between SMA activation and severity of EA or memory bias, each of which were uniquely related to SMA activation on the CORT but not Placebo day. Greater SMA activation was associated with less negative memory bias for pictures encoded on the CORT day. The increase in SMA activation observed may have been “protective” in contributing to the CORT-driven normalization of emotional memory in women with severe EA.
Several lines of research support the interpretation that SMA may mediate relations between stress neuromodulators and behavior. Recent retrograde tracing studies in nonhuman primates identified SMA and adjacent Cingulate Motor Areas (CMAs) (56) as the cortical regions most densely projecting to adrenal medulla (24). Comparable regions in rodents (i.e., dorsomedial prefrontal areas) are necessary for GC (57) and noradrenergic (58) regulation of adrenal output, and stress alters the role of these circuits in behavior (59). SMA, along with pre-SMA and CMAs, are associated with integrating cognitive and affective inputs, especially negative affect, in motor planning (60) and ultimately guiding response selection (61). SMA recruitment may reflect a less passive, more action-oriented (62–64) cognitive response to negative stimuli. Human neuroimaging studies have found SMA involvement in directed emotion regulation (65) and in first-person emotional memory (66), supporting a role in embodied motor influence on emotional memory formation.
Severity of childhood EA also moderated CORT’s effects on thalamic activity centered in the pulvinar nucleus. Lesion and neuroimaging studies suggest a role for the pulvinar in emotional gating of attention and binding salient emotional features in working memory, and alterations of these processes in mood disorders (67, 68). Future research should investigate whether CORT’s effects on emotional cognition are related to SMA, PMC, and thalamic activation.
Mechanisms of Cortisol Neural Signaling
Endogenous cortisol levels measured during Placebo did not differ by EA severity, which suggests that neurocognitive effects of cortisol may be altered even if circulating cortisol concentrations are relatively unaffected. Mechanisms that govern neuronal response to GCs are extremely complex, and recent research has elucidated many factors that alter GC effects on neuronal function (7, 13, 69). Extensive description of mechanisms underlying variation in GC cellular signaling is outside the scope of this paper and reviewed elsewhere (8, 70, 71). Briefly, genetic and functional variations in MRs and GRs, and many other mechanisms such as FKBP5 functioning, affect GC cellular signaling (8, 70, 71). Moreover, early life adversity is associated with alterations in both MR and GR function in relation to neuroplasticity and memory (72, 73). The current study does not address whether GC action at MRs or GRs (or any other intracellular or membrane-bound mechanisms) are responsible for observed effects. However, the memory encoding task was conducted in the evening when endogenous cortisol levels are low and presumably GRs (with low affinity for cortisol) and MRs (with high affinity for cortisol) were not fully occupied by circulating cortisol prior to drug administration (8). We can speculate that the normalization of emotional memory bias in women with history of severe EA may be due to CORT’s actions at MRs (8), although findings may be due to CORT’s effects at GRs or other mechanisms.
Central noradrenergic activation and peripheral sympathetic activation moderate GC effects on emotional memory (26, 74). Interestingly, SMA has recently been identified as the cortical region most densely projecting to adrenal medulla in nonhuman primates, suggesting a key role in controlling adrenal medulla (sympathetic) output (24). We found that sAA levels (indexing sympathetic activation) were related to SMA activation during CORT, but did not account for the relation between SMA and EA or memory bias. These findings are suggestive of sympathetic nervous system involvement in cortisol’s effects on activation in SMA, but this involvement does not fully account for the association between SMA activation and psychological variables under study.
Implications for Psychiatric Treatment
The MR agonist fludrocortisone may be beneficial in augmenting treatment for non-psychotic depression (8, 75), whereas GR antagonism (e.g., with mifepristone) may be beneficial in psychotic depression (76). While these treatment-related findings are promising, efforts to identify effective psychiatric medications directly targeting GC signaling have been largely unsuccessful (77). This may be partially attributable to the sheer number and complexity of factors affecting neuronal and cognitive responses to GCs (7, 8, 70, 78). Greater success may stem from investigating how severity of early life adversity moderates efficacy of experimental therapeutics. Prior research shows that depression associated with prior experience of aversive caregiving requires a different treatment regimen than depression in the absence of early adversity (79, 80). The current study paradigm can be adapted to investigate mechanisms and potential therapeutic efficacy of corticosteroid receptor ligands, and whether their neural actions vary based upon childhood adversity.
Limitations
Although our overall study was well-powered (N=75), our primary findings reflected differences for the small subgroup of women (n=15) with severe EA. The findings need to be replicated with a larger sample of individuals with severe EA with a wide range of severity of affective pathology. Results may have differed if women with a wider age range, or more severe depression, or men were included (21), or if other methods regarding dose and timing were used (64). Our study is not adequately powered to test whether PTSD, anxiety, or depression specifiers of melancholia or atypical depression moderate CORT’s effects. Finally, though the findings suggest that pharmacologically elevated cortisol may be beneficial in depressed women with EA, the findings do not necessarily suggest that acutely heightened endogenous cortisol due to a stressor would be beneficial.
Summary
Consistent with NIMH RDoC framework, our study integrates across multiple levels of information (cognitive, hormonal, neural). In women with varying levels of depression, EA moderated CORT’s effects on neural activation during memory encoding for emotional stimuli, and depression-related memory bias for these stimuli. The findings suggest that increasing cortisol signaling may be neurocognitively beneficial in women with depression who experienced aversive caregiving in childhood. These findings support past research suggesting that presence or absence of childhood maltreatment is etiologically important in depression, which should be taken into account when developing experimental therapeutics targeting cortisol signaling (79, 80). These findings also support previous research suggesting that aversive caregiving has the potential to fundamentally alter effects of GCs on neurocognitive function into adulthood (4, 6, 10).
Supplementary Material
Acknowledgments
This research was funded by grants to H. Abercrombie from NIMH (R01MH094478) and the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation; a Training Program in Emotion Research grant (5T32MH018931-25, PI: Richard J. Davidson) and a Dissertation Completion Fellowship to C. Frost; and a NCCIH grant (T32AT003378) to E. Walsh. Data were collected with the assistance of the ICTR Mobile Research Team, which is supported by the Clinical and Translational Science Award (CTSA) program through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The NIH had no further role in study design; in the collection, analysis, and interpretation of the data; in the writing of the report; or in the decision to submit the article for publication.
We thank all of the volunteers who participated in this study, as well as A. Blumenfeld, C. Siwik, M. Dennison, A. Ehlers, C. Ernstoff, S. Goldberg, M. Kalambokidis, A. Lang, J. Nelson, E. Osterbauer, R. Svoboda, R. Vohnoutka, A. Winter, Lane Neuroimaging Laboratory staff, and ICTR Mobile Research Team for assistance with data collection. We thank Christine Heim, Marilyn J. Essex, Ned H. Kalin, and Richard J. Davidson for consultation and advice. We thank Clemens Kirschbaum’s laboratory for conducting salivary cortisol and alpha-amylase assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov: Depression, Adversity, and Stress Hormones (DASH) Study; https://clinicaltrials.gov/ct2/show/NCT03195933; NCT03195933.
FINANCIAL DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
EA significantly moderated effects of CORT when menstrual phase and age were included in the model, F(2,50)=6.40, p<.005.
Posthoc test remained significant when menstrual phase and age were included in the model, F(2,62)=3.99, p<.03.
References
- 1.Drury SS, Sánchez MM, Gonzalez A. When mothering goes awry: Challenges and opportunities for utilizing evidence across rodent, nonhuman primate and human studies to better define the biological consequences of negative early caregiving. Horm Behav. 2016;77:182–192. doi: 10.1016/j.yhbeh.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carr CP, Martins CMS, Stingel AM, Lemgruber VB, Juruena MF. The role of early life stress in adult psychiatric disorders: A systematic review according to childhood trauma subtypes. J Nerv Ment Dis. 2013;201:1007–1020. doi: 10.1097/NMD.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 3.Martins CMS, Von Werne Baes C, Tofoli SM, Juruena MF. Emotional abuse in childhood is a differential factor for the development of depression in adults. J Nerv Ment Dis. 2014;202:774–782. doi: 10.1097/NMD.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 4.Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, et al. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cattaneo A, Macchi F, Plazzotta G, Veronica B, Bocchio-Chiavetto L, Riva MA, et al. Inflammation and neuronal plasticity: a link between childhood trauma and depression pathogenesis. Frontiers in cellular neuroscience. 2015;9:1–12. doi: 10.3389/fncel.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moriceau S, Raineki C, Holman JD, Holman JG, Sullivan RM. Enduring neurobehavioral effects of early life trauma mediated through learning and corticosterone suppression. Front Behav Neurosci. 2009;3:1–13. doi: 10.3389/neuro.08.022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Ast VA, Cornelisse S, Marin MF, Ackermann S, Garfinkel SN, Abercrombie HC. Modulatory mechanisms of cortisol effects on emotional learning and memory: Novel perspectives. Psychoneuroendocrinology. 2013;38:1874–1882. doi: 10.1016/j.psyneuen.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Kloet ER, Otte C, Kumsta R, Kok L, Hillegers MHJ, Hasselmann H, et al. Stress and depression: a crucial role of the mineralocorticoid receptor. J Neuroendocrinol. 2016;28:1–12. doi: 10.1111/jne.12379. [DOI] [PubMed] [Google Scholar]
- 9.Herbert J, Goodyer IM, Grossman AB, Hastings MH, de Kloet ER, Lightman SL, et al. Do corticosteroids damage the brain? J Neuroendocrinol. 2006;18:393–411. doi: 10.1111/j.1365-2826.2006.01429.x. [DOI] [PubMed] [Google Scholar]
- 10.Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, Joels M. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol Learn Mem. 2009;92:292–300. doi: 10.1016/j.nlm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 12.Morris SE, Rumsey JM, Cuthbert BN. Rethinking mental disorders: the role of learning and brain plasticity. Restorative neurology and neuroscience. 2014;32:5–23. doi: 10.3233/RNN-139015. [DOI] [PubMed] [Google Scholar]
- 13.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 14.Abercrombie HC, Speck NS, Monticelli RM. Endogenous cortisol elevations are related to memory facilitation only in individuals who are emotionally aroused. Psychoneuroendocrinology. 2006;31:187–196. doi: 10.1016/j.psyneuen.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Wolf OT. Stress and memory in humans: twelve years of progress? Brain Res. 2009;1293:142–154. doi: 10.1016/j.brainres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- 17.Kuehl LK, Wolf OT, Driessen M, Schlosser N, Fernando SC, Wingenfeld K. Effects of cortisol on the memory bias for emotional words? A study in patients with depression and healthy participants using the Directed Forgetting task. J Psychiatr Res. 2017;92:191–198. doi: 10.1016/j.jpsychires.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Abercrombie HC, Jahn AL, Davidson RJ, Kern S, Kirschbaum C, Halverson J. Cortisol’s effects on hippocampal activation in depressed patients are related to alterations in memory formation. J Psychiatr Res. 2011;45:15–23. doi: 10.1016/j.jpsychires.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gotlib IH, Krasnoperova E. Biased information processing as a vulnerability factor for depression. Behav Ther. 1998;29:603–617. [Google Scholar]
- 20.Wingenfeld K, Wolf OT. Effects of cortisol on cognition in major depressive disorder, posttraumatic stress disorder and borderline personality disorder - 2014 Curt Richter Award Winner. Psychoneuroendocrinology. 2015;51:282–295. doi: 10.1016/j.psyneuen.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology. 2001;26:711–720. doi: 10.1016/s0306-4530(01)00025-7. [DOI] [PubMed] [Google Scholar]
- 22.van Stegeren AH, Roozendaal B, Kindt M, Wolf OT, Joels M. Interacting noradrenergic and corticosteroid systems shift human brain activation patterns during encoding. Neurobiol Learn Mem. 2010;93:56–65. doi: 10.1016/j.nlm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 23.van Stegeren AH. Imaging stress effects on memory: a review of neuroimaging studies. Can J Psychiatry. 2009;54:16–27. doi: 10.1177/070674370905400105. [DOI] [PubMed] [Google Scholar]
- 24.Dum RP, Levinthal DJ, Strick PL. Motor, cognitive, and affective areas of the cerebral cortex influence the adrenal medulla. Proc Natl Acad Sci U S A. 2016;113:9922–9927. doi: 10.1073/pnas.1605044113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Carroll RE, Drysdale E, Cahill L, Shajahan P, Ebmeier KP. Memory for emotional material: a comparison of central versus peripheral beta blockade. J Psychopharmacol. 1999;13:32–39. doi: 10.1177/026988119901300104. [DOI] [PubMed] [Google Scholar]
- 26.Segal SK, Simon R, McFarlin S, Alkire M, Desai A, Cahill LF. Glucocorticoids interact with noradrenergic activation at encoding to enhance long-term memory for emotional material in women. Neuroscience. 2014;277:267–272. doi: 10.1016/j.neuroscience.2014.06.059. [DOI] [PubMed] [Google Scholar]
- 27.Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology. 2009;34:486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T. The long-term health consequences of child physical abuse, emotional abuse, and neglect: A systematic review and meta-analysis. PLoS Med. 2012;9:1–31. doi: 10.1371/journal.pmed.1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibb BE, Abela JRZ. Emotional Abuse, Verbal Victimization, and the Development of Children’s Negative Inferential Styles and Depressive Symptoms. Cognitive Ther Res. 2007;32:161–176. [Google Scholar]
- 30.Gibb BE, Alloy LB, Abramson LY, Rose DT, Whitehouse WG, Donovan P, et al. History of Childhood Maltreatment, Negative Cognitive Styles, and Episodes of Depression in Adulthood. Cognitive Ther Res. 2001;25:425–556. [Google Scholar]
- 31.Shapero BG, Black SK, Liu RT, Klugman J, Bender RE, Abramson LY, et al. Stressful life events and depression symptoms: The effect of childhood emotional abuse on stress reactivity. J Clin Psychol. 2014;70:209–223. doi: 10.1002/jclp.22011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 33.Caspi A, Moffitt TE, Thornton A, Freedman D, Amell JW, Harrington H, et al. The life history calendar: A research and clinical assessment method for collecting retrospective event-history data. Int J Method Psych. 1996;6:101–114. [Google Scholar]
- 34.Insel TR. The NIMH Research Domain Criteria (RDoC) Project: Precision medicine for psychiatry. Am J Psychiatry. 2014;171:395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- 35.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 36.Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 37.Roelofs J, van Breukelen G, de Graaf LE, Beck AT, Arntz A, Huibers MJH. Norms for the Beck Depression Inventory (BDI-II) in a large Dutch community sample. J Psychopathol Behav. 2013;35:93–98. [Google Scholar]
- 38.van Minnen A, Wessel I, Verhaak C, Smeenk J. The relationship between autobiographical memory specificity and depressed mood following a stressful life event: a prospective study. The British journal of clinical psychology / the British Psychological Society. 2005;44:405–415. doi: 10.1348/014466505X29648. [DOI] [PubMed] [Google Scholar]
- 39.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Instruction manual and affective ratings Technical Report A-5. Gainsville, FL: The Center for Research in Psychophysiology, University of Florida; 2001. [Google Scholar]
- 40.Abercrombie HC, Wirth MM, Hoks RM. Inter-individual differences in trait negative affect moderate cortisol’s effects on memory formation: Preliminary findings from two studies. Psychoneuroendocrinology. 2012;37:693–701. doi: 10.1016/j.psyneuen.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 42.Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, et al. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45:S173–186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 43.Reeder SB, Pineda AR, Wen Z, Shimakawa A, Yu H, Brittain JH, et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn Reson Med. 2005;54:636–644. doi: 10.1002/mrm.20624. [DOI] [PubMed] [Google Scholar]
- 44.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- 45.Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. fMRI clustering and false-positive rates. Proc Natl Acad Sci U S A. 2017;114:E3370–E3371. doi: 10.1073/pnas.1614961114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. FMRI clustering in AFNI: False-positive rates redux. Brain Connect. 2017;7:152–171. doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joëls M, Krugers HJ. LTP after stress: up or down? Neural Plast. 2007;2007:93202. doi: 10.1155/2007/93202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. P Natl Acad Sci USA. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heim C, Newport DJ. Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Rohleder N, Wolf JM, Wolf OT. Glucocorticoid sensitivity of cognitive and inflammatory processes in depression and posttraumatic stress disorder. Neurosci Biobehav Rev. 2010;35:104–114. doi: 10.1016/j.neubiorev.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Menke A, Arloth J, Putz B, Weber P, Klengel T, Mehta D, et al. Dexamethasone stimulated gene expression in peripheral blood is a sensitive marker for glucocorticoid receptor resistance in depressed patients. Neuropsychopharmacology. 2012;37:1455–1464. doi: 10.1038/npp.2011.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jarcho MR, Slavich GM, Tylova-Stein H, Wolkowitz OM, Burke HM. Dysregulated diurnal cortisol pattern is associated with glucocorticoid resistance in women with major depressive disorder. Biol Psychol. 2013;93:150–158. doi: 10.1016/j.biopsycho.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roozendaal B, McReynolds JR, Van der Zee EA, Lee S, McGaugh JL, McIntyre CK. Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J Neurosci. 2009;29:14299–14308. doi: 10.1523/JNEUROSCI.3626-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cerebral Cortex. 2004;14:647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- 55.Canli T, Zhao Z, Desmond JE, Glover G, Gabrieli JDE. fMRI identifies a network of structures correlated with retention of positive and negative emotional memory. Psychobiology. 1999;27:441–452. [Google Scholar]
- 56.Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 57.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. Journal of Neuroscience. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Radley JJ, Williams B, Sawchenko PE. Noradrenergic innervation of the dorsal medial prefrontal cortex modulates hypothalamo-pituitary-adrenal responses to acute emotional stress. J Neurosci. 2008;28:5806–5816. doi: 10.1523/JNEUROSCI.0552-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Radley JJ. Toward a limbic cortical inhibitory network: implications for hypothalamic-pituitary-adrenal responses following chronic stress. Front Behav Neurosci. 2012;6:1–10. doi: 10.3389/fnbeh.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 62.Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the Human Medial Frontal Cortex in Task Switching: A Combined fMRI and TMS Study. J Neurophysiol. 2002;87:2577–2592. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- 63.Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat Neurosci. 2007;10:240–248. doi: 10.1038/nn1830. [DOI] [PubMed] [Google Scholar]
- 64.Vogel S, Fernández G, Joëls M, Schwabe L. Cognitive adaptation under stress: A case for the mineralocorticoid receptor. Trends Cogn Sci. 2016;20:192–203. doi: 10.1016/j.tics.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Domes G, Schulze L, Bottger M, Grossmann A, Hauenstein K, Wirtz PH, et al. The neural correlates of sex differences in emotional reactivity and emotion regulation. Hum Brain Mapp. 2010;31:758–769. doi: 10.1002/hbm.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eich E, Nelson AL, Leghari MA, Handy TC. Neural systems mediating field and observer memories. Neuropsychologia. 2009;47:2239–2251. doi: 10.1016/j.neuropsychologia.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 67.Arend I, Henik A, Okon-Singer H. Dissociating emotion and attention functions in the pulvinar nucleus of the thalamus. Neuropsychology. 2015;29:191–196. doi: 10.1037/neu0000139. [DOI] [PubMed] [Google Scholar]
- 68.Okon-Singer H, Hendler T, Pessoa L, Shackman AJ. The neurobiology of emotion-cognition interactions: fundamental questions and strategies for future research. Front Hum Neurosci. 2015;9:1–14. doi: 10.3389/fnhum.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Joëls M, Karst H, DeRijk R, de Kloet ER. The coming out of the brain mineralocorticoid receptor. Trends Neurosci. 2008;31:1–7. doi: 10.1016/j.tins.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 70.Pariante CM. Risk factors for development of depression and psychosis. Glucocorticoid receptors and pituitary implications for treatment with antidepressant and glucocorticoids. Ann N Y Acad Sci. 2009;1179:144–152. doi: 10.1111/j.1749-6632.2009.04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Provencal N, Binder EB. The neurobiological effects of stress as contributors to psychiatric disorders: focus on epigenetics. Current Opinion in Neurobiology. 2015;30:31–37. doi: 10.1016/j.conb.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 72.Vogel S, Gerritsen L, van Oostrom I, Arias-Vásquez A, Rijpkema M, Joëls M, et al. Linking genetic variants of the mineralocorticoid receptor and negative memory bias: Interaction with prior life adversity. Psychoneuroendocrinology. 2014;40:181–190. doi: 10.1016/j.psyneuen.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 73.Turecki G, Meaney MJ. Effects of the social environment and stress on glucocorticoid receptor gene methylation: A systematic review. Biol Psychiatry. 2016;79:87–96. doi: 10.1016/j.biopsych.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roozendaal B, Okuda S, de Quervain DJ, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 75.Otte C, Wingenfeld K, Kuehl LK, Kaczmarczyk M, Richter S, Quante A, et al. Mineralocorticoid Receptor Stimulation Improves Cognitive Function and Decreases Cortisol Secretion in Depressed Patients and Healthy Individuals. Neuropsychopharmacology. 2015;40:386–393. doi: 10.1038/npp.2014.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Flores BH, Kenna H, Keller J, Solvason HB, Schatzberg AF. Clinical and biological effects of mifepristone treatment for psychotic depression. Neuropsychopharmacology. 2006;31:628–636. doi: 10.1038/sj.npp.1300884. [DOI] [PubMed] [Google Scholar]
- 77.Schatzberg AF. Development of new psychopharmacological agents for depression and anxiety. Psychiatr Clin North Am. 2015;38:379–393. doi: 10.1016/j.psc.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 78.Kalafatakis K, Russell GM, Zarros A, Lightman SL. Temporal control of glucocorticoid neurodynamics and its relevance for brain homeostasis, neuropathology and glucocorticoid-based therapeutics. Neurosci Biobehav Rev. 2016;61:12–25. doi: 10.1016/j.neubiorev.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 79.Williams LM, Debattista C, Duchemin AM, Schatzberg AF, Nemeroff CB. Childhood trauma predicts antidepressant response in adults with major depression: data from the randomized international study to predict optimized treatment for depression. Translational psychiatry. 2016;6:1–7. doi: 10.1038/tp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A. 2003;100:14293–14296. doi: 10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miller R, Stalder T, Jarczok M, Almeida DM, Badrick E, Bartels M, et al. Corrigendum to “The CIRCORT database: Reference ranges and seasonal changes in diurnal salivary cortisol derived from a meta-dataset comprised of 15 field studies” [PNEC 73C (2016) 16-23] Psychoneuroendocrinology. 2017;76:226–227. doi: 10.1016/j.psyneuen.2016.11.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


